Abstract
Invadopodia are dynamic protrusions in motile tumor cells whose function is to degrade extracellular matrix so that cells can enter into new environments. Invadopodia are specifically identified by microscopy as proteolytic invasive protrusions containing TKS5 and cortactin. The increasing complexity in models for the study of invadopodia, including engineered 3D environments, explants, or animal models in vivo, entails a higher level of microenvironment complexity as well as cancer cell heterogeneity. Such experimental setups are rich in information and offer the possibility of contextualizing invadopodia and other motility-related structures. That is, they hold the promise of revealing more realistic microenvironmental conditions under which the invadopodium assembles and functions or in which tumor cells switch to a different cellular phenotype (focal adhesion, lamellipodia, proliferation, and apoptosis). For such an effort, we need a systemic approach to microscopy, which will integrate information from multiple modalities. While the individual technologies needed to achieve this are mostly available, data integration and standardization is not a trivial process. In a systems microscopy approach, microscopy is used to extract information on cell phenotypes and the microenvironment while -omics technologies assess profiles of cancer cell and microenvironment genetic, transcription, translation, and protein makeups. Data are classified and linked via in silico modeling (including statistical and mathematical models and bioinformatics). Computational considerations create predictions to be validated experimentally by perturbing the system through use of genetic manipulations and molecular biology. With such a holistic approach, a deeper understanding of function of invadopodia in vivo will be reached, opening the potential for personalized diagnostics and therapies.
Keywords: metastasis, invadopodia, intravasation, microenvironment, motility, invasion, microscopy, systems microscopy, machine learning, mathematical modeling, holistic approach
Introduction
Invadopodia are extracellular matrix (ECM)-degrading membrane protrusions1 present in invasive/motile cancer cells and used to degrade components of extracellular matrix in order to cross into new environments.2 Invadopodia were identified in a number of invasive (solid) cancer cell lines, such as breast, head and neck, gastric, prostate, fibrosarcoma, and melanoma.3 Recent evidence has demonstrated direct molecular links between invadopodium assembly and metastasis in mouse models4 and human patients.5 As metastasis is the primary reason for mortality of cancer patients and current chemotherapeutics primarily target proliferation, research into possible ways of targeting invadopodium is of the essence. Signaling circuitry of the invadopodium involves > 50 structural and regulatory proteins,1 many of them kinases and proteases amenable for inhibition by clinically approved drugs.
Under physiological conditions, a similar type of protrusion, podosomes, occur in monocytic cells2,6 (e.g., macrophages, osteoclasts), endothelial cells,7 and immature dendritic cells.8 In addition, using developmental models, protrusions similar to invadopodia were recently characterized in motile cells of neural crest9 and intestine of zebrafish,10 as well as during organogenesis of C. elegans11 (in anchor cell) and in urochodates.12 While the general function of enabling migration across obstacles may arise in many species and under various conditions (development, inflammation, and cancer), there are clear differences in signaling and dynamics between invadopodia, podosomes, and invasive protrusions present during development. As the level of evolutionary similarity is unclear for now, translating conclusions between models is challenging and a direct study of invadopodia in tumors is irreplaceable for the development of new treatments.
In this commentary we will identify some of the challenges of studying invadopodium formation in the context of their natural environment and propose disciplinary integration as one of the needed directions for the future.
The Importance of Microscopy in the Study of Invadopodia
Accumulating knowledge on molecular mechanisms of invadopodium assembly continuously adds to its complexity, as well as its challenging identification. We now know that invadopodia are not necessarily unique in their ECM-degrading function or enrichment in structural proteins compared with other motility-related compartments. Cortactin, a structural protein enriched in invadopodia, is also enriched within lamellipodia and within endosomal vesicles,13 making it essential but not a specific marker for invadopodia. In addition, ECM degradation cannot be used as a unique marker either, as focal adhesions in cancer cells also degrade ECM, though to a smaller extent.14 The importance of using multiple markers in invadopodium identification was heightened with the shift from 2D to 3D experiments15 and studies in complex environments,16 due to the heterogeneity of cell shapes and the lack of ventral orientation of invadopodia standardly seen in 2D, whereas invadopodia are seen at the tips of leading edge protrusions in 3D.17 In summary, only a combination of morphology, structure, and functionality confirms invadopodia, and this can only be done by including direct visualization of cells via multicolor microscopy.
