Abstract
High level microsatellite instability (MSI-H) occurs in about 15% of colorectal cancer (CRCs), either as sporadic cancers or in the context of hereditary non-polyposis cancer (HNPCC) or Lynch syndrome. In MSI-H CRC, mismatch repair deficiency leads to insertion/deletion mutations at coding microsatellites (cMS) and thus to the translation of frameshift peptides (FSPs). FSPs are potent inductors of T cell responses in vitro and in vivo. The present study aims at the identification of FSP-specific humoral immune responses in MSI-H CRC and Lynch syndrome.
Sera from patients with history of MSI-H CRC (n=69), healthy Lynch syndrome mutation carriers (n=31) and healthy controls (n=52) were analyzed for antibodies against FSPs using peptide ELISA. Reactivities were measured against FSPs derived from genes frequently mutated in MSI-H CRCs, AIM2, TGFBR2, CASP5, TAF1B, ZNF294, and MARCKS.
Antibody reactivity against FSPs was significantly higher in MSI-H CRC patients than in healthy controls (p=0.036, Mann-Whitney) and highest in patients with shortest interval between tumor resection and serum sampling. Humoral immune responses in patients were most frequently directed against FSPs derived from mutated TAF1B (11.6%, 8/69) and TGFBR2 (10.1%, 7/69). Low level FSP-specific antibodies were also detected in healthy mutation carriers.
Our results show that antibody responses against FSPs are detectable in MSI-H CRC patients and healthy Lynch syndrome mutation carriers. Based on the high number of defined FSP antigens, measuring FSP-specific humoral immune responses is a highly promising tool for future diagnostic application in MSI-H cancer patients.
Keywords: antibodies, frameshift peptides, immune responses, Lynch syndrome, microsatellite instability
Introduction
High level microsatellite instability (MSI-H) occurs in about 15% of colorectal cancers (CRCs) and results from defects in the DNA mismatch repair system (MMR). MSI-H CRCs accumulate numerous insertion/deletion mutations at short repetitive DNA sequences, termed microsatellites [1–3]. MSI-H CRCs may develop sporadically or in the context of the hereditary non-polyposis colorectal cancer (HNPCC) or Lynch syndrome [4,5]. Clinically, they are characterized by a better prognosis and a lower frequency of distant metastases as compared to microsatellite stable CRCs [6]. A dense infiltration with lymphocytes is a typical feature of MSI-H CRCs, suggesting a pronounced immunogenicity [7–10].
The immunogenicity of MSI-H CRCs is attributed to a wealth of frameshift neopeptides (FSPs) that may be generated following insertion or deletion mutations affecting microsatellites in gene-encoding regions (coding microsatellites, cMS). These FSPs are a direct consequence of MMR deficiency and hence represent tumor antigens that may be recognized as “non-self” by the immune system and may elicit an immune response of the host [11].
FSP sequences have been predicted in a genome-wide bioinformatics approach using the human genome sequence databases [12]. A list of candidate genes most frequently mutated in MSI-H CRC that may be translated into FSPs has been recently published ([13], www.seltarbase.org). The in silico prediction of FSP antigens provided the basis for detailed in vivo analyses that revealed a high frequency of FSP-specific T cell responses in patients with MSI-H CRC and healthy Lynch syndrome mutation carriers [14], thus confirming that FSPs predicted in silico in fact are relevant tumor antigens in vivo.
The detection of humoral immune responses against FSPs may provide the basis for a serological test to identify or monitor patients with MSI-H tumors or Lynch syndrome. Against classical tumor antigens like p53, Her2/neu, or NY-ESO-1, serum antibody frequencies ranged between 10 and 20% [15]. The prevalence of humoral immune responses against FSPs has not been analyzed so far. Up to now, there is only an anecdotal report on antibodies specific for one FSP in a single patient suffering from a Lynch syndrome-associated CRC [16].
