Abstract
Background and purpose
To understand factors related to increases in serum free fatty acid levels (FFA) and association with delayed cerebral ischemia (DCI) after subarachnoid hemorrhage (SAH).
Methods
Serial measurement of systemic oxygen consumption (VO2) by indirect calorimetry (IDC) and FFA levels by liquid chromatography/mass spectrometry in the first 14 days after ictus in 50 consecutive SAH patients. Multivariable GEE models identified associations with FFA levels in the first 14 days after SAH and Cox proportional hazards model utilized to identified associations with time to DCI.
Results
There were 187 measurements in 50 SAH patients (mean age: 56+/−14 years old, 66% women) with a median Hunt Hess Score 3. Adjusting for Hunt Hess grade and daily caloric intake, n-6 and n-3 FFA levels were both associated with VO2 and the modified Fisher score. Fourteen (28%) patients developed DCI on median post bleed day 7. The modified Fisher score (P = 0.01), mean n-6: n-3 FFA ratio (P = 0.02), and mean VO2 level (P = 0.04) were higher in patients that developed DCI. In a Cox proportional hazards model the mean n-6:n-3 FFA ratio (P<0.001), younger age (P = 0.05) and modified Fisher scale (P = 0.004) were associated with time to DCI.
Conclusions
Injury severity and VO2 hypermetabolism are associated with higher n - FFA levels, and an increased n-6:n-3 FFA ratio is associated with DCI. This may indicate a role for interventions that modulate both VO2 and FFA levels to reduce the occurrence of DCI.
Keywords: subarachnoid hemorrhage, vasospasm, fatty acids, oxygen consumption
INTRODUCTION
Cerebrovascular vasospasm, which occurs most commonly between 4 and 14 days post subarachnoid hemorrhage, results in delayed cerebral ischemia (DCI) in approximately 21% of SAH patients and is a leading cause of long term morbidity1, 2. Vasospasm is thought to be the end result of the activation of inflammatory cytokines that affect the reactivity and relaxation of smooth muscles in cerebral vessels. Despite advances in our knowledge of risk factors, prevention and treatment protocols have not significantly altered incidence or sequelae of vasospasm3.
Acute brain injury results in a disturbance in the normal metabolic mechanisms due to sympathetic nervous system activation and systemic inflammatory response resulting in a metabolic state that can promote secondary complications. Studies in other critical illnesses have not only demonstrated the importance of the metabolic response and related sequelae, but have also begun to demonstrate the possible benefit of immune modulating nutritional support4, 5.
The production of serum free fatty acids may represent common pathway by which hypermetabolism influences nutritional status and complications after SAH. The role of lipid peroxidation after SAH has been recognized in both laboratory and clinical settings6. This process directly stimulates smooth muscle contraction by exerting cytotoxic effects on the vessel wall and by generating an inflammatory response involving metabolites of arachidonic acid, an n-6 FFA6, 7.
We recently demonstrated a direct relationship between systemic oxygen consumption (VO2) and inflammation after SAH and further found that acute elevation in VO2 was an independent predictor of delayed cerebral ischemia8. While many factors after acute brain injury can influence changes in VO2, an increase in lipid peroxidation may be the end result of the hypermetabolic state.
In this study we sought to understand the relationship between systemic oxygen consumption and levels of both n-6 and n-3 free fatty acid levels after SAH. We hypothesized that higher levels of n-6 and n-3 FFAs would be related to higher systemic oxygen consumption and further that levels of n-6 FFAs would mediate the relationship between VO2 and DCI in the first 2 weeks after SAH.
METHODS
Patient Selection and Data Collection
This is an analysis of a consecutive group of patients that underwent analysis of serum free fatty acids in addition to comprehensive nutritional assessments (n = 50) of a previously reported prospective observational study8 of aneurysmal subarachnoid hemorrhage (SAH) patients admitted to the Neurological Intensive Care Unit (NICU) at Columbia University Medical Center (CUMC). The criteria for study inclusion have been previously published8. The clinical care for SAH patients at CUMC has been described previously9 and conforms to guidelines set forth by the American Heart Association10.
