Abstract
Osteoclasts are the cells responsible for physiological bone resorption. A specific organization of their most prominent cytoskeletal structures, podosomes, is crucial for the degradation of mineralized bone matrix. Each podosome is constituted of an F-actin-enriched central core surrounded by a loose F-actin network, called the podosome cloud. In addition to intrinsic actin dynamics, podosomes are defined by their adhesion to the extracellular matrix, mainly via core-linking CD44 and cloud-linking integrins. These properties allow podosomes to collectively evolve into different patterns implicated in migration and bone resorption. Indeed, to resorb bone, osteoclasts polarize, actively secrete protons, and proteases into the resorption pit where these molecules are confined by a podosome-containing sealing zone. Here, we review recent advancements on podosome structure and regulatory pathways in osteoclasts. We also discuss the distinct functions of different podosome patterns during the lifespan of a single osteoclast.
Keywords: osteoclasts, podosomes, sealing zone, actin, bone degradation, migration, actin rings
Osteoclasts (OC) are multi-nucleated giant cells deriving from the fusion of mononucleated precursors. Their normal physiological function is bone degradation. However, in certain pathological conditions such as age-related osteoporosis, tumor osteolysis, or the inflammatory disease rheumatoid arthritis, OCs are excessively differentiated and/or highly active leading to increased, pathological bone loss.1-4 Conversely, a significant reduction of either osteoclastogenesis or OC function leads to osteopetrosis, a bone disease characterized by high bone mass.5 Hence, understanding how OCs differentiate and how they degrade bone is necessary to decipher bone physiology and pathology. In this review, we will briefly discuss how OCs are differentiated and how they polarize and degrade the stiffest physiological matrix: bone. We will mostly detail advancements pertaining to the organization of the OC actin cytoskeleton that is mandatory for bone resorption and resorption-related functions.
Osteoclasts are Multinucleated Cells from the Monocytic Lineage
OCs derive from the differentiation and fusion of mononucleated hematopoietic precursors of the myeloid lineage, which, in turn, originate from granulocyte/macrophage progenitors (GMPs). GMPs can be induced by Macrophage colony-stimulating factor (M-CSF) to differentiate into monocytes (Mo), dendritic cells (DCs), macrophages, and OCs.6 The identification of the key osteoclastogenic cytokine Receptor Activator of NFκB ligand (RANKL) in 19987-10 accompanied by technological advancements, mainly Fluorescence-Activated Cell Sorting (FACS), has allowed the isolation of specific human and murine GMP-derived monoclonal populations and the investigation of their osteoclastogenic potential ex vivo. Strikingly, all the data so far obtained have described wide osteoclastogenic plasticity across the myeloid lineage of which very early myeloid precursors as well as committed Mo, macrophages, and DCs can differentiate into OCs.11-17
It is now established that M-CSF induces GMPs or GMP-derived cells to differentiate into OCs precursors that express RANK, the receptor of RANKL, and Triggering receptor expressed by myeloid cells-2 (TREM2).18,19 Upon recognition of RANKL by RANK and activation of Immunoreceptor tyrosine-based activation motif (ITAM) family receptors, OC precursors undergo further differentiation to mononuclear pre-OCs with a high activity of the transcription factor Nuclear factor of activated T cells, cytoplasmic 1 (NFATc1), and a high expression of resorption-related genes, e.g., Tartrate Resistant Acidic Phosphatase (TRAP), cathepsin K, and αvβ3 integrin.18,20,21 Finally, pre-OCs fuse to give rise to multinucleated OCs, the mature bone-resorbing cells. This process of OCs differentiation is regulated by various transcription factors and exogenous factors at different stages. While other immune cytokines such as TNF-α have been shown to regulate osteoclastogenesis, it is noteworthy that mice deficient of c-Fms (the M-CSF receptor), RANKL, and RANK do not have OCs.22,23 Indeed, osteoclastogenesis but also bone resorption are regulated: by OBs through expression of RANKL and its antagonist osteoprotegerin (OPG); by osteocytes through RANKL expression; and by immune cells though expression of M-CSF, RANKL, TNF-α, and different interleukins.20,24
Bone Degradation: Polarization, Acidification, and Proteolysis
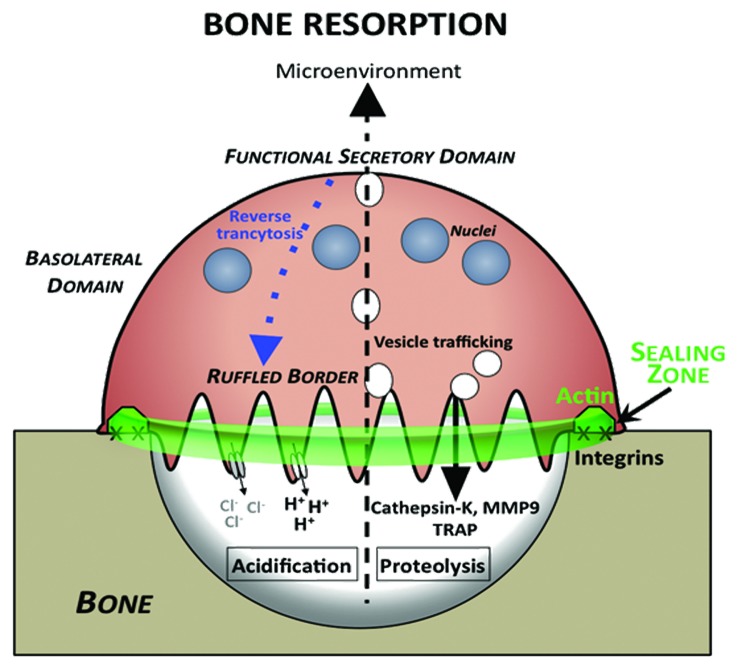
Once a differentiated, multinucleated OC adheres on the mineralized bone matrix it can start resorbing bone. It first becomes polarized and its membrane is reorganized into four distinct and unique domains: the sealing zone (SZ) that is tightly adherent to the matrix; the membrane-rich ruffled border (RB) centrally positioned relative to the SZ; the basolateral domain (BD); and the functional secretory domain (FSD) at the basal pole of the cell, distal to the matrix25 (Fig. 1).
Figure 1. Bone-resorbing osteoclasts are polarized. In contact with bone, OCs exhibit functional membrane domains defining apico-basal polarity. At the bone surface level they form the actin-rich sealing zone (green) around integrins, such as αVβ3. Sealing zone delineates the ruffled border (RB) where bone degradation takes place. The ruffled border is formed as a consequence of trafficking of vesicles in the endosomal pathway, and therefore, has characteristics of a late endosomal membrane. H+ protons are generated through the activity of carbonic anhydrase II and excreted in the resorption lacuna through V-ATPase, leading to acidification and mineral dissolution. In addition, proteases are secreted in the lacuna through the ruffled border leading to degradation of organic matrix components. Simultaneously, degradation products from the resorption process are removed from the resorption lacuna by a transcytotic pathway and released at the functional secretory domain (FSD). A reverse pathway from the functional secretion domain to the ruffled border has been identified (blue).
The function of the RB, surrounded by the SZ, is to acidify the subjacent resorption lacuna, therefore dissolving the mineral phase of bone and to secrete proteases that will degrade the organic phase of bone.25 Bone degradation thus both occurs through and results in the release of minerals (e.g., calcium, phosphate) and peptides into the resorption lacuna. These degradation products are then transmitted to the OC FSD via transcytosis, and eventually into the microenvironment to contribute to general homeostasis. Interestingly, bone resorption also leads to the emission of signaling molecules such as TGF-β, formerly embedded in the matrix, into the bone microenvironment.26,27 The RB is a convoluted membrane with a high surface area that forms as a consequence of active and directed transport of vesicles that fuse with the plasma membrane in the basal pole of the OC, facing the bone matrix26,28,29 (Fig. 1).
In order for bone degradation to be efficient, the OC tightly regulates the acidification of the resorption lacuna (pH 4.5) where it also maintains a high protease concentration.30-34 What enables the OC to maintain these conditions is establishing the SZ. The SZ is a circular adhesive superstructure comprised of a multitude of dot-like, actin-containing structural units called podosomes. Podosomes and podosome-related invadopodia, presented in a variety of cell types from distinct lineages, can directly degrade extracellular matrices.35-37 In OCs, however, the role of the podosomes is to collectively form the SZ, thus to surround and isolate the RB. Hence, the latter is the bone-degrading organelle per se in OCs.38
How the SZ is organized starting from the establishment and subsequent patterning of singular structural units, i.e., podosomes, is key to understanding bone degradation, and consequently, bone remodeling.
Podosome Formation in Osteoclasts
At very early stages of their RANKL- and M-CSF-induced differentiation in vitro, OCs form integrin-based and actin-rich adhesive structures called podosomes. Podosomes are circular structures that are around 1 µm-wide and that rise from the plasma membrane into the cytoplasm reaching a height of around 0.6 µm.39-41 They participate in OC adhesion, spreading, migration, and bone degradation. In fact, genetic depletions in mouse models of podosome components or regulatory proteins leading to their disruption have serious consequences on the bone phenotype (will be discussed below).
Although the existence of podosomes has not yet been demonstrated in vivo due to physical limitations of imaging methods, their formation in OCs seeded on their natural substrate, i.e., bone, has been documented by electronic microscopy and fluorescence microscopy.42 Hence, their formation in vivo is highly plausible.
The role of integrins
Integrins are transmembrane matrix receptors that are present at, but not restricted to, the base of podosomes and constitute a link between the extracellular matrix and the actin cytoskeleton. These heterodimeric receptors are essential for podosome-related OC function, such as adhesion and resorption.43 The importance of integrins to bone physiology and OC function has been evaluated through extensive molecular studies of αvβ3 integrin as well as the matrix-degradation defects observed in Glanzmann patients and in mice carrying lack-of-function mutations in the β3 integrin gene.44-46 The reduced resorption activity from which suffer β3−/− OCs is thought to be caused by loss of αvβ3-mediated signaling that regulates cell polarity and cytoskeletal organization.47 Likely, other integrins, namely β1-containing dimers, are also involved in OC function. Two recent reports using β1-deficient OC and β1-deficient Src-transformed murine fibroblasts point to the important role of the β1 subunit in the formation of podosomes and their related adhesive structures called invadopodia, respectively.43,48 Some studies of integrin expression have shown that αv, α2, β1, and β3 subunits are found in osteoclasts from human bone tissue.49
In addition to their expression and localization to the cell membrane, another major factor in the podosome-organizing capacity of integrins is their activation. Indeed, the deletion of kindlin-3, an intracellular activator of integrins, in mice leads to a severe deregulation of podosomes in OC and to significant reductions of OC-mediated bone resorption. This genetic deletion of kindlin-3 in mice mimics osteopetrosis observed in patients with mutations in the kindlin-3 gene, due to a block into their resorptive capacities.43 It has been shown that loss of αv, β1, and β3 subunits, through kindlin-3 inactivation, ends up in a more severe osteopetrotic phenotype than β3 mutants alone.