MSI-H CRC represents an ideal tumor entity for studying tumor antigen-specific humoral immune responses and designing serum antibody assays because the abundance of FSPs and their predictability by bioinformatics. The present cross-sectional study is the first step towards a comprehensive systematic evaluation of FSP-specific antibodies in MSI-H CRC patients, healthy Lynch syndrome mutation carriers, and healthy controls.
Materials and Methods
Patients and healthy controls
A total number of 152 sera were analyzed for antibodies against FSPs, obtained from 69 Lynch syndrome patients with history of MSI-H CRC (termed MSI-H CRC patients), 31 healthy Lynch syndrome mutation carriers and 52 healthy controls. Controls were age- and gender-matched to the MSI-H CRC patients. The median age of the patients was 50 years, of the mutation carriers 38 years and of the healthy controls 48 years (Table 1). Sera from MSI-H CRC patients and Lynch syndrome mutation carriers were collected at the University Hospitals of Heidelberg and Munich, Germany in the framework of the German HNPCC Consortium funded by the “Deutsche Krebshilfe”. Time after tumor resection in the MSI-H CRC patients was between 2 months and 16 years with a median of 3.6 years (interquartile range 1.8 to 6.0 years) (Table 1). Healthy Lynch syndrome mutation carriers took part in a clinical surveillance program (yearly colonoscopy and, where applicable, gynecologic examination), and only individuals without evidence for any cancer or preneoplastic lesion were included in this group. Healthy control sera were obtained from anonymized specimens of occupational employee examinations performed at the University Hospital of Mannheim, Germany. Sera were stored at −70°C in aliquots to minimize freeze-thaw cycles. All procedures were approved by the institutional ethics committee.
Table 1.
MSI-H CRC patients, healthy Lynch syndrome mutation carriers and healthy controls.
| patients | mutation carriers |
healthy controls |
|
|---|---|---|---|
| total n | 69 | 31 | 52 |
| median age (years) | 50 | 38 | 48 |
| age range | 28–78 | 25–75 | 27–80 |
| % male | 61.8 | 41.9 | 61.0 |
| UICC stage | |||
| 1 | 10 (14.5%) | - | - |
| 2 | 20 (29.0%) | - | - |
| 3 | 14 (20.3%) | - | - |
| unknown | 25 (36.2%) | - | - |
| time after tumor resection | |||
| 2 months | 5 (7.2%) | - | - |
| 3 months to 12 months | 7 (10.1%) | - | - |
| 13 months to 48 months | 19 (27.5%) | - | - |
| 49 months to 96 months | 20 (29.0%) | - | - |
| >96 months | 7 (10.1%) | - | - |
| unknown | 11 (15.9%) | - | - |
Peptides
FSPs derived from six coding microsatellite-containing genes were selected according to the following criteria: high coding microsatellite mutation frequency in MSI-H CRC [12,13] (www.seltarbase.org), and the presence of T cell immune responses in patients [14]. FSP sequences translated from mutated complementary DNA sequences are referred to as ‘gene name (minus number of deleted nucleotides)’ in the text. Based on these criteria, the FSPs AIM2(-1), CASP5(-1), MARCKS(-1), TAF1B(-1), TGFBR2(-1) and ZNF294(-1) were selected. FSPs encompassed wild type and frameshift sequences enabling detection of antibody reactions against the junction regions. In addition to TGFBR2(-1) that covers 49 amino acids, two shorter overlapping peptides were designed (TGFBR2(-1)-N and TGFBR2(-1)-C). A detailed list of FSP sequences is provided in Table 2. Peptides were obtained from the Peptide Synthesis Facility of the German Cancer Research Center (DKFZ) in Heidelberg, Germany, purified by high-performance liquid chromatography (HPLC), and analyzed by mass spectrometry. Peptides were dissolved to 5 mg/ml in DMSO and stored at −70°C.
Table 2.
