Abstract
After transient exposure to the gaseous hormone ethylene, dark-grown cucumber (Cucumis sativus) hypocotyls developed unusual features. Upon ethylene's removal, the developing epidermis showed significant increases in cell division rates, producing an abundance of guard cells and trichomes. These responses to ethylene depended on the stage of development at the time of ethylene exposure. In the upper region of the hypocotyl, where cells were least differentiated at the onset of ethylene treatment, complex, multicellular protuberances formed. Further down the hypocotyl, where stomata and trichomes were beginning to develop at the onset of ethylene exposure, an increase in the number of stomata and trichomes was observed. Stomatal complexes developing after the ethylene treatment had a significant increase in the number of stomatal subsidiary cells and the number of cells per trichome increased. Analysis of division patterns in stomatal complexes indicated that exposure to ethylene either suspended or altered cell fate. Ethylene also altered cell division polarity, resulting in aberrant stomatal complexes and branched trichomes. To our knowledge, the results of this study demonstrate for the first time that transient treatment with physiological concentrations of ethylene can alter cell fate and increase the propensity of cells to divide.
Ethylene regulates a variety of physiological and biochemical processes in plants, such as fruit ripening, senescence, abscission, sex determination, root initiation, and cell elongation (Abeles et al., 1992). Ethylene also regulates the size and stature of plants in response to various environmental cues (Abeles, 1973; Kieber, 1997). Its effects on dark-grown seedling development, described initially as the triple response, include increased radial growth of the root and stem, reduced root and stem elongation, and abnormal horizontal stem growth (Neljubow, 1901; Knight et al., 1910). The term triple response, as it is now commonly used, includes exaggeration in the curvature of the apical hook instead of abnormal horizontal growth (Bleecker et al., 1988; Guzman and Ecker, 1990 and references therein). The triple response of Arabidopsis has been exploited for characterizing the ethylene signal transduction system by identifying numerous mutants that either phenocopy the triple response in the absence of exogenous ethylene or fail to respond properly to ethylene (Guzman and Ecker, 1990).
The dramatic stimulation of radial swelling of stems and roots by ethylene has led to physiological studies and cellular analyses of this phenomenon (Burg and Burg, 1966; Lang et al., 1982; Bleecker et al., 1988; Abeles et al., 1992; Baskin and Williamson, 1992; Kieber, 1997). Depending on the timing of ethylene's application, the physiological condition, seedling age, and the plant species concerned, either promotive (Ku et al., 1970; Lehman et al., 1996; Smalle et al., 1997) or inhibitory (Kieber et al., 1993) effects on organ elongation have been reported. For young developing dicot seedlings, ethylene has generally been reported to be a negative regulator of elongation growth (Pratt and Goeschl, 1969; Guzman and Ecker, 1990; Abeles et al., 1992, and references therein). Relatively few studies have examined ethylene's stimulatory properties, which include root hair (Chadwick and Burg, 1967; Ecker, 1995; Tanimoto et al., 1995) and adventitious root formation (Zimmerman and Hitchcock, 1933; Angeles et al., 1986; Abeles, 1973) and the generation of aerenchyma during hypoxic stress (Drew et al., 1979, 1981; Jackson et al., 1981).
In contrast to numerous studies of ethylene's effects on organ expansion, very few studies have considered ethylene's effects on cell division. As seedling growth accompanies cell division in the apical meristem, suppression of seedling growth by ethylene implies that the gaseous hormone inhibits both cell elongation and division. Inhibition of DNA synthesis by ethylene was reported in meristems of shoots and roots of pea (Pisum sativum; Burg and Burg, 1968; Apelbaum and Burg, 1972; Eisinger and Burg, 1972) and in wounded tissue of potato (Solanum tuberosum; Sato et al., 1976). Because ethylene's formative effects should include changes in cell division control, we are especially interested in the effects of ethylene on those cells that remain competent for cell division, such as the progenitors of trichomes and stomata.
In this study, we examined how ethylene treatments alter cell division patterns in the progenitors of guard cells and trichomes of etiolated seedlings of cucumber (Cucumis sativus). We document for the first time to our knowledge that after exposure to ethylene at levels likely to be encountered during bouts of stress, cell division polarity is altered and that there is an increased propensity for cell division, organogenesis, and histogenesis.
RESULTS
Most of ethylene's known effects on plant development have been observed during continuous ethylene treatments. We wanted to examine the consequence of transient exposure to ethylene, and therefore documented the structural changes that took place following exposure to exogenous ethylene. We chose the hypocotyl because it provides a range of developmental stages, with differentiation progressing from the base upwards. In hypocotyls, postembryonic division is confined to cells that give rise to stomatal complexes and trichomes so it is possible to quantify changes in cell division that are dependent on a given treatment.
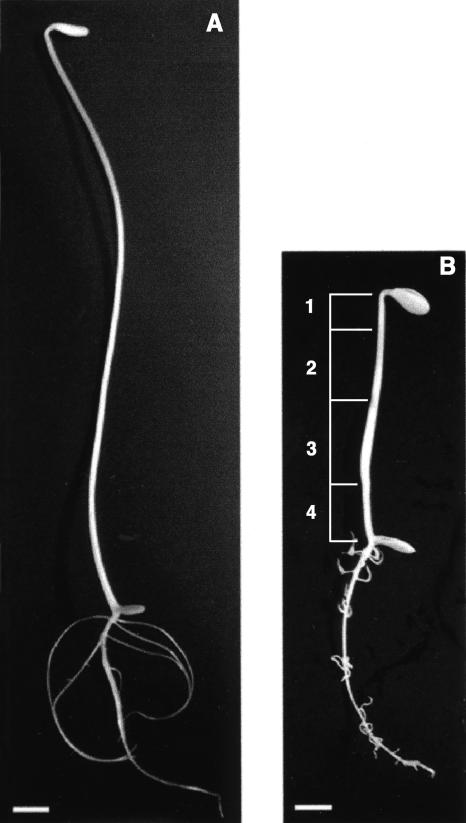
We first examined the effects of ethylene on stomatal development along the entire length of the hypocotyl. We grew seedlings in the dark in airtight containers throughout the treatment period. Three days after planting in normal air, we injected 10 μL L−1 ethylene into the containers and after 2 d, ventilated them to restore normal air for a further 2 d. Control seedlings were grown continuously in the containers and injected with normal air and ventilated at the same times as the ethylene-treated seedlings. Compared to untreated seedlings (Fig. 1A), ethylene-treated seedlings had relatively short hypocotyls, approximately 70 to 85 mm in length (Fig. 1B). During the 2-d ethylene treatment, cell growth was greatest in the lower mid region (Fig. 1B, zone 3), which consequently underwent radial expansion over the course of exposure to ethylene. Stomatal development progresses apically from the base of the hypocotyl, which can be divided into four developmental zones (Table I). It is known from a previous study (Kazama and Mineyuki, 1997) that at the time of ethylene's application to 3-d-old seedlings, guard cells have already formed and cell expansion is complete in the lowest portion of the hypocotyl (Fig. 1B, zone 4), guard mother cell division and cell expansion are most active in the lower midzone (Fig. 1B, zone 3), and stomatal progenitor cell commitment and division are taking place in the uppermost zone (Fig. 1B, zones 1 and 2). The consequences of the transient ethylene treatment in these different zones are summarized in Table I.
