Abstract
Cell invasion of the extracellular matrix is prerequisite to cross tissue migration of tumor cells in cancer metastasis, and vascular smooth muscle cells in atherosclerosis. The tumor suppressor p53, better known for its roles in the regulation of cell cycle and apoptosis, has ignited much interest in its function as a suppressor of cell migration and invasion. How p53 and its gain-of-function mutants regulate cell invasion remains a puzzle and a challenge for future studies. In recent years, podosomes and invadopodia have also gained center stage status as veritable apparatus specialized in cell invasion. It is not clear, however, whether p53 regulates cell invasion through podosomes and invadopodia. In this review, evidence supporting a negative role of p53 in podosomes formation in vascular smooth muscle cells will be surveyed, and signaling nodes that may mediate this regulation in other cell types will be explored.
Keywords: p53, podosomes, invadopodia, cell invasion, cell migration, cancer, atherosclerosis, smooth muscle cells
Introduction
There is ample evidence to indicate that the tumor suppressor p53 and its mutants are key players in the regulation of cell migration and invasion in human cancer cells. Relatively little is known, however, whether p53 acts by regulating the formation of podosomes and invadopodia in cell invasion. There are excellent recent reviews on p53 in the regulation of cell invasion1-3 and on the biology of podosomes and invadopodia.4-12 In this review, I will describe some recent work on (1) the roles of p53 in cell migration and invasion in general; (2) evidence that supports a role for p53 in suppressing podosomes formation in vascular smooth muscle cells; and (3) nodes that can potentially mediate p53 regulation of podosomes and invadopodia in other cell types.
Regulation of Cell Migration and Invasion of Cancer Cells by p53 and its Gain-of-Function Mutants
Since its discovery over 30 years ago by Lane and Crawford,13 and Linzer and Levine,14 p53 is arguably the most widely studied tumor suppressor that functions mainly as a transcription factor, but also has transcription-independent roles (for review, see Lane and Levine15). Point mutations clustered mostly within the DNA-binding domain occur in about 50% of all human cancers. Many p53 mutants (mutp53) not only lose their wild-type function as a tumor suppressor, they acquire a gain-of-function in cell migration and invasion, often by inhibiting the closely related isoforms, p63 and p73.2In cancers expressing wild-type p53 (wtp53), its function is usually compromised due to deregulation of its regulatory pathways.3
In unstressed cells, p53 is kept at a low level by Mdm2, an E3 ubiquitin ligase, which targets p53 for degradation. In response to a variety of stresses, such as DNA-damage, p53 levels are increased substantially due to Mdm2 inhibition, and the p53 protein is stabilized to form active homo-tetramers. Forming a complex with a number of transcriptional cofactors, p53 induces DNA repair, cell cycle arrest, senescence, or apoptosis depending on the severity of the damage, a protective mechanism necessary to ensure that defective genes are not passed on to the next generation.
It is becoming clear that p53 plays crucial roles in the regulation of many cellular functions other than cell cycle-related pathways.1 Among the more recently discovered functions, wtp53 and mutp53 are involved in the regulation of cell migration and invasion in cancer cell metastasis.3 A survey of p53-regulated human genes reveals many regulators of cell migration and invasion, including proteins involved in endothelial-mesenchymal-transition (EMT) and the extra cellular matrix (ECM) composition and invasion, growth factor receptors, and adhesion signaling.1,16,17 Some of these migration and invasion regulators have been identified as direct transcriptional targets of wtp53 or mutp53, as well as indirect effectors of p53, forming nodes of a network of positive and negative feedback pathways.
Does p53 Suppress Cell Invasion by Inhibiting the Formation of Podosomes and Invadopodia?
Podosomes and invadopodia
Podosomes and invadopodia, often collectively called invadosomes, are actin-based membrane protrusions employed by mesenchymal cells in adhesion and invasion.4-12 It has been shown that location of the N-WASP/WASP upstream activators, Nck1 and Grb2, may provide molecular distinction between invadopodia and podosomes.18 They are sites of integrin-ECM contacts and signaling that are crucial for the recruitment and secretion of matrix metalloproteinases (MMPs) for ECM degradation, hence invasion. Podosomes are dynamic structures with lifetimes ranging from 2–10 min. They are formed spontaneously in cells of hematopoietic origin such as macrophages, osteoclasts, and dendritic cells. Growth factors, such as PDGF and EGF, induce podosomes in many cell types, including vascular smooth muscle cells, endothelial cells, and epithelial cells. In Src-transformed fibroblasts and vascular smooth muscle cells, podosomes aggregate to form rosettes where individual podosomes are connected by contractile actin-stress fibers. Similar, but morphologically distinct structures found in metastatic cancer cells are called invadopodia. They are fewer in number per cell, generally have longer lifetimes, and are more invasive than podosomes. Apart from their distinct structures, podosomes and invadopodia share many common molecular components, including actin-binding proteins such as Arp2/3, N-Wasp, cortactin, caldesmon, cofilin, and fascin; focal adhesion molecules such as talin, FAK, PAX, and integrin, as well as many signaling kinases and phosphatases.
p53 suppresses Src-induced podosome formation in vascular smooth muscle cells
We have shown that p53 and Src are mutually antagonistic in podosome/rosette formation and ECM invasion in rat vascular smooth muscle cells and fibroblasts.19 Expression of exogenous wtp53 or activation of endogenous p53 inhibits Src-induced podosome formation by upregulating the expression of the PTEN tumor suppressor and suppressing the pro-invasion Src-effector, STAT3. In contrast, shRNA-knockdown of p53 exacerbates the Src-induced invasive phenotype.20 Furthermore, p53 upregulates the expression of caldesmon, an actin-binding protein that localizes to podosomes and inhibits their formation.21,22 Reciprocally, constitutively active Src (SrcY527F) suppresses p53 and caldesmon expression, but enhances STAT3 expression, resulting in the formation of podosomes/rosettes, and invasive phenotypes.20,21
Caldesmon is an actin- and Ca2+-calmodulin-binding protein; its expression in vascular smooth muscle cells is changed from the heavy (h-caldesmon) to the light (l-caldesmon) isoform during the switch from the differentiated to synthetic and migratory phenotype in atherosclerosis.23,24 It is well documented that caldesmon stabilizes actin-stress fibers and inhibits myosin II-contractility in the absence of Ca2+-calmodulin. Thus, caldesmon may act as a negative regulator of podosome formation25,26 by stabilizing stress fibers and/or by inhibiting Arp2/3-mediated actin polymerization.27 This is consistent with finding that caldesmon expression is repressed in some invasive cancer cells and Src-induced cells,24 but enhanced by wtp53 in smooth muscle cells;21 and caldesmon was identified as a possible p53 transcription target by chromatin-immunoprecipitation (ChIP) analysis.17
Quintavalle et al.28 have recently shown that PDGF-induced podosome formation in smooth muscle cells requires Src-mediated downregulation of miR-143/145, likely via suppression of p53. They have identified two potential p53-binding sites at the promoter region encoding miR-143/145, suggesting that they are possible p53 transcriptional targets. In cancer cells, however, p53 activity promotes post-transcriptional maturation of miR-143 and -145,29 suggesting that p53 upregulation of miR-143/145 can be transcriptional or post-transcriptional, dependent on the cell type. Quintavalle et al.28 further identified PDGFRα and PKC-ε as negative targets of miR-143 and fascin as a miR-145 target, and showed that both PKC-ε and fascin localized to podosomes. In cancer cells, fascin stabilizes actin bundles in invadopodia.30 These findings add to our understanding of smooth muscle cell migration and invasion in atherosclerosis because miRNA143/145, like caldesmon, are key regulators of the switch of vascular smooth muscle cells from a contractile to a motile and proliferative phenotype.31 Thus, miR-143/145 are excellent candidates to mediate p53 function in the regulation of podosomes, although evidence supporting a direct link remains to be established.
Src provides a major link between p53 and podosome signaling
Results obtained from studies on vascular smooth muscle cells clearly point to Src as the key player that connects p53 and podosome signaling.20,21,28 This is probably not surprising since Src is recognized as a central hub in invadosome signaling that leads to activation of phosphoinositide 3-kinase (PI3K) activity, and many Src-substrates are involved in the biogenesis of podosomes and invadopodia.5,32,33 The precise mechanism that regulates the interplay between p53 and Src remains to be determined, however. Since Src has not been identified as a p53 transcriptional target, nor p53 a substrate of Src, they most likely act indirectly on each other through the PTEN, PI3K-Akt, STAT3, PKC pathways, or miR-143/145 as illustrated in Figure 1.