The recruitment of adhesion plaque molecules
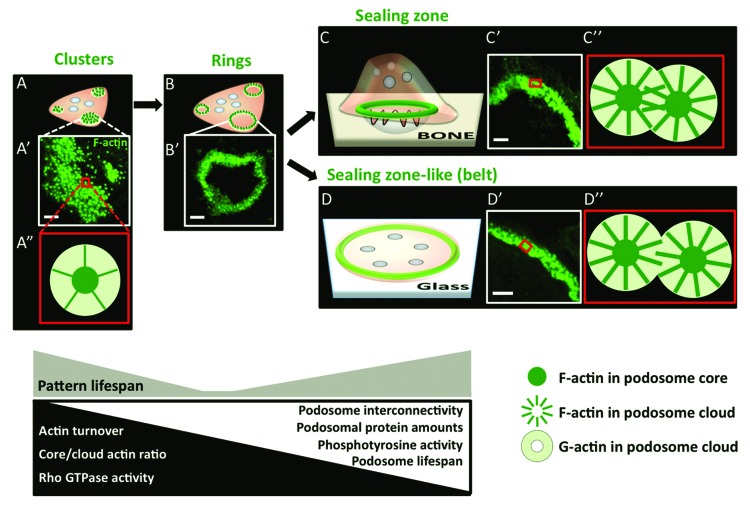
Besides integrins, other structural proteins are recruited to build the podosome. These proteins are described as cytoskeletal adaptors that organize F-actin. We have found it suitable to collectively call them “plaque proteins.” One of these proteins is talin, which binds the cytodomain of β3 integrin subunit and contributes to its inside-out activation.50,51 Talin is an elongated antiparallel flexible dimer52 capable of also binding other podosome components, such as F-actin and vinculin.53 In turn, vinculin is able to bind F-actin, α-actinin, and paxillin.54,55 The sequestration of these F-actin anchoring/docking proteins to several concentric integrin sites at the immediate vicinity of the plasma membrane, an interface usually called the “adhesion plaque,” indicates that podosome assembly occurs in a bottom-up direction. Furthermore, the actin filaments that are anchored by these integrin-associated proteins are crosslinked by myosin II and α-actinin and organize into co-axial segments linking concentric integrin sites.42 This peripheral region of the podosome is called the podosome cloud. At the center of these radial segments, i.e., of the podosome cloud, the F-actin network characterized by a higher order of density is called the “podosome core” (Fig. 2).
Figure 2. Current model of dynamic podosome patterning in OCs. Podosomes (green) are collectively arranged into clusters (A), rings (B), and then finally into either sealing zones (SZ) on glass (C) or SZ-like structures (also called “belts”) on non-mineralized substrates (D). In the cluster, podosomes are grouped in close vicinity as can be seen by immunofluorescence (IF) in an area of the cell (A’) and have a “relaxed” architecture (A”). Podosome clusters evolve to transient ring patterns. The SZ ensures proper bone resorption by confining the degradation molecules secreted by the OC ruffled border into the resorption lacuna. The SZ-like structure is not associated with a functional activity and is at the extreme periphery of the cell. There is an increase in podosome density and interconnectivity in the SZ (C”) and the SZL (D”) compared with the cluster. Kinetic, biochemical, and structural properties that accompany the patterning process are also displayed in this scheme. Scale bar in all micrographs is 5 µm.
Molecular mapping of the podosome subdomains: The cloud and the core
The high F-actin density in the podosome core is largely due to oriented and highly dynamic actin nucleation and branching machinery composed of cortactin,56 the Arp2/3 complex, (Neuronal-) Wiskott Aldrich Syndrome Protein (N-)WASP, and WASP-associated protein (WIP).57,58 The latter is necessary for the formation of the actin core for several reasons. First, by binding both to cortactin and actin monomers (G-actin), WIP recruits G-actin to cortactin-activated Arp2/3 nucleation sites proximal to the cytoplasmic membrane.59 Second, WIP interacts and activates actin-organizing molecules (N) WASP, Nck, and myosin.58,60,61 Third, WIP protects WASP from proteasomal degradation.62 The importance of WIP is underlined through the cytoskeletal phenotype of WIP−/− murine OCs, which produce podosomes that lack the core domain.63 Because WIP colocalizes with the podosome core, this phenotype suggests that the podosome cloud could form, at least partially independently of the podosome core.
The study of WIP in OCs has underlined the importance of an unexpected podosome component: CD44. CD44 is a cell-surface transmembrane proteoglycan that binds to hyaluronic acid, collagen, osteopontin, and laminin.64 The matrix-dependent activation of CD44 can rescue podosome core formation in WIP−/− OCs and increases overall adhesion of these cells to their substrate. The need for outside-in activation of CD44 has been demonstrated by addition of an activating antibody and testing its podosome-inducing capacity on different substrates.63 Surprisingly, inside-out activation of CD44 has not been investigated so far and the detailed molecular mechanism by which CD44 supports podosome core formation remains unclear. However, the non-overlapping localizations and functions of αvβ3 and CD44 served as decisive proof for the distinction between the two podosome domains: the cloud and the core.
In parallel to the core-specific function of CD44, the tyrosine kinase Src has podosome cloud-specific function. In fact, Src−/− OCs exhibit podosomes with cores but without clouds.65 The SH2 or SH3 domains of Src are necessary for the docking of Src to the podosome cloud. This localization of Src can therefore allow it to exert its tyrosine kinase activity on podosomal proteins. Both the localization of Src and its enzymatic activity are essential for full podosome assembly.65
Finally, a novel podosomal subdomain has been reported in macrophages: the podosome “cap.” The existence of this domain has been concluded from the localization of the formin FRL1 on top of the podosome core.35,66 Its potential role, however, is still to be elucidated and its presence in OCs should be confirmed.
Spatio-temporal order of podosome assembly
The exact spatio-temporal sequence of recruitment of proteins to the site of nascent podosomes has not been fully dissected. In consequence, the chronological priority of cloud vs. core formation during normal podosome assembly is still debated. A study made by Luxenburg and colleagues, however, suggested that the first step of podosome assembly is the recruitment of cloud-component β3 integrin followed by paxillin and the core-component cortactin.67 This step, which represents the formation of the adhesion plaque at the membrane-cytosol interface, is followed by the apparition of F-actin at the podosome core simultaneously with recruitment of α-actinin. Finally, β3 integrin would then further enrich at the podosome cloud. Although this data consolidates some aspects of the current podosome formation model, such as down-to-up assembly and inside-out activation of integrins, it raises a serious question: how are the site and the direction of actin nucleation in the core determined in the absence of integrins? A part of the answer could lie in the fact that, besides β3, other integrins subunits, such as αv, β1, and β2 and other non-integrin receptors, such as CD44, are present at podosome site.43,63 These receptors, which have not been kinetically accounted for, could therefore participate in initial docking of the adhesion plaque and F-actin.
Internal podosome dynamics: Interplay between polymerization and contractility
Once the podosome is “constructed,” it has a lifespan of several minutes (2–10 min). This seemingly long duration is however coupled with highly dynamic modulation, which is owed to intrinsic physical properties of its components.
First, the scaffold of the podosome, i.e., F-actin, is a polymer that constitutively undergoes fast treadmilling68 and is entirely renewed every 20–60 s in OC podosomes, i.e., at least 2.5 times within the podosome lifespan.39 This fast actin turnover requires the presence of polymerization-regulating molecules such as gelsolin. This protein is in fact an actin-capping and -severing protein with cleaves fast growing filament ends, and thus, creates new nucleation sites.69,70 Gelsolin localization at podosomes has been extensively documented71-73 and its indispensability to podosome formation in OCs has been confirmed.74 Moreover, gelsolin depletion in mice results in mild osteopetrosis indicating a decreased bone resorption.74 The presence of cofilin, another actin-severing protein at podosome sites, has recently been described.75,76 Cofilin is characterized with a lower affinity for actin compared with gelsolin70 but how it regulates podosomes is still unknown.
A second level of podosome dynamics is provided by acto-myosin contractility63 and elastic properties of vinculin and talin55 all present in the podosome cloud. These molecules convey to podosomes a property called “mechanosensitivity,” which can be defined by modulation of contractility in parallel to F-actin polymerization in the core and, therefore, the adaptation of size and stiffness of the entire podosome to the extracellular matrix.40,77 Mathematical modeling of gelsolin-mediated actin polymerization in podosomes has predicted that F-actin growth can itself be a size-limiting factor of podosome core growth.78 It is therefore conceivable that contractility and actin polymerization can interdependently modulate podosome mechanosensitivity.
Osteoclast-specific podosome properties
Podosomes are not exclusively present in OCs. Different cell types from a variety of lineages also exhibit these cytoskeletal structures. They include endothelial cells,79 smooth muscle cells,80 and cells from the monocytic lineage such as DCs and macrophages.41,81,82 The fact that podosome-presenting cells have different physiological abilities shows the high adaptability of podosomes to different microenvironments. Although the pool of structural proteins that build the podosome is conserved between different cell types, the signals that transduce podosome formation are often different. It is therefore not surprising that some experimental observations depict differences between podosomes in different cells. This section will review some of these differences within the monocytic lineage, in other words, between podosomes of OCs, DCs, and macrophages.
