Abstract
Basement membranes are thin sheets of self-assembled extracellular matrices that are essential for embryonic development and for the homeostasis of adult tissues. They play a role in structuring, protecting, polarizing, and compartmentalizing cells, as well as in supplying them with growth factors. All basement membranes are built from laminin and collagen IV networks stabilized by nidogen/perlecan bridges. The precise composition of basement membranes, however, varies between different tissues. Even though basement membranes represent physical barriers that delimit different tissues, they are breached in many physiological or pathological processes, including development, the immune response, and tumor invasion. Here, we provide a brief overview of the molecular composition of basement membranes and the process of their assembly. We will then illustrate the heterogeneity of basement membranes using two examples, the epithelial basement membrane in the gut and the vascular basement membrane. Finally, we examine the different strategies cells use to breach the basement membrane.
Keywords: basement membrane, laminin, collagen, invadopodia, invasion
Introduction
Basement membranes (BMs) are thin sheets of self-assembled extracellular matrix (ECM). They are found in nearly all tissues and have a wide range of functions. They provide structural and adhesion support to cells, coating the basal side of epithelial and endothelial cells and surrounding muscles, adipocytes, and peripheral nerve axons. They also serve as a reservoir for growth factors, such as transforming growth factor β (TGFβ), fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), and heparin epidermal growth factor (HB-EGF), which play a role in cell survival, migration, and proliferation.1 BMs protect tissues from disruptive mechanical stresses and play a role in compartmentalizing different cell types. More specifically, BMs promote the attachment of epidermis to dermis, establish epithelial cell polarization, control the selectivity of glomeral filtration, stabilize the sarcomeres in skeletal muscle, and prevent the spread of cancer cells to adjacent stroma.2 Nevertheless, some cells possess or gain the ability to breach BMs in many physiological and pathological conditions, such as development, immune response, and metastasis. It is therefore crucial to understand if there is a link between the diversity of BMs, their functions and mechanisms for their breaching.
Molecular Composition of BMs
Transmission electron microscopy has shown that BMs are typically between 50–100 nm thick. However, the thickness of BMs measured by atomic force microscopy appears on average four-times greater. The difference could be due to loss of water during the preparation of samples for electron microscopy resulting in decreased thickness of BM.3 BMs are composed of two interconnected polymer networks made of collagen IV and laminin that have combined pore size in the order of 10 nm.4 Besides collagen IV and laminins, the other major components of BMs are glycoproteins: nidogens and the heparan sulfate proteoglycans such as perlecan and/or agrin.5
Not all basement membranes are the same, however. They have heterogeneous molecular compositions in different organs, which reflect their unique biological functions. Minor components, such as fibulin, collagen type XV, XVIII, VI, SPARC, BM90, and others contribute to BM tissue specificity.5 Additional variations in the final structure, signaling, and stability of BMs arise from differences in the assembly, receptor binding, and cross-linking of laminin and collagen isoforms.2 Here, we provide a brief overview of major components of BMs. For more details, refer to references 6 and 7.
Laminin
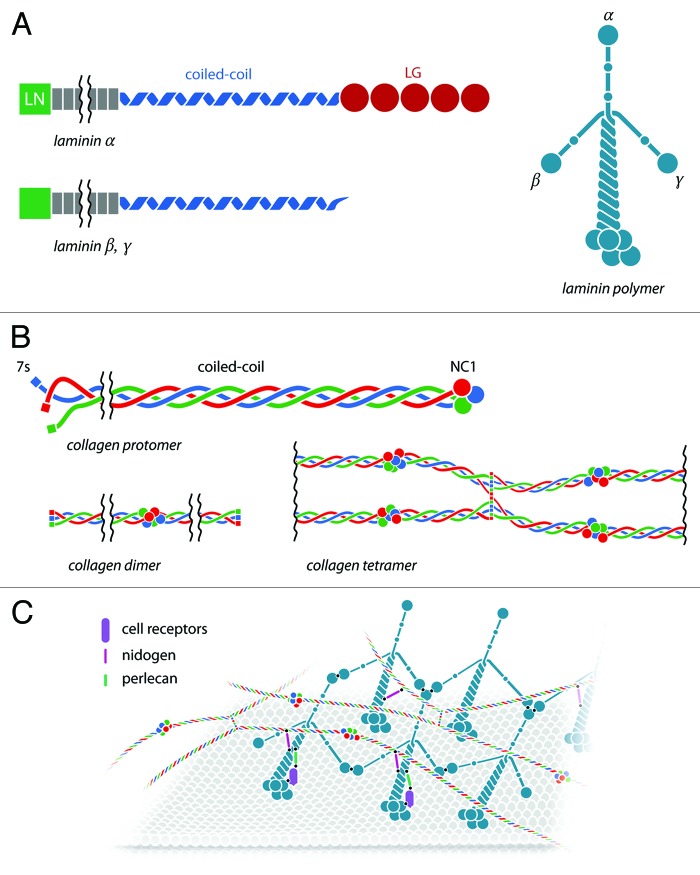
Laminin is the most abundant non-collagenous protein in the BM. Each laminin is a heterotrimer consisting of α, β, and γ-chains (Fig. 1A). The α-chain is the biggest, averaging 160 nm in length. The β- and γ-chains are smaller, with short 60 and 40 nm length arms, respectively. In general, laminins are comprised of an N-terminal LN domain (exceptions are α4 and γ2), which plays a role in laminin polymerization. The coiled-coil region is responsible for the assembly of the heterodimers. In the case of α chains, a C-terminal LG domain is responsible for cell surface adhesion and receptor interactions.
Figure 1. Major components and the assembly of basement membranes. (A) Schematic representation of α, β, and γ laminin chains. The domains on each chain are indicated: LN domain is important for laminin polymerization; coil-coil domain—for the assembly of the heterodimers; and C-terminal LG domain of α chains—for cell surface adhesion. (B) Schematic representation of collagen type IV. The domains of collagen IV are indicated: N-terminal 7S domain, important for collagen network formation, a triple helical domain—for formation of heterotrimers (protomers) and a C-terminal NC1 domain—for formation of dimers. (C) BM assembly. Laminin and collagen type IV are secreted in the extracellular milieu. Laminin binds to cell surface receptors, such as integrins, via the LG domain of the α-chain. Intermolecular binding between LN domains of α, β, and γ-chains of the adjacent laminins results in the formation of a distinct network. Type IV collagen protomers also self-assemble and form a depicted network. Nidogen and perlecan come and serve as binding bridges between the two networks to form a dense mesh. Minor components are not illustrated.
Different combinations of α, β, and γ-chains lead to the generation of 15 different heterotrimers such as laminin-111 (α1β1γ1), 211(α2β1γ1), that have different roles in tissue structure and cell behavior.8 Chains are joined together via a triple α-helical coiled-coil domain, from which the N termini of each of the three chains emerge as three arms. Mutations in the laminin LN domains result in defects in laminin polymerization and contribute to numerous developmental abnormalities.2 Deletion of laminin γ1, for example, leads to early embryonic lethality due to a failure to assemble BM.9 Furthermore, fragments of laminin that are unable to polymerize are still deposited into the ECM, where they act as metastasis-promoting factors.10
Type IV Collagen
Type IV collagen is a non-fibrillar collagen whose abundance increases with age comprising more than 50% of adult BMs.3 It is a heterotrimer of approximately 400 nm long. Collagen IV differs from fibrillar collagens (such as types I, II, and III) due to its distinct capacity to self-assemble into networks.