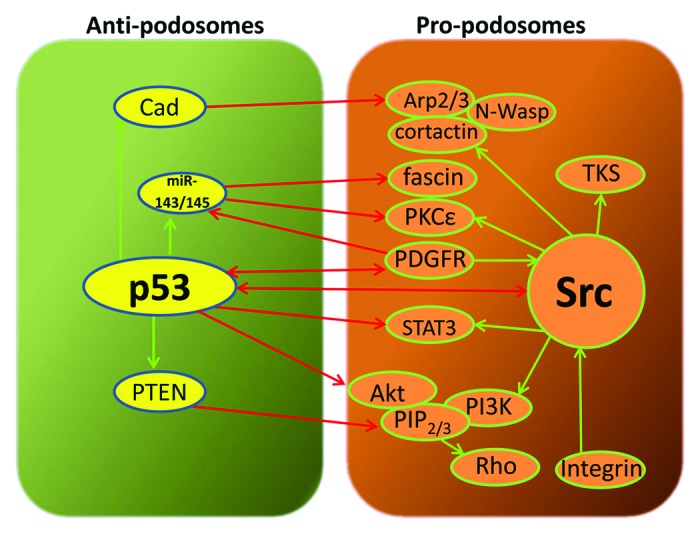
Figure 1. The anti- and pro-podosome pathways staged by p53 and Src, respectively, in vascular smooth muscle cells. p53 and Src play antagonistic roles in the regulation of podosome formation. The mechanism regulating the p53–Src interplay is not fully understood. Recent data suggest that p53 suppresses podosome formation by upregulating caldesmon, PTEN, and miR-143/145, which in turn, inhibit pro-podosome signaling nodes downstream of Src. Thus, caldesmon inhibits Arp2/3-mediated actin polymerization or stabilizes actin-stress fibers; PTEN antagonizes PI(3,4,5)P3 and PI(3,4)P2 formation and inhibits RhoGTPases; miR143/145 suppresses fascin, PKCε, and PDGFRα in the pro-podosome pathways. p53 also acts by downregulating the expression of Src and its downstream effectors, such as STAT3 and Akt. Red and green arrows represent inhibition and activation relationship, respectively.
Src-PI3K-Akt and p53-PTEN
PTEN, a lipid-phosphatase that antagonizes the formation of PI(3,4,5)P3 and P(3,4)P2, has been verified as a positive transcription target of p53.16 Upregulation of PTEN by p53 would act to inhibit the Src-PI3K-Akt pro-podosome pathway. Moreover, PTEN possesses a protein–phosphatase activity that appears to play a role in cell migration.34,35 Recently, PTEN has been shown to inactivate Src by dephosphorylating the critical Y416 site in the activation loop.36 On the other hand, the PI3K–Akt has the potential to downregulate p53 activity because mitogen activation of PI3K and Akt has been shown to result in phosphorylation of Mdm2, allowing it to translocate to the nucleus, where it enhances p53 degradation and reduces its transcriptional activity.37 Although we have shown a good correlation between Src-induced activation of Akt and podosome formation in smooth muscle cells, how Akt promotes podosome formation is not clear. To this end, it is critical to identify Akt substrates involved in the regulation of podosome signaling.
Src-STAT3 and p53
Phosphorylation of the oncogenic transcription factor, STAT3, by Src, can lead to its translocation to the nucleus, where STAT3 inhibits p53 transcription activity by binding to its promoter.38,39 In reverse, wtp53, but not mutp53, has been reported to suppress the activity of STAT3, thus creating a mutual negative feedback loop.40 Thus, promotion of podosome formation by Src may be mediated by STAT3, and it is important to identify other downstream effectors of STAT3 that may contribute to podosome formation for a better understanding of its role in cell invasion.
Src-PKC and p53
Interaction between the ECM and integrin-αv upregulates PKCα and suppresses p53 in melanoma cells by promoting the exit of p53 from the nucleus when cells are grown in a 3-D collagen matrix.41 This is in line with the notion that Src, in collaboration with PKC, plays a major role in regulating the assembly of invadopodia in response to integrin stimulation via mechanotransduction.32
Potential Mediators of p53-Suppression of Podosomes and Invadopodia in Normal and Cancer Cells
Numerous proteins have been identified that have some type of link to either p53 or to invadosomes. Interestingly, a subset of these nodes has connections to both p53 and invadosomes, and may provide important links between p53 and invadosomes, as summarized in Table 1. In most cases though, evidence has yet to be found to show that these nodes actually act as mediators in p53-invadosome signaling, and thus, represent excellent prospects for future studies.
Table 1. Potential mediators of p53 regulation of invadosomes formation and cell invasion.
| Protein sub‐groups | Nodes with connections to both p53 and invadosomes | Interactions with p53* | Effects of nodes on invadosome formation and/or cell invasion | ||
|---|---|---|---|---|---|
| Transcriptional regulation by p53 | Non‐transcriptional regualtion by p53 | Effects of nodes on p53 expression and/or activity | |||
| ECM proteins and MMPs | Fibronectin | ↓(48) | ↓(47) | NA | ↑ (42, 45, 46) |
| MT1‐MMP | ↓ (55) | NA | NA | ↑ (50, 53, 54) | |
| MMP‐2 | ↓ or ↑ (55) | NA | NA | ↑ (49) | |
| Focal adhesion and associated proteins | Integrin | ↓ (63,64) | ↑ by mutp53 (65,66) | ↓ (41,67) | ↑ (56,59‐62) |
| Supervillin | NA | NA | ↓ (71) | ↑(70); ↓ (69) | |
| FAK | ↓ (77) | NA | ↓ (78, 79) | ↑ (73, 74); ↓ (75, 76) |
|
| Calpain | NA | NA | ↓ (90‐92) | ↑ (89); ↓(85‐ 87) |
|
| Growth factors and receptors | EGFR | ↓ (16) | NA | ↓ (95) | ↑ (5) |
| MET | ↓ (16, 98) | NA | ↓ (98) | ↑ (5) | |
| PDGF | ↓ (99) | NA | ↓ (100, 101) | ↑ (28) | |
| TGF‐β | NA | NA | ↓ (103) | ↑ (102) | |
| Signaling molecules | Src | NA | ↓ (19, 20) | ↓ (19, 20, 21) | ↑ (6,20,21,28) |
| PTEN | ↑ (16) | NA | NA | ↓ (20, 34) | |
| PKCε | NA | ↓ (28) | NA | ↑ (28) | |
| STAT3 | NA | ↓(40) | ↓ (38,39) | ↑ (20) | |
| Rho GTPases and regulators of actin polymerization | Cdc42 | NA | ↓(113) | NA | ↑ (107,109‐111); ↓(112) |
| RhoA | NA | ↓ (3, 123, 126); ↑ by mutp53 (124) | NA | ↑ (110, 118, 119, 122); ↓ (120‐122) | |
| RhoC | ↑ (108,125,128) | ↑ (125) | NA | ↓ (127) | |
| Rac | NA | ↓ (132, 133) | NA | ↑ (129,130, 131); ↓ (122,131) | |
| RhoE (Rn3) | ↑ (134) | ↑(134) | NA | ↑ (135) | |
| Caldesmon | ↑ (17, 21, 24) | NA | NA | ↓ (25,26) | |
| Fascin | NA | ↓ (28) | NA | ↑ (30) | |
| miRNAs | miRNA‐143/145 | ↑ (28) | ↑(29) | NA | ↓ (28) |
Protein nodes are listed as functional sub-groups that have been shown to have connections to both p53 and invadosomes. They represent potential mediators of p53-dependent downregulation of cell invasion via podosome and invadopodia formation in cancer and vascular smooth muscle cells. *Listed here are references (in brackets) that have provided evidence for three different modes of interaction between protein nodes and p53: (1) proteins as transcriptional targets of p53; (2) post-transcriptional protein expression and activity regulated by p53; and (3) effect of proteins on p53 expression and stability, providing a potential feedback loop. NA, evidence not available to my knowledge; ↓, downregulation; ↑, upregulation.
Extracellular matrix proteins and MMPs
It is well documented that the composition and rigidity of the ECM play a crucial role in controlling the number, lifetime, and invasiveness of invadosomes, which are considered as mechanosensors.6,10,42-44 For example, liver microvascular endothelial cells produce podosomes constitutively and can be modulated by matrix stiffness.45 Density of gelatin substrates correlates positively with an increase in the number and ECM-degrading capacity of invadopodia in breast cancer cells via myosin II-dependent contractility and tension.46 Forced expression of wtp53, not mutp53, in p53-null mouse fibroblasts results in a significant decrease in fibronectin in the ECM, matrix fibrils, and the number and size of focal contacts.47 This is consistent with the finding that wtp53 downregulates fibronectin expression in different cell lines;48 however, the physiological significance and mechanism by which p53 may regulate the ECM structure remain to be established.
