Abstract
Transient cerebral ischemia leads to endoplasmic reticulum (ER) stress. However, the contributions of ER stress to cerebral ischemia are not clear. To address this issue, the ER stress activators tunicamycin (TM) and thapsigargin (TG) were administered to transient middle cerebral artery occluded (tMCAO) mice and oxygen-glucose deprivation-reperfusion (OGD-Rep.)-treated neurons. Both TM and TG showed significant protection against ischemia-induced brain injury, as revealed by reduced brain infarct volume and increased glucose uptake rate in ischemic tissue. In OGD-Rep.-treated neurons, 4-PBA, the ER stress releasing mechanism, counteracted the neuronal protection of TM and TG, which also supports a protective role of ER stress in transient brain ischemia. Knocking down the ER stress sensor Eif2s1, which is further activated by TM and TG, reduced the OGD-Rep.-induced neuronal cell death. In addition, both TM and TG prevented PARK2 loss, promoted its recruitment to mitochondria, and activated mitophagy during reperfusion after ischemia. The neuroprotection of TM and TG was reversed by autophagy inhibition (3-methyladenine and Atg7 knockdown) as well as Park2 silencing. The neuroprotection was also diminished in Park2+/− mice. Moreover, Eif2s1 and downstream Atf4 silencing reduced PARK2 expression, impaired mitophagy induction, and counteracted the neuroprotection. Taken together, the present investigation demonstrates that the ER stress induced by TM and TG protects against the transient ischemic brain injury. The PARK2-mediated mitophagy may be underlying the protection of ER stress. These findings may provide a new strategy to rescue ischemic brains by inducing mitophagy through ER stress activation.
Keywords: cerebral ischemia, endoplasmic reticulum stress, mitophagy, neuroprotection, PARK2
Introduction
Focal cerebral ischemia is one of the leading causes of mortality worldwide with limited therapeutic approaches. Cerebral ischemia disrupts the endoplasmic reticulum (ER) function in neuronal cells, which is known as ER stress.1,2 The contribution of ER stress to ischemic brain injury is still not conclusive. ER stress may ultimately leads to neuronal cell death by inducing calcium overload,3 protein synthesis cessation,4 and apoptosis.5 Reagents that ameliorate ischemia-induced ER stress have a protective effect on ischemic brain,6,7 implying that ER stress could be a crucial mechanism responsible for ischemic brain injury.4
On the other hand, ER stress activates an adaptive program termed unfolded protein response to deal with the accumulated malfolded proteins and thus relieve the cerebral ischemia-induced ER dysfunction.8,9 These responses suppress protein translation and upregulate ER chaperones, which are sensed by PKR-like EIF2AK3/eIF2α kinase/PERK (eukaryotic translation initiation factor 2-α kinase 3), EIF2S1 (eukaryotic initiation factor 2, subunit 1 α), ERN1/IRE1 (endoplasmic reticulum to nucleus signaling 1) and XBP1 (X-box binding protein 1) signaling, respectively.10 Manipulations of these ER stress sensors altered cell vulnerability to injury.11,12 A recent study has shown that pretreatment with ER stressors limits ischemia-induced cardiocyte injury.13 Therefore, it is very likely that ER stress may protect against ischemia-induced brain damage, although the underlying mechanisms remain unclear.1
Autophagy is a bulk degradation process which removes intracellular organelles and proteins in a lysosome-dependent manner. ER is closely associated with autophagy, since ER is proposed to be the potential membrane source of autophagosomes,14 and provides calcium signaling for autophagy induction.15,16 Moreover, a variety of studies indicates that autophagy compensates for disposal of accumulated proteins result from ER stress,17-19 suggesting that ER stress may be a trigger for autophagy induction. In the context of cerebral ischemia, the role of autophagy is under debate. Although several studies have proposed its detrimental roles,20,21 our recent findings suggest that autophagy may have different actions in permanent and transient focal ischemia.22 The circulation restored after ischemia may be a critical determinant for switching the actions of autophagy from detrimental to protective. In particular, we found that in the reperfusional phase after ischemia, the damaged mitochondria are cleared by autophagy-related mechanisms, which are termed mitophagy, and consequently reduced mitochondria-dependent neuronal cell apoptosis.22 This study suggests a neuroprotective role of mitophagy and implies that activation of mitophagy may be a potential therapeutic strategy against transient cerebral ischemia.
Therefore, in the present study, we hypothesize that ER stress might protect neurons against ischemia-reperfusion-induced injury through activation of mitophagy. We further investigate the mechanisms by which ER stress induces mitophagy in the context of cerebral ischemia.
Results
Tunicamycin and thapsigargin protected against ischemia-reperfusion (I-R)-induced brain injury
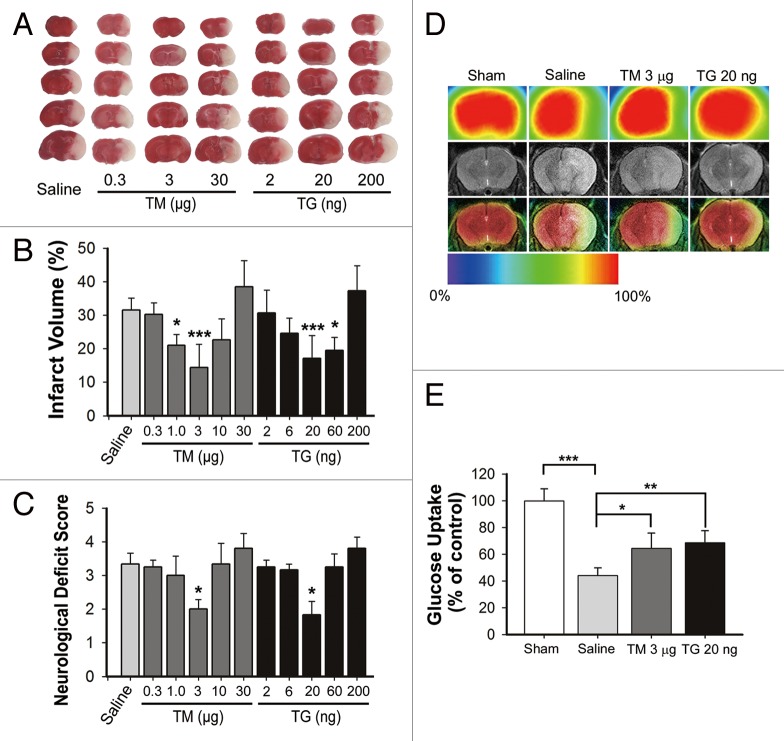
Tunicamycin (TM) and thapsigargin (TG) were icv injected at the indicated dosage prior to transient middle cerebral artery occlusion (tMCAO). The brain infarct volumes were determined by TTC assay 24 h after surgery. The TM and TG dose-dependently reduced the brain infarct within the range of 0.3 to 3 µg and 2 to 20 ng, and the maximal protection was achieved at 3 µg and 20 ng, respectively. However, there was no protection at a higher dosage (Fig. 1A and B). Accordingly, the neurological deficient scores mirrored the results of infarct volume, indicating the protection of TM and TG (Fig. 1C). These data suggested a neuroprotective role of ER stress in reperfusion after ischemia.
Figure 1. Tunicamycin (TM) and thapsigargin (TG) protected against ischemia-reperfusion-induced brain injury. Mice were subjected to middle cerebral artery occlusion for 1 h, and reperfusion was allowed by removing the monofilament suture. The indicated dosages of TM or TG were injected icv at the onset of reperfusion. (A) Animals were euthanized 24 h after MCAO and infarct volumes were determined by TTC staining in the bar charts (mean ± SD, n = 6). (B) Representative TTC-stained brain slices from each group are shown. (C) The neurological deficit scores of each group are presented. (D) Representative coregistration of MicroPET and MRI images of mice. Three micrograms TM, 20 ng TG or the same saline volume was administered icv at the onset of reperfusion, respectively; 24 h after reperfusion, T2-weighted MRI scanning mouse brain was obtained. 18F-FDG (activity ~300 Ci/mmol) was administered to mice under halothane anesthesia. After a quiet uptake period of 60 min, a 20 min static acquisition of MicroPET scan was performed. (E) Ratio of region was normalized to contralateral brain uptake (mean ± SD, n = 4). Statistical analysis was performed with one-way ANOVA followed by the Dunnett t test; *P < 0.05, **P < 0.01, ***P < 0.001 vs. saline (B and C) or indicated group (E).