There are six genetically different α-chains (α1–α6), which can assemble into three different heterotrimers (protomers). The most common variant, found in almost all BMs, consists of two α1 subunits and one α2 subunit. Each α chain contains an N-terminal 7S domain that is important for the formation of collagen networks; a middle, triple helical collagenous domain; and a C-terminal globular domain (NC1), which plays a role in the assembly of the trimeric structure (Fig. 1B).
The assembly of collagen starts with the interaction between the NC1 domains of all three α-chains, followed by zippering of the middle triple helical domains resulting in a fully assembled protomer. In the next step, two protomers associate via their NC1 domains to form dimers. Finally, four protomers interact via their 7S domains to form a tetramer, a complex that is considered to be the nucleus of the collagen IV scaffold.
This nucleus further evolves into a collagen IV suprastructure as a result of end-to-end interactions and lateral connections between collagen IV protomers.11 Although the BM can be assembled in collagen IV α1/α2-knockout mice, it is unstable and leads to prenatal lethality.12 Collagen IV is also important for the stability of the microvasculature.13-15 Bioactive fragments released by the proteolytic cleavage of type IV collagen are involved in the regulation of several physiological and pathological processes such as development, angiogenesis, tumor growth, and metastasis. These fragments are called matricryptins and have antiangiogenic and antitumoral activity.16
Nidogens
Nidogens (or entactins) are glycoproteins that consist of three globular domains. They bind to type IV collagen, laminin, and perlecan. The two isoforms are ubiquitously expressed in all BMs with nidogen2 being predominant in vascular BMs.5 The BM can be assembled in the absence of nidogens.17 However, the early lethality of nidogen 1/2 double knockout mice suggests that nidogens are essential for the stabilization of the BM during late embryogenesis and for its proper function in adult tissues when mechanical stresses increase.5 Nidogens are mainly produced by fibroblasts.
Perlecan
Perlecan is a 200 nm long heparan sulfate proteoglycan whose structure resembles a string of pearls. It accumulates in tissue borders, where it plays a role in separating tissue layers. It is also involved in stocking growth factors, via the heparan sulfate chains.18 Perlecan is important in angiogenesis, neurogenesis, and chondrogenesis and may play a critical role during vascular BM remodeling. Upon proteolytic degradation, its fragments can interact with pro- and anti-angiogenic regulators, promoting angiogenesis and potentially tumor growth.5
BM Assembly
The primary sequences of collagen IV and laminin contain the information that promotes intermolecular self-assembly.2 In vitro experiments reveal that collagen IV and laminin can self-assemble into lattice-like networks.19 Additional links are then established: nidogens bridge laminin and collagen IV networks, while perlecan connects nidogen to laminin.20 Although these interactions are crucial for BM assembly in vitro, they do not explain how these processes occur in vivo.
In vivo, cells secrete laminins, which diffuse into the extracellular space. Given that the correct self-assembly of a BM depends upon the relative concentrations of its components, BM-assembling cells must possess a mechanism for regulating the local concentration of the laminin they secrete. Studies of aggregates of pluripotent stem cells show that cell-surface receptors, such as β1 integrin or dystrogly, can assist the formation of laminin polymers on the cell surfaces. Compared with wild-type cells, cells lacking β1 integrin or dystroglycan deposit reduced amounts of laminin.21,22 Similarly, mice carrying mutations in certain cell surface receptors, like β1 integrins, show various defects in their BMs.22-24 Thus, the assembly of a BM starts with laminin–cell receptor interactions (Fig. 1C).
In the first step on BM formation, the LG domains of laminin molecules anchor to the selected cell surface via sulfate glycolipids, integrins, and α-dystroglycan. This increases the local concentration of laminin beyond the level necessary for polymerization to take place.25-28 Once sufficiently close together, the LN domains of α, β, and γ-chains of adjacent laminins bind to each other, leading to the formation of ternary complexes.29,30 The LN domain of the laminin α-subunit can also bind to sulfatides and integrins, generating a second cell–matrix attachment point that forces laminin polymers to orient themselves parallel to the cell surface they are outlining.
In the second step, collagen IV, nidogen, perlecan, and agrin coalesce on the nascent laminin scaffold. Although all these molecules are able to bind to the cell surface, they seem unable to assemble into BM in the absence of laminins.25 Collagen IV polymerizes and forms a second network. Unlike the laminin network, it is covalently stabilized. Nidogen binds to the laminin γ-subunit and to collagen IV, forming a non-covalent high-affinity stabilizing bridge. Finally, agrin and perlecan bind to both the laminin coiled-coil domain and to nidogen, as well as to cell surface molecules, thus creating collateral linkages to additional receptors.
In conclusion, two different classes of interactions drive the assembly of the BM components. On one hand, BM components interact with each other and self-assemble, and on the other, they interact with cell surface receptors.25 These two different types of interactions are coordinated to generate a BM on competent cell surfaces.
The cells that are competent to assemble BM do not necessarily secrete all its components. Neighboring cells of a different type to the BM-forming cells often play a role in generation of BM components. For example, epithelial BMs form specifically beneath epithelial cells but their constituent proteins are partly synthesized by mesenchymal cells that do not bind to the BM.31,32 The reason why some cells are able to assemble BM while others are not is probably related to the cell surface molecules they carry. It is likely, for example, that mesenchymal cells lack the surface molecules that anchor the laminin LG domain, meaning that they do not favor the accumulation and self-assembly of laminin molecules. If laminin-binding molecules, such as sulfatides, are experimentally added to the plasma membrane of fibroblasts and these cells are then supplied with high concentrations of laminin, they assemble BMs on their surfaces.