Another reason for using microscopy in the study of invadopodia is the need for time-resolved measurements. Such a need exists due to the fact that the invadopodium is a dynamic structure. It was shown that assembly of invadopodia is a reversible process, and even when all initial structural components are in place, not all invadopodia will have the same lifetime or reach the fully functional stage.18 As a result, not all invadopodia fully mature to degrade ECM. To make a distinction between mature, fully functional invadopodia and those that disassemble prior to reaching the functional stage, the latter were termed invadopodium precursors.1,18 Details of reversibility and conditions under which reversibility of the invadopodium precursors takes place in vivo are not clear as of yet and they may be determined by local concentrations of ligands in the microenvironment outside the cell, neighboring cells, or even stochastic noise in gene expression within the cell. In contrast, invadopodia, which do reach maturity and degrade ECM, are likely to disassemble following the degradation. In either of these cases, the invadopodium is under a constant change and it is not a permanent phenotype within a cell. Examples of changes among motility-related cellular phenotypes, or “phenotypic switches,” were previously shown by artificially inducing permanent changes in gene expression, although it is likely such changes occur dynamically within a cell throughout its life cycle. One such study demonstrated that FAK levels could switch between focal adhesion and invadopodium production in motile breast cancer cells.19 Further, depletion of individual WASP-family proteins, which can activate actin polymerization, WAVE2, or N-WASP, selectively eliminates lamellipodia or invadopodia.20 To take such thinking further, phenotype switching may also lead the cell to exit motility altogether and enter phenotypes of mitosis, apoptosis, or senescence, an example of which may be found in go-or-grow model, which suggests an existence of a switch between cell proliferation and migration.21
Challenges of Spatial and Temporal Heterogeneity
As mentioned above, directed by the growing amount of evidence that cell microenvironments can dominate cell behavior, many studies of motility in cancer are now based on more complex, 3D environments, which contain multiple ECM components and multiple cell types or they utilize in vivo animal models. The main challenge of such approaches is the introduction of new levels of heterogeneity, such as heterogeneity of microenvironments surrounding cancer cells and (epi)genetic heterogeneity of cancer cells.
Heterogeneity and complexity of microenvironments
Use of purified ECM components, such as collagen I or fibronectin, and abundance of growth factors in the cell culture medium, offers simple and mostly homogeneous environment for cancer cells but cannot always emulate all the intricacies or behaviors detected in tissues. The introduction of more complex experimental models, including cell spheroids,22 co-cultures, organotypic cultures,23 tissue explants, engineered complex environments,24 and in vivo models, assumes a spatial and temporal heterogeneity of cell environment, and hence, may lead to variable cell behaviors. Spatially, the local concentrations of growth factors, chemokines, and ECM-components that each tumor cell encounters are different, and temporally, they change as cells move or degrade the ECM. For example, tumor cells in a necrotic center, where microenvironment is more hypoxic, behave differently than those in the proliferating edge.22 Next, direct contact with microenviroment components such as macrophages25 or ECM26 can change tumor cell behavior.
Finally, the phenotype of each cancer cell is a combined effect of several fluctuating signals from the surroundings,27 including signals from other cancer cells. Hence, to understand spatio-temporal context of invadopodia and other motility-related structures in complex environments, it is important to study in concert multiple microenvironment parameters and link these to invadopodia and/or other present phenotypes. For such a study to be successful, a large amount of data from different modalities needs to be collected, simultaneously or sequentially, and in turn, processed using machine-learning and statistical modeling techniques. In in vitro models, standard imaging technologies such as confocal microscopy can be used while microenvironmental conditions are easily controlled, making the systematic analysis of multiple parameters straight forward. In in vivo models, recent advances in multiparametric monitoring of microenvironment at high-resolution28-30 are paving the way for direct observations. While it is very challenging to control the in vivo experimental conditions, a natural heterogeneity of animal models can be used to our benefit, as it inherently provides a wide range of micronvironmental conditions.