All study patients underwent serial assessments of FFA, inflammatory and metabolic parameters during the first 14 days after SAH. Each assessment was conducted once during four pre defined time periods or phases: post bleed day (PBD) 0 – 3; PBD 4 – 7; PBD 8 – 10; PBD 11 – 14. All parameters were measured during the same 24 hour period within each phase. Data collection was considered complete in instances when patients died or were discharged from the hospital prior to completion of the four phases. The clinical ICU team was blinded to all IDC, FFA and high sensitivity C-reactive protein (hsCRP) measurements.
SAH data collection
This study was conducted in parallel to data collection for our Subarachnoid Hemorrhage Outcomes Project (SHOP), which has been previously described in detail9, 11. Briefly, SHOP is a prospective outcomes database that since July 1996 has collected data regarding admission and in-hospital characteristics, as well as long-term global outcome in all SAH patients admitted to the NICU at CUMC. Delayed cerebral ischemia (DCI) was defined as either the presence of symptomatic vasospasm or the presence of an infarction on CT scan attributable to vasospasm1. Symptomatic vasospasm was defined as clinical deterioration (i.e. a new focal deficit, decrease in level of consciousness, or both) in the presence of confirmed vasospasm determined by CT Angiography or cerebral angiography. Decreased level of consciousness was defined as a 2-point drop in the Glasgow Coma Score in a 24-hour period. All patients that experience clinical deterioration underwent CT angiography to determine the presence of vasospasm and rule out other causes of deterioration (e.g., fever, hydrocephalus, rebleeding, cerebral edema), followed by medical and/or interventional therapy as indicated. All endpoints were classified according to a priori criteria and adjudicated weekly at a SHOP database meeting. The adjudication process involved a consensus agreement of each endpoint by neurocritical care faculty (NB, KL, JC, SAM) after a complete review of source documentation, imaging, and laboratory tests.
Laboratory measurements
Serum samples were assayed for hsCRP using an enzyme-linked immunoassay (BioCheck, Inc.: normal range < 3.0 mg/L). All other laboratory measures were measured daily as part of routine laboratory testing and recorded as part of the assessment for inflammation and infectious disease status.
Free Fatty Acid Measurement
Serum free fatty acid (FFA) measurements were performed by a liquid chromatography/mass spectrometry (LC/MS) method. Serum samples extracted using a modified Folch protocol12. Briefly, 3 mL of 2:1 chloroform:methanol (v/v) and 50 μL of 0.25 mM deuterated palmitic acid in methanol as internal standard was added to 100 μL of serum in a clean glass tube. The mixture was vortexed well and centrifuged at 3,000 g for 10 min to separate phases. The lower chloroform phase was transferred to another clean glass tube using a Pasteur pipette. Two mL of chloroform added to the remaining upper aqueous phase and mixed well and again centrifuged at 3,000 g for 10 min to separate phases. The lower chloroform phases pooled and evaporated to dryness under nitrogen. The lipid extract was reconstituted in 5 mL of methanol and 500 μL of the redissolved lipid extract transferred to an autosampler vial (Waters, Milford, MA USA) for processing by liquid chromatography mass spectrometry (LC/MS).
LC/MS measurements of specific n-fatty acid concentrations were carried out on a Waters Xevo TQ MS ACQUITY UPLC system (Waters, Milford, MA, USA). The system was controlled by MassLynx Software 4. 1 (Waters Corporation, Milford, MA). Samples in LC/MS vials were maintained at 4°C in the autosampler until 5 μL injected onto a Waters ACQUITY UPLC BEH C18 column (2.1 × 100 mm, 1.7 μm particle size, Waters), and a 2.1 × 5 mm guard column using the same packing material. The column was maintained at 40 °C. The flow rate was 300 μL/min in a binary gradient mode initiated using 15% solvent A [100% H2O] and 85% solvent B [100% Methanol]. The concentration of solvent B was increased linearly to 100% over 8 min and maintained at 100% until 10 min. The gradient was then reequilibrated to 15% solvent A and 85% solvent B over 2 min before the next sample was injected. Negative ESI-MS mass spectrometry using the selected ion recording (SIR) mode was performed, employing the following parameters: capillary voltage −3.8 kV; source temperature 150 °C; desolvation temperature 500 °C; and desolvation gas flow 1000 L/hr. Specific n-6 and n-3 compounds extracted are listed in Table 1.