Diversity of BMs
The composition of BMs is extremely diverse and dynamic. It varies between tissues and also changes with the tissue’s physiological and pathophysiological state and age.3 It remains unclear, however, whether and how these compositional differences translate into specific BM functions. Here, we illustrate the heterogeneity of BMs with two examples, the epithelial BM in the gut and the vascular BM. For detailed analyses of the specificity of BM in different tissues, we refer the reader to the comprehensive reviews of Yurchenko,2 Kalluri,11 and Halfter3 and Liliensiek.33
Epithelial BM in the Gut
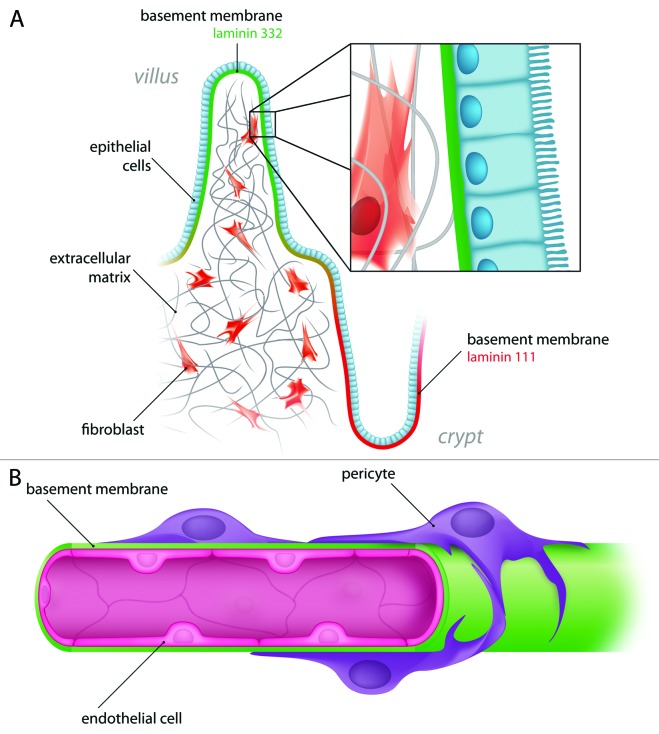
The epithelium of the small intestine is composed of a single layer of cells. It lines the villi, which project into the lumen of the gut, and the crypts, which descend into the underlying connective tissue (Fig. 2A). As with all epithelia, the gut BM lies under the basal surface of the epithelium. Interestingly, the composition of the BM changes during gut development and along the crypt–villus axis.34 For example, the β1γ1 chains are homogenously localized in both crypts and villi, but the α1 and α2 chains are restricted to the crypts, with α1 being present from early development and α2 appearing only around birth when crypt growth occurs.
Figure 2. Examples of different basement membranes. (A) Intestinal epithelial BM. The differences in composition within the same type of BM, in one particular tissue, is shown with the crypt BM vs. the villus BM, suggesting different functions for these BMs. (B) Endothelial basement membrane. In between endothelial cells and pericytes, the vascular BM assures the integrity of the blood vessel and also the contact between stroma and circulatory cells.
The expression of laminin-332 is concomitant with the formation of villi. Its highest level of expression occurs and at the villi's tips, which correlates with its localization in the BM undelaying epithelial cells at the tips of the villi. Although the physiological significance of laminin variants is not clear, the presence of laminin-322 specifically in the villi, where active cell migration occurs, suggests that this laminin variant could have a role in cell migration. Since ternary interactions between the LN domains of α-β-γ chains is strictly required for the self-assembly of laminin polymers and BM in general,29,35 it is likely that laminin-332, whose γ chain lack the LN domain, is assembled through an alternative mechanism. Deletion of the LN domain, for example, from the α chain, results in laminin-poor, collagen IV-rich ECM.2 This suggests that BMs containing laminin-322 could also have special properties.
To determine whether BM components originate from epithelial or mesenchymal cells, Kedinger, et al.34 generated hybrid intestines composed of mice endoderm and chick mesenchyme, and vice versa. These were then grafted for different periods of time and analyzed immunocytochemically using species-specific antibodies. This technique showed that while perlecan is produced by the epithelium, nidogen and collagen IV are mostly mesenchymal products.
In the case of laminin, the situation is more complex: different laminin chains are produced by different cells. In general, during embryonic development, laminin α5, β1, and γ1 chains have a dual, epithelial, and mesenchymal origin; α2 and β3 originate from the mesenchyme; and α1 is mostly produced by the epithelium. Interestingly, γ2 shows a shift in its cellular expression: at early stages, it is expressed by epithelial cells and then at later stages by mesenchymal cells. Thus, the epithelial BM in the gut is produced by the coordinated action of epithelial cells and stromal fibroblasts.
The intestine is characterized by rapid cell renewal of the epithelium, as cells differentiate and migrate from the crypts toward the villus tips, while remaining attached to the BM.36 There is evidence that fibroblasts, present on the other side of the BM, migrate along with the epithelial cells toward the villus tips.37 This observation led to the speculation that the epithelium, the BM and the fibroblasts migrate synchronously as a unit along the villus-crypt axis. Because the entire epithelium is renewed every three days in mice, this would suggest that the BM is also renewed every three days. This hypothesis has been challenged by the observation that the BM turns over focally over a period of weeks after being labeled with fluorescent antibodies.38 Dynamic studies are needed to solve this discrepancy.
Transmission electron microscopy studies have shown that the intestinal BM have distinctive morphological features during gut development in rodents.39 A week before birth, a continuous BM separates the epithelium from the underlying mesenchyme. Several days before birth, when villi start to form, gaps in the BM are observed. In those regions where BM is discontinuous, epithelial cells and fibroblasts sit opposite one another and epithelial cells project pseudopod-like protrusions through the gaps in the BM, making contact with the fibroblasts. Those cellular protrusions and BM gaps persist until 10 d post-birth, when the BM becomes again continuous. Besides being a place of communication between epithelial and mesenchymal cells, these scattered BM defects could also be associated with leukocyte trafficking.38 Through these pores, immune cells can migrate from the lamina propria into the epithelium and can directly contact the enterocytes or sample antigens.40-42
Vascular BMs
The vascular BM assures integrity and robustness of blood vessels throughout different tissues. It also plays a crucial role in the trans-BM migration of different cell types during development, the immune response, and cancer.
Capillaries are formed of two cell types: endothelial cells, which line the lumen, and pericytes, which dot the exterior. The BM is sandwiched between these two cell types, which are tightly connected to each other via a large number of long protrusions.43,44 Unlike endothelial cells, which form a continuous, well-organized monolayer, pericytes form a discontinuous layer on the BM (Fig. 2B). Both cell types produce the components of the vascular BM, so the discontinuous nature of the pericyte coverage results in the formation of regions in the BM that have patchy expression of collagen IV and laminins.45-47 Those regions, termed low expression regions, often serve as gates for migrating leukocytes during inflammation.
Vascular BMs differ significantly in their thickness, pore size, and fiber diameter depending on the location and physical properties of the vessel. For the extensive review about specificity of vascular BMs, see Kalluri.11 In tumors, the blood vessel BM is often described as incomplete or absent.48 However, other studies suggest that it is present but morphologically abnormal.49,50 For example, although the vascular BM in pancreatic tumors, as identified by collagen IV, laminin, and nidogen immunostaining, outlines blood vessels in a similar fashion to normal tissue, it still shows numerous structural abnormalities. Its association with endothelial cells and pericytes is much looser than in normal vessels. Electron microscopy studies have revealed the presence of multiple BM layers.49
During angiogenesis, the BM is either degraded, to enable sprout formation and endothelial cell migration,51 or remodeled continuously as endothelial sprouts form and new vessels grow.52 Migrating endothelial cells can degrade collagen IV in vitro.51 In more recent studies, collagen IV-positive sheets were detected on endothelial sprouts, suggesting that BM is deposited where sprouts grow and regress.49 Altogether, these results indicate that in angiogenesis, both mechanisms of degradation and continuous remodeling of the BM might occur. Further studies are required to determine whether these two mechanisms operate simultaneously.
