Abstract
Over 20 years ago, protrusive, F-actin-based membrane structures, termed invadopodia, were identified in highly metastatic cancer cell lines. Invadopodia penetrate artificial or explanted extracellular matrices in 2D culture conditions and have been hypothesized to facilitate the migration of cancer cells through basement membrane, a thin, dense, barrier-like matrix surrounding most tissues. Despite intensive study, the identification of invadopodia in vivo has remained elusive and until now their possible roles during invasion or even existence have remained unclear. Studies in remarkably different cellular contexts—mouse tumor models, zebrafish intestinal epithelia, and C. elegans organogenesis—have recently identified invadopodia structures associated with basement membrane invasion. These studies are providing the first in vivo insight into the regulation, function, and role of these fascinating subcellular devices with critical importance to both development and human disease.
Keywords: basement membrane, cancer, cell invasion, development, in vivo, invadopodia
Introduction
Basement membrane is a thin, dense, sheet-like form of extracellular matrix that underlies all epithelia and encapsulates muscle, fat, and glial cells.1-3 The basement membrane arose at the emergence of animal multicellularity4,5 and is a key regulator of many cellular and tissue-level functions, including cell polarity, differentiation, organ shape, and tissue compartmentalization.6-11 Despite its dense and highly cross-linked structure, leukocytes and numerous migrating cells in development traffic through basement membranes.12-14 For example, during the epithelial-to-mesenchymal transitions (EMTs) that occur in gastrulation and neural crest migration, cells acquire the ability to invade through the epithelial basement membrane to initiate their migrate to distant sites.15-17 In a similar manner, cancer cells are also thought to acquire the ability to breach basement membrane to enable metastasis, the most lethal step in cancer progression.18 Largely because of its importance in cancer, there has been significant interest in understanding the mechanisms cells utilize to invade (or transmigrate) basement membrane barriers. A better understanding of the mechanisms cells use to cross basement membrane should facilitate strategies to therapeutically modulate invasive cellular behavior.19
A considerable amount of attention has focused on the potential role of invadopodia, F-actin-based, highly protrusive, matrix-degrading membrane structures, in directing cancer cell invasion through basement membrane.20-23 Invadopodia depend upon integrin for their formation and are often enriched with the actin regulators Arp2/3 and Wasp, the signaling protein Src, the scaffolding proteins cortactin and Tks5, and the matrix metalloproteinase MT1-MMP.23 Another membrane-associated structure highly similar to invadopodia, called podosomes, has also been identified. Podosomes, however, are generally associated with non-transformed cells involved with matrix remodeling events, such as osteoclasts and vascular smooth muscle cells, and not basement membrane invasion.23-29 The term invadosomes has recently been used to encompass both structures,21,30-32 recognizing that invadopodia and podosomes (and possibly other protrusive membrane structures) likely represent more of a continuum than completely distinct entities.33
Invadopodia were first identified over 20 y ago in transformed fibroblasts and human cancer cell lines and have since been observed in primary tumor cells from human patients.34-37 Reflecting the growing interest in these structures, more than half of the approximately 350 scholarly articles on invadopodia have been published within the last 3 y. Although recent advances have been made in analyzing invadopodia-like protrusions in 3D culture conditions,19,38 most mechanistic studies on invadopodia have been performed with cancer cell lines in 2D cell culture environments. The advantages in imaging, biochemical approaches, and genetic and pharmacological perturbations in 2D conditions have led to remarkable advances in our understanding of invadopodia.22,39-42 These findings include the identification of different components of invadopodia,43-45 elucidation of the stages of invadopodia formation,46-49 identification of genes associated with cancer metastasis that regulate invadopodia formation,19,21,50-52 understanding of the trafficking of proteases to invadopodia,53-57 and examination of invadopodia membrane dynamics.58 Key insights have also been gained into the regulation of their formation by growth factors, integrin activation, and the microenvironment, including hypoxia, matrix stiffness, and metabolism.28,59-61 Despite intensive study, one of the greatest gaps in the field has been whether these structures actually exist in vivo, and if so, how they facilitate basement membrane transmigration.21,62,63 In this review, we present recent work from our lab and others that have indentified invadopodia in vivo. Although the term invadopodia is typically used to describe invasive structures in cancer cells, we propose that invadopodia are components of a normal and likely ancient invasion program utilized by cells to pass through basement membrane during development that is co-opted by transformed cells. Further, we suggest that work in model systems such as zebrafish and C. elegans offers new and powerful approaches to understand the role of invadopodia in basement membrane transmigration, as well as insights into the physiological cues and intrinsic programs that govern their formation and function.
The Challenge of Studying Invadopodia In Vivo
The greatest impediment to identifying invadopodia in vivo has been the difficulty of visualizing interactions at the cell–basement membrane interface during the process of invasion. In tumor models, cancer cell invasion events are highly dynamic, rare, and unpredictable; it is thus challenging to catch cells in the act of invading.64 Further, invasion events often occur deep in complex tissues and require simultaneous visualization of the invasive cell and basement membrane. As live-cell fluorescent-based basement membrane reporters are currently not available in vertebrate models, imaging basement membrane has been limited to ex vivo assays and fixed samples,65-68 thus hindering the ability to detect and examine the dynamics of invadopodia in native settings.
Cancer Cells Generate Invadopodia Protrusions In Vivo
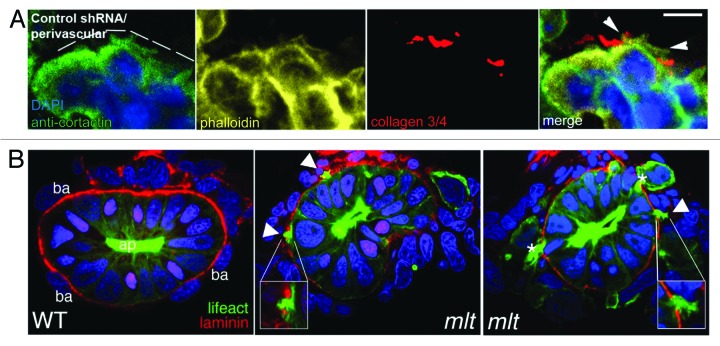
Despite the challenges of in vivo studies, a significant body of work has shed remarkable insight into cancer cell invasion in physiological settings.20,62,64,69,70 The Condeelis and Segall groups have pioneered intravital (optical live-animal) imaging techniques, as well as a tumor model where GFP-expressing MTLn3 cells (MTLn3-GFP), a highly invasive rat mammary adenocarcinoma cell line that forms invadopodia in vitro, are injected into the mammary gland of immunocompromised mice and rats and allowed to form tumors.20,71 Tumors derived from MTLn3 cells metastasize to lung, allowing tumor dissemination and invasion to be studied. Live-cell imaging and intravenous injection of rhodamine dextran have permitted visualization of MTLn3-GFP in the act of invading into vasculature, where these cells must pass through the vascular basement membrane.72 Recently, utilizing cryosectioning and immunolocalization, protrusions from MTLn3-GFP adjacent to blood vessels have been identified that contain the invadopodia markers cortactin and N-WASP (key F-actin regulators; Fig. 1A).73 The formation of these protrusions also correlates with areas of collagen I degradation, an interstitial matrix component (Fig. 1A), consistent with the possibility that these structures mediate invasion through extracellular matrix. Intriguingly, reduction of N-WASP, which regulates invadopodia in vitro,74 resulted in reduced protrusions, and correlated with a dramatic reduction in collagen degradation and circulating tumor cells, consistent with an inability of these cells to enter the vasculature. Mouse–tumor model studies with Ras transformed HMLE cells expressing Twist (an immortalized human mammary epithelial cell line), have also revealed punctate subcellular invadopodia-like structures within tumor cells.75 Inhibition of invadopodia formation through targeting of the PDGF receptor α and the scaffold protein Tks5 inhibited the local invasive ability of the HMLE cells expressing Twist and the tumors remained encapsulated. Stable knockdown of Tks5 also resulted in reduced metastasis in a mouse model for metastatic lung adenocarcinoma.52 These results strongly support the idea that invadopodia are critical for cancer cell dissemination in vivo and suggest that these structures might be mediating basement membrane transmigration.
