Summary
NADPH oxidases play key roles in immunity and inflammation that go beyond the production of microbicidal reactive oxygen species (ROS). The past decade has brought a new appreciation for the diversity of roles played by ROS in signaling associated with inflammation and immunity. NADPH oxidase activity affects disease outcome during infections by human pathogenic fungi, an important group of emerging and opportunistic pathogens that includes Candida, Aspergillus and Cryptococcus species. Here we review how alternative roles of NADPH oxidase activity impact fungal infection and how ROS signaling affects fungal physiology. Particular attention is paid to roles for NADPH oxidase in immune migration, immunoregulation in pulmonary infection, neutrophil extracellular trap formation, autophagy and inflammasome activity. These recent advances highlight the power and versatility of spatiotemporally controlled redox regulation in the context of infection, and point to a need to understand the molecular consequences of NADPH oxidase activity in the cell.
Chronic granulomatous disease due to NADPH oxidase deficiency was one of the first Mendelian traits linked to a gene and assigned a physiological basis (Babior, 2004, Nauseef, 2008, Segal et al., 2012). For much of the past fifty years, Occam’s razor has balanced on its edge a single role for the phagocyte NADPH oxidase (Phox): production of superoxide radicals and thus reactive oxygen and nitrogen species that damage and kill invading microbes. It is manifestly clear that ROS and RNS created through Phox activity are microbicidal, but recent work is expanding the role of NADPH oxidases beyond strict and direct microbicidal functions. New work in the past ten years has focused attention on other roles for reactive oxygen species in autophagy, extracellular trap formation, metabolic transformations, and signaling (Steinberg et al., 2007, Dupre-Crochet et al., 2013, Nathan et al., 2013). Moreover, NADPH oxidase may even change an organism’s gut physiology by depleting oxygen and creating localized hypoxia at sites of inflammation (Campbell et al., 2014). In addition to our new appreciation for the creative capacity of ROS, there is also new insight into how ROS levels are modulated. Superoxide and hydrogen peroxide are produced by NADPH oxidases and other enzymatic activities, and intracellular control of redox has emerged as an important post-translational tool (Aguirre et al., 2005, Heller et al., 2011).
Human fungal pathogens are clinically relevant in both developed economies (largely in iatrogenically immunocompromised hosts) and underdeveloped countries (chiefly in the context of HIV/AIDS) (Brown et al., 2012a, Brown et al., 2012b, Brown et al., 2014). The emerging nature of fungal disease in humans has meant that there is a great unknown in terms of the mechanisms whereby these pathogens colonize and cause morbidity. In the United States and Europe, Candida and Aspergillus species are responsible for the greatest number of opportunistic infections (Hidron et al., 2008, Brown et al., 2012a). Among HIV/AIDS patients, Cryptococcus neoformans is the most deadly culprit (Brown et al., 2014). Incongruously, the phagocyte oxidase plays a largely protective role against both Candida and Aspergillus (Pollock et al., 1995, Aratani et al., 2002b, Aratani et al., 2002a), whereas it can play a detrimental role in immunity to C. neoformans (Snelgrove et al., 2006). It is likely that these differences stem from the relative contributions of direct effects (i.e. oxidative damage) and indirect effects (i.e. regulation of adaptive T-cell responses) of Phox activity in immunity. This dichotomy highlights the potential for NADPH oxidase to play multiple roles in response to fungal pathogens. In addition to the ROS that fungi are exposed to during immune attack, fungi also respond to endogenously-produced ROS in several ways, including in the regulation of key differentiation events (Figure 1). The ability of pathogenic microbes to respond to both endogenous and host-derived ROS adds a new and potentially important dimension to host-pathogen interaction.
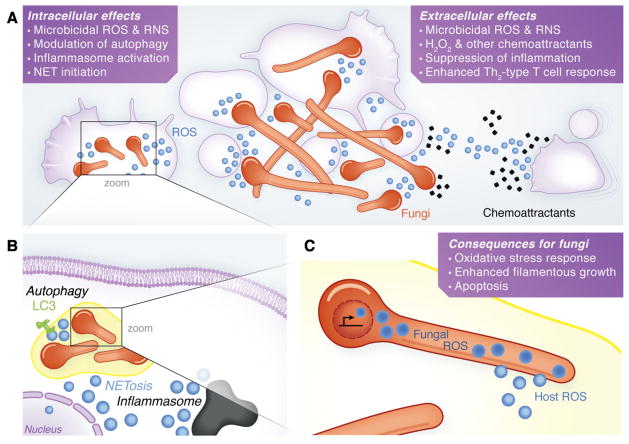
Fig. 1. Roles of NADPH oxidases and ROS in the context of fungal infection.
A. Host cells at the site of infection, including phagocytes and epithelial cells, create reactive oxygen species (ROS) through activation of NADPH oxidase complexes. (Left) Within phagocytes, these ROS have intracellular effects on both phagocyte physiology [e.g. autophagy, inflammasome, neutrophil extracellular traps (NETs)] and microbial physiology (damaging effects). (Right) ROS directly cause extracellular damage and act as chemoattractants, and their indirect effects on phagocyte signalling and adaptive immune responses lead to chemoattraction of immune cells to the site of infection, as well as programming of the inflammatory and adaptive immune responses locally and systemically.
B. Within the phagocyte, ROS drive recruitment of LC3 to the phagosome and activate autophagy, in most cases are required for NETosis, and lead to inflammasome activation.
C. Within the infecting fungus, ROS derived from either host or fungus can trigger the oxidative stress response, can enhance filamentous growth, or can activate apoptosis of the fungi.
Thus, NADPH oxidases and the ROS they produce can drive changes in both the host and the pathogenic fungi causing infection. In this Review, we will focus on recent work to highlight the roles of NADPH oxidases and ROS in fungal virulence and antifungal immunity of human fungal pathogens. First we will look at links between NADPH oxidases and immune migration, then cover unexpected physiological roles of NADPH oxidases in antifungal immunity, and finally examine the roles of ROS in the physiology and virulence of fungal pathogens (Figure 1).
Alternative roles of NADPH oxidases in tissue homeostasis and immunity to fungi
There has been a recent expansion in our perspective on the ways in which NADPH oxidases can impact immunity. These alternative roles have been comprehensively reviewed recently (Deken et al., 2013, Paiva et al., 2014, van der Vliet et al., 2014) so here we will focus on their impacts on fungal infection. NADPH oxidases have important roles in tissue homeostasis and can direct and participate in chemotaxis. Recent work in the zebrafish model suggests that these activities may be important in promoting early fungal containment to limit invasive growth. In the context of anti-fungal immunity, Phox activity has also been connected with activation of three potentially effective immune weapons: neutrophil extracellular traps (NETs), autophagy, and the inflammasome. We will cover each of these immune mechanisms in turn, with particular attention to recent developments in the field.
Immune migration and tissue homeostasis
Work in the last ten years has established that NADPH oxidase-derived reactive oxygen species play an important role in attracting immune cells to the sites of damage and inflammation (van der Vliet et al., 2014). Although the phagocyte NADPH oxidase (Phox) is the best-studied vertebrate member of this enzyme class, it is the activity of alternate NADPH oxidase enzyme complexes that has been most closely linked to immune migration in vivo. Influx of macrophages to damaged tissue has been linked to Nox1 and Nox4 in endothelial tissue, as well as Duox in epithelial tissue (Kvietys et al., 2012). Similar pathways are active in Drosophila development and C. elegans gut immunity, suggesting that regulated ROS production is not only a “damage” signal, but is part and parcel of creating and maintaining a multicellular organism (Hurd et al., 2012).