First, given that dynamic actin polymerization and depolymerization are necessary to maintain all podosomes, the genetic ablation of gelsolin, a high-affinity actin-severing protein that promotes actin turnover, in OCs results in their inability to form podosomes and SZs74 but has visibly no effect on podosome formation in DCs.83 Second, several studies have described that podosomes in mature OCs seeded on culture-treated glass collectively evolve into circular patterns called rings and SZ-like structures.38 In human and murine macrophages seeded on 2D substrates, podosomes also collectively organize into circular structures called “rosettes.”84 However, when seeded on the same 2D substrate, DCs podosomes do not exhibit such circular distributions but rather sustain their individual aspect.85 Third, cell migration has been shown to be mainly exerted by podosome rings in OCs but not in macrophages where it is driven by many individual podosomes neither in DCs where it is promoted by podosome clusters. These observations suggest that there are yet unrevealed molecular pathways that induce the regulation of the actin cytoskeleton in OCs to adequately support bone resorption, the ultimate function of OCs. Furthermore, the role of WIP to integral podosome formation seems to be more indispensable to DCs compared with OCs. WIP−/− DCs do not exhibit podosomes at all,86 while WIP−/− OCs do form podosome clouds (i.e., podosomes without the core), although overall podosome formation is decreased compared with wild-type OCs.63
Interplay between the actin-containing podosomes and other cytoskeletal structures, such as microtubules, can also be differently regulated between cells. While chemical microtubule depolymerization by nocodazole, severely abolishes podosome formation in macrophages,87 treatment with the same chemical at higher concentrations only partially blocks podosome formation in OCs but dramatically abrogates their collective patterning.88 Recently, it has been shown that in osteoclasts, microtubule dynamic instability plays an important role in podosome patterning through interacting molecular partners, such as cortactin, c-Src, and EB-1.89
Podosome Patterning in Osteoclasts
Structural and kinetic characteristics of podosome patterns
Regardless of the debated importance of podosomes and adhesion in general to the OC differentiation process itself, these structures are undoubtedly crucial for the support of the mature function of OCs: bone resorption. As early as individual podosomes form within an OC, they are collectively and sequentially organized into patterns along the life of the same cell. These patterns evolve from apparently random groups of podosomes called “clusters” to circle of podosomes “rings” and, eventually, to more massive circular structures, i.e., either “SZ-like structures” (SZL, also known as “belts”) or “sealing zones” (SZ) when adherent on glass or plastic or on mineralized matrix, respectively38,39,42,90 (Fig. 2).
The first pattern formed by a group of podosomes is the cluster. Several clusters can exist within an OC. At this point, individual podosomes have a “relaxed” conformation: the diameter of the core is ~300 nm, that of the cloud is 3 µm, and the average distance between the cores of two podosomes is 750 nm.42 Podosomes being in this vicinity, the radial F-actin filaments of neighboring podosome clouds overlap in a common area yet without directly linking the cores to each other, suggesting the existence of some topological crosstalk between podosomes.42 The cluster pattern can last up to several hours in an OC while the lifespan of an individual podosome within the cluster is around 3 min39,91 (Fig. 2). This means that podosome patterns are sustained by spatial commitment of de novo podosome formation through yet unknown mechanisms. Several non-exclusive hypotheses could explain this spatial confinement: (1) a local abundance of matrix-embedded or membrane-anchored molecules that directly stimulate podosome formation; (2) a wider microenvironment-induced cell-homing mechanism that induces migration or resorption in a given direction; (3) a cytoplasmic enrichment of podosomal proteins “left over” from disassembled podosomes, thus shifting the balance from a diffusion-rate stochastic podosome formation anywhere at the cell–matrix interface in the favor of the region containing previous component.
As clusters grow, de novo podosomes are positioned toward the periphery of the clusters, thus making transient circular structures that lasts for a few minutes: the rings (Fig. 2). Although individual podosomes within a given pattern are not displaced, it has been suggested that, as a consequence of actin nucleation and polymerization at the base of the podosome core, nascent podosomes repel each other while growing.78 The lifespan of individual podosomes in rings shortens down from 3 min to 1 min,92 thus conveying a more dynamic aspect to this pattern compared with clusters. The shorter podosome lifespan is correlated with a fast ring expansion rate at 2 µm/s.39 A more detailed characterization of ring properties has been technically challenging because of the transient nature of this structure. However, what is visible by microscopy of fluorescently labeled actin is that if rings stabilize, i.e., exceed their typical lifespan without changing that of individual podosomes,92 rings can give rise either to the SZ or to the SZL (Fig. 2). When OCs are adherent to a non-mineralized substrate (glass, plastic), rings are transformed into SZL by fusing together and positioning podosomes at the very periphery of the OC.42 In contrast, when OCs are on bone or on dentine, rings give rise to SZs by growing individually and making a thicker and more central and stable “super-ring.” Several SZ can be found in the same resorbing OC.
The SZ and SZL are the most mature podosome patterns. The role of the SZ is to ensure osteoclastic bone resorption by confining OC-secreted degradation molecules at the vicinity of the bone matrix. We have shown that their formation is substrate-exclusive, meaning that the SZ forms only on mineralized substrates such as bone or hydroxyapatite-coated glass.90 However, Fuller et al. (2010) have provided experimental data indicating that the SLZ, observed on glass or plastic, is associated with the same functional changes as the SZ, and the difference between the two structures might be attributable to the greater spreading caused by smooth surfaces.93
The overall transition from clusters to SZs is marked not only by a collective displacement of podosomes but also by different internal actin dynamics and increased interconnectivity between podosomes (Fig. 2). The amounts of actin and other structural proteins such as paxillin, vinculin, and α-actinin recruited per podosome increases 3- to 4-fold in this transition, and neighboring podosomes become more tightly packed with a core-to-core distance that increases 2-fold on average, reaching 480 nm in the SZL and 210 nm in the SZ.42 With the increase of intimacy between podosomes, the density of the F-actin radial fibers that make the podosome cloud increases (Fig. 2). This allows for clouds to form a continuous circular band with a thickness of 2–3 µm in the SZL and 3–6 µm in the SZ. The podosome cloud is therefore “accorded” 70–80% of all the actin present in the podosome (with 20–30% of podosomal actin in the core) knowing that, at the cluster stage, the cloud only contained about 30% of actin present in the podosome.94
Although the rate of actin turnover might change from one pattern to another, it has been shown that individual actin turnover rates in the cloud and in the ring are similar and vary simultaneously.39 Even if these two podosomal subdomains might have different molecular architectures, the joint actin turnover rate attests for a common regulation of actin polymerization.
Finally, all the above-mentioned podosomal patterns can appear within the lifetime of a single OC but the frequency of their formation varies as a function of OC maturation. In the early stages of osteoclastogenesis, the cluster is the most frequent pattern, making around 65% of all patterns. At the final stages of OC maturation, the SZL becomes the major pattern, constituting more than 60% of all patterns in OCs.39 This observation reveals that OCs, in order to organize these patterns, not only respond to instantaneous extracellular cues but also commit very early along their differentiation to end-line functions such as resorption by reaching mature podosomal patterns. The fact that this differential patterning frequency occurs on the same substrate makes it tempting to speculate that the long-term regulation is directly dependent on genetic factors. Given that the frequency of SZL patterns coincides with increase OC fusion events, it would be interesting to investigate whether multinucleation is a direct genetic determinant of SZ formation.
Cluster-dependent spreading
It is currently widely accepted that the SZ is the result of podosome patterning into clusters then rings. While the SZ has a long-described function, to seal the resorption pit, and the SZL has not been affiliated with a any specific cellular process, a question rises about the cellular functions of the initial and intermediate podosome patterns, i.e., the cluster and ring, respectively. Do clusters and rings serve any cellular purpose(s) besides giving rise to the SZ? Recently published in vitro studies have provided partial answers to this question.
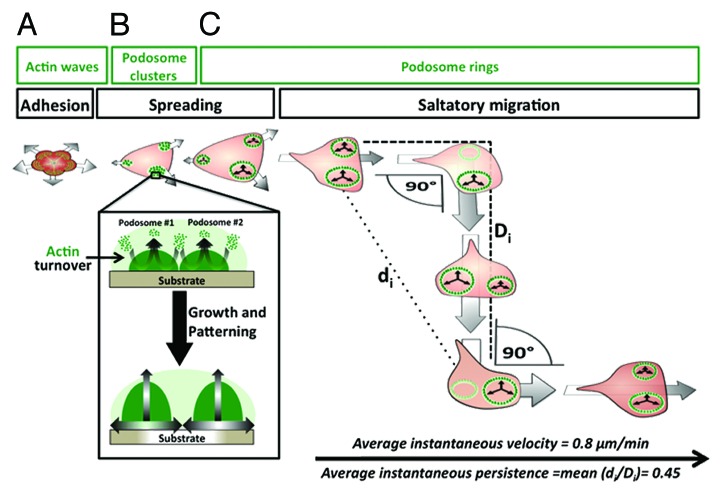
In an adherent cell such as the OC, the cell–matrix interplay is continuous but involves several different processes: adhesion initiation, spreading, and migration. Time-lapse microscopy of OCs undergoing de novo adhesion onto a substrate has shown that as soon as these giant multinucleated cells contact the matrix, they first fully spread before they can start migrating in a given direction. In the first phase of adhesion, corresponding to approximately the first 10–20 min of contact with its substrate, the OC becomes attached to it but does not display podosomes.95 Instead, its actin cytoskeleton adopts the form of intracellular waves of unorganized actin that are pooled in lateral membrane protrusions. This coincides with the initial, minimal outward spreading of the plasma membrane. The subsequent major phase of OC spreading is concomitant with podosome formation and cluster patterning.95 Indeed, after 10–20 min of contact with the substrate, given the fact that actin recruitment in podosomes occurs at the level of the adhesion plaque at the immediate vicinity of the cell membrane, it would mean that podosome assembly and, podosome core growth in a bottom-up manner exerts a force on the membrane itself, pushing it away from the center of the cell, and thus, inducing spreading.40,78,95,96 Conversely, the inhibition of integrins in an already-spread OC results in two sequential phases of detachment: first, within the first seconds, podosomes disassemble but the cell remains spread, then, the cell retracts and detaches from the substrate.95 These observations have allowed the conclusion that podosomes are not the only mediators of adhesion in OCs, at least in a short period of time, but that they greatly enhance and maintain it. Indeed, while previous investigations of OC adhesion have been performed on culture-treated substrates, it has been shown that OCs can adhere when seeded on a substrate, which does not allow podosome formation, i.e., devoid of either integrin ligands or more specifically αvβ3integrin ligand.75,93
Ring-driven saltatory migration
An adherent and fully spread OC forms podosomes that grow in a confined space during their assembly phase. The growth of podosomes in close vicinity within a cluster leads to them “pushing” each other, thus resulting in a ring pattern. While rings expand by rapid podosome assembly and disassembly in an outward radial direction, they apply traction forces on the matrix, thus steering a part of the cell that becomes a leading edge. This cluster-to-ring transition corresponds with the initiation of OC migration (Fig. 3). Within one OC, at least two podosome rings grow simultaneously, hence creating two leading edges at opposite directions of the cell. Rings on one edge eventually dissociate, whereas the most stable ring determines the migratory direction95 (Fig. 3). These events translate into a cyclic biphasic migration model comprised of: the straightforward movement phase, followed by the angular “jump” of the entire cell of a characteristic deviation of 90 °C95 (Fig. 3). In the normal situation, the OC migrates at an average instantaneous velocity of 0.8 µm/s and with a track persistence value of 0.45 (0 being a constant rotation of the cell around itself and 1 being a perfectly straight line)97 (Fig. 3).