Figure 1. In vivo invadopodia in rat and zebrafish transformed cells. (A) Immunohistofluorescence of MTLn3-derived perivascular tumors (DAPI, blue; phallodin, yellow; anti-cortactin, green; dotted line indicates boundary with vasculature, which is encapsulated with a basement membrane) indicate that protrusions (arrowheads) are associated with sites of collagen degradation (an antibody specific for degraded collagen I, Collagen ¾, red). (B) Cross sectional images of a zebrafish larvae showing intestinal epithelia in wild-type (left) and mlt mutants (center and right). Basement membrane is shown in red (laminin immunostain) and nuclei are stained blue (DAPI). Actin-rich (green, lifeact) protrusions in mlt intestine are associated with sites of basement membrane loss (arrowheads and insets). An asterisk labels an epithelial cell invading into the tissue stroma through a cleared region of the basement membrane. Scale bar is 10 µm. Images in (A) reprinted with permission, ©Gligorijevic, B., et al. 2012. Originally published in J Cell Sci. doi#10.1242/jcs.092726. Images in (B) reprinted with permission, ©Sieler, C., et al. 2012. Originally published in PLOS Biol. doi#10.1371/journal.pbio.1001386.
Invadopodia-Like Protrusions in Transformed Zebrafish Intestinal Epithelium
Recent work by the Pack group has modeled epithelial cell invasion in transformed cells in zebrafish.76 Intriguingly, zebrafish harboring a mutation that results in constitutive activity of the smooth muscle myosin heavy chain gene (mlt mutants) show a striking invasive remodeling of the neighboring intestinal epithelium. Constitutive activation of mlt in the smooth muscles causes an increase in smooth muscle contractile tone. Unregulated smooth muscle contractile tone is thought to result in the production of reactive oxygen species, which non-autonomously stimulates invasive remodeling in the neighboring intestinal epithelium.76 This invasive remodeling includes the appearance of cellular protrusions that contain the invadopodia markers cortactin and Src.76 These protrusions extend through regions of the intestinal epithelial basement membrane that are devoid of the basement membrane protein laminin, which is consistent with a potential role of invadopodia breaching the basement membrane (Fig. 1B). Further, their formation is dependent on Tks5, as well as the non-receptor tyrosine kinase Src, an upstream regulator of invadopodia formation.23,77
Recent studies have suggested that loss or disruptions of basement membrane may stimulate protrusions and invasive behavior.8,78,79 Thus, contractions from the smooth musculature, which is tightly associated with the intestinal epithelia, could tear the basement membrane, inducing epithelial invasive behavior and escape. While this possibility was not formally excluded in this study, a constitutively activate form of Src expressed specifically within the intestinal epithelium in otherwise wild-type animals, led to ectopic F-actin-rich protrusions that were present in fixed samples at sites of apparent breaks in basement membrane.76 This result adds weight to the notion that the invadopodia-like protrusions from the intestinal epithelial cells may be actively breaching the basement membrane. Interestingly, Src was not sufficient to promote escape of these cells from the epithelium, thus Src might be a specific regulator of invadopodia and does not stimulate a full EMT transition. The work in zebrafish offers further evidence that invadopodia are present in vivo and may mediate breaches in basement membrane.
Invadopodia Breach Basement Membrane During C. elegans Organogenesis
During uterine–vulval development in the nematode worm C. elegans, a specialized uterine cell called the anchor cell (AC), breaches the juxtaposed uterine and vulval epithelial basement membranes to initiate uterine–vulval attachment.80,81 AC invasion is highly stereotyped, occurring over a narrow developmental window. The major structural components of basement membrane (laminin and type IV collagen) as well as minor components (SPARC, hemicentin, and fibulin) have been functionally tagged with GFP or GFP-derivatives in C. elegans.82-86 Furthermore, specific proteins of interest can be tagged and expressed within the AC.87 The ability to easily manipulate the orientation of the worm permits imaging of AC invasion from the lateral viewpoint, as well as within the plane of the cell–basement membrane interface (Fig. 2). The combination of these perspectives has facilitated the first real-time, high-resolution imaging of cell–basement membrane interactions during invasion in vivo.12
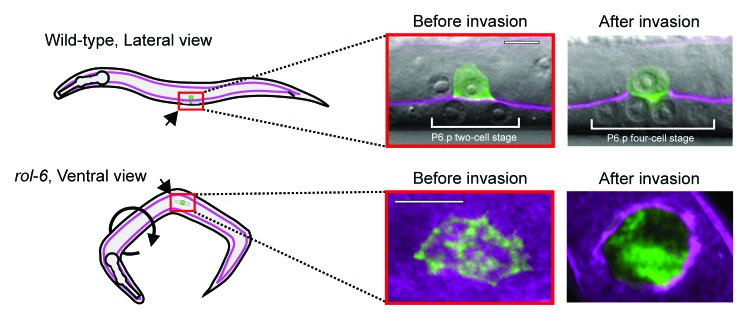
Figure 2.C. elegans anchor cell invasion imaged laterally or ventrally. The AC is viewed from two different perspectives for time-lapse imaging, depicted in the schematic (left), and examples of the images from these perspectives are shown (right). The basement membrane is visualized with laminin::GFP (magenta) and F-actin (green) with an AC-specific F-actin probe (cdh-3 > mCherry::moeABD). Fluorescence is overlaid on differential interference contrast images. Wild-type animals (top) lie on their side, resulting in lateral imaging of the AC-basement membrane interaction. Roller mutant animals (bottom) orient randomly, permitting ventral imaging within the plane of the basement membrane. Confocal slices through the AC-basement membrane interface are shown at 2X magnification relative to the lateral view panels. Subsequent panels in this review of the AC are labeled with their perspective used for imaging. Scale Bars, 5 µm. This figure is reprinted with permission, ©Hagedorn, E.J., et al. 2013. Originally published in J Cell Biol. doi#10.1083/jcb.201301091.
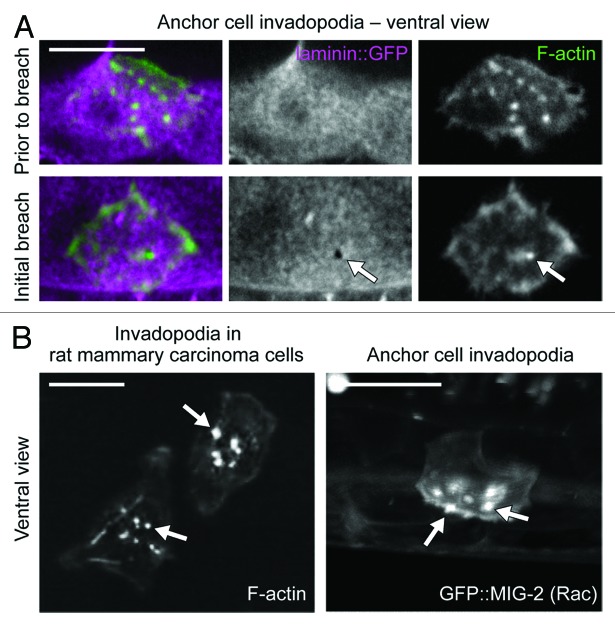
Using live-cell imaging of F-actin and actin regulators, we have recently found that the AC breaches the basement membrane with invadopodia (Fig. 3A).88 Prior to invasion, these F-actin-rich foci, which form at the cell–basement membrane interface, look strikingly similar to invadopodia from cancer cells generated upon contact with gelatin and other artificial matrices plated on glass slides (Fig. 3B). The C. elegans invadopodia also have molecular markers shared by cancer cell invadopodia, including the membrane anchored Rac GTPases, the actin regulatory protein Ena/VASP, and the phospholipid PI(4,5)P2.44,89 Similar to cancer cell invadopodia, formation of these structures in the AC is dependent on integrin function.88,90,91 Confirming their ability to breach basement membrane, time-lapse analysis indicated that these structures depress basement membrane during turnover, and one always presages and then occupies the first visible breach in the basement membrane (Fig. 3A). These studies confirm that invadopodia breach basement membrane in vivo and strongly suggest that invadopodia are a conserved subcellular device utilized in normal development to penetrate basement membrane barriers.