Figure 1.
Transient ethylene exposure alters development of dark-grown cucumber seedlings. A, A seedling grown in normal air for 7 d has typical etiolated growth. B, After a 2-d-long exposure to 10 μL L−1 ethylene at day 3, followed by 2 d in normal air, the hypocotyl is approximately one-half the length of control seedlings (A) and is swollen in the lower midzone (zone 3) where expansion was greatest during ethylene treatment. Note that root development is also altered, with lateral roots having altered orientation with respect to gravity. The consequences of transient ethylene treatment on four developmentally distinct regions of the hypocotyl, indicated by numbers, are described in Table I. Bars = 10 mm.
Table I.
Effects on stomatal development of ethylene treatment between days 3 and 5
| Hypocotyl Zone (as Defined in Fig. 1B) | Processes Active during Ethylene Treatment | Consequence of Transient Ethylene Exposure | Figure Reference |
|---|---|---|---|
| Apical zone (1) | Meristemoid and stomatal progenitor cell commitment | Protuberances | 2, A and B |
| Subsidiary cells | |||
| Increased stomatogenesis | |||
| Upper midzone (2) | Guard mother cell commitment | Subsidiary cells | 4C |
| Stomatal twins | 4, E and F | ||
| Aberrant stomatal division planes | 4E | ||
| Lower midzone (3) | Ordinary cell expansion | Radial swelling | 1B |
| Guard mother cell division | Aberrant stomatal division planes | 4, G–J | |
| Basal zone (4) | Guard cell differentiation | Guard cell redivision | 4K |
Snorkel-Like Protuberances Form in the Region with Greatest Development Potential
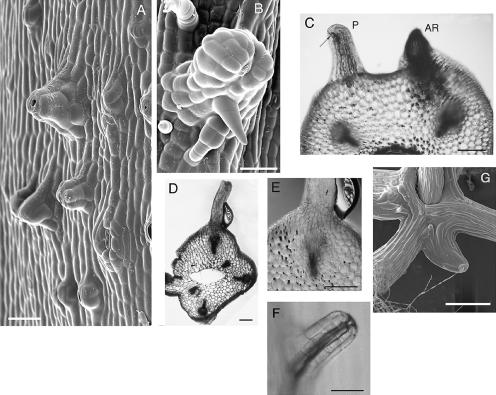
Once ethylene was removed from the containers, the upper region of the hypocotyl (Fig. 1B, zones 1 and 2) continued elongating with no apparent radial swelling. Within 2 d of removing ethylene, conspicuous protuberances developed in the uppermost (apical) zone (Fig. 1B, zone 1; Fig. 2, A and B). Scanning electron micrographs of the protuberances revealed that they always terminated with at least one stoma (Fig. 2A) and commonly developed trichomes (Fig. 2B). Protuberances ranged from 20 to several thousand cells in size. In some experiments we achieved a sustained release of ethylene over longer periods of time (up to 7 d) by enclosing a ripe apple (Malus domestica) within the container from the time of seed planting (Fig. 2D). In these cases the protuberances were generally larger than those produced after the 2-d pure ethylene exposure. Unlike adventitious roots, which commonly develop in dark-grown cucumber hypocotyls under high humidity (Fig. 2C), protuberances showed no discontinuity with the surrounding epidermis and cortex tissues (Fig. 2, D and E). Air-filled cavities were detected during the early stages of protuberance development (Fig. 2F). Notably, the longer ethylene treatments using a ripe apple also resulted in large cavities in the center of the hypocotyl (Fig. 2D), though these cavities may have been present before removing the apple.
Figure 2.
A–F, Protuberances generated in the apical zone of cucumber hypocotyls after transient exposure to ethylene. Protuberances shown in A–C and F were formed in response to a 2-d exposure to 10 μL L−1 ethylene at day 3, followed by 2 (A and F) or 4 (B and C) d in normal air. Protuberance shown in D and E was produced after one week exposure to ethylene produced by including a ripe apple in the container from time of planting, followed by 5 d incubation in normal air. A and B, Scanning electron micrographs of protuberances. A, Early stage protuberances from the apical zone 2 d after ethylene removal. Terminal stomata are evident. B, Mature protuberance with trichomes 4 d after ethylene removal. Bars = 50 μm in A and B. C, A transverse hand section from a seedling 4 d after ethylene removal includes both a protuberance (P), which terminates with a stoma (arrow), and an adventitious root (AR). Bar = 200 μm. D, A hand section cut longitudinally through a protuberance formed after 7 d coculture with an apple, followed by 5 d in normal air. Note vascular tissue subjacent to the protuberance and the central air-filled cavity. Bar = 300 μm. E, Higher magnification image of D shows continuity of the protuberance with the surrounding epidermis and vascular tissue. Bar = 300 μm. F, An early stage protuberance (2 d after ethylene removal) has a snorkel-like appearance with an air-filled chamber (dark region) beneath the terminal stoma. Bar = 50 μm. G, Protuberance formation in the upper hypocotyl of a 2-week-old Arabidopsis seedling that had been exposed to 10 μL L−1 ethylene during its first week of growth. Bar = 500 μm.