Peptide Sequences. Neopeptide sequences are underlined.
| gene | repeat | mutation frequency |
peptide length |
peptide sequence |
|---|---|---|---|---|
| AIM2(-1) | A(10) | 51.6% | 20 | VIKAKKKHREVKRTNSSQLV |
| TGFBR2(-1) | A(10) | 74.8% | 49 | LEDAASPKCIMKEKKSLVRLSSCVPVALMSAMTTSSSQKNITPAILTCC |
| TGFBR2(-1)-N | A(10) | 74.8% | 27 | LEDAASPKCIMKEKKSLVRLSSCVPVA |
| TGFBR2(-1)-C | A(10) | 74.8% | 31 | RLSSCVPVALMSAMTTSSSQKNITPAILTCC |
| CASP5(-1) | A(10) | 44.7% | 25 | QLRCWNTWAKMFFMVFLIIWQNTMF |
| TAF1B(-1) | A(11) | 74.6% | 40 | PNTQIKALNRGLKKKTILKKAGIGMCVKVSSIFFINKQKP |
| ZNF294(-1) | A(11) | 60.0% | 41 | SSLKSSKKKMVRLDLLMRYLKAIKRMKNVYLQKERRLKAGN |
| MARCKS(-1) | A(11) | 72.7% | 23 | TPSPSNETPKKKRSAFPSRSLSS |
| control peptide p16INK4a_76–105 | - | - | 30 | ATLTRPVHDAAREGFLDTLVVLHRAGARLD |
Peptide ELISA
Serum antibodies were detected using a newly developed peptide ELISA. Peptides were coated to 96 well polystyrol microtiter plates “Maxisorp” (Nunc, Roskilde, Denmark) at a concentration of 40 µg/ml in PBS overnight at 4°C. After coating, plates were washed 4 times with PBS (0.05% Tween) and blocked for 1 h with 0.5% casein in PBS. Peptide binding to the microtiter plates and optimal saturating peptide concentration were assessed using an alkaline phosphatase – peptide competition assay according to a previously published protocol [17]. To monitor individual background reactivity of each serum, a peptide derived from the p16INK4a protein (p16_76–105) was used, against which no antibody reactivity was found in a large cohort of individuals [18]. Each serum was diluted 1:100 in blocking buffer (0.5% casein in PBS) and tested in duplicates for the presence of antibodies against all FSPs and the control peptide. As a reference for inter-plate variance, one control serum was included on every plate, and peptide-specific ODs of the control serum were used for normalization. Diluted sera (50 µl/well) were incubated for 1 h, and after a wash step plates were incubated with HRP-labeled rabbit anti-human-IgG antibody (Jackson Immunoresearch, West Grove, PA; 1:10,000 in blocking buffer) for 1 h. After washing, 50 µl/well of TMB substrate (Sigma, Deisenhofen, Germany) was added and the enzyme reaction was stopped after 30 minutes by adding 50 µl/well of 1N H2SO4. Absorption was measured at 450 nm (reference wavelength 595 nm). Pre-absorption of serum antibodies for specificity control was done by incubating sera (1:100 in blocking buffer) with 1 to 100 µg/ml peptide at 4°C over night before the ELISA was performed according to the protocol.
Statistics
Raw ODs (ODraw) of all sera were normalized by subtracting ODs of negative control peptide (ODcontrol peptide) and were adjusted for plate variations using a peptide- and plate-specific normalization factor defined by the control serum (ODneg).
OD= (ODraw−ODcontrol peptide)/NF
with NF=ODneg(plate)/ x̃(ODneg(all))
OD is the mean of the duplicate measurements. The cut-off was calculated for each antigen individually (90th percentile + 2 interquartile ranges in all healthy control sera). All sera with ODs equal or greater than the cut-off were considered positive for the respective antigen.
ELISA ODs in the different groups were compared using Mann-Whitney test. All statistical analyses have been performed using the SPSS software package.