The dual-specific oxidase Duox is a predominantly epithelial enzyme that has been implicated in leukocyte chemotaxis to wounds, cancerous cells and infected tissue (Feng et al., 2010, Feng et al., 2012, Deken et al., 2013). Groundbreaking work in the zebrafish wounding model has since proven to be applicable in other models of wounding and inflammation (van der Vliet et al., 2014). Current molecular models suggest a post-transcriptional signaling pathway that includes activity of intracellular calcium transients, purine receptors and hydrogen peroxide generation to propagate signals (van der Vliet et al., 2014). The proximal biochemical mechanisms by which hydrogen peroxide drive leukocyte attraction are still unknown, although elegant work in the zebrafish model led to the discovery that a key thiol in the Lyn tyrosine kinase cell autonomously regulates neutrophil chemotaxis in zebrafish and their human counterparts (Yoo et al., 2011).
In addition to recruiting to wound sites, NADPH oxidases also work in multiple cell types in the lung to drive macrophages, neutrophils and eosinophils into allergic airways (van der Vliet, 2011). Contributions from Duox1/2, Nox1, Nox2, Nox3, and Nox4 combine to upregulate ROS production in lung tissue. It is notable that these different NADPH oxidase complexes function in a number of cell types, including epithelial, endothelial, muscle, and immune cells. Thus, NADPH oxidase-produced ROS can enhance immune migration combinatorially.
Initial work in the Drosophila, roundworm, and zebrafish models has also uncovered a role for the evolutionarily well-conserved Duox enzyme in producing microbicidal hydrogen peroxide and directing gut immunity (Bae et al., 2010, Flores et al., 2010, Hoeven et al., 2011, Deken et al., 2013, Jain et al., 2013, Lee et al., 2013, Strengert et al., 2013). Notably, ablation of Duox makes flies, worms and fish more sensitive to gut pathogens. Follow-up studies in mice have shown increased susceptibility to Helicobacter felis and influenza A virus in the absence of Duox activity (Grasberger et al., 2013, Strengert et al., 2013). This suggests that Duox-mediated mucosal immunity is conserved from worms to mammals.
Immune recruitment to fungal infection in the zebrafish
Hydrogen peroxide-driven phagocyte influx to inflammatory sites suggests the possibility that it may also play a role in recruitment of immune cells to sites of infection. Accordingly, recent work in the zebrafish has examined the contributions of NADPH oxidases to infection-driven leukocyte migration. Independent work from two leading laboratories suggests that NADPH oxidases play minimal or redundant roles in phagocyte recruitment to sites of bacterial infection. There is no apparent contribution of NADPH oxidase-produced hydrogen peroxide to neutrophil recruitment in response to Gram-positive or Gram-negative pathogens in the otic vesicle (Deng et al., 2011). Nor is there any apparent role for p22phox in leukocyte recruitment to mycobacterial granulomas (Yang et al., 2012). The NADPH oxidase-independent early chemoattraction of neutrophils to sites of bacterial infection indicates that, in contrast to the wound response, other mechanisms such as lipid chemoattractants and chemokines play predominant roles in early chemoattraction to these bacterial infections.
In contrast to the inconsequential or redundant role played by NADPH oxidases in early response to bacteria, both Phox and Duox were shown to play positive roles in containment of fungi by phagocytes in the zebrafish hindbrain ventricle infection model of candidemia (Brothers et al., 2013). The reduced phagocyte recruitment and lack of early containment strongly increased susceptibility to infection, as the unengulfed fungi germinated hyphae that caused extensive tissue damage and morbidity. The mechanism(s) whereby Phox and Duox promote early recruitment of phagocytes to C. albicans are still unknown. Notably, although Phox and Duox are more highly expressed in phagocytes or the epithelium, respectively, the compartment(s) within which they function in these circumstances are still not known. While both Phox and Duox are required for early responses in the hindbrain ventricle, early recruitment of neutrophils to a mucosal C. albicans infection in the swimbladder is insensitive to NADPH oxidase inhibition by diphenyleneiodonium. Thus, the requirement for NADPH oxidase activity appears to be tissue-specific and limited to only some infections.
The unusual requirements of both Phox and Duox for phagocyte recruitment to C. albicans infection in the hindbrain ventricle suggests that this tissue and/or the fungus provide an unusual stimulus that is sensitive to ROS signaling. Further work with a C. albicans mutant that does not efficiently make the switch from yeast to hypha suggests that fungi may actively inhibit other modes of chemoattraction (Brothers et al., 2013). Specifically, it was found that recruitment to and containment of this “yeast-locked” knockout of the EDT1 gene was unaffected by DPI inhibition. Either this mutant promoted chemoattraction in a novel way or it failed to limit NADPH oxidase-independent chemoattraction. Consistent with the latter possibility, inactivated wildtype yeast drive phagocyte recruitment even with DPI inhibition, suggesting that an active process linked to the morphogenetic switch is responsible for limiting Nox-independent chemoattraction (Barker and Wheeler, unpublished).
Support for the idea that phagocyte NADPH oxidase can also play an important role in directing chemotaxis in mammals comes from work with purified chemoattractants in vitro and in vivo. Work in vitro with mouse and human neutrophils and in vivo with mouse neutrophils clearly implicates the Phox complex in chemotaxis towards fMLP and TNFα (Hattori et al., 2010a, Hattori et al., 2010b). This was the first demonstration of a signaling role for Phox within migrating phagocytes, revealing that activation of Phox promotes directional movement. Independently, it was also found that macrophage chemotaxis to purified M-CSF requires Phox in the macrophages (Chaubey et al., 2013). Taken together this work suggests that Phox is important in mammalian neutrophils and macrophages for active chemotaxis in vivo.
An immunomodulatory role to limit tissue damage in fungal infection
In humans suffering from chronic granulomatous disease (CGD) due to loss of phagocyte oxidase activity, patients suffer from granulomatous lesions and inflammatory bowel disease, the consequences of an overly robust immune responses (Schappi et al., 2008, Segal et al., 2012). Sterile inflammation models have shown that Phox −/− knockout mice have a stronger initial recruitment of neutrophils to chemoattractants (Segal et al., 2012). Intravital imaging in the zebrafish has also elucidated a dampening effect of phagocyte NADPH oxidase on neutrophil influx to inflammatory lesions through myeloperoxidase-mediated signal inactivation (Pase et al., 2012, Robertson et al., 2014).
Recent work has sought to determine how this hyperactive response relates to immunity to fungal infection, focusing on A. fumigatus, which causes the most frequent and debilitating invasive fungal infections in CGD patients. It has been found that, in addition to roles in mediating leukocyte attraction to fungi, NADPH oxidase also limits lung-damaging immune infiltrates in pulmonary fungal infection. Segal and co-workers found that phagocyte oxidase plays an important role in dampening neutrophil activity and recruitment through, in part, activation of the Nrf2 transcriptional repressor (Segal et al., 2010, Grimm et al., 2011). This provides important mechanistic insight into how phagocyte oxidase can have a dampening role in detrimental neutrophil infiltration. The Phox −/− mice are also more susceptible to infection, despite the exaggerated neutrophilic infiltrate, and this increased susceptibility may be due to both increased fungal proliferation and increased toxicity from neutrophilic activity. It remains an open but important question whether this ability of Phox to dampen neutrophil responses requires its activity in the myeloid compartment or in the stroma. Although the expression of Phox components is greatest in phagocytes, this enzyme complex clearly plays important roles in other cell types (Bedard et al., 2007a, Bedard et al., 2007b, Kvietys et al., 2012).