Heterogeneity of cancer cells and the use of -omics
Depending on the experimental model and the scale of the question (molecular, cellular, tissue, whole animal, and population), researchers are also challenged with cancer cells’ heterogeneity, either genetically encoded or epigenetic (in response to the microenvironment). Genetic and epigenetic heterogeneity translate to phenotypic and functional heterogeneities. In cell cultures, we can create homogeneous environments and avoid epigenetic heterogeneity, but due to the high genomic instability (ie. high mutation rate) in cancer, even clonal cell lines slowly change their genetic profiles over time, causing inter-cellular heterogeneity. When the cell lines are injected into animals to create xenograft tumors, additional epigenetic heterogeneity is unavoidable. Cell–cell and cell–ECM interactions create unique microenvironments, which are also highly heterogeneous, inducing additional fluctuations in gene expression and modifications in (post-)transcriptional or translational profiles. Many imaging studies nowadays use transgenic mouse models, which have a full repertoire of immune cells and form spontaneous tumors, exhibiting all tumor progression stages.28,30 Use of transgenic animals introduces additional intra-tumor (subclonal) heterogeneity as a result of branched tumor evolution.31 Finally, heterotransplants of primary cell lines or patient tissue in animals exhibit yet another level of heterogeneity, intra-tumoral, originating in diverse genetic backgrounds of the patients. The build-up of different levels of heterogeneity may lead to experimental outcomes different or opposite from intuition, sometimes even cases where all major components of the system are well-described.32
Interestingly, even with all the levels of heterogeneity that we observe in cancer, the number of cell phenotypes we detect, both in culture models and primary tumors, is limited, and the underlying mechanisms of cell motility and proliferation are conserved. This means that even with infinite combinations of chemical and mechanical signals at different levels, there are only a few phenotypes that cancer cells will assume throughout their life cycle. In a similar fashion, functions of all known driver genes (~140 altered by mutations)33 funnel into 12 signaling pathways, which control cell fate, cell survival, and genome maintenance.33 In summary, these observations suggest that the vast complexity has an underlying order and rules we are yet to discover.
So far, we were focusing on studies of individual signals or phenotypes, in a reductionist manner. But what if we were to look at cell phenotypes in the context of their immediate microenvironments in a holistic manner? That is, determining the combined effect of all observed microenvironmental parameters on cell phenotypic fate. What are the microenvironmental conditions under which invadopodia assemble and function in complex environments and/or in vivo? Ability to link cell phenotypes, including invadopodia, to the cell’s microenvironment as well as to the genetic profile, transcriptome, and proteome using RNA and DNA sequencing or mass spectrometry, all on small samples or single cells, will bring us closer to the understanding of context-specificity of cellular phenotypes, and possibly, to the rules of cellular decision-making. Inclusion of advanced statistical models for data analysis or mathematical modeling has a great potential to offer us with more mechanistic insights and many holistic/systemic approaches are quickly becoming standard in the fields of development and neuroscience. With such insights, we may be able to predict and potentially control invadopodium assembly and function. Based on the evidence we have so far on the link of invadopodia to metastasis,1,4,5 identifying new targets in invadopodium assembly or targeting their switch into other cellular phenotypes (adhesion, migration, cell division, and apoptosis) may be an ideal strategy of preventing cancer progression.
Next we will explore how systems-level approaches can be integrated with the imaging studies of tumor cell motility and invasion.
Systems Microscopy—A Holistic Approach to Invadopodium Function, Motility, and Invasion
As portrayed in Figure 1, “systems microscopy” of motile cells is defined here as the intersection of four different fields of research with overlapping roles in creating hypotheses, constructing experimental design and data interpretation. Initial observations that initiate research commonly come from the classical molecular and cell biology. As previously described, microscopy is essential for phenotype characterization in motile cells and its development continuously yields new breakthroughs in cell phenotype research. Images gathered in vivo provide both phenomenology which leads to engineering more precise, complex in vitro models as well as offers medium-throughput acquisition at single-cell resolution. Sequencing and other -omics technologies provide (epi)genetic cell profiles and the link between tumor cells phenotype and microenvironment characteristics. Finally, in silico modeling offers integration of different levels of information. Statistical modeling and bioinformatics handles analysis of high-throughput data, while mathematical modeling (multiscale, dynamical systems, etc.) are an ideal tool for abstracting fundamental processes and developing predictions of cellular behavior. The computational and theoretical considerations can further be used to create and support new hypotheses that are further validated experimentally.
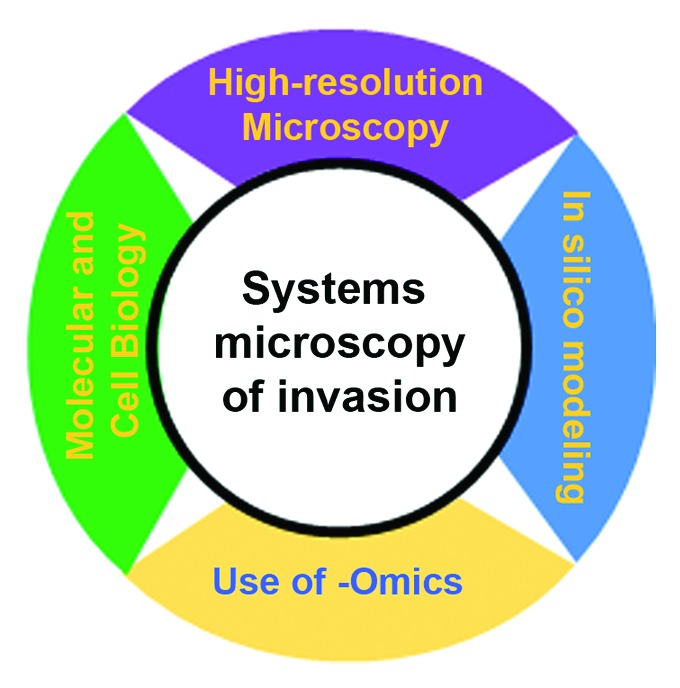
Figure 1. Proposed workflow for systems microscopy of invadopodia, cell motility, and invasion.