Results
Detection of antibodies against FSPs
All 152 sera were tested in a peptide ELISA against six FSPs in duplicates blinded to the disease status. Raw ODs were normalized to the serum-specific background as well as to the plate-dependent background. Normalized ODs ranged from 0.000 to 0.3446 (median=0.0026, interquartile range=0.000–0.0070). There was a good agreement between duplicate measurements (coefficient of variation = 10.2%). To demonstrate the specificity of reactions, blocking experiments were conducted in sera with high ODs by preincubation of the serum with the reacting peptide. Preincubation with the relevant peptide reduced the OD substantially, while preincubation with other peptides did not have an effect (Supplemental figure 1).
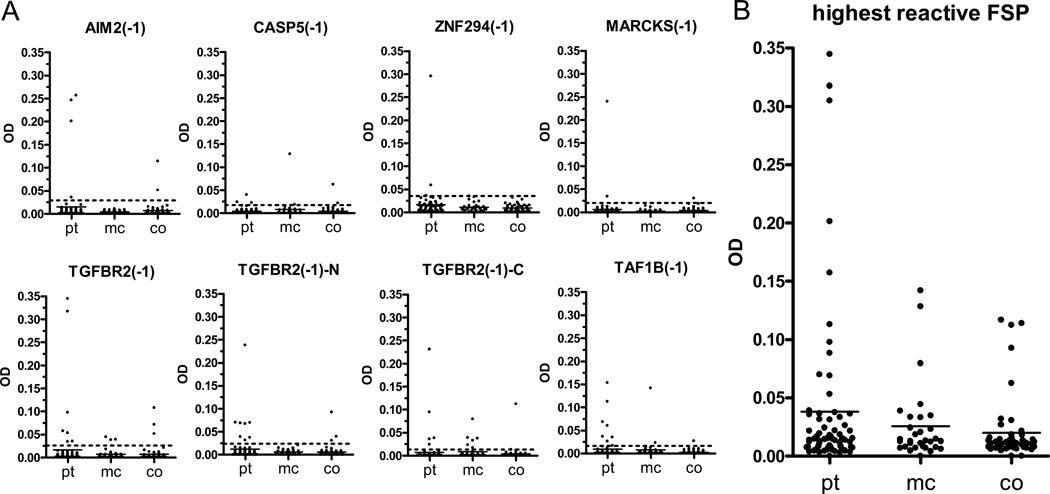
OD distribution of MSI-H CRC patients, Lynch syndrome mutation carriers, and healthy controls is displayed for each peptide in Figure 1A. When the FSP with highest OD was selected for each serum (Figure 1B), a significantly higher FSP-specific antibody reactivity was observed in patients compared to healthy controls (p=0.036; MSI-H CRC patients n=69, median OD=0.0144, interquartile range=0.0078–0.0325; controls n=52, median OD=0.0114, interquartile range=0.0079–0.0154). Antibody reactivity in mutation carriers also tended to be higher than in healthy controls (p=0.241; mutation carriers n=31, median OD=0.0128, interquartile range=0.0076–0.0332). No association of FSP antibodies with age of the individuals was observed.
Figure 1.
A) ELISA ODs for the different FSPs in MSI-H CRC patients (pt, n=69), healthy Lynch syndrome mutation carriers (mc, n=31) and healthy controls (co, n=52). Cut-offs are indicated by dotted lines. B) OD of the highest reactive FSP for each serum.