The double-edged sword of phagocyte NADPH oxidase activity in fungal infection is also brought out in surprising work that implicates Phox-produced ROS in exacerbation of Cryptococcus neoformans lung infection. C. neoformans is a primary fungal pathogen that typically causes life-threatening disease in immunocompromised hosts. This predilection has made it a devastating infection among HIV/AIDS patients in Africa, where it competes with tuberculosis for the most important AIDS-associated lethal infection. Using first a Phox −/− mouse and then treating mice intranasally with an antioxidant, it was found that mice were protected against intranasal cryptococcal infection by abrogating NADPH oxidase activity or reducing ROS (Snelgrove et al., 2006). The loss of Phox-derived ROS was associated with a more protective Th1-type response and containment in pulmonary granulomatous lesions. Thus, counter intuitively, loss of this key phagocyte weapon rendered the mice more resistant to fungal infection. This work identifies important indirect consequences of Phox activity on downstream events, pointing to its influence on T-helper cell activity and adaptive immunity.
It is notable that Phox plays anti-protective roles in two fungal lung infections by promoting detrimental neutrophil-mediated inflammation in Aspergillus infection and limiting protective Th1 responses to cryptococcosis. Taken together, these studies highlight the important role of Phox and ROS in dampening immune responses, especially in the lung. Interestingly, there appears to be an important lung-specific activity for Phox, as proinflammatory response to TNFα is affected more in Phox knockout animals in the lung than other tissues (Zhang et al., 2011). The mechanistic basis for tissue-specific activity of Phox against infection is still unknown but represents an important area in the context of therapy.
Neutrophil extracellular traps
Granulocytes from vertebrates from fish to man can undergo a process called neutrophil extracellular trap (NET) formation, or NETosis (Yipp et al., 2013). NETs can play an important role in limiting the spread of infection and/or directly damaging microbes (Ermert et al., 2009). Both in vitro and in vivo experiments have implicated Phox as a key player in production of NETs, although results have differed somewhat depending on experimental setup (Yipp et al., 2013). Human neutrophil NETs are stimulated by C. albicans recognition and limit C. albicans proliferation in vitro (Urban et al., 2006). These NETs contain calprotectin, which is important in their ability to damage and kill fungi (Urban et al., 2009). Intriguingly, calprotectin (S100A8/A9) is a chemoattractant that is also associated with symptomatic Candida vaginitis (Peters et al., 2014). Although these studies implicate NETs in protection against C. albicans, it is currently not known if C. albicans stimulates NET formation that protects against candidiasis in vivo.
A. fumigatus also stimulates NET formation, and a series of studies from several laboratories has established the requirement of Phox for NET formation both in vivo in the mouse lung and in vitro in the Petri dish (Bruns et al., 2010, Rohm et al., 2014). NETs were also shown to inhibit growth of A. fumigatus in vitro (McCormick et al., 2010). Remarkably, gene therapy in an X-CGD patient was monitored with respect to NET formation and reconstitution Phox function was found to correlate with restored NET formation (Bianchi et al., 2009). Neutrophils from this patient were also used to implicate calprotectin in Phox-dependent human NET formation (Bianchi et al., 2011). The recent work from Urban and colleagues further demonstrated that Phox is required for NET induction in a pulmonary aspergillosis model (Rohm et al., 2014). Remarkably, in this hyphal infection model CGD neutrophils failed to efficiently undergo apoptosis, which could potentially contribute to hyperinflammation. These elegant studies combine to implicate Phox-dependent NET formation in control of pulmonary A. fumigatus infection in human disease.
Autophagy
Autophagy is an important cellular mechanism both for cellular recycling and pathogen containment. ROS have been linked to activation of the autophagy pathway in both cellular recycling and in response to intracellular microbes (Huang et al., 2009, Scherz-Shouval et al., 2011). Key early experiments showing that NADPH oxidase activity is required for efficient recruitment of autophagic proteins to microbe-containing phagosomes used the fungal particle zymosan (Sanjuan et al., 2007, Huang et al., 2009). This work was followed up by several groups who have shown that LC3 accumulation on fungi-containing phagosomes is dependent on ROS production through NADPH oxidase.
In 2012, two groups independently showed that the autophagy reporter protein LC3 is recruited to phagosomes containing live C. albicans and C. neoformans. In one case, it was shown that the recognition of C. albicans by Dectin-1 in RAW264.7 mouse macrophages leads to LC3 recruitment to phagosomes, which facilitates presentation of fungal antigens to CD4+ T cells (Ma et al., 2012). LC3 recruitment was shown to require the Dectin-1 receptor, the Syk tyrosine kinase, NADPH oxidase-derived ROS, and the activity of Atg5. Intriguingly, LC3 recruitment was not linked to intracellular fungal containment or killing. In the other work, myeloid expression of Atg5 was shown to mediate resistance to intravenous C. albicans infection but not intraperitoneal or intratracheal C. neoformans infection (Nicola et al., 2012). Intriguingly, knockdown of Atg5 enhanced C. neoformans intramacrophage survival but mice lacking Atg5 in myeloid cells exhibited decreased pathology compared to littermate controls. An altered macrophage polarization profile in the Atg5 myeloid-specific knockout mice may help to explain the decreased pathology. The disconnect between in vitro activity and in vivo activity suggests that autophagy may be playing other important roles in immune responses—beyond containment of intracellular pathogens.
Phagosomes of ingested Aspergillus fumigatus spores have also been shown to recruit LC3 in macrophages (Kyrmizi et al., 2013). Similar to the case for C. albicans, this localization requires the β-glucan receptor Dectin-1 and NADPH oxidase. However, autophagy does contribute to preventing germination and proliferation of intracellular fungi. Remarkably, treatment of monocytes with hydrocortisone ex vivo, or isolation of monocytes from patients treated with hydrocortisone, results in reduced containment of A. fumigatus. This finding uncovers a surprising ability of hydrocortisone to block signaling through the Dectin-1 pathway and forges a mechanistic connection between autophagy and corticosteroid-mediated immunosuppression in susceptibility to fungal disease.
The zebrafish model has also been used to examine links among NADPH oxidase activity, autophagy and C. albicans infection. A transgenic line expressing the GFP-LC3 fusion protein has been found to report robustly on autophagic flux, and was used to demonstrate recruitment of LC3 to bacteria-containing phagosomes in vivo for the first time (He et al., 2009, Meijer et al., 2014). Remarkably, C. albicans-containing phagosomes only rarely recruit significant levels of GFP-LC3 in vivo, and this low level recruitment is not abolished by either the antioxidant vitamin E or the NADPH oxidase inhibitor diphenyleneiodonium (Brothers et al., 2013). This suggests that phagosomal recruitment may be less important in the context of vertebrate C. albicans infection.
In agreement with a more nuanced role for autophagy in vivo, recent work argues that autophagy is not crucially important for human control of C. albicans infection (Smeekens et al., 2013). These authors found that myeloid-specific knockout of Atg7 in mice did not affect infection outcome. In addition, no associations were found between fungal infection and single-nucleotide polymorphisms in autophagy pathway genes in humans. Similar to what was found for RAW264.7 mouse macrophages, there was no significant contribution of autophagy to phagocytosis or killing of C. albicans by human monocytes.
Taken together, these studies support a nuanced view of autophagy and suggest that some components of the autophagy machinery may be more important than others when considered in the whole animal. Further, these combined findings are consistent with other work that has defined roles for autophagy in immune signaling as well as phagosome maturation.
Inflammasome
Autophagy is closely linked to inflammasome activity, which regulates production of mature IL-1 and IL-18, activates pyroptosis, and plays an important role in resistance to fungal infection (Gross et al., 2011, Skeldon et al., 2011, Rodgers et al., 2014). Inflammasomes can be activated by NADPH oxidase activation, but it may be a negative role played by Phox that is a key event in controlling immune response to fungi.