Podosomes and invadopodia degrade ECM proteins using mainly three members of the MMPs, MT1-MMP (MMP-14), MMP-2, and MMP-9.49-51 How these MMPs are recruited and secreted by podosomes and invadopodia is not fully understood, although recent studies have offered some answers. Using human bronchial epithelial cells, Xiao et al. have shown that the atypical PKCζ regulates the recruitment of MMP-9 to phorbo-ester-induced podosomes for its release and activation, perhaps via the MEK/ERK and JNK pathways.51,52 Wiesner et al.53 have identified Rab5a, Rab8a, and Rab14 of the RabGTPase family as major regulators of MT1–MMP trafficking of primary human macrophages. Most recently, Monteiro et al.54 showed that exocytosis of MT1–MMP through late endosomes occurs at invadopodia, requiring the WASP- and Scar-homolog protein, WASH, and the exocyst complexes. In human umbilical vein endothelial cells, p190 RhoGAP plays an important role in the regulation of MMP2 activation, as well as cell–surface presentation of MT1–MMP expression.49
In a study to investigate the effects of key tumor suppressors on cancer progression, Delassus et al. studied the effects of raising the cellular level of wtp53 on the mRNA expression of MMPs in several invasive cancer cells.55 In PC-3 human prostate cancer cells, raising wtp53 level suppresses mRNA expression of MMP-2 and MT1-MMP by about 60- and 20-folds, respectively, over control cells. However, p53 has little effect on MMP-2 and MT1-MMP expression in MDA–MB-231 human breast cancer cells, but upregulates MMP-2 expression in human ovarian cancer cells. These results offer insights into the complexity of the p53–MMPs regulation in different cancer types, most likely involving multiple signaling pathways, which may be upregulated in some cancers and downregulated in others.
Focal adhesion and associated proteins
Communication between focal adhesions and the ECM via integrin is important for cell survival, cell motility, and invasion. Adhesion signaling is a key factor in invadopodia maturation, resulting in the recruitment and exocytosis of MMPs and ECM degradation.5
Integrins
Integrins are transmembrane adhesion proteins that belong to a family comprising 18 α and eight β subunits, which form 24 distinct heterodimers.56-58 The β1 integrin subunit, which constitutes the largest integrin subfamily, promotes metastasis in a number of tumor models through poorly understood mechanisms. It has been shown that α3β1 and α6β1 integrins localize to the core of invadopodium in highly metastatic melanoma cells.59-61 More recently, Beaty et al. have shown that β1 integrin is required in MDA–MB-231 breast cancer cells for invadopodia maturation via its interaction with the tyrosine kinase Arg, which phosphorylates cortactin at Y-421 and releases it from cofilin. This leads to generation of cofilin-dependent actin barb ends and cortactin-N-WASP-Arp2/3-dependent actin branching in invadopodia maturation and MMP secretion.62
Although there is no evidence that integrin is a transcription target of p53, there are convincing data showing that p53 downregulates integrin signaling. For instance, pharmacological activation of p53 by Nutlin-3a inhibits the expression of integrin α5β1, the fibronectin receptor that determines malignant properties of colon carcinoma.63 In contrast, inhibiting p53 function using the chemical inhibitor, PFT-α, increases the expression of β1-integrin and promotes cell migration in endothelial cells.64 This is consistent with the finding that mutp53 promotes cell motility and invasion via enhanced β1-integrin recycling,65 and trafficking of integrin and EGFR that depend on the Rab-coupling protein and inhibition of p63.66 There are also data showing that integrin suppresses p53 activity. For example, when highly expressed, the α5 integrin subunit compromises temozolomide-induced p53 activity in human glioblastoma cells.67 Similarly, integrin-αv in a collagen 3-D matrix suppresses p53 activity by upregulating PKCα, facilitating p53 translocation from the nucleus to the cytoplasm in melanoma cells.41 In view of these findings, it is reasonable to speculate that p53 suppresses invadopodia maturation and ECM degradation by downregulating integrin, especially the β1 subunit. Moreover, there appears to be a bilateral p53-integrin negative feedback loop in cell migration and invasion regulation.
Supervillin
Supervillin is a member of the villin/gelsolin family of actin–cytoskeleton organizing proteins. It is a multi-domain protein that interacts with F-actin, MLCK, activated myosin IIA, Tks5, and cortactin.68 It has been shown recently that supervillin decreases podosome lifetimes in primary macrophages involving myosin II-associated contractility.69 Interestingly, supervillin localizes preferentially to successor podosomes and promotes their turnover, consistent with the observation that podosome numbers are inversely correlated with supervillin protein levels. However, in MDA-MB-231 breast cancer cells and in Src-COS-7 cells, overexpression of supervillin induces the loss of focal adhesion function and increases the number of invadopodia and podosomes per cell.70 These data suggest that supervillin has multiple or even opposite roles in the formation and turnover of podosomes and invadopodia that are cell type- and context-dependent, probably through its interaction with different podosomal proteins such as Tks5, and cortactin at different points of the lifecycle of podosomes.
It is not known whether p53 regulates supervillin expression or function. However, supervillin isoform-1 and -4 suppress p53 expression to increase survival of U2OS osteosarcoma and HeLa cells.71 Whether supervillin also negatively affects p53 expression in cell invasion and invadosomes formation is not known and requires further studies.
FAK
FAK is a non-receptor tyrosine kinase, originally identified as a Src substrate that localizes to focal adhesions. FAK is critical for normal cell motility, adhesion, and cell cycle progression, and for tumor invasion and survival.72 Autophosphorylation of FAK at Y397 causes its interaction with Src and the p83 subunit of PI3K, targets it to focal adhesion, and promotes cell motility. Src phosphorylation at Y576 and Y577 fully activates FAK. The activated Src/FAK complex mediates the phosphorylation of many focal adhesion proteins, including p130Cas.
FAK is required for the assembly of podosome rosettes in Src-transformed fibroblasts and carcinoma cells by suppressing RhoA and ROCK activity and formation of vimentin intermediate filaments, and by promoting p130Cas phosphorylation. However, it is dispensable for formation of individual dot-shaped podosomes.73 PYK2, another member of the FAK family, is essential for podosome belt formation as well as for bone resorption in osteoclasts.74 FAK is present in invadopodia and is essential for invadopodia activity in response to signals conveyed by ECM-rigidity via a myosin II-FAK-p130Cas pathway.46 Interestingly, FAK is not required for invadopodia formation in some cell types. Thus, siRNA-knockdown of FAK has no effect on invadopodia formation and ECM degradation, and overexpression of FAK actually suppresses invadopodia formation in KM12C colon cancer cells.75 In breast cancer cells, FAK acts as a negative regulator of invadopodia formation, while depletion of FAK induces invadopodia formation.76
p53 has been shown to bind to the FAK promoter and inhibit its transcriptional activity in vitro and in vivo in metastatic breast and colon cancer cells.77 Recently, it has been shown that FAK can be translocated to the nucleus where it promotes Mdm2-mediated p53 degradation through its N-terminal FERM domain.78,79 These recent results suggest that there exists a mutual negative feedback loop involving FAK and p53.
Calpain
The calpain family of Ca2+-dependent thiol-proteases plays a crucial role in the regulation of the dynamics of cell migration and invasion.80 There is good in vitro evidence that calpain is involved in the turnover of focal adhesions by fragmentation of its components such as talin,81 FAK,82 paxillin,83 vinculin, and β-integrin;84 and the degradation of cortactin in podosomes.85 Calpain may also play a role in cell invasion by promoting the turnover of podosomes in dendritic cells86 and osteoclasts.87 In myeloid cells, WASP promotes calpain-dependent protein degradation during podosome disassembly.88 In contrast, calpain-mediated fragmentation of the Src antagonist, PTP1B, results in the activation of Src-induced invadopodia formation in breast cancer cells.89 Thus, dependent on the cell type, calpain appears to be able to promote the turnover or formation of podosomes and invadopodia.
There are reports suggesting that calpain may play a role in regulating p53 stability and protein expression, although there is no evidence that calpain is a p53 transcription target. p53 has been shown to be a calpain substrate in vitro,90 and inhibition of calpains by calpastatin, the physiological inhibitor, leads to an increase in p53 expression in MCF7 human breast cancer cells.91 More recent data have shown that semaphorin 3A-induced growth cone collapse in hippocampal neurons may be mediated by calpain degradation of p53, leading to the activation of ROCK and actin cytoskeleton remodelling.92 Whether calpain may play a role in p53-mediated regulation of cell invasion and podosomes/invadopodia signaling is not known and requires further studies.
Growth factor receptors
Growth factor receptors and integrin are key signal transducers relating outside-in signals crucial for invasion and formation of invadosomes. Activated by their cognate growth factors, these receptors act on signaling nodes such as PI3K-AKT, Src, RhoGTPases, PKC, and ERKs.5 Epidermal growth factor receptor (EGFR), hepatocyte growth factor receptor (MET), and transforming growth factor α (TGF-α) have been validated experimentally as p53-regulated genes.16
EGFR is overexpressed in many human cancers, e.g. it is overexpressed in more than 60% of human lung cancer,93 and both mutp53 and EGFR are overexpressed in advanced stage colorectal cancer patients.94 In primary lung tumors, p53 suppresses EGFR indirectly by upregulation of desmocollin 3, a member of the desmosomal cadherin family.95 The ΔNp63 isoform of p53 induces EGFR in pancreatic cancer.96
MET is overexpressed in p53-deficient tumor cells in mice and Li-Fraumeni patients.97 Recently, Hwang et al.98 showed that wtp53 downregulates MET in ovarian epithelial cells either indirectly by activating miR-34 or directly by suppressing MET promoter activity.