Table 1. Specific n - fatty acids (FAs) extracted using liquid chromatography/mass spectroscopy.
List of specific n – 6 and n – 3 fatty acids extracted from 187 samples in 50 patients.
| n-6 FFAs | n-3 FFAs | ||
|---|---|---|---|
|
| |||
| Compound | Name | Compound | Name |
|
| |||
| C 18:2 | Linoleic Acid | C 18:3 | α-Linolenic Acid |
| C 18:3 | γ-Linolenic Acid | C 20:4 | Eicosatetraenoic Acid |
| C 20:2 | Eicosadienoic Acid | C 20:5 | Eicosapentaenoic Acid |
| C 20:4 | Arachidonic Acid | C 22:6 | Docosahexaenoic Acid |
Oxygen consumption measurements
Details regarding the method by which we performed indirect calorimetry (IDC) studies have been previously published8, 13. Each IDC assessment was based upon a steady state, which was defined as a 20 – 30 minute interval during which average minute oxygen consumption (VO2) and carbon dioxide production (VCO2) changed by <5% and <10%, respectively. Continuous measurements were averaged every 60 seconds throughout the entire IDC session.
Statistical analysis
Continuous variables were assessed for normality. Normally distributed data was reported as a mean and standard deviation. Nonparametric data was reported and analyzed as a median with 25% and 75% percentile values. Categorical variables were reported as count and proportions in each group. Generalized estimating equation (GEE) analyses were performed with an identity link function and an exchangeable within-group correlation structure to determine the relationship between FFA and VO2 as well as factors that may predict either FFA or VO2 measurements. Multivariate models were built by entering in those factors found to have a P value ≤ 0.1 on univariate analysis. Among similar variables that were highly intercorrelated, only the variable with the largest Wald Chi square value and smallest P value in the GEE analysis was used as a candidate variable in the final multivariate model. Factors that were found on univariate analysis to be associated (P < 0.1) with DCI were entered into a backward Wald Cox proportional hazards model to calculate adjusted hazards ratios and corresponding 95% CI for developing DCI. Poor outcome at 3 months post hemorrhage was defined as a modified Rankin scale score >=4. For all tests, significance was set at P < 0.05. All analyses were performed with SPSS V16.0 (Chicago, Illinois).
Informed consent
Given the minimal risk of this study and utilization of residual blood and urine for laboratory assessments, this study was conducted with a waiver of consent when necessary. Data was linked with the SHOP database, which utilizes a tiered consent process, whereby consent was obtained from those patients that were able to provide consent at the time of injury. In neurologically impaired patients, family members were approached for assent to participation in the study. In cases where capacity was regained, patients were directly approached for consent. This process of consent and the conduct of both studies was approved by the Institutional Review Board and were consistent with guiding principles for research involving humans14.
RESULTS
Baseline characteristics
Of the 65 SAH patients admitted during the study period, 50 met inclusion criteria for study (mean age 56 +/−14 years old, 66%women). Six were excluded for arriving late, 5 for early withdrawal of care, 4 for inability to perform IDC. The mean BMI 28+/− 6 kg/m2 and the median admission Hunt Hess grade was 3 (IQR: 2, 4) and modified Fisher score 3 (IQR: 3,4). Admission variables categorized by DCI status are shown in Table 2. Patients who developed DCI were more likely to have a poor outcome (modified Rankin score≥ 4) at 3 months than those who did not develop DCI (71% v. 36 %, P = 0.03).
Table 2. Baseline Characteristics of SAH Patients (N= 50).
Admission characteristics of SAH patients separated by delayed cerebral ischemia status.
