Abstract
Disulfide exchange is an important bioconjugation tool, enabling chemical modification of peptides and proteins containing free cysteines. We previously reported the synthesis of a macromer bearing an activated disulfide and its incorporation into hydrogels. Despite their ability to diffuse freely into hydrogels, larger proteins were unable to undergo in-gel disulfide exchange. In order to understand this phenomenon, we synthesized four different activated disulfide-bearing model compounds (Mn = 300 Da-10 kDa) and quantified their rate of disulfide exchange with a small peptide (glutathione), a moderate-sized protein (β-lactoglobulin), and a large protein (bovine serum albumin) in four different pH solutions (6.0, 7.0, 7.4, and 8.0) to mimic biological systems. Rate constants of exchange depend significantly on the size and accessibility of the thiolate. pH also significantly affects the rate of reaction, with the faster reactions occurring at higher pH. Surprisingly, little difference in exchange rates is seen between macromolecular disulfides of varying size (Mn = 2 kDa – 10kDa), although all undergo exchange more slowly than their small molecule analogue (MW = 300 g/mol). The maximum exchange efficiencies (% disulfides exchanged after 24 h) are not siginificantly affected by thiol size or pH, but somewhat affected by disulfide size. Therefore, while all three factors investigated (pH, disulfide size and thiolate size) can influence the exchange kinetics and extent of reaction, the size of the thiolate and its accessibility plays the most significant role.
Keywords: Disulfide exchange, sterics, photodegradable, polymer, protein, peptide
Introduction
Biomolecules commonly possess free functional groups such as thiols, amines, alcohols and carboxylic acids which can participate in bioconjugation reactions. One important method of biomolecule conjugation is disulfide exchange between a thiol on a biomolecule and an activated disulfide present on another molecule. In 1978 researchers used protein thiolation to create proteinprotein conjugates.1 Jou and colleagues later described synthetic schemes for creating disulfide bonds linking protein antigens and cell membranes.2 By 1993 Woghiren and colleagues had modified polyethylene glycol with a pyridine disulfide for reaction with cysteine residues of proteins, finally forming protein-polymer conjugates for potential therapeutic delivery.3 Currently, disulfide exchange is the basis for the preparation of a range of conjugates focused on targeted or controlled delivery of therapeutics. Cysteine-containing proteins, drugs, or small molecules are reversibly linked or covalently tethered to plain polymers4,5 or polymers with advanced functions such as pH sensitivity,6 temperature sensitivity,7 enzyme sensitivity,8 membrane penetrability,6 and photodegradability9. Disulfide exchange is a well understood biological mechanism in the context of protein tertiary structure stabilization, but when applied to create new bioconjugates, scientists need to understand what factors influence the rates and efficiencies of disulfide exchange between proteins or peptides and disulfides linked to uniquely synthesized polymers.
In a recent report, we attached an activated disulfide linkage to photodegradable macromers, and used this linkage to incorporate active biomolecules (glutathione, CRGDSC, transforming growth factor-β1, bovine serum albumin) into hydrogels, and subsequently controlled their release with light.9 Surprisingly, despite the ability to diffuse freely into and out of the hydrogel, large proteins were unable to undergo disulfide exchange in the gel. Increasing the distance between the network backbone and the activated disulfide improved exchange, but it was still only limited to ∼10% using bovine serum albumin. We were therefore interested in the origin of this limitation. Is the longer polymer chain sterically shielding the activated disulfide from the thiolate anion? Or is the thiolate anion buried within the protein and sterically hindered from reacting with the activated disulfide?
Sterics likely play an important role in exchange rates, but variations in both the primary structure of peptides and proteins as well as higher order folding and 3D shape make it difficult to understand the origin of the limitation in disulfide exchange. Shaked and colleagues anticipated the role of sterics in rates of disulfide exchange within proteins but paired only low molecular weight thiols with disulfide bonds within different proteins (disulfide bonds whose locations were not well known at the time).10 They concluded that sterics were not the basis of differences in exchange rates for those proteins and thiols, but rather local electrostatic effects. However, Zavialov and colleagues later found that sterics do affect the rate of disulfide exchange in this situation with a dimerized heat-shock protein, Hsp25, and glutathione as the thiolate. The disulfide bond in Hsp25 is buried in a pocket and the thiolate anion does not have easy access.11 While it may be straightforward to visualize a small thiolate anion being sterically restricted from entering a pocket in a protein, if the thiolate is present on the surface of a large protein and the disulfide bond is at the end of a long polymer how does the role of sterics change? Here we investigated the role of both the peptide/protein and the disulfide in order to understand the effects of each on the kinetics of disulfide exchange. Specifically, we paired varying sizes of free-thiolcontaining biomolecules (glutathione (GSH), bovine serum albumin (BSA), and β-lactoglobulin (βLG)) with different sized activated-disulfide, and observed changes in rate of exchange. The utility of disulfide exchange for labeling diverse biomolecules or sequestering them into gels relies on the elucidation of these particular interactions.
Experimental
Materials
N-ethyl-N′-(3-dimethylaminopropyl)carbodiimide HCl (EDC) (CreoSalus), dichloromethane (DCM) (Fisher Scientific, 99.9%), (4-dimethyl-aminopyridine (DMAP) (Acros Organics, 99%), succinic anhydride (Acros Organics, 99%), magnesium sulfate anhydrous (EMD), poly(ethylene glycol) (Mn = 10,000) (Sigma Aldrich), poly(ethylene glycol) (Mn = 4,000) (Mallinckrodt Chemicals), poly(ethylene glycol) (Mn = 2,000) (Fluka), poly(ethylene) glycol (Mn = 400) (Aldrich), glutathione (GSH, reduced, Acros, 98%), β- lactoglobulin (Sigma Aldrich >90%), bovine serum albumin (BSA, Heat Shock treated, Biotech Grade, Fisher BioReagents, 99%) dithiothreitol (DTT, Fisher BioReagents, 99%). DCM was distilled from CaH2 under N2 and stored under N2 in a dry, airfree flask. All other chemicals were used as received. 2-(Pyridin-2-yldisulfanyl)ethyl succinate (SUCC-SS-Pyr) and 4-(2-methoxy-5-nitro-4-(1-(4-oxo-4-(2-(pyridin-2-yldisulfanyl)ethoxy)butanamido)ethyl)phenoxy)butanoic acid (PDG-SS-Pyr)were synthesized as previously reported.9
Techniques
All reactions were performed under an N2 atmosphere using a Schlenk line unless noted otherwise. 1H NMR spectra (δ ppm) were recorded on a Bruker Biospin Ultrashield 300MHz NMR Spectrometer.
