Abstract
To investigate the intercellular control of glutathione synthesis and its influence on leaf redox state in response to short-term chilling, genes encoding γ-glutamylcysteine synthetase (γ-ECS) and glutathione synthetase (GSH-S) were cloned from maize (Zea mays) and specific antibodies produced. These tools were used to provide the first information on the intercellular distribution of γ-ECS and GSH-S transcript and protein in maize leaves, in both optimal conditions and chilling stress. A 2-d exposure to low growth temperatures (chill) had no effect on leaf phenotype, whereas return to optimal temperatures (recovery) caused extensive leaf bleaching. The chill did not affect total leaf GSH-S transcripts but strongly induced γ-ECS mRNA, an effect reversed during recovery. The chilling-induced increase in γ-ECS transcripts was not accompanied by enhanced total leaf γ-ECS protein or extractable activity. In situ hybridization and immunolocalization of leaf sections showed that γ-ECS and GSH-S transcripts and proteins were found in both the bundle sheath (BS) and the mesophyll cells under optimal conditions. Chilling increased γ-ECS transcript and protein in the BS but not in the mesophyll cells. Increased BS γ-ECS was correlated with a 2-fold increase in both leaf Cys and γ-glutamylcysteine, but leaf total glutathione significantly increased only in the recovery period, when the reduced glutathione to glutathione disulfide ratio decreased 3-fold. Thus, while there was a specific increase in the potential contribution of the BS cells to glutathione synthesis during chilling, it did not result in enhanced leaf glutathione accumulation at low temperatures. Return to optimal temperatures allowed glutathione to increase, particularly glutathione disulfide, and this was associated with leaf chlorosis.
Stress survival strategies are essential for sedentary organisms, and evolution has conferred on plants a high degree of plasticity that underpins survival in a constantly changing environment (Pastori and Foyer, 2002). One of the most important and fluctuating environmental factors limiting the geographic distribution of plant species is temperature, and the rapidity with which plants can respond to temperature changes is key to growth and reproductive success (Levitt, 1962). Plants vary greatly in their ability to tolerate low growth temperatures, and they have different capacities for regulating metabolism and maintaining redox homeostasis at low temperatures. Species can be classified according to the threshold below which chilling injury is observed. Chilling-sensitive plants usually show decreased photosynthesis and growth between 10°C and 15°C (Levitt, 1962). Many cereals are able to withstand long periods of subzero temperatures by virtue of the process called cold acclimation (Hughes and Dunn, 1990). However, maize (Zea mays), a C4 species of subtropical origin, is chilling sensitive and shows little or no cold acclimation traits.
The chilling sensitivity of maize leaves is likely related to their high degree of cellular specialization. Maize leaves have Kranz anatomy and show extreme cellular differentiation between bundle sheath (BS) and mesophyll (M) cells, which have specialized metabolic roles. The Benson-Calvin cycle is restricted to the BS cells, minimizing photorespiration and allowing increased carbon, nitrogen, and water use efficiency. However, BS cells produce relatively little reductant, and, thus, antioxidant enzymes that require NADH or NADPH are restricted to the maize M cells, rendering BS proteins more susceptible to oxidative damage (Kingston-Smith and Foyer, 2000). The extreme structural and functional specialization of the BS and M cells requires rapid intercellular exchange of metabolites (Leegood, 1985), including antioxidants (Doulis et al., 1997), and this poses important problems for the operation and regulation of photosynthesis and antioxidant metabolism at low temperatures (Kingston-Smith et al., 1999). We have extensively investigated the mechanism of the sensitivity of photosynthesis to chilling in the H99 variety (Doulis et al., 1997; Kingston-Smith et al., 1999; Kingston-Smith and Foyer, 2000; Pastori et al., 2000a, 2000b), which we have chosen to study because it is a chilling-sensitive, dent-type maize that is relatively easy to transform (Van Breusegem, 1998).