To verify the observation, the glucose uptake ratio in different groups was measured by micro positron emission tomography (microPET) assay, which indicates tissue viability. The tMCAO treatment resulted in significant brain injury as reflected by decreased glucose uptake, which could be partly reversed either by 3 µg TM or 20 ng TG. These data further confirmed the neuroprotection revealed by the TTC assay (Fig. 1D and E).
TM and TG alleviated OGD-Rep.-induced cell apoptosis
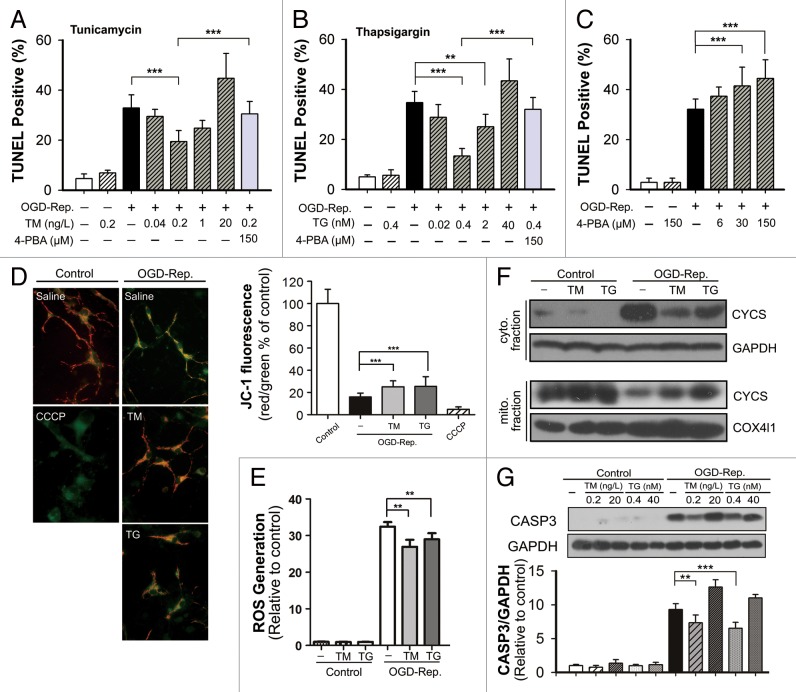
The primary cultures of neuronal cells were subjected to oxygen-glucose deprivation (OGD) treatment for 2 h. The reperfusion was performed by refreshing the cells with normal medium, which contains the indicated concentrations of TM or TG. As the results show in Figure 2, both TM and TG reversed the OGD-Rep.-induced cell apoptosis at a dosage of 0.2 ng/L and 0.4 nmol/L, respectively. Similar to the results in vivo, higher concentrations did not show the neuroprotection. Moreover, the neuroprotection can be partly compromised by cotreatment with 4-PBA, a compound that ameliorates ER stress, while 4-PBA alone aggravated cell apoptosis induced by OGD-Rep. To determine the involvement of mitochondria-dependent apoptosis, mitochondrial membrane potential (Δψm) as well as reactive oxygen species (ROS) generation were examined. The results showed that both TM and TG partly reversed the Δψm loss and ROS generation revealed by JC-1 and DCFH-DA staining, respectively. Furthermore, both TM and TG treatment counteracted the CYCS release from mitochondria to cytosol. Cleaved CASP3 (caspase 3) was regulated accordingly, which also reflected that TM and TG protected against I-R-induced apoptotic cell death (Fig. 2).
Figure 2. Tunicamycin (TM) and thapsigargin (TG) attenuated oxygen-glucose deprivation-reperfusion (OGD-Rep.)-induced apoptosis in primary neuronal cell cultures. Primary cultured neurons were subjected to OGD for 2 h, and treated with the indicated dosage of TM, TG, or 4-PBA during reperfusion. After 24 h of reperfusion, the cell apoptosis rate with treatment of TM (A), TG (B) or 4-PBA alone (C) was were determined by TUNEL and DAPI staining. (D) The mitochondrial membrane potential was detected by JC-1 staining. (E) Reactive oxygen species generation was detected by DCFH-DA staining. (F) CYCS levels in both cytosol (cyto.) and mitochondria (mito.) fractions were determined by western blot. (G) Cleaved-CASP3 expression was determined by western blot and semiquantitative levels are shown in the bar chart (mean ± SD, n = 3). Statistical comparisons were performed with one-way ANOVA followed by the Dunnett t test; **P < 0.01 and ***P < 0.001 vs. the indicated group.
TM and TG further activated I-R-induced mitophagy
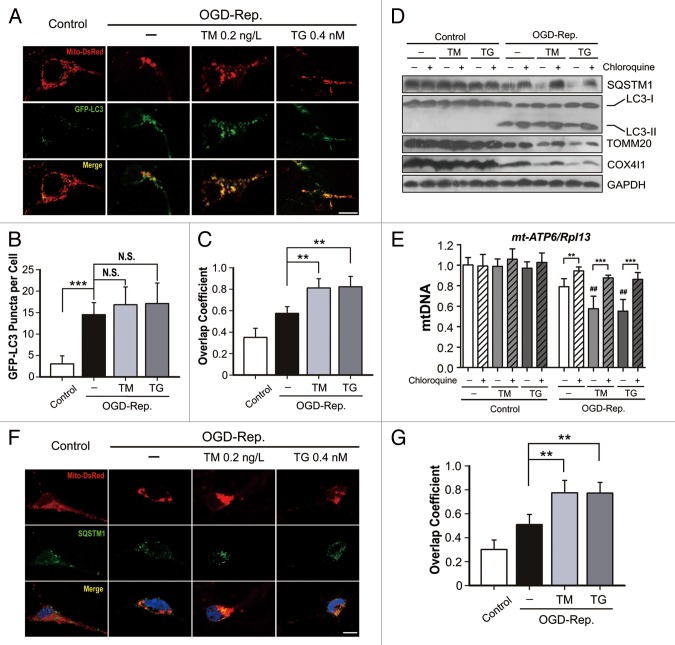
We have previously reported that autophagy can be activated by I-R, which subsequently protects from ischemic brain injury by inducing mitophagy. To explore the involvement of autophagy in ER-stress-mediated neuroprotection, GFP-LC3 puncta in primary neuronal cells were counted after OGD-Rep. treatment. As shown in Figure 3B, TM and TG only slightly increased puncta number, and LC-3II expression was not further increased as determined by western blot. However, both TM and TG significantly reduced the SQSTM1 level. Recent investigations have found that SQSTM1 is selectively recruited to mitochondria during mitophagy,23,24 which implies that mitophagy is selectively induced by ER stress. To address this, the overlap between mitochondria and GFP-LC3-labled autophagosomes was quantified. It was found that both TM and TG further increased the overlap coefficient between mitochondria and autophagosomes (Fig. 3A and C). Furthermore, both of the mitochondrial markers TOMM20 and COX4I1, and the mt-Atp6/Rpl13 ratio, which reflect the relative amount of mitochondria, were found significantly decreased compared with OGD-Rep. alone group (Fig. 3D and E). To confirm that the mitochondria loss was autophagic, the lysosome inhibitor chloroquine was employed, which significantly reversed the reduction in TOMM20, COX4I1 and mt-Atp6/Rpl13. (The semiquantitative analysis of western blot bands is shown in Figure S1.The possible contributions of reduced transcription to TOMM20 and COX4I1 protein loss can be excluded by qRT-PCR, which revealed that both Tomm20 and Cox4i1 mRNA were not further reduced with TG and TM treatment during reperfusion (Fig. S2). To confirm that mitophagy was activated by ER stress, SQSTM1 and mitochondria were visualized by immunostaining and Mito-DsRed labeling, respectively. The results showed increased overlap between SQSTM1 and mitochondria following TM and TG treatment. Overall, these results indicated that I-R-induced mitophagy can be further augmented with TM and TG treatment.