We also observed analogous structures in the upper regions of Arabidopsis hypocotyls after seedlings were exposed to ethylene during the early stages of development (Fig. 2G). The phenomenon is therefore not a peculiarity of cucumber seedlings.
Transient Exposure to Ethylene Stimulates Extra Stomatogenesis
In the upper midzone (Fig. 1B, zone 2), where guard mother cell commitment was taking place at the time of ethylene exposure, protuberances did not form, but the number of stomata was increased significantly. To quantify this effect, we measured stomatal frequency (defined as the number of stomata for every 100 epidermal cells; Kazama and Mineyuki, 1997) of hypocotyls. On day 5, that is, immediately after 2 d continuous ethylene treatment, the stomatal frequency was less than 0.01% ± 0.01%, but by day 7 (after an additional 2 d in normal air) we detected stomatal frequencies as high as 3.0% ± 1.1%. Seedlings left in ethylene continuously over the 4-d period showed no sign of cell division (data not shown). Stomatal frequencies in 7-d untreated control seedlings were only 0.02% ± 0.01% (Kazama and Mineyuki, 1997). This suggests that ethylene stimulated many ordinary cells to enter the stomatal development pathway.
Ethylene Stimulates Subsidiary Cell Development
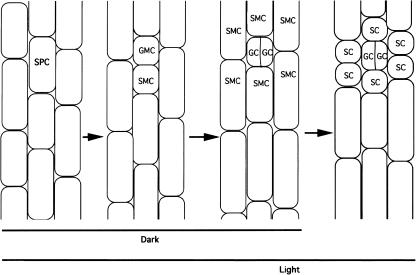
In addition to the increased incidence of stomata, exposure to ethylene increased the number of nonspecialized epidermal cells in the stomatogenesis-competent zones. The pattern of stomatogenesis in light- and dark-grown seedlings is compared schematically in Figure 3. Stomata developing in normal air in the dark consist on average of a pair of guard cells subtended by one undivided subsidiary mother cell, which was the sister cell of the original guard mother cell (Figs. 3 and 4A). By comparison, 5 to 10 subsidiary cells surround each stoma in light-grown seedlings (Fig. 3). After transient ethylene treatment, however, we detected many extra cells adjacent to guard cells, especially in the upper and lower midzones (Fig. 1B, zones 2 and 3; Fig. 4, B–D). The mean number of cells in stomatal complexes, including guard and other epidermal cells, was 12 (n = 30–50 for three separate experiments) in the dark-grown seedlings, more than the usual number of subsidiary cells found in light-grown seedlings. Nearest the hypocotyl base (Fig. 1B, zone 4), stomata were found in normal abundance but some guard cells, distinguished by their morphology and chloroplasts, had undergone extra divisions (Fig. 4K).
Figure 3.
Cell division patterns during stomatogenesis in dark-grown (first three stages) and light-grown (all four stages) cucumber hypocotyls. SPC, stomatal progenitor cell; GMC, guard mother cell; GC, guard cell; SMC, subsidiary mother cell; SC, subsidiary cell. In light-grown seedlings, transverse division of the stomatal precursor cell produces an apical guard mother cell and a basal subsidiary mother cell. Division of all cells surrounding the guard mother cell generates six subsidiary cells. In contrast, in dark-grown seedlings, the basal sibling of the GMC, as well as other surrounding subsidiary mother cells, do not divide and the stomatal development arrests at the third stage.
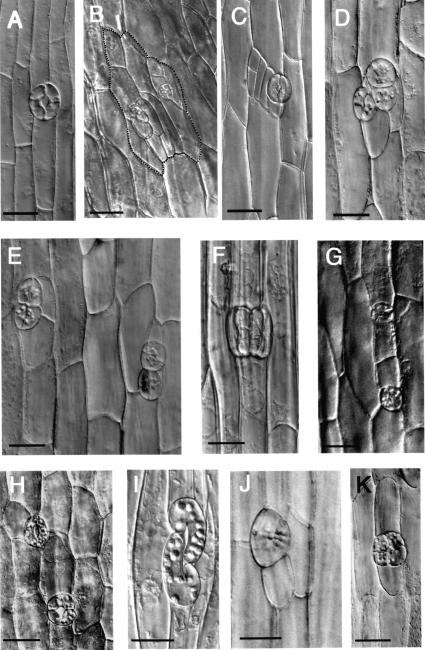
Figure 4.
Cell division patterns are altered in stomatogenesis-competent cell files after transient exposure to ethylene (3 d normal air, 2 d 10 μL L−1 ethylene, 2 d normal air). A, Normal stoma from a 7-d-old cucumber hypocotyl grown in normal air in the dark has longitudinal division plane and lacks subsidiary cells. B–K, Stomatal complexes formed 2 d after a 2-d ethylene treatment in 7-d-old dark-grown seedlings. B–F, Aberrant stomatal complexes in upper midzone (Fig. 1B, zone 2) include two stomata with subsidiary cells (B), cluster of lateral cells (C), stomatal cluster (D), and stomatal twins (E and F). G–J, Extra division and aberrant division plane orientations detected in radial swelling lower midzone (Fig. 1B, zone 3). G, Two oppositely oriented stomata have formed as a result of extra transverse division of the stomata progenitor cell. H, The upper stoma is oriented correctly but the lower stoma has formed from a transversely dividing guard mother cell. I, This aberrant stoma has formed by an oblique guard mother cell division plane followed by guard cell differentiation. J, Periclinal division results in a single guard cell at the hypocotyl surface, whose identity is clear by the presence of chloroplasts. A second guard cell (not shown) sits directly beneath this guard cell. K, Redivided guard cells from the basal zone of the hypocotyl (Fig. 1B, zone 4). Bars = 10 μm.