Chilling resistance requires effective up-regulation of the antioxidant system because limitations on leaf metabolism at low temperature promote increased electron transport to oxygen and hence increased production of active oxygen species, including superoxide, H2O2, and hydroxyl radicals (Foyer and Harbinson, 1994; Fryer et al., 1998). Avoidance of active oxygen species-induced cell death and senescence requires efficient antioxidant protection (Foyer and Noctor, 2000). Among the battery of antioxidants found in leaves, the tripeptide thiol glutathione (GSH; γ-Glu-Cys-Gly) is strongly implicated in chilling tolerance, particularly in maize (Kocsy et al., 1996, 2000a, 2000b, 2001a, 2001b). Glutathione is the major thiol redox buffer in the soluble phase of most aerobic cells and in plants is involved in redox signaling, modulation of enzyme activity, control of root development, and in processes that modify and transport hormones and other endogenous compounds and xenobiotics, via formation of glutathione S-conjugates (May et al., 1998a; Noctor and Foyer, 1998a). Especially at low temperatures, the cyclic interconversion of dithiols and disulfides is key to cell defense processes (Levitt, 1962; Kunert and Foyer, 1993).
In all organisms studied so far, biosynthesis of GSH occurs from constituent amino acids via γ-glutamylcysteine synthetase (γ-ECS) and glutathione synthetase (GSH-S; Hell and Bergmann, 1988, 1990; Meister, 1988; Rennenberg, 1997). In recent years, studies in model systems have increased our understanding of how glutathione synthesis is controlled. Genes encoding the above enzymes have been identified from a number of C3 dicotyledonous plants such as Arabidopsis, tomato (Lycopersicon esculentum), Brassica juncea, and Medicago trunculata. In Arabidopsis γ-ECS appears to be encoded by a single gene, gsh1, while the gene that encodes GSH-S produces both cytosolic and plastidic isoforms by alternative mRNA splicing. All the plant enzymes display high sequence homology. Plant transformation and expression studies in C3 plants have identified two main factors likely to control the accumulation of GSH: γ-ECS abundance and Cys availability (Noctor et al., 1996, 1998a, 1998b, 2002; Xiang and Oliver, 1998; Xiang and Bertrand, 2000; Xiang et al., 2001). Despite the importance of this thiol in chilling stress, there is limited knowledge of the factors that determine or limit glutathione accumulation and redox potential in these conditions. In maize, our knowledge of the regulation of glutathione biosynthesis during the chilling response is limited by the lack of gene sequences for any C4 or monocotyledonous species and by the absence of expression data for γ-ECS and GSH-S during chilling. The regulation of glutathione synthesis in maize is further complicated by data that suggest that, whereas Cys synthesis occurs exclusively in the BS, GSH-S activity is located primarily in the M cells (Burgener et al., 1998). No data has yet appeared on γ-ECS localization in maize leaves.
Leaf glutathione contents correlate with chilling resistance in maize (Kocsy et al., 1996, 2000a, 2000b, 2001a, 2001b). Furthermore, our previous data suggest that one factor in the sensitivity of maize to both short-term and long-term chilling may be restrictions over glutathione reduction and cycling between cell types (Doulis et al., 1997; Pastori et al., 2000a). The aims of this study were (1) to investigate the intercellular distribution of the expression of γ-ECS and GSH-S in maize leaves and (2) to explore how these enzymes and glutathione content and redox state respond to short-term chilling stress.
RESULTS
Cloning γ-ECS and GSH-S from Maize
To investigate the control of the intercellular distribution of GSH synthesis in maize and its response to chilling, maize γ-ECS and GSH-S cDNAs were cloned using a PCR-based approach. The cDNA obtained for γ-ECS (EMBL Nucleotide Sequence Database accession no. AJ302783) was 1,664 bp in length with an open reading frame coding for 437 amino acids. The cloned maize γ-ECS shares a high degree of identity with the homologous amino acid sequences from tomato (83%), Arabidopsis (82%), and M. trunculata (82%). The deduced amino acid sequence predicted a 51-kD protein with a pI of 5.5. Conserved features of the predicted proteins from other species are also present in the maize sequence (i.e. an active site Cys residue and glutathione disulfide [GSSG] binding site). The 1,608-bp GSH-S cDNA (EMBL Nucleotide Sequence Database accession no. AJ302784) isolated from maize shows approximately 69% identity with the predicted B. juncea GSH-S protein. The open reading frame between nucleotides 278 and 1,515 encodes a protein of 409 amino acids that contains the conserved active site Cys residues and Gly binding motif.