Figure 3. AC invadopodia breach the basement membrane and are similar to cancer cell invadopodia. (A) A ventral view time-lapse series of AC invadopodia (actin, green) and the basement membrane (laminin, magenta). Prior to a detectable breach of the basement membrane (top), the AC has many punctate F-actin structures. Upon breach of the basement membrane (bottom), a single F-actin-rich structure occupies the gap in the basement membrane (arrow). (B) Phallodin staining of F-actin in MtLn3 plated on fibronectin- and gelatin-coated coverslips labels invadopodia (left). An AC-specific translational reporter for a C. elegans Rac ortholog (right), GFP::MIG-2, labels invadopodia in a lateral image tilted 45 degrees back. These two models allow for resolution of individual invadopodia. AC scale bars, 5 um; cancer cell scale bar, 20 um. Left panel of (B) is reprinted with permission**, ©Yamaguchi, H., et al. 2005. Originally published in J Cell Biol. 168(3):441–52.
One of the more interesting aspects of AC invadopodia is how rapidly they turnover and puncture basement membrane. Whereas invadopodia in cancer cells can persist over an hour,21 the average lifetime of AC invadopodia is approximately 1 min, with the invadopodium that breaches the basement membrane persisting for approximately 5 min.88 The reasons and significance of these dynamics between in vitro and AC invadopodia are unclear, and may reflect differences in the microenvironment (signaling or matrix properties) or intrinsic factors within the cells. It is notable that the entire process of AC invasion through the juxtaposed uterine and vulval basement membranes is completed in only 90 min.80,87 In contrast, breast and colon cancer cells plated on native basement membranes in ex vivo assays take days to complete invasion.65,68 It seems likely that these ex vivo assays may not recapitulate the in vivo signaling environment that mediates rapid invasion. Alternatively, cancer cells might be more inefficient in their ability to invade basement membrane.
Over 50 proteins as well as specific phosphoinositide lipids are known to be associated with invadopodia in cancer cells.43,44 Using genetic, expression, and RNAi based screens, we have identified over 100 genes that regulate AC invasion or are expressed specifically in the AC.87,92-95 It will be important to determine their possible connection to AC invadopodia, as this will likely increase our understanding of the regulation of invadopodia as well as identify pathways that function in parallel with invadopodia to promote invasion. Most proteins strongly associated with invadopodia in cancer cells are encoded in the C. elegans genome and many of these have been identified in screens for genes promoting AC invasion (Table 1). Several important regulators, however, are absent. These include the actin regulator and adaptor protein cortactin, the Tks4/5 adaptor proteins, and the transmembrane matrix metalloproteinase MT1-MMP (Table 1).42,44,96 Other proteins may functionally compensate for these absences. For example, similar to cortactin, the highly conserved Abp1 actin-binding protein also activates the Arp2/3 complex.97,98 In addition, C. elegans encodes a predicted GPI membrane anchored matrix metalloproteinase, zmp-1, which is specifically expressed in the AC at the time of invasion. Although loss of zmp-1 does not disrupt invasion in isolation, it is possible that one or more of the five other matrix metalloproteinases in C. elegans function redundantly with ZMP-1 during invasion.99 Finally, it will be important to understand the potential role of PI(4,5)P2 and other phosphosphionositides in AC invadopodia. In cancer cells, these lipids have been proposed to play important roles in recruiting and activating actin regulators within invadopodia, as well as linking the F-actin-based invadopodium core to the plasma membrane.47,100
Table 1. Major cancer cell invadopodia proteins and C. elegans orthologs.
| Human protein | C. elegans ortholog | Category | Known role in AC invasion |
|---|---|---|---|
| Src127 | SRC-1/2 | Tyrosine kinase | |
| Tks4/5128 | none | Src associated adaptor protein | |
| Cortactin129 | none | Cytoskeleton regulation | |
| Abl family kinases130,131 | ABL-1 | Tyrosine kinase | |
| MMPs56,132 | ZMP-1–6 | Matrix degradation | +87 |
| β1 and β3 integrins133,134 | PAT-3 | Cell adhesion | +91 |
| Actin135 | ACT-1 | Cell structure and signaling scaffold | +91 |
| Fascin136 | FASN-1 | Cytoskeleton regulation | |
| Arp2/374 | ARX-1–7 | Cytoskeleton regulation | +92 |
| Cofilin74 | UNC-60 | Cytoskeleton regulation | +92 |
| WASp family137 | WSP-1 | Cytoskeleton regulation | |
| Ena/VASP/Mena138 | UNC-34 | Cytoskeleton regulation | +93 |
| PKC139 | PKC-1/2 | Serine/Threonine kinase | |
| Fak/Pyk2140,141 | KIN-32 | Tyrosine kinase | |
| Paxilin139,142 | PXL-1 | Cytoskeleton regulation | |
| Nck74 | NCK-1 | Scaffold protein | |
| PI3K143 | AAP-1 | Kinase | |
| Rho family GTPases144-146 | RHO-1 | Cytoskeleton regulation | |
| RAC-2, MIG-2, CED-10 | Cytoskeleton regulation | +93 | |
| CDC-42 | Cytoskeleton regulation | +92 |
Invadopodia Are Only One Component of the Invasion Process In Vivo
One of the advantages of examining invadopodia at high resolution in vivo is elucidating the role they play in the context of the complete process of invasion. By following the fate of breached AC invadopodia in C. elegans, we have also discovered that soon after penetrating the basement membrane, a large protrusion forms at the site of breach, crosses the basement membrane, and intercalates between cells of the underlying vulval epithelium (Fig. 4). Formation of this protrusion correlates with cessation of invadopodia formation, which likely prevents or reduces multiple breaching events. The C. elegans ortholog of the vertebrate netrin receptor DCC (UNC-40) plays a critical role in mediating the morphogenetic transition from invadopodia-driven basement membrane breaching to the formation of a protrusion that extends through the basement membrane.88,93 During BM breaching by invadopodia, UNC-40 traffics to the initial breach and directs the formation of the invasive protrusion by recruiting and concentrating the same F-actin effectors that generate invadopodia (Fig. 4).88 UNC-40 accumulation at the breach site appears to act as a molecular sink that depletes F-actin regulators at invadopodia—in the absence of UNC-40, invadopodia persist, multiple breaching events occur, and a large invasive protrusion fails to form (Fig. 4). This elegant mechanism shuts down invadopodia and focuses invasion through a single basement breach. The switch from invadopodia to invasive protrusion-driven changes probably involves mechanisms to dynamically add membrane and actin regulators that facilitate extension of the actin network. Furthermore, in cell culture, microtubules and the intermediate filament protein vimentin are required to extend invasive protrusions.68 Mechanisms regulating an invadopodia-to-invasive protrusions switch likely occur in other invasive cells as electron microscopy and immunofluorescence studies have observed single protrusions from cancer cells crossing basement membrane in ex vivo invasion assays.65,68 Whether these are also regulated by the netrin receptor DCC is unknown, but is possible given the strong association of netrin with metastatic cancers and invasive behavior in cell culture.101-104 Importantly, after breaching basement membrane (a 2D flat, sheet-like surface), cancer cells often enter interstitial tissue, a largely acellular environment dominated by a less dense 3D network of fibrillar collagens.105 This environment appears to trigger the formation of invadopodia-like structures that promote removal of interstitial matrix at points of cell restriction.38,106-108 Thus, invadopodia dynamics are likely rapidly tuned to the matrix environment encountered by invasive cells.