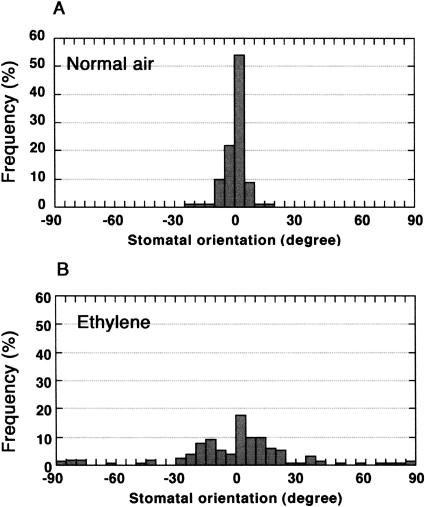
We detected a high proportion of unusual and defective stomata in the upper midzone (Fig. 1B, zone 2), and to a lesser extent, in the lower midzone (zone 3). Stomatal twins (Fig. 4, E and F) were very common, suggesting that processes inhibiting the formation of adjacent stomata were perturbed by ethylene. Guard cell division planes were also frequently aberrant in the midzones (Fig. 1B, zones 2 and 3; Fig. 4, G–J). Figure 4G shows two oppositely oriented stomata, formed as a result of extra transverse divisions of the stomatal progenitor cell. In some cases, the normally longitudinal guard mother cell division planes (Fig. 4A) were transverse (Fig. 4H), oblique (Fig. 4I), or even periclinal (Fig. 4J). Quantitative analysis showed that while stomata were consistently oriented almost parallel to the longitudinal axis in the seedlings grown without exogenous ethylene (Fig. 5A), stomatal orientation deviated greatly from the longitudinal axis in seedlings exposed to ethylene (Fig. 5B).
Figure 5.
Transient ethylene treatment alters subsequent guard cell orientation. A, In normal air control (7-d seedlings), the division plane between guard cells is predominantly longitudinal and never transverse to the long axis of the hypocotyl (n = 91). B, After a 2-d ethylene treatment at 10 μL L−1 on day 3 followed by 2 d in normal air, guard cell orientation is highly variable (n = 154).
Ethylene Exposure Stimulates Extra Cell Numbers and Branching in Trichomes
Trichome formation, like stomatogenesis, is a feature of postembryo hypocotyl development that involves cell division. Under normal conditions, trichomes develop either as hair-form or secretory trichomes. The hair-form trichomes point downward and terminate with a fourth cone-shaped cell (Figs. 6A and 7A). Secretory trichomes are much smaller and point toward the top of the hypocotyl, with a three-celled stalk and a four-celled globular head (Fig. 7B).
Figure 6.
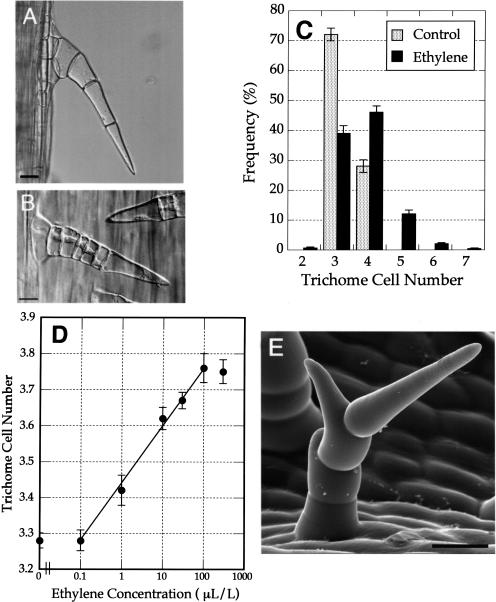
Ethylene treatment increases cell numbers and induces branching of trichomes. Seedlings were either grown for 5 d under normal air (A) or for 3 d in normal air, 1 day in 100 μL L−1 ethylene, followed by 1-d incubation in normal air (B and E). A, Mature trichomes grown under normal air. B, Additional cells are produced in developing trichomes after transient ethylene exposure. C, Frequency distribution histograms show that trichome cell numbers vary more in ethylene-treated seedlings than in those grown in normal air. Note that trichomes with five or more cells are only produced in the ethylene-treated seedlings. (n = 20 seedlings for control; n = 20 seedlings for ethylene treatment). D, A dose response curve demonstrates a strong correlation between ethylene concentration and eventual cell number. Mean trichome cell numbers (±sd) are shown. E, Scanning electron micrograph of a branched trichome stimulated after ethylene exposure. For each treatment, a total of 20 seedlings were examined, and a minimum of 50 trichomes per seedling analyzed. Bars = 10 μm.
Figure 7.
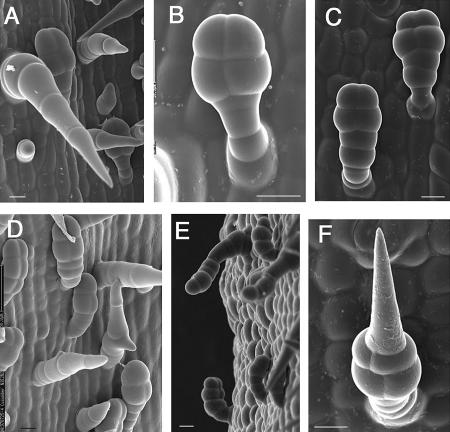
Scanning electron micrographs of unusual trichomes induced by treatment of 3-d-old seedlings with 100 μL L−1 ethylene for 1 d followed by 2 d in normal air. Under normal conditions, trichomes develop either as hair-form or secretory trichomes. A, The hair-form trichomes, point downward and terminate with a fourth cone-shaped cell. B, Secretory trichomes are relatively small and point toward the top of the hypocotyl, with a four-celled stalk and a four-celled globular head. C and D, Secretory trichomes developed with extra cells, either in the stalk (C) or head region (D). D–F, Trichomes of mixed character, including upward-bending hair-form trichomes (D), downward-bending secretory trichomes (E), and trichomes with both globular heads and cone-shaped end cells (F). Bars = 20 μm.
To determine ethylene's effects on trichome development, we examined trichomes after exposing dark-grown seedlings to ethylene at a range of concentrations from 0.1 to 300 μL L−1 for 24 h on day 3. Transient ethylene exposure stimulated trichome formation, increased the number of cells per trichome, stimulated trichome branching, and altered the fate of trichomes to specialize as secretory or nonsecretory trichomes. In untreated control seedlings, hair-form trichomes had a maximum of four cells (Fig. 6A), whereas seedlings that had been exposed to 100 μL L−1 ethylene had hair-form trichomes consisting of as many as seven cells (Fig. 6, B and C). The mean number of cells per trichome after ethylene treatment was apparently only moderately higher than in the control treatments, but this statistic reflects the fact that the total population of trichomes also included many two- and three-celled immature trichomes, whose formation was also initiated after ethylene treatment (Fig. 6, B and C). The mean number of cells per trichome increased in proportion to the concentration of ethylene applied within the range of 0.1 μL L−1 to 100 μL L−1 (Fig. 6D). The correlation factor of the dose response curve was R = 0.99, indicating that the increase in trichome cell number was dependent on ethylene concentration. Branching of trichomes was observed in 38.2% of the trichomes (n = 386) of seedlings exposed to ethylene (10 μL L−1) for 48 h following 2-d incubation in normal air (Fig. 6E). No branched trichomes were detected in control seedlings.