Although the emergence of systems microscopy has been predicted a few years ago,34 due to the numerous challenges in data standardization and integration, and the need for work in large teams, creating a modular platform encompassing four disciplines may take another decade. However, the process is underway and the advantages can be demonstrated by several studies, which incorporate systems-level approaches to imaging and cell biology of invadopodia and motility:
In the last decade, Condeelis group has published a series of studies establishing the link between in vivo tumor cell phenotypes present in metastatic tumors and the underlying genotypes, making the first steps toward the molecular mechanism of tumor cell motility in vivo.35-38 This was done by a combination of intravital multiphoton microscopy, in vivo collection of migratory cells, as well as genomics to dissect phenotypes of directional migration in single cells35-37 and multicellular streams.38 Gene expression signatures of migratory cells which accompanied the changes in cell phenotypes were conserved in a number of animal models, including xenografts and patient transplants, and were further demonstrated to be predictors of clinical outcome.37
The Courtneidge group has devised a high-content screening assay for activators and inhibitors of invadopodium formation.39 They labeled and imaged actin and nuclei in cancer cells; images were then processed automatically for a series of parameters, including size, morphology, and number of nuclei and invadopodia. Compounds that modify invadopodium number without inducing cytotoxicity were chosen and validated, leading to the discovery of Cdk family role in invadopodium and adhesion formation.
Weaver and colleagues have recently found that PI3K and PICα, interacting regulators of cytoskeleton, control focal adhesion transition to invadopodia. To reach this conclusion, they have used integration of cluster analysis of primary tumors, literature-mining-based network analysis, and microscopy of cancer cell lines with different PI3K mutants.40
In collaboration with Quaranta and Anderson groups, Weaver et al. have also made steps toward the integrated cell-decision model. In particular, they have combined microscopy-based measurements of ECM degradation, proliferation, and cell velocities with mathematical models of cell phenotypic changes and game theory modeling, putting an evolutionary spin on tumor progression.41 Their study suggests that the invasiveness of cancer cells increases as their proliferation and migration evolves to be less dependent on perturbations in microenvironmental parameters such as oxygen, nutrients, chemokines, free space, and ECM architecture.
Lauffenburger’s group has published a series of systems-level studies on how the balance among multiple parameters, both internal (e.g., receptor density) as well as environmental (matrix stiffness, composition, etc.) affects cell phenotype. Using computational modeling of cancer cell motility integrated with speed and force measurements, they have shown that migration speed is a balancing equation between drag force (dependent on matrix stiffness), adhesive forces (dependent on number of adhesion receptors), and protrusive forces, also unequivocally demonstrating different mechanisms of migration in 2D and 3D environments.42,43
Sahai’s lab has taken the approach of mathematically modeling different modes (phenotypes) of migratory cells and how they are controlled by matrix geometry. They made use of agent-based modeling to correctly predict the effect of different combinations of kinase inhibitors and integrin depletion both in cell culture and in vivo, which was demonstrated using intravital imaging in mouse model.44
These studies demonstrate feasibility of integration between microscopy and computational considerations, such as mathematical modeling or network analysis, demonstrating that systems-level studies hold promise to generating novel insights and hypotheses. For further reading, a recent review covers use of engineering in developing novel biomaterials and in multi-scale modeling of invasion.24 In addition to these subjects, a number of technologies, platforms, and databases that were developed for both invasion and other applications (development, neuroscience) may in the future be essential for augmenting classical biology studies of invadopodia and motility into integrative, systems-level research. For example, great effort has been invested to automate image acquisition, annotation, processing, and analysis in live cells or whole embryos. Some of the most notable work has been done on development of automated RNAi and drug screening techniques.45 Technology for automated dynamic proteomics includes fluorescent tagging of > 1000 proteins from the library to asses heterogeneity and dynamics of drug response in individual cancer cells. Next, algorithms such as Focal Adhesion Analysis Software (FAAS), for multi-parametric, high-throughput analysis, tracking, and quantification of focal adhesions46or Cellcognition,47 for phenotypic classification using machine-learning and Hidden Markov Models (HMM), were recently produced. The emerging field of Bioimage informatics (or image-based systems biology), with overview of useful software tools and databases has been recently covered in Nature Methods.48 Intravital microscopy has taken steps toward higher imaging throughput,28 ability to image abdominal organs,49 and do immunofluorescent procedures in vivo.50 Moreover, we can now relocate tumor microenvironments using internal landmarks,51 photo-tatooing,52 or photoconversion,53,54 which allows for longitudinal studies, correlative histology, and may be used to link genotype/transcriptome and phenotype in the future experimental pipelines. To that end, transcriptomics can now be done on single cell level.55