We used in silico analyses to test both sequences for targeting, including mitochondrial targeting (see http://www.cbs.dtu.dk/services/TargetP/). For both γ-ECS and GSH-S sequences, probability values were well below those predicted for targeting to chloroplasts or mitochondria. While other published γ-ECS protein sequences show a putative transit peptide in the N terminus of the protein and a highly conserved Ile-X-Ala↓Ala cleavage motif (van Heyne, 1983), 5′ RACE analysis clearly indicates that the 5′ end of the cDNA is complete, suggesting the absence of a transit peptide. As for the maize γ-ECS cDNA, no transit peptide could be predicted from the GSH-S amino acid sequence.
A bacterial artificial chromosome library generated by partial HindIII digestion of genomic DNA from the maize Flint inbred line F2 was then screened to identify further possible members of the gene families. This library contains 86,850 clones with an average insert size of 90 kb and covers the genome approximately 3.2 times (Ripoll et al., 2000). Once positive clones were isolated, the inserts were digested with HindIII, HindIII plus EcoRI, BamHI, and SalI, then hybridized with the corresponding homologous probe. Fragments that hybridized to the γ-ECS or GSH-S probes were subcloned in pGEM-3Zf (Promega, Madison, WI) and partially sequenced to confirm the identity. The probes used to identify the positive clones for γ-ECS and GSH-S were identical to those used in the northern-blot experiments. After two rounds of screening of the whole library and further confirmation by dot blotting, five positive clones for γ-ECS (889H4, 802A9, 782G11, 598D3, and 857G10) and two for GSH-S (389A11 and 785B1) were obtained. The fingerprints obtained by restriction enzyme digestion of the inserts and subsequent hybridization with homologous probes showed that the five clones of γ-ECS represented two or three different copies of the γ-ECS gene. The two clones corresponding to GSH-S contained identical copies of the gene. Partial sequencing confirmed the identity of the fragments isolated.
Expression and Localization of γ-ECS and GSH-S Transcripts under Chilling
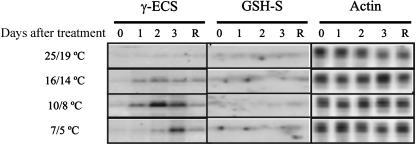
We investigated GSH synthesis in young maize plants that were maintained at optimal temperature, subjected to a short-term chill, or subjected to a short-term chill followed by a recovery period at optimal temperature. As Figure 1 shows, leaf bleaching was only observed after return to optimal growth temperatures. Measurement of chlorophyll contents showed that these did not change during the chill itself but had declined 60% after 3-d recovery (data not shown). Having cloned the cDNAs for γ-ECS and GSH-S, we examined how the corresponding transcripts isolated from whole maize leaves responded to the treatments detailed in the legend to Figure 1. The expression of both transcripts was uniformly low in maize leaves under optimal growth conditions (Fig. 2). The abundance of leaf γ-ECS transcripts was increased by exposure to 10°C (day)/8°C (night). Transfer to 16°C/14°C also increased γ-ECS transcript levels, while after transfer to 7°C/5°C these transcripts were only increased after 3 d, suggesting a delay in the induction of gene expression under these conditions. Following return to optimal growth conditions, the abundance of leaf γ-ECS transcripts decreased to values observed in controls that had not been exposed to low growth temperatures (Fig. 2). In contrast to γ-ECS transcripts, those for GSH-S were difficult to detect under all conditions, and no evidence was obtained for induction at 10°C/8°C (Fig. 2). Analyses of plants exposed to less or more severe chilling conditions did not show induction of GSH-S transcripts (Fig. 2). Unlike the chilling-enhanced accumulation of γ-ECS transcripts in maize leaves, the chilling treatments used here diminished γ-ECS expression in roots (data not shown).
Figure 1.
Moderate short-term chilling causes bleaching of maize leaves only after return to optimum temperatures. Top, whole plants. Bottom, second leaf. A, Plants maintained at optimal growth temperatures (25°C [day]/19°C [night]). B, Plants after 2 d of exposure to suboptimal growth temperatures (10°C/8°C). C, Plants after 2 d of exposure to suboptimal growth temperatures (10°C/8°C), followed by 2 d of subsequent recovery at optimal temperature (25°C/19°C).
Figure 2.