PDGF, which is the most potent chemoattractant of vascular smooth muscle cells, has been shown also to induce podosome formation after prolonged activation of its receptor.28 PDGFRβ expression is suppressed by p53 and p73 that bind directly to its promoter.99 Silber et al. have shown recently that the p53 target, miR-34a, is downregulated by PDGFRα in glioblastoma and in reciprocal, PDGFRα is a negative target of miR-34a.100 Interestingly, transactivation of monomeric PDGFRα suppresses p53 expression and induces prolonged activation of Akt in fibroblasts.101
TGF-β stimulates EMT leading to cell invasion and invadopodia formation in cancer cells. This is mediated by Hic-5, a focal adhesion protein, and RhoC.102 Phosphorylation of p53 by MAPK promotes its interaction with TGF-β-activated Smad2/3, forming a complex that regulates some TGFβ target genes.103 Ras-activated mutp53 form a ternary complex with Smad and p63, thus inhibiting the normal protective function of p63.104
RhoGTPases
The Rho family of small GTPases are key regulators of actin cytoskeleton, cell cycle progression,105 and the formation of invadosomes.106,107 There are some 20 members of the Rho family, which are subdivided into the classical subclass, including cdc42, Rac, RhoA, and RhoC, and the atypical subclass of constitutively active members such as the Rnd family (Rnd1,2,3 [RhoE]). p53 plays an important role in the regulation of expression and activities of a number of Rho members, hence cytoskeleton organization and cell invasion.108
Cdc42
Of all the RhoGTPases, Cdc42 seems to be the major regulator of podosomes and invadopodia biology in a variety of cell types.107 In vascular endothelial cells, podosome formation is induced by constitutively active cdc42 (V12Cdc42), but is not associated with a migratory nor with a proliferative phenotype.109,110 In metastatic cancer cells, cdc42 acting downstream of EGFR is required for invadopodia formation by recruiting N-WASP to Arp2/3 at the actin polymerization sites by the adaptor protein Nck1 and the WASP-interacting protein, WIP.111 In human macrophages, cdc42 localizes to the core of podosomes; however, microinjection of constitutively active cdc42, V12Cdc42, induces the disassembly of podosomes.112 These results suggest that an optimal level of cdc42 activity is critical for the maintenance of steady-state podosome population and function in invasion.
While there is no evidence that cdc42 is a direct p53 transcriptional target, overexpression of wtp53 or activation of endogenous p53 in mouse embryonic fibroblasts suppresses cdc42-induced filopodia formation and prevents cell polarization; in contrary, p53-deficient cells form constitutive filopodia.113 It is conceivable that p53 may act by controlling PI3K-dependent formation of PIPs that in turn regulate GEFs or GAPs of cdc42. While cdc42 is a promising candidate in mediating p53 effects on podosomes/invadopodia signaling, detailed mechanisms require further studies.
RhoA
RhoA expression is critical for cell migration, invasion, and cancer metastasis,108,114-117 but its role in the formation of podosomes and invadopodia is less clear. Active RhoA has been found to be necessary for podosome formation in fibroblasts, osteoclasts, and endothelial cells.110,118,119 However, limiting RhoA activity promotes Src-induced podosome formation in fibroblasts,120 and activated RhoA can inhibit podosome formation by activating myosin II contractility and stress fiber stabilization.121 Furthermore, either inhibition or excess RhoA activity blocks podosome formation in macrophage-derived multinucleated cells.122 These data echo what have been observed with cdc42, underscoring the importance of activity level of these Rho members in podosome regulation.
There is a general consensus that loss of wtp53 leads to upregulation of RhoA activity and activation of its main effector, Rho kinase (ROCK), in a number of cell types.3 Thus, inhibition of RhoA–ROCK activities by p53 may provide a mechanism by which p53 downregulates podosome formation. In addition, by suppressing RhoA and ROCK, p53 may also inhibit ROCK-dependent amoeboid migration.123 This is consistent with the report that mutp53, acquiring a gain-of-function, promotes amoeboid migration by activating RhoA-ROCK.124 How p53 regulates RhoA–ROCK is not clear, however. Their interaction is most likely indirect, perhaps via RhoGEF and RhoGAP, since transcription of RhoA is regulated by the Myc transcription factor, not p53.125 Alternatively, p53 acting via its transcription target, miR-31, can suppress RhoA expression.126
RhoC
RhoC is a crucial regulator of tumor cell invasion and it is highly upregulated at the mRNA level in invasive tumor cells. Recent studies have shown that RhoC plays a critical role in invadopodia formation.127 RhoC is activated by p190RhoGEF in areas surrounding invadopodia, leading to activation of ROCK and LIM kinase, phosphorylation and inactivation of cofilin resulting in the inhibition of actin polymerization. In the core of the invadopodium, RhoC is inhibited by p190RhoGAP, resulting in activation of cofilin and focused actin polymerization and protrusion.127 Interestingly, RhoC and LIM kinase2 (LIMK2) are positive p53 transcriptional targets.108,125,128 It is tempting to speculate that upregulation of RhoC, LIMK1 and LIMK2 by p53 would lead to cofilin phosphorylation, inhibition of actin polymerization, and invadopodia formation.
Rac
Rac, one of the key regulators of actin cytoskeleton structures and a downstream effector of PDGF, has been shown to be a promoter of podosomes and invadopodia formation in a number of cell types. Rac1 promotes Src-induced podosome formation in aortic smooth muscle cells,129 and expression of the dominant-negative N17Rac1 inhibits VEGF-induced podosome formation in endothelial cells.130 Interestingly, microinjection of either N17Rac1 or activated Rac1 leads to disassembly of podosomes in macrophages.122,131 Wheeler et al. showed that macrophages from Rac2-/- mice do not form podosomes; however, Rac1, not Rac2, is required for ECM invasion, suggesting a distinct role of Rac1 and Rac2 in podosome formation and maturation.131
Although Rac is not a transcription target of p53, Guo et al.132 showed that deletion of p53 in MEF cells results in elevated activities of PI3K and Rac1, without affecting their protein expression, leading to a significant increase in cell migration.133 This finding suggests that enhanced capacity of cell migration induced by loss of p53 may be mediated by upregulation of the PI3K pathway that acts upstream of Rac1.
RhoE (Rnd3)
The atypical RhoE (Rnd3) is constitutively active due to a very low GTPase activity. It was identified as a positively regulated p53-target gene that inhibits ROCK-1-mediated apoptosis, thus acting as an antagonist to p53 in cell survival signaling.134 Most recently, Georgess et al. have shown that RhoE is required for maintaining fast actin turnover in podosomes during osteoclasts migration and bone resorption, and RhoE activates cofilin by inhibiting its ROCK-mediated phosphorylation.135 This is consistent with findings that p53 upregulates Notch1 leading to inhibition of ROCK1/2.136 Together, these data show that upregulation of RhoE by p53 results in ROCK inhibition that may lead to either cell survival or podosome formation in different cell context and cell types. Interestingly, this would suggest that RhoE may function as an antagonist rather than a mediator of p53-suppression of podosome formation.
In sum, there is good evidence suggesting that members of the Rho family, especially cdc42, RhoA, RhoC, and Rac1, could be important mediators of p53 downregulation of podosome/invadopodia formation. These data also reflect the complexity of Rho members in podosomes and invadopodia signaling that involves tightly regulated cross-talk among members, optimal expression and activity levels, and timely subcellular localization. p53 can potentially regulate Rho members by directly affecting their transcription, e.g. RhoC and RhoE; by regulating transcription of miRNAs such as miR-31, miR-21, and miR-138 that target RhoA, RhoB, and RhoC, respectively;108 or by controlling the expression of GEFs and GAPs via the PI3K pathways.132 Future work is needed to determine how different Rho members act on similar or different targets, and how they are coordinated to mediate p53-associated suppression of cell invasion and podosome formation.
Perspective and Conclusion
Exciting data have appeared in the literature in recent years strongly suggesting that wtp53 and its mutants play crucial roles in the regulation of invasion of cancer cells and vascular smooth muscle cells, implicating their involvement in cancer metastasis and atherosclerosis. Many unresolved questions remain to be addressed concerning the underlying mechanisms. For example, how are p53-mediated regulation of cell cycle progression, cell migration, and invasion coupled and coordinated? Does p53 suppress cell invasion by inhibiting EMT, podosomes, and invadopodia, and/or amoeboid type of invasion? Do gain-of-function of p53 mutants promote cell invasion via upregulation of podosomes and invadopodia formation? On the technical side, it is important to ask how overexpression or knockdown of p53 in cell models may affect cell survival/death and cell invasion, and to address their cause-and-effect relationship.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgements
Mak AS is funded by the Canadian Institute of Health Research and Heart and Stroke Foundation of Ontario. Infrastructure supports provided by the Protein Function Discovery facilities at Queen’s University are gratefully acknowledged. I thank Graham Côtè and Andrew Craig for reading the manuscript.