Finally, a data-mining approach directly applicable to the field of cell motility recently led to assembling the integrin adhesome network, revealing separate functional subnets in integrin control of cytoskeletal organization, adhesion, and migration.56
The biggest challenge for the future is to create a universal platform which can cover all sub-disciplines. One of the technical problems we are facing is how to standardize the interface between the fields of inquiry, allowing an easy connection between machine learning, stochastic modeling, agent-based modeling, as well as mathematical models at cellular, and population levels. While crossing modalities and analyzing microscopy images has become pretty standard due to the efforts of Open Microscopy Consortium (OME, https://www.openmicroscopy.org/site), in analyzing -omics data, a special care needs to be taken that data are abstracted in a proper manner, preserving the information for the potentially unknown, new ways to probe the source of data. Further challenges involve educating the next generation of young scientists in biology, microscopy, as well as quantitative approaches, and building infrastructure, which houses both microscopy and -omics equipment.
All developments discussed lead us toward integration of molecular and cell biology and microscopy with the use of complex environments (both engineered and in vivo) and computational considerations. We will next illustrate how systems microscopy approach may contribute to both the basic and translational research of invadopodia and cancer cell motility.
What the Future Might Bring
The spatio-temporal context of invadopodia and other motility-related structures in complex environments is essential in order to deepen our understanding of cancer cell motility process. Information on microenvironmental parameters, which may catalyze or simply allow presence of different phenotypes or phenotypic switching, will allow us to move the focus from the differences (between lamellipodia, invadopodia, podosomes, adhesions, etc.) to the universalities and commonalities of motility-related structures. Due to the abundance of questions we have about motility, most studies tend to focus on individual compartments in the cell. However, all motility-related structures are a part of the same continuous motility cycle, and apparent differences actually originate from the momentary communication of the cell with the surrounding environment. Viewing the entire motility cycle holistically may help us to derive the underlying principles of cell decision-making. This would open up the possibility of predicting cellular behavior for any range of local conditions, by data-fitting into statistical models and machine-learning classifications or abstracting essential components of the process into mechanistic models. Ideally, such efforts may end in a unified cellular-decision model.
Another important avenue in which invadopodia contextualization is of essence is a direct application to early diagnosis of breast cancer metastasis, and in turn, development of new metastasis treatments. Due to the small size of invadopodia and a lack of specific molecular markers, which would undoubtedly point to their location, screening for invadopodia in complex environments such as the animal or human tissue sections is a challenging task. Monitoring and understanding the spatio-temporal contexts in which invadopodia are likely to be present will make invadopodium localization and quantification in vivo possible. Going further, correlating invadopodium appearance with metastatic potential in patients would allow for development of personalized treatments. For example, based on the increasing counts of invadopodia in biopsies, we will be able to predict the timing of the peak counts and determine when is best to treat patients with invadopodium-targeting drugs.57 Such an approach may greatly help in reducing off-target effects and immune system perturbations. On the more speculative side, our increased capacity to characterize tumor microenvironments and draw their limits may result in ability to target individual microenvironments in localized treatments.
To that end, systems microscopy will not only contribute to the understanding of basic biological processes, but also brings us closer to individualized medicine.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Gligorijevic B is funded by NIH 1K99CA172360 and Condeelis J by CA100324 and CA1664468, latter shared with Bergman A.