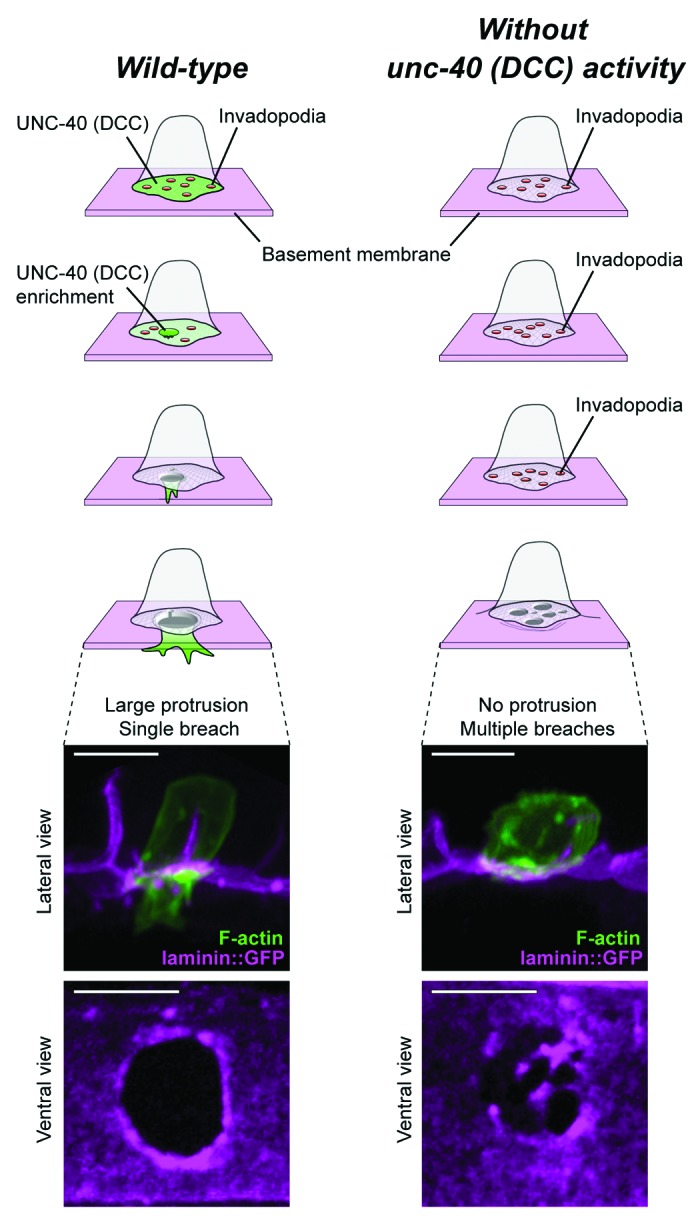
Figure 4. The netrin receptor DCC (UNC-40) mediates invadopodia to invasive protrusion transition. In wild-type animals (left), invadopodia (red circles) are dynamic and rapidly turn over until one breaches the basement membrane. UNC-40 (green) localizes to the initial breach and mediates the transition from many invadopodia to a single invasive protrusion that extends through the single gap in the basement membrane. The invasive protrusion opens a single gap in the basement membrane, allowing the basal membrane of the AC to contact the underlying vulval cells. A lateral view image shows the invasive protrusion extending (actin, green) through a single gap in the basement membrane (laminin, magenta). A ventral view image shows the widened gap through the basement membrane. In the absence of UNC-40 (right), invadopodia persist after the initial breach in the basement membrane, an invasive protrusion fails to form, and multiple breaches through the basement membrane occur. A lateral view image shows the lack of invasive protrusion formation in the AC and a ventral view images shows the multiple breaches through the basement membrane. Scale bars, 5 um. This figure is adapted from and reprinted with permission, ©Morrissey, M.A., et al., 2013. Originally published in Worm. doi#20.4262/worm.26169 and ©Hagedorn, E.J., et al. 2013. Originally published in J Cell Biol. doi#10.1083/jcb.201301091.
The AC also specifically modifies the composition of the basement membrane targeted for invasion. It has been known for sometime that cancer cells secrete normal as well as novel basement membrane proteins and matrix-modifying enzymes.109-113 How this may specifically contribute to invasion and tumor metastasis is poorly understood. Just prior to invasion, the AC secretes the conserved extracellular matrix protein hemicentin specifically under the AC in the basement membrane that will be crossed.84,87 Although the precise function of hemicentin is unclear, deposition of hemicentin assists basement removal during invasion.87 Active modification of the basement membrane might change its structural properties to facilitate invadopodia penetration and precise removal.
One possible function for hemicentin is to promote physical displacement of the basement membrane. Although it has been generally assumed that basement membrane is dissolved by proteases during invasion,114 optical highlighting of basement membrane components using a photoconvertible Dendra tag83,115 has revealed that the basement membrane under the AC is removed, in part, by physical means.88 The protrusion generated by the AC appears to generate forces that physically shift the basement membrane. Given that the protease zmp-1 is expressed throughout AC invasion, zmp-1 and other proteases might assist invasion by weakening the structural make-up of the basement membrane to facilitate physical displacement. Further sensitized screening, as well as profiling gene expression in the AC using single cell isolation techniques,116 will help reveal mechanisms of how basement membrane is modified to assist in cell invasion events.
Summary and Outlook
The recent studies highlighted here have established that invadopodia and invadopodia-like protrusions exist in vivo. Although examination of invadopodia and cell invasion in natural settings are inherently more challenging due to limitations in visual analysis, experimental manipulations, and examination of large numbers of events, these studies will be essential in establishing how invadopodia are regulated and function during invasion. It is already clear from in vivo studies in zebrafish and C. elegans that while invadopodia mediate basement membrane breaching, they are not sufficient to facilitate basement membrane crossing or full removal of this barrier. The use of model systems such as zebrafish and C. elegans, with their visual accessibility, simple tissue architecture, transgenics, and amenability to genetic analysis, offers a potent complementary approach to tumor models to answer some of the most pressing questions concerning invadopodia and cell invasion. These include the identities of the signaling pathways that control their formation and regulation, and the cell-intrinsic factors, including transcriptional networks that facilitate invadopodia generation in cells. Identification of other models of invadopodia-basement membrane breaching events in vivo will also be valuable in elucidating the range of invadopodia dynamics and activity in breaching basement membrane. Perhaps most importantly, in vivo studies will be critical in determining how widespread the use of invadopodia are in breaching basement membrane. For example, are invadopodia used in EMT events in development and cancer to facilitate basement membrane crossing? While several studies have implicated invadopodia with EMT,75,117 direct visualization of their activity and formation is lacking. Moreover, there is evidence that cells cross basement membrane through a variety of mechanisms, including downregulation of basement membrane receptors,118 physical forces that shift or breach the basement membrane,79,83,119 entry through preformed portals,66,67,120,121 and remodeling events that create absences or holes in the basement membrane.62,122-124 These studies will be important in evaluating whether targeting invadopodia would be an efficient approach to blocking cancer metastasis. The observation of invadopodia in nematodes, which diverged from the lineage that gave rise to vertebrates approximately 600 million y ago,125,126 argues strongly that invadopodia are ancient and likely widely utilized structures for basement membrane transmigration. We expect the expansion of in vivo studies will be essential in clarifying the utilization of invadopodia in invasive cell contexts and reveal novel aspects of the regulation and function of these fascinating basement membrane-piercing structures.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank A Schindler and M Morrissey for helpful comments, and A Weaver for discussions on invadopodia. This work was supported by F32 GM103148 to Kelley LC, and The Pew Scholars Program in the Biomedical Sciences, and NIH Grants GM079320 and GM100083 to Sherwood DR.