Several fungi trigger NLRP3 inflammasome activation, including dermatophytes and invasive opportunistic pathogens (Hise et al., 2009, Joly et al., 2010, Gross et al., 2011, Li et al., 2013, Pietrella et al., 2013, Mao et al., 2014). NLRP3 activation by A. fumigatus in THP-1 monocytes requires activation of the β-glucan receptor Dectin-1 and NADPH oxidase activation, emphasizing the role of Phox in this process (Said-Sadier et al., 2010). In the context of both mucosal and disseminated candidiasis, NLRP3 seems to play an important protective role (Hise et al., 2009, Tomalka et al., 2011). This includes both regulating innate immunity and adaptive immunity (Hise et al., 2009, Tomalka et al., 2011, van de Veerdonk et al., 2011). In addition to NLRP3, the NLRC4 inflammasome also activates in response to C. albicans and plays a protective role against oropharyngeal candidiasis, primarily in the stroma (Tomalka et al., 2011).
New work highlights another role for inflammasome activity in C. albicans interaction with macrophages in vitro. Namely, the NLRP3 inflammasome has been implicated in activating pyroptosis of macrophages in response to C. albicans that have germinated within them (Wellington et al., 2012, Uwamahoro et al., 2014, Wellington et al., 2014). It had been previously thought that fungal germination itself destroyed macrophages, but this new work from two laboratories shows that changes to the fungal cell surface upon this morphotypic switching event trigger NLRP3 activation and death of the macrophage. These studies are also in agreement with previous work that first identified the hyphal-specific activation of inflammasome activation (Cheng et al., 2011). Although the role for NADPH oxidase activity in NLRP3 inflammasome-mediated pyroptosis is unclear, its requirement for NLRP3 activation downstream of A. fumigatus recognition suggests it might play a similar role.
The inflammasome is also implicated in damaging hyperinflammatory states, such as the gastrointestinal granulomas and inflammatory bowel disease (IBD) seen in some human CGD patients. Exciting new work shows that IL-1 production through the inflammasome is a key mediator of the hyperinflammatory state, both in CGD-associated IBD and in pulmonary A. fumigatus infection (de Luca et al., 2014). It appears that the phagocyte NADPH oxidase normally limits a positive feedback loop between inflammasome IL-1 production and autophagy both in vivo and in vitro. The mechanism whereby NADPH oxidase downregulates autophagy and upregulates IL-1 production is not yet known. Importantly, blockade of IL-1R activation through a clinically approved drug (anakinra) can short-circuit this loop in vitro and in vivo in both mice and in CGD patients to ameliorate gut inflammation. Furthermore, anakinra also reduced gut inflammation in experimental IBD that did not involve loss of phagocyte NADPH oxidase, suggesting that this drug may be effective in treating the majority of IBD patients that do not have defective Phox. Consistent with previous work that suggests Phox exacerbates pulmonary fungal infection, use of anakinra in Phox −/− mice reduced fungal load in A. fumigatus infection. While the target tissue for anakinra action in the context of IBD or A. fumigatus infection has not been positively identified in vivo, previous work suggests that CGD monocytes play an important role in exacerbated IL-1 production (Meissner et al., 2010). These studies highlight the power of IL-1 and provoke a number of new and interesting questions about the relationships among NADPH oxidase activity, IL-1 and IL-17 production in the context of fungal infection (Mills et al., 2013).
Role of reactive oxygen species in fungal development
As discussed above, ROS play important roles in mammalian signaling. Similarly, ROS from various sources, including internally-generated and host-generated, regulate important processes in fungal cells that have been implicated in pathogenesis. Some fungi have one or more NADPH oxidases that play various roles in development, as recently reviewed in (Takemoto et al., 2007, Scott et al., 2008). Even those fungi that don’t encode NADPH oxidases encounter ROS generated from metabolism or non-respiratory enzymatic activity, or from a mammalian host or neighboring bacterial or fungal species. The potential sources of ROS encountered during the life of the fungus can be demonstrated using C. albicans as an example. C. albicans is thought to generally reside in the presence of mammalian hosts either as a commensal or a pathogen. In the presence of host-associated microbiota in the mouth, intestinal or female reproductive tract, C. albicans encounters hydrogen peroxide-producing microbes such as Lactobacillus species (Collins et al., 1980, Fitzsimmons et al., 1994). Normal fungal metabolism generates endogenous ROS, and Miramón and colleagues (Miramon et al., 2012) found, using GFP-reporters, that both phagocytosed and non-phagocytosed C. albicans induces genes indicative of ROS exposure in the presence of immune cells. In addition, a small molecule signal produced by C. albicans can also impact intracellular ROS levels. Antifungal therapy can also promote intracellular ROS levels (Delattin et al., 2014). In this section, we will highlight regulated genes in intracellular ROS in C. albicans, and the consequences of ROS in the control of developmental processes including morphogenesis, biofilm formation, and apoptosis. We will also briefly mention ROS in the regulation of fungal processes in other fungi.
Candida albicans produces a quorum-sensing molecule called farnesol. This small molecule accumulates in culture supernatants to concentrations that can repress hyphal growth despite the presence of hypha-inducing cues (Hornby et al., 2001). Farnesol acts, at least in part, through direct inhibition of adenylate cyclase activity (Davis-Hanna et al., 2008, Hall et al., 2011). The consequences of decreased cAMP signaling, due to inhibition of adenylate cyclase, include the induction of stress response genes such as those that are protective against reactive oxygen species (e.g. catalase) (Deveau et al., 2010). This induced protection to ROS upon exposure to farnesol may be advantageous because farnesol itself can induce ROS in C. albicans (Westwater et al., 2005) and farnesol-induced ROS may contribute to apoptosis (Shirtliff et al., 2009). The ROS generated by farnesol may be a consequence of altered metabolic activity, perhaps caused by interaction with the electron transport chain, as has been demonstrated in S. cerevisiae (Machida et al., 1998, Machida et al., 1999). Farnesol, which is also produced when cells grow as biofilms (Martins et al., 2007), may inhibit the further accumulation of cells in the community (Ramage et al., 2002). Though it is not yet known if ROS generated by farnesol also modulate the activity of the Ras1-controlled signaling pathway in C. albicans, low concentrations of hydrogen peroxide appear to directly impact Ras activity in Paracoccidioides brasiliensis Pb18, potentially explaining the observed stimulation of hyphal growth by ROS in this fungus (Haniu et al., 2013).
Hydrogen peroxide can also modulate the morphology of C. albicans. Nasution et al. (Nasution et al., 2008) found that both exogenous hydrogen peroxide and endogenously-produced ROS induced hyphal growth in cells within colonies. It is not yet known if ROS signaling pathways play a role in morphogenesis, or if the change in morphology is an indirect effect of oxidizing molecules, but it is interesting to note that the authors also found increased levels of ROS in cells grown with serum, a potent inducer of hypha formation. Increased ROS levels in hyphae may be due to the repression of ROS scavenging enzymes that are repressed upon increased cAMP signaling (Harcus et al., 2004, Davis-Hanna et al., 2008). Alternatively, increases in respiration that are often concomitant with filamentation may lead to increased levels of ROS (Morales et al., 2013). Work by Srinivasa and colleagues (Srinivasa et al., 2012) showed that filamentation induced by H2O2 lead specifically to growth in the pseudohyphal morphology, and this morphogenic change involves the multiple pathways including the Cek1-Cph1-dependent MAP kinase pathway. ROS are also predicted to impact the formation of chlamydospores, thick walled structures that may contribute to stress resistance in C. albicans, in part through the activity of the Hog1 MAP kinase (Alonso-Monge et al., 2003).