References
- 1.Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12:413–26. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–17. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Monsky WL, Lin CY, Aoyama A, Kelly T, Akiyama SK, Mueller SC, Chen WT. A potential marker protease of invasiveness, seprase, is localized on invadopodia of human malignant melanoma cells. Cancer Res. 1994;54:5702–10. [PubMed] [Google Scholar]
- 4.Gligorijevic B, Wyckoff J, Yamaguchi H, Wang Y, Roussos ET, Condeelis J. N-WASP-mediated invadopodium formation is involved in intravasation and lung metastasis of mammary tumors. J Cell Sci. 2012;125:724–34. doi: 10.1242/jcs.092726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, Yang J. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. 2011;19:372–86. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oser M, Dovas A, Cox D, Condeelis J. Nck1 and Grb2 localization patterns can distinguish invadopodia from podosomes. Eur J Cell Biol. 2011;90:181–8. doi: 10.1016/j.ejcb.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varon C, Tatin F, Moreau V, Van Obberghen-Schilling E, Fernandez-Sauze S, Reuzeau E, Kramer I, Génot E. Transforming growth factor β induces rosettes of podosomes in primary aortic endothelial cells. Mol Cell Biol. 2006;26:3582–94. doi: 10.1128/MCB.26.9.3582-3594.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns S, Thrasher AJ, Blundell MP, Machesky L, Jones GE. Configuration of human dendritic cell cytoskeleton by Rho GTPases, the WAS protein, and differentiation. Blood. 2001;98:1142–9. doi: 10.1182/blood.V98.4.1142. [DOI] [PubMed] [Google Scholar]
- 9.Murphy DA, Diaz B, Bromann PA, Tsai JH, Kawakami Y, Maurer J, Stewart RA, Izpisúa-Belmonte JC, Courtneidge SAA. A Src-Tks5 pathway is required for neural crest cell migration during embryonic development. PLoS One. 2011;6:e22499. doi: 10.1371/journal.pone.0022499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seiler C, Davuluri G, Abrams J, Byfield FJ, Janmey PA, Pack M. Smooth muscle tension induces invasive remodeling of the zebrafish intestine. PLoS Biol. 2012;10:e1001386. doi: 10.1371/journal.pbio.1001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagedorn EJ, Yashiro H, Ziel JW, Ihara S, Wang Z, Sherwood DR, Sherwood DR. Integrin acts upstream of netrin signaling to regulate formation of the anchor cell’s invasive membrane in C. elegans. Dev Cell. 2009;17:187–98. doi: 10.1016/j.devcel.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooley J, Whitaker S, Sweeney S, Fraser S, Davidson B. Cytoskeletal polarity mediates localized induction of the heart progenitor lineage. Nat Cell Biol. 2011;13:952–7. doi: 10.1038/ncb2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaksonen M, Peng HB, Rauvala H. Association of cortactin with dynamic actin in lamellipodia and on endosomal vesicles. J Cell Sci. 2000;113:4421–6. doi: 10.1242/jcs.113.24.4421. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, McNiven MA. Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAK-p130Cas complex. J Cell Biol. 2012;196:375–85. doi: 10.1083/jcb.201105153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoumacher M, Goldman RD, Louvard D, Vignjevic DM. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J Cell Biol. 2010;189:541–56. doi: 10.1083/jcb.200909113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tolde O, Rösel D, Veselý P, Folk P, Brábek J. The structure of invadopodia in a complex 3D environment. Eur J Cell Biol. 2010;89:674–80. doi: 10.1016/j.ejcb.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Magalhaes MA, Larson DR, Mader CC, Bravo-Cordero JJ, Gil-Henn H, Oser M, Chen X, Koleske AJ, Condeelis J. Cortactin phosphorylation regulates cell invasion through a pH-dependent pathway. J Cell Biol. 2011;195:903–20. doi: 10.1083/jcb.201103045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oser M, Yamaguchi H, Mader CC, Bravo-Cordero JJ, Arias M, Chen X, Desmarais V, van Rheenen J, Koleske AJ, Condeelis J. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J Cell Biol. 2009;186:571–87. doi: 10.1083/jcb.200812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan KT, Cortesio CL, Huttenlocher A. FAK alters invadopodia and focal adhesion composition and dynamics to regulate breast cancer invasion. J Cell Biol. 2009;185:357–70. doi: 10.1083/jcb.200809110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarmiento C, Wang W, Dovas A, Yamaguchi H, Sidani M, El-Sibai M, Desmarais V, Holman HA, Kitchen S, Backer JM, et al. WASP family members and formin proteins coordinate regulation of cell protrusions in carcinoma cells. J Cell Biol. 2008;180:1245–60. doi: 10.1083/jcb.200708123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gil-Henn H, Patsialou A, Wang Y, Warren MS, Condeelis JS, Koleske AJ. Arg/Abl2 promotes invasion and attenuates proliferation of breast cancer in vivo. Oncogene. 