Figure 3. Osteoclast adhesion, spreading, and migration. (A) When the osteoclasts first adheres to its substrate, it is contracted and radial waves of disorganized actin are consistent with the initiation of its spreading. (B) At the early stages of spreading, podosomes form at the periphery of the osteoclast and cluster at the edges of the cell. The dynamic turnover of actin within each podosome leads to podosome growth. Growing neighboring podosomes apply forces on one another and, to the substrate if possible, thus leading to further spreading of the cell periphery. Individual podosome growth during osteoclast spreading also leads to their collective patterning from clusters into circular superstructures called podosome rings. (C) Podosome rings continue to display the actin dynamics and podosome growth observed in clusters. The expansion of individual rings generates forces that drive osteoclast displacement. A typical mode of osteoclast motility on two-dimensional substrates, called saltatory migration, is a biphasic cycle, namely due to ring assembly and disassembly in different areas of the cell. In the first phase, podosome rings expanding at the same side of the cell, drive osteoclast displacement in a straightforward direction. The second phase is consistent with the disassembly/collapse of one or more rings and the continued expansion of the remaining ring leads to a 90 °C-angular turn in direction. Soon after the angular turn, new rings are formed and determine the new leading edge of the osteoclast, thus restarting a second phase of straightforward movement. The latter ends with a new angular turn and the biphasic cycle of migration is therefore maintained for several rounds, allowing the osteoclast to cover a wide area of the substrate. Saltatory osteoclast migration can be characterized by quantifiable parameters, such as an average velocity of 0.8 µm/min and an average instantaneous persistence of 0.45. This mode of migration as well as its quantified parameters have been characterized in osteoclasts migration on culture-treated dishes.
Podosome-driven migration seems to be the main mode of OC motility when these cells can actually form podosomes, i.e., when these cells are seeded on culture-treated substrates or on bone. Interestingly, a recent report investigating OC differentiation and migration on non-functionalized substrates, i.e., substrates without integrin ligands, has shown that OCs devoid of podosomes can still efficiently migrate and differentiate.75
Podosome Regulatory Pathways in Osteoclasts
Ring-driven migration and SZ formation are essential to OC-mediated bone resorption. Podosomes are the structural units of the ring and the SZ and, although the intrinsic molecular composition of podosomes is well described, the signaling pathways that govern their collective organization into these superstructures remains relatively uncovered. This section will focus on the essential studies that have elucidated the molecular mechanisms involved in podosome patterning.
Tyrosine protein kinases—Focus on Src
During the collective patterning of podosomes into the SZ or the SZL, the apparent amounts of structural proteins such as actin, vinculin, cortactin, α-actinin, and paxillin increase within the individual podosome. The increase of these proteins is inversely correlated with global tyrosine phosphorylation in podosomes.94,98 This residue-specific post-translational modification has been associated with dynamic changes in adhesion structures.
In OCs, Src localizes to podosomes and is important for their assembly as well as their patterning.65 In addition, Src−/− mice exhibit a severe osteopetrosis due to dysfunctional OCs.99 The dual importance of Src involves structural/docking activity and kinase activity. The first is necessary for the proper formation of the actin cloud in podosomes probably by binding the cytoplasmic tail of β3 and Pyk2 at the same time.65,100 Podosome cluster to podosome belt transition is impaired in both Src−/− and Pyk2 −/− osteoclasts, resulting in defective bone resorption.65,101 Interestingly, although Src kinase activity has been shown important for podosome initiation, it is most crucial for podosome patterning by phosphorylating several downstream targets regulating actin dynamics in osteoclasts.65 In contrast, Pyk2 catalytic activity is not required for such transition but rather controls microtubule-dependent belt formation through inhibition of RhoA–mDia2 pathway.101 Upon integrin activation, Cbl adaptator proteins are also recruited by the Src-Pyk2 complex to promote podosome belt formation.102 Dynamin overexpression increases osteoclast-mediated bone resorption and migration. Cbl was shown to indirectly bind to dynamin and form a complex at the podosome belt. In addition, Src kinase activity is able to disrupt this complex promoting dynamin self-assembly and GTPase activity. In turn, dynamin GTPase activity reduces Pyk2 Y402 phosphorylation, which induces Src dissociation from the complex, providing a possible negative feedback of the Src signaling cascade and suggesting a coordinated role of Src, Cbl, and dynamin in regulating actin remodeling.103,104 Moreover, Src can activate another tyrosine kinase Syk, which is involved in podosomal rearrangement by facilitating Rac GTPase activation.105 Indeed, mice lacking Src, Syk, or Vav3 expression suffer from increased bone mass due to defective OC-resorption.99,105-107 Another target of Src is cortactin, which induces slow actin turnover in podosomes in its phosphorylated form, and thus, contributes to SZL formation.92,108 Finally, Src expression has been shown to increase during osteoclastogenesis,109 thus suggesting that it is needed for mature podosome patterns.110 When other tyrosine kinases that are important for podosome patterning such as Hck are deleted, OCs compensate for this deletion by overexpressing Src, which in fact re-establishes mature podosome patterning, i.e., SZL formation, and above-average OC resorptive capacity. However, Src is not expressed in OC precursors, and therefore, its overexpression cannot rescue defected precursor migration toward bone. As a result, Hck−/− mice, despite overexpressing Src in OCs, are ostepetrotic.109
It is widely assumed that tyrosine phosphorylation of downstream mediators of integrins (e.g., c-Src, Pyk2) and of podosome-associated proteins, is an essential mechanism for collective podosome patterning. In contrast, reversible tyrosine phosphorylation is also a regulatory process, which establishes the general phosphorylation status of key proteins playing a role in podosomal organization, but its investigation has been long neglected. Indeed, the use of non-specific phosphatase inhibitors (orthovanadate or bisphosphonates) revealed the importance of protein tyrosine phosphatases (PTP) in promoting OC-mediated bone resorption. PTP epsilon (PTPε) is highly expressed in OCs and has been reported to activate Src by removing the inhibitory phosphorylation at Y527, and thus, allowing Src protein unfolding and subsequent activation. Mice lacking PTPε display a bone osteopetrotic phenotype due to defective resorption. In fact, PTPε-deficient OC podosomes mostly conserve an individual aspect and only a fraction of these show SZL patterning. A poor interconnecting radial actin network, as well as increased stability and lifespan, characterized these podosomes.111 Additionally, the receptor PTP CD45 also supports OC-mediated resorption; CD45-deficient OC have abnormal morphology and reduced migration, which is correlated with a decreased Src activity; this in vitro phenotype is in accordance with defective trabecular remodeling in CD45-deficient mice.112 Furthermore, PTP-PEST overexpression in RAW-264.7-derived OCs promotes activation of Src by dephosphorylation at Y527, resulting in an increased phosphorylation of Y421 cortactin and WASP at Y294. All these studies report positive regulation of bone resorption by PTP acting on Src activation and podosome patterning. Inversely, no PTP has been yet reported to restrain OC activity by regulating the actin cytoskeleton. However, PTP can be negative regulators of other OC processes such as RANKL-mediated proliferation and differentiation. PTP SHP-deficient mice present a low bone mass phenotype explained by an increased pool of OC progenitors showing an elevated fusion index resulting in a high number of enlarged osteoclasts containing up to 100 nuclei. In addition, SHP-deficient osteoclasts are hyper-resorptive showing a prolonged lifespan.113 The activity of PTPs, their substrate specificity, and tissue-specific pattern is poorly documented and is clearly a field waiting to be explored in bone physiology.
Rho and Rac GTPases
This family of molecular switches mediates signal transduction and cytoskeletal remodeling related to a broad spectrum of cellular processes in all cell types.114,115 These molecules have been extensively studied in vitro and in vivo, but the specificity of the OC cytoskeleton raises the difficulty in extrapolating their roles into OCs. The comprehension of the roles of these proteins has been coincidently further complicated by several technical difficulties encountered by investigators as will be briefly discussed in the following paragraphs.
The importance of Rho to podosome organization and OC-mediated bone resorption has first been commented by Zhang and colleagues. In their study, the microinjection of C3 exoenzyme (a Clostridium botulinum toxin, which inhibits RhoA/B/C) in murine OC-like cells resulted in the disassembly of the SZL structure after 20 min.116 The treatment of avian OCs with C3 lead, in the first 15 min, to transient growth of podosomes eventually resulted in their complete dissolution 2 h after the treatment.117 Also, avian macrophage polykaryons treated with membrane-permeable C3 suffered from total podosomes disassembly.118 This data shows a positive contribution of Rho to podosome stability. However, when the reversed experiments were performed, i.e., when avian OCs were transduced using a constitutively active Rho, they also suffered from podosome disassembly after 30 min.117 The converging results of Rho overactivation and inhibition suggest the necessity for a precise and time-dependent Rho activation levels during podosome formation and patterning.
Even more, a discrepancy is observed between the C3-mediated podosome disassembly in avian macrophage polykaryons and C3-mediated stabilization of podosome rings and SZ disassembly.88,118 Whether this experimental contradiction is species-dependent and/or due to technical differences should be investigated.