| Characteristic1 | Delayed Cerebral Ischemia | P value3 | |
|---|---|---|---|
| No (n = 36) | Yes (n = 14) | ||
| Age, years | 57 (13) | 54 (16) 0.5 | |
| Women | 21 (58) | 12 (86) | 0.2 |
| Race | 0.4 | ||
| Black | 8 (22) | 2 (14) | |
| White, non Hispanic | 14 (39) | 3 (21) | |
| White, Hispanic | 13 (36) | 7 (50) | |
| Asian | 1 (3) | 2 (14) | |
| Body Mass Index, kg/m2 | 28 (6) | 27 (6) | 0.4 |
| Aneurysm size, mm | 6 (4,9) | 6 (4,8) | 0.8 |
| Aneurysm Clipping | 25 (69) | 11 (79) | 0.7 |
| APACHE2 2 Score | 17 (8) | 17 (8) | 1.0 |
| Temperature, °F | 98 (2) | 99 (1) | 0.3 |
| Glucose (mmol/L) | 9 (2) | 10 (2) | 0.4 |
| Glasgow Coma Scale Score | 13 (7,15) | 10 (8,15) | 0.8 |
| Hunt Hess Score | 0.9 | ||
| 1 & 2 | 11 (31) | 2 (14) | |
| 3 | 9 (25) | 4 (29) | |
| 4 | 10 (28) | 5 (36) | |
| 5 | 6 (17) | 3 (21) | |
| Modified Fisher Score | 0.01 | ||
| 1 | 4 (11) | -- | |
| 2 | 7 (19) | -- | |
| 3 | 18 (50) | 5 (36) | |
| 4 | 7 (19) | 9 (64) | |
All continuous measures are shown as mean (SD). Glasgow coma scale score and aneurysm size shown as median (25thile, 75thile). Categorical variables shown as n(%).
Acute Physiologic and Chronic Health Evaluation score.
P value representative of Fisher’s exact test or Chi square test for categorical variables and Independent t – test for continuous variables, with the exception of aneurysm size and Glasgow Coma Scale for which Mann-Whitney U test was performed.
Indirect Calorimetry Measurements
There were 187 measurements in 50 patients with a 14 day mean VO2 of 240 +/− 70 ml/min per patient. There were 86 measurements done during mechanical ventilation with 46 of these measurements done while patients were on propofol (median dose: 30 mcg/kg/min, IQR: 16 mcg/kg/min – 50 mcg/kg/min). The median daily caloric intake during the day of each IDC measurement was 7.7 calories/kg (IQR: 1.2 calories/kg, 16.6 calories/kg). The median hsCRP during each IDC assessments was 46.1 mg/L (IQR: 19.1 mg/L, 116.5 mg/L).
n-Free Fatty Acid (FFA) measurements
The overall 14 day mean n-6 FFA and n-3 FFA levels were 206.3 +/− 79.3 μmol/L and 19.2 +/− 6.4 μmol/L, respectively, with a 14 day mean n-6:n-3 FFA ratio of 12.1 +/− 5.1. The use of propofol was not associated with a mean difference in level of n-6 FFA (247.6+/−84.5 μmol/L v. 187.4 +/− 22.7 μmol/L, P = 0.2), n-3 FFA (21.0 +/− 5.8 μmol/L v 18.0 +/− 5.7 μmol/L, P =0.5) and n-6: n-3 ratio (12.5+/− 5.8 v. 11.5 +/− 4.5, P =0.5). The amount of fat delivered in enteral nutrition and via propofol infusions did not correlate with n-6 FFA (Spearman’s rho = 0.1, P = 0.5) or n-3 FFA (Spearman’s Rho = 0.03, P = 0.7) levels. In separate multivariate GEE models, n-6 FFA levels and n-3 FFA levels were each found to be associated with VO2, hsCRP, and the modified Fisher scale (Table 3). The mean 14 day n – 6 FFA, mean 14 day n-3 FFA, and mean n-6:n-3 FFA ratio were not associated with poor outcome (modified Rankin scale score ≥ 4) at 3 months after hemorrhage.
Table 3. Factors associated with n-Free Fatty Acid levels after SAH.
Multivariate GEE analyses for factors associated with n-6 and n-3 free fatty acid levels.
| Factor | β Coefficient | Wald Chi Square | P value |
|---|---|---|---|
| n-6 Free Fatty Acid | |||
| Hunt Hess Grade | 23.5 | 1.86 | 0.17 |
| modified Fisher Scale | 93.0 | 6.73 | 0.01 |
| hsCRP1 (mg/L) | 0.65 | 3.95 | 0.04 |
| VO22 (ml/min) | 2.0 | 11 | 0.001 |
| Caloric intake (calories/kg) | −0.84 | 0.22 | 0.64 |
| n-3 Free Fatty Acid | |||
| Hunt Hess Grade | 2.54 | 1.74 | 0.19 |
| modified Fisher Scale | 10.4 | 8.1 | 0.01 |
| hsCRP (mg/L) | 0.07 | 4.24 | 0.03 |
| VO2 (ml/min) | 0.26 | 15.67 | <0.001 |
| Caloric intake (calories/kg) | −0.02 | 0.01 | 0.91 |
high sensitivity C – reactive Protein.