Synthesis
PEG400-SS-Pyr
PEG 400-bis-4-(4-acetyl-2-methoxy-5-nitrophenoxy)butanoate: 4-(4-acetyl-2-methoxy-5-nitrophenoxy)butanoic acid (3.37mmol), poly(ethylene glycol) 400 (1.5mmol), EDC (3.86mmol), DMAP (0.57mmol) were dissolved in DCM (20mL) and stirred overnight at room temperature reaction was complete and confirmed by 1H NMR. The reaction was diluted with DCM and washed with water three times and then passed through a basic alumina plug to get rid of excess 4-(4-acetyl-2-methoxy-5-nitrophenoxy)butanoic acid and water. The product was dried by lyophilization. Product is a dark yellow liquid (67% yield). 1H NMR.(CDCl3): δ=7.46 (s, Ar-H ortho to Ar-NO2), δ=7.26 (s, Ar-H meta to Ar-NO2), δ=4.12 (t, Ar-O-(CH2)3C(O)OCH2CH2(OCH2CH2)7CH2CH2OC(O)(CH2)3-O-Ar), δ=4.03 (t, Ar-O-CH2—), δ=3.83 (s, Ar-OCH3), δ=3.50 (m, —CH2CH2O—), δ=2.73 (t, Ar-OCH2CH2CH2C(O)O—), δ=2.36 (s, Ar-C(O)CH3), δ=2.06 (m, Ar-OCH2CH2CH2C(O)O—)
PEG 400-bis-4-(4-(1-hydroxyethyl)-2-methoxy-5-nitrophenoxy)butanoate: PEG 400-bis-4-(4-acetyl-2-methoxy-5-nitrophenoxy)butanoate (0.25mmol), NaBH4 (1.58mmol), were dissolved in DCM (20mL) and stirred overnight at 4 °C to room temperature until reaction was complete and confirmed by 1H NMR. The reaction was diluted with DCM and washed with water three times and then dried with magnesium sulfate. The product was collected by rotary evaporation. The product is a dark yellow liquid (81% yield). 1H NMR.(CDCl3): δ=7.55 (s, Ar-H ortho to Ar-NO2), δ=7.29 (s, Ar-H meta to Ar-NO2), δ=5.54 (m, Ar-CHCH3), δ=4.24 (t, Ar-O-(CH2)3C(O)OCH2CH2(OCH2CH2) 7CH2CH2OC(O)(CH2)3-O-Ar), δ=4.11 (t, Ar-O-CH2—), δ=3.97 (s, Ar-OCH3), δ=3.63 (m, —CH2CH2O—), δ=2.58 (t, Ar-OCH2CH2CH2C(O)O—), δ=2.18 (m, Ar-OCH2CH2CH2C(O)O—), δ=1.54 (d, Ar-CHCH3)
PEG400-bis-4-(2-methoxy-5-nitro-4-(1-((4-oxo-4-(2-(pyridin-2-yldisulfanyl)ethoxy)butanoyl)oxy)ethyl)phenoxy)butanoate: 2-(Pyridin-2-yldisulfanyl)ethyl succinate (0.050mmol), DMAP (0.09mmol), EDC (0.16mmol), and PEG 400-bis-4-(4-(1-hydroxyethyl)-2-methoxy-5-nitrophenoxy)butanoate (0.087mmol), were diluted in 20mL of DCM and stirred with activated molecular sieves and and stirred overnight at room temperature reaction was complete and confirmed by 1H NMR. The product was dried by rotary evaporation and dissolved in water to rid of the excess 2-(Pyridin-2-yldisulfanyl)ethyl succinate. The product was then dried by lyophalization. The product is a dark-yellow liquid (18%) 1H NMR.(CDCl3): δ=8.45 (d, C5H5N ortho from the N), δ=7.67 (m, C5H5N para from the N), δ=7.64 (d, C5H5N meta from N closer to SS), δ=7.56 (s, Ar-H ortho to Ar-NO2), δ=7.09 (m, C5H5N meta from N farther to SS), δ=7.01 (s, Ar-H meta to Ar-NO2), δ=6.45 (m, Ar-CHCH3), δ=4.31 (t, Ar-O-(CH2)3C(O)OCH2CH2(OCH2CH2) 7CH2CH2OC(O)(CH2)3-O-Ar), δ=4.25 (t, Ar-O-CH2—), δ=3.97 (s, Ar-OCH3), δ=3.71 (d, C5H5N-SSCH2CH2OC(O)—), δ=3.61 (m, —CH2CH2O—), δ=3.01 (d, C5H5N-SSCH2CH2OC(O)CH2CH2C(O)O-Ar), δ=2.61 (d, C5H5N-SSCH2CH2OC(O)CH2CH2C(O)O-Ar), δ=2.54 (t, Ar-OCH2CH2CH2C(O)O—), δ=2.17 (m, Ar-OCH2CH2CH2C(O)O—), δ=1.61 (d, Ar-C(O)CH3)
PEG2000-SS-Pyr
PEG 2000-bis-4-(4-acetyl-2-methoxy-5-nitrophenoxy)butanoate: 4-(4-acetyl-2-methoxy-5-nitrophenoxy)butanoic acid (2.22mmol), poly(ethylene glycol) 2000 (1.00mmol), EDC (2.53mmol), DMAP (0.40mmol) were dissolved in DCM (20mL) and stirred overnight at room temperature reaction was complete and confirmed by 1H NMR. The reaction was diluted with DCM and washed with sodium bicarbonate and brine three times each and then passed through a basic alumina plug to get rid of excess 4-(4-acetyl-2-methoxy-5-nitrophenoxy)butanoic acid. The product was then precipitated into cold diethyl ether and collected with a fritted filter. It was dried by lyophilization. Product is a yellow tinted powder (84% yield) 1H NMR.(CDCl3): δ=7.59 (s, Ar-H ortho to Ar-NO2), δ=6.73 (s, Ar-H meta to Ar-NO2), δ=4.24 (t, Ar-O-CH2)3C(O)OCH2CH2(OCH2CH2)43CH2CH2OC(O)(CH2)3-O-Ar), δ=4.14 (t, Ar-O-CH2—), δ=3.94 (s, Ar-OCH3), δ=3.62 (m, —CH2CH2O—), δ=2.57 (t, Ar-OCH2CH2CH2C(O)O—), δ=2.47 (s, Ar-C(O)CH3), δ=2.18 (m, Ar-OCH2CH2CH2C(O)O—)
PEG 2000-bis-4-(4-(1-hydroxyethyl)-2-methoxy-5-nitrophenoxy)butanoate: PEG 4000-bis-4-(4-acetyl-2-methoxy-5-nitrophenoxy)butanoate (0.84mmol), NaBH4 (7.02mmol), were dissolved in DCM (20mL) and stirred overnight at 4 °C to room temperature until reaction was complete by 1H NMR. The reaction was diluted with DCM and washed with sodium bicarbonate and brine three times each and then dried rotary evaporation. The product is a yellow powder (50% yield). 1H NMR.(CDCl3): δ=7.50 (s, Ar-H ortho to Ar-NO2), δ=7.28 (s, Ar-H meta to Ar-NO2), δ=5.49 (m, Ar-CHCH3), δ=4.20 (t, Ar-O-(CH2)3C(O)OCH2CH2(OCH2CH2) 43CH2CH2OC(O)(CH2)3-O-Ar), δ=4.06 (t, Ar-O-CH2—), δ=3.92 (s, Ar-OCH3), δ=3.59 (m, —CH2CH2O—), δ=2.53 (t, Ar-OCH2CH2CH2C(O)O—), δ=2.13 (m, Ar-OCH2CH2CH2C(O)O—), δ=1.47 (d, Ar-CHCH3)