Overcoming the BM Barrier
The BM acts as a formidable barrier to diffusing macromolecules and migrating cells because of its lateral organization and intrinsic biomechanical properties. However, the existence of small pores in BMs suggests that different cell types could communicate by exchanging diffusible factors53,54 During postnatal gut development, epithelial cells make numerous physical contacts with mesenchymal cells through the larger gaps in the BMs.40 However, those gaps in the BMs and protrusions of the epithelial cells are only rarely observed in the adult mice. Although different cells contact each other by poking long cellular protrusions through BM gaps, the density of the covalently cross-linked BM mesh means it is unlikely that entire cells could squeeze through the existing gaps (see review by Weiss55). Several cell types, however, are able to cross BMs. These include trophoblasts, neural crest cells, leukocytes, and cancer cells. Breaching of the BM in those cases is regulated in a cell-specific manner by a mechanism that comprises proteolytic capacity and cellular deformability.46 Local degradation of the BM components is achieved by specialized cellular protrusions, termed invadopodia. Those finger-like, F-actin-rich structures form at the ventral side of the cell and serve as a localized source of matrix degrading proteases including matrix metalloproteinases (MMPs). However, it has been also proposed that BM transmigration can occur, in addition, through mechanisms independent of proteolytic digestion.55,56
Breaching the BM During Development
During mammalian gastrulation, embryonic epithelial cells breach the BM, acquire mesenchymal characteristics, and populate the intervening space to form the mesoderm. Similar BM invasion processes are conserved in development and are observed in different organisms. These include imaginal disk eversion in Drosophila melanogaster or invasion of anchor cell during Caenorhabditis elegans morphogenesis.57,58
Sherwood and colleagues have used anchor cell invasion in Caenorhabditis elegans as an in vivo model of BM transmigration. This model provides visual evidence of the interaction between the invading cell and the BM59 (Fig. 3A). Sherwood, et al. demonstrated anchor cells use invadopodia-like structures to breach the BM and so connect the uterine and vulval epithelia.60 Other studies, performed in the zebrafish, showed that intestinal epithelial cells also form invadopodia-like protrusions and invade the stroma in response to a physical signal arising from smooth muscle contractility in the larval intestine.61 Thus, invadopodia-like protrusions are conserved subcellular structures used for BM breaching during development. Using chick embryo as a model system to study epithelial-to-mesenchymal transition (EMT) in gastrulation, Nakaya and colleagues showed that destabilization of microtubules at the epiblast’s basal cell cortex weaken the interactions between epiblasts and BMs, which leads to BM breakdown.62,63 Thus, during EMT, cells lose connections with the BM while they are still connected to the rest of the epiblasts as a continuous sheet. Whether invadopodia are involved in BM breakdown during gastrulation still needs to be investigated.
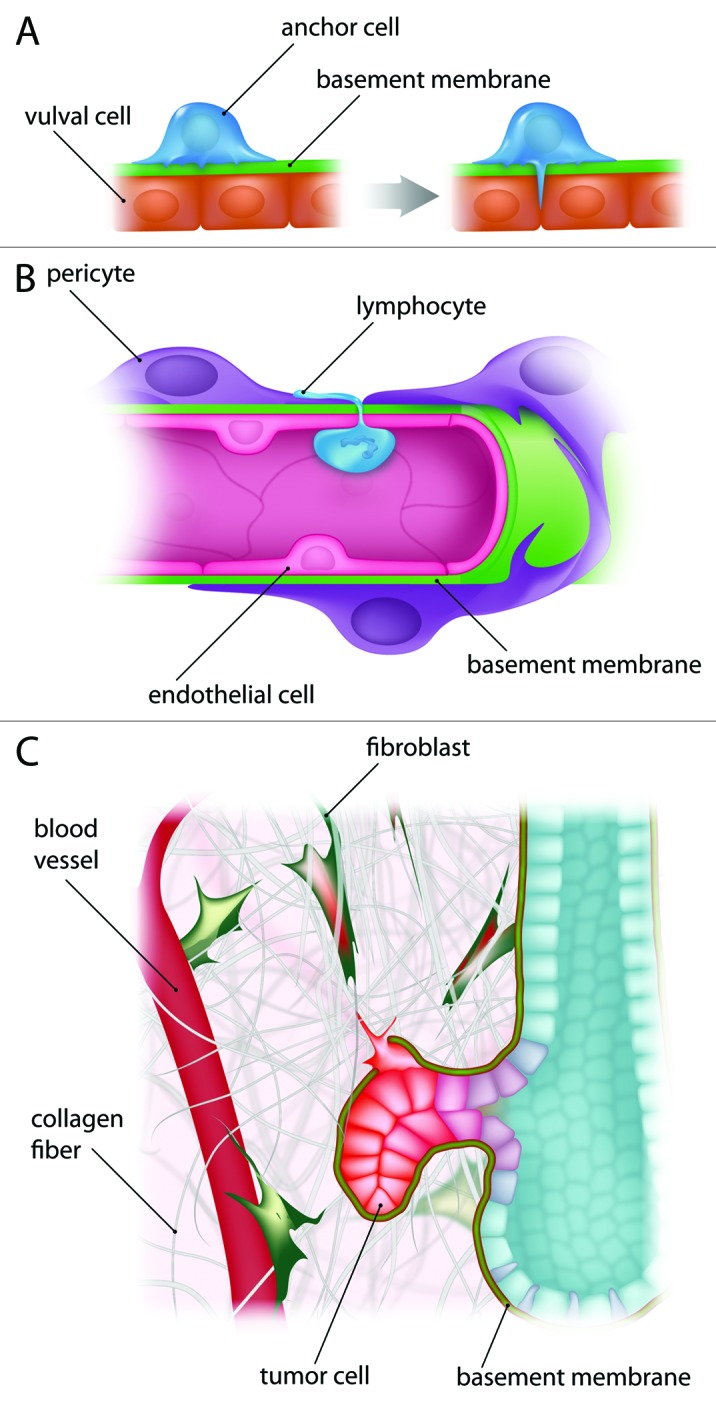
Figure 3. Breaching the BMs in several processes. (A) BM breaching in development. The anchor cell invades the BM during hermaphrodite development in Caenorhabditis elegans. This process requires invadopodia-like structures to initiate BM breaching. (B) Vascular BM invasion. Immune cells can pass through the BM, a process called trans-endothelial migration. For example, lymphocytes breach the BM in order to access a tissue during the inflammatory response. (C) BM invasion during cancer metastasis. Cancer cells breach the BM in order to reach the blood circulation. They might form invadopodia, which have been shown in vitro assays to be required for BM proteolytic degradation.
Immune Cells Passing Through the BM
During inflammation, leukocytes adhere to the vascular lumen, roll, and then migrate through the endothelium. Once underneath the endothelial cells, they extend cellular protrusions to search for “low expression regions” in the vascular BM and permissive sites in the pericyte monolayer. This allows them to exit vessels and to migrate through the extravascular tissue. This process is termed trans-endothelial migration (Fig. 3B).
How leukocytes breach the BM and whether proteolytic invadopodia-like structures are involved remains unclear. Both neutrophils and monocytes use “low expression regions” on the BM as preferred sites for penetration. While neutrophils enlarge those regions using proteases, monocytes squeeze through the BM without remodeling it.64-66
The migration of leukocytes through the vascular BM could also occur through biochemically permissive sites. For example, BM rich in laminin-511 (α5β1γ1) is considered to be anti-migratory, whereas BM composed of laminin-411 (α4β1γ1) promotes migration.67 Mice engineered to lack the laminin α-4 chain are not only deficient in laminin-411, but also show compensatory upregulation of the laminin α-5 chain.68,69 Trans-endothelial migration is reduced in these mice. Because the laminin α-4 chain lacks an LN domain, its assembly into polymers is defective and probably results in sparser BMs that are easier to penetrate. In contrast to these findings, in vitro studies have shown that laminin-511 is more potent as a stimulator of lymphocyte migration than other laminin isoforms, including laminin-411.70 Further efforts are needed to understand those discrepancies.