systemic oxygen consumption
Delayed cerebral ischemia
Fourteen (28%) patients developed DCI on median post bleed day 7 (IQR: 6, 10). The mean VO2 (284+/−50 ml/min v. 235+/− 65, P = 0.04), as well as the mean n-6 levels (278 +/− 103 μmol/L v. 170 +/− 89 μmol/L, P = 0.03) and mean n-6: n-3 FFA ratio (15 +/−7 v. 10+/−2, P = 0.02) were all higher in patients that developed DCI. There was no difference in the mean n-3 FFA levels between DCI and non DCI patients (18 +/− 9 μmol/L v. 21 +/− 12 μmol/L, P = 0.6). The 14 day mean n-6: n-3 FFA ratio had a higher area under the curve for predicting DCI than the 14 day mean n-6 FFA level (Figure 1). An n-6:n3 FFA ratio of ≥ 8.8 was found to have a sensitivity of 93% and specificity of 80% for predicting DCI. The median time from n – FFA and VO2 measurement and diagnosis of DCI was 24 hours [range: 12 hours – 48 hours]. In a backward Wald Cox proportional hazards model adjusting for VO2 and sex, the modified Fisher score (P = 0.004), younger age (P = 0.05) and n-6:n3 FFA ratio (P <0.001) predicted time to DCI (Table 4).
Figure 1.
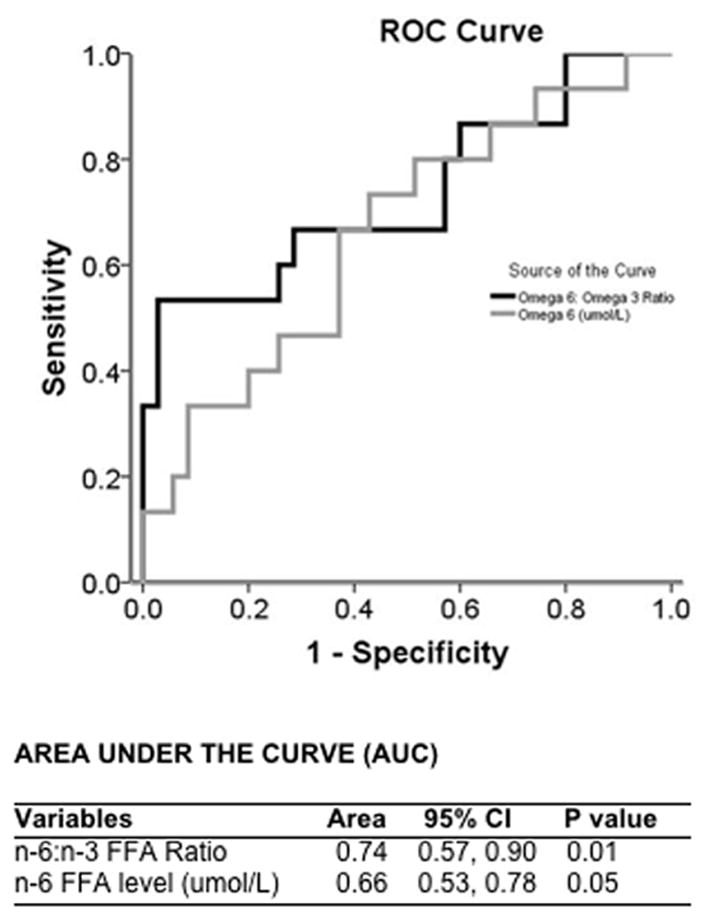
ROC analysis predicting delayed cerebral ischemia
Table 4.
Multivariable Cox Proportional Hazards Model predicting time to DCI
| Factor | Hazards Ratio | 95% CI | P value |
|---|---|---|---|
| n-6:n-3 FFA Ratio1,2 | 2.93 | 1.76, 4.88 | <0.001 |
| Modified Fisher Scale | 2.83 | 1.39, 5.74 | 0.004 |
| Age3 | 0.83 | 0.69, 0.99 | 0.05 |
FFA – Free Fatty Acid.