Prevalence of antibodies against FSPs
To determine antibody frequencies, an individual cut-off was defined for each FSP (dotted lines in Figure 1A). Highest antibody frequencies were found in MSI-H CRC patients and in healthy Lynch syndrome mutation carriers: 11.6% (8/69) of the patients reacted against TAF1B(-1) and 11.6% (8/69) reacted against TGFBR2(-1)-N. Highest frequency in healthy mutation carriers was found against TGFBR2(-1)-C with 19.4% (6/31) (Table 3). There was a substantial agreement between antibody positivity against the TGFBR2(-1) 49mer peptide and either of the two shorter constructs TGFBR2(-1)-N or TGFBR2(-1)-C (95.4% agreement, kappa=0.76, p<0.001). Additionally, for TAF1B(-1) a peptide construct containing one additional amino acid at its N-terminal wild-type sequence was available. Agreement of ELISA results between these two constructs was also substantial (97.4% agreement, kappa=0.77, p<0.001).
Table 3.
Frequency of antibodies against single FSPs and FSP combinations.
| peptide | patients | healthy mutation carriers |
healthy controls |
|---|---|---|---|
| AIM2(-1) | 5.8% (4/69) | 0.0% (0/31) | 3.8% (2/52) |
| CASP5(-1) | 4.3% (3/69) | 6.5% (2/31) | 1.9% (1/52) |
| ZNF294(-1) | 4.3% (3/69) | 0.0% (0/31) | 0.0% (0/52) |
| MARCKS(-1) | 2.9% (2/69) | 0.0% (0/31) | 1.9% (1/52) |
| TGFBR(-1) | 10.1% (7/69) | 9.7% (3/31) | 5.8% (3/52) |
| TGFBR(-1)-N | 11.6% (8/69) | 0.0% (0/31) | 5.8% (3/52) |
| TGFBR(-1)-C | 7.2% (5/69) | 19.4% (6/31) | 3.8% (2/52) |
| TAF1B(-1) | 11.6% (8/69) | 6.5% (2/31) | 1.9% (1/52) |
| all tested FSPs | 29.0% (20/69) | 29.0% (9/31) | 15.4% (8/52) |
Most of the seropositive individuals reacted against only one FSP (81.8%, 27/32). Antibodies against multiple FSPs were found in 3 MSI-H CRC patients (one each against two, four, and six FSPs), in 2 healthy controls (one against two and one against three FSPs), and in one healthy Lynch syndrome mutation carrier (against two FSPs).
FSP antibody frequency in MSI-H CRC patients and healthy Lynch syndrome mutation carriers increased to a frequency of 29.0% (20/69 patients, 9/31 mutation carriers) when reactions against at least one of the FSPs were considered, compared to 15.4% (8/52) in healthy controls (Table 3).
FSP-specific antibodies in MSI-H CRC patients in association with time after tumor resection and stage
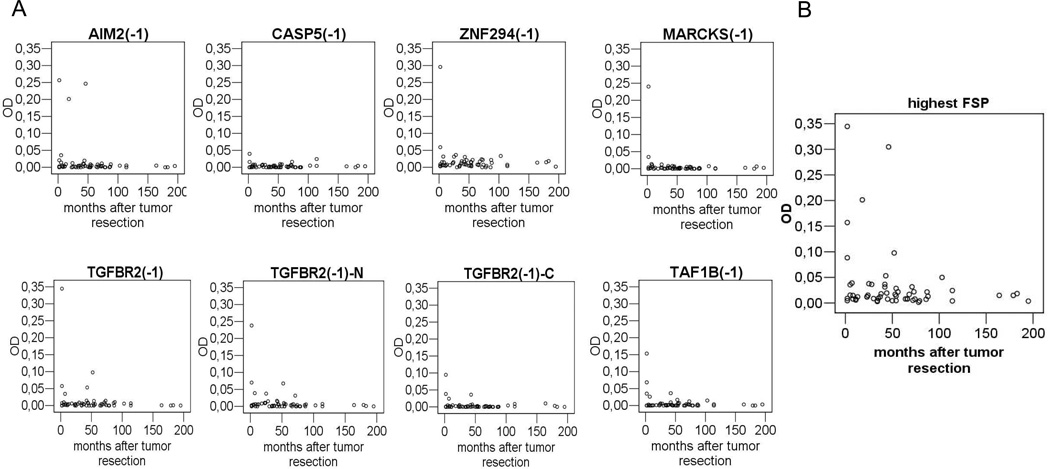
There was an association between the presence of antibodies against FSPs in MSI-H CRC patients and time after tumor resection. Antibody frequency (all FSPs) was 60.0% (3/5) in patients whose tumor resection was less than 3 months ago, 28.6% (2/7) in patients with resection 3 to 12 months ago and 27.7% (10/36) in patients with resection more than one year ago. In all MSI-H CRC patients with antibodies against multiple peptides (n=3) tumor resection was less than 3 months ago. High level FSP antibody responses (OD>0.1) against MARCKS(-1), TAF1B(-1), TGFBR2(-1) and ZNF294(-1) were restricted to patients that underwent surgery within the last 3 months (Figure 2). Additionally, a trend towards an association of FSP antibodies with higher UICC stage was observed. 42.9% (6/14) of patients with metastasis to regional lymph nodes (UICC III) had antibodies against any of the FSPs while only 13.3% (4/30) of patients with local disease (UICC I or II) had antibodies (Fishers exact test p=0.051).