PEG2000-bis-4-(2-methoxy-5-nitro-4-(1-((4-oxo-4-(2-(pyridin-2-yldisulfanyl)ethoxy)butanoyl)oxy)ethyl)phenoxy)butanoate: 2-(Pyridin-2-yldisulfanyl)ethyl succinate (0.065mmol), DMAP (0.0090mmol), EDC (0.13mmol), and PEG 2000-bis-4-(4-(1-hydroxyethyl)-2-methoxy-5-nitrophenoxy)butanoate (0.021mmol), were diluted in 20mL of DCM and stirred with activated molecular sieves overnight at room temperature. The reaction was diluted with DCM and washed with sodium bicarbonate, brine, and water three times each and dried in magnesium sulfate. The product was then dried by rotary evaporation and then precipitated in diethyl ether. Product was dried by lyophilization. The product is a light yellow powder (40%) 1H NMR.(CDCl3): δ=8.46 (d, C5H5N ortho from the N), δ=7.67 (m, C5H5N para from the N), δ=7.64 (d, C5H5N meta from N closer to SS), δ=7.56 (s, Ar-H ortho to Ar-NO2), δ=7.09 (m, C5H5N meta from N farther to SS), δ=7.01 (s, Ar-H meta to Ar-NO2), δ=6.45 (m, Ar-CHCH3), δ=4.32 (t, Ar-O-(CH2)3C(O)OCH2CH2(OCH2CH2) 43CH2CH2OC(O)(CH2)3-O-Ar), δ=4.24 (t, Ar-O-CH2—), δ=3.97 (s, Ar-OCH3), δ=3.86 (d, C5H5N -SSCH2CH2OC(O)—), δ=3.63 (m, —CH2CH2O—), δ=3.01 (d, C5H5N-SSCH2CH2OC(O)CH2CH2C(O)O-Ar), δ=2.61 (d, C5H5N-SSCH2CH2OC(O)CH2CH2C(O)O-Ar), δ=2.57 (t, Ar-OCH2CH2CH2C(O)O—), δ=2.17 (m, Ar-OCH2CH2CH2C(O)O—), δ=1.59 (d, Ar-C(O)CH3)
PEG4000-SS-Pyr
PEG 4000-bis-4-(4-acetyl-2-methoxy-5-nitrophenoxy)butanoate: 4-(4-acetyl-2-methoxy-5-nitrophenoxy)butanoic acid (0.57mmol), poly(ethylene glycol) 4000 (0.25mmol), EDC (0.64mmol), DMAP (0.08mmol) were dissolved in DCM (15mL) and stirred overnight at room temperature reaction was complete and confirmed by 1H NMR. The reaction was diluted with DCM and passed through a basic alumina plug to get rid of excess 4-(4-acetyl-2-methoxy-5-nitrophenoxy)butanoic acid. The product was then precipitated into cold diethyl ether and collected with a fritted filter. It was then dried by lyophilization. Product is a yellow-tinted powder (61% yield)
PEG 4000-bis-4-(4-(1-hydroxyethyl)-2-methoxy-5-nitrophenoxy)butanoate: PEG 4000-bis-4-(4-acetyl-2-methoxy-5-nitrophenoxy)butanoate (0.065mmol), NaBH4 (0.13mmol), were dissolved in DCM (20mL) and stirred overnight at 4 °C to room temperature until reaction was complete by 1H NMR. The reaction was diluted with DCM and washed with water three times and then dried with magnesium sulfate. The product was then dried by rotary evaporation. The product is a dark yellow powder (96% yield). 1H NMR.(CDCl3): δ=7.53 (s, Ar-H ortho to Ar-NO2), δ=7.30 (s, Ar-H meta to Ar-NO2), δ=5.53 (m, Ar-CHCH3), δ=4.21 (t, Ar-O-(CH2)3C(O)OCH2CH2(OCH2CH2) 89CH2CH2OC(O)(CH2)3-O-Ar), δ=4.10 (t, Ar-O-CH2—), δ=3.94 (s, Ar-OCH3), δ=3.61 (m, — CH2CH2O —), δ=2.52 (t, Ar- OCH2CH2CH2C (O)O—), δ=2.12 (m, Ar- OCH2CH2CH2C (O)O—), δ=1.51 (d, Ar- CHCH3)
PEG 4000-bis-4-(2-methoxy-5-nitro-4-(1-((4-oxo-4-(2-(pyridin-2-yldisulfanyl)ethoxy)butanoyl)oxy)ethyl)phenoxy)butanoate: 2-(Pyridin-2-yldisulfanyl)ethyl succinate (0.031mmol), DMAP (0.025mmol), EDC (0.054mmol), and PEG 4000-bis-4-(4-(1-hydroxyethyl)-2-methoxy-5-nitrophenoxy)butanoate (0.0090mmol), were diluted in 20mL of DCM and stirred with activated molecular sieves overnight at room temperature until reaction was complete by 1H NMR. The reaction was diluted with DCM and washed with sodium bicarbonate and brine three times each and dried in magnesium sulfate. The product was then dried by rotary evaporation and then precipitated in diethyl ether. Product was placed in lyophalizer to be dried overnight. The product is a light yellow powder (56%) 1H NMR.(CDCl3): δ=8.45 (d, C5H5N ortho from the N), δ=7.66 (m, C5H5N para from the N), δ=7.64 (d, C5H5N meta from N closer to SS), δ=7.55 (s, Ar-H ortho to Ar-NO2), δ=7.09 (m, C5H5N meta from N farther to SS), δ=7.01 (s, Ar-H meta to Ar-NO2), δ=6.46 (m, Ar-CHCH3), δ=4.32 (t, Ar-O-(CH2)3C(O)OCH2CH2(OCH2CH2) 89CH2CH2OC(O)(CH2)3-O-Ar), δ=4.08 (t, Ar-O-CH2—), δ=3.97 (s, Ar-OCH3), δ=3.87 (d, C5H5N -SSCH2CH2OC(O)—), δ=3.63 (m, —CH2CH2O—), δ=3.00 (d, C5H5N-SSCH2CH2OC(O)CH2CH2C(O)O-Ar), δ=2.63 (d, C5H5N-SSCH2CH2OC(O)CH2CH2C(O)O-Ar), δ=2.54 (t, Ar-OCH2CH2CH2C(O)O—), δ=2.18 (m, Ar-OCH2CH2CH2C(O)O—), δ=1.59 (d, Ar-C(O)CH3)
PEG10000-SS-Pyr