The role of reactive oxygen species in biofilm differentiation in S. cerevisiae and C. albicans was nicely reviewed in by Čáp et al. (Cap et al., 2012). The authors first discuss the potential benefits of hormesis, a process by which low concentrations of a stressor or toxin enhance survival upon exposure to higher concentrations of this stress and even other stresses. In addition, ROS impact biofilm development in other ways. Heterogeneity in ROS exposure in biofilms can create variability of signaling pathways within the population, and can contribute to localized cell death or changes in metabolism. C. albicans cells undergoes an apoptosis-like process in response to ROS (Phillips et al., 2006).
While we focus on C. albicans in this review, A. fumigatus and C. neoformans are also influenced by ROS. As examples, in C. neoformans, Rac1 and Rac2 play roles in the localization of ROS (Ballou et al., 2013), either through the localization of Nox proteins, as has been reported for Cdc42 in A. nidulans (Rolke et al., 2008) or through the regulation of Nox activity, as has been reported in Claviceps purpurea (Semighini et al., 2008) or Epichloë festucae (Takemoto et al., 2011). In these fungi, the proper localization of intracellular ROS is critical for establishing and maintaining polarized growth. Future studies will determine how NADPH-generated ROS, endogenous ROS produced by other pathways, and extracellular ROS work together to regulate key processes in these and other fungi.
Conclusion and perspectives
As detailed here, study of NADPH oxidase activity and the mechanistic consequences of spatiotemporally controlled ROS production have led to a greater understanding of fungal infection. NADPH oxidases play both effector and signaling roles in infection, which can lead to protective responses and dampened tissue damage. Phox-associated immunomodulation can promote protection (as in the case of resistance to C. albicans or A. fumigatus) or damage and exacerbated disease (as in pulmonary C. neoformans infection). Similarly, study of fungal infection can lead to insight into novel signaling roles of NADPH oxidases in immunity. This is illustrated by discussion of recent work that provokes new questions about autophagy-inflammasome connectivity and chemotaxis mechanisms.
Overall, the last few years have seen a shift in the view of ROS from microbicidal molecules to short-lived and targeted second messengers that regulate crucial signaling pathways in the immunocompetent host. In addition, it is also clear that ROS are produced and used as signals by eukaryotic pathogens. Thus, pathogenic fungi may respond to host-derived ROS by activating morphogenetic switching and thereby increased virulence.
The development of improved techniques for visualizing the presence of diverse reactive oxygen species in real time and for discerning the structural and biochemical effects of ROS on proteins, lipids, sugars and DNA will likely clarify how ROS work (Finkel, 2011, van der Vliet, 2011, Enyedi et al., 2013). This, in turn, will advance our understanding of the complex homeostatic and immune roles of NADPH oxidases, which may lead to more nuanced approaches to treatment of inflammatory dysregulation and fungal infections.
Acknowledgments
The authors gratefully acknowledge their funding from the National Institutes of Health (Grant # R15AI094406 to RTW & # R01AI091702, R01GM108492, and P30GM106394 to DAH) and USDA (Project # ME0-H-1-00517-13 to RTW).
References
- Aguirre J, Rios-Momberg M, Hewitt D, Hansberg W. Reactive oxygen species and development in microbial eukaryotes. Trends in microbiology. 2005;13:111–118. doi: 10.1016/j.tim.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Alonso-Monge R, Navarro-Garcia F, Roman E, Negredo AI, Eisman B, Nombela C, Pla J. The Hog1 mitogen-activated protein kinase is essential in the oxidative stress response and chlamydospore formation in Candida albicans. Eukaryot Cell. 2003;2:351–361. doi: 10.1128/EC.2.2.351-361.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, Suzuki K, et al. Critical role of myeloperoxidase and nicotinamide adenine dinucleotide phosphate-oxidase in high-burden systemic infection of mice with Candida albicans. The Journal of infectious diseases. 2002a;185:1833–1837. doi: 10.1086/340635. [DOI] [PubMed] [Google Scholar]
- Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, Suzuki K, et al. Relative contributions of myeloperoxidase and NADPH-oxidase to the early host defense against pulmonary infections with Candida albicans and Aspergillus fumigatus. Medical mycology. 2002b;40:557–563. doi: 10.1080/mmy.40.6.557.563. [DOI] [PubMed] [Google Scholar]
- Babior BM. NADPH oxidase. Current opinion in immunology. 2004;16:42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Bae YS, Choi MK, Lee WJ. Dual oxidase in mucosal immunity and host-microbe homeostasis. Trends in immunology. 2010;31:278–287. doi: 10.1016/j.it.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Ballou ER, Selvig K, Narloch JL, Nichols CB, Alspaugh JA. Two Rac paralogs regulate polarized growth in the human fungal pathogen Cryptococcus neoformans. Fungal genetics and biology : FG & B. 2013;57:58–75. doi: 10.1016/j.fgb.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiological reviews. 2007a;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Bedard K, Lardy B, Krause KH. NOX family NADPH oxidases: not just in mammals. Biochimie. 2007b;89:1107–1112. doi: 10.1016/j.biochi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Bianchi M, Hakkim A, Brinkmann V, Siler U, Seger RA, Zychlinsky A, Reichenbach J. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood. 2009;114:2619–2622. doi: 10.1182/blood-2009-05-221606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M, Niemiec MJ, Siler U, Urban CF, Reichenbach J. Restoration of anti-Aspergillus defense by neutrophil extracellular traps in human chronic granulomatous disease after gene therapy is calprotectin-dependent. The Journal of allergy and clinical immunology. 2011;127:1243–1252. e1247. doi: 10.1016/j.jaci.2011.01.021. [DOI] [PubMed] [Google Scholar]
- Brothers KM, Gratacap RL, Barker SE, Newman ZR, Norum A, Wheeler RT. NADPH oxidase-driven phagocyte recruitment controls Candida albicans filamentous growth and prevents mortality. PLoS pathogens. 2013;9:e1003634. doi: 10.1371/journal.ppat.1003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Science translational medicine. 2012a;4:165rv113. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- Brown GD, Denning DW, Levitz SM. Tackling human fungal infections. Science. 2012b;336:647. doi: 10.1126/science.1222236. [DOI] [PubMed] [Google Scholar]
- Brown GD, Meintjes G, Kolls JK, Gray C, Horsnell W, et al. Working Group from the EARMW. AIDS-related mycoses: the way forward. Trends in microbiology. 2014;22:107–109. doi: 10.1016/j.tim.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns S, Kniemeyer O, Hasenberg M, Aimanianda V, Nietzsche S, Thywissen A, et al. Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS pathogens. 2010;6:e1000873. doi: 10.1371/journal.ppat.1000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EL, Bruyninckx WJ, Kelly CJ, Glover LE, McNamee EN, Bowers BE, et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity. 2014;40:66–77. doi: 10.1016/j.immuni.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cap M, Vachova L, Palkova Z. Reactive oxygen species in the signaling and adaptation of multicellular microbial communities. Oxidative medicine and cellular longevity. 2012;2012:976753. doi: 10.1155/2012/976753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaubey S, Jones GE, Shah AM, Cave AC, Wells CM. Nox2 is required for macrophage chemotaxis towards CSF-1. PloS one. 2013;8:e54869. doi: 10.1371/journal.pone.0054869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SC, van de Veerdonk FL, Lenardon M, Stoffels M, Plantinga T, Smeekens S, et al. The dectin-1/inflammasome pathway is responsible for the induction of protective T-helper 17 responses that discriminate between yeasts and hyphae of Candida albicans. Journal of leukocyte biology. 2011;90:357–366. doi: 10.1189/jlb.1210702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins EB, Aramaki K. Production of Hydrogen peroxide by Lactobacillus acidophilus. J Dairy Sci. 1980;63:353–357. doi: 10.3168/jds.S0022-0302(80)82938-9. [DOI] [PubMed] [Google Scholar]