Figure 2.
A) ELISA ODs for the single FSPs in MSI-H CRC patients in relation to time after tumor resection. B) OD of the highest reactive FSP for each serum in MSI-H CRC patients in relation to time after tumor resection.
Discussion
With the present study we demonstrate that individuals affected by MSI-H CRC or Lynch syndrome generate humoral immune responses against MMR deficiency-induced FSPs. Antibodies directed against FSPs were detectable in Lynch syndrome patients with history of MSI-H CRC. In addition, low level antibody reactions were also observed in healthy Lynch syndrome mutation carriers without tumor history. Reactions were directed against a variety of FSPs, suggesting that different FSPs are generated in MSI-H cells and elicit a humoral response of the host’s immune system. Similarly, we previously reported a high frequency of T cell-based immune responses against a broad spectrum of FSP antigens [14].
FSP-specific antibodies were measured using a newly developed peptide ELISA with a high analytical specificity and reproducibility. Specificity was confirmed by pre-absorption of antibodies with peptides encompassing the respective FSP regions. Cut-offs were calculated for each antigen individually on the basis of the OD distribution in control sera, aiming at a low frequency of seropositivity in healthy controls.
In our study, highest ELISA ODs were found in MSI-H CRC patients with the shortest interval between tumor resection and serum sampling. This finding suggests that high antibody levels are strongly related to high antigen loads and may drop shortly after removal of the cancer. An association of antibodies with antigen load and decline of antibody levels after surgical tumor removal has previously been reported for some of the known tumor antigens, e.g. p53 and NY-ESO-1. Accordingly, the application of antibody serology for the detection of disease recurrence has been suggested [19–21]. In line with these observations is the report of a patient with serum antibodies directed against a neopeptide derived from one MSI-H tumor-specific FSP (CDX2) prior to surgical resection, but not after 7 years of disease-free follow-up [16]. Thus, it is conceivable that the frequency of antibodies directed against FSPs is much higher in patients before or at the time of tumor resection. Longitudinal studies are currently initiated to monitor in depth antibody responses preoperatively and during different time points of follow-up in Lynch syndrome patients.
Low level antibody reactivity against FSPs was present in healthy mutation carriers without evidence of a prevalent tumor. Similar to the observation of FSP-specific T cell responses in healthy Lynch syndrome mutation carriers [14], this suggests an exposure of the immune system towards FSPs which might be generated by mismatch repair-deficient cells not developing into cancer. This hypothesis is supported by the observation that no colorectal tumor was detected in any of the mutation carriers seropositive for FSPs in our study.