Figure 3. Tunicamycin (TM) and thapsigargin (TG) further activated oxygen-glucose deprivation-reperfusion (OGD-Rep.)-induced mitophagy in primary cultures of neuronal cells. Primary neuronal cultures were subjected to OGD for 2 h, and treated with 0.4 nmol/L TG or 0.2 ng/L TM at the onset of reperfusion. (A) Neuronal cells were previously transfected with GFP-LC3 and Mito-DsRed by viral vector infection. After 1 h of reperfusion, fluorescent images were captured by confocal microscopy. Images show representative examples from 3 independent experiments. (B) Columns represent the number of GFP-LC3-positive puncta per cell. At least 5 random fields from one section and 3 to 6 sections were averaged in each independent experiment. The bar chart shows mean ± SD values of puncta number from at least 3 independent experiments; at least 50 cells were counted in each group. (C) Columns represent the Manders overlap coefficient of Mito-DsRed and GFP-LC3. At least 44 cells from 3 independent experiments for each group were included. (D) After 6 h of reperfusion, the LC3, SQSTM1, TOMM20 and COX4I1 protein levels were determined by western blot analysis in the presence or absence of chloroquine. (E) Six hours after reperfusion, relative mitochondrial DNA (mtDNA) levels indicated as the mt-Atp6 (mitochondria-encoded DNA)/Rpl13 (nucleus-encoded DNA) ratio were assessed by real-time PCR. (F) Cells were previously labeled with Mito-DsRed and 6 h after reperfusion, SQSTM1 was determined by immunostaining. (G) The columns represent the Manders overlap coefficient of Mito-DsRed and SQSTM1. At least 29 cells from 3 independent experiments for each group were included. The experiments were repeated independently at least 3 times. The data are expressed as mean ± SD. Statistical comparisons were performed with one-way ANOVA followed by the Dunnett t test. **P < 0.01, ***P < 0.001 vs. the indicated group. ##P < 0.01 vs. OGD-Rep. alone group.
Autophagy suppression compromised the neuroprotection of TM and TG
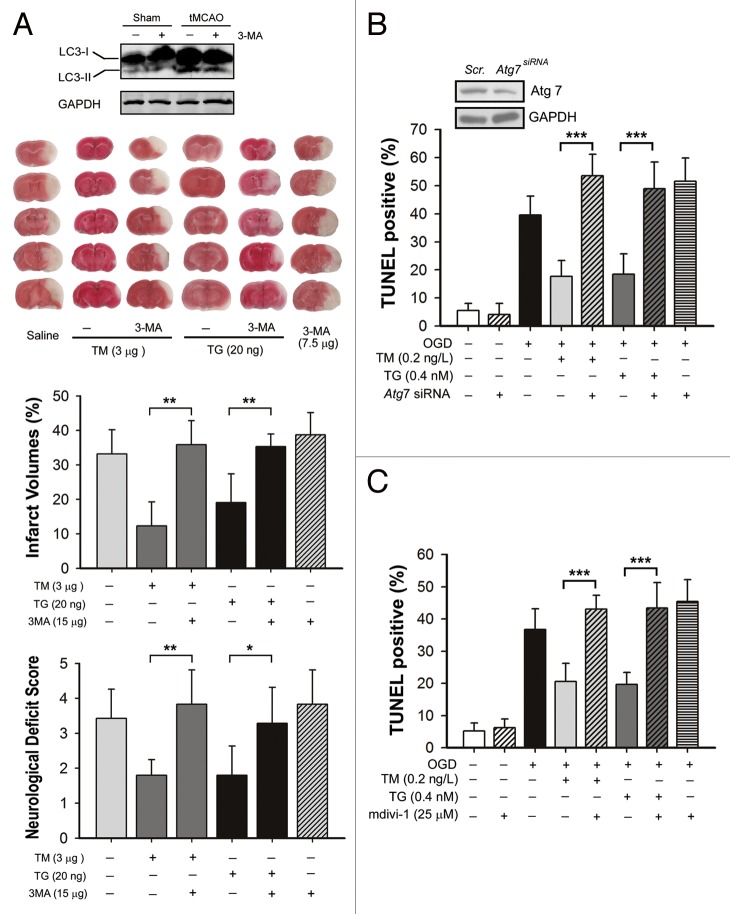
To further clarify whether the activated mitophagy is involved in the neuroprotection of TM and TG, autophagy was blocked either by 3-MA or Atg7 knockdown. As shown in Figure 4A, icv injection of 7.5 µg 3-MA reduced tMCAO-induced autophagy as revealed by LC3-II level. Importantly, combined administration of 3-MA almost completely blocked the neuroprotection conferred by TM and TG. Furthermore, the autophagy process in cultured neurons was blocked by knocking down Atg7, a gene essential for autophagy induction, leading to the antiapoptosis effects of TM and TG significantly reversed (Fig. 4B). In addition, mdivi-1 was administered to specifically inhibit the mitophagy process by blocking DNM1L, which is involved in mitochondrial fission. As shown in Figure 4C, mdivi-1 impaired the antiapoptosis effects of TM and TG. These data suggested that reinforcing ER stress during reperfusion protects the neuronal cells by, at least partly, inducing mitophagy.
Figure 4. Mitophagy inhibition blocked the protection of tunicamycin (TM) and thapsigargin (TG). (A) Mice were subjected to middle cerebral artery occlusion for 1 h, infarct volumes and neurological deficit scores were measured 24 h after reperfusion. 7.5 µg of 3-methyladenine (3-MA) was icv injected either with 3 µg TM, 20 ng TG or alone at the onset of reperfusion. (B) Atg7 was knocked down in primary cultured mice cortical neurons by pretreating cells with a lentivirus containing Atg7-shRNA (Atg7 siRNA); control cells were treated with scrambled shRNA (Scr.). The silencing effect was identified by western blot (upper panel). Cells were then subject to 2 h OGD followed by 24 h of reperfusion, and all the reagents indicated were administered at the onset of reperfusion. Cell apoptosis rate was determined by TUNEL staining after 24 h of reperfusion. (C) Cells were treated with 25 µmol/L mdivi-1 at the onset of reperfusion with or without TM and TG. TUNEL staining was performed 24 h after reperfusion. The data are expressed as mean ± SD. Statistical comparisons were performed with one-way ANOVA followed by the Dunnett t test. *P < 0.05, **P < 0.01, ***P < 0.001 vs. the indicated group.
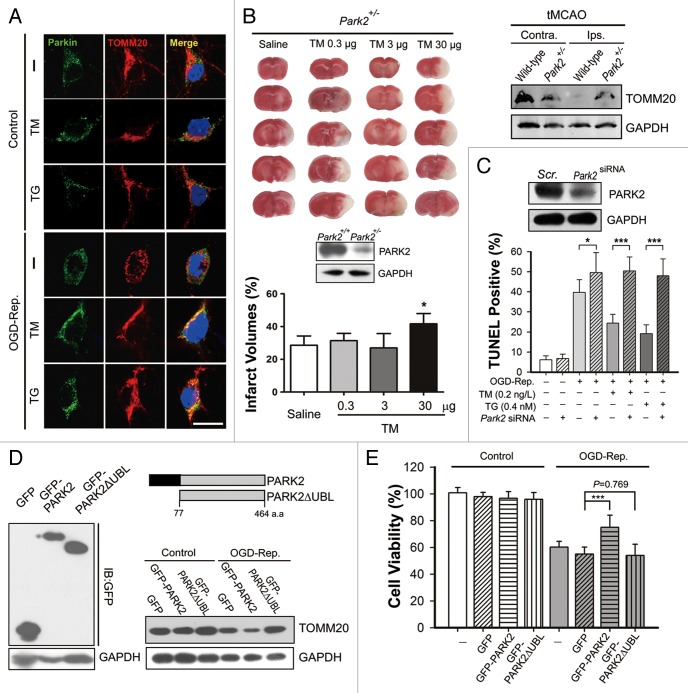
PARK2 was responsible for the neuroprotection of TM and TG
PARK2 has been demonstrated to play a crucial role in mitophagy induction in mammalian cells. We previously provided evidence indicating that PARK2 may be involved in the mitophagy process of neuronal cells in the context of I-R.22 Here, we found that both TM and TG increased the recruitment of PARK2 to mitochondria in OGD-Rep.-treated neurons, but not in intact ones (Fig. 5A). The neuroprotection of TM and TG can be significantly counteracted by PARK2 knockdown in primary cultured neurons (Fig. 5C). Notably, the neuroprotective effect of TM was greatly attenuated in heterozygous Park2 knockout mice (Park2+/−), in which mitochondria clearance was largely impaired as reflected by the relative TOMM20 level, which was retained (Fig. 5B). Conversely, GFP-PARK2 plasmid-transfected Neuro2a cells showed decreased vulnerability to OGD-Rep. insult. The protection was not observed in cells transfected with GFP-PARK2ΔUBL, which encodes a mutant PARK2 protein without the ubiquitin E3 ligase activity (Fig. 5E). Consistently, PARK2 transfection but not PARK2ΔUBL transfection, enhanced mitophagy, as reflected by a decreased TOMM20 level, suggesting that the E3 ligase function of PARK2 was essential for cells survival against ischemic injury (Fig. 5D). Therefore, these results indicated that PARK2-induced mitophagy may be required for ER stress-induced neuroprotection.