- Davis-Hanna A, Piispanen AE, Stateva LI, Hogan DA. Farnesol and dodecanol effects on the Candida albicans Ras1-cAMP signalling pathway and the regulation of morphogenesis. Mol Microbiol. 2008;67:47–62. doi: 10.1111/j.1365-2958.2007.06013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Luca A, Smeekens SP, Casagrande A, Iannitti R, Conway KL, Gresnigt MS, et al. IL-1 receptor blockade restores autophagy and reduces inflammation in chronic granulomatous disease in mice and in humans. Proc Natl Acad Sci U S A. 2014;111:3526–3531. doi: 10.1073/pnas.1322831111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deken XD, Corvilain B, Dumont JE, Miot F. Roles of DUOX-Mediated Hydrogen Peroxide in Metabolism, Host Defense, and Signaling. Antioxidants & redox signaling. 2013 doi: 10.1089/ars.2013.5602. [DOI] [PubMed] [Google Scholar]
- Delattin N, Cammue BP, Thevissen K. Reactive oxygen species-inducing antifungal agents and their activity against fungal biofilms. Future medicinal chemistry. 2014;6:77–90. doi: 10.4155/fmc.13.189. [DOI] [PubMed] [Google Scholar]
- Deng Q, Yoo SK, Cavnar PJ, Green JM, Huttenlocher A. Dual roles for Rac2 in neutrophil motility and active retention in zebrafish hematopoietic tissue. Developmental cell. 2011;21:735–745. doi: 10.1016/j.devcel.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveau A, Piispanen AE, Jackson AA, Hogan DA. Farnesol induces hydrogen peroxide resistance in Candida albicans yeast by inhibiting the Ras-cyclic AMP signaling pathway. Eukaryot Cell. 2010;9:569–577. doi: 10.1128/EC.00321-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupre-Crochet S, Erard M, Nubetae O. ROS production in phagocytes: why, when, and where? Journal of leukocyte biology. 2013;94:657–670. doi: 10.1189/jlb.1012544. [DOI] [PubMed] [Google Scholar]
- Enyedi B, Zana M, Donko A, Geiszt M. Spatial and temporal analysis of NADPH oxidase-generated hydrogen peroxide signals by novel fluorescent reporter proteins. Antioxidants & redox signaling. 2013;19:523–534. doi: 10.1089/ars.2012.4594. [DOI] [PubMed] [Google Scholar]
- Ermert D, Zychlinsky A, Urban C. Fungal and bacterial killing by neutrophils. Methods in molecular biology. 2009;470:293–312. doi: 10.1007/978-1-59745-204-5_21. [DOI] [PubMed] [Google Scholar]
- Feng Y, Renshaw S, Martin P. Live imaging of tumor initiation in zebrafish larvae reveals a trophic role for leukocyte-derived PGE(2) Current biology : CB. 2012;22:1253–1259. doi: 10.1016/j.cub.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Santoriello C, Mione M, Hurlstone A, Martin P. Live imaging of innate immune cell sensing of transformed cells in zebrafish larvae: parallels between tumor initiation and wound inflammation. PLoS biology. 2010;8:e1000562. doi: 10.1371/journal.pbio.1000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. Signal transduction by reactive oxygen species. The Journal of cell biology. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimmons N, Berry DR. Inhibition of Candida albicans by Lactobacillus acidophilus: evidence for the involvement of a peroxidase system. Microbios. 1994;80:125–133. [PubMed] [Google Scholar]
- Flores MV, Crawford KC, Pullin LM, Hall CJ, Crosier KE, Crosier PS. Dual oxidase in the intestinal epithelium of zebrafish larvae has anti-bacterial properties. Biochemical and biophysical research communications. 2010;400:164–168. doi: 10.1016/j.bbrc.2010.08.037. [DOI] [PubMed] [Google Scholar]
- Grasberger H, El-Zaatari M, Dang DT, Merchant JL. Dual oxidases control release of hydrogen peroxide by the gastric epithelium to prevent Helicobacter felis infection and inflammation in mice. Gastroenterology. 2013;145:1045–1054. doi: 10.1053/j.gastro.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm MJ, Vethanayagam RR, Almyroudis NG, Lewandowski D, Rall N, Blackwell TS, Segal BH. Role of NADPH oxidase in host defense against aspergillosis. Medical mycology. 2011;49(Suppl 1):S144–149. doi: 10.3109/13693786.2010.487077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross O, Thomas CJ, Guarda G, Tschopp J. The inflammasome: an integrated view. Immunological reviews. 2011;243:136–151. doi: 10.1111/j.1600-065X.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- Hall RA, Turner KJ, Chaloupka J, Cottier F, De Sordi L, Sanglard D, et al. The quorum-sensing molecules farnesol/homoserine lactone and dodecanol operate via distinct modes of action in Candida albicans. Eukaryot Cell. 2011;10:1034–1042. doi: 10.1128/EC.05060-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haniu AE, Maricato JT, Mathias PP, Castilho DG, Miguel RB, Monteiro HP, et al. Low concentrations of hydrogen peroxide or nitrite induced of Paracoccidioides brasiliensis cell proliferation in a Ras-dependent manner. PloS one. 2013;8:e69590. doi: 10.1371/journal.pone.0069590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcus D, Nantel A, Marcil A, Rigby T, Whiteway M. Transcription profiling of cyclic AMP signaling in Candida albicans. Mol Biol Cell. 2004;15:4490–4499. doi: 10.1091/mbc.E04-02-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori H, Subramanian KK, Sakai J, Jia Y, Li Y, Porter TF, et al. Small-molecule screen identifies reactive oxygen species as key regulators of neutrophil chemotaxis. Proc Natl Acad Sci U S A. 2010a;107:3546–3551. doi: 10.1073/pnas.0914351107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori H, Subramanian KK, Sakai J, Luo HR. Reactive oxygen species as signaling molecules in neutrophil chemotaxis. Communicative & integrative biology. 2010b;3:278–281. doi: 10.4161/cib.3.3.11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Bartholomew CR, Zhou W, Klionsky DJ. Assaying autophagic activity in transgenic GFP-Lc3 and GFP-Gabarap zebrafish embryos. Autophagy. 2009;5:520–526. doi: 10.4161/auto.5.4.7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller J, Tudzynski P. Reactive oxygen species in phytopathogenic fungi: signaling, development, and disease. Annual review of phytopathology. 2011;49:369–390. doi: 10.1146/annurev-phyto-072910-095355. [DOI] [PubMed] [Google Scholar]
- Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, Fitzgerald KA. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell host & microbe. 2009;5:487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeven R, McCallum KC, Cruz MR, Garsin DA. Ce-Duox1/BLI-3 generated reactive oxygen species trigger protective SKN-1 activity via p38 MAPK signaling during infection in C. elegans. PLoS pathogens. 2011;7:e1002453. doi: 10.1371/journal.ppat.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornby JM, Jensen EC, Lisec AD, Tasto JJ, Jahnke B, Shoemaker R, et al. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl Environ Microbiol. 2001;67:2982–2992. doi: 10.1128/AEM.67.7.2982-2992.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Canadien V, Lam GY, Steinberg BE, Dinauer MC, Magalhaes MA, et al. Activation of antibacterial autophagy by NADPH oxidases. Proc Natl Acad Sci U S A. 2009;106:6226–6231. doi: 10.1073/pnas.0811045106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd TR, DeGennaro M, Lehmann R. Redox regulation of cell migration and adhesion. Trends in cell biology. 2012;22:107–115. doi: 10.1016/j.tcb.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain C, Pastor K, Gonzalez AY, Lorenz MC, Rao RP. The role of Candida albicans AP-1 protein against host derived ROS in in vivo models of infection. Virulence. 2013;4:67–76. doi: 10.4161/viru.22700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly S, Sutterwala FS. Fungal pathogen recognition by the NLRP3 inflammasome. Virulence. 2010;1:276–280. doi: 10.4161/viru.1.4.11482. [DOI] [PubMed] [Google Scholar]