Current approaches of serum antibody detection in cancer are focusing on potential diagnostic applications [22]. Here, combinations of tumor antigens can enhance diagnostic sensitivity of serological assays in cancer patients [23]. Lynch syndrome is ideal for monitoring humoral immune response in cancer, as diagnostic or prediagnostic sensitivity can be longitudinally monitored in high risk individuals that are clearly defined by germ line mutations in DNA mismatch repair genes. Coding microsatellites are abundant in the human genome and already about 40 bioinformatically predicted cMS-containing genes have been validated in cancer tissue showing a mutation frequency in MSI-H CRC of 50% or higher (www.seltarbase.org). Thus, it is very well conceivable that the analysis of humoral immune responses against an extended panel of FSPs will lead to a high cumulative antibody frequency and diagnostic accuracy, the more so as the majority of seropositive patients in this study only showed reaction against one or two FSPs.
There is data suggesting a biological relevance of serum antibodies, e.g. by blocking growth factor receptors like Her2/neu [24]. In the present study, we cannot address a potential functional role of seroreactivity against FSPs. Recently FSPs have attracted considerable interest as agents for vaccination in MSI-H cancer patients and Lynch syndrome mutation carriers. For the selection of immunogenic FSP antigens appropriate for vaccination, it should be taken into account that only a subset of FSPs seems to be generated at a level sufficient for mediating effective anti-tumor immune responses [25], By providing information about the potential of different FSPs to induce antibody responses, our study adds valuable information about the immunogenicity of FSPs that should be considered in future strategies in design of FSP-based vaccination.
In conclusion, we present the first systematic analysis of humoral immune responses against FSPs. Our data indicate that humoral immune responses against a variety of different FSP antigens are detectable in MSI-H CRC patients with Lynch syndrome. Antibody levels were highest in patients with short interval between tumor resection and serum sampling. Low level antibody responses were already present in healthy Lynch syndrome mutation carriers without tumor history. This study paves the way for future diagnostic and therapeutic applications based on the detection of FSP-specific antibodies in Lynch syndrome mutation carriers and MSI-H cancer patients in general.
Supplementary Material
Acknowledgements
This work was funded by a grant from the “Deutsche Krebshilfe”.
Abbreviations
- CRC
colorectal cancer
- ELISA
enzyme-linked immunosorbent assay
- HNPCC
hereditary non-polyposis cancer
- MSI-H
high level microsatellite instability
- FSP
frameshift-derived peptide
- MMR
mismatch repair
- PBS
phosphate-buffered saline
References
- 1.Ionov Y, Peinado MA, Malkhosyan S, et al. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363:558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- 2.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260:816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 3.Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348:919–932. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- 4.Quehenberger F, Vasen HF, van Houwelingen HC. Risk of colorectal and endometrial cancer for carriers of mutations of the hMLH1 and hMSH2 gene: correction for ascertainment. J Med Genet. 2005;42:491–496. doi: 10.1136/jmg.2004.024299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783–5788. doi: 10.1200/JCO.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson P, Lin KM, Rodriguez-Bigas MA, et al. Colorectal carcinoma survival among hereditary nonpolyposis colorectal carcinoma family members. Cancer. 1998;83:259–266. [PubMed] [Google Scholar]