Figure 5. PARK2 is involved in the neuroprotection conferred by tunicamycin (TM) and thapsigargin (TG). (A) Primary cultures of mouse cortical neurons were subjected to 2 h of OGD and 1 h of reperfusion. Dosages of 0.2 ng/L TM or 0.4 nmol/L TG were administered simultaneously with reperfusion. Control cells experienced the same treatment without OGD. PARK2 (green) and the mitochondrial marker TOMM20 (red) were stained by immunocytochemistry and the images were taken by confocal microscopy. (B) The PARK2 level in wild-type and Park2+/− mice cortex was measured by western blot (lower panel). The Park2+/− mice were subjected to tMCAO as described and the indicated dosage of TM was icv injected at the onset of reperfusion. The infarct volumes were determined 24 h after reperfusion. The TOMM20 level in both wild-type and the Park2+/− mouse cortex after 6 h of tMCAO, either the ipsilateral (Ips.) or contralateral (Contra.), was examined by western blot (right upper panel). (C) In primary cultures of cortical neurons, PARK2 was knocked down by shRNA against mouse Park2 mRNA. The silencing effect was identified by western blot (upper panel). TUNEL staining was performed after 2 h of OGD followed by 24 h of reperfusion. (D and E) Neuro2a cells were subjected to 4 h of OGD followed by 24 h of reperfusion. (D) Neuro2a cells were either transfected with plasmids encoding pEGFP, pEGFP-PARK2 or pEGFP-PARK2ΔUBL. The transfection effects were identified by western blot against GFP (left panel). The schematic diagram shows the PARK2 and PARK2ΔUBL amino acid sequence (right upper panel). After 24 h of reperfusion, the TOMM20 level of the indicated groups was examined by western blot. (E) Cells had been transfected with the indicated plasmids 24 h previously, and cell viability was examined by MTT assay after 24 h of reperfusion. The data are expressed as mean ± SD. Statistical comparisons were performed with one-way ANOVA followed by the Dunnett t test. *P < 0.05, **P < 0.01, ***P < 0.001 vs. the indicated group.
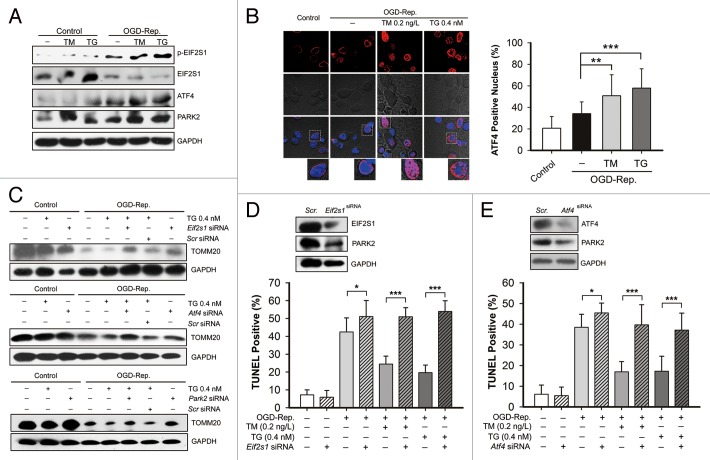
The EIF2S1-ATF4 signaling pathway was involved in TM and TG-induced PARK2 regulation
To further explore the mechanisms by which TM and TG upregulate PARK2 expression, the EIF2S1-ATF4 signaling pathway was investigated. Both TM and TG further increased OGD-Rep.-induced EIF2S1 phosphorylation and ATF4 expression. Simultaneously, PARK2 was also further upregulated (Fig. 6A). In addition, immunocytochemical staining revealed that both TM and TG promoted the translocation of ATF4 to the cell nucleus, which indicated ATF4 activation (Fig. 6B). To further confirm that the EIF2S1-ATF4 signaling pathway is responsible for mitophagy induction, Eif2s1, Atf4, and Park2 were silenced by shRNA. It was shown that either Eif2s1, Atf4, or Park2 knockdown reversed the TG-induced enhancement of mitochondria loss in the context of OGD-Rep., as revealed by the expression of TOMM20 (Fig. 6C). Both the Eif2s1 and Atf4 knockdown downregulated PARK2 in intact neurons, which further suggested that PARK2 expression may be regulated by EIF2S1-ATF4 signaling. Supportively, Eif2s1 and Atf4 silencing both blocked the protection of either TM or TG, as revealed by an increased apoptotic cell ratio (Fig. 6D and E). Interestingly, it seems that Eif2s1 and Atf4 silencing reduced PARK2 expression in neurons in steady-state conditions. The dose- and time-dependent effect was observed in the neurons, after lentivirus-mediated knockdown. (Fig. S3) Taken together, these data suggested that EIF2S1-ATF4 signaling may be involved in PARK2 upregulation following TM and TG-induced ER stress, and subsequently protects the cells by mitophagy induction.
Figure 6. The EIF2S1-ATF4 signaling pathway is involved in tunicamycin (TM) and thapsigargin (TG)-activated PARK2-mediated mitophagy in ischemic neurons. Primary cultures of mouse cortical neurons were subjected to 2 h of OGD followed by different periods of reperfusion. Dosages of 0.2 ng/L TM or 0.4 nmol/L TG were treated simultaneously with reperfusion for 3 h. (A) The levels of EIF2S1, phosphorylated EIF2S1 (p-EIF2S1), ATF4 and PARK2 were determined by western blot, and the GAPDH level was taken as loading control. (B) Representative images show that cells were immunostained for ATF4 (Red) and the cell nucleus was labeled by DAPI (blue). The ATF4-positive nucleus ratio in each indicated group was calculated and is shown in the right bar chart. At least 5 random fields from one section and 3 to 6 sections were averaged in each independent experiment. (C–E) EIF2S1, ATF4, and PARK2 in cultures of primary neurons, were previously knocked down by transfecting their specific shRNA, respectively. The control cells were transfected with scrambled shRNA. (C) The TOMM20 levels in the indicated groups were determined by western blot, and GAPDH was detected as loading control. (D) The effects of Eif2s1 knockdown was confirmed by western blot (upper panel). The cell apoptosis rate in each group was examined by TUNEL staining after 24 h of reperfusion. (E) The effects of Atf4 knockdown were confirmed by western blot (upper panel). The cell apoptosis rate in each group was examined by TUNEL staining after 24 h of reperfusion. The data are expressed as mean ± SD. Statistical comparisons were performed with one-way ANOVA followed by the Dunnett t test. *P < 0.05, **P < 0.01, ***P < 0.001 vs. indicated group.
Discussion
Although it has been more than a decade since ER stress has been identified in ischemic brains, its contributions, especially to focal cerebral ischemia, are not fully elucidated. In the present investigation, we found that ER stress inducers both tunicamycin (TM) and thapsigargin (TG) at a low dosage, protected against ischemia-reperfusion (I-R)-induced brain injury. Remarkably, OGD-Rep.-induced neuronal death was significantly ameliorated by silencing Eif2s1, an ER stress sensor. In addition, we further provided evidence indicating that TM and TG treatment during reperfusion induced mitophagy. Mitophagy inhibition by 3-MA, mdivi-1, and Atg7 silencing significantly attenuated the protection of TM and TG. Furthermore, the protection was also partly counteracted either in Park2 knockdown neurons or in heterozygous Park2 knockout mice (Park2+/−). Taken together, these findings indicated the protective role of ER stress in the context of cerebral I-R, and it is very likely that the PARK2-dependent mitophagy may be involved in its protective effects.
