Abstract
The transcription factor TWIST1 is a basic helix-loop-helix protein that regulates epithelial-mesenchymal transition (EMT) in early embryonic morphogenesis, cancer development, and cancer metastasis. The regulation of TWIST1 remains poorly understood. Recently, we found that autophagy deficiency stabilizes TWIST1 protein through SQSTM1/p62 accumulation. SQSTM1 binds with TWIST1 to inhibit TWIST1 degradation in both autophagosomes and proteasomes. SQSTM1-mediated TWIST1 stabilization promotes EMT in vitro, and tumor growth and metastasis in mice. We propose autophagy as a new mechanism to control the TWIST1 protein levels and activity in cancer development and progression.
Keywords: autophagosome, autophagy, EMT, melanoma, metastasis, p62, proteasome, skin cancer, tumor growth, TWIST1
Epithelial-mesenchymal transition is a critical process for early embryonic morphogenesis, cancer development, and cancer metastasis. One of the essential EMT regulators is the basic helix-loop-helix protein and transcription factor TWIST1. TWIST1 induces loss of CDH1/E-cadherin-mediated cell-cell adhesion and thus facilitates EMT. The protein level of TWIST1 is tightly controlled by proteasomal degradation.
Macroautophagy (hereafter autophagy) is a catabolic process for proteolytic degradation of cellular proteins, cytoplasm, and organelles in lysosomes. Autophagy dysfunction contributes to the pathogenesis of multiple human diseases, such as neurodegeneration, microbial infection, metabolic diseases, cardiovascular diseases, aging, and cancer. The protein SQSTM1 serves as both a selective autophagy substrate and an autophagy receptor protein. SQSTM1 is increased in several human cancers and induced by the oncogene RRAS, suggesting that SQSTM1 is oncogenic.
As a homeostatic process, autophagy can serve both oncogenic and tumor-suppressive roles depending on the tumorigenic context. The ability of autophagy to restrict inflammation and DNA damage may limit tumorigenesis and tumor progression. SQSTM1 accumulation resulting from autophagy defects is sufficient to activate NFKB signaling and promote tumorigenesis. The defective clearance of SQSTM1 in autophagy deficiency leads to the constitutive activation of the redox-regulatory transcription factor NFE2L2/NRF2 and contributes to development of liver cancer. Conversely, autophagy may promote tumor formation and metastasis at later stages by protecting tumor cells from environmental stresses. Our recent studies demonstrate that autophagy deficiency promotes cell proliferation and migration through SQSTM1-dependent stabilization of TWIST1 (Fig. 1), but not other EMT factors such as SNAI1/Snail, SNAI2/Slug, ZEB1, or TWIST2.
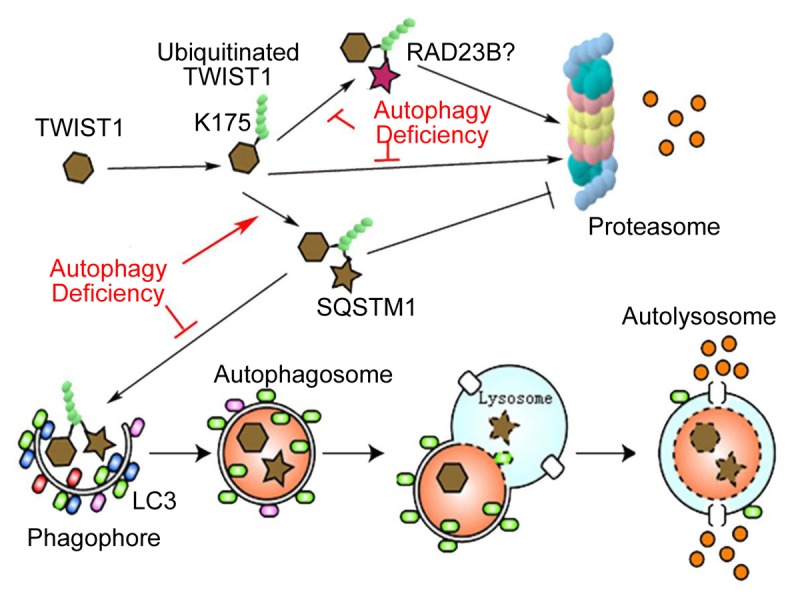
Figure 1. Schematic for the regulation of TWIST1 by SQSTM1 through autophagy and the proteasome. This figure has been modified from Qiang L, Zhao BZ, Ming M, Wang N, He TC, Hwang S, Thorburn A, He YY. Regulation of cell proliferation and migration by p62 through stabilization of Twist1. Proc Natl Acad Sci USA 2014; 111:9241–6; PMID:24927592; 10.1073/pnas.1322913111 by permission of the publisher.
In addition to the autophagosome-lysosome pathway, the proteasomal protein degradation system plays important roles in regulating stability of many short-lived proteins. Although these 2 systems often respond to different physiological or pathological cues, they do interact to regulate protein stabilities. Indeed, during autophagy stimulation SQSTM1 and TWIST1 colocalize in autophagosomes. However, in autophagy-deficient cells, SQSTM1 binds to TWIST1 and inhibits degradation of TWIST1. Our findings indicate that SQSTM1 inhibits TWIST1 protein degradation in both autophagosomes and proteasomes. Autophagy suppresses TWIST1 and EMT by controlling the protein levels of SQSTM1 (Fig. 1).
This regulation is relevant to human cancers, since we found that SQSTM1 is upregulated in human skin squamous cell carcinoma. SQSTM1 is also reported to be upregulated in human melanoma and several other human cancers. In addition, we found that SQSTM1 upregulation promotes tumor growth and metastasis of human squamous cell carcinoma cells in mice in a TWIST1-dependent manner. We have also shown that autophagy suppression increases while SQSTM1 knockdown decreases tumor growth of human melanoma cells in mice.
At the molecular level, we found that the ubiquitin-associated domain of SQSTM1 is required for SQSTM1 binding to TWIST1 (Fig. 1). Lysine 175 (K175) is critical for TWIST1 binding to SQSTM1 (Fig. 1). SQSTM1 interacts with polyubiquitinated TWIST1 at K175 and inhibits TWIST1 degradation through the autophagy and proteasome pathways. Thus, either autophagy suppression or SQSTM1 upregulation increases the TWIST1 protein level and activity in EMT.
To determine the physiological relevance of the SQSTM1-TWIST1 interaction in EMT, we tested the hypothesis that SQSTM1 plays a critical role in TGFB-EGF-induced EMT. Indeed, we found that TGFB-EGF induces SQSTM1 expression. Such SQSTM1 induction is crucial for TGFB-EGF-induced EMT and TWIST1 upregulation at the protein levels, while it does not affect TWIST1 mRNA levels. These findings indicate that TWIST1 regulation by SQSTM1 is critical for EMT in a physiological context.
Given that TWIST1 is vital for cancer development, progression, and metastasis and that the incidence of several cancers including both human skin squamous cell carcinoma and melanoma continues to rise at an alarming rate every year, our findings not only add new molecular insights into the pathogenesis of cancer formation and metastasis, but also suggest that targeting SQSTM1-mediated TWIST1 stabilization is a promising preventive and therapeutic strategy to prevent and treat cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the National Institutes of Health (NIH)/National Institute on Environmental Health Sciences Grant ES016936 (to YYH), the American Cancer Society Grant RSG-13-078-01 (to YYH), the University of Chicago Cancer Research Center (P30 CA014599), the CTSA (UL1 TR000430), and the University of Chicago Friends of Dermatology Endowment Fund.