Transient ethylene treatment resulted in a range of unusual trichomes. Some secretory trichomes developed with extra cells, either in the stalk (Fig. 7C) or head region (Fig. 7D). In other cases, trichomes were of mixed character, including downward-bending secretory trichomes (Fig. 7E), upward-bending hair-form trichomes (Fig. 7D), and trichomes with both globular heads and cone-shaped end cells (Fig. 7F).
DISCUSSION
To date, most ethylene research has focused on the effects of ethylene on gross morphology during continuous application. In contrast, our study examined the consequences of seedling development to short-term ethylene exposure, which simulates ethylene signaling in plants as they respond to many environmental signals and stresses, which are often transient.
Our study looked beyond seedling morphology to examine how short-term ethylene exposure alters cell differentiation patterns. The hypocotyl epidermis is ideal for this because the only postembryonic divisions are those giving rise to stomata and trichomes. Hypocotyl development, which includes epidermal cell expansion as well as the differentiation of stomata and trichomes, begins at the base and progresses toward the cotyledons (Kazama and Mineyuki, 1997). This predictable developmental continuum means that for a brief period around 3 d postgermination, there is a range of cells at all stages of the same differentiation pathway. Thus, by exposing dark-grown cucumber seedlings to ethylene at day 3, we were able to document the relative effects of a 2-d ethylene exposure on immature, uncommitted cells in the apical zone, on expanding and dividing cells in the midzones and on fully differentiated cells at the basal zone of the hypocotyl. We found that the uncommitted cells in the upper region of the hypocotyl were stimulated to the greatest extent, resulting in increased stomata and trichome development and the generation of organ-like protuberances. In the upper midzone, where the progenitors of stomata were forming at day 3, cells were stimulated into extra rounds of cell division, producing abnormal clustering of stomata. In the lower midzone, the most obvious effect of the treatment was altered expansion polarity but this occurred during the 2-d ethylene treatment itself. Aberrant division patterns, however, were observed in this region, resulting in unusually oriented stomata and branched trichomes. Finally, ethylene exposure had relatively little effect on the mature cells of the basal zone but some already differentiated guard cells, identified by their distinctive morphology and chloroplasts, underwent extra cell divisions.
The results of our study demonstrate that ethylene exposure has considerable promotive effects on cell development that are only manifested after the initial signal subsides. These effects differ dramatically from the responses of cells during ethylene exposure. They include increased propensity for cells to divide, altered cell fate and altered division polarity. These three effects are now discussed.
Ethylene Exposure Suppresses Cell Division But Potentiates Cells for Further Division
In a companion study, we report that continuous ethylene treatment suppresses cell division but that removing ethylene triggers an exceptional burst of cell division activity (Dan et al., 2003). In the same study, we demonstrate that endoreduplication is stimulated in a subset of epidermal cells during exposure to ethylene. Early studies demonstrated that ethylene suppresses cell division and mitotic DNA synthesis in apical meristems (Apelbaum and Burg, 1972). However, more recent work on light-grown hypocotyls of Arabidopsis demonstrates that ethylene can both promote hypocotyl enlargement (Smalle et al., 1997) and stimulate endoreduplication (Gendreau et al., 1999), although which cells are affected was not determined.
Ethylene also promotes rapid stem elongation and adventitious root formation in submerged amphibious plants. In deepwater rice (Oryza sativa), apparent stimulation of cell division by ethylene has also been reported (Métraux and Kende, 1984) but may not be the causal factor for cell division per se. This is because ethylene is believed to increase the responsiveness of the internode to gibberellin, which stimulates cell division of the intercalary meristem at the base of internode (Raskin and Kende, 1984; Sauter and Kende, 1992) by up-regulating mitotic cyclins and cyclin-dependent kinases (Lorbiecke and Sauter, 1998). Submergence of deepwater rice also stimulates adventitious root growth through cell cycle regulatory gene expression (Lorbiecke and Sauter, 1999). In the same study, treatment of rice stem cuttings with ethephon also promoted the growth of adventitious roots, suggesting that ethylene can also promote cell division.
Ethylene May Suspend Cell Fate Determination
Our results show that ethylene extends the period during which cells remain competent for division. As a result, cells that would normally cease dividing instead retain the capacity for further rounds of division upon removal of the ethylene block. It is well known that continuous exposure to auxin and cytokinin stimulates proliferation of plant tissues (den Boer and Murray, 2000) to produce dedifferentiated cell masses. Subsequent alteration in the ratio of auxin to cytokinin supplied to these cultured masses of cells determines whether the dedifferentiated cells develop into shoots or roots (John et al., 1993). Our study suggests that in intact plants, ethylene has a similar capacity to promote cell proliferation and suspend fate determination. This is not restricted to those cells that normally retain a capacity for further division, such as stomatal progenitor cells, guard mother cells, subsidiary mother cells, and trichoblasts. The stimulation of extra trichomes and stomata indicates that many cells that would otherwise differentiate as unspecialized epidermal cells are reprogrammed during ethylene treatment. This suspension of cell fate could be achieved through the prevention of the terminal differentiation of pavement cells. We also determined that ethylene can induce division in terminally differentiating guard cells. Division of formed guard cells has recently been observed using other treatment regimes including rapid temperature shifts (Hall et al., 1997; H. Kazama, unpublished data).