A series of studies have indicated the detrimental role of ER stress in ischemic brains.6,10,25-27 Ischemia-induced energy depletion rapidly disrupts ER calcium homeostasis and subsequently impairs protein folding, which ultimately can lead to cell death.28 ER function can be partly recovered upon reperfusion.29 As expected, both p-EIF2S1 and the spliced Xbp1 mRNA level induced by ischemia were continuously mitigated along with reperfusion, suggesting a relief of ER stress (Fig. S4). However, ER stress activation by TM and TG during reperfusion attenuated the ischemic brain injury either in mice or in cultured neuronal cells along with the upregulation of p-EIF2S1 and ATF4, which verified the activation of ER stress (Fig. 1; Fig. 6A). To further assess the neuroprotection of the ER stressor, we knocked down Eif2s1, which has been demonstrated to be the primary ER stress sensor in cerebral ischemia.30 We showed that the beneficial effects of TM and TG were almost completely abrogated, and Eif2s1 siRNA alone also intensified cell apoptosis (Fig. 6D), indicating that EIF2S1 phosphorylation can be a therapeutic target for cerebral ischemia.1,7,8 In addition, similar results were observed by knocking down the EIF2S1 downstream effector Atf4 (Fig. 6E). These data revealed that ER stress has a protective role in the context of cerebral I-R, and inhibition of ER stress relief during reperfusion is beneficial to the ischemic brain. In contrast, neither TM nor TG showed neuroprotection in a 24 h prolonged MCAO mouse model (data not shown). Permanent focal ischemia results in persistent ER stress and causes cell demise by CASP12 upregulation.31,32 Therefore, it seems that the reperfusion process relieves the ER stress and thus provides the therapeutic opportunities for ER stressors. The present data showed the pleiotropic contributions of ER stress to ischemic brain besides its detrimental effects during ischemia. Notably, both TM and TG showed their benefits only at a relative low dosage, whereas a higher dosage induced apoptosis (Fig. 3) and enlarged brain infarct volume (Fig. 1). Thus, these findings clearly highlighted the benefits of the appropriate extent of ER stress to ischemic neuronal cells during reperfusion.13
ER stress is known to trigger autophagy in several cell types;19,33-35 we then explored whether autophagy is involved in the protective effects of ER stress. It was shown that autophagy can be activated by OGD-Rep., and we interestingly found that TM and TG further reduced SQSTM1 level without enhancing LC3-II. Although SQSTM1 is considered as a general macroautophagy marker, recent investigations indicate that SQSTM1 is selectively recruited to mitochondria in the PARK2-dependent mitophagy process.23,24 These data imply that SQSTM1 is more likely to be recruited to mitochondria than other autophagic cargoes. Therefore, our data suggested that mitophagy rather than general autophagy was further activated by ER stressors in the reperfusional phase after ischemia (Fig. 3). Mitophagy removed the damaged mitochondria and subsequently prevented the neuronal cells from going into apoptosis. Additionally, both PARK2 and mitochondrial fission are known to be required for mitophagy during cerebral I-R.22 Here we show that suppression of autophagy either by 3-MA or Atg7 silencing counteracted the neuroprotective effect of ER stressors both in vivo and in vitro (Fig. 4A and B). Moreover, we found that either the mitochondrial fission inhibitor mdivi-1 (Fig. 4C) or Park2 shRNA treatment (Fig. 5C) reversed the antiapoptosis effects of TM and TG in primary cultured neurons, and importantly, the neuroprotective effect of the ER stressor was impaired in Park2+/− mice. These data strongly indicated that mitophagy is required for the neuroprotective effects of ER stressors. Our previous study has shown that mitophagy can be triggered by reperfusion rather than ischemia. This can partly explain why the ER stressor only showed protection in I-R rather than ischemia alone models. We showed that ER stressors further upregulated PARK2 expression and recruitment to mitochondria following OGD-Rep. (Fig. 5A; Fig. 6A), which suggested that PARK2 might be responsible for ER stress-induced mitophagy in our models. The mechanisms underlying ER stress-induced mitophagy remain not fully understood. A very recent investigation has reported that autophagosomes form at the contact sites of mitochondria and ER.36 Thus, mitochondria may have the priority to be recognized by autophagosomes under ER stress, which might be taken as a model to provide insight into the mechanisms of selective autophagy.
The contributions of PINK1-PARK2 to mitophagy have been intensively studied recently. However, their involvement in ischemic brains is largely unknown. It has been reported that PARK2 was downregulated after ischemic stroke.37 We found that park2 null mice (park2−/−) were more vulnerable to ischemic brain injury, leading to a high mortality rate within 24 h after ischemia (7 of 8, data not shown), which indicated that PARK2 may be crucial for neuronal survival after I-R. We have previously found that Park2 knockdown impairs OGD-Rep.-induced mitophagy.22 In the present research, it was shown that I-R-induced mitophagy in ischemic brain was largely attenuated in Park2+/− mice (Fig. 5B), further indicating the involvement of PARK2 in I-R-induced mitophagy. Conversely, wild-type, but not the UBL-deletion Park2 mutant, partly rescued ischemia-induced cell death in the transfection experiments, and promoted mitophagy. Therefore, the E3 ligase capacity of PARK2 seems to be required for its protection against ischemic neuronal injury. Although it has been demonstrated that some important proteins are turned over by PARK2 and thus prevent neurodegeneration,38 PARK2-mediated proteasomal degradation of proteins was not involved in stress-induced cell death.39 Therefore, PARK2-mediated direct clearance of impaired mitochondria may be the potential mechanism in the context of acute ischemic injury. Nevertheless, the role of PARK2 in degrading protein aggregation has been demonstrated,40-42 which can be triggered by ischemic stress and contributes to cellular damage.43 Therefore, mitophagy-independent contributions of PARK2 cannot be completely excluded. We next provided evidence indicating that ER stress activated the EIF2S1-ATF4 signaling cascade and subsequently induced PARK2 expression.39 It was found that PARK2 is more prone to be upregulated by ER stress in neurons compared with other cell types.44 Although the cell specific mechanisms were not fully elucidated, our data at least suggest that ER stress conferred brain protection in our model, may be largely due to its direct actions on neurons. In addition, we interestingly found that both Atf4 and Eif2s1 silencing reduced PARK2 expression in intact neurons in a dose- and time-dependent manner (Fig. S3). Therefore, in nonstressed neurons the involvement of EIF2S1-ATF4 signaling in the regulation of PARK2 expression cannot be excluded. Nevertheless, this regulation in steady-state may not be sufficient to trigger mitophagy since the PARK2 translocation to mitochondria, rather than its expression alone, is more crucial.
In summary, the present study suggested that moderate ER stress during reperfusion protected against ischemic brain injury by reinforcing mitophagy. The EIF2S1-ATF4-signaling-regulated PARK2 expression may be involved in mitophagy regulation. These findings implied that ER stress-induced mitophagy can be a potential therapeutic target for cerebral ischemia treatment.
Materials and Methods
Animals
Male C57BL/6 mice weighing 22 to 25 g were used. The park2−/− mice (C57BL/6 strain background) were kindly provided by Prof Zhuohua Zhang. For the culture of primary cortical neurons, pregnant C57BL/6 mice with embryonic (E17) fetuses were used. All experiments were approved by and conducted in accordance with the ethical guidelines of the Zhejiang University Animal Experimentation Committee and were in complete compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Efforts were made to minimize any pain or discomfort, and the minimum number of animals was used.
Transient MCAO mouse model and drugs administration
Male C57BL/6 mice were anesthetized by intraperitoneal injection of choral hydrate (350 mg/kg). Cerebral blood flow (CBF) was determined in the territory of the middle cerebral artery (MCA) by laser Doppler flowmetry (Moor Instruments, Devon, UK). A flexible fiber-optic probe was affixed to the skull over the cortex supplied by the proximal part of the right MCA (2 mm caudal to bregma and 6 mm lateral to midline). Animals with < 80% reduction in CBF in the core of the MCA territory were excluded from the study.
Transient focal cerebral ischemia was induced by MCAO as described previously with minor modifications.45,46 Briefly, a 6–0 nylon monofilament suture was advanced 10 mm into the internal carotid to occlude the origin of the MCA. Reperfusion was allowed after 60 min by monofilament removal. Body temperature was maintained at 37 °C by a heat lamp (FHC, Bowdoinham) during surgery and for 2 h after the start of reperfusion. Mice were given an intracerebroventricular injection of 7.5 µg 3-MA (Sigma, M9281) or the indicated dosage of tunicamycin (Sigma, T7765) or thapsigargin (Sigma, T9033) at the onset of reperfusion. 3-MA was dissolved in normal saline by heating the solution to 60–70 °C immediately before injection.20 Control mice were injected with the same volume of saline.
To determine infarct volume, mice were anesthetized and sacrificed by decapitation at 24 h after surgery and coronal brain slices at 2-mm intervals were stained with TTC. Infarcted areas were analyzed using Image-Pro Plus 7.0 and determined by the indirect method, which corrects for edema. The percentage of the corrected infarct volume was calculated by dividing the infarct volume by the total contralateral hemispheric volume, and this ratio was then multiplied by 100.45 Neurologic deficit scores were evaluated at 24 h of reperfusion as follows: 0, no deficit; 1, flexion of the contralateral forelimb on lifting of the whole animal by the tail; 2, circling to the contralateral side; 3, falling to the contralateral side; and 4, no spontaneous motor activity.