References
- 1.Aylon Y, Oren M. New plays in the p53 theater. Curr Opin Genet Dev. 2011;21:86–92. doi: 10.1016/j.gde.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oren M, Rotter V. Mutant p53 gain-of-function in cancer. Cold Spring Harb Perspect Biol. 2010;2:a001107. doi: 10.1101/cshperspect.a001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller PA, Vousden KH, Norman JC. p53 and its mutants in tumor cell migration and invasion. J Cell Biol. 2011;192:209–18. doi: 10.1083/jcb.201009059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta. 2007;1773:642–52. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoshino D, Branch KM, Weaver AM. Signaling inputs to invadopodia and podosomes. J Cell Sci. 2013;126:2979–89. doi: 10.1242/jcs.079475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12:413–26. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luxenburg C, Winograd-Katz S, Addadi L, Geiger B. Involvement of actin polymerization in podosome dynamics. J Cell Sci. 2012;125:1666–72. doi: 10.1242/jcs.075903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Artym VV, Matsumoto K, Mueller SC, Yamada KM. Dynamic membrane remodeling at invadopodia differentiates invadopodia from podosomes. Eur J Cell Biol. 2011;90:172–80. doi: 10.1016/j.ejcb.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bravo-Cordero JJ, Hodgson L, Condeelis J. Directed cell invasion and migration during metastasis. Curr Opin Cell Biol. 2012;24:277–83. doi: 10.1016/j.ceb.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linder S, Wiesner C, Himmel M. Degrading devices: invadosomes in proteolytic cell invasion. Annu Rev Cell Dev Biol. 2011;27:185–211. doi: 10.1146/annurev-cellbio-092910-154216. [DOI] [PubMed] [Google Scholar]
- 11.Ayala I, Baldassarre M, Giacchetti G, Caldieri G, Tetè S, Luini A, Buccione R. Multiple regulatory inputs converge on cortactin to control invadopodia biogenesis and extracellular matrix degradation. J Cell Sci. 2008;121:369–78. doi: 10.1242/jcs.008037. [DOI] [PubMed] [Google Scholar]
- 12.Gimona M, Buccione R, Courtneidge SA, Linder S. Assembly and biological role of podosomes and invadopodia. Curr Opin Cell Biol. 2008;20:235–41. doi: 10.1016/j.ceb.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Lane DP, Crawford LV. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979;278:261–3. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- 14.Linzer DI, Levine AJ. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979;17:43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- 15.Lane D, Levine A. p53 Research: the past thirty years and the next thirty years. Cold Spring Harb Perspect Biol. 2010;2:a000893. doi: 10.1101/cshperspect.a000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9:402–12. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- 17.Wei CL, Wu Q, Vega VB, Chiu KP, Ng P, Zhang T, Shahab A, Yong HC, Fu Y, Weng Z, et al. A global map of p53 transcription-factor binding sites in the human genome. Cell. 2006;124:207–19. doi: 10.1016/j.cell.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 18.Oser M, Dovas A, Cox D, Condeelis J. Nck1 and Grb2 localization patterns can distinguish invadopodia from podosomes. Eur J Cell Biol. 2011;90:181–8. doi: 10.1016/j.ejcb.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mak AS. p53 regulation of podosome formation and cellular invasion in vascular smooth muscle cells. Cell Adh Migr. 2011;5:144–9. doi: 10.4161/cam.5.2.14375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukhopadhyay UK, Mooney P, Jia L, Eves R, Raptis L, Mak AS. Doubles game: Src-Stat3 versus p53-PTEN in cellular migration and invasion. Mol Cell Biol. 2010;30:4980–95. doi: 10.1128/MCB.00004-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukhopadhyay UK, Eves R, Jia L, Mooney P, Mak AS. p53 suppresses Src-induced podosome and rosette formation and cellular invasiveness through the upregulation of caldesmon. Mol Cell Biol. 2009;29:3088–98. doi: 10.1128/MCB.01816-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eves R, Webb BA, Zhou S, Mak AS. Caldesmon is an integral component of podosomes in smooth muscle cells. J Cell Sci. 2006;119:1691–702. doi: 10.1242/jcs.02881. [DOI] [PubMed] [Google Scholar]
- 23.Wang CL. Caldesmon and the regulation of cytoskeletal functions. Adv Exp Med Biol. 2008;644:250–72. doi: 10.1007/978-0-387-85766-4_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayanagi T, Sobue K. Diversification of caldesmon-linked actin cytoskeleton in cell motility. Cell Adh Migr. 2011;5:150–9. doi: 10.4161/cam.5.2.14398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka J, Watanabe T, Nakamura N, Sobue K. Morphological and biochemical analyses of contractile proteins (actin, myosin, caldesmon and tropomyosin) in normal and transformed cells. J Cell Sci. 1993;104:595–606. doi: 10.1242/jcs.104.2.595. [DOI] [PubMed] [Google Scholar]
- 26.Yoshio T, Morita T, Kimura Y, Tsujii M, Hayashi N, Sobue K. Caldesmon suppresses cancer cell invasion by regulating podosome/invadopodium formation. FEBS Lett. 2007;581:3777–82. doi: 10.1016/j.febslet.2007.06.073. [DOI] [PubMed] [Google Scholar]
- 27.Yamakita Y, Oosawa F, Yamashiro S, Matsumura F. Caldesmon inhibits Arp2/3-mediated actin nucleation. J Biol Chem. 2003;278:17937–44. doi: 10.1074/jbc.M208739200. [DOI] [PubMed] [Google Scholar]
- 28.Quintavalle M, Elia L, Condorelli G, Courtneidge SA. MicroRNA control of podosome formation in vascular smooth muscle cells in vivo and in vitro. J Cell Biol. 2010;189:13–22. doi: 10.1083/jcb.200912096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki HI, Miyazono K. Dynamics of microRNA biogenesis: crosstalk between p53 network and microRNA processing pathway. J Mol Med (Berl) 2010;88:1085–94. doi: 10.1007/s00109-010-0650-1. [DOI] [PubMed] [Google Scholar]
- 30.Machesky LM, Li A. Fascin: Invasive filopodia promoting metastasis. Commun Integr Biol. 2010;3:263–70. doi: 10.4161/cib.3.3.11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 32.Destaing O, Block MR, Planus E, Albiges-Rizo C. Invadosome regulation by adhesion signaling. Curr Opin Cell Biol. 2011;23:597–606. doi: 10.1016/j.ceb.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Courtneidge SA. Cell migration and invasion in human disease: the Tks adaptor proteins. Biochem Soc Trans. 2012;40:129–32. doi: 10.1042/BST20110685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poon JS, Eves R, Mak AS. Both lipid- and protein-phosphatase activities of PTEN contribute to the p53-PTEN anti-invasion pathway. Cell Cycle. 2010;9:4450–4. doi: 10.4161/cc.9.22.13936. [DOI] [PubMed] [Google Scholar]
- 35.Leslie NR, Maccario H, Spinelli L, Davidson L. The significance of PTEN’s protein phosphatase activity. Adv Enzyme Regul. 2009;49:190–6. doi: 10.1016/j.advenzreg.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Zhang S, Huang WC, Li P, Guo H, Poh SB, Brady SW, Xiong Y, Tseng LM, Li SH, Ding Z, et al. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat Med. 2011;17:461–9. doi: 10.1038/nm.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Brate A, Giannakakou P. The importance of p53 location: nuclear or cytoplasmic zip code? Drug Resist Updat. 2003;6:313–22. doi: 10.1016/j.drup.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Niu G, Wright KL, Ma Y, Wright GM, Huang M, Irby R, Briggs J, Karras J, Cress WD, Pardoll D, et al. Role of Stat3 in regulating p53 expression and function. Mol Cell Biol. 2005;25:7432–40. doi: 10.1128/MCB.25.17.7432-7440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu Y, Zhang K, Li C, Yao Y, Tao D, Liu Y, Zhang S, Ma Y. Piwil2 suppresses p53 by inducing phosphorylation of signal transducer and activator of transcription 3 in tumor cells. PLoS One. 2012;7:e30999. doi: 10.1371/journal.pone.0030999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin J, Tang H, Jin X, Jia G, Hsieh JT. p53 regulates Stat3 phosphorylation and DNA binding activity in human prostate cancer cells expressing constitutively active Stat3. Oncogene. 2002;21:3082–8. doi: 10.1038/sj.onc.1205426. [DOI] [PubMed] [Google Scholar]