Hazards ratio represents the risk increase for every standard deviation (5.1) increase in ratio.
Hazards ratio represents the risk decrease for every 5 year increase in age.
DISCUSSION
We found that higher n-6 and n-3 FFA levels were associated with higher O2 consumption and severity of initial hemorrhage15. Additionally, an increased ratio of n-6: n-3 FFAs was independently associated with DCI after accounting for hemorrhage severity. These findings indicate that lipid peroxidation may mediate the relationship between hypermetabolism and DCI.
Lipid peroxidation after experimental SAH has been extensively studied, and metabolites of n-6 FFAs such as arachidonic acid have been implicated in the pathophysiology of vasospasm and related complications6, and has led to the development of a class of steroids that target lipid peroxidation. Tirilazad, a non-glucocorticoid 21 amino-steroid free radical scavenger with a mechanism of action believed to be an inhibition of iron-dependent lipid peroxidation was studied in several controlled trials following promising results in primate vasospasm models. The results from these multiple clinical trials demonstrated a consistent reduction in vasospasm and vasospasm related complications, whereas there was an inconsistent effect on global outcomes16, 17. Despite these clinical therapeutic trials, few studies have focused on determinants of lipid peroxidation and serum levels of FFA in SAH patients.
Similar to a study demonstrating FFA elevation in the cerebrospinal fluid of SAH patients7, we found that hemorrhage severity was associated with the extent of FFA elevation in the serum. However, our study is unique in the assessment of VO2 and its potential role in the production of FFAs. Our study design precludes determining causality, but it is plausible that the hypermetabolic state was influenced by inflammatory status; leading to increases fat utilization and rise in serum FFAs levels. In studies of non brain injured critically ill patients, an inflammation-mediated hypermetabolic state has been linked to increases in FFAs, with subsequent development of nutritional interventions designed to modulate the immune response and blunt downstream sequelae4, 18, 19. We recently reported the strong relationship between hsCRP and VO2 after SAH, and demonstrated the ability of rises in VO2 to predict the occurrence of DCI8. The current analyses point to the possibility that n-6 FFAs may mediate the relationship between VO2 and DCI.
Our analysis found that the ratio of n-6: n-3 FFAs ratio was more highly associated with DCI than just absolute levels of n-6 FFAs alone. The imbalance between these classes of FFAs may play a role in subsequent injury. Metabolic balance among different classes of fatty acids has been shown to be necessary for optimal cellular function. Moreover, n-6 FFAs and n-3 FFAs compete in metabolic pathways that impact cellular responses to physiologic stress. The resultant competition for cyclooxygenases and lipoxygenases determines which types of eicosanoids will be synthesized and thus potentially influence inflammatory and vascular responses.
Because membrane n-6 and n-3 fatty acids are derived from the diet, tissue imbalances might be corrected by either decreasing n-6 fatty acid intake and/or by increasing n-3 fatty acid intake. As seen in our study, traditional enteral nutritional formulations do not impact FFA levels. The administration of n-3 fatty acids may help establish a causal link in SAH patients by modulation of both responses given their competition for lipoxygenase and cyclooxygenase and resultant reduction and opposing effect on the inflammatory modulators, which are the metabolic products of arachidonic acid (n-6 FFA) when acted on by these enzymes. Formulations enriched with n-3 fatty acids have already been shown to modulate the inflammatory response and improve physiologic profiles in ARDS patients4, 20.
In a recent prospective pilot randomized clinical trial of SAH patients, eicosapentaenoic acid (EPA), a n – 3 fatty acid, was orally administered at a daily dose of 1800 mg between days day 4 to day 14 and compared to placebo in terms of the frequency of symptomatic vasospasm, and cerebral infarction21. Serum levels of EPA increased significantly and were associated with a decreased frequency of symptomatic vasospasm related deterioration and infarcts. Findings of this pilot study need further confirmation with additional data regarding VO2 as well as EPA levels and n-6 FFAs levels to better understand the mechanism by which n-3 FFAs may reduce the occurrence of DCI and improve outcome after SAH.