The microPET and MRI scanning
MRI and microPET (evaluation of regional cerebral glucose metabolism, rCMR) were taken as previous reported.47 Briefly, 24 h after the MCAO surgery of mice, MRI scan was taken on a 3.0-T scanner system (Signa, Excite HD, GE, USA). After scout images were obtained, a 3D FSPGR T2-weighted image of the mouse brain was acquired. The same mouse was taken to Micropet scan immediately after done of MRI. 18F-FDG (activity ~300 Ci/mmol) was administered to mice under halothane anesthesia. After a quiet uptake period of 60 min, the mice were placed in a spread prone position under halothane anesthesia (5% induction and 1.5% maintenance), and scanned with the MicroPET R4 (Concorde Microsystems, Knoxville, TN, USA) consisting a 15-cm diameter ring of 96 position-sensitive γ-ray scintillation detectors, providing a 10.8-cm transaxial and a 7.8-cm axial field of view, with image resolution < 1.8 mm. A 20 min static acquisition was performed in three-dimensional mode, and images were reconstructed by a maximum-a-posteriori probability algorithm with a pixel size of 0.4 × 0.4 × 1.2 mm.3
The PET and MRI images were coregistered and analyzed by IDL (ver. 6.2, Research Systems, CO, USA) and ASIPro VM (6.0.5.0, Concorde Microsystems Inc.) software. The ipsilateral brain of the mice was assigned as regions of interest (ROIs), and both the ipsilateral and contralateral rCMR value was evaluated.
Cell culture, OGD procedures, and cell apoptosis determination
The primary cortical neuronal culture was performed as described.48 Briefly, the dissected cortex from E17 fetal mice was treated with 0.125% trypsin in Hank’s buffer (in mmol/L: 137 NaCl, 5.4 KCl, 0.4 KH2PO4, 0.34 Na2PO4·7H2O, 10 glucose and 10 HEPES) for 12 min at 37 °C and dissociated by repeated passage through a series of fire-polished Pasteur pipettes. Approximate 2 × 105 cells/cm2 were seeded onto poly-l-lysine (10 µg/ml)-coated plates and dishes. The neurons were grown in Neurobasal medium (Invitrogen) supplemented with 2% B27 (Invitrogen), 10 U/ml penicillin, 10 U/ml streptomycin, and 0.5 mmol/L glutamine at 37 °C in a humidified atmosphere with 5% CO2. Cultures were maintained for 8 d before treatment and routinely observed under a phase-contrast inverted microscope.
The murine neuronal cell line Neuro2a was routinely cultured in DMEM containing 10% fetal bovine serum (Gibco), 10 U/ml penicillin, 10 U/ml streptomycin, and 0.5 mM glutamine at 37 °C in a humidified atmosphere with 5% CO2.
For OGD treatment, cells were rinsed once with warm glucose-free DMEM (Gibco), and then refreshed with O2- and glucose-free DMEM (prebalanced in an O2-free chamber at 37 °C). Cells were then immediately placed in a sealed chamber (MIC-101, Billups-Rothenburg) loaded with mixed gas containing 5% CO2 and 95% N2 for 5 min at 25 L/min. Primary neurons were incubated at 37 °C for 2 h before reperfusion, while for Neuro2a cells, the OGD duration was 4 h. For reperfusion, cells were refreshed with normal culture medium. The indicated concentrations of tunicamycin, thapsigargin, 3-MA, and mdivi-1 (Santa Cruz Biotechnology, sc-215291) were dissolved in normal culture medium and added to the cells at the onset of reperfusion. Control cells were given equal refreshment but were incubated in glucose-containing DMEM for 2 h and 4 h for OGD-Rep.
Cell apoptosis rate was determined by the in situ cell death detecting kit (Roche 11684795910) based on the TUNEL assay. Briefly, cells were previously seeded on poly-l-lysine-treated coverslips. After treatment, fixed cells were labeled with TUNEL according to the protocol provided by the datasheet, and cell nucleuses were stained with DAPI. Coverslips were observed by flurorescence microscopy. For each coverslip, 5 random fields were captured by a microscope camera (Fluoview FV1000, Olympus, Tokyo, Japan). The total cell number was counted using DAPI staining, and the average TUNEL-positive cell ratio was calculated. For each time, 4 to 6 slips were used for the indicated group, and the experiments were repeated 3 times independently.
Transfection and RNA silence
To achieve a high efficient transfection in primary cultured neurons, an adeno-associated virus (AAVs) containing GFP-LC3 (Hanbio, Shanghai, China) was employed. For Mito-DsRed delivery, the Mito-DsRed DNA sequence was cloned from pDsRed2-Mito (Clontech) and inserted into pLVX-puro plasmid, and transfected into HEK293T cells with a set of plasmids for lentiviral packaging (Clontech, 631247). Both the lentivirus and/or the AAVs were incubated with neurons at 6 DIV. For Neuro2a cells, the plasmids were transfected by employing the X-tremeGENE HP transfection reagent (Roche) following the specific protocol for this cell line.
Small interfering RNA (siRNA) targeting mice Atg7 (5′-GCAUCAUCUU UGAAGUGAA-3′)49 and scrambled control siRNA (5′-AUGAAGTGA AUUGCUCAA-3′) were synthesized by GenePharm (Shanghai). Primary neurons were transfected on d 5 with 20 nmol Atg7 siRNA or scrambled siRNA using Lipofectamine RNAiMAX (Invitrogen). After transfection in antibiotic-free medium for 8 h, cells were refreshed with normal medium. The efficacy of the Atg7 knockdown was assessed by western blot using antibodies against ATG7 (Epitomics, 5146-1). Experiments were performed 72 h after transfection. For Park2, Eif2s1, and Atf4 siRNA, short hairspin RNA (shRNA) targeting mouse Park2 (5′-CCA UCA CUUCAGGAUC CUU-3′), Eif2s1 (5′-TGCAGAAGTG GATGGAGA T-3′), Atf4 (5′-GGATGACACA TGTGATCTT-3′) and scrambled control shRNA (5′-AUG AACGTGAAUU GCUCAA-3′) were constructed into a pLVX-shRNA1 plasmid for lentiviral packaging (Clontech, 631247). The lentivirus was collected 48 h after transfection; the titer was determined and subsequently, the lentivirus was administered to primary cultured neurons (6 DIV) at a ratio of 50:1. The silencing efficiency was confirmed by immunoblot of target proteins 48 h after infection.
Immunoblot
The brain tissues and cells were homogenized in RIPA buffer (20 mmol/L TRIS-HCl pH 7.5, 150 mmol/L NaCl, 1 mmol/L EDTA, 1% Triton X-100 (SANGON, T0694), 0.5% sodium dexoycholate (Sigma, 30970), 1 mmol/L PMSF (Amresco, 1001010530), and 10 µg/ml leupeptin (Amresco, 2147483647). For cytosolic and mitochondrial protein extraction, an isolation kit (Beyotime, C3601) was used. An aliquot of 40 µg protein from each sample was separated using SDS-PAGE and transferred to a nitrocellulose membrane, which was then blocked with 5% nonfat milk in PBS (pH 7.4). The membranes were incubated with primary antibodies against LC3 (1:1,000; MBL, PM036), SQSTM1 (1:1,000; CST, 5114), cleaved CASP3 (1:1,000; CST, 9661), CYCS (1:800; CST, 9665), COX4I1 (1:1,000; CST, 4850S), TOMM20 (1:1,000; Anbo Biotechnology, c16678), PARK2 (1:1,000; Sigma, P5748), ATF4 (1:1,000; CST, 11815), EIF2S1 (1:1,000; CST, 9722), Phospho-EIF2S1 (1:1,000; CST, 9721) or GAPDH (1:3,000; KangChen, KC-5G4) 4 °C overnight. Secondary antibodies conjugated with HRP against either rabbit or mouse IgG (1:5,000, CST, 7071 and 7072) were performed for 2 h at room temperature and blots were exposed to a chemiluminescent detection system using the SuperSignal West Pico Substrate (34077, Pierce) and exposed to film. Digital images were quantified using densitometric measurements by Quantity-One software (Bio-Rad).
JC-1 staining and reactive oxygen species (ROS) determination
The mitochondria membrane potential was determined by JC-1 staining. After 6 h incubation with the indicated concentrations of TM and TG, primary cultured neurons were then incubated with 1 μg/ml JC-1 (Sigma, T4069) for 15 min in 37 °C. Cell staining was observed using a fluorescence microscopy (DMI 3000B, Leica, Wetzlar, Germany). At least 3 random fields of views were captured from each sample, data were calculated as red/green fluorescence analyzed by Image-Pro-Plus 7.0 software.