PEG 10000-bis-4-(4-acetyl-2-methoxy-5-nitrophenoxy)butanoate: 4-(4-acetyl-2-methoxy-5-nitrophenoxy)butanoic acid (0.44mmol), poly(ethylene glycol) 10000 (0.20mmol), EDC (0.55mmol), DMAP (0.13mmol) were dissolved in DCM (20mL) and stirred overnight at room temperature reaction was complete and confirmed by 1H NMR. The reaction was diluted with DCM and washed with sodium bicarbonate and brine three times each and then passed through a basic alumina plug to get rid of excess 4-(4-acetyl-2-methoxy-5-nitrophenoxy)butanoic acid. The product was then precipitated into cold diethyl ether then dried by lyophilization. Product is a yellow-tinted powder (40% yield) 1H NMR.(CDCl3): δ=7.50 (s, Ar-H ortho to Ar-NO2), δ=6.67 (s, Ar-H meta to Ar-NO2), δ=4.15 (t, Ar-O- (CH2)3C(O)OCH2CH2(OCH2CH2)225CH2CH2OC(O)(CH2)3-O-Ar), δ=4.06 (t, Ar-O-CH2—), δ=3.86 (s, Ar-OCH3), δ=3.54 (m, — CH2CH2O —), δ=2.49 (t, Ar- OCH2CH2CH2C (O)O—), δ=2.39 (s, Ar-C(O) CH3), δ=2.10 (m, Ar- OCH2CH2CH2C (O)O—)
PEG 10000-bis-4-(4-(1-hydroxyethyl)-2-methoxy-5-nitrophenoxy)butanoate: PEG 10000-bis-4-(4-acetyl-2-methoxy-5-nitrophenoxy)butanoate (0.0798mmol), NaBH4 (1.42mmol), were dissolved in DCM (20mL) and stirred overnight at 4 °C to room temperature until reaction was complete and confirmed by 1H NMR. The reaction was diluted with DCM and washed with sodium bicarbonate and brine three times each and then dried by rotary evaporation followed by lyophilization. The product is a light yellow powder (46% yield). 1H NMR.(CDCl3): δ=7.56 (s, Ar-H ortho to Ar-NO2), δ=7.31 (s, Ar-H meta to Ar-NO2), δ=5.56 (m, Ar-CHCH3), δ=4.24 (t, Ar-O-(CH2)3C(O)OCH2CH2(OCH2CH2) 225CH2CH2OC(O)(CH2)3-O-Ar), δ=4.11 (t, Ar-O-CH2—), δ=3.97 (s, Ar-OCH3), δ=3.63 (m, — CH2CH2O —), δ=2.52 (t, Ar- OCH2CH2CH2C(O)O—), δ=2.12 (m, Ar- OCH2CH2CH2C (O)O—), δ=1.53 (d, Ar- CHCH3)
PEG10000-bis-4-(2-methoxy-5-nitro-4-(1-((4-oxo-4-(2-(pyridin-2-yldisulfanyl)ethoxy)butanoyl)oxy)ethyl)phenoxy)butanoate: 2-(Pyridin-2-yldisulfanyl)ethyl succinate (0.040mmol), DMAP (0.0082mmol), EDC (0.13mmol), and PEG 10000-bis-4-(4-(1-hydroxyethyl)-2-methoxy-5-nitrophenoxy)butanoate (0.0094mmol), were diluted in 20mL of DCM and stirred with activated molecular sieves overnight until reaction was complete and confirmed by 1H NMR. The reaction was diluted with DCM and washed with sodium bicarbonate, brine, and water three times each and dried in magnesium sulfate. The product was then dried by rotary evaporation and then precipitated in diethyl ether. Product was placed in lyophalizer to be dried overnight. The product is a light yellow powder (73%) 1H NMR.(CDCl3): δ=8.43 (d, C5H5N ortho from the N), δ=7.65 (m, C5H5N para from the N), δ=7.63 (d, C5H5N meta from N closer to SS), δ=7.53 (s, Ar-H ortho to Ar-NO2), δ=7.07 (m, C5H5N meta from N farther to SS), δ=6.99 (s, Ar-H meta to Ar-NO2), δ=6.44 (m, Ar- C5H5N), δ=4.30 (t, Ar-O-(CH2)3C(O)OCH2CH2(OCH2CH2) 225CH2CH2OC(O)(CH2)3-O-Ar), δ=4.22 (t, Ar-O-CH2—), δ=3.95 (s, Ar-OCH3), δ=3.84 (d, C5H5N - SSCH2CH2OC (O)—), δ=3.61 (m, — CH2CH2O —), δ=2.97 (d, C5H5N-SSCH2CH2OC(O)CH2CH2C(O)O-Ar), δ=2.59 (d, C5H5N-SSCH2CH2OC(O)CH2CH2C(O)O-Ar), δ=2.54 (t, Ar-OCH2CH2CH2C(O)O—), δ=2.14 (m, Ar- OCH2CH2CH2C (O)O—), δ=1.57 (d, Ar-C(O) CH3)
Exchange experiments
All exchange experiments were prepared and performed at room temperature. Stock solutions of PEG 2K-bis-(4-(2-methoxy-5-nitro-4-(1-((4-oxo-4-(2-(pyridin-2- yldisulfanyl)ethoxy)butanoyl)oxy)ethyl)phenoxy)butanoate) (0.1mM, (0.2mM disulfide groups)), PEG 4K-bis-(4-(2-methoxy-5-nitro-4-(1-((4-oxo-4-(2-(pyridin-2-yldisulfanyl)ethoxy)butanoyl)oxy)ethyl)phenoxy)butanoate) (0.1mM, (0.2mM disulfide groups)), PEG 10K-bis-(4-(2-methoxy-5-nitro-4-(1-((4-oxo-4-(2-(pyridin-2-yldisulfanyl)ethoxy)butanoyl)oxy)ethyl)phenoxy)butanoate) (0.1mM, (0.2mM disulfide groups)), and 2-(pyridin-2-yldisulfanyl)ethyl succinate (0.2mM) were pre-dissolved in PBS (100mM) with a pH of either 6.0, 7.0, 7.4, or 8.0. Each solution was combined 1:1 volume to volume with stock solutions of thiol-containing proteins BSA, βLG, or GSH (0.2mM) and tested for release of pyridine-2-thione (7568.9 M-1 cm-1 at 340nm, calculated from a calibration curve generated on the same machine) in a costar 3370 96 well flat bottom assay plate measured by a DTX 880 Multimode Detector plate reader. Measurements were taken for 24 hours at 17 s intervals for the first hour and 828 s intervals for the remaining 23 hours. Each experiment was performed five times. βLG exists naturally in dimeric form, thus the solutions were reduced using DTT (0.95 eq) before combining with the activated disulfide.12
Statistics
Kinetic rate constants of exchange (kexch) were determined for all conditions from each of the five trials independently performed by three individuals. A four-way anova was performed for both kinetic rate constants and maximum exchange with 2-way interactions. The four factors were replicate, pH, protein, and macromer. All data are presented as means over five replicates, with error bars representing standard error of the mean.