- 41.Smith SD, Enge M, Bao W, Thullberg M, Costa TD, Olofsson H, Gashi B, Selivanova G, Strömblad S. Protein kinase Cα (PKCα) regulates p53 localization and melanoma cell survival downstream of integrin αv in three-dimensional collagen and in vivo. J Biol Chem. 2012;287:29336–47. doi: 10.1074/jbc.M112.341917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collin O, Na S, Chowdhury F, Hong M, Shin ME, Wang F, Wang N. Self-organized podosomes are dynamic mechanosensors. Curr Biol. 2008;18:1288–94. doi: 10.1016/j.cub.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Labernadie A, Thibault C, Vieu C, Maridonneau-Parini I, Charrière GM. Dynamics of podosome stiffness revealed by atomic force microscopy. Proc Natl Acad Sci U S A. 2010;107:21016–21. doi: 10.1073/pnas.1007835107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saltel F, Daubon T, Juin A, Ganuza IE, Veillat V, Génot E. Invadosomes: intriguing structures with promise. Eur J Cell Biol. 2011;90:100–7. doi: 10.1016/j.ejcb.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 45.Juin A, Planus E, Guillemot F, Horakova P, Albiges-Rizo C, Génot E, Rosenbaum J, Moreau V, Saltel F. Extracellular matrix rigidity controls podosome induction in microvascular endothelial cells. Biol Cell. 2013;105:46–57. doi: 10.1111/boc.201200037. [DOI] [PubMed] [Google Scholar]
- 46.Alexander NR, Branch KM, Parekh A, Clark ES, Iwueke IC, Guelcher SA, Weaver AM. Extracellular matrix rigidity promotes invadopodia activity. Curr Biol. 2008;18:1295–9. doi: 10.1016/j.cub.2008.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alexandrova A, Ivanov A, Chumakov P, Kopnin B, Vasiliev J. Changes in p53 expression in mouse fibroblasts can modify motility and extracellular matrix organization. Oncogene. 2000;19:5826–30. doi: 10.1038/sj.onc.1203944. [DOI] [PubMed] [Google Scholar]
- 48.Iotsova V, Stehelin D. Down-regulation of fibronectin gene expression by the p53 tumor suppressor protein. Cell Growth Differ. 1996;7:629–34. [PubMed] [Google Scholar]
- 49.Guegan F, Tatin F, Leste-Lasserre T, Drutel G, Genot E, Moreau V. p190B RhoGAP regulates endothelial-cell-associated proteolysis through MT1-MMP and MMP2. J Cell Sci. 2008;121:2054–61. doi: 10.1242/jcs.025817. [DOI] [PubMed] [Google Scholar]
- 50.Poincloux R, Lizárraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J Cell Sci. 2009;122:3015–24. doi: 10.1242/jcs.034561. [DOI] [PubMed] [Google Scholar]
- 51.Xiao H, Bai XH, Wang Y, Kim H, Mak AS, Liu M. MEK/ERK pathway mediates PKC activation-induced recruitment of PKCζ and MMP-9 to podosomes. J Cell Physiol. 2013;228:416–27. doi: 10.1002/jcp.24146. [DOI] [PubMed] [Google Scholar]
- 52.Xiao H, Bai XH, Kapus A, Lu WY, Mak AS, Liu M. The protein kinase C cascade regulates recruitment of matrix metalloprotease 9 to podosomes and its release and activation. Mol Cell Biol. 2010;30:5545–61. doi: 10.1128/MCB.00382-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiesner C, El Azzouzi K, Linder S. A specific subset of RabGTPases controls cell surface exposure of MT1-MMP, extracellular matrix degradation and three-dimensional invasion of macrophages. J Cell Sci. 2013;126:2820–33. doi: 10.1242/jcs.122358. [DOI] [PubMed] [Google Scholar]
- 54.Monteiro P, Rossé C, Castro-Castro A, Irondelle M, Lagoutte E, Paul-Gilloteaux P, Desnos C, Formstecher E, Darchen F, Perrais D, et al. Endosomal WASH and exocyst complexes control exocytosis of MT1-MMP at invadopodia. J Cell Biol. 2013 doi: 10.1083/jcb.201306162. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Delassus GS, Cho H, Hoang S, Eliceiri GL. Many new down- and up-regulatory signaling pathways, from known cancer progression suppressors to matrix metalloproteinases, differ widely in cells of various cancers. J Cell Physiol. 2010;224:549–58. doi: 10.1002/jcp.22157. [DOI] [PubMed] [Google Scholar]
- 56.Ganguly KK, Pal S, Moulik S, Chatterjee A. Integrins and metastasis. Cell Adh Migr. 2013;7:251–61. doi: 10.4161/cam.23840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huck L, Pontier SM, Zuo DM, Muller WJ. beta1-integrin is dispensable for the induction of ErbB2 mammary tumors but plays a critical role in the metastatic phase of tumor progression. Proc Natl Acad Sci U S A. 2010;107:15559–64. doi: 10.1073/pnas.1003034107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–23. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 59.Mueller SC, Chen WT. Cellular invasion into matrix beads: localization of beta 1 integrins and fibronectin to the invadopodia. J Cell Sci. 1991;99:213–25. doi: 10.1242/jcs.99.2.213. [DOI] [PubMed] [Google Scholar]
- 60.Mueller SC, Ghersi G, Akiyama SK, Sang QX, Howard L, Pineiro-Sanchez M, Nakahara H, Yeh Y, Chen WT. A novel protease-docking function of integrin at invadopodia. J Biol Chem. 1999;274:24947–52. doi: 10.1074/jbc.274.35.24947. [DOI] [PubMed] [Google Scholar]
- 61.Destaing O, Planus E, Bouvard D, Oddou C, Badowski C, Bossy V, Raducanu A, Fourcade B, Albiges-Rizo C, Block MR. β1A integrin is a master regulator of invadosome organization and function. Mol Biol Cell. 2010;21:4108–19. doi: 10.1091/mbc.E10-07-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beaty BT, Sharma VP, Bravo-Cordero JJ, Simpson MA, Eddy RJ, Koleske AJ, Condeelis J. β1 integrin regulates Arg to promote invadopodial maturation and matrix degradation. Mol Biol Cell. 2013;24:1661–75, S1-11. doi: 10.1091/mbc.E12-12-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Janouskova H, Ray AM, Noulet F, Lelong-Rebel I, Choulier L, Schaffner F, Lehmann M, Martin S, Teisinger J, Dontenwill M. Activation of p53 pathway by Nutlin-3a inhibits the expression of the therapeutic target α5 integrin in colon cancer cells. Cancer Lett. 2013;336:307–18. doi: 10.1016/j.canlet.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 64.Qiu J, Wang G, Hu J, Peng Q, Zheng Y. Id1-induced inhibition of p53 facilitates endothelial cell migration and tube formation by regulating the expression of beta1-integrin. Mol Cell Biochem. 2011;357:125–33. doi: 10.1007/s11010-011-0882-6. [DOI] [PubMed] [Google Scholar]
- 65.Muller PA, Caswell PT, Doyle B, Iwanicki MP, Tan EH, Karim S, Lukashchuk N, Gillespie DA, Ludwig RL, Gosselin P, et al. Mutant p53 drives invasion by promoting integrin recycling. Cell. 2009;139:1327–41. doi: 10.1016/j.cell.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 66.Selivanova G, Ivaska J. Integrins and mutant p53 on the road to metastasis. Cell. 2009;139:1220–2. doi: 10.1016/j.cell.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 67.Janouskova H, Maglott A, Leger DY, Bossert C, Noulet F, Guerin E, Guenot D, Pinel S, Chastagner P, Plenat F, et al. Integrin α5β1 plays a critical role in resistance to temozolomide by interfering with the p53 pathway in high-grade glioma. Cancer Res. 2012;72:3463–70. doi: 10.1158/0008-5472.CAN-11-4199. [DOI] [PubMed] [Google Scholar]
- 68.Nebl T, Pestonjamasp KN, Leszyk JD, Crowley JL, Oh SW, Luna EJ. Proteomic analysis of a detergent-resistant membrane skeleton from neutrophil plasma membranes. J Biol Chem. 2002;277:43399–409. doi: 10.1074/jbc.M205386200. [DOI] [PubMed] [Google Scholar]