For ROS determination, primary neuronal cells were cultured in 48-well plates. A dosage of 5 μmol/L 2,7-Dichlorodihydrofluorescein diacetate (DCFH-DA; Sigma, D6883) was added to the cell cultures immediately after treatment. The intracellular fluorescence was detected by a microplate reader with excitation wavelength 488 nm and emission wavelength at 520 nm (DTX880, Beckman Coulter, USA) as previously described.50 These experiments were repeated 3 times independently.
Immunocytochemistry and confocal microscopy
For microscopy examination, primary neurons were seeded onto poly-l-lysine-treated glass coverslips (Warner Instruments, 64-0702) and transfected with GFP-LC3 and/or Mito-DsRed as aforementioned. After OGD, cells were reperfused for the indicated duration. Cells were then fixed with 4% paraformaldehyde for GFP-LC3 and Mito-DsRed detecting. For immunostaining, cells were incubated with antibodies against SQSTM1 (1:100; Abcam, ab56416), PARK2 (1:200; Sigma, P5748) or TOMM20 (1:100; Anbo Biotechnology, C16678). Secondary antibodies labbled with Alexa Fluor 488 or 596 (abcam, ab150073 or ab150108) were subsequently added to the cells. Coverslips were observed on a confocal microscope (Fluoview FV1000, Olympus, Tokyo, Japan). The Manders overlap efficiency was measured and analyzed as described, by Image Pro-Plus 7.0 software.51 Five randomly selected fields from one coverslip were included to get an average, and experiments were repeated independently at least 3 times.
Real-time PCR
After 6 h of OGD-reperfusion, total cellular DNA was extracted with a DNeasy Blood and Tissue kit (Tiangen, DP304-03). Aliquots of 20 ng total DNA were used for PCRs to detect the mitochondrial gene mt-Atp6 and the genomic gene Rpl13, as we previously described.22 The amount of gene was calculated and normalized by the standard curve. Relative expression was presented as the mt-Atp6/Rpl13 ratio.
Statistical analysis
All data were collected and analyzed in a blind manner. Data are presented as mean ± SD. One-way ANOVA (analysis of variance) with the Dunnett T3 post-hoc test was applied for multiple comparisons. P < 0.05 was considered statistically significant.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was funded by the National Basic Research of China 973 Program (2011CB504403), the National Natural Science Foundation of China (81030061, 81273506, 81173040, 81102429, 81273490, 81221003 and 81373393), and the Program for Zhejiang Leading Team of S&T Innovation Team (2011R50014). The authors are grateful to Dr Zhuohua Zhang for providing the park2 gene knockout mice. We are grateful to the Imaging Facilities, Zhejiang University School of Medicine for the help in confocal microscopy. We are grateful to Dr Hanmin Shen and Dr Guanghui Wang for reading the manuscript.
Glossary
Abbreviations:
- 3-MA
3-methyladenine
- ATF4
activating transcription factor 4
- EIF2S1
eukaryotic translation initiation factor 2, subunit 1 alpha
- icv
intracerebroventricular
- ip
intraperitoneal
- I-R
ischemia/reperfusion
- MAP1LC3 (LC3)
microtubule-associated protein 1 light chain 3
- mdivi-1
mitochondrial division inhibitor-1
- mtDNA
mitochondrial DNA
- OGD-Rep.
oxygen and glucose deprivation-reperfusion
- tMCAO
transient middle cerebral artery occlusion
- TG
thapsigargin
- TM
tunicamycin
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick-end labeling
References
- 1.Roussel BD, Kruppa AJ, Miranda E, Crowther DC, Lomas DA, Marciniak SJ. Endoplasmic reticulum dysfunction in neurological disease. Lancet Neurol. 2013;12:105–18. doi: 10.1016/S1474-4422(12)70238-7. [DOI] [PubMed] [Google Scholar]
- 2.Paschen W. Endoplasmic reticulum dysfunction in brain pathology: critical role of protein synthesis. Curr Neurovasc Res. 2004;1:173–81. doi: 10.2174/1567202043480125. [DOI] [PubMed] [Google Scholar]
- 3.Chen X, Kintner DB, Luo J, Baba A, Matsuda T, Sun D. Endoplasmic reticulum Ca2+ dysregulation and endoplasmic reticulum stress following in vitro neuronal ischemia: role of Na+-K+-Cl- cotransporter. J Neurochem. 2008;106:1563–76. doi: 10.1111/j.1471-4159.2008.05501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paschen W, Doutheil J. Disturbances of the functioning of endoplasmic reticulum: a key mechanism underlying neuronal cell injury? J Cereb Blood Flow Metab. 1999;19:1–18. doi: 10.1097/00004647-199901000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci U S A. 2005;102:12005–10. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qi X, Hosoi T, Okuma Y, Kaneko M, Nomura Y. Sodium 4-phenylbutyrate protects against cerebral ischemic injury. Mol Pharmacol. 2004;66:899–908. doi: 10.1124/mol.104.001339. [DOI] [PubMed] [Google Scholar]
- 7.Sokka AL, Putkonen N, Mudo G, Pryazhnikov E, Reijonen S, Khiroug L, Belluardo N, Lindholm D, Korhonen L. Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neurosci. 2007;27:901–8. doi: 10.1523/JNEUROSCI.4289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakka VP, Gusain A, Raghubir R. Endoplasmic reticulum stress plays critical role in brain damage after cerebral ischemia/reperfusion in rats. Neurotox Res. 2010;17:189–202. doi: 10.1007/s12640-009-9110-5. [DOI] [PubMed] [Google Scholar]
- 9.Rissanen A, Sivenius J, Jolkkonen J. Prolonged bihemispheric alterations in unfolded protein response related gene expression after experimental stroke. Brain Res. 2006;1087:60–6. doi: 10.1016/j.brainres.2006.02.095. [DOI] [PubMed] [Google Scholar]
- 10.Badiola N, Penas C, Miñano-Molina A, Barneda-Zahonero B, Fadó R, Sánchez-Opazo G, Comella JX, Sabriá J, Zhu C, Blomgren K, et al. Induction of ER stress in response to oxygen-glucose deprivation of cortical cultures involves the activation of the PERK and IRE-1 pathways and of caspase-12. Cell Death Dis. 2011;2:e149. doi: 10.1038/cddis.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. doi: 10.1016/S1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 12.Ibuki T, Yamasaki Y, Mizuguchi H, Sokabe M. Protective effects of XBP1 against oxygen and glucose deprivation/reoxygenation injury in rat primary hippocampal neurons. Neurosci Lett. 2012;518:45–8. doi: 10.1016/j.neulet.2012.04.053. [DOI] [PubMed] [Google Scholar]
- 13.Petrovski G, Das S, Juhasz B, Kertesz A, Tosaki A, Das DK. Cardioprotection by endoplasmic reticulum stress-induced autophagy. Antioxid Redox Signal. 2011;14:2191–200. doi: 10.1089/ars.2010.3486. [DOI] [PubMed] [Google Scholar]
- 14.Tooze SA, Yoshimori T. The origin of the autophagosomal membrane. Nat Cell Biol. 2010;12:831–5. doi: 10.1038/ncb0910-831. [DOI] [PubMed] [Google Scholar]
- 15.Decuypere JP, Kindt D, Luyten T, Welkenhuyzen K, Missiaen L, De Smedt H, Bultynck G, Parys JB. mTOR-Controlled Autophagy Requires Intracellular Ca(2+) Signaling. PLoS One. 2013;8:e61020. doi: 10.1371/journal.pone.0061020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crawford SE, Estes MK. Viroporin-mediated calcium-activated autophagy. Autophagy. 2013;9:797–8. doi: 10.4161/auto.23959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–31. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding WX, Ni HM, Gao W, Hou YF, Melan MA, Chen X, Stolz DB, Shao ZM, Yin XM. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J Biol Chem. 2007;282:4702–10. doi: 10.1074/jbc.M609267200. [DOI] [PubMed] [Google Scholar]
- 19.Fouillet A, Levet C, Virgone A, Robin M, Dourlen P, Rieusset J, Belaidi E, Ovize M, Touret M, Nataf S, et al. ER stress inhibits neuronal death by promoting autophagy. Autophagy. 2012;8:915–26. doi: 10.4161/auto.19716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen YD, Sheng R, Zhang LS, Han R, Zhang X, Zhang XD, Han F, Fukunaga K, Qin ZH. Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy. 2008;4:762–9. doi: 10.4161/auto.6412. [DOI] [PubMed] [Google Scholar]