Results
In a previous report,9 we described the synthesis of PEG526-methacrylate-4-(2-methoxy-5-nitro-4-(1-(4-oxo-4-(2-(pyridin-2-yldisulfanyl)ethoxy)butanoyl)oxy))butanoate, and its use for tethering thiol-containing biomolecules into hydrogels, allowing their subsequent (controlled) photorelease. Complete disulfide exchange occurs between this macromer and peptides/proteins in solution (prior to gel fabrication). The peptides were also able to undergo disulfide in-gel exchange (post-polymerization), but bovine serum albumin (BSA) did not undergo exchange after extended incubation in the gel (48 h). Because BSA freely diffuses into and out of the gel within a few hours, we presumed the photodegradable tether was sterically inaccessible to larger proteins. Using a longer linker, PEG10K-methacrylate-4-(2-methoxy-5-nitro-4-(1-((4-oxo-4-(2-(pyridin-2-yldisulfanyl)ethoxy)butanoyl)oxy) butanoate, allowed limited exchange (10% after 48 h), reinforcing that physical accessibility of the disulfide plays a role in the rate of exchange. However, it is unclear whether the sterics of the disulfide tether dominate the exchange kinetics, or the accessibility of the thiol on BSA does.
To further explore these steric interactions, we synthesized three different macromolecular model compounds representing the photodegradable macromer with the activated disulfide and compared their exchange rate to that of 2-(pyridin-2-yldisulfanyl)ethyl succinate (SUCC-SS-Pyr), the small molecule analogue of the group undergoing exchange. To generate the macromolecular model compounds, poly(ethylene glycol) chains of varying molecular weight (Mn=400, 2K, 4K and 10K Da) were coupled on both ends to a photodegradable ortho-nitrobenzyl group (o-NB, to mimic our previous linking strategy). The benzylic alcohol of the o-NB group was coupled to SUCC-SS-Pyr, to produce bifunctional macromolecules containing terminal activated disulfides. In solution, the disulfide bond can undergo thiol-disulfide exchange to release pyridine-2-thione, which is easily quantified via UV-Vis Spectroscopy.
We monitored the exchange kinetics in solution between these model compounds and thiol-containing biomolecules of various sizes. A stock solution (0.2 mM –SS-Pyr) was made for each disulfide molecule as well as each thiol-containing biomolecule, in PBS (pH 6.0, 7.0, 7.4, 8.0). GSH (MW = 307 g/mol) and BSA (MW = 66,800 Da) both naturally present a free thiol in solution, while βLG (MW = 18,300 Da) exists as a disulfide-linked protein dimer.12 Therefore, the disulfide linkages in βLG were reduced using dithiothreitol prior to exchange experiments to expose a thiol on each monomer. We verified that the DTT was consumed prior to the exchange experiments and did not alter the exchange kinetics (Supporting Information Table S1).
Each disulfide solution was combined with a thiol solution 1:1 (volume to volume) at room temperature and monitored for the release of pyridine-2-thione over the course of 24h. Because disulfide exchange follows first order reaction kinetics, the rate constant of the exchange can easily be obtained from the slope of a semi-logarithmic plot of disulfide consumption versus time. The rate constants of exchange obtained for each experiment are summarized in Table 1.
Table 1. Rate constant of exchange for thiol-containing biomolecules with activated disulfides with different sizes. (± standard deviations).
| kexch× 105, s-1 | |||
|---|---|---|---|
| GSH | βLG | BSA | |
| SUCC-SS-Pyr pH = 6.0 | 289 ± 270 | 97 ± 41 | 16 ± 8 |
| pH = 7.0 | 638 ± 567 | 263 ± 68 | 85 ± 39 |
| pH = 7.4 | 899 ± 487 | 508 ± 309 | 161 ± 33 |
| pH = 8.0 | 3392 ± 2482 | 986 ± 941 | 191 ± 87 |
| PEG2K-PDG-SS-Pyr pH = 6.0 | 745 ± 46 | 52 ± 14 | 11 ± 3 |
| pH = 7.0 | 193 ± 108 | 153 ± 46 | 71 ± 40 |
| pH = 7.4 | 324 ± 66 | 299 ± 55 | 136 ± 33 |
| pH = 8.0 | 602 ± 53 | 464 ± 58 | 186 ± 65 |
| PEG4K-PDG-SS-Pyr pH = 6.0 | 100 ± 45 | 118 ± 47 | 17 ± 9 |
| pH = 7.0 | 192 ± 85 | 197 ± 73 | 68 ± 24 |
| pH = 7.4 | 322 ± 168 | 259 ± 82 | 97 ± 29 |
| pH = 8.0 | 392 ± 142 | 229 ± 107 | 118 ± 66 |
| PEG10K-PDG-SS-Pyr pH = 6.0 | 96 ± 72 | 45 ± 6 | 8 ± 3 |
| pH = 7.0 | 198 ± 116 | 157 ± 51 | 47 ± 12 |
| pH = 7.4 | 308 ± 102 | 275 ± 89 | 112 ± 39 |
| pH = 8.0 | 506 ± 122 | 378 ± 61 | 190 ± 56 |
Figure 1 summarizes the effects of several reaction variables (pH, disulfide size, thiol size) on the rate constants of exchange (kexch).
Figure 1.
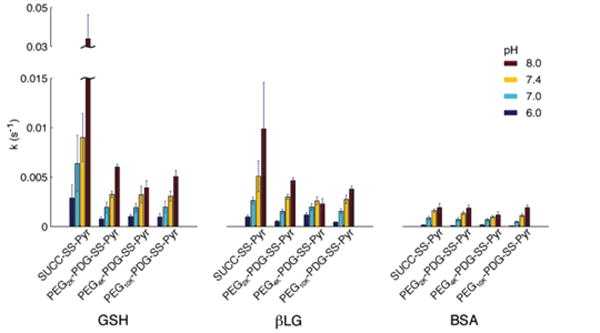
Kinetic rate constants of exchange (kexch) for the reaction between thiols of varying size (GSH, βLG and BSA) with small molecule (SUCC-SS-Pyr) and macromolecular (PEG2K-PDG-SS-Pyr, PEG4K-PDG-SS-Pyr, PEG10K-PDG-SS-Pyr) disulfides at varying pH (pH = 6.0
 , pH = 7.0
, pH = 7.0
 , pH = 7.4
, pH = 7.4
 and pH= 8.0
and pH= 8.0
 ), organized by thiol size.