Our study has several strengths. First, all measurements and data collection were conducted prospectively, with the clinical team blinded to results from IDC testing and FFA assessments. This eliminated any influence the knowledge of metabolic or inflammatory measurements may have had upon diagnostic or therapeutic management, especially as it pertained to DCI. We carefully recorded and analyzed all pharmacological and physiological parameters that may have confounded the relationship between VO2 and FFAs. Finally, we used prospectively documented on the day of occurrence a strict, validated definition for our primary endpoint, delayed cerebral ischemia1, which has been previously validated as a strong predictor of outcome after SAH. There are limitations to this study, however. Though we had 187 measurements, there were only 50 patients studied, and therefore our results may not fully describe the relationship between n-FFAs and nutritional intake. We believe that these results do provide preliminary evidence for the importance of the interrelationship between hypermetabolism, lipid peroxidation and delayed cerebral ischemia after SAH, and warrant further study. Serial measurements of FFAs and VO2 have not been previously reported in SAH patients, and provide for novel analyses; however, we did not make these measurements daily, potentially biasing our analyses regarding their interrelationship and that with DCI. The median time difference between measurement and diagnosis was brief, and therefore the effect, if any, of a time delay between measurement and DCI diagnosis should be minimal.
In summary we believe there is evidence for an interrelationship between VO2, the increase in n-6 FFAs and n-6/n-3 FFA ratio, and DCI after SAH. This suggests that immunomodulatory interventions using n-3 FFAs may reduce the incidence and impact of DCI after SAH, and provides a rationale for further studies to test this approach.
Acknowledgments
Funding:
The project described was supported by Grant Number UL1 RR024156 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at NCRR Website. Information on Re-engineering the Clinical Research Enterprise can be obtained from NIH Roadmap website.
The authors would like to acknowledge the laboratory of William S. Blaner, PhD in the Division of Preventive Medicine and Nutrition, Department of Internal Medicine at Columbia University College of Physicians and Surgeons for their work on isolating n –free fatty acids from serum samples.
Footnotes
Disclosures
There are no disclosures from any of the co authors related to this publication
References
- 1.Frontera JA, Fernandez A, Schmidt JM, Claassen J, Wartenberg KE, Badjatia N, et al. Defining vasospasm after subarachnoid hemorrhage: What is the most clinically relevant definition? Stroke. 2009;40:1963–1968. doi: 10.1161/STROKEAHA.108.544700. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt JM, Wartenberg KE, Fernandez A, Claassen J, Rincon F, Ostapkovich ND, et al. Frequency and clinical impact of asymptomatic cerebral infarction due to vasospasm after subarachnoid hemorrhage. J Neurosurg. 2008;109:1052–1059. doi: 10.3171/JNS.2008.109.12.1052. [DOI] [PubMed] [Google Scholar]
- 3.Pluta RM, Butman JA, Schatlo B, Johnson DL, Oldfield EH. Subarachnoid hemorrhage and the distribution of drugs delivered into the cerebrospinal fluid. Laboratory investigation. J Neurosurg. 2009;111:1001–1007. 1001–1004. doi: 10.3171/2009.2.JNS081256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer P, Shapiro H, Theilla M, Anbar R, Singer J, Cohen J. Anti-inflammatory properties of n--3 fatty acids in critical illness: Novel mechanisms and an integrative perspective. Intensive Care Med. 2008;34:1580–1592. doi: 10.1007/s00134-008-1142-4. [DOI] [PubMed] [Google Scholar]
- 5.Weitzel LR, Wischmeyer PE. Glutamine in critical illness: The time has come, the time is now. Crit Care Clin. 2010;26:515–525. ix–x. doi: 10.1016/j.ccc.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Sehba FA, Bederson JB. Mechanisms of acute brain injury after subarachnoid hemorrhage. Neurol Res. 2006;28:381–398. doi: 10.1179/016164106X114991. [DOI] [PubMed] [Google Scholar]