- 21.Adhami F, Liao G, Morozov YM, Schloemer A, Schmithorst VJ, Lorenz JN, Dunn RS, Vorhees CV, Wills-Karp M, Degen JL, et al. Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am J Pathol. 2006;169:566–83. doi: 10.2353/ajpath.2006.051066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Yan H, Yuan Y, Gao J, Shen Z, Cheng Y, Shen Y, Wang RR, Wang X, Hu WW, et al. Cerebral ischemia-reperfusion-induced autophagy protects against neuronal injury by mitochondrial clearance. Autophagy. 2013;9:1321–33. doi: 10.4161/auto.25132. [DOI] [PubMed] [Google Scholar]
- 23.Geisler S, Holmström KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12:119–31. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 24.Huang C, Andres AM, Ratliff EP, Hernandez G, Lee P, Gottlieb RA. Preconditioning involves selective mitophagy mediated by Parkin and p62/SQSTM1. PLoS One. 2011;6:e20975. doi: 10.1371/journal.pone.0020975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paschen W. Dependence of vital cell function on endoplasmic reticulum calcium levels: implications for the mechanisms underlying neuronal cell injury in different pathological states. Cell Calcium. 2001;29:1–11. doi: 10.1054/ceca.2000.0162. [DOI] [PubMed] [Google Scholar]
- 26.Reimertz C, Kögel D, Rami A, Chittenden T, Prehn JH. Gene expression during ER stress-induced apoptosis in neurons: induction of the BH3-only protein Bbc3/PUMA and activation of the mitochondrial apoptosis pathway. J Cell Biol. 2003;162:587–97. doi: 10.1083/jcb.200305149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11:372–80. doi: 10.1038/sj.cdd.4401378. [DOI] [PubMed] [Google Scholar]
- 28.Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47:122–9. doi: 10.1016/j.ceca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Paschen W, Aufenberg C, Hotop S, Mengesdorf T. Transient cerebral ischemia activates processing of xbp1 messenger RNA indicative of endoplasmic reticulum stress. J Cereb Blood Flow Metab. 2003;23:449–61. doi: 10.1097/00004647-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Althausen S, Mengesdorf T, Mies G, Oláh L, Nairn AC, Proud CG, Paschen W. Changes in the phosphorylation of initiation factor eIF-2alpha, elongation factor eEF-2 and p70 S6 kinase after transient focal cerebral ischaemia in mice. J Neurochem. 2001;78:779–87. doi: 10.1046/j.1471-4159.2001.00462.x. [DOI] [PubMed] [Google Scholar]
- 31.Mouw G, Zechel JL, Gamboa J, Lust WD, Selman WR, Ratcheson RA. Activation of caspase-12, an endoplasmic reticulum resident caspase, after permanent focal ischemia in rat. Neuroreport. 2003;14:183–6. doi: 10.1097/00001756-200302100-00004. [DOI] [PubMed] [Google Scholar]
- 32.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 33.Appenzeller-Herzog C, Hall MN. Bidirectional crosstalk between endoplasmic reticulum stress and mTOR signaling. Trends Cell Biol. 2012;22:274–82. doi: 10.1016/j.tcb.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Zhao Y, Hu J, Xiao J, Qu L, Wang Z, Ma D, Chen Y. A novel ER-localized transmembrane protein, EMC6, interacts with RAB5A and regulates cell autophagy. Autophagy. 2013;9:150–63. doi: 10.4161/auto.22742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheng R, Liu XQ, Zhang LS, Gao B, Han R, Wu YQ, Zhang XY, Qin ZH. Autophagy regulates endoplasmic reticulum stress in ischemic preconditioning. Autophagy. 2012;8:310–25. doi: 10.4161/auto.18673. [DOI] [PubMed] [Google Scholar]
- 36.Hamasaki M, Furuta N, Matsuda A, Nezu A, Yamamoto A, Fujita N, Oomori H, Noda T, Haraguchi T, Hiraoka Y, et al. Autophagosomes form at ER-mitochondria contact sites. Nature. 2013;495:389–93. doi: 10.1038/nature11910. [DOI] [PubMed] [Google Scholar]
- 37.Mengesdorf T, Jensen PH, Mies G, Aufenberg C, Paschen W. Down-regulation of parkin protein in transient focal cerebral ischemia: A link between stroke and degenerative disease? Proc Natl Acad Sci U S A. 2002;99:15042–7. doi: 10.1073/pnas.232588799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vincow ES, Merrihew G, Thomas RE, Shulman NJ, Beyer RP, MacCoss MJ, Pallanck LJ. The PINK1-Parkin pathway promotes both mitophagy and selective respiratory chain turnover in vivo. Proc Natl Acad Sci U S A. 2013;110:6400–5. doi: 10.1073/pnas.1221132110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bouman L, Schlierf A, Lutz AK, Shan J, Deinlein A, Kast J, Galehdar Z, Palmisano V, Patenge N, Berg D, et al. Parkin is transcriptionally regulated by ATF4: evidence for an interconnection between mitochondrial stress and ER stress. Cell Death Differ. 2011;18:769–82. doi: 10.1038/cdd.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olzmann JA, Li L, Chudaev MV, Chen J, Perez FA, Palmiter RD, Chin LS. Parkin-mediated K63-linked polyubiquitination targets misfolded DJ-1 to aggresomes via binding to HDAC6. J Cell Biol. 2007;178:1025–38. doi: 10.1083/jcb.200611128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olzmann JA, Chin LS. Parkin-mediated K63-linked polyubiquitination: a signal for targeting misfolded proteins to the aggresome-autophagy pathway. Autophagy. 2008;4:85–7. doi: 10.4161/auto.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimura H, Hattori N, Kubo Si, Mizuno Y, Asakawa S, Minoshima S, Shimizu N, Iwai K, Chiba T, Tanaka K, et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25:302–5. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- 43.Hu BR, Martone ME, Jones YZ, Liu CL. Protein aggregation after transient cerebral ischemia. J Neurosci. 2000;20:3191–9. doi: 10.1523/JNEUROSCI.20-09-03191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang HQ, Imai Y, Kataoka A, Takahashi R. Cell type-specific upregulation of Parkin in response to ER stress. Antioxid Redox Signal. 2007;9:533–42. doi: 10.1089/ars.2006.1522. [DOI] [PubMed] [Google Scholar]
- 45.Fan YY, Zhang XN, He P, Shen Z, Shen Y, Wang XF, Hu WW, Chen Z. Transient lack of glucose but not O2 is involved in ischemic postconditioning-induced neuroprotection. CNS Neurosci Ther. 2013;19:30–7. doi: 10.1111/cns.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan YY, Hu WW, Dai HB, Zhang JX, Zhang LY, He P, Shen Y, Ohtsu H, Wei EQ, Chen Z. Activation of the central histaminergic system is involved in hypoxia-induced stroke tolerance in adult mice. J Cereb Blood Flow Metab. 2011;31:305–14. doi: 10.1038/jcbfm.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao F, Guo Y, Zhang H, Wang S, Wang J, Wu JM, Chen Z, Ding MP. Anterior thalamic nucleus stimulation modulates regional cerebral metabolism: an FDG-MicroPET study in rats. Neurobiol Dis. 2009;34:477–83. doi: 10.1016/j.nbd.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Zhang CH, Fan YY, Wang XF, Xiong JY, Tang YY, Gao JQ, Shen Z, Song XH, Zhang JY, Shen Y, et al. Acidic preconditioning protects against ischemia-induced brain injury. Neurosci Lett. 2012;523:3–8. doi: 10.1016/j.neulet.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 49.Du L, Hickey RW, Bayir H, Watkins SC, Tyurin VA, Guo F, Kochanek PM, Jenkins LW, Ren J, Gibson G, et al. Starving neurons show sex difference in autophagy. J Biol Chem. 2009;284:2383–96. doi: 10.1074/jbc.M804396200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X, Wu J, Dou Y, Xia B, Rong W, Rimbach G, Lou Y. Asiatic acid protects primary neurons against C2-ceramide-induced apoptosis. Eur J Pharmacol. 2012;679:51–9. doi: 10.1016/j.ejphar.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 51.Zinchuk V, Zinchuk O, Okada T. Quantitative colocalization analysis of multicolor confocal immunofluorescence microscopy images: pushing pixels to explore biological phenomena. Acta Histochem Cytochem. 2007;40:101–11. doi: 10.1267/ahc.07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.