), organized by thiol size.
Effect of pH
For each combination of thiol and disulfide, kexch increases with increasing pH. pH alone is not a good predictor of the exchange kinetics, however, as both the size of the thiol and the size of the disulfide substrate can affect the kinetics of exchange.
Effect of thiol size
As the size of the thiol-bearing molecule increases (GSH<βLG <BSA), kexch decreases. However, this effect becomes less pronounced as reaction pH decreases. For example, at pH = 8.0, kexch for the SUCC-SS-Pyr and GSH (kexch = 3.4 × 10-2 s-1) is more than triple kexchfor SUCC-SS-Pyr and βLG (kexch =9.9 × 10-3 s-1) and an order of magnitude greater than that for SUCC-SS-Pyr and BSA (kexch =1.9 × 10-3 s-1). As pH decreases from 8.0 to 6.0, kexch decreases significantly for all three reactions, and remains lowest for the larger thiol, however, the absolute differences in kexch are much less pronounced.
Effect of size of disulfide substrate
When pH and thiol size are held constant, it is clear that the size of the disulfide-bearing substrate also influences exchange kinetics, although this effect appears to be more subtle than that of pH or thiol size. The rate constant of exchange is lower for the macromolecular PEGX-PDG-SS-Pyrs than for the small molecule analogue SUCC-SS-Pyr, when the thiol is either GSH or βLG. However, for both these thiols, no significant differences in the rate of exchange exist between PEG2K-PDG-SS-Pyr, PEG4K-PDG-SS-Pyr and PEG10K-PDG-SS-Pyr, within any given pH. This can be seen clearly in Figure 2. Interestingly, the size of the disulfide appears to have no effects on the rate constant of exchange when the thiol is large and somewhat hindered, as seen in the data for BSA.
Figure 2.
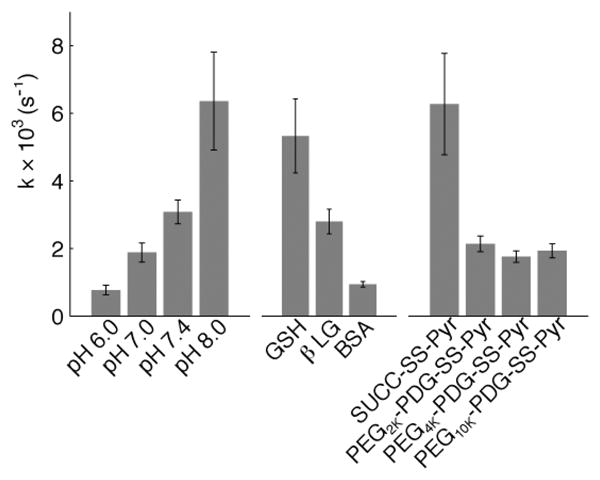
Effect of experimental variables (pH, thiol size and disulfide size) on the kinetic rate constants of exchange (kexch); pH data averaged over all thiols and all disulfide sizes; thiol data averaged over all pHs and all disulfide sizes; disulfide data averaged over all thiols and all pHs.
The kinetic rate constants of exchange are greater overall with increasing pH (averaged over all thiols and disulfides), smaller overall with increasing thiol size (averaged over all pH and disulfides), and smaller with the addition of PEG to the disulfide, but not statistically different between each PEG chain (averaged over all pH and thiols) (Figure 2). We synthesized PEG400-PDG-SS-Pyr to investigate when the rate constant of disulfide exchange became independent of macromolecular size, however, PEG400-PDG-SS-Pyr is not water-soluble and thus the exchange experiments could not be performed.
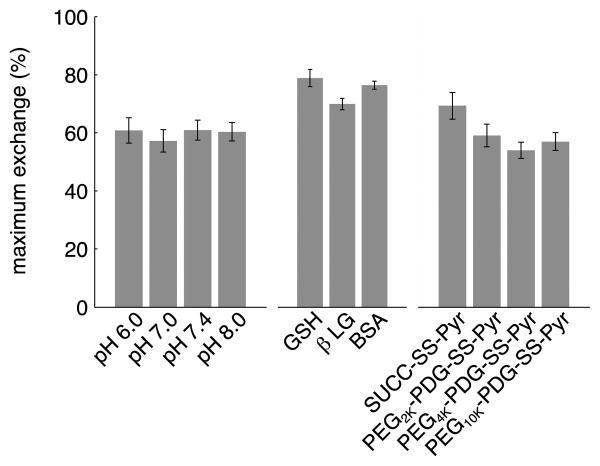
In most experiments, the thiol-disulfide reactions do not reach 100% completion in the 24 h exchange interval, and most seem to level off or reach an equilibrium extent of exchange within two hours. This may be due to disulfide bond formation by the thiols during the course of the experiment, or to steric effects (thiol or disulfide size). We first verified the concentration of free thiols in solution at different time points. The three thiols were reconstituted in pH 7.4 buffer at 0.2mM. DTT was added to βLG at 0.95 equivalents immediately after reconstitution and allowed to react for 30 minutes. The number of free thiols in each solution was measured using Ellman's reagent after 30 minutes, 1 h 45 min, and 24 h (the length of the exchange experiment) to determine extent of free dimerization in solution (Supporting Information Table S2-S3). We quantified the thiol concentration for both the stock solution (0.2 mM, based on peptide/protein mass) and the concentration used in the exchange experiments (0.1 mM). For the stock solutions (0.2 mM), neither GSH nor βLG show any evidence of dimerization; in contrast, BSA only shows ∼40% free thiol. BSA is known to be a mixture of monomer, dimer, and sometimes higher order tetramer and hexamer. The fraction of each depends on vendor, purification method, and lot. The solutions were allowed to incubate at room temperature at 0.2mM, while some of each solution was used for exchange experiments diluted to 1mM for 24 hrs. These unreacted solutions were measured again with Ellman's Reagent after 1h 45 min and again after 24 h. Based on the Ellman's results, no significant dimerization occurs for any of the solutions after 1h 45 min. After 24 h, the concentration of thiols in the GSH and βLG decreased to roughly 10% while the BSA was relatively unchanged (decreasing by only 4%). The rate constants are fairly high for GSH and βLG, such that all of the reactions are complete within the first two hours. Thus the dimerization of those thiols should be insignificant in the calculation of rate constants of disulfide exchange. BSA, though being mostly oxidized/dimerized at the beginning of the experiment, is relatively stable to further dimerization over the course of the exchange experiment. These results indicate the maximum extent of exchange we can observe for BSA is approximately 40%, while it should be close to 100% for GSH and βLG. We therefore analyzed the effect of each of the other variables (pH, thiol size, disulfide size) on the maximum amount of exchange reached in 24 h. The data is depicted in Figure 3.