- 7.Pilitsis JG, Coplin WM, O’Regan MH, Wellwood JM, Diaz FG, Fairfax MR, et al. Free fatty acids in human cerebrospinal fluid following subarachnoid hemorrhage and their potential role in vasospasm: A preliminary observation. J Neurosurg. 2002;97:272–279. doi: 10.3171/jns.2002.97.2.0272. [DOI] [PubMed] [Google Scholar]
- 8.Badjatia N, Carpenter A, Fernandez L, Schmidt J, Mayer S, Claassen J, Lee K, Connolly E, Seres D, Elkind M. Relationship between c-reactive protein, systemic oxygen consumption and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Stroke. 2011 doi: 10.1161/STROKEAHA.111.614685. In press. [DOI] [PubMed] [Google Scholar]
- 9.Wartenberg KE, Schmidt JM, Claassen J, Temes RE, Frontera JA, Ostapkovich N, et al. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med. 2006;34:617–623. doi: 10.1097/01.ccm.0000201903.46435.35. quiz 624. [DOI] [PubMed] [Google Scholar]
- 10.Bederson JB, Connolly ES, Jr, Batjer HH, Dacey RG, Dion JE, Diringer MN, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A statement for healthcare professionals from a special writing group of the stroke council, american heart association. Stroke. 2009;40:994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 11.Frontera JA, Fernandez A, Schmidt JM, Claassen J, Wartenberg KE, Badjatia N, et al. Impact of nosocomial infectious complications after subarachnoid hemorrhage. Neurosurgery. 2008;62:80–87. doi: 10.1227/01.NEU.0000311064.18368.EA. discussion 87. [DOI] [PubMed] [Google Scholar]
- 12.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 13.Badjatia N, Strongilis E, Gordon E, Prescutti M, Fernandez L, Fernandez A, et al. Metabolic impact of shivering during therapeutic temperature modulation: The bedside shivering assessment scale. Stroke. 2008;39:3242–3247. doi: 10.1161/STROKEAHA.108.523654. [DOI] [PubMed] [Google Scholar]
- 14.American Physiological Society. Guiding principles for research involving animals and human beings. American Journal of Physiology - Cell Physiology. 2002;282:3. doi: 10.1152/ajpregu.00279.2002. [DOI] [PubMed] [Google Scholar]
- 15.Claassen J, Bernardini GL, Kreiter K, Bates J, Du YE, Copeland D, et al. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: The fisher scale revisited. Stroke. 2001;32:2012–2020. doi: 10.1161/hs0901.095677. [DOI] [PubMed] [Google Scholar]
- 16.Jang YG, Ilodigwe D, Macdonald RL. Metaanalysis of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage. Neurocritical Care. 2009;10:141–147. doi: 10.1007/s12028-008-9147-y. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki H, Kanamaru K, Kuroki M, Sun H, Waga S, Miyazawa T. Effects of tirilazad mesylate on vasospasm and phospholipid hydroperoxides in a primate model of subarachnoid hemorrhage. Stroke. 1999;30:450–455. doi: 10.1161/01.str.30.2.450. discussion 455–456. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Li W, Li N, Li J. N--3 fatty acids-supplemented parenteral nutrition decreases hyperinflammatory response and attenuates systemic disease sequelae in severe acute pancreatitis: A randomized and controlled study. JPEN J Parenter Enteral Nutr. 2008;32:236–241. doi: 10.1177/0148607108316189. [DOI] [PubMed] [Google Scholar]
- 19.Kelley DS, Siegel D, Fedor DM, Adkins Y, Mackey BE. Dha supplementation decreases serum c-reactive protein and other markers of inflammation in hypertriglyceridemic men. Journal of Nutrition. 2009;139:495–501. doi: 10.3945/jn.108.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pontes-Arruda A, Demichele S, Seth A, Singer P. The use of an inflammation-modulating diet in patients with acute lung injury or acute respiratory distress syndrome: A meta-analysis of outcome data. JPEN J Parenter Enteral Nutr. 2008;32:596–605. doi: 10.1177/0148607108324203. [DOI] [PubMed] [Google Scholar]
- 21.Shirao S, Fujisawa H, Kudo A, Kurokawa T, Yoneda H, Kunitsugu I, et al. Inhibitory effects of eicosapentaenoic acid on chronic cerebral vasospasm after subarachnoid hemorrhage: Possible involvement of a sphingosylphosphorylcholine-rho-kinase pathway. Cerebrovasc Dis. 2008;26:30–37. doi: 10.1159/000135650. [DOI] [PubMed] [Google Scholar]