References
- 1.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–33. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 2.Halfter W, Candiello J, Hu H, Zhang P, Schreiber E, Balasubramani M. Protein composition and biomechanical properties of in vivo-derived basement membranes. Cell Adh Migr. 2013;7:64–71. doi: 10.4161/cam.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hohenester E, Yurchenco PD. Laminins in basement membrane assembly. Cell Adh Migr. 2013;7:56–63. doi: 10.4161/cam.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hynes RO. The evolution of metazoan extracellular matrix. J Cell Biol. 2012;196:671–9. doi: 10.1083/jcb.201109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozbek S, Balasubramanian PG, Chiquet-Ehrismann R, Tucker RP, Adams JC. The evolution of extracellular matrix. Mol Biol Cell. 2010;21:4300–5. doi: 10.1091/mbc.E10-03-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pastor-Pareja JC, Xu T. Shaping cells and organs in Drosophila by opposing roles of fat body-secreted Collagen IV and perlecan. Dev Cell. 2011;21:245–56. doi: 10.1016/j.devcel.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmussen JP, Reddy SS, Priess JR. Laminin is required to orient epithelial polarity in the C. elegans pharynx. Development. 2012;139:2050–60. doi: 10.1242/dev.078360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egea J, Erlacher C, Montanez E, Burtscher I, Yamagishi S, Hess M, Hampel F, Sanchez R, Rodriguez-Manzaneque MT, Bösl MR, et al. Genetic ablation of FLRT3 reveals a novel morphogenetic function for the anterior visceral endoderm in suppressing mesoderm differentiation. Genes Dev. 2008;22:3349–62. doi: 10.1101/gad.486708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gnanaguru G, Bachay G, Biswas S, Pinzón-Duarte G, Hunter DD, Brunken WJ. Laminins containing the β2 and γ3 chains regulate astrocyte migration and angiogenesis in the retina. Development. 2013;140:2050–60. doi: 10.1242/dev.087817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashimoto J, Kariya Y, Miyazaki K. Regulation of proliferation and chondrogenic differentiation of human mesenchymal stem cells by laminin-5 (laminin-332) Stem Cells. 2006;24:2346–54. doi: 10.1634/stemcells.2005-0605. [DOI] [PubMed] [Google Scholar]
- 11.Spenlé C, Simon-Assmann P, Orend G, Miner JH. Laminin α5 guides tissue patterning and organogenesis. Cell Adh Migr. 2013;7:90–100. doi: 10.4161/cam.22236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagedorn EJ, Sherwood DR. Cell invasion through basement membrane: the anchor cell breaches the barrier. Curr Opin Cell Biol. 2011;23:589–96. doi: 10.1016/j.ceb.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowe RG, Weiss SJ. Breaching the basement membrane: who, when and how? Trends Cell Biol. 2008;18:560–74. doi: 10.1016/j.tcb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Tech. 2008;71:357–70. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakaya Y, Sheng G. EMT in developmental morphogenesis. Cancer Lett. 2013;341:9–15. doi: 10.1016/j.canlet.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 16.Cheung M, Chaboissier MC, Mynett A, Hirst E, Schedl A, Briscoe J. The transcriptional control of trunk neural crest induction, survival, and delamination. Dev Cell. 2005;8:179–92. doi: 10.1016/j.devcel.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Nakaya Y, Sukowati EW, Wu Y, Sheng G. RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat Cell Biol. 2008;10:765–75. doi: 10.1038/ncb1739. [DOI] [PubMed] [Google Scholar]
- 18.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–92. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi H. Pathological roles of invadopodia in cancer invasion and metastasis. Eur J Cell Biol. 2012;91:902–7. doi: 10.1016/j.ejcb.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Condeelis J, Segall JE. Intravital imaging of cell movement in tumours. Nat Rev Cancer. 2003;3:921–30. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- 21.Linder S, Wiesner C, Himmel M. Degrading devices: invadosomes in proteolytic cell invasion. Annu Rev Cell Dev Biol. 2011;27:185–211. doi: 10.1146/annurev-cellbio-092910-154216. [DOI] [PubMed] [Google Scholar]
- 22.Weaver AM. Invadopodia: specialized cell structures for cancer invasion. Clin Exp Metastasis. 2006;23:97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
- 23.Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12:413–26. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zambonin-Zallone A, Teti A, Carano A, Marchisio PC. The distribution of podosomes in osteoclasts cultured on bone laminae: effect of retinol. J Bone Miner Res. 1988;3:517–23. doi: 10.1002/jbmr.5650030507. [DOI] [PubMed] [Google Scholar]
- 25.Davies WA, Stossel TP. Peripheral hyaline blebs (podosomes) of macrophages. J Cell Biol. 1977;75:941–55. doi: 10.1083/jcb.75.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarone G, Cirillo D, Giancotti FG, Comoglio PM, Marchisio PC. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp Cell Res. 1985;159:141–57. doi: 10.1016/S0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- 27.Wolosewick JJ. Distribution of actin in migrating leukocytes in vivo. Cell Tissue Res. 1984;236:517–25. doi: 10.1007/BF00217218. [DOI] [PubMed] [Google Scholar]
- 28.Hoshino D, Branch KM, Weaver AM. Signaling inputs to invadopodia and podosomes. J Cell Sci. 2013;126:2979–89. doi: 10.1242/jcs.079475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buccione R, Orth JD, McNiven MA. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol. 2004;5:647–57. doi: 10.1038/nrm1436. [DOI] [PubMed] [Google Scholar]
- 30.Linder S. Invadosomes at a glance. J Cell Sci. 2009;122:3009–13. doi: 10.1242/jcs.032631. [DOI] [PubMed] [Google Scholar]
- 31.Destaing O, Block MR, Planus E, Albiges-Rizo C. Invadosome regulation by adhesion signaling. Curr Opin Cell Biol. 2011;23:597–606. doi: 10.1016/j.ceb.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Saltel F, Daubon T, Juin A, Ganuza IE, Veillat V, Génot E. Invadosomes: intriguing structures with promise. Eur J Cell Biol. 2011;90:100–7. doi: 10.1016/j.ejcb.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 33.McNiven MA. Breaking away: matrix remodeling from the leading edge. Trends Cell Biol. 2013;23:16–21. doi: 10.1016/j.tcb.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mueller SC, Chen WT. Cellular invasion into matrix beads: localization of beta 1 integrins and fibronectin to the invadopodia. J Cell Sci. 1991;99:213–25. doi: 10.1242/jcs.99.2.213. [DOI] [PubMed] [Google Scholar]
- 35.Chen WT. Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J Exp Zool. 1989;251:167–85. doi: 10.1002/jez.1402510206. [DOI] [PubMed] [Google Scholar]
- 36.Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007;67:4227–35. doi: 10.1158/0008-5472.CAN-06-3928. [DOI] [PubMed] [Google Scholar]
- 37.Kramer RH, Bensch KG, Wong J. Invasion of reconstituted basement membrane matrix by metastatic human tumor cells. Cancer Res. 1986;46:1980–9. [PubMed] [Google Scholar]
- 38.Yu X, Machesky LM. Cells assemble invadopodia-like structures and invade into matrigel in a matrix metalloprotease dependent manner in the circular invasion assay. PLoS One. 2012;7:e30605. doi: 10.1371/journal.pone.0030605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma VP, Entenberg D, Condeelis J. High-resolution live-cell imaging and time-lapse microscopy of invadopodium dynamics and tracking analysis. Methods Mol Biol. 2013;1046:343–57. doi: 10.1007/978-1-62703-538-5_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weaver AM, Page JM, Guelcher SA, Parekh A. Synthetic and tissue-derived models for studying rigidity effects on invadopodia activity. Methods Mol Biol. 2013;1046:171–89. doi: 10.1007/978-1-62703-538-5_10. [DOI] [PubMed] [Google Scholar]
- 41.Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–17. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Buccione R, Caldieri G, Ayala I. Invadopodia: specialized tumor cell structures for the focal degradation of the extracellular matrix. Cancer Metastasis Rev. 2009;28:137–49. doi: 10.1007/s10555-008-9176-1. [DOI] [PubMed] [Google Scholar]