Finally, the SZL-stabilizing role of Rho is dependent on the decrease of its GTPase activity during OC maturation. Indeed, lower levels of active Rho allow for less activation of its effector, mDia. In fact, active mDia can activate HDAC6, and thus, lead to deacetylation and destabilization of microtubules (MTs). Therefore, lower levels of Rho allow the stabilization of MTs by maintaining their acetylation, resulting in enhanced OC spreading, and SZL formation at the cell periphery.88
Rac1 and Rac2 are both expressed in OCs. These proteins are generally involved in organization of the cytoskeleton and are also important components of the NADPH, the enzyme that generates free radicals. The NADPH-related function of Rac has not been related with podosome organization.
A study of Rac functions in OCs using a murine Cre-recombinase-based genetic depletion model has depicted distinct roles of each of the two Rac proteins during OC precursor chemotaxis and differentiation in vivo.119 These results have been contested by Croke et al. (2011) claiming the insufficient depletion of Rac1 and Rac2 genes.120 In their study, Croke et al. (2011) have shown that Rac1 and Rac2 are not involved in osteoclastogenesis but have overlapping roles in podosome assembly and SZL formation by localizing Arp3 at podosome sites during osteoclastogenesis. In a Rac2−/− context, the deletion of Rac1 results in podosome disassembly, the absence of SZs, and diminished bone resorption but only if Rac1 deletion occurs at early myeloid precursor stage (under the Lysosome M promoter common to macrophages and granulocytes). Surprisingly, the abnormalities first observed in OCs due to Rac double knockout, were not reproduced when a different promoter corresponding to Cathepsin-K+ differentiated OCs drove Rac1 deletion. Cathepsin-K promoter expression is specific to late differentiated OCs; therefore, the authors proposed that during the differentiation process, late Rac−/− OCs still fuse with early Cathepsin K negative (i.e., Rac1 positive) mononucleated precursors, thus compensating Rac KO.120 Whether Rac1 and Rac2 play identical roles remains to be established by testing if Rac2 would compensate for the Rac-1 KO in the reverse experiment. The importance of Rac1/2 to the OC cytoskeleton and bone resorption is, however, confirmed by targeting them via intra-cellular blocking antibodies.121
Rac activity in OCs is regulated by its Guanine Exchange Factors (GEFs) Dock5 and vav3.105,122 Dock5 is increasingly expressed along osteoclastogenesis and localizes to podosomes in SZLs. The deletion of Dock5 in mice results in an osteopetrotic phenotype explained by decreased Rac activity, absence of podosome formation, and SZL patterning, leading to reduced adhesion and bone resorption.122 Distinctly from Dock5, the other Rac-specific GEF in OCs, vav3, is stably expressed during osteoclastogenesis but, like Dock5, promotes cell spreading and SZ formation.105 The two Rac GEFs still exert distinct functions. Vav3 controls Rac activation during the early events of OC adhesion while Dock5, in association with p130Cas, rather appears to activate Rac later during the adhesion processes.105,123,124 Physiologically, Vav3-null mice are osteopetrotic and protected from PTH- and RANK-stimulated bone loss indicating a role of this GEF in physiologically regulated bone remodeling.
Coupling Osteoclast Migration with Resorption
In a bone-adherent OC, the description of the straightforward development of patterns from single podosomes to clusters that induce spreading, then to rings that drive migration and, ultimately, to SZs that sustain bone resorption is currently accepted (Figs. 2 and 3). However, it is also widely accepted that, during its lifetime, the fully differentiated OC is capable of resorbing several areas on the bone surface, which means that this cell has the capacity to couple resorption with migration. This capacity has been made clear by two observations. First, the resorption tracks made by OC cultured on bone, dentine, ivory, or hydroxyapatite slices consist of several individual resorption lacunae that partially overlap to collectively form what is referred to as trails of resorption pits.125-128 Second, in vivo optical imaging of mature OCs has demonstrated the presence of two distinct states of the OC: motile and non-motile.129
Based on non-overlapping data provided by the literature, these observations imply two completely independent states of OC functions. These are resorption and migration, respectively.90 During bone resorption, the OC is static129 and is characterized by apico-basal polarity. The latter is defined by the formation of a RB, an FSD, a SZ, and the secretion of bone-degrading molecules.130 Once done with degrading a specific spot of bone, the OC then entirely dismisses the first state and adopts a migratory state characterized by the formation of a loose podosome ring and a “front-to-back” migratory polarity, i.e., with leading and trailing edges.95 Within the second migratory state, the RB and the FSD are completely lost and the bulk of the OC cytoplasm is at the leading edge.95 Finally, having migrated to a new area to be degraded, the OC reestablishes its resorption state (Fig. 4). This alternation between the two distinct polarizations concomitant with the loss of resorption-specific organelles, e.g., the RB, FSD, and SZ would implicate a loss of the degradative molecules already secreted and highly concentrated within the resorption lacuna. The functional loss of secreted protons and proteases is not accounted for in this SZ-ring alternation model, nor is the presence of heterogeneous matrix signals, which would differentially promote either the resorption state or the migration.
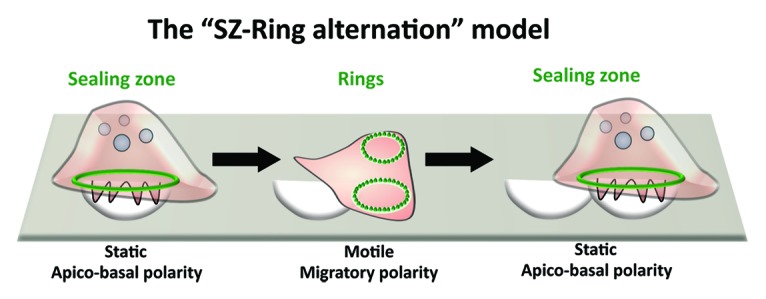
Figure 4. Osteoclast migration and resorption. Typically, osteoclasts resorb bone by forming resorption pit trails. In the current model for bone degradation and migration (SZ-Ring alternation), bone-resorbing osteoclasts are apico-basally polarized with a sealing zone. When resorption stops, they flatten and start migration by becoming polarized with a leading and a trailing edge. Then, osteoclast will stop again and start a new cycle of resorption.
Finally, live imaging tools and powerful mouse genetic models are now available for both in vitro and in vivo investigations of the many unresolved aspects of the OC function.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank all team members, past and present, for their invaluable contributions. Georgess D has been supported by fellowships from T3Net (Marie Curie Training Program) and by Fondation ARC. Blangy A has been supported by research grants from the Institut National du Cancer (grant # INCa-4361) and the Agence Nationale de la Recherche (ANR grant # ANR-2011-BLAN-006).
References
- 1.Bartl R. Osteoporosis: diagnosis, prevention, therapy. New York: Springer, 2009. [Google Scholar]
- 2.Harre U, Schett G. Bone research in 2012: the ups and downs of bone in health and rheumatic disease. Nat Rev Rheumatol. 2013;9:67–8. doi: 10.1038/nrrheum.2012.219. [DOI] [PubMed] [Google Scholar]
- 3.Harre U, Georgess D, Bang H, Bozec A, Axmann R, Ossipova E, Jakobsson PJ, Baum W, Nimmerjahn F, Szarka E, et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Invest. 2012;122:1791–802. doi: 10.1172/JCI60975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–19. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 5.Lazner F, Gowen M, Pavasovic D, Kola I. Osteopetrosis and osteoporosis: two sides of the same coin. Hum Mol Genet. 1999;8:1839–46. doi: 10.1093/hmg/8.10.1839. [DOI] [PubMed] [Google Scholar]
- 6.Geissmann F. The origin of dendritic cells. Nat Immunol. 2007;8:558–60. doi: 10.1038/ni0607-558. [DOI] [PubMed] [Google Scholar]
- 7.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. doi: 10.1016/S0092-8674(00)81569-X. [DOI] [PubMed] [Google Scholar]
- 8.Fuller K, Wong B, Fox S, Choi Y, Chambers TJ. TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J Exp Med. 1998;188:997–1001. doi: 10.1084/jem.188.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuzaki K, Udagawa N, Takahashi N, Yamaguchi K, Yasuda H, Shima N, Morinaga T, Toyama Y, Yabe Y, Higashio K, et al. Osteoclast differentiation factor (ODF) induces osteoclast-like cell formation in human peripheral blood mononuclear cell cultures. Biochem Biophys Res Commun. 1998;246:199–204. doi: 10.1006/bbrc.1998.8586. [DOI] [PubMed] [Google Scholar]
- 10.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallois A, Lachuer J, Yvert G, Wierinckx A, Brunet F, Rabourdin-Combe C, Delprat C, Jurdic P, Mazzorana M. Genome-wide expression analyses establish dendritic cells as a new osteoclast precursor able to generate bone-resorbing cells more efficiently than monocytes. J Bone Miner Res. 2010;25:661–72. doi: 10.1359/jbmr.090829. [DOI] [PubMed] [Google Scholar]
- 12.Rivollier A, Mazzorana M, Tebib J, Piperno M, Aitsiselmi T, Rabourdin-Combe C, Jurdic P, Servet-Delprat C. Immature dendritic cell transdifferentiation into osteoclasts: a novel pathway sustained by the rheumatoid arthritis microenvironment. Blood. 2004;104:4029–37. doi: 10.1182/blood-2004-01-0041. [DOI] [PubMed] [Google Scholar]
- 13.Servet-Delprat C, Arnaud S, Jurdic P, Nataf S, Grasset MF, Soulas C, Domenget C, Destaing O, Rivollier A, Perret M, et al. Flt3+ macrophage precursors commit sequentially to osteoclasts, dendritic cells and microglia. BMC Immunol. 2002;3:15. doi: 10.1186/1471-2172-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Speziani C, Rivollier A, Gallois A, Coury F, Mazzorana M, Azocar O, Flacher M, Bella C, Tebib J, Jurdic P, et al. Murine dendritic cell transdifferentiation into osteoclasts is differentially regulated by innate and adaptive cytokines. Eur J Immunol. 2007;37:747–57. doi: 10.1002/eji.200636534. [DOI] [PubMed] [Google Scholar]