- 7.Dolcetti R, Viel A, Doglioni C, et al. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol. 1999;154:1805–1813. doi: 10.1016/S0002-9440(10)65436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckowitz A, Knaebel HP, Benner A, et al. Microsatellite instability in colorectal cancer is associated with local lymphocyte infiltration and low frequency of distant metastases. Br J Cancer. 2005;92:1746–1753. doi: 10.1038/sj.bjc.6602534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerjea A, Bustin SA, Dorudi S. The immunogenicity of colorectal cancers with high-degree microsatellite instability. World J Surg Oncol. 2005;3 doi: 10.1186/1477-7819-3-26. 26- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lynch HT, Drescher KM, de la Chapelle A. Immunology and the Lynch syndrome. Gastroenterology. 2008;134:1246–1249. doi: 10.1053/j.gastro.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saeterdal I, Bjorheim J, Lislerud K, et al. Frameshift-mutation-derived peptides as tumor-specific antigens in inherited and spontaneous colorectal cancer. Proc Natl Acad Sci U S A. 2001;98:13255–13260. doi: 10.1073/pnas.231326898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woerner SM, Benner A, Sutter C, et al. Pathogenesis of DNA repair-deficient cancers: a statistical meta-analysis of putative Real Common Target genes. Oncogene. 2003;22:2226–2235. doi: 10.1038/sj.onc.1206421. [DOI] [PubMed] [Google Scholar]
- 13.Woerner SM, Kloor M, von Knebel DM, et al. Microsatellite instability in the development of DNA mismatch repair deficient tumors. Cancer Biomark. 2006;2:69–86. doi: 10.3233/cbm-2006-21-208. [DOI] [PubMed] [Google Scholar]
- 14.Schwitalle Y, Kloor M, Eiermann S, et al. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology. 2008;134:988–997. doi: 10.1053/j.gastro.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Reuschenbach M, von Knebel DM, Wentzensen N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother. 2009;58:1535–1544. doi: 10.1007/s00262-009-0733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishikawa T, Fujita T, Suzuki Y, et al. Tumor-specific immunological recognition of frameshift-mutated peptides in colon cancer with microsatellite instability. Cancer Res. 2003;63:5564–5572. [PubMed] [Google Scholar]
- 17.Steinitz M, Baraz L. A rapid method for estimating the binding of ligands to ELISA microwells. J Immunol Methods. 2000;238:143–150. doi: 10.1016/s0022-1759(00)00160-5. [DOI] [PubMed] [Google Scholar]
- 18.Reuschenbach M, Waterboer T, Wallin KL, et al. Characterization of humoral immune responses against p16, p53, HPV16 E6 and HPV16 E7 in patients with HPV-associated cancers. Int J Cancer. 2008;123:2626–2631. doi: 10.1002/ijc.23837. [DOI] [PubMed] [Google Scholar]
- 19.Korangy F, Ormandy LA, Bleck JS, et al. Spontaneous tumor-specific humoral and cellular immune responses to NY-ESO-1 in hepatocellular carcinoma. Clin Cancer Res. 2004;10:4332–4341. doi: 10.1158/1078-0432.CCR-04-0181. [DOI] [PubMed] [Google Scholar]
- 20.Jäger E, Stockert E, Zidianakis Z, et al. Humoral immune responses of cancer patients against"Cancer-Testis" antigen NY-ESO-1: correlation with clinical events. Int J Cancer. 1999;84:506–510. doi: 10.1002/(sici)1097-0215(19991022)84:5<506::aid-ijc10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Shimada H, Takeda A, Arima M, et al. Serum p53 antibody is a useful tumor marker in superficial esophageal squamous cell carcinoma. Cancer. 2000;89:1677–1683. [PubMed] [Google Scholar]
- 22.Lu H, Goodell V, Disis ML. Humoral immunity directed against tumor-associated antigens as potential biomarkers for the early diagnosis of cancer. J Proteome Res. 2008;7:1388–1394. doi: 10.1021/pr700818f. [DOI] [PubMed] [Google Scholar]
- 23.Zhang JY, Casiano CA, Peng XX, et al. Enhancement of antibody detection in cancer using panel of recombinant tumor-associated antigens. Cancer Epidemiol Biomarkers Prev. 2003;12:136–143. [PubMed] [Google Scholar]
- 24.Montgomery RB, Makary E, Schiffman K, et al. Endogenous anti-HER2 antibodies block HER2 phosphorylation and signaling through extracellular signal-regulated kinase. Cancer Res. 2005;65:650–656. [PubMed] [Google Scholar]
- 25.Speetjens FM, Kuppen PJ, Morreau H, et al. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology. 2008;135:711–712. doi: 10.1053/j.gastro.2008.04.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.