Breaching the BM in Cancer Invasion
During epithelial tumor formation, cancer cells, like normal epithelial cells, are retained within the boundaries of BM and are therefore separated from the adjacent stroma. The BM therefore serves as a barrier for cell invasion. As the tumor progresses, the stroma undergoes changes in both ECM and cell type composition. There is an increase in the stiffness of the stroma and an increase in the numbers of several cell types such as fibroblasts, macrophages, and pericytes.71 It is not known whether the composition or structural stability of the BM changes by this point.
Eventually, a combination of events, involving cancer and stromal cell interaction and communication through the BM, leads to tumor invasion. At this point, the BM is breached and the cancer cells invade and migrate through the tumor stroma until they reach the blood or lymphatic vessels. To disseminate and metastasize, cancer cells also need to overcome two vascular BMs. First, they must breach the BM to enter the blood vessel (intravasation). Then, they must breach a second BM to exit the blood vessel (extravasation) and seed a specific organ to form a secondary tumor.72 Overcoming the different BM barriers is therefore crucial for cancer metastasis (Fig. 3C).
Breaching the BM by cancer and/or stromal cells might involve three distinct and possibly complementary mechanisms: proteolytic degradation of the BM, local displacement of the BM by mechanical forces, and abnormal BM synthesis. Evidence exists to support the existence of all three mechanisms.
Degradation of the BM involves the formation of invadopodia, which provide a localized source of matrix-degrading proteases. These include matrix metalloproteinases (MMPs) such as MT1-MMP (MMP14) and the serine protease seprase (FAP).73,74 The role of invadopodia in matrix degradation has been extensively studied in cell culture models but their role in cell invasion still remains to be determined in vivo.75
Non-proteolytic means for the invasion of cancer cells through BM-like matrices have been also suggested, involving amoeboid movement of the cells through the mesh of collagen fibers.76 Similarly, other studies showed that cancer cells can adopt a rounded bleb-associated mode of motility through 3D BM-like matrices, via a Rho signaling depending pathway, without proteolysis.77
Finally, reports indicate that BM components are synthesized in lower amounts around invasive tumors.78,79 For example, the stage and grade of many tumors correlate positively with a loss of the laminin-332 γ2 chain from the BM and its retention in the cell cytoplasm.78 Other studies show that in carcinomas, collagen VII and laminin-511 are almost absent from the BM while laminin-322 accumulates along the invading edges of carcinomas.79 It is therefore possible that the spreading of the cancer cells to adjacent tissues is achieved at least in part by reduced BM deposition, rather than as result of its proteolytic degradation or physical disruption. BM imperfections arising from abnormal manufacture could represent “hot spots” for cancer cell invasion.
Model Systems for Cancer Cells BM Invasion
Isolating such a thin and highly dense matrix as the BM for ex vivo studies is extremely difficult. This limitation, coupled with the problem of imaging BM invasion in vivo, has led to alternative solutions, which use matrices that closely resemble the BM. The one most commonly used is Matrigel, a protein mixture secreted Engelbreth-Holm-Swarm (EHS) mouse sarcoma cells. Although its exact composition is unknown, it consists of approximately 60% laminin, 30% collagen IV, and 8% nidogen. Matrigel is often used in invasion assays, such as Boyden chambers, where cells are plated on top of a thin layer of Matrigel, which itself lies on top of a plastic membrane perforated by small pores.80 It can also be used as a matrix for embedding cells to study cell migration and invasion in 3D, or even as a structural support for cells that will subsequently be injected in the animals.
Although the molecular composition of Matrigel closely resembles that of the BM, a key limitation that is frequently overlooked is that the physical properties of the extracellular proteins are altered during the procedure used to separate the gel from the EHS cells that produce it. Matrigel lacks the complex mix of covalent cross-links characteristic of collagen IV networks in vivo, which could explain why migrating cells penetrate it more easily than native BM.55 In addition, Matrigel is comprised mainly of a laminin isoform found rarely in adult tissues.81
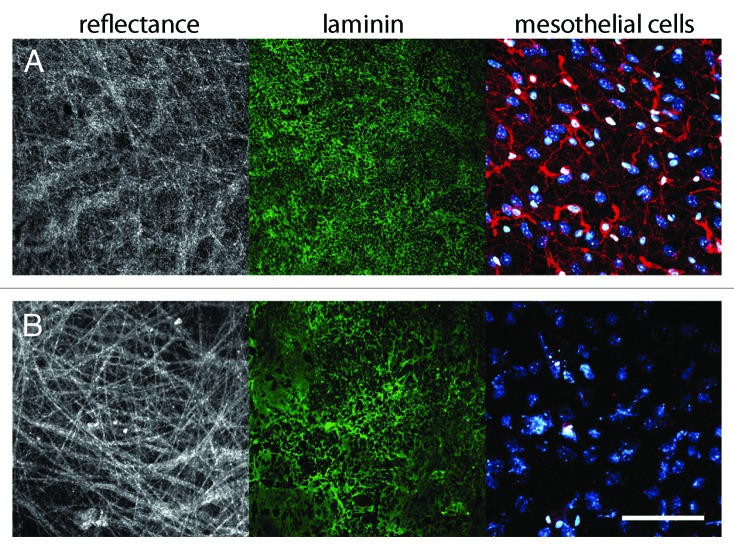
Since Matrigel does not recapitulate the structural properties of physiological BMs, it is questionable whether it can be used to define the proteolytic mechanisms involved in invasion. To overcome those caveats, Weiss and colleagues used mesentery explants as a model system to study cancer cell invasion ex vivo55 (Fig. 4). The mesentery is the double layer of peritoneum that connects the intestine to the abdominal wall. It is composed of two BMs, and fulfills the basic requirements for cancer cell invasion analysis: (1) it is produced and assembled in vivo, thus its molecular composition and structural barrier effectiveness are preserved; and (2) it can be easily explanted to allow ex vivo experimentation.82
Figure 4. Native mesentery basement membrane assay as a model to study BM invasion. (A) Untreated mouse mesentery. Collagen fibers revealed by reflectance (white), laminin revealed by immunostaining (green), DNA and actin cytoskeleton of mesothelial cells revealed by DAPI (cyan), and phalloidin-Cy3 labeling (red). (B) Isolated mesentery is washed and treated with ammonium hydroxide to remove mesothelial cells. The treatment of the mesentery with ammonium hydroxide removes efficiently all mesothelial cells while preserving the integrity of the BM structure, thus making it suitable for invasion studies. Color code as in (A). Scale bar, 200 μm.