2013;32:2622–30. doi: 10.1038/onc.2012.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alessandri K, Sarangi BR, Gurchenkov VV, Sinha B, Kießling TR, Fetler L, Rico F, Scheuring S, Lamaze C, Simon A, et al. Cellular capsules as a tool for multicellular spheroid production and for investigating the mechanics of tumor progression in vitro. Proc Natl Acad Sci U S A. 2013;110:14843–8. doi: 10.1073/pnas.1309482110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 24.Zaman MH. The role of engineering approaches in analysing cancer invasion and metastasis. Nat Rev Cancer. 2013;13:596–603. doi: 10.1038/nrc3564. [DOI] [PubMed] [Google Scholar]
- 25.Roh-Johnson M, Bravo-Cordero JJ, Patsialou A, Sharma VP, Guo P, Liu H, Hodgson L, Condeelis J. Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene. 2013;1:1–10. doi: 10.1038/onc.2013.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leight JL, Wozniak MA, Chen S, Lynch ML, Chen CS. Matrix rigidity regulates a switch between TGF-β1-induced apoptosis and epithelial-mesenchymal transition. Mol Biol Cell. 2012;23:781–91. doi: 10.1091/mbc.E11-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim BJ, Hannanta-anan P, Chau M, Kim YS, Swartz MA, Wu M. Cooperative roles of SDF-1α and EGF gradients on tumor cell migration revealed by a robust 3D microfluidic model. PLoS One. 2013;8:e68422. doi: 10.1371/journal.pone.0068422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egeblad M, Ewald AJ, Askautrud HA, Truitt ML, Welm BE, Bainbridge E, Peeters G, Krummel MF, Werb Z. Visualizing stromal cell dynamics in different tumor microenvironments by spinning disk confocal microscopy. Dis Model Mech. 2008;1:155–67, discussion 165. doi: 10.1242/dmm.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andresen V, Alexander S, Heupel WM, Hirschberg M, Hoffman RM, Friedl P. Infrared multiphoton microscopy: subcellular-resolved deep tissue imaging. Curr Opin Biotechnol. 2009;20:54–62. doi: 10.1016/j.copbio.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Entenberg D, Wyckoff J, Gligorijevic B, Roussos ET, Verkhusha VV, Pollard JW, Condeelis J. Setup and use of a two-laser multiphoton microscope for multichannel intravital fluorescence imaging. Nat Protoc. 2011;6:1500–20. doi: 10.1038/nprot.2011.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah SP, Morin RD, Khattra J, Prentice L, Pugh T, Burleigh A, Delaney A, Gelmon K, Guliany R, Senz J, et al. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature. 2009;461:809–13. doi: 10.1038/nature08489. [DOI] [PubMed] [Google Scholar]
- 32.Zhao B, Pritchard JR, Lauffenburger DA, Hemann MT. Addressing Genetic Tumor Heterogeneity through Computationally Predictive Combination Therapy. Cancer Discov. 2014;4:166–74. doi: 10.1158/2159-8290.CD-13-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr., Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–58. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Dévédec SE, Yan K, de Bont H, Ghotra V, Truong H, Danen EH, Verbeek F, van de Water B. Systems microscopy approaches to understand cancer cell migration and metastasis. Cell Mol Life Sci. 2010;67:3219–40. doi: 10.1007/s00018-010-0419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang W, Wyckoff JB, Frohlich VC, Oleynikov Y, Hüttelmaier S, Zavadil J, Cermak L, Bottinger EP, Singer RH, White JG, et al. Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular profiling. Cancer Res. 2002;62:6278–88. [PubMed] [Google Scholar]
- 36.Wang W, Goswami S, Lapidus K, Wells AL, Wyckoff JB, Sahai E, Singer RH, Segall JE, Condeelis JS. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res. 2004;64:8585–94. doi: 10.1158/0008-5472.CAN-04-1136. [DOI] [PubMed] [Google Scholar]
