Abstract
Autophagosomes arise in yeast and animals from the sealing of a cup-shaped double-membrane precursor, the phagophore. The concerted action of about 30 evolutionarily conserved autophagy related (ATG) proteins lies at the core of this process. However, the mechanisms allowing phagophore generation and its differentiation into a sealed autophagosome are still not clear in detail, and very little is known in plants. This is due in part to the scarcity of structurally informative, real-time imaging data of ATG proteins at the phagophore site. Among these, the ATG5 complex directs anchoring of ATG8 to the phagophore, an event required for membrane expansion. Detailed real-time and 3D imaging of ATG5, ATG8, and an ER marker at the expanding phagophore allowed us to propose a model for autophagosome formation in plants. This model implies tight connections of the growing phagophore with the outer face of the cortical endoplasmic reticulum and prompts new questions on the mechanism of autophagosome biogenesis.
Keywords: autophagosome, Arabidopsis, phagophore, endoplasmic reticulum, autophagy-related protein 5
From a cell biologist's perspective, autophagosome biogenesis is a particularly attractive example of compartment formation. It can be tightly induced experimentally; is, in the appropriate conditions, dispensable for cell survival and therefore amenable to genetic analysis; and must involve original mechanisms allowing the constitution of a sealed double membrane surrounding distinct types of cargo. During macroautophagy (autophagy hereafter), autophagosomes allow the degradation of apparently randomly entrapped cytoplasmic portions for reattribution of resources, after fusion to lytic compartments of the cell and subsequent degradation and efflux.
Although intensive research efforts in yeast and mammals have allowed the proposition of a hierarchical order of action for the ATG proteins during autophagosome formation, one present challenge is the full integration of this knowledge with the fine localization, on differentiating autophagosomal structures, of the molecules involved. In the first phase of such a differentiation process, local membranes are enriched in phosphatidylinositol 3-phosphate (PtdIns3P) by the PtdIns 3-kinase activity of the VPS34 complex. These PtdIns3P-rich membranes are required for assembly of the ATG12–ATG5-ATG16 complexes at the phagophore assembly site. Subsequently, growth of the phagophore is thought to depend upon anchoring of ubiquitin-like ATG8 to the phagophore membrane. This occurs via a lipidation reaction catalyzed by the ubiquitin-like-conjugating enzyme ATG3, once activated by its association with ATG5-containing complexes. Finally, the phagophore is sealed to form the autophagosome, which will subsequently fuse with the vacuole (in yeast and plants) or, in mammalian cells, with the lysosome.
It is possible that the site of formation and possibly membrane sources for the phagophore depend on physiological conditions, cell type, and organism. In yeast, imaging of early autophagosomal markers has established that autophagosomes form in close proximity of the vacuolar membrane, while, in mammalian cells, they preferentially form at ER-mitochondria contact sites, but also at the plasma membrane. In plants, very little information is presently available on the site of autophagosome formation and, more generally, on early autophagosomal structures, despite the fact that autophagy plays an important role in several agronomically relevant traits.
Since localization of the ATG5 complex should define precisely the site of ATG8–PE insertion into the membrane of the phagophore, high resolution imaging of ATG5 in plants should reveal useful information on the structure of the expanding phagophore and on the subcellular site of autophagosome formation.