Could altered cell to cell signaling during ethylene exposure account for the apparent developmental regression of cells? Asymmetric division of the progenitors of guard mother cells in dicot leaves is considered to be crucial for subsequent differentiation into guard mother, subsidiary, and guard cells (Zhao and Sack, 1999). Unlike leaf stomatogenesis, cell divisions that give rise to the guard mother cell in cucumber hypocotyls are symmetric, producing two cells of equal size. This crucial division is, however, asymmetric in cell fate, with signaling between cells of different fates or from underlying cortex tissue likely to be important. Thus, if the period of ethylene treatment negates directional signaling between adjacent cells or tissues, guard mother cell fate determination could be delayed. The formation of stomatal twins, for example, can be explained by the disturbance of stomatal progenitor cell polarity. As illustrated in Figure 4, these cells that enter the stomatal development pathway normally divide transversely relative to the hypocotyl axis and the basal cell becomes a subsidiary cell. Over-and-under stomatal twins form when the both of the daughter cells convert to guard mother cells. Side-by-side stomatal twins form when the stomatal progenitor cell divides longitudinally instead of transversely and, as in the case of over-under twins, both daughter cells convert into guard mother cells. During normal development, the apical daughter cell invariably becomes the guard mother cell. Transient ethylene exposure led, in some cases, to a reversal of this fate.
Stomatal patterning in Arabidopsis hypocotyls is, like root hairs, highly dependent on cell positioning (Berger et al., 1998) and it is likely that the same transcription factors regulate cell fate specification. Like root hairs, stomata also only develop in analogous hypocotyl cell files. Ethylene is known to promote ectopic root hair formation. We have demonstrated that ethylene can promote extra stomata and trichome formation in hypocotyls, though we have no evidence that a greater number of cell files contribute cells to these developmental pathways. The increase in stomata and trichome numbers could be manifested indirectly, by altering cell polarity and therefore the positioning of epidermal cells relative to underlying cortex cells or apoplast.
Altered Cell Division Polarity
Transient ethylene exposure also altered division planes, and generated aberrant stomatal complexes and branched trichomes. Interestingly, transient red light exposure to otherwise dark-grown seedlings can also produce aberrant stomata (Kazama and Mineyuki, 1997), suggesting that red light- and ethylene-signaling pathways may converge. Our experiments on stomatal development were carried out in the absence of any light stimuli.
Our results show that ethylene signaling impinges on the mechanisms that control division planes and tissue patterning. Mutational approaches are beginning to identify elements that control these processes (Otegui and Staehelin, 2000; Geisler et al., 2000; Smith et al., 2001; Nadeau and Sack, 2002). Our findings show that transient ethylene exposure can mimic some of these mutant phenotypes like the too many mouths mutation of Arabidopsis (Geisler et al., 2000). Similarly, Serna and Fenoll (1997) altered the normal spacing of stomata by limiting gas exchange in Arabidopsis cultures, conditions that are now demonstrated to increase ethylene concentrations (Buer et al., 2003).
The formation of protuberances in the upper zone of cucumber hypocotyls is compelling evidence that ethylene not only stimulates proliferation and alters cell fate but may also play a pivotal role in the direction of organ development. The protuberances form at right angles to the hypocotyl growth axis, suggesting that a new growth polarity is established in the presence of ethylene. Hypocotyl protuberances can also be stimulated when carrot (Daucus carota) seedlings are agitated in the presence of abscisic acid (ABA) and, when agitation ceases, these eventually develop into somatic embryos (Nishiwaki et al., 2000). Stimulation of cell proliferation by ABA alone is at odds with ABA's stimulation of the cyclin-dependent kinase inhibitor ICK (Wang et al., 1998), which suppresses G1 to S transitions. Mechanical stimulation is therefore crucial in this experimental system for protuberance initiation, and it is tempting to speculate that ethylene is generated under these conditions.
The protuberances, which always form with stomata at their tips, are reminiscent of ray parenchyma, whose frequency and development in woody tissues are stimulated by ethylene. According to the ethylene aeration hypothesis, the centrifugal flow of ethylene through ray initials promotes the differentiation of vascular rays in radial directions (Lev-Yadun and Aloni, 1995). The protuberances could help to vent ethylene from interior portions of the hypocotyls, as suggested for ray cells.
Finally, one simple explanation for the very rapid responses observed is that the expression of ethylene receptors is up-regulated during ethylene exposure. Removal of ethylene gas from the experimental containers would result in an unusually large proportion of unbound ethylene receptors, creating a state that could counteract the generally negative effects ethylene has on normal development. Investigating this possibility and the other novel phenomena described in this study should pave the way for identifying new aspects of ethylene physiology, and impinge on future strategies to identify new sensory, regulatory, and transcription factors in ethylene signaling.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Cucumber (Cucumis sativus) L. cv Aonagajibai seeds purchased from Takii Seed (Kyoto) were imbibed for 2 h and planted in Smithers-Oasis growing medium (nutrient free medium, H–TC276, Nippon Soda, Tokyo) or in 0.35% (w/v) plain agar medium (Bacto-agar, DIFCO Laboratories, Detroit), and grown at 25°C ± 1°C in total darkness unless otherwise mentioned. Two different growth cabinets were used (Koitotron KG206, Koito Industries, Tokyo, or a Sherer CEL15 cabinet, Sherer-Pennant, Seven Hill, Australia) with no difference in results. For ethylene treatments and controls, seedlings were cultured in 825-ml glass, airtight containers whose acrylic lids were drilled to accommodate gas chromatography-grade silicon plugs (Shimazu, Kyoto). Four seedlings were grown in each container. Three days after germination, reagent-grade ethylene (Nippon Sanso, Tokyo) was injected through the plug using a hypodermic syringe. Equivalent volumes of normal air were injected for control treatments. Gas chromatograph mass spectrometric analysis demonstrated that ethylene levels were steady over at least 7 d after a single injection (data not shown). A small block of ice was placed on top of the lid to facilitate circulation of gas within the container. For experiments requiring a sustained release of ethylene over a long term, a ripe apple, expected to release between 10 and 50 μL L−1 ethylene, was placed inside a 20-L airtight acrylic container at time of planting. For experiments investigating trichomes, seedlings were exposed to red light for 30 min on day 2 to induce trichome development. For quantitative analysis, each experiment was repeated at least 10 times. All other treatments were repeated at least three times. Arabidopsis seedlings were grown in petri dishes as described (Sugimoto et al., 2000).
Light Microscopic Observation of Seedlings
For morphological studies, epidermal strips were peeled from the hypocotyl with fine forceps. Microscopic observations were performed with an Olympus microscope (New Vanox S AHBS-s, Olympus, Tokyo) equipped with DIC optics.