- 37.Patsialou A, Wang Y, Lin J, Whitney K, Goswami S, Kenny PA, Condeelis JS. Selective gene-expression profiling of migratory tumor cells in vivo predicts clinical outcome in breast cancer patients. Breast Cancer Res. 2012;14:R139. doi: 10.1186/bcr3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patsialou A, Bravo-Cordero JJ, Wang Y, Entenberg D, Liu H, Clarke M, Condeelis JS. Intravital multiphoton imaging reveals multicellular streaming as a crucial component of in vivo cell migration in human breast tumors. IntraVital. 2013;2:e25294. doi: 10.4161/intv.25294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quintavalle M, Elia L, Price JH, Heynen-Genel S, Courtneidge SA. A cell-based high-content screening assay reveals activators and inhibitors of cancer cell invasion. Sci Signal. 2011;4:ra49. doi: 10.1126/scisignal.2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoshino D, Jourquin J, Emmons SW, Miller T, Goldgof M, Costello K, Tyson DR, Brown B, Lu Y, Prasad NK, et al. Network analysis of the focal adhesion to invadopodia transition identifies a PI3K-PKCα invasive signaling axis. Sci Signal. 2012;5:ra66. doi: 10.1126/scisignal.2002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson ARA, Hassanein M, Branch KM, Lu J, Lobdell NA, Maier J, Basanta D, Weidow B, Narasanna A, Arteaga CL, et al. Microenvironmental independence associated with tumor progression. Cancer Res. 2009;69:8797–806. doi: 10.1158/0008-5472.CAN-09-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zaman MH, Trapani LM, Sieminski AL, Mackellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci U S A. 2006;103:10889–94. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer AS, Hughes-Alford SK, Kay JE, Castillo A, Wells A, Gertler FB, Lauffenburger DA. 2D protrusion but not motility predicts growth factor-induced cancer cell migration in 3D collagen. J Cell Biol. 2012;197:721–9. doi: 10.1083/jcb.201201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tozluoğlu M, Tournier AL, Jenkins RP, Hooper S, Bates PA, Sahai E. Matrix geometry determines optimal cancer cell migration strategy and modulates response to interventions. Nat Cell Biol. 2013;15:751–62. doi: 10.1038/ncb2775. [DOI] [PubMed] [Google Scholar]
- 45.Ghotra VPS, He S, de Bont H, van der Ent W, Spaink HP, van de Water B, Snaar-Jagalska BE, Danen EH. Automated whole animal bio-imaging assay for human cancer dissemination. PLoS One. 2012;7:e31281. doi: 10.1371/journal.pone.0031281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berginski ME, Vitriol EA, Hahn KM, Gomez SM. High-resolution quantification of focal adhesion spatiotemporal dynamics in living cells. PLoS One. 2011;6:e22025. doi: 10.1371/journal.pone.0022025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Held M, Schmitz MH, Fischer B, Walter T, Neumann B, Olma MH, Peter M, Ellenberg J, Gerlich DW. CellCognition: time-resolved phenotype annotation in high-throughput live cell imaging. Nat Methods. 2010;7:747–54. doi: 10.1038/nmeth.1486. [DOI] [PubMed] [Google Scholar]
- 48.Focus on Bioimage Informatics. Nat Methods. 2012;9:627–763. doi: 10.1038/nmeth.2102. [DOI] [PubMed] [Google Scholar]
- 49.Ritsma L, Steller EJA, Beerling E, Loomans CJM, Zomer A, Gerlach C, Vrisekoop N, Seinstra D, van Gurp L, Schäfer R, et al. Intravital microscopy through an abdominal imaging window reveals a pre-micrometastasis stage during liver metastasis. Sci Transl Med. 2012;4:ra145. doi: 10.1126/scitranslmed.3004394. [DOI] [PubMed] [Google Scholar]
- 50.Kilarski WW, Güç E, Teo JCM, Oliver SR, Lund AW, Swartz MA. Intravital immunofluorescence for visualizing the microcirculatory and immune microenvironments in the mouse ear dermis. PLoS One. 2013;8:e57135. doi: 10.1371/journal.pone.0057135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WEF, Goldbrunner R, Herms J, Winkler F. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010;16:116–22. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- 52.Ritsma L, Vrisekoop N, van Rheenen J. In vivo imaging and histochemistry are combined in the cryosection labelling and intravital microscopy technique. Nat Commun. 2013;4:2366–70. doi: 10.1038/ncomms3366. [DOI] [PubMed] [Google Scholar]
- 53.Kedrin D, Gligorijevic B, Wyckoff J, Verkhusha VV, Condeelis J, Segall JE, van Rheenen J. Intravital imaging of metastatic behavior through a mammary imaging window. Nat Methods. 2008;5:1019–21. doi: 10.1038/nmeth.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gligorijevic B, Condeelis J. Stretching the timescale of intravital imaging in tumors. Cell Adh Migr. 2009;3:313–5. doi: 10.4161/cam.3.4.9581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu AR, Neff NF, Kalisky T, Dalerba P, Treutlein B, Rothenberg ME, Mburu FM, Mantalas GL, Sim S, Clarke MF, et al. Quantitative assessment of single-cell RNA-sequencing methods. Nat Methods. 2014;11:41–6. doi: 10.1038/nmeth.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–67. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eckert MA, Yang J. Targeting invadopodia to block breast cancer metastasis. Oncotarget. 2011;2:562–8. doi: 10.18632/oncotarget.301. [DOI] [PMC free article] [PubMed] [Google Scholar]