Using this assay, we have shown that the invasive human colon cancer cell line HCT116 needs more than a week to invade native mouse mesenteric BM,83 while the same cell line, in otherwise identical culture conditions, invades Matrigel overnight.84 The technical details of how to use mesenteric BM for invasion studies are described by Schoumacher, et al.85
Conclusions
Scientific interest in the mechanisms cells employ to escape their tissue of origin to reach distant sites is growing rapidly. It is therefore essential to develop a better understanding of BM biology and its role as a barrier to cell migration. While it has long been demonstrated that cells can squeeze through the different extracellular spaces during their journey, how they escape their primary site remains largely mysterious. In cancer biology, it is now clear that escaping the primary site involves breaching the BM, either by protelolytic degradation or by alternative, non-proteolytic means. Finding parallels between tumor cell behavior and the mechanisms normal cells use to cross the BM physiological processes such as development is one possible approach for determining the machinery implicated in tumor dissemination.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- BM
basement membrane
- ECM
extracellular matrix
References
- 1.Iozzo RV, Zoeller JJ, Nyström A. Basement membrane proteoglycans: modulators Par Excellence of cancer growth and angiogenesis. Mol Cells. 2009;27:503–13. doi: 10.1007/s10059-009-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol. 2011;3:3. doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halfter W, Candiello J, Hu H, Zhang P, Schreiber E, Balasubramani M. Protein composition and biomechanical properties of in vivo-derived basement membranes. Cell Adh Migr. 2013;7:64–71. doi: 10.4161/cam.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yurchenco PD, Ruben GC. Basement membrane structure in situ: evidence for lateral associations in the type IV collagen network. J Cell Biol. 1987;105:2559–68. doi: 10.1083/jcb.105.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LeBleu VS, Macdonald B, Kalluri R. Structure and function of basement membranes. Exp Biol Med (Maywood) 2007;232:1121–9. doi: 10.3181/0703-MR-72. [DOI] [PubMed] [Google Scholar]
- 6.Kruegel J, Miosge N. Basement membrane components are key players in specialized extracellular matrices. Cell Mol Life Sci. 2010;67:2879–95. doi: 10.1007/s00018-010-0367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yurchenco PD, Patton BL. Developmental and pathogenic mechanisms of basement membrane assembly. Curr Pharm Des. 2009;15:1277–94. doi: 10.2174/138161209787846766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JCR, et al. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–32. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Yurchenco PD, Amenta PS, Patton BL. Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol. 2004;22:521–38. doi: 10.1016/j.matbio.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Tran M, Rousselle P, Nokelainen P, Tallapragada S, Nguyen NT, Fincher EF, Marinkovich MP. Targeting a tumor-specific laminin domain critical for human carcinogenesis. Cancer Res. 2008;68:2885–94. doi: 10.1158/0008-5472.CAN-07-6160. [DOI] [PubMed] [Google Scholar]
- 11.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–33. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 12.Pöschl E, Schlötzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619–28. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- 13.Gould DB, Phalan FC, Breedveld GJ, van Mil SE, Smith RS, Schimenti JC, Aguglia U, van der Knaap MS, Heutink P, John SWM. Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science. 2005;308:1167–71. doi: 10.1126/science.1109418. [DOI] [PubMed] [Google Scholar]
- 14.Gould DB, Phalan FC, van Mil SE, Sundberg JP, Vahedi K, Massin P, Bousser MG, Heutink P, Miner JH, Tournier-Lasserve E, et al. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N Engl J Med. 2006;354:1489–96. doi: 10.1056/NEJMoa053727. [DOI] [PubMed] [Google Scholar]
- 15.Gould DB, Marchant JK, Savinova OV, Smith RS, John SWM. Col4a1 mutation causes endoplasmic reticulum stress and genetically modifiable ocular dysgenesis. Hum Mol Genet. 2007;16:798–807. doi: 10.1093/hmg/ddm024. [DOI] [PubMed] [Google Scholar]
- 16.Ricard-Blum S. [Matricryptins derived from non fibrillar collagens, MMP-2 and SPARC are involved in the control of angiogenesis] J Soc Biol. 2003;197:41–4. [PubMed] [Google Scholar]
- 17.Böse K, Nischt R, Page A, Bader BL, Paulsson M, Smyth N. Loss of nidogen-1 and -2 results in syndactyly and changes in limb development. J Biol Chem. 2006;281:39620–9. doi: 10.1074/jbc.M607886200. [DOI] [PubMed] [Google Scholar]
- 18.Farach-Carson MC, Warren CR, Harrington DA, Carson DD. Border patrol: Insights into the unique role of perlecan/heparan sulfate proteoglycan 2 at cell and tissue borders. Matrix Biol J Int Soc Matrix Biol 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yurchenco PD, O’Rear JJ. Basal lamina assembly. Curr Opin Cell Biol. 1994;6:674–81. doi: 10.1016/0955-0674(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 20.Battaglia C, Mayer U, Aumailley M, Timpl R. Basement-membrane heparan sulfate proteoglycan binds to laminin by its heparan sulfate chains and to nidogen by sites in the protein core. Eur J Biochem. 1992;208:359–66. doi: 10.1111/j.1432-1033.1992.tb17195.x. [DOI] [PubMed] [Google Scholar]
- 21.Aumailley M, Pesch M, Tunggal L, Gaill F, Fässler R. Altered synthesis of laminin 1 and absence of basement membrane component deposition in (beta)1 integrin-deficient embryoid bodies. J Cell Sci. 2000;113:259–68. doi: 10.1242/jcs.113.2.259. [DOI] [PubMed] [Google Scholar]
- 22.Henry MD, Campbell KP. A role for dystroglycan in basement membrane assembly. Cell. 1998;95:859–70. doi: 10.1016/S0092-8674(00)81708-0. [DOI] [PubMed] [Google Scholar]
- 23.Fässler R, Pfaff M, Murphy J, Noegel AA, Johansson S, Timpl R, Albrecht R. Lack of beta 1 integrin gene in embryonic stem cells affects morphology, adhesion, and migration but not integration into the inner cell mass of blastocysts. J Cell Biol. 1995;128:979–88. doi: 10.1083/jcb.128.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki T, Brakebusch C, Engel J, Timpl R. Mac-2 binding protein is a cell-adhesive protein of the extracellular matrix which self-assembles into ring-like structures and binds beta1 integrins, collagens and fibronectin. EMBO J. 1998;17:1606–13. doi: 10.1093/emboj/17.6.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn. 2000;218:213–34. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 26.Kalb E, Engel J. Binding and calcium-induced aggregation of laminin onto lipid bilayers. J Biol Chem. 1991;266:19047–52. [PubMed] [Google Scholar]