Cryo-Scanning Electron Microscopy
Hypocotyl segments were attached to mounting plates with a 50:50 mixture of Tissue-Tek tissue freezing medium (Miles Scientific, Naperville, IL) and colloidal graphite (Agar Aids, Stansted, Essex, United Kingdom) and rapidly frozen by plunging them into liquid nitrogen slush at −230°C. The plate with attached sample was then inserted into the preparation chamber of an Oxford CT1500 Cryo Preparation System and slowly warmed to −80°C to sublimate ice crystals from the specimen surface. The cryochamber temperature was adjusted to −169°C and, by introducing a plasma of argon gas to the chamber, the specimen was sputter-coated with 100 Å gold particles for approximately 3 min, resulting in a 10-nm coating. The frozen sample was then transferred to a cryo stage at −185°C, which fitted inside the chamber of a Cambridge Instruments S360 scanning electron microscope (SEM). The electron optics system of the SEM was optimized for high resolution, but with sufficient depth-of-field to enable the entire object to be focused. This involved the use of a final aperture, 30 μm in diameter, a working distance of approximately 18 mm, electron beam current of 80 pA and an accelerating voltage of 15 kV. The sample was maintained at approximately −165°C throughout the SEM viewing operation. Images were recorded electronically as TIF files and as photographic negatives on Kodak PXP 6057 black and white film.
Measurement of Division Planes
To view newly formed cell walls in stomatal complexes and in trichomes, freshly peeled epidermal strips were incubated with 0.05% (w/v) aniline blue (Schmidt gmbH, Keonigen, Netherlands) in phosphate-buffered saline buffer at pH 8.5. After 5 min incubation in the dark, fluorescence microscopic observation was performed under violet light irradiation with an Olympus microscope (New Vanox S AHBS-s, Olympus). Images were recorded on photographic negatives on Fujicolor Superia 400 film (Fuji Photo Film, Tokyo). The angular deviation of the cell division plane from the normal longitudinal orientation was measured on digitized images using the public domain NIH Image program (developed at the United States National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image).
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Acknowledgments
We thank Prof. B.E. Gunning for valuable suggestions, Dr. M.E. Galway for critical reading of the manuscript, and Dr. R. Heady from the Australian National University Electron Microscopy Unit for excellent technical assistance with the SEM.
This work was supported by a grant in aid from the Ministry of Education, Science and Culture, Japan (grant no. 11874120 to H.K.), and by the Australian Research Council Discovery Project (grant no. DP0208872 to G.O.W.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.031088
References
- Abeles FB (1973) Ethylene in Plant Biology. Academic Press, New York and London
- Abeles FB, Morgan PW, Saltveit ME, Jr (1992) Ethylene in Plant Biology, Ed 2. Academic Press, San Diego
- Angeles G, Evert RF, Kozlowski TT (1986) Development of lenticels and adventitious roots in flooded Ulmus americana seedlings. Can J For Res 16: 585–590 [Google Scholar]
- Apelbaum A, Burg SP (1972) Effect of ethylene on cell division and deoxyribonucleic acid synthesis in Pisum sativum. Plant Physiol 50: 117–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin TI, Williamson RE (1992) Ethylene, microtubules and root morphology in wild-type and mutant Arabidopsis seedlings. In DD Randal, RE Sharp, AJ Novacky, DG Blevins, eds, Current Topics in Plant Biochemistry and Physiology. Vol 11. Interdisciplinary Plant Biochemistry and Physiology Program, Columbia, MO, pp 119–130
- Berger F, Linstead P, Dolan L, Haseloff J (1998) Stomata patterning on the hypocotyl of Arabidopsis thaliana is controlled by genes involved in the control of root patterning. Dev Biol 194: 226–234 [DOI] [PubMed] [Google Scholar]
- Bleecker A, Estelle M, Somerville C, Kende H (1988) Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 241: 1086–1089 [DOI] [PubMed] [Google Scholar]
- Buer CS, Wasteneys GO, Masle J (2003) Ethylene modulates root-wave responses in arabidopsis. Plant Physiol 132: 1085–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg SP, Burg EA (1966) The interaction between auxin and ethylene and its role in plant growth. Proc Natl Acad Sci USA 55: 262–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg SP, Burg EA (1968) Ethylene formation in pea seedlings; its relation to the inhibition of bud growth caused by indol-3-acetic acid. Plant Physiol 43: 1069–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick AV, Burg SP (1967) An explanation of the inhibition of root growth caused by indole-3-acetic acid. Plant Physiol 42: 415–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan H, Imaseki H, Wasteneys GO, Kazama H (2003) Ethylene stimulates endoreduplication but inhibits cytokinesis in cucumber hypocotyl epidermis. Plant Physiol 133: 1726–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Boer BGW, Murray JAH (2000) Control of plant growth and development through manipulation of cell cycle genes. Curr Opin Biotech 11: 138–145 [DOI] [PubMed] [Google Scholar]
- Drew MC, Jackson MB, Giffard SC (1979) Ethylene-promoted adventitious rooting and development of cortical air spaces (aerenchyma) in roots may be adaptive responses to flooding in Zea mays L. Planta 147: 83–86 [DOI] [PubMed] [Google Scholar]
- Drew MC, Jackson MB, Giffard SC, Campbell R (1981) Inhibition by silver ions of gas space (aerenchyma) formation in adventitious roots of Zea mays L subjected to exogenous ethylene or to oxygen deficiency. Planta 153: 217–224 [DOI] [PubMed] [Google Scholar]
- Ecker JR (1995) The ethylene signal transduction pathway in plants. Science 268: 667–675 [DOI] [PubMed] [Google Scholar]