- 69.Bhuwania R, Cornfine S, Fang Z, Krüger M, Luna EJ, Linder S. Supervillin couples myosin-dependent contractility to podosomes and enables their turnover. J Cell Sci. 2012;125:2300–14. doi: 10.1242/jcs.100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crowley JL, Smith TC, Fang Z, Takizawa N, Luna EJ. Supervillin reorganizes the actin cytoskeleton and increases invadopodial efficiency. Mol Biol Cell. 2009;20:948–62. doi: 10.1091/mbc.E08-08-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fang Z, Luna EJ. Supervillin-mediated suppression of p53 protein enhances cell survival. J Biol Chem. 2013;288:7918–29. doi: 10.1074/jbc.M112.416842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 73.Pan YR, Chen CL, Chen HC. FAK is required for the assembly of podosome rosettes. J Cell Biol. 2011;195:113–29. doi: 10.1083/jcb.201103016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gil-Henn H, Destaing O, Sims NA, Aoki K, Alles N, Neff L, Sanjay A, Bruzzaniti A, De Camilli P, Baron R, et al. Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2(-/-) mice. J Cell Biol. 2007;178:1053–64. doi: 10.1083/jcb.200701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vitale S, Avizienyte E, Brunton VG, Frame MC. Focal adhesion kinase is not required for Src-induced formation of invadopodia in KM12C colon cancer cells and can interfere with their assembly. Eur J Cell Biol. 2008;87:569–79. doi: 10.1016/j.ejcb.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 76.Chan KT, Cortesio CL, Huttenlocher A. FAK alters invadopodia and focal adhesion composition and dynamics to regulate breast cancer invasion. J Cell Biol. 2009;185:357–70. doi: 10.1083/jcb.200809110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Golubovskaya VM, Cance W. Focal adhesion kinase and p53 signal transduction pathways in cancer. Front Biosci (Landmark Ed) 2010;15:901–12. doi: 10.2741/3653. [Landmark Ed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, Larocque N, Fisher SJ, Schlaepfer DD, Ilic D. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell. 2008;29:9–22. doi: 10.1016/j.molcel.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lim ST. Nuclear FAK: a new mode of gene regulation from cellular adhesions. Mol Cells. 2013;36:1–6. doi: 10.1007/s10059-013-0139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Franco SJ, Huttenlocher A. Regulating cell migration: calpains make the cut. J Cell Sci. 2005;118:3829–38. doi: 10.1242/jcs.02562. [DOI] [PubMed] [Google Scholar]
- 81.Franco SJ, Rodgers MA, Perrin BJ, Han J, Bennin DA, Critchley DR, Huttenlocher A. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat Cell Biol. 2004;6:977–83. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- 82.Chan KT, Bennin DA, Huttenlocher A. Regulation of adhesion dynamics by calpain-mediated proteolysis of focal adhesion kinase (FAK) J Biol Chem. 2010;285:11418–26. doi: 10.1074/jbc.M109.090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cortesio CL, Boateng LR, Piazza TM, Bennin DA, Huttenlocher A. Calpain-mediated proteolysis of paxillin negatively regulates focal adhesion dynamics and cell migration. J Biol Chem. 2011;286:9998–10006. doi: 10.1074/jbc.M110.187294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Glading A, Lauffenburger DA, Wells A. Cutting to the chase: calpain proteases in cell motility. Trends Cell Biol. 2002;12:46–54. doi: 10.1016/S0962-8924(01)02179-1. [DOI] [PubMed] [Google Scholar]
- 85.Perrin BJ, Amann KJ, Huttenlocher A. Proteolysis of cortactin by calpain regulates membrane protrusion during cell migration. Mol Biol Cell. 2006;17:239–50. doi: 10.1091/mbc.E05-06-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Calle Y, Carragher NO, Thrasher AJ, Jones GE. Inhibition of calpain stabilises podosomes and impairs dendritic cell motility. J Cell Sci. 2006;119:2375–85. doi: 10.1242/jcs.02939. [DOI] [PubMed] [Google Scholar]
- 87.Marzia M, Chiusaroli R, Neff L, Kim NY, Chishti AH, Baron R, Horne WC. Calpain is required for normal osteoclast function and is down-regulated by calcitonin. J Biol Chem. 2006;281:9745–54. doi: 10.1074/jbc.M513516200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Macpherson L, Monypenny J, Blundell MP, Cory GO, Tomé-García J, Thrasher AJ, Jones GE, Calle Y. Tyrosine phosphorylation of WASP promotes calpain-mediated podosome disassembly. Haematologica. 2012;97:687–91. doi: 10.3324/haematol.2011.048868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cortesio CL, Chan KT, Perrin BJ, Burton NO, Zhang S, Zhang ZY, Huttenlocher A. Calpain 2 and PTP1B function in a novel pathway with Src to regulate invadopodia dynamics and breast cancer cell invasion. J Cell Biol. 2008;180:957–71. doi: 10.1083/jcb.200708048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kubbutat MH, Vousden KH. Proteolytic cleavage of human p53 by calpain: a potential regulator of protein stability. Mol Cell Biol. 1997;17:460–8. doi: 10.1128/mcb.17.1.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pariat M, Carillo S, Molinari M, Salvat C, Debüssche L, Bracco L, Milner J, Piechaczyk M. Proteolysis by calpains: a possible contribution to degradation of p53. Mol Cell Biol. 1997;17:2806–15. doi: 10.1128/mcb.17.5.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Qin Q, Liao G, Baudry M, Bi X. Role of calpain-mediated p53 truncation in semaphorin 3A-induced axonal growth regulation. Proc Natl Acad Sci U S A. 2010;107:13883–7. doi: 10.1073/pnas.1008652107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Normanno N, Maiello MR, De Luca A. Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs): simple drugs with a complex mechanism of action? J Cell Physiol. 2003;194:13–9. doi: 10.1002/jcp.10194. [DOI] [PubMed] [Google Scholar]
- 94.Theodoropoulos GE, Karafoka E, Papailiou JG, Stamopoulos P, Zambirinis CP, Bramis K, Panoussopoulos SG, Leandros E, Bramis J. P53 and EGFR expression in colorectal cancer: a reappraisal of ‘old’ tissue markers in patients with long follow-up. Anticancer Res. 2009;29:785–91. [PubMed] [Google Scholar]
- 95.Cui T, Chen Y, Yang L, Knösel T, Huber O, Pacyna-Gengelbach M, Petersen I. The p53 target gene desmocollin 3 acts as a novel tumor suppressor through inhibiting EGFR/ERK pathway in human lung cancer. Carcinogenesis. 2012;33:2326–33. doi: 10.1093/carcin/bgs273. [DOI] [PubMed] [Google Scholar]
- 96.Danilov AV, Neupane D, Nagaraja AS, Feofanova EV, Humphries LA, DiRenzo J, Korc M. DeltaNp63alpha-mediated induction of epidermal growth factor receptor promotes pancreatic cancer cell growth and chemoresistance. PLoS One. 2011;6:e26815. doi: 10.1371/journal.pone.0026815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rong S, Donehower LA, Hansen MF, Strong L, Tainsky M, Jeffers M, Resau JH, Hudson E, Tsarfaty I, Vande Woude GF. Met proto-oncogene product is overexpressed in tumors of p53-deficient mice and tumors of Li-Fraumeni patients. Cancer Res. 1995;55:1963–70. [PubMed] [Google Scholar]
- 98.Hwang CI, Matoso A, Corney DC, Flesken-Nikitin A, Körner S, Wang W, Boccaccio C, Thorgeirsson SS, Comoglio PM, Hermeking H, et al. Wild-type p53 controls cell motility and invasion by dual regulation of MET expression. Proc Natl Acad Sci U S A. 2011;108:14240–5. doi: 10.1073/pnas.1017536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang W, Wetterskog D, Matsumoto Y, Funa K. Kinetics of repression by modified p53 on the PDGF beta-receptor promoter. Int J Cancer. 2008;123:2020–30. doi: 10.1002/ijc.23735. [DOI] [PubMed] [Google Scholar]
- 100.Silber J, Jacobsen A, Ozawa T, Harinath G, Pedraza A, Sander C, Holland EC, Huse JT. miR-34a repression in proneural malignant gliomas upregulates expression of its target PDGFRA and promotes tumorigenesis. PLoS One. 2012;7:e33844. doi: 10.1371/journal.pone.0033844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lei H, Velez G, Kazlauskas A. Pathological signaling via platelet-derived growth factor receptor alpha involves chronic activation of Akt and suppression of p53. Mol Cell Biol. 2011;31:1788–99. doi: 10.1128/MCB.01321-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pignatelli J, Tumbarello DA, Schmidt RP, Turner CE. Hic-5 promotes invadopodia formation and invasion during TGF-β-induced epithelial-mesenchymal transition. J Cell Biol. 2012;197:421–37. doi: 10.1083/jcb.201108143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cordenonsi M, Montagner M, Adorno M, Zacchigna L, Martello G, Mamidi A, Soligo S, Dupont S, Piccolo S. Integration of TGF-beta and Ras/MAPK signaling through p53 phosphorylation. Science. 2007;315:840–3. doi: 10.1126/science.1135961. [DOI] [PubMed] [Google Scholar]