Figure 3.

Maximum extent of disulfide exchange for the reaction between thiols of varying size (GSH, βLG and BSA) with small molecule (SUCC-SS-Pyr) and macromolecular (PEG2K-PDG-SS-Pyr, PEG4K-PDG-SS-Pyr, PEG10K-PDG-SS-Pyr) disulfides at varying pH (pH = 6.0
 , pH = 7.0
, pH = 7.0
 , pH = 7.4
, pH = 7.4
 and pH= 8.0
and pH= 8.0
 ), organized by thiol size.
), organized by thiol size.
The maximum extent of exchange occurs between the smallest disulfide, SUCC-SS-Pyr and the smallest thiol, GSH. No other combinations underwent complete exchange within 24 h. A somewhat lower extent of exchange was observed between GSH and each of the three PEGX-PDG-SS-Pyrs, with no significant differences seen due to macromer size. Although the overall extent of exchange is slightly lower for experiments utilizing βLG compared to GSH, the same overall trend is observed – the greatest extent of exchange occurs with SUCC-SS-Pyr, and a slightly lower extent of exchange is observed for the PEGX-PDG-SS-Pyrs. The extent of exchange is limited to less than 50% for the largest thiol, BSA. The size of the activated disulfide has no apparent effect, as the extent of exchange is limited even for the smallest disulfide, SUCC-SS-Pyr. This is likely due to the lower concentration of thiols available in the BSA sample, which the Ellman's test indicated has significant oxidation prior to the exchange experiment.
Figure 4 summarizes the influence of each of the three reaction variables – pH, thiol size and disulfide size – on the maximum extent of exchange. pH has no significant effect on the maximum extent of reaction after 24 h (averaged over all thiols and disulfides). If the initial extent of oxidation is accounted for (e.g. only 40% of thiols in BSA sample are available for exchange), then the size of the thiol does not significantly affect the maximum extent of exchange (averaged over all pH and disulfides). The size of the disulfide substrate has a subtle effect on the overall maximum extent of exchange (averaged over all pH and thiols). Overall, while each of these factors affect the kinetics of exchange, none has a large effect on the extent of reaction after 24 h.
Figure 4.
Effect of experimental variables (pH, thiol size and disulfide size) on the maximum extent of exchange; pH data averaged over all thiols and all disulfide sizes; thiol data averaged over all pHs and all disulfide sizes (maximum extent based on initial thiol concentration available for exchange); disulfide data averaged over all thiols and all pHs.
Discussion
From the data, steric interactions at both the local level (thiolate anion) and the global level (overall size of the biomolecule) play a role in the exchange kinetics. The mechanism and structural transitions during disulfide exchange are well established. The disulfide exchange is a first order SN2 reaction with a thiolate anion attacking the disulfide bond. This attack must come directly along the S-S bond axis.13 The general trend of larger biomolecules exhibiting lower rates of disulfide exchange may be due to the inability of the free thiol to reach the disulfide bond from the necessary angle. The amino acids adjacent to the cysteine in each biomolecule may affect the steric interaction between the thiolate and the disulfide, in addition to the overall 3D shape of the biomolecule with respect to the location of the thiol. Glutathione is a tripeptide with the sequence glutamic acid-cysteine-glycine, notably with a gamma linkage between glutamic acid and cysteine. The free cysteine in βLG is flanked by glutamine and leucine, and the free cysteine in BSA is flanked by glutamine and proline. The tripeptide sequence for each is depicted in Scheme 2. At the local level, the thiol of GSH is the least hindered. However, it is difficult to discern which protein has the more hindered thiol. While the leucine residue in βLG is a bit larger and more hydrophobic than the proline in BSA, the proline introduces a kink that may result in a more shielded thiol. It is therefore imperative to consider the larger 3D structure of each of these biomolecules.
Scheme 2.
Tripeptide sequence including the thiol participating in disulfide exchange for glutathione, βLG and BSA.
GSH is a small molecule that diffuses freely in solution. Since the thiol is unencumbered, it can easily exchange with the smallest disulfide, SUCC-SS-Pyr. The exchange rate with the much larger activated disulfides, PEGx-PDG-SS-Pyrs, is not statistically significantly different, indicating that the disulfide linkage on this larger molecule is not sterically restricted from small thiolate approach.
The larger protein, βLG has a free thiol, Cys-121,12 that is relatively unencumbered at the surface of the monomer but still must attack in a specific direction. The free thiol, Cys-121, of the monomer state is typically responsible for βLG's dimerization but when separated also quickly forms non-native disulfide bonds leading to aggregation.14 Its strong role in aggregation indicates that Cys-121 is very accessible at the surface of the monomer. The overall relative size of βLG apparently impacts its rate of exchange, as kexch is always smaller for βLG compared to GSH, and kexch is smaller for exchange with the PEG-PDG-Pyrs compared to SUCC-SS-Pyr.
BSA is the largest protein studied here. Its free thiol, Cys-34,15 lies within a cleft of the protein 6 Å from the surface16. Steric conditions in this cleft are typically very important for disulfide exchange. In fact, many studies of albumin, both human and bovine, show that conditions such as heat,17 pH (slightly acidic or slightly basic)16 and ligand binding (such as fatty acids)18 can cause conformational changes that expose the free thiol at Cys-34 and reduce steric barriers to thiol/disulfide exchange at that location. At a neutral pH, the Cys-34 is turned inward and may become exposed at higher or lower pH. Others have reported low efficiency of disulfide exchange between BSA and disulfide containing polymers at low (6.0) pH, possibly due to the cysteine residues being mostly non-oxidized at that pH.19 Our data is consistent with this report, as we observe both lower kexch values for BSA at pH 6.0 than at pH 7.0, 7.4 and 8.0, and we also observe an overall lower maximum extent of reaction at pH 6.0 than the higher pH experiments; quantification of free thiol indicates that a significant portion of it is oxidized at the beginning of the experiment. To achieve disulfide exchange two conditions must be met, a) the thiolate anion must be present and b) it must have appropriate access to the disulfide bond to attack with the required orbital overlap for an SN2 reaction. The activated disulfide must fit into the cleft of BSA in the correct orientation for the S-S bond to line up with the free thiol. An increase in pH can cause conformational changes in the protein that open up the thiol site, although steric barriers to disulfide exchange presented by the cleft are not eliminated completely. That is, regardless of pH, the thiol in BSA is sterically hindered in the SN2 attack on any tethered disulfide, and thus the exchange between BSA and PEGx-PDG-SS-Pyrs was no different than with SUCC-SS-Pyr.