- Kvietys PR, Granger DN. Role of reactive oxygen and nitrogen species in the vascular responses to inflammation. Free radical biology & medicine. 2012;52:556–592. doi: 10.1016/j.freeradbiomed.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrmizi I, Gresnigt MS, Akoumianaki T, Samonis G, Sidiropoulos P, Boumpas D, et al. Corticosteroids block autophagy protein recruitment in Aspergillus fumigatus phagosomes via targeting dectin-1/Syk kinase signaling. Journal of immunology. 2013;191:1287–1299. doi: 10.4049/jimmunol.1300132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KA, Kim SH, Kim EK, Ha EM, You H, Kim B, et al. Bacterial-derived uracil as a modulator of mucosal immunity and gut-microbe homeostasis in Drosophila. Cell. 2013;153:797–811. doi: 10.1016/j.cell.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Li H, Wu S, Mao L, Lei G, Zhang L, Lu A, et al. Human pathogenic fungus Trichophyton schoenleinii activates the NLRP3 inflammasome. Protein & cell. 2013;4:529–538. doi: 10.1007/s13238-013-2127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Becker C, Lowell CA, Underhill DM. Dectin-1-triggered recruitment of light chain 3 protein to phagosomes facilitates major histocompatibility complex class II presentation of fungal-derived antigens. The Journal of biological chemistry. 2012;287:34149–34156. doi: 10.1074/jbc.M112.382812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida K, Tanaka T. Farnesol-induced generation of reactive oxygen species dependent on mitochondrial transmembrane potential hyperpolarization mediated by F(0)F(1)-ATPase in yeast. FEBS Lett. 1999;462:108–112. doi: 10.1016/s0014-5793(99)01506-9. [DOI] [PubMed] [Google Scholar]
- Machida K, Tanaka T, Fujita K, Taniguchi M. Farnesol-induced generation of reactive oxygen species via indirect inhibition of the mitochondrial electron transport chain in the yeast Saccharomyces cerevisiae. J Bacteriol. 1998;180:4460–4465. doi: 10.1128/jb.180.17.4460-4465.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Zhang L, Li H, Chen W, Wang H, Wu S, et al. Pathogenic fungus Microsporum canis activates the NLRP3 inflammasome. Infection and immunity. 2014;82:882–892. doi: 10.1128/IAI.01097-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins M, Henriques M, Azeredo J, Rocha SM, Coimbra MA, Oliveira R. Morphogenesis control in Candida albicans and Candida dubliniensis through signaling molecules produced by planktonic and biofilm cells. Eukaryot Cell. 2007;6:2429–2436. doi: 10.1128/EC.00252-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick A, Heesemann L, Wagener J, Marcos V, Hartl D, Loeffler J, et al. NETs formed by human neutrophils inhibit growth of the pathogenic mold Aspergillus fumigatus. Microbes and infection / Institut Pasteur. 2010;12:928–936. doi: 10.1016/j.micinf.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Meijer AH, van der Vaart M, Spaink HP. Real-time imaging and genetic dissection of host-microbe interactions in zebrafish. Cellular microbiology. 2014;16:39–49. doi: 10.1111/cmi.12236. [DOI] [PubMed] [Google Scholar]
- Meissner F, Seger RA, Moshous D, Fischer A, Reichenbach J, Zychlinsky A. Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood. 2010;116:1570–1573. doi: 10.1182/blood-2010-01-264218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KH, Dungan LS, Jones SA, Harris J. The role of inflammasome-derived IL-1 in driving IL-17 responses. Journal of leukocyte biology. 2013;93:489–497. doi: 10.1189/jlb.1012543. [DOI] [PubMed] [Google Scholar]
- Miramon P, Dunker C, Windecker H, Bohovych IM, Brown AJ, Kurzai O, Hube B. Cellular responses of Candida albicans to phagocytosis and the extracellular activities of neutrophils are critical to counteract carbohydrate starvation, oxidative and nitrosative stress. PloS one. 2012;7:e52850. doi: 10.1371/journal.pone.0052850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales DK, Grahl N, Okegbe C, Dietrich L, Jacobs N, Hogan D. Control of Candida albicans metabolism and biofilm formation by Pseudomonas aeruginosa phenazines. mBio. 2013 doi: 10.1128/mBio.00526-12. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasution O, Srinivasa K, Kim M, Kim YJ, Kim W, Jeong W, Choi W. Hydrogen peroxide induces hyphal differentiation in Candida albicans. Eukaryot Cell. 2008;7:2008–2011. doi: 10.1128/EC.00105-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nature reviews Immunology. 2013;13:349–361. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauseef WM. Biological roles for the NOX family NADPH oxidases. The Journal of biological chemistry. 2008;283:16961–16965. doi: 10.1074/jbc.R700045200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola AM, Albuquerque P, Martinez LR, Dal-Rosso RA, Saylor C, De Jesus M, et al. Macrophage autophagy in immunity to Cryptococcus neoformans and Candida albicans. Infection and immunity. 2012;80:3065–3076. doi: 10.1128/IAI.00358-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva CN, Bozza MT. Are reactive oxygen species always detrimental to pathogens? Antioxidants & redox signaling. 2014;20:1000–1037. doi: 10.1089/ars.2013.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pase L, Layton JE, Wittmann C, Ellett F, Nowell CJ, Reyes-Aldasoro CC, et al. Neutrophil-delivered myeloperoxidase dampens the hydrogen peroxide burst after tissue wounding in zebrafish. Current biology : CB. 2012;22:1818–1824. doi: 10.1016/j.cub.2012.07.060. [DOI] [PubMed] [Google Scholar]
- Peters BM, Yano J, Noverr MC, Fidel PL., Jr Candida vaginitis: when opportunism knocks, the host responds. PLoS pathogens. 2014;10:e1003965. doi: 10.1371/journal.ppat.1003965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AJ, Crowe JD, Ramsdale M. Ras pathway signaling accelerates programmed cell death in the pathogenic fungus Candida albicans. Proc Natl Acad Sci U S A. 2006;103:726–731. doi: 10.1073/pnas.0506405103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrella D, Pandey N, Gabrielli E, Pericolini E, Perito S, Kasper L, et al. Secreted aspartic proteases of Candida albicans activate the NLRP3 inflammasome. European journal of immunology. 2013;43:679–692. doi: 10.1002/eji.201242691. [DOI] [PubMed] [Google Scholar]
- Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nature genetics. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- Ramage G, Saville SP, Wickes BL, Lopez-Ribot JL. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol. 2002;68:5459–5463. doi: 10.1128/AEM.68.11.5459-5463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson AL, Holmes GR, Bojarczuk AN, Burgon J, Loynes CA, Chimen M, et al. A zebrafish compound screen reveals modulation of neutrophil reverse migration as an anti-inflammatory mechanism. Science translational medicine. 2014;6:225ra229. doi: 10.1126/scitranslmed.3007672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers MA, Bowman JW, Liang Q, Jung JU. Regulation where autophagy intersects the inflammasome. Antioxidants & redox signaling. 2014;20:495–506. doi: 10.1089/ars.2013.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohm M, Grimm MJ, D’Auria AC, Almyroudis NG, Segal BH, Urban CF. NADPH oxidase promotes neutrophil extracellular trap formation in pulmonary aspergillosis. Infection and immunity. 2014 doi: 10.1128/IAI.00096-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolke Y, Tudzynski P. The small GTPase Rac and the p21-activated kinase Cla4 in Claviceps purpurea: interaction and impact on polarity, development and pathogenicity. Mol Microbiol. 2008;68:405–423. doi: 10.1111/j.1365-2958.2008.06159.x. [DOI] [PubMed] [Google Scholar]
- Said-Sadier N, Padilla E, Langsley G, Ojcius DM. Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PloS one. 2010;5:e10008. doi: 10.1371/journal.pone.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- Schappi MG, Jaquet V, Belli DC, Krause KH. Hyperinflammation in chronic granulomatous disease and anti-inflammatory role of the phagocyte NADPH oxidase. Seminars in immunopathology. 2008;30:255–271. doi: 10.1007/s00281-008-0119-2. [DOI] [PubMed] [Google Scholar]
- Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends in biochemical sciences. 2011;36:30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Scott B, Eaton CJ. Role of reactive oxygen species in fungal cellular differentiations. Current opinion in microbiology. 2008;11:488–493. doi: 10.1016/j.mib.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Segal BH, Grimm MJ, Khan AN, Han W, Blackwell TS. Regulation of innate immunity by NADPH oxidase. Free radical biology & medicine. 2012;53:72–80. doi: 10.1016/j.freeradbiomed.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal BH, Han W, Bushey JJ, Joo M, Bhatti Z, Feminella J, et al. NADPH oxidase limits innate immune responses in the lungs in mice. PloS one. 2010;5:e9631. doi: 10.1371/journal.pone.0009631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semighini CP, Harris SD. Regulation of apical dominance in Aspergillus nidulans hyphae by reactive oxygen species. Genetics. 2008;179:1919–1932. doi: 10.1534/genetics.108.089318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtliff ME, Krom BP, Meijering RA, Peters BM, Zhu J, Scheper MA, et al. Farnesol-induced apoptosis in Candida albicans. Antimicrob Agents Chemother. 2009;53:2392–2401. doi: 10.1128/AAC.01551-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeldon A, Saleh M. The inflammasomes: molecular effectors of host resistance against bacterial, viral, parasitic, and fungal infections. Frontiers in microbiology. 2011;2:15. doi: 10.3389/fmicb.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens SP, Malireddi RK, Plantinga TS, Buffen K, Oosting M, Joosten LA, et al. Autophagy is redundant for the host defense against systemic Candida albicans infections. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2013 doi: 10.1007/s10096-013-2002-x. [DOI] [PubMed] [Google Scholar]
- Snelgrove RJ, Edwards L, Williams AE, Rae AJ, Hussell T. In the absence of reactive oxygen species, T cells default to a Th1 phenotype and mediate protection against pulmonary Cryptococcus neoformans infection. Journal of immunology. 2006;177:5509–5516. doi: 10.4049/jimmunol.177.8.5509. [DOI] [PubMed] [Google Scholar]
- Srinivasa K, Kim J, Yee S, Kim W, Choi W. A MAP kinase pathway is implicated in the pseudohyphal induction by hydrogen peroxide in Candica albicans. Molecules and cells. 2012;33:183–193. doi: 10.1007/s10059-012-2244-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg BE, Grinstein S. Unconventional roles of the NADPH oxidase: signaling, ion homeostasis, and cell death. Science’s STKE : signal transduction knowledge environment. 2007:pe11. doi: 10.1126/stke.3792007pe11. [DOI] [PubMed] [Google Scholar]
- Strengert M, Jennings R, Davanture S, Hayes P, Gabriel G, Knaus UG. Mucosal Reactive Oxygen Species Are Required for Antiviral Response: Role of Duox in Influenza A Virus Infection. Antioxidants & redox signaling. 2013 doi: 10.1089/ars.2013.5353. [DOI] [PubMed] [Google Scholar]
- Takemoto D, Kamakura S, Saikia S, Becker Y, Wrenn R, Tanaka A, et al. Polarity proteins Bem1 and Cdc24 are components of the filamentous fungal NADPH oxidase complex. Proc Natl Acad Sci U S A. 2011;108:2861–2866. doi: 10.1073/pnas.1017309108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto D, Tanaka A, Scott B. NADPH oxidases in fungi: diverse roles of reactive oxygen species in fungal cellular differentiation. Fungal genetics and biology : FG & B. 2007;44:1065–1076. doi: 10.1016/j.fgb.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Tomalka J, Ganesan S, Azodi E, Patel K, Majmudar P, Hall BA, et al. A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. PLoS pathogens. 2011;7:e1002379. doi: 10.1371/journal.ppat.1002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS pathogens. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cellular microbiology. 2006;8:668–676. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- Uwamahoro N, Verma-Gaur J, Shen HH, Qu Y, Lewis R, Lu J, et al. The pathogen Candida albicans hijacks pyroptosis for escape from macrophages. MBio. 2014;5:e00003–00014. doi: 10.1128/mBio.00003-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk FL, Joosten LA, Shaw PJ, Smeekens SP, Malireddi RK, van der Meer JW, et al. The inflammasome drives protective Th1 and Th17 cellular responses in disseminated candidiasis. European journal of immunology. 2011;41:2260–2268. doi: 10.1002/eji.201041226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vliet A. Nox enzymes in allergic airway inflammation. Biochimica et biophysica acta. 2011;1810:1035–1044. doi: 10.1016/j.bbagen.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vliet A, Janssen-Heininger YM. Hydrogen peroxide as a damage signal in tissue injury and inflammation: murderer, mediator, or messenger? Journal of cellular biochemistry. 2014;115:427–435. doi: 10.1002/jcb.24683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellington M, Koselny K, Krysan DJ. Candida albicans morphogenesis is not required for macrophage interleukin 1beta production. MBio. 2012;4:e00433–00412. doi: 10.1128/mBio.00433-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellington M, Koselny K, Sutterwala FS, Krysan DJ. Candida albicans triggers NLRP3-mediated pyroptosis in macrophages. Eukaryot Cell. 2014;13:329–340. doi: 10.1128/EC.00336-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwater C, Balish E, Schofield DA. Candida albicans-conditioned medium protects yeast cells from oxidative stress: a possible link between quorum sensing and oxidative stress resistance. Eukaryot Cell. 2005;4:1654–1661. doi: 10.1128/EC.4.10.1654-1661.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CT, Cambier CJ, Davis JM, Hall CJ, Crosier PS, Ramakrishnan L. Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages. Cell host & microbe. 2012;12:301–312. doi: 10.1016/j.chom.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yipp BG, Kubes P. NETosis: how vital is it? Blood. 2013;122:2784–2794. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- Yoo SK, Starnes TW, Deng Q, Huttenlocher A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature. 2011;480:109–112. doi: 10.1038/nature10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WJ, Wei H, Tien YT, Frei B. Genetic ablation of phagocytic NADPH oxidase in mice limits TNFalpha-induced inflammation in the lungs but not other tissues. Free radical biology & medicine. 2011;50:1517–1525. doi: 10.1016/j.freeradbiomed.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]