- 104.Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B, Solari A, Bobisse S, Rondina MB, Guzzardo V, et al. A Mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell. 2009;137:87–98. doi: 10.1016/j.cell.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 105.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–69. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 106.Ory S, Brazier H, Pawlak G, Blangy A. Rho GTPases in osteoclasts: orchestrators of podosome arrangement. Eur J Cell Biol. 2008;87:469–77. doi: 10.1016/j.ejcb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 107.van Helden SF, Hordijk PL. Podosome regulation by Rho GTPases in myeloid cells. Eur J Cell Biol. 2011;90:189–97. doi: 10.1016/j.ejcb.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 108.Croft DR, Olson MF. Transcriptional regulation of Rho GTPase signaling. Transcription. 2011;2:211–5. doi: 10.4161/trns.2.5.16904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moreau V, Tatin F, Varon C, Anies G, Savona-Baron C, Génot E. Cdc42-driven podosome formation in endothelial cells. Eur J Cell Biol. 2006;85:319–25. doi: 10.1016/j.ejcb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 110.Moreau V, Tatin F, Varon C, Génot E. Actin can reorganize into podosomes in aortic endothelial cells, a process controlled by Cdc42 and RhoA. Mol Cell Biol. 2003;23:6809–22. doi: 10.1128/MCB.23.19.6809-6822.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T, et al. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441–52. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Linder S, Nelson D, Weiss M, Aepfelbacher M. Wiskott-Aldrich syndrome protein regulates podosomes in primary human macrophages. Proc Natl Acad Sci U S A. 1999;96:9648–53. doi: 10.1073/pnas.96.17.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gadéa G, Lapasset L, Gauthier-Rouvière C, Roux P. Regulation of Cdc42-mediated morphological effects: a novel function for p53. EMBO J. 2002;21:2373–82. doi: 10.1093/emboj/21.10.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Timpson P, McGhee EJ, Morton JP, von Kriegsheim A, Schwarz JP, Karim SA, Doyle B, Quinn JA, Carragher NO, Edward M, et al. Spatial regulation of RhoA activity during pancreatic cancer cell invasion driven by mutant p53. Cancer Res. 2011;71:747–57. doi: 10.1158/0008-5472.CAN-10-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mizuarai S, Yamanaka K, Kotani H. Mutant p53 induces the GEF-H1 oncogene, a guanine nucleotide exchange factor-H1 for RhoA, resulting in accelerated cell proliferation in tumor cells. Cancer Res. 2006;66:6319–26. doi: 10.1158/0008-5472.CAN-05-4629. [DOI] [PubMed] [Google Scholar]
- 116.Xia M, Land H. Tumor suppressor p53 restricts Ras stimulation of RhoA and cancer cell motility. Nat Struct Mol Biol. 2007;14:215–23. doi: 10.1038/nsmb1208. [DOI] [PubMed] [Google Scholar]
- 117.Chan CH, Lee SW, Li CF, Wang J, Yang WL, Wu CY, Wu J, Nakayama KI, Kang HY, Huang HY, et al. Deciphering the transcriptional complex critical for RhoA gene expression and cancer metastasis. Nat Cell Biol. 2010;12:457–67. doi: 10.1038/ncb2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Berdeaux RL, Díaz B, Kim L, Martin GS. Active Rho is localized to podosomes induced by oncogenic Src and is required for their assembly and function. J Cell Biol. 2004;166:317–23. doi: 10.1083/jcb.200312168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chellaiah MA, Soga N, Swanson S, McAllister S, Alvarez U, Wang D, Dowdy SF, Hruska KA. Rho-A is critical for osteoclast podosome organization, motility, and bone resorption. J Biol Chem. 2000;275:11993–2002. doi: 10.1074/jbc.275.16.11993. [DOI] [PubMed] [Google Scholar]
- 120.Schramp M, Ying O, Kim TY, Martin GS. ERK5 promotes Src-induced podosome formation by limiting Rho activation. J Cell Biol. 2008;181:1195–210. doi: 10.1083/jcb.200801078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Burgstaller G, Gimona M. Actin cytoskeleton remodelling via local inhibition of contractility at discrete microdomains. J Cell Sci. 2004;117:223–31. doi: 10.1242/jcs.00839. [DOI] [PubMed] [Google Scholar]
- 122.Ory S, Munari-Silem Y, Fort P, Jurdic P. Rho and Rac exert antagonistic functions on spreading of macrophage-derived multinucleated cells and are not required for actin fiber formation. J Cell Sci. 2000;113:1177–88. doi: 10.1242/jcs.113.7.1177. [DOI] [PubMed] [Google Scholar]
- 123.Qin Q, Baudry M, Liao G, Noniyev A, Galeano J, Bi X. A novel function for p53: regulation of growth cone motility through interaction with Rho kinase. J Neurosci. 2009;29:5183–92. doi: 10.1523/JNEUROSCI.0420-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gadea G, de Toledo M, Anguille C, Roux P. Loss of p53 promotes RhoA-ROCK-dependent cell migration and invasion in 3D matrices. J Cell Biol. 2007;178:23–30. doi: 10.1083/jcb.200701120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Croft DR, Crighton D, Samuel MS, Lourenco FC, Munro J, Wood J, Bensaad K, Vousden KH, Sansom OJ, Ryan KM, et al. p53-mediated transcriptional regulation and activation of the actin cytoskeleton regulatory RhoC to LIMK2 signaling pathway promotes cell survival. Cell Res. 2011;21:666–82. doi: 10.1038/cr.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Valastyan S, Reinhardt F, Benaich N, Calogrias D, Szász AM, Wang ZC, Brock JE, Richardson AL, Weinberg RA. A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell. 2009;137:1032–46. doi: 10.1016/j.cell.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 127.Bravo-Cordero JJ, Oser M, Chen X, Eddy R, Hodgson L, Condeelis J. A novel spatiotemporal RhoC activation pathway locally regulates cofilin activity at invadopodia. Curr Biol. 2011;21:635–44. doi: 10.1016/j.cub.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hsu FF, Lin TY, Chen JY, Shieh SY. p53-Mediated transactivation of LIMK2b links actin dynamics to cell cycle checkpoint control. Oncogene. 2010;29:2864–76. doi: 10.1038/onc.2010.40. [DOI] [PubMed] [Google Scholar]
- 129.Furmaniak-Kazmierczak E, Crawley SW, Carter RL, Maurice DH, Côté GP. Formation of extracellular matrix-digesting invadopodia by primary aortic smooth muscle cells. Circ Res. 2007;100:1328–36. doi: 10.1161/CIRCRESAHA.106.147744. [DOI] [PubMed] [Google Scholar]
- 130.Wang J, Taba Y, Pang J, Yin G, Yan C, Berk BC. GIT1 mediates VEGF-induced podosome formation in endothelial cells: critical role for PLCgamma. Arterioscler Thromb Vasc Biol. 2009;29:202–8. doi: 10.1161/ATVBAHA.108.174391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wheeler AP, Wells CM, Smith SD, Vega FM, Henderson RB, Tybulewicz VL, Ridley AJ. Rac1 and Rac2 regulate macrophage morphology but are not essential for migration. J Cell Sci. 2006;119:2749–57. doi: 10.1242/jcs.03024. [DOI] [PubMed] [Google Scholar]
- 132.Guo F, Gao Y, Wang L, Zheng Y. p19Arf-p53 tumor suppressor pathway regulates cell motility by suppression of phosphoinositide 3-kinase and Rac1 GTPase activities. J Biol Chem. 2003;278:14414–9. doi: 10.1074/jbc.M300341200. [DOI] [PubMed] [Google Scholar]
- 133.Guo F, Zheng Y. Rho family GTPases cooperate with p53 deletion to promote primary mouse embryonic fibroblast cell invasion. Oncogene. 2004;23:5577–85. doi: 10.1038/sj.onc.1207752. [DOI] [PubMed] [Google Scholar]
- 134.Ongusaha PP, Kim HG, Boswell SA, Ridley AJ, Der CJ, Dotto GP, Kim YB, Aaronson SA, Lee SW. RhoE is a pro-survival p53 target gene that inhibits ROCK I-mediated apoptosis in response to genotoxic stress. Curr Biol. 2006;16:2466–72. doi: 10.1016/j.cub.2006.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 135.Georgess D, Mazzorana M, Terrado J, Delprat C, Chamot C, Guasch RM, Pérez-Roger I, Jurdic P, Machuca-Gayet I. Comparative transcriptomics reveal RhoE as a novel regulator of actin dynamics in bone-resorbing osteoclasts. Mol Biol Cell. 2013 doi: 10.1091/mbc.E13-07-0363. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lefort K, Mandinova A, Ostano P, Kolev V, Calpini V, Kolfschoten I, Devgan V, Lieb J, Raffoul W, Hohl D, et al. Notch1 is a p53 target gene involved in human keratinocyte tumor suppression through negative regulation of ROCK1/2 and MRCKalpha kinases. Genes Dev. 2007;21:562–77. doi: 10.1101/gad.1484707. [DOI] [PMC free article] [PubMed] [Google Scholar]