Figure 5 gives a general illustration of the steric components for some representative pairings along with their exchange rate constants to help visualize their steric interactions. While previous reports20,11,10 attributed exchange rate variations primarily to thiolate sterics, we find the relationship between thiolate size and disulfide size to be a bit more intricate. When the protein is small (GSH), the thiolate can easily access activated disulfides for rapid exchange. Exchange is most rapid for the two smallest molecules – GSH and SUCC-SS-Pyr – and somewhat slower for the larger disulfides, although no significant differences are seen in the exchange kinetics for the macromolecular disulfides. A similar effect overall is seen for the next largest thiol, the protein βLG; faster exchange occurs with the small molecule disulfide than the macromolecular disulfides. The sterics of the thiolate also play a role in the exchange, as kexch is always lower for βLG than that seen for GSH. When the protein is very large and has added local steric barriers (BSA) the sterics of the thiolate anion dominate the exchange so we see that the rate constant is equally slow for both the smallest and the largest disulfide. This steric barrier is reinforced by the stability of the free thiol of BSA (the non-oxidized portion present at the beginning of the reaction); that is, the remaining free thiols are unable to further dimerize during the course of the reaction, indicating they are sterically restricted from doing so (unlike GSH and βLG, which dimerize in the absence of a disulfide with which to react).
Figure 5.

Representative pairings of disulfides and thiols illustrate the potential steric interactions responsible for varying exchange rates.
Conclusions
By studying the exchange kinetics between different sized thiols and different model compounds based on a disulfide-bearing macromer, we have determined the influence of thiol size and steric accessibility on its exchange with different disulfides at several pHs. Kinetic rate constants of exchange increase with increasing pH, but the maximum extent of exchange is not affected by pH in the range tested. Small molecule disulfides react faster and more completely than macromolecular disulfides when the thiol is small (GSH) to moderate molecular weight (βLG) and relatively accessible, but no difference was seen in the exchange kinetics between different molecular weight macromolecular disulfides (Mn = 2kDa-10kDa). Larger proteins (βLG and BSA), and particularly proteins with additional local steric barriers (BSA) react more slowly than smaller peptides (GSH). With decreasing pH and increasing protein size, the disulfide size becomes less important in determining the kinetics of disulfide exchange. Overall, the sterics of the thiols are more influential than disulfide size and pH in determining the rate and extent of disulfide exchange.
Supplementary Material
Scheme 1.
Disulfide exchange between pyridine-disulfide succinate (SUCC-SS-Pyr) or pyridine-disulfide-PDG-PEG (PEG-PDG-SS-Pyr) and a free thiol (RSH).
Acknowledgments
Funding for this work was provided by UCLA HSSEAS Start-up funds and the National Institutes of Health through the NIH Director's New Innovator Award Program, 1-DP2-OD008533.
Footnotes
Author Contributions: The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
References
- 1.Carlsson J, Drevin H, Axen R. Biochem J. 1978;173:723. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jou YH, Bankert RB. P Natl Acad Sci-Biol. 1981;78:2493. doi: 10.1073/pnas.78.4.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woghiren C, Sharma B, Stein S. Bioconjugate Chem. 1993;4:314. doi: 10.1021/bc00023a002. [DOI] [PubMed] [Google Scholar]
- 4.Wong LJ, Boyer C, Jia ZF, Zareie HM, Davis TP, Bulmus V. Biomacromolecules. 2008;9:1934. doi: 10.1021/bm800197v. [DOI] [PubMed] [Google Scholar]
- 5.Bontempo D, Heredia KL, Fish BA, Maynard HD. J Am Chem Soc. 2004;126:15372. doi: 10.1021/ja045063m. [DOI] [PubMed] [Google Scholar]
- 6.Bulmus V, Woodward M, Lin L, Murthy N, Stayton P, Hoffman A. J Control Release. 2003;93:105. doi: 10.1016/j.jconrel.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Heredia KL, Bontempo D, Ly T, Byers JT, Halstenberg S, Maynard HD. D J Am Chem Soc. 2005;127:16955. doi: 10.1021/ja054482w. [DOI] [PubMed] [Google Scholar]
- 8.Choh SY, Cross D, Wang C. Biomacromolecules. 2011;12:1126. doi: 10.1021/bm101451k. [DOI] [PubMed] [Google Scholar]
- 9.Griffin DR, Schlosser JS, Lam SF, Nguyen TH, Maynard HD, Kasko AM. Biomacromolecules. 2013;14:1199–1207. doi: 10.1021/bm400169d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaked Z, Szajewski RP, Whitesides GM. Biochemistry-Us. 1980;19:4156. doi: 10.1021/bi00559a004. [DOI] [PubMed] [Google Scholar]
- 11.Zavialov AV, Gaestel M, Korpela T, Zav'yalov VP. Bba-Protein Struct M. 1998;1388:123. doi: 10.1016/s0167-4838(98)00172-1. [DOI] [PubMed] [Google Scholar]
- 12.Brownlow S, Cabral JHM, Cooper R, Flower DR, Yewdall SJ, Polikarpov I, North ACT, Sawyer L. Structure. 1997;5:481. doi: 10.1016/s0969-2126(97)00205-0. [DOI] [PubMed] [Google Scholar]
- 13.Fernandes PA, Ramos MJ. Chemistry-a European Journal. 2004;10:257. doi: 10.1002/chem.200305343. [DOI] [PubMed] [Google Scholar]
- 14.Yagi M, Sakurai K, Kalidas C, Batt CA, Goto Y. J Biol Chem. 2003;278:47009. doi: 10.1074/jbc.M308592200. [DOI] [PubMed] [Google Scholar]
- 15.Sugio S, Kashima A, Mochizuki S, Noda M, Kobayashi K. Protein Eng. 1999;12:439. doi: 10.1093/protein/12.6.439. [DOI] [PubMed] [Google Scholar]
- 16.Brahma A, Mandal C, Bhattacharyya D. Bba-Proteins Proteom. 2005;1751:159. doi: 10.1016/j.bbapap.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Takeda K, Wada A, Yamamoto K, Moriyama Y, Aoki K. J Protein Chem. 1989;8:653. doi: 10.1007/BF01025605. [DOI] [PubMed] [Google Scholar]
- 18.Torres MJ, Turell L, Botti H, Antmann L, Carballal S, Ferrer-Sueta G, Radi R, Alvarez B. Arch Biochem Biophys. 2012;521:102. doi: 10.1016/j.abb.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyer C, Liu J, Wong L, Tippett M, Bulmus V, Davis TP. J Polym Sci Pol Chem. 2008;46:7207. [Google Scholar]
- 20.Nagy IB, Dancs A, Koczan G, Mezo G, Hudecz F. J Bioact Compat Pol. 2000;15:139. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.