- 43.Hoshino D, Jourquin J, Emmons SW, Miller T, Goldgof M, Costello K, Tyson DR, Brown B, Lu Y, Prasad NK, et al. Network analysis of the focal adhesion to invadopodia transition identifies a PI3K-PKCα invasive signaling axis. Sci Signal. 2012;5:ra66. doi: 10.1126/scisignal.2002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klemke RL. Trespassing cancer cells: ‘fingerprinting’ invasive protrusions reveals metastatic culprits. Curr Opin Cell Biol. 2012;24:662–9. doi: 10.1016/j.ceb.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Attanasio F, Caldieri G, Giacchetti G, van Horssen R, Wieringa B, Buccione R. Novel invadopodia components revealed by differential proteomic analysis. Eur J Cell Biol. 2011;90:115–27. doi: 10.1016/j.ejcb.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Kelley LC, Ammer AG, Hayes KE, Martin KH, Machida K, Jia L, Mayer BJ, Weed SA. Oncogenic Src requires a wild-type counterpart to regulate invadopodia maturation. J Cell Sci. 2010;123:3923–32. doi: 10.1242/jcs.075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma VP, Eddy R, Entenberg D, Kai M, Gertler FB, Condeelis J. Tks5 and SHIP2 regulate invadopodium maturation, but not initiation, in breast carcinoma cells. Curr Biol. 2013;23:2079–89. doi: 10.1016/j.cub.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–43. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- 49.Oser M, Yamaguchi H, Mader CC, Bravo-Cordero JJ, Arias M, Chen X, Desmarais V, van Rheenen J, Koleske AJ, Condeelis J. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J Cell Biol. 2009;186:571–87. doi: 10.1083/jcb.200812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Razidlo GL, Schroeder B, Chen J, Billadeau DD, McNiven MA. Vav1 as a Central Regulator of Invadopodia Assembly. Curr Biol. 2013 doi: 10.1016/j.cub.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brisson L, Driffort V, Benoist L, Poet M, Counillon L, Antelmi E, Rubino R, Besson P, Labbal F, Chevalier S, et al. NaV1.5 Na⁺ channels allosterically regulate the NHE-1 exchanger and promote the activity of breast cancer cell invadopodia. J Cell Sci. 2013;126:4835–42. doi: 10.1242/jcs.123901. [DOI] [PubMed] [Google Scholar]
- 52.Li CM, Chen G, Dayton TL, Kim-Kiselak C, Hoersch S, Whittaker CA, Bronson RT, Beer DG, Winslow MM, Jacks T. Differential Tks5 isoform expression contributes to metastatic invasion of lung adenocarcinoma. Genes Dev. 2013;27:1557–67. doi: 10.1101/gad.222745.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poincloux R, Lizárraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J Cell Sci. 2009;122:3015–24. doi: 10.1242/jcs.034561. [DOI] [PubMed] [Google Scholar]
- 54.Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N, Tyska MJ, Weaver AM. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 2013;5:1159–68. doi: 10.1016/j.celrep.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Monteiro P, Rossé C, Castro-Castro A, Irondelle M, Lagoutte E, Paul-Gilloteaux P, Desnos C, Formstecher E, Darchen F, Perrais D, et al. Endosomal WASH and exocyst complexes control exocytosis of MT1-MMP at invadopodia. J Cell Biol. 2013;203:1063–79. doi: 10.1083/jcb.201306162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen WT, Wang JY. Specialized surface protrusions of invasive cells, invadopodia and lamellipodia, have differential MT1-MMP, MMP-2, and TIMP-2 localization. Ann N Y Acad Sci. 1999;878:361–71. doi: 10.1111/j.1749-6632.1999.tb07695.x. [DOI] [PubMed] [Google Scholar]
- 57.Paz H, Pathak N, Yang J. Invading one step at a time: the role of invadopodia in tumor metastasis. Oncogene. 2013 doi: 10.1038/onc.2013.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Artym VV, Matsumoto K, Mueller SC, Yamada KM. Dynamic membrane remodeling at invadopodia differentiates invadopodia from podosomes. Eur J Cell Biol. 2011;90:172–80. doi: 10.1016/j.ejcb.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lucien F, Brochu-Gaudreau K, Arsenault D, Harper K, Dubois CM. Hypoxia-induced invadopodia formation involves activation of NHE-1 by the p90 ribosomal S6 kinase (p90RSK) PLoS One. 2011;6:e28851. doi: 10.1371/journal.pone.0028851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Horssen R, Buccione R, Willemse M, Cingir S, Wieringa B, Attanasio F. Cancer cell metabolism regulates extracellular matrix degradation by invadopodia. Eur J Cell Biol. 2013;92:113–21. doi: 10.1016/j.ejcb.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 61.Alexander NR, Branch KM, Parekh A, Clark ES, Iwueke IC, Guelcher SA, Weaver AM. Extracellular matrix rigidity promotes invadopodia activity. Curr Biol. 2008;18:1295–9. doi: 10.1016/j.cub.2008.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beerling E, Ritsma L, Vrisekoop N, Derksen PW, van Rheenen J. Intravital microscopy: new insights into metastasis of tumors. J Cell Sci. 2011;124:299–310. doi: 10.1242/jcs.072728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sibony-Benyamini H, Gil-Henn H. Invadopodia: the leading force. Eur J Cell Biol. 2012;91:896–901. doi: 10.1016/j.ejcb.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 64.Sahai E. Illuminating the metastatic process. Nat Rev Cancer. 2007;7:737–49. doi: 10.1038/nrc2229. [DOI] [PubMed] [Google Scholar]
- 65.Hotary K, Li XY, Allen E, Stevens SL, Weiss SJ. A cancer cell metalloprotease triad regulates the basement membrane transmigration program. Genes Dev. 2006;20:2673–86. doi: 10.1101/gad.1451806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pflicke H, Sixt M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J Exp Med. 2009;206:2925–35. doi: 10.1084/jem.20091739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang S, Voisin MB, Larbi KY, Dangerfield J, Scheiermann C, Tran M, Maxwell PH, Sorokin L, Nourshargh S. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med. 2006;203:1519–32. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schoumacher M, Goldman RD, Louvard D, Vignjevic DM. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J Cell Biol. 2010;189:541–56. doi: 10.1083/jcb.200909113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol. 2005;17:559–64. doi: 10.1016/j.ceb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 70.Alexander S, Weigelin B, Winkler F, Friedl P. Preclinical intravital microscopy of the tumour-stroma interface: invasion, metastasis, and therapy response. Curr Opin Cell Biol. 2013;25:659–71. doi: 10.1016/j.ceb.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 71.Wyckoff JB, Jones JG, Condeelis JS, Segall JE. A critical step in metastasis: in vivo analysis of intravasation at the primary tumor. Cancer Res. 2000;60:2504–11. [PubMed] [Google Scholar]
- 72.Wang W, Wyckoff JB, Frohlich VC, Oleynikov Y, Hüttelmaier S, Zavadil J, Cermak L, Bottinger EP, Singer RH, White JG, et al. Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular profiling. Cancer Res. 2002;62:6278–88. [PubMed] [Google Scholar]
- 73.Gligorijevic B, Wyckoff J, Yamaguchi H, Wang Y, Roussos ET, Condeelis J. N-WASP-mediated invadopodium formation is involved in intravasation and lung metastasis of mammary tumors. J Cell Sci. 2012;125:724–34. doi: 10.1242/jcs.092726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T, et al. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441–52. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, Yang J. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. 2011;19:372–86. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seiler C, Davuluri G, Abrams J, Byfield FJ, Janmey PA, Pack M. Smooth muscle tension induces invasive remodeling of the zebrafish intestine. PLoS Biol. 2012;10:e1001386. doi: 10.1371/journal.pbio.1001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frame MC. Newest findings on the oldest oncogene; how activated src does it. J Cell Sci. 2004;117:989–98. doi: 10.1242/jcs.01111. [DOI] [PubMed] [Google Scholar]
- 78.Nguyen-Ngoc KV, Cheung KJ, Brenot A, Shamir ER, Gray RS, Hines WC, Yaswen P, Werb Z, Ewald AJ. ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium. Proc Natl Acad Sci U S A. 2012;109:E2595–604. doi: 10.1073/pnas.1212834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hiramatsu R, Matsuoka T, Kimura-Yoshida C, Han SW, Mochida K, Adachi T, Takayama S, Matsuo I. External mechanical cues trigger the establishment of the anterior-posterior axis in early mouse embryos. Dev Cell. 2013;27:131–44. doi: 10.1016/j.devcel.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 80.Sherwood DR, Sternberg PW. Anchor cell invasion into the vulval epithelium in C. elegans. Dev Cell. 2003;5:21–31. doi: 10.1016/S1534-5807(03)00168-0. [DOI] [PubMed] [Google Scholar]