- 15.Wakkach A, Mansour A, Dacquin R, Coste E, Jurdic P, Carle GF, Blin-Wakkach C. Bone marrow microenvironment controls the in vivo differentiation of murine dendritic cells into osteoclasts. Blood. 2008;112:5074–83. doi: 10.1182/blood-2008-01-132787. [DOI] [PubMed] [Google Scholar]
- 16.Horowitz MC, Lorenzo JA. The origins of osteoclasts. Curr Opin Rheumatol. 2004;16:464–8. doi: 10.1097/01.bor.0000127825.05580.eb. [DOI] [PubMed] [Google Scholar]
- 17.Jacome-Galarza CE, Lee SK, Lorenzo JA, Aguila HL. Identification, characterization, and isolation of a common progenitor for osteoclasts, macrophages, and dendritic cells from murine bone marrow and periphery. J Bone Miner Res. 2013;28:1203–13. doi: 10.1002/jbmr.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otero K, Shinohara M, Zhao H, Cella M, Gilfillan S, Colucci A, Faccio R, Ross FP, Teitelbaum SL, Takayanagi H, et al. TREM2 and β-catenin regulate bone homeostasis by controlling the rate of osteoclastogenesis. J Immunol. 2012;188:2612–21. doi: 10.4049/jimmunol.1102836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao B, Ivashkiv LB. Negative regulation of osteoclastogenesis and bone resorption by cytokines and transcriptional repressors. Arthritis Res Ther. 2011;13:234. doi: 10.1186/ar3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakashima T, Takayanagi H. New regulation mechanisms of osteoclast differentiation. Ann N Y Acad Sci. 2011;1240:E13–8. doi: 10.1111/j.1749-6632.2011.06373.x. [DOI] [PubMed] [Google Scholar]
- 21.Takayanagi H. The role of NFAT in osteoclast formation. Ann N Y Acad Sci. 2007;1116:227–37. doi: 10.1196/annals.1402.071. [DOI] [PubMed] [Google Scholar]
- 22.Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–24. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pettit AR, Ji H, von Stechow D, Müller R, Goldring SR, Choi Y, Benoist C, Gravallese EM. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol. 2001;159:1689–99. doi: 10.1016/S0002-9440(10)63016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Brien CA, Nakashima T, Takayanagi H. Osteocyte control of osteoclastogenesis. Bone. 2013;54:258–63. doi: 10.1016/j.bone.2012.08.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coxon FP, Taylor A. Vesicular trafficking in osteoclasts. Semin Cell Dev Biol. 2008;19:424–33. doi: 10.1016/j.semcdb.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Mulari M, Vääräniemi J, Väänänen HK. Intracellular membrane trafficking in bone resorbing osteoclasts. Microsc Res Tech. 2003;61:496–503. doi: 10.1002/jemt.10371. [DOI] [PubMed] [Google Scholar]
- 27.Yadav VK, Oury F, Tanaka KF, Thomas T, Wang Y, Cremers S, Hen R, Krust A, Chambon P, Karsenty G. Leptin-dependent serotonin control of appetite: temporal specificity, transcriptional regulation, and therapeutic implications. J Exp Med. 2011;208:41–52. doi: 10.1084/jem.20101940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abu-Amer Y, Ross FP, Schlesinger P, Tondravi MM, Teitelbaum SL. Substrate recognition by osteoclast precursors induces C-src/microtubule association. J Cell Biol. 1997;137:247–58. doi: 10.1083/jcb.137.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulari MT, Zhao H, Lakkakorpi PT, Väänänen HK. Osteoclast ruffled border has distinct subdomains for secretion and degraded matrix uptake. Traffic. 2003;4:113–25. doi: 10.1034/j.1600-0854.2003.40206.x. [DOI] [PubMed] [Google Scholar]
- 30.Karsdal MA, Henriksen K, Sørensen MG, Gram J, Schaller S, Dziegiel MH, Heegaard AM, Christophersen P, Martin TJ, Christiansen C, et al. Acidification of the osteoclastic resorption compartment provides insight into the coupling of bone formation to bone resorption. Am J Pathol. 2005;166:467–76. doi: 10.1016/S0002-9440(10)62269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rousselle AV, Heymann D. Osteoclastic acidification pathways during bone resorption. Bone. 2002;30:533–40. doi: 10.1016/S8756-3282(02)00672-5. [DOI] [PubMed] [Google Scholar]
- 32.Silver IA, Murrills RJ, Etherington DJ. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res. 1988;175:266–76. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]
- 33.Baron R, Neff L, Louvard D, Courtoy PJ. Cell-mediated extracellular acidification and bone resorption: evidence for a low pH in resorbing lacunae and localization of a 100-kD lysosomal membrane protein at the osteoclast ruffled border. J Cell Biol. 1985;101:2210–22. doi: 10.1083/jcb.101.6.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linder S, Wiesner C, Himmel M. Degrading devices: invadosomes in proteolytic cell invasion. Annu Rev Cell Dev Biol. 2011;27:185–211. doi: 10.1146/annurev-cellbio-092910-154216. [DOI] [PubMed] [Google Scholar]
- 36.Rottiers P, Saltel F, Daubon T, Chaigne-Delalande B, Tridon V, Billottet C, Reuzeau E, Génot E. TGFbeta-induced endothelial podosomes mediate basement membrane collagen degradation in arterial vessels. J Cell Sci. 2009;122:4311–8. doi: 10.1242/jcs.057448. [DOI] [PubMed] [Google Scholar]
- 37.Saltel F, Daubon T, Juin A, Ganuza IE, Veillat V, Génot E. Invadosomes: intriguing structures with promise. Eur J Cell Biol. 2011;90:100–7. doi: 10.1016/j.ejcb.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 38.Jurdic P, Saltel F, Chabadel A, Destaing O. Podosome and sealing zone: specificity of the osteoclast model. Eur J Cell Biol. 2006;85:195–202. doi: 10.1016/j.ejcb.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 39.Destaing O, Saltel F, Géminard JC, Jurdic P, Bard F. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol Biol Cell. 2003;14:407–16. doi: 10.1091/mbc.E02-07-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Labernadie A, Thibault C, Vieu C, Maridonneau-Parini I, Charrière GM. Dynamics of podosome stiffness revealed by atomic force microscopy. Proc Natl Acad Sci U S A. 2010;107:21016–21. doi: 10.1073/pnas.1007835107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linder S, Wiesner C, Himmel M. Degrading devices: invadosomes in proteolytic cell invasion. Annu Rev Cell Dev Biol. 2011;27:185–211. doi: 10.1146/annurev-cellbio-092910-154216. [DOI] [PubMed] [Google Scholar]
- 42.Luxenburg C, Geblinger D, Klein E, Anderson K, Hanein D, Geiger B, Addadi L. The architecture of the adhesive apparatus of cultured osteoclasts: from podosome formation to sealing zone assembly. PLoS One. 2007;2:e179. doi: 10.1371/journal.pone.0000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt S, Nakchbandi I, Ruppert R, Kawelke N, Hess MW, Pfaller K, Jurdic P, Fässler R, Moser M. Kindlin-3-mediated signaling from multiple integrin classes is required for osteoclast-mediated bone resorption. J Cell Biol. 2011;192:883–97. doi: 10.1083/jcb.201007141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gluckman E, Esperou H, Devergie A, Traineau R, Leverger G, Schaison G. Pediatric bone marrow transplantation for leukemia and aplastic anemia. Report of 222 cases transplanted in a single center. Nouv Rev Fr Hematol. 1989;31:111–4. [PubMed] [Google Scholar]
- 45.Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Culleré M, Ross FP, Coller BS, Teitelbaum S, Hynes RO. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–38. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McHugh KP, Hodivala-Dilke K, Zheng MH, Namba N, Lam J, Novack D, Feng X, Ross FP, Hynes RO, Teitelbaum SL. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J Clin Invest. 2000;105:433–40. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faccio R, Novack DV, Zallone A, Ross FP, Teitelbaum SL. Dynamic changes in the osteoclast cytoskeleton in response to growth factors and cell attachment are controlled by beta3 integrin. J Cell Biol. 2003;162:499–509. doi: 10.1083/jcb.200212082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Destaing O, Planus E, Bouvard D, Oddou C, Badowski C, Bossy V, Raducanu A, Fourcade B, Albiges-Rizo C, Block MR. β1A integrin is a master regulator of invadosome organization and function. Mol Biol Cell. 2010;21:4108–19. doi: 10.1091/mbc.E10-07-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nesbitt S, Nesbit A, Helfrich M, Horton M. Biochemical characterization of human osteoclast integrins. Osteoclasts express alpha v beta 3, alpha 2 beta 1, and alpha v beta 1 integrins. J Biol Chem. 1993;268:16737–45. [PubMed] [Google Scholar]
- 50.Calderwood DA. Integrin activation. J Cell Sci. 2004;117:657–66. doi: 10.1242/jcs.01014. [DOI] [PubMed] [Google Scholar]
- 51.Zou W, Izawa T, Zhu T, Chappel J, Otero K, Monkley SJ, Critchley DR, Petrich BG, Morozov A, Ginsberg MH, et al. Talin1 and Rap1 are critical for osteoclast function. Mol Cell Biol. 2013;33:830–44. doi: 10.1128/MCB.00790-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winkler J, Lünsdorf H, Jockusch BM. Energy-filtered electron microscopy reveals that talin is a highly flexible protein composed of a series of globular domains. Eur J Biochem. 1997;243:430–6. doi: 10.1111/j.1432-1033.1997.0430a.x. [DOI] [PubMed] [Google Scholar]
- 53.Papagrigoriou E, Gingras AR, Barsukov IL, Bate N, Fillingham IJ, Patel B, Frank R, Ziegler WH, Roberts GC, Critchley DR, et al. Activation of a vinculin-binding site in the talin rod involves rearrangement of a five-helix bundle. EMBO J. 2004;23:2942–51. doi: 10.1038/sj.emboj.7600285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jockusch BM, Rüdiger M. Crosstalk between cell adhesion molecules: vinculin as a paradigm for regulation by conformation. Trends Cell Biol. 1996;6:311–5. doi: 10.1016/0962-8924(96)10022-2. [DOI] [PubMed] [Google Scholar]