- 27.Yurchenco PD, Tsilibary EC, Charonis AS, Furthmayr H. Laminin polymerization in vitro. Evidence for a two-step assembly with domain specificity. J Biol Chem. 1985;260:7636–44. [PubMed] [Google Scholar]
- 28.Yurchenco PD, Cheng YS, Colognato H. Laminin forms an independent network in basement membranes. J Cell Biol. 1992;117:1119–33. doi: 10.1083/jcb.117.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKee KK, Harrison D, Capizzi S, Yurchenco PD. Role of laminin terminal globular domains in basement membrane assembly. J Biol Chem. 2007;282:21437–47. doi: 10.1074/jbc.M702963200. [DOI] [PubMed] [Google Scholar]
- 30.Yurchenco PD, Cheng YS. Self-assembly and calcium-binding sites in laminin. A three-arm interaction model. J Biol Chem. 1993;268:17286–99. [PubMed] [Google Scholar]
- 31.Ekblom P, Ekblom M, Fecker L, Klein G, Zhang HY, Kadoya Y, Chu ML, Mayer U, Timpl R. Role of mesenchymal nidogen for epithelial morphogenesis in vitro. Development. 1994;120:2003–14. doi: 10.1242/dev.120.7.2003. [DOI] [PubMed] [Google Scholar]
- 32.Kedinger M, Lefebvre O, Duluc I, Freund JN, Simon-Assmann P. Cellular and molecular partners involved in gut morphogenesis and differentiation. Philos Trans R Soc Lond B Biol Sci. 1998;353:847–56. doi: 10.1098/rstb.1998.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liliensiek SJ, Nealey P, Murphy CJ. Characterization of endothelial basement membrane nanotopography in rhesus macaque as a guide for vessel tissue engineering. Tissue Eng Part A. 2009;15:2643–51. doi: 10.1089/ten.tea.2008.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kedinger M, Duluc I, Fritsch C, Lorentz O, Plateroti M, Freund JN. Intestinal epithelial-mesenchymal cell interactions. Ann N Y Acad Sci. 1998;859:1–17. doi: 10.1111/j.1749-6632.1998.tb11107.x. [DOI] [PubMed] [Google Scholar]
- 35.McKee KK, Capizzi S, Yurchenco PD. Scaffold-forming and Adhesive Contributions of Synthetic Laminin-binding Proteins to Basement Membrane Assembly. J Biol Chem. 2009;284:8984–94. doi: 10.1074/jbc.M809719200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madara JL, Stafford J, Dharmsathaphorn K, Carlson S. Structural analysis of a human intestinal epithelial cell line. Gastroenterology. 1987;92:1133–45. doi: 10.1016/s0016-5085(87)91069-9. [DOI] [PubMed] [Google Scholar]
- 37.Parker FG, Barnes EN, Kaye GI. The pericryptal fibroblast sheath. IV. Replication, migration, and differentiation of the subepithelial fibroblasts of the crypt and villus of the rabbit jejunum. Gastroenterology. 1974;67:607–21. [PubMed] [Google Scholar]
- 38.Trier JS, Allan CH, Abrahamson DR, Hagen SJ. Epithelial basement membrane of mouse jejunum. Evidence for laminin turnover along the entire crypt-villus axis. J Clin Invest. 1990;86:87–95. doi: 10.1172/JCI114720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathan M, Hermos JA, Trier JS. Structural features of the epithelio-mesenchymal interface of rat duodenal mucosa during development. J Cell Biol. 1972;52:577–88. doi: 10.1083/jcb.52.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nossol C, Diesing AK, Kahlert S, Kersten S, Kluess J, Ponsuksili S, Hartig R, Wimmers K, Dänicke S, Rothkötter HJ. Deoxynivalenol affects the composition of the basement membrane proteins and influences en route the migration of CD16(+) cells into the intestinal epithelium. Mycotoxin Res. 2013;29:245–54. doi: 10.1007/s12550-013-0177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palay SL, Karlin LJ. An electron microscopic study of the intestinal villus. I. The fasting animal. J Biophys Biochem Cytol. 1959;5:363–72. doi: 10.1083/jcb.5.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rescigno M, Rotta G, Valzasina B, Ricciardi-Castagnoli P. Dendritic cells shuttle microbes across gut epithelial monolayers. Immunobiology. 2001;204:572–81. doi: 10.1078/0171-2985-00094. [DOI] [PubMed] [Google Scholar]
- 43.Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–98. doi: 10.1016/S0008-6363(96)00063-6. [DOI] [PubMed] [Google Scholar]
- 44.Nourshargh S, Hordijk PL, Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11:366–78. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- 45.Voisin M-B, Woodfin A, Nourshargh S. Monocytes and neutrophils exhibit both distinct and common mechanisms in penetrating the vascular basement membrane in vivo. Arterioscler Thromb Vasc Biol. 2009;29:1193–9. doi: 10.1161/ATVBAHA.109.187450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Voisin M-B, Pröbstl D, Nourshargh S. Venular basement membranes ubiquitously express matrix protein low-expression regions: characterization in multiple tissues and remodeling during inflammation. Am J Pathol. 2010;176:482–95. doi: 10.2353/ajpath.2010.090510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Z-H, Ding K-F, Yu J-K, Zhai X-H, Ruan S-Q, Wang S-W, Zhu Y-L, Zheng S, Zhang S-Z. Proteomic analysis of primary colon cancer-associated fibroblasts using the SELDI-ProteinChip platform. J Zhejiang Univ Sci B. 2012;13:159–67. doi: 10.1631/jzus.B1100266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paku S. Current concepts of tumor-induced angiogenesis. Pathol Oncol Res. 1998;4:62–75. doi: 10.1007/BF02904699. [DOI] [PubMed] [Google Scholar]
- 49.Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2003;163:1801–15. doi: 10.1016/S0002-9440(10)63540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farnoud MR, Lissak B, Kujas M, Peillon F, Racadot J, Li JY. Specific alterations of the basement membrane and stroma antigens in human pituitary tumours in comparison with the normal anterior pituitary. An immunocytochemical study. Virchows Arch A Pathol Anat Histopathol. 1992;421:449–55. doi: 10.1007/BF01606873. [DOI] [PubMed] [Google Scholar]
- 51.Kalebic T, Garbisa S, Glaser B, Liotta LA. Basement membrane collagen: degradation by migrating endothelial cells. Science. 1983;221:281–3. doi: 10.1126/science.6190230. [DOI] [PubMed] [Google Scholar]
- 52.Egginton S, Zhou AL, Brown MD, Hudlická O. Unorthodox angiogenesis in skeletal muscle. Cardiovasc Res. 2001;49:634–46. doi: 10.1016/S0008-6363(00)00282-0. [DOI] [PubMed] [Google Scholar]
- 53.Takeuchi T, Gonda T. Distribution of the pores of epithelial basement membrane in the rat small intestine. J Vet Med Sci. 2004;66:695–700. doi: 10.1292/jvms.66.695. [DOI] [PubMed] [Google Scholar]
- 54.Mestres P, Gomez LL, Lopez TN, del Rosario G, Lukas SW, Hartmann U. The basement membrane of the isolated rat colonic mucosa. A light, electron and atomic force microscopy study. Ann Anat - Anat Anz [Internet] [cité 2014 mars 23]; Available from: http://www.sciencedirect.com/science/article/pii/S0940960214000144 [DOI] [PubMed]