- 81.Schindler AJ, Sherwood DR. Morphogenesis of the caenorhabditis elegans vulva. Wiley Interdiscip Rev Dev Biol. 2013;2:75–95. doi: 10.1002/wdev.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kao G, Huang CC, Hedgecock EM, Hall DH, Wadsworth WG. The role of the laminin beta subunit in laminin heterotrimer assembly and basement membrane function and development in C. elegans. Dev Biol. 2006;290:211–9. doi: 10.1016/j.ydbio.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 83.Ihara S, Hagedorn EJ, Morrissey MA, Chi Q, Motegi F, Kramer JM, Sherwood DR. Basement membrane sliding and targeted adhesion remodels tissue boundaries during uterine-vulval attachment in Caenorhabditis elegans. Nat Cell Biol. 2011;13:641–51. doi: 10.1038/ncb2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vogel BE, Hedgecock EM. Hemicentin, a conserved extracellular member of the immunoglobulin superfamily, organizes epithelial and other cell attachments into oriented line-shaped junctions. Development. 2001;128:883–94. doi: 10.1242/dev.128.6.883. [DOI] [PubMed] [Google Scholar]
- 85.Fitzgerald MC, Schwarzbauer JE. Importance of the basement membrane protein SPARC for viability and fertility in Caenorhabditis elegans. Curr Biol. 1998;8:1285–8. doi: 10.1016/S0960-9822(07)00540-4. [DOI] [PubMed] [Google Scholar]
- 86.Hesselson D, Newman C, Kim KW, Kimble J. GON-1 and fibulin have antagonistic roles in control of organ shape. Curr Biol. 2004;14:2005–10. doi: 10.1016/j.cub.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 87.Sherwood DR, Butler JA, Kramer JM, Sternberg PW. FOS-1 promotes basement-membrane removal during anchor-cell invasion in C. elegans. Cell. 2005;121:951–62. doi: 10.1016/j.cell.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 88.Hagedorn EJ, Ziel JW, Morrissey MA, Linden LM, Wang Z, Chi Q, Johnson SA, Sherwood DR. The netrin receptor DCC focuses invadopodia-driven basement membrane transmigration in vivo. J Cell Biol. 2013;201:903–13. doi: 10.1083/jcb.201301091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yamaguchi H, Yoshida S, Muroi E, Kawamura M, Kouchi Z, Nakamura Y, Sakai R, Fukami K. Phosphatidylinositol 4,5-bisphosphate and PIP5-kinase Ialpha are required for invadopodia formation in human breast cancer cells. Cancer Sci. 2010;101:1632–8. doi: 10.1111/j.1349-7006.2010.01574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Destaing O, Planus E, Bouvard D, Oddou C, Badowski C, Bossy V, Raducanu A, Fourcade B, Albiges-Rizo C, Block MR. β1A integrin is a master regulator of invadosome organization and function. Mol Biol Cell. 2010;21:4108–19. doi: 10.1091/mbc.E10-07-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hagedorn EJ, Yashiro H, Ziel JW, Ihara S, Wang Z, Sherwood DR. Integrin acts upstream of netrin signaling to regulate formation of the anchor cell’s invasive membrane in C. elegans. Dev Cell. 2009;17:187–98. doi: 10.1016/j.devcel.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matus DQ, Li XY, Durbin S, Agarwal D, Chi Q, Weiss SJ, Sherwood DR. In vivo identification of regulators of cell invasion across basement membranes. Sci Signal. 2010;3:ra35. doi: 10.1126/scisignal.2000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ziel JW, Hagedorn EJ, Audhya A, Sherwood DR. UNC-6 (netrin) orients the invasive membrane of the anchor cell in C. elegans. Nat Cell Biol. 2009;11:183–9. doi: 10.1038/ncb1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schindler AJ, Sherwood DR. The transcription factor HLH-2/E/Daughterless regulates anchor cell invasion across basement membrane in C. elegans. Dev Biol. 2011;357:380–91. doi: 10.1016/j.ydbio.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ziel JW, Matus DQ, Sherwood DR. An expression screen for RhoGEF genes involved in C. elegans gonadogenesis. Gene Expr Patterns. 2009;9:397–403. doi: 10.1016/j.gep.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stylli SS, Kaye AH, Lock P. Invadopodia: at the cutting edge of tumour invasion. J Clin Neurosci. 2008;15:725–37. doi: 10.1016/j.jocn.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 97.Olazabal IM, Machesky LM. Abp1p and cortactin, new “hand-holds” for actin. J Cell Biol. 2001;154:679–82. doi: 10.1083/jcb.200105061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kessels MM, Engqvist-Goldstein AE, Drubin DG. Association of mouse actin-binding protein 1 (mAbp1/SH3P7), an Src kinase target, with dynamic regions of the cortical actin cytoskeleton in response to Rac1 activation. Mol Biol Cell. 2000;11:393–412. doi: 10.1091/mbc.11.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Altincicek B, Fischer M, Fischer M, Lüersen K, Boll M, Wenzel U, Vilcinskas A. Role of matrix metalloproteinase ZMP-2 in pathogen resistance and development in Caenorhabditis elegans. Dev Comp Immunol. 2010;34:1160–9. doi: 10.1016/j.dci.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 100.Yamaguchi H, Oikawa T. Membrane lipids in invadopodia and podosomes: key structures for cancer invasion and metastasis. Oncotarget. 2010;1:320–8. doi: 10.18632/oncotarget.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dumartin L, Quemener C, Laklai H, Herbert J, Bicknell R, Bousquet C, Pyronnet S, Castronovo V, Schilling MK, Bikfalvi A, et al. Netrin-1 mediates early events in pancreatic adenocarcinoma progression, acting on tumor and endothelial cells. Gastroenterology. 2010;138:1595–606, e1-8. doi: 10.1053/j.gastro.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 102.Kaufmann S, Kuphal S, Schubert T, Bosserhoff AK. Functional implication of Netrin expression in malignant melanoma. Cell Oncol. 2009;31:415–22. doi: 10.3233/CLO-2009-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ramesh G, Berg A, Jayakumar C. Plasma netrin-1 is a diagnostic biomarker of human cancers. Biomarkers. 2011;16:172–80. doi: 10.3109/1354750X.2010.541564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shimizu A, et al. Netrin-1 Promotes Glioblastoma Cell Invasiveness and Angiogenesis by Multiple Pathways Involving Activation of RhoA, Cathepsin B and CREB. J Biol Chem. 2012;288:2210, 22. doi: 10.1074/jbc.M112.397398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Even-Ram S, Yamada KM. Cell migration in 3D matrix. Curr Opin Cell Biol. 2005;17:524–32. doi: 10.1016/j.ceb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 106.Li A, Dawson JC, Forero-Vargas M, Spence HJ, Yu X, König I, Anderson K, Machesky LM. The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion. Curr Biol. 2010;20:339–45. doi: 10.1016/j.cub.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 108.Tolde O, Rösel D, Veselý P, Folk P, Brábek J. The structure of invadopodia in a complex 3D environment. Eur J Cell Biol. 2010;89:674–80. doi: 10.1016/j.ejcb.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 109.Mercurio AM, Rabinovitz I. Towards a mechanistic understanding of tumor invasion--lessons from the alpha6beta 4 integrin. Semin Cancer Biol. 2001;11:129–41. doi: 10.1006/scbi.2000.0364. [DOI] [PubMed] [Google Scholar]
- 110.Fujita M, Khazenzon NM, Bose S, Sekiguchi K, Sasaki T, Carter WG, Ljubimov AV, Black KL, Ljubimova JY. Overexpression of beta1-chain-containing laminins in capillary basement membranes of human breast cancer and its metastases. Breast Cancer Res. 2005;7:R411–21. doi: 10.1186/bcr1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marinkovich MP. Tumour microenvironment: laminin 332 in squamous-cell carcinoma. Nat Rev Cancer. 2007;7:370–80. doi: 10.1038/nrc2089. [DOI] [PubMed] [Google Scholar]