- Eisinger WR, Burg SP (1972) Ethylene-induced pea internode swelling. Its relation to ribonucleic acid metabolism, wall protein synthesis, and cell wall structure. Plant Physiol 50: 510–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler M, Nadeau J, Sack FD (2000) Oriented asymmetric divisions that generate the stomatal spacing pattern in Arabidopsis are disrupted by the too many mouths mutation. Plant Cell 12: 2075–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendreau E, Orbovic V, Höfte H, Traas J (1999) Gibberellin and ethylene control endoreduplication levels in the Arabidopsis thaliana hypocotyl. Planta 209: 513–516 [DOI] [PubMed] [Google Scholar]
- Guzman P, Ecker JR (1990) Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2: 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RD, Riksenbruinsma T, Weyens G, Lefebvre M, Dunwell JM, Vantunen A, Krens FA (1997) Sugar beet guard cell protoplasts demonstrate a remarkable capacity for cell division enabling applications in stomatal physiology and molecular breeding. J Exp Bot 48: 255–263 [Google Scholar]
- Jackson MB, Drew MC, Giffard SC (1981) Effects of applying ethylene to the root system of Zea mays on growth and nutrient concentration in relation to flooding tolerance. Physiol Plant 52: 23–28 [Google Scholar]
- John PCL, Zhang K, Dong C, Diederich L, Wightman F (1993) P34(cdc2) related proteins in control of cell cycle progression, the switch between division and differentiation in tissue development, and stimulation of division by auxin and cytokinin. Aust J Plant Physiol 20: 503–526 [Google Scholar]
- Kazama H, Mineyuki Y (1997) Alteration of division polarity and preprophase band orientation in stomatogenesis by light. J Plant Res 110: 489–493 [Google Scholar]
- Kieber JJ (1997) The ethylene signal transduction pathway in Arabidopsis. J Exp Bot 48: 211–218 [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann K, Ecker JR (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of Raf family of protein kinases. Cell 72: 427–441 [DOI] [PubMed] [Google Scholar]
- Knight LI, Rose RC, Crocker W (1910) Effects of various gases and vapors upon etiolated seedlings of the sweet pea. Science 31: 635–636 [Google Scholar]
- Ku HS, Suge H, Rappaport L, Pratt HK (1970) Stimulation of rice coleoptile growth by ethylene. Planta 90: 333–339 [DOI] [PubMed] [Google Scholar]
- Lang JM, Eisinger WR, Green PB (1982) Effects of ethylene on the orientation of microtubules and cellulose microfibrils in pea epicotyl cells with polylamellate cell walls. Protoplasma 110: 5–14 [Google Scholar]
- Lehman A, Black R, Ecker JR (1996) Hookless1, an ethylene response gene, is required for differential cell elongation in the arabidopsis hypocotyl. Cell 85: 183–194 [DOI] [PubMed] [Google Scholar]
- Lev-Yadun S, Aloni R (1995) Differentiation of the ray system in woody plants. Bot Rev 61: 45–84 [Google Scholar]
- Lorbiecke R, Sauter M (1998) Induction of cell growth and cell division in the intercalary meristem of submerged and deepwater rice (Oryza sativa L.). Planta 204: 140–145 [Google Scholar]
- Lorbiecke R, Sauter M (1999) Adentitious root growth and cell-cycle induction in deepwater rice. Plant Physiol 119: 21–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métraux J-P, Kende H (1984) The cellular basis of the elongation response of submerged deep-water rice. Planta 160: 73–77 [DOI] [PubMed] [Google Scholar]
- Nadeau JA, Sack FD (2002) Control of Stomatal Distribution on the Arabidopsis Leaf Surface. Science 296: 1697–1700 [DOI] [PubMed] [Google Scholar]
- Neljubow D (1901) Ueber die horizontale nutation der stengel von Pisum sativum und einiger Anderer. Pflanzen Beih Bot Zentralbl 10: 128–139 [Google Scholar]
- Nishiwaki M, Fujino K, Koda Y, Masuda K, Kikuta Y (2000) Somatic embryogenesis induced by the simple application of abscisic acid to carrot (Daucus carota L.) seedlings in culture. Planta 211: 756–759 [DOI] [PubMed] [Google Scholar]
- Otegui M, Staehelin LA (2000) Cytokinesis in flowering plants: more than one way to divide a cell. Curr Opin Plant Biol 3: 493–502 [DOI] [PubMed] [Google Scholar]
- Pratt HK, Goeschl JD (1969) Physiological roles of ethylene in plants. Annu Rev Plant Physiol 20: 541–584 [Google Scholar]
- Raskin I, Kende H (1984) Role of gibberellin in the growth of deepwater rice. Plant Physiol 76: 947–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Watanabe A, Imaseki H (1976) Effect of ethylene on DNA synthesis in potato tuber discs. Plant Cell Physiol 17: 1255–1262 [Google Scholar]
- Sauter M, Kende H (1992) Gibberellin-induced growth and regulation of the cell division cycle in deepwater rice. Planta 188: 362–368 [DOI] [PubMed] [Google Scholar]
- Serna L, Fenoll C (1997) Tracing the ontogeny of stomatal clusters in arabidopsis with molecular markers. Plant J 12: 747–755 [DOI] [PubMed] [Google Scholar]
- Smalle J, Haegman M, Kurepa J, van Montagu M, van der Straeten D (1997) Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proc Natl Acad Sci USA 94: 2756–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LG, Gerttula SM, Han SC, Levy J (2001) TANGLED1: A microtubule binding protein required for the spatial control of cytokinesis in maize. J Cell Biol 152: 231–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K, Williamson RE, Wasteneys GO (2000) New techniques enable comparative analysis of microtubule orientation, wall texture and growth rate in intact roots of Arabidopsis thaliana. Plant Physiol 124: 1493–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto M, Roberts K, Dolan L (1995) Ethylene is a positive regulator of root hair development in Arabidopsis thaliana. Plant J 8: 943–948 [DOI] [PubMed] [Google Scholar]
- Wang H, Qi QG, Schorr P, Cutler AJ, Crosby WL, Fowke LC (1998) ICK1, a cyclin-dependent protein kinase inhibitor from Arabidopsis thaliana interacts with both cdc2a and cycd3, and its expression is induced by abscisic acid. Plant J 15: 501–510 [DOI] [PubMed] [Google Scholar]
- Zhao LM, Sack FD (1999) Ultrastructure of stomatal development in Arabidopsis (Brassicaceae) leaves. Am J Bot 86: 929–939 [PubMed] [Google Scholar]
- Zimmerman PW, Hitchcock AE (1933) Initiation and stimulation of adventitious roots caused by unsaturated hydrocarbon gases. Contrib Boyce Thompson Inst 5: 351–369 [Google Scholar]