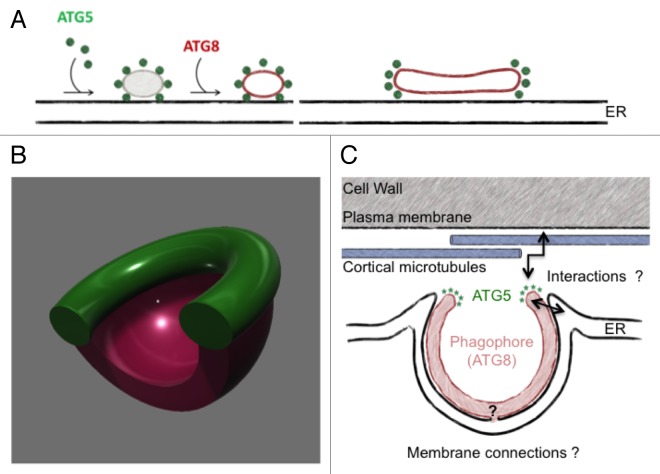
We imaged ATG5 fluorescent reporters in root epidermal cells of Arabidopsis seedlings, coupling the analysis with ATG8 reporters and a marker of the endoplasmic reticulum. Evolving autophagosomal structures were followed in real-time, after rapid induction of macroautophagy by nutrient starvation. First, similar to what is known in yeast and animal models, we observed that an ATG5-GFP reporter, which we show has retained sufficient biological activity to sustain autophagosome formation in the absence of endogenous Atg5, labels transiently expanding phagophores, leaving the autophagosomal particle before it enters the plant vacuole. However, we also noticed that ATG5 decorates a toroidal domain of the expanding phagophore (or a yet unidentified structure closely associated with it), rather than being spread on the whole surface of the autophagosomal particle. Confocal imaging in real-time of the ATG5 toroidal domain during autophagosome differentiation, and correlative light-electron microscopy observations, allowed us to propose a working model for autophagosome formation during plant macroautophagy, in which the ATG5 complex decorates the high curvature edge of a cisternal-like early phagophore anchored to the outer face (that is, facing the plasma membrane) of cortical ER, as depicted in Figure 1A.
Figure 1. A working model for autophagosome formation in plants. (A) After a dot-like, sub-resolution phase, ATG5 labels a toroidal domain at the edge of a cisternal-like expanding phagophore at the outer surface of the cortical endoplasmic reticulum. As the phagophore expands in the 3 dimensions, the ATG5 domain surrounds the phagophore's aperture, as schematized by the 3D model in (B). (C) Some of the open questions prompted by the model.
Somehow surprisingly, such toroidal localization of ATG5 had not been previously described in other models. It is not known whether this reflects species-specific differences in phagophore differentiation, or if such torus-like domain were not detected in other models for technical reasons. Notably, the plant autophagosomal structures we described can be rather large when compared with those observed in other models, reaching often 2 μm in diameter. This facilitated generation of morphological information by confocal microscopy, and also underscores the interest of a multiorganismal approach in the autophagy field. Interestingly, labeling of a dot-like portion of the phagophore by ATG5 has been recently reported in yeast, suggesting that confinement of ATG5, and therefore probably of ATG8 lipidation, to specific domains of the phagophore may be a common theme. Moreover, in mammals, the autophagosome associated, PtdIns3P-binding protein ZFYVE1/DFCP1 defines ring-like structures, which, however, do not colocalize with ATG5, on endoplasmic reticulum-related membranes at the phagophore site. Such ER structures could be functionally related to the ATG5 tori we observe on plant phagophores. It is also tempting to speculate that ATG5 complexes located at the phagophore aperture (Fig. 1B) might, in addition to allowing insertion of ATG8–PE into the membrane of the phagophore, also mediate its connection to underlying ER membranes, and control sealing of the phagophore, in a process regulated by interactions with the cargo. Many additional open questions are associated with this working model of autophagosome formation. Some are schematized in Figure 1C: they include the possible tethering of the phagophore to cellular structures such as cortical microtubules or the plasma membrane, and the existence of a membrane continuum between the phagophore and compartments of the plant endomembrane system.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work has benefited from the facilities and expertise of the Imagif Cell Biology Unit of the Gif campus (www.imagif.cnrs.fr), which is supported by the Conseil Général de l'Essonne. We acknowledge support by France-BioImaging infrastructure and Saclay Plant Science, for French National Research Agency grants “Investments for the future” ANR-10-INSB-04-01, and ANR-11-IDEX-0003-02, respectively.