- 55.Turner CE, Glenney JR, Jr., Burridge K. Paxillin: a new vinculin-binding protein present in focal adhesions. J Cell Biol. 1990;111:1059–68. doi: 10.1083/jcb.111.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu H, Parsons JT. Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J Cell Biol. 1993;120:1417–26. doi: 10.1083/jcb.120.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calle Y, Chou HC, Thrasher AJ, Jones GE. Wiskott-Aldrich syndrome protein and the cytoskeletal dynamics of dendritic cells. J Pathol. 2004;204:460–9. doi: 10.1002/path.1651. [DOI] [PubMed] [Google Scholar]
- 58.Monypenny J, Chou HC, Bañón-Rodríguez I, Thrasher AJ, Antón IM, Jones GE, Calle Y. Role of WASP in cell polarity and podosome dynamics of myeloid cells. Eur J Cell Biol. 2011;90:198–204. doi: 10.1016/j.ejcb.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kinley AW, Weed SA, Weaver AM, Karginov AV, Bissonette E, Cooper JA, Parsons JT. Cortactin interacts with WIP in regulating Arp2/3 activation and membrane protrusion. Curr Biol. 2003;13:384–93. doi: 10.1016/S0960-9822(03)00107-6. [DOI] [PubMed] [Google Scholar]
- 60.Antón IM, Jones GE, Wandosell F, Geha R, Ramesh N. WASP-interacting protein (WIP): working in polymerisation and much more. Trends Cell Biol. 2007;17:555–62. doi: 10.1016/j.tcb.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 61.Krzewski K, Chen X, Orange JS, Strominger JL. Formation of a WIP-, WASp-, actin-, and myosin IIA-containing multiprotein complex in activated NK cells and its alteration by KIR inhibitory signaling. J Cell Biol. 2006;173:121–32. doi: 10.1083/jcb.200509076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de la Fuente MA, Sasahara Y, Calamito M, Antón IM, Elkhal A, Gallego MD, Suresh K, Siminovitch K, Ochs HD, Anderson KC, et al. WIP is a chaperone for Wiskott-Aldrich syndrome protein (WASP) Proc Natl Acad Sci U S A. 2007;104:926–31. doi: 10.1073/pnas.0610275104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chabadel A, Bañon-Rodríguez I, Cluet D, Rudkin BB, Wehrle-Haller B, Genot E, Jurdic P, Anton IM, Saltel F. CD44 and beta3 integrin organize two functionally distinct actin-based domains in osteoclasts. Mol Biol Cell. 2007;18:4899–910. doi: 10.1091/mbc.E07-04-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goodison S, Urquidi V, Tarin D. CD44 cell adhesion molecules. Mol Pathol. 1999;52:189–96. doi: 10.1136/mp.52.4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Destaing O, Sanjay A, Itzstein C, Horne WC, Toomre D, De Camilli P, Baron R. The tyrosine kinase activity of c-Src regulates actin dynamics and organization of podosomes in osteoclasts. Mol Biol Cell. 2008;19:394–404. doi: 10.1091/mbc.E07-03-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mersich AT, Miller MR, Chkourko H, Blystone SD. The formin FRL1 (FMNL1) is an essential component of macrophage podosomes. Cytoskeleton (Hoboken) 2010;67:573–85. doi: 10.1002/cm.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luxenburg C, Winograd-Katz S, Addadi L, Geiger B. Involvement of actin polymerization in podosome dynamics. J Cell Sci. 2012;125:1666–72. doi: 10.1242/jcs.075903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wanger M, Keiser T, Neuhaus JM, Wegner A. The actin treadmill. Can J Biochem Cell Biol. 1985;63:414–21. doi: 10.1139/o85-060. [DOI] [PubMed] [Google Scholar]
- 69.Yin HL, Albrecht JH, Fattoum A. Identification of gelsolin, a Ca2+-dependent regulatory protein of actin gel-sol transformation, and its intracellular distribution in a variety of cells and tissues. J Cell Biol. 1981;91:901–6. doi: 10.1083/jcb.91.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Southwick FS. Gelsolin and ADF/cofilin enhance the actin dynamics of motile cells. Proc Natl Acad Sci U S A. 2000;97:6936–8. doi: 10.1073/pnas.97.13.6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zallone AZ, Teti A, Primavera MV, Naldini L, Marchisio PC. Osteoclasts and monocytes have similar cytoskeletal structures and adhesion property in vitro. J Anat. 1983;137:57–70. [PMC free article] [PubMed] [Google Scholar]
- 72.Marchisio PC, Cirillo D, Naldini L, Primavera MV, Teti A, Zambonin-Zallone A. Cell-substratum interaction of cultured avian osteoclasts is mediated by specific adhesion structures. J Cell Biol. 1984;99:1696–705. doi: 10.1083/jcb.99.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duong LT, Lakkakorpi P, Nakamura I, Rodan GA. Integrins and signaling in osteoclast function. Matrix Biol. 2000;19:97–105. doi: 10.1016/S0945-053X(00)00051-2. [DOI] [PubMed] [Google Scholar]
- 74.Chellaiah M, Kizer N, Silva M, Alvarez U, Kwiatkowski D, Hruska KA. Gelsolin deficiency blocks podosome assembly and produces increased bone mass and strength. J Cell Biol. 2000;148:665–78. doi: 10.1083/jcb.148.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Touaitahuata H, Planus E, Albiges-Rizo C, Blangy A, Pawlak G. Podosomes are dispensable for osteoclast differentiation and migration. Eur J Cell Biol. 2013;92:139–49. doi: 10.1016/j.ejcb.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 76.Blangy A, Touaitahuata H, Cres G, Pawlak G. Cofilin activation during podosome belt formation in osteoclasts. PLoS One. 2012;7:e45909. doi: 10.1371/journal.pone.0045909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van den Dries K, Meddens MBM, de Keijzer S, Shekhar S, Subramaniam V, Figdor CG, Cambi A. Interplay between myosin IIA-mediated contractility and actin network integrity orchestrates podosome composition and oscillations. Nat Commun. 2013;4:1412. doi: 10.1038/ncomms2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu S, Biben T, Wang X, Jurdic P, Géminard JC. Internal dynamics of actin structures involved in the cell motility and adhesion: Modeling of the podosomes at the molecular level. J Theor Biol. 2011;270:25–30. doi: 10.1016/j.jtbi.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 79.Moreau V, Tatin F, Varon C, Génot E. Actin can reorganize into podosomes in aortic endothelial cells, a process controlled by Cdc42 and RhoA. Mol Cell Biol. 2003;23:6809–22. doi: 10.1128/MCB.23.19.6809-6822.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaverina I, Stradal TE, Gimona M. Podosome formation in cultured A7r5 vascular smooth muscle cells requires Arp2/3-dependent de-novo actin polymerization at discrete microdomains. J Cell Sci. 2003;116:4915–24. doi: 10.1242/jcs.00818. [DOI] [PubMed] [Google Scholar]
- 81.Calle Y, Burns S, Thrasher AJ, Jones GE. The leukocyte podosome. Eur J Cell Biol. 2006;85:151–7. doi: 10.1016/j.ejcb.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 82.Linder S, Nelson D, Weiss M, Aepfelbacher M. Wiskott-Aldrich syndrome protein regulates podosomes in primary human macrophages. Proc Natl Acad Sci U S A. 1999;96:9648–53. doi: 10.1073/pnas.96.17.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hammarfjord O, Falet H, Gurniak C, Hartwig JH, Wallin RP. Gelsolin-independent podosome formation in dendritic cells. PLoS One. 2011;6:e21615. doi: 10.1371/journal.pone.0021615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cougoule C, Van Goethem E, Le Cabec V, Lafouresse F, Dupré L, Mehraj V, Mège JL, Lastrucci C, Maridonneau-Parini I. Blood leukocytes and macrophages of various phenotypes have distinct abilities to form podosomes and to migrate in 3D environments. Eur J Cell Biol. 2012;91:938–49. doi: 10.1016/j.ejcb.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 85.Bhuwania R, Cornfine S, Fang Z, Krüger M, Luna EJ, Linder S. Supervillin couples myosin-dependent contractility to podosomes and enables their turnover. J Cell Sci. 2012;125:2300–14. doi: 10.1242/jcs.100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bañón-Rodríguez I, Monypenny J, Ragazzini C, Franco A, Calle Y, Jones GE, Antón IM. The cortactin-binding domain of WIP is essential for podosome formation and extracellular matrix degradation by murine dendritic cells. Eur J Cell Biol. 2011;90:213–23. doi: 10.1016/j.ejcb.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 87.Linder S, Hüfner K, Wintergerst U, Aepfelbacher M. Microtubule-dependent formation of podosomal adhesion structures in primary human macrophages. J Cell Sci. 2000;113:4165–76. doi: 10.1242/jcs.113.23.4165. [DOI] [PubMed] [Google Scholar]
- 88.Destaing O, Saltel F, Gilquin B, Chabadel A, Khochbin S, Ory S, Jurdic P. A novel Rho-mDia2-HDAC6 pathway controls podosome patterning through microtubule acetylation in osteoclasts. J Cell Sci. 2005;118:2901–11. doi: 10.1242/jcs.02425. [DOI] [PubMed] [Google Scholar]
- 89.Biosse Duplan M, Zalli D, Stephens S, Zenger S, Neff L, Oelkers JM, Lai FP, Horne W, Rottner K, Baron R. Microtubule dynamic instability controls podosome patterning in osteoclasts through EB1, cortactin, and Src. Mol Cell Biol. 2014;34:16–29. doi: 10.1128/MCB.00578-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saltel F, Destaing O, Bard F, Eichert D, Jurdic P. Apatite-mediated actin dynamics in resorbing osteoclasts. Mol Biol Cell. 2004;15:5231–41. doi: 10.1091/mbc.E04-06-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Luxenburg C, Addadi L, Geiger B. The molecular dynamics of osteoclast adhesions. Eur J Cell Biol. 2006;85:203–11. doi: 10.1016/j.ejcb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 92.Luxenburg C, Parsons JT, Addadi L, Geiger B. Involvement of the Src-cortactin pathway in podosome formation and turnover during polarization of cultured osteoclasts. J Cell Sci. 2006;119:4878–88. doi: 10.1242/jcs.03271. [DOI] [PubMed] [Google Scholar]