- 112.Oikawa Y, Hansson J, Sasaki T, Rousselle P, Domogatskaya A, Rodin S, Tryggvason K, Patarroyo M. Melanoma cells produce multiple laminin isoforms and strongly migrate on α5 laminin(s) via several integrin receptors. Exp Cell Res. 2011;317:1119–33. doi: 10.1016/j.yexcr.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 113.Hamasaki H, Koga K, Aoki M, Hamasaki M, Koshikawa N, Seiki M, Iwasaki H, Nakayama J, Nabeshima K. Expression of laminin 5-γ2 chain in cutaneous squamous cell carcinoma and its role in tumour invasion. Br J Cancer. 2011;105:824–32. doi: 10.1038/bjc.2011.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 115.Gurskaya NG, Verkhusha VV, Shcheglov AS, Staroverov DB, Chepurnykh TV, Fradkov AF, Lukyanov S, Lukyanov KA. Engineering of a monomeric green-to-red photoactivatable fluorescent protein induced by blue light. Nat Biotechnol. 2006;24:461–5. doi: 10.1038/nbt1191. [DOI] [PubMed] [Google Scholar]
- 116.Zhang S, Kuhn JR. Cell isolation and culture. WormBook. 2013:1–39. doi: 10.1895/wormbook.1.157.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pignatelli J, Tumbarello DA, Schmidt RP, Turner CE. Hic-5 promotes invadopodia formation and invasion during TGF-β-induced epithelial-mesenchymal transition. J Cell Biol. 2012;197:421–37. doi: 10.1083/jcb.201108143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nakaya Y, Sukowati EW, Sheng G. Epiblast integrity requires CLASP and Dystroglycan-mediated microtubule anchoring to the basal cortex. J Cell Biol. 2013;202:637–51. doi: 10.1083/jcb.201302075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wong CW, Song C, Grimes MM, Fu W, Dewhirst MW, Muschel RJ, Al-Mehdi AB. Intravascular location of breast cancer cells after spontaneous metastasis to the lung. Am J Pathol. 2002;161:749–53. doi: 10.1016/S0002-9440(10)64233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Madsen CD, Sahai E. Cancer dissemination--lessons from leukocytes. Dev Cell. 2010;19:13–26. doi: 10.1016/j.devcel.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 121.Nourshargh S, Hordijk PL, Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11:366–78. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- 122.Stoletov K, Kato H, Zardouzian E, Kelber J, Yang J, Shattil S, Klemke R. Visualizing extravasation dynamics of metastatic tumor cells. J Cell Sci. 2010;123:2332–41. doi: 10.1242/jcs.069443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stoletov K, Montel V, Lester RD, Gonias SL, Klemke R. High-resolution imaging of the dynamic tumor cell vascular interface in transparent zebrafish. Proc Natl Acad Sci U S A. 2007;104:17406–11. doi: 10.1073/pnas.0703446104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kelley LC, Lohmer LL, Hagedorn EJ, Sherwood DR. Traversing the basement membrane in vivo: A diversity of strategies. J Cell Biol. 2014;204:291–302. doi: 10.1083/jcb.201311112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Peterson KJ, Lyons JB, Nowak KS, Takacs CM, Wargo MJ, McPeek MA. Estimating metazoan divergence times with a molecular clock. Proc Natl Acad Sci U S A. 2004;101:6536–41. doi: 10.1073/pnas.0401670101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Aris-Brosou S, Yang Z. Bayesian models of episodic evolution support a late precambrian explosive diversification of the Metazoa. Mol Biol Evol. 2003;20:1947–54. doi: 10.1093/molbev/msg226. [DOI] [PubMed] [Google Scholar]
- 127.Rohrschneider LR, Eisenman RN, Leitch CR. Identification of a Rous sarcoma virus transformation-related protein in normal avian and mammalian cells. Proc Natl Acad Sci U S A. 1979;76:4479–83. doi: 10.1073/pnas.76.9.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Abram CL, Seals DF, Pass I, Salinsky D, Maurer L, Roth TM, Courtneidge SA. The adaptor protein fish associates with members of the ADAMs family and localizes to podosomes of Src-transformed cells. J Biol Chem. 2003;278:16844–51. doi: 10.1074/jbc.M300267200. [DOI] [PubMed] [Google Scholar]
- 129.Wu H, Parsons JT. Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J Cell Biol. 1993;120:1417–26. doi: 10.1083/jcb.120.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mader CC, Oser M, Magalhaes MA, Bravo-Cordero JJ, Condeelis J, Koleske AJ, Gil-Henn H. An EGFR-Src-Arg-cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion. Cancer Res. 2011;71:1730–41. doi: 10.1158/0008-5472.CAN-10-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Smith-Pearson PS, Greuber EK, Yogalingam G, Pendergast AM. Abl kinases are required for invadopodia formation and chemokine-induced invasion. J Biol Chem. 2010;285:40201–11. doi: 10.1074/jbc.M110.147330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Monsky WL, Kelly T, Lin CY, Yeh Y, Stetler-Stevenson WG, Mueller SC, Chen WT. Binding and localization of M(r) 72,000 matrix metalloproteinase at cell surface invadopodia. Cancer Res. 1993;53:3159–64. [PubMed] [Google Scholar]
- 133.Mueller SC, Chen WT. Cellular invasion into matrix beads: localization of beta 1 integrins and fibronectin to the invadopodia. J Cell Sci. 1991;99:213–25. doi: 10.1242/jcs.99.2.213. [DOI] [PubMed] [Google Scholar]
- 134.Zambonin-Zallone A, Teti A, Grano M, Rubinacci A, Abbadini M, Gaboli M, Marchisio PC. Immunocytochemical distribution of extracellular matrix receptors in human osteoclasts: a beta 3 integrin is colocalized with vinculin and talin in the podosomes of osteoclastoma giant cells. Exp Cell Res. 1989;182:645–52. doi: 10.1016/0014-4827(89)90266-8. [DOI] [PubMed] [Google Scholar]
- 135.Marchisio PC, Cirillo D, Teti A, Zambonin-Zallone A, Tarone G. Rous sarcoma virus-transformed fibroblasts and cells of monocytic origin display a peculiar dot-like organization of cytoskeletal proteins involved in microfilament-membrane interactions. Exp Cell Res. 1987;169:202–14. doi: 10.1016/0014-4827(87)90238-2. [DOI] [PubMed] [Google Scholar]
- 136.Li A, Dawson JC, Forero-Vargas M, Spence HJ, Yu X, König I, Anderson K, Machesky LM. The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion. Curr Biol. 2010;20:339–45. doi: 10.1016/j.cub.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mizutani K, Miki H, He H, Maruta H, Takenawa T. Essential role of neural Wiskott-Aldrich syndrome protein in podosome formation and degradation of extracellular matrix in src-transformed fibroblasts. Cancer Res. 2002;62:669–74. [PubMed] [Google Scholar]
- 138.Philippar U, Roussos ET, Oser M, Yamaguchi H, Kim HD, Giampieri S, Wang Y, Goswami S, Wyckoff JB, Lauffenburger DA, et al. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev Cell. 2008;15:813–28. doi: 10.1016/j.devcel.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC. An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18:4440–9. doi: 10.1038/sj.onc.1202827. [DOI] [PubMed] [Google Scholar]
- 140.Chan KT, Cortesio CL, Huttenlocher A. FAK alters invadopodia and focal adhesion composition and dynamics to regulate breast cancer invasion. J Cell Biol. 2009;185:357–70. doi: 10.1083/jcb.200809110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Duong LT, Rodan GA. PYK2 is an adhesion kinase in macrophages, localized in podosomes and activated by beta(2)-integrin ligation. Cell Motil Cytoskeleton. 2000;47:174–88. doi: 10.1002/1097-0169(200011)47:3<174::AID-CM2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 142.Mueller SC, Yeh Y, Chen WT. Tyrosine phosphorylation of membrane proteins mediates cellular invasion by transformed cells. J Cell Biol. 1992;119:1309–25. doi: 10.1083/jcb.119.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nakahara H, Otani T, Sasaki T, Miura Y, Takai Y, Kogo M. Involvement of Cdc42 and Rac small G proteins in invadopodia formation of RPMI7951 cells. Genes Cells. 2003;8:1019–27. doi: 10.1111/j.1365-2443.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- 144.Berdeaux RL, Díaz B, Kim L, Martin GS. Active Rho is localized to podosomes induced by oncogenic Src and is required for their assembly and function. J Cell Biol. 2004;166:317–23. doi: 10.1083/jcb.200312168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Head JA, Jiang D, Li M, Zorn LJ, Schaefer EM, Parsons JT, Weed SA. Cortactin tyrosine phosphorylation requires Rac1 activity and association with the cortical actin cytoskeleton. Mol Biol Cell. 2003;14:3216–29. doi: 10.1091/mbc.E02-11-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Linder S, Nelson D, Weiss M, Aepfelbacher M. Wiskott-Aldrich syndrome protein regulates podosomes in primary human macrophages. Proc Natl Acad Sci U S A. 1999;96:9648–53. doi: 10.1073/pnas.96.17.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]