- 55.Rowe RG, Weiss SJ. Breaching the basement membrane: who, when and how? Trends Cell Biol. 2008;18:560–74. doi: 10.1016/j.tcb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 56.Huber AR, Weiss SJ. Disruption of the subendothelial basement membrane during neutrophil diapedesis in an in vitro construct of a blood vessel wall. J Clin Invest. 1989;83:1122–36. doi: 10.1172/JCI113992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sherwood DR. Cell invasion through basement membranes: an anchor of understanding. Trends Cell Biol. 2006;16:250–6. doi: 10.1016/j.tcb.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 58.Srivastava A, Pastor-Pareja JC, Igaki T, Pagliarini R, Xu T. Basement membrane remodeling is essential for Drosophila disc eversion and tumor invasion. Proc Natl Acad Sci U S A. 2007;104:2721–6. doi: 10.1073/pnas.0611666104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sherwood DR, Sternberg PW. Anchor cell invasion into the vulval epithelium in C. elegans. Dev Cell. 2003;5:21–31. doi: 10.1016/S1534-5807(03)00168-0. [DOI] [PubMed] [Google Scholar]
- 60.Hagedorn EJ, Ziel JW, Morrissey MA, Linden LM, Wang Z, Chi Q, Johnson SA, Sherwood DR. The netrin receptor DCC focuses invadopodia-driven basement membrane transmigration in vivo. J Cell Biol. 2013;201:903–13. doi: 10.1083/jcb.201301091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seiler C, Davuluri G, Abrams J, Byfield FJ, Janmey PA, Pack M. Smooth muscle tension induces invasive remodeling of the zebrafish intestine. PLoS Biol. 2012;10:e1001386. doi: 10.1371/journal.pbio.1001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakaya Y, Sukowati EW, Wu Y, Sheng G. RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat Cell Biol. 2008;10:765–75. doi: 10.1038/ncb1739. [DOI] [PubMed] [Google Scholar]
- 63.Nakaya Y, Sukowati EW, Sheng G. Epiblast integrity requires CLASP and Dystroglycan-mediated microtubule anchoring to the basal cortex. J Cell Biol. 2013;202:637–51. doi: 10.1083/jcb.201302075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reichel CA, Rehberg M, Bihari P, Moser CM, Linder S, Khandoga A, Krombach F. Gelatinases mediate neutrophil recruitment in vivo: evidence for stimulus specificity and a critical role in collagen IV remodeling. J Leukoc Biol. 2008;83:864–74. doi: 10.1189/jlb.1007666. [DOI] [PubMed] [Google Scholar]
- 65.Wang S, Voisin M-B, Larbi KY, Dangerfield J, Scheiermann C, Tran M, Maxwell PH, Sorokin L, Nourshargh S. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med. 2006;203:1519–32. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yadav R, Larbi KY, Young RE, Nourshargh S. Migration of leukocytes through the vessel wall and beyond. Thromb Haemost. 2003;90:598–606. doi: 10.1160/TH03-04-0220. [DOI] [PubMed] [Google Scholar]
- 67.Hallmann R, Horn N, Selg M, Wendler O, Pausch F, Sorokin LM. Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev. 2005;85:979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- 68.Wondimu Z, Geberhiwot T, Ingerpuu S, Juronen E, Xie X, Lindbom L, Doi M, Kortesmaa J, Thyboll J, Tryggvason K, et al. An endothelial laminin isoform, laminin 8 (alpha4beta1gamma1), is secreted by blood neutrophils, promotes neutrophil migration and extravasation, and protects neutrophils from apoptosis. Blood. 2004;104:1859–66. doi: 10.1182/blood-2004-01-0396. [DOI] [PubMed] [Google Scholar]
- 69.Wu C, Ivars F, Anderson P, Hallmann R, Vestweber D, Nilsson P, Robenek H, Tryggvason K, Song J, Korpos E, et al. Endothelial basement membrane laminin alpha5 selectively inhibits T lymphocyte extravasation into the brain. Nat Med. 2009;15:519–27. doi: 10.1038/nm.1957. [DOI] [PubMed] [Google Scholar]
- 70.Gorfu G, Virtanen I, Hukkanen M, Lehto V-P, Rousselle P, Kenne E, Lindbom L, Kramer R, Tryggvason K, Patarroyo M. Laminin isoforms of lymph nodes and predominant role of alpha5-laminin(s) in adhesion and migration of blood lymphocytes. J Leukoc Biol. 2008;84:701–12. doi: 10.1189/jlb.0108048. [DOI] [PubMed] [Google Scholar]
- 71.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 72.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Poincloux R, Lizárraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J Cell Sci. 2009;122:3015–24. doi: 10.1242/jcs.034561. [DOI] [PubMed] [Google Scholar]
- 74.Weaver AM. Invadopodia: specialized cell structures for cancer invasion. Clin Exp Metastasis. 2006;23:97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
- 75.Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12:413–26. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gadea G, de Toledo M, Anguille C, Roux P. Loss of p53 promotes RhoA-ROCK-dependent cell migration and invasion in 3D matrices. J Cell Biol. 2007;178:23–30. doi: 10.1083/jcb.200701120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5:711–9. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- 78.Kang S-G, Ha Y-R, Ko Y-H, Kang S-H, Joo K-J, Cho H-Y, Park H-S, Kim C-H, Kwon S-Y, Kim J-J, et al. Effect of laminin 332 on motility and invasion in bladder cancer. Kaohsiung J Med Sci. 2013;29:422–9. doi: 10.1016/j.kjms.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 79.Lohi J, Oivula J, Kivilaakso E, Kiviluoto T, Fröjdman K, Yamada Y, Burgeson RE, Leivo I, Virtanen I. Basement membrane laminin-5 is deposited in colorectal adenomas and carcinomas and serves as a ligand for alpha3beta1 integrin. APMIS. 2000;108:161–72. doi: 10.1034/j.1600-0463.2000.d01-40.x. [DOI] [PubMed] [Google Scholar]
- 80.Schoumacher M, Louvard D, Vignjevic D. Cytoskeleton networks in basement membrane transmigration. Eur J Cell Biol. 2011;90:93–9. doi: 10.1016/j.ejcb.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 81.Virtanen I, Gullberg D, Rissanen J, Kivilaakso E, Kiviluoto T, Laitinen LA, Lehto VP, Ekblom P. Laminin alpha1-chain shows a restricted distribution in epithelial basement membranes of fetal and adult human tissues. Exp Cell Res. 2000;257:298–309. doi: 10.1006/excr.2000.4883. [DOI] [PubMed] [Google Scholar]
- 82.Witz CA, Montoya-Rodriguez IA, Cho S, Centonze VE, Bonewald LF, Schenken RS. Composition of the extracellular matrix of the peritoneum. J Soc Gynecol Investig. 2001;8:299–304. doi: 10.1016/S1071-5576(01)00122-8. [DOI] [PubMed] [Google Scholar]
- 83.Schoumacher M, Goldman RD, Louvard D, Vignjevic DM. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J Cell Biol. 2010;189:541–56. doi: 10.1083/jcb.200909113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vignjevic D, Schoumacher M, Gavert N, Janssen K-P, Jih G, Laé M, Louvard D, Ben-Ze’ev A, Robine S. Fascin, a novel target of beta-catenin-TCF signaling, is expressed at the invasive front of human colon cancer. Cancer Res. 2007;67:6844–53. doi: 10.1158/0008-5472.CAN-07-0929. [DOI] [PubMed] [Google Scholar]
- 85.Schoumacher M, Glentis A, Gurchenkov VV, Vignjevic DM. Basement membrane invasion assays: native basement membrane and chemoinvasion assay. Methods Mol Biol. 2013;1046:133–44. doi: 10.1007/978-1-62703-538-5_8. [DOI] [PubMed] [Google Scholar]