- 93.Fuller K, Ross JL, Szewczyk KA, Moss R, Chambers TJ. Bone is not essential for osteoclast activation. PLoS One. 2010;5:e12837. doi: 10.1371/journal.pone.0012837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Luxenburg C, Winograd-Katz S, Addadi L, Geiger B. Involvement of actin polymerization in podosome dynamics. J Cell Sci. 2012;125:1666–72. doi: 10.1242/jcs.075903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu S, Planus E, Georgess D, Place C, Wang X, Albiges-Rizo C, Jurdic P, Géminard JC. Podosome rings generate forces that drive saltatory osteoclast migration. Mol Biol Cell. 2011;22:3120–6. doi: 10.1091/mbc.E11-01-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van den Dries K, Meddens MB, de Keijzer S, Shekhar S, Subramaniam V, Figdor CG, Cambi A. Interplay between myosin IIA-mediated contractility and actin network integrity orchestrates podosome composition and oscillations. Nat Commun. 2013;4:1412. doi: 10.1038/ncomms2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Georgess D, Mazzorana M, Terrado J, Delprat C, Chamot C, Guasch RM, Pérez-Roger I, Jurdic P, Machuca-Gayet I. Comparative transcriptomics reveal RhoE as a novel regulator of actin dynamics in bone-resorbing osteoclasts. Mol Biol Cell. 2013 doi: 10.1091/mbc.E13-07-0363. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luxenburg C, Parsons JT, Addadi L, Geiger B. Involvement of the Src-cortactin pathway in podosome formation and turnover during polarization of cultured osteoclasts. J Cell Sci. 2006;119:4878–88. doi: 10.1242/jcs.03271. [DOI] [PubMed] [Google Scholar]
- 99.Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-O. [DOI] [PubMed] [Google Scholar]
- 100.Zou W, Kitaura H, Reeve J, Long F, Tybulewicz VL, Shattil SJ, Ginsberg MH, Ross FP, Teitelbaum SL. Syk, c-Src, the alphavbeta3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J Cell Biol. 2007;176:877–88. doi: 10.1083/jcb.200611083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gil-Henn H, Destaing O, Sims NA, Aoki K, Alles N, Neff L, Sanjay A, Bruzzaniti A, De Camilli P, Baron R, et al. Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2(-/-) mice. J Cell Biol. 2007;178:1053–64. doi: 10.1083/jcb.200701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Purev E, Neff L, Horne WC, Baron R. c-Cbl and Cbl-b act redundantly to protect osteoclasts from apoptosis and to displace HDAC6 from beta-tubulin, stabilizing microtubules and podosomes. Mol Biol Cell. 2009;20:4021–30. doi: 10.1091/mbc.E09-03-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bruzzaniti A, Neff L, Sanjay A, Horne WC, De Camilli P, Baron R. Dynamin forms a Src kinase-sensitive complex with Cbl and regulates podosomes and osteoclast activity. Mol Biol Cell. 2005;16:3301–13. doi: 10.1091/mbc.E04-12-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bruzzaniti A, Neff L, Sandoval A, Du L, Horne WC, Baron R. Dynamin reduces Pyk2 Y402 phosphorylation and SRC binding in osteoclasts. Mol Cell Biol. 2009;29:3644–56. doi: 10.1128/MCB.00851-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Faccio R, Teitelbaum SL, Fujikawa K, Chappel J, Zallone A, Tybulewicz VL, Ross FP, Swat W. Vav3 regulates osteoclast function and bone mass. Nat Med. 2005;11:284–90. doi: 10.1038/nm1194. [DOI] [PubMed] [Google Scholar]
- 106.Lowell CA, Soriano P. Knockouts of Src-family kinases: stiff bones, wimpy T cells, and bad memories. Genes Dev. 1996;10:1845–57. doi: 10.1101/gad.10.15.1845. [DOI] [PubMed] [Google Scholar]
- 107.Horne WC, Neff L, Chatterjee D, Lomri A, Levy JB, Baron R. Osteoclasts express high levels of pp60c-src in association with intracellular membranes. J Cell Biol. 1992;119:1003–13. doi: 10.1083/jcb.119.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Martinez-Quiles N, Ho HY, Kirschner MW, Ramesh N, Geha RS. Erk/Src phosphorylation of cortactin acts as a switch on-switch off mechanism that controls its ability to activate N-WASP. Mol Cell Biol. 2004;24:5269–80. doi: 10.1128/MCB.24.12.5269-5280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vérollet C, Gallois A, Dacquin R, Lastrucci C, Pandruvada SN, Ortega N, Poincloux R, Behar A, Cougoule C, Lowell C, et al. Hck contributes to bone homeostasis by controlling the recruitment of osteoclast precursors. FASEB J. 2013;27:3608–18. doi: 10.1096/fj.13-232736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Horne WC, Sanjay A, Bruzzaniti A, Baron R. The role(s) of Src kinase and Cbl proteins in the regulation of osteoclast differentiation and function. Immunol Rev. 2005;208:106–25. doi: 10.1111/j.0105-2896.2005.00335.x. [DOI] [PubMed] [Google Scholar]
- 111.Granot-Attas S, Luxenburg C, Finkelshtein E, Elson A. Protein tyrosine phosphatase epsilon regulates integrin-mediated podosome stability in osteoclasts by activating Src. Mol Biol Cell. 2009;20:4324–34. doi: 10.1091/mbc.E08-11-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shivtiel S, Kollet O, Lapid K, Schajnovitz A, Goichberg P, Kalinkovich A, Shezen E, Tesio M, Netzer N, Petit I, et al. CD45 regulates retention, motility, and numbers of hematopoietic progenitors, and affects osteoclast remodeling of metaphyseal trabecules. J Exp Med. 2008;205:2381–95. doi: 10.1084/jem.20080072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Takeshita S, Namba N, Zhao JJ, Jiang Y, Genant HK, Silva MJ, Brodt MD, Helgason CD, Kalesnikoff J, Rauh MJ, et al. SHIP-deficient mice are severely osteoporotic due to increased numbers of hyper-resorptive osteoclasts. Nat Med. 2002;8:943–9. doi: 10.1038/nm752. [DOI] [PubMed] [Google Scholar]
- 114.Hall A. Rho family GTPases. Biochem Soc Trans. 2012;40:1378–82. doi: 10.1042/BST20120103. [DOI] [PubMed] [Google Scholar]
- 115.Vega FM, Ridley AJ. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582:2093–101. doi: 10.1016/j.febslet.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 116.Zhang D, Udagawa N, Nakamura I, Murakami H, Saito S, Yamasaki K, Shibasaki Y, Morii N, Narumiya S, Takahashi N, et al. The small GTP-binding protein, rho p21, is involved in bone resorption by regulating cytoskeletal organization in osteoclasts. J Cell Sci. 1995;108:2285–92. doi: 10.1242/jcs.108.6.2285. [DOI] [PubMed] [Google Scholar]
- 117.Chellaiah MA, Soga N, Swanson S, McAllister S, Alvarez U, Wang D, Dowdy SF, Hruska KA. Rho-A is critical for osteoclast podosome organization, motility, and bone resorption. J Biol Chem. 2000;275:11993–2002. doi: 10.1074/jbc.275.16.11993. [DOI] [PubMed] [Google Scholar]
- 118.Ory S, Munari-Silem Y, Fort P, Jurdic P. Rho and Rac exert antagonistic functions on spreading of macrophage-derived multinucleated cells and are not required for actin fiber formation. J Cell Sci. 2000;113:1177–88. doi: 10.1242/jcs.113.7.1177. [DOI] [PubMed] [Google Scholar]
- 119.Wang Y, Lebowitz D, Sun C, Thang H, Grynpas MD, Glogauer M. Identifying the relative contributions of Rac1 and Rac2 to osteoclastogenesis. J Bone Miner Res. 2008;23:260–70. doi: 10.1359/jbmr.071013. [DOI] [PubMed] [Google Scholar]
- 120.Croke M, Ross FP, Korhonen M, Williams DA, Zou W, Teitelbaum SL. Rac deletion in osteoclasts causes severe osteopetrosis. J Cell Sci. 2011;124:3811–21. doi: 10.1242/jcs.086280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Razzouk S, Lieberherr M, Cournot G. Rac-GTPase, osteoclast cytoskeleton and bone resorption. Eur J Cell Biol. 1999;78:249–55. doi: 10.1016/S0171-9335(99)80058-2. [DOI] [PubMed] [Google Scholar]
- 122.Vives V, Laurin M, Cres G, Larrousse P, Morichaud Z, Noel D, Côté JF, Blangy A. The Rac1 exchange factor Dock5 is essential for bone resorption by osteoclasts. J Bone Miner Res. 2011;26:1099–110. doi: 10.1002/jbmr.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vives V, Laurin M, Cres G, Larrousse P, Morichaud Z, Noel D, Côté JF, Blangy A. The Rac1 exchange factor Dock5 is essential for bone resorption by osteoclasts. J Bone Miner Res. 2011;26:1099–110. doi: 10.1002/jbmr.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nagai Y, Osawa K, Fukushima H, Tamura Y, Aoki K, Ohya K, Yasuda H, Hikiji H, Takahashi M, Seta Y, et al. p130Cas, Crk-associated substrate, plays important roles in osteoclastic bone resorption. J Bone Miner Res. 2013;28:2449–62. doi: 10.1002/jbmr.1936. [DOI] [PubMed] [Google Scholar]
- 125.Susa M, Luong-Nguyen N-H, Cappellen D, Zamurovic N, Gamse R. Human primary osteoclasts: in vitro generation and applications as pharmacological and clinical assay. J Transl Med. 2004;2:6. doi: 10.1186/1479-5876-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Novack DV, Faccio R. Osteoclast motility: putting the brakes on bone resorption. Ageing Res Rev. 2011;10:54–61. doi: 10.1016/j.arr.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hefti T, Frischherz M, Spencer ND, Hall H, Schlottig F. A comparison of osteoclast resorption pits on bone with titanium and zirconia surfaces. Biomaterials. 2010;31:7321–31. doi: 10.1016/j.biomaterials.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 128.Schilling AF, Linhart W, Filke S, Gebauer M, Schinke T, Rueger JM, Amling M. Resorbability of bone substitute biomaterials by human osteoclasts. Biomaterials. 2004;25:3963–72. doi: 10.1016/j.biomaterials.2003.10.079. [DOI] [PubMed] [Google Scholar]
- 129.Kikuta J, Wada Y, Kowada T, Wang Z, Sun-Wada GH, Nishiyama I, Mizukami S, Maiya N, Yasuda H, Kumanogoh A, et al. Dynamic visualization of RANKL and Th17-mediated osteoclast function. J Clin Invest. 2013;123:866–73. doi: 10.1172/JCI65054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Coxon FP, Taylor A. Vesicular trafficking in osteoclasts. Semin Cell Dev Biol. 2008;19:424–33. doi: 10.1016/j.semcdb.2008.08.004. [DOI] [PubMed] [Google Scholar]