Expression of γ-ECS and GSH-S in maize leaves. Northern-blot analysis was used to measure maize leaf γ-ECS and GSH-S transcripts in plants maintained either at optimal growth temperatures (25°C/19°C) on day 0 or exposed for 3 d (lanes 1, 2, and 3) to either 16°C (day)/14°C (night), 10°C/8°C, or 7°C/5°C temperatures. In each case, one group of plants was then examined after 2 d of recovery (R) at optimal growth temperatures. Actin was used as the internal control.
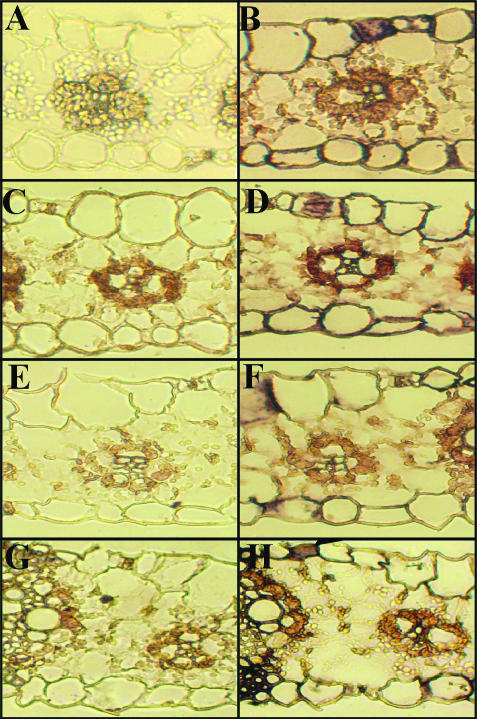
Given that the most marked induction of global leaf γ-ECS transcript was observed at 10°C/8°C, we used this condition in the experiments described below. First, we examined the intercellular distribution of γ-ECS and GSH-S transcripts and its response to chilling. Figure 3 shows a representative hybridization of γ-ECS and GSH-S transcripts in transverse section of maize leaves. Examination of numerous hybridizations with different sections revealed that: (1) Under optimal growth conditions, γ-ECS transcripts were most abundant in the epidermal cells, including the stomatal guard cells, and in the M cells (Fig. 3B). (2) After chilling, the clearest change in staining was located to the BS cells, where γ-ECS transcripts showed a clear increase (Fig. 3, compare B and D), which was reversible during the recovery period (Fig. 3, compare D and F). In the M cells, however, some decrease in the signal was observed in sections of chilled leaves (Fig. 3D), followed by reappearance of signal during the recovery phase (Fig. 3F). (3) Staining for GSH-S transcripts was observed in most cell types at optimal temperatures (Fig. 3H), and no clear change was apparent following the temperature treatments examined here (data not shown).
Figure 3.
The intercellular distribution of γ-ECS and GSH-S transcripts in maize leaves. Left, hybridization with sense control sequence. Right, hybridization with antisense probe. In situ localization was performed for both transcripts under optimal conditions (A and B, γ-ECS; G and H, GSH-S). In addition, the localization of γ-ECS transcripts is shown after short-term chilling (C and D) and following recovery from chilling (E and F). A and B, γ-ECS transcripts; plants maintained at 25°C/19°C. C and D, γ-ECS transcripts; plants grown at 25°C/19°C and transferred to 10°C/8°C for 2 d (chill). E and F, as in C and D, but 2 d after subsequent transfer back to 25°C/19°C (recovery). G and H, GSH-S transcripts; plants maintained at 25°C/19°C.
γ-ECS and GSH-S Protein and Activities Are Not Induced by Short-Term Chilling
Following the analysis of transcripts, we examined the effect of chilling on protein and extractable enzyme activities. Despite the clear increase in leaf γ-ECS transcripts (Fig. 2) associated with enhanced signal in the BS cells (Fig. 3), chilling did not increase global leaf γ-ECS protein (data not shown). Likewise, no chilling-induced increase in extractable γ-ECS activity was observed (Table I). The extractable activity of GSH-S was in 4-fold excess over that of γ-ECS and was not changed by chilling (Table I), in agreement with unchanged GSH-S transcripts (Fig. 2) and protein abundance (data not shown). Glutathione reductase (GR) activities were also similar in chilled and control plants (Table 1).
Table I.
The extractable activities of leaf enzymes involved in glutathione synthesis and reduction are not changed by short-term chilling of maize leaves
| γ-ECS | GSH-S | GR | |
|---|---|---|---|
| Control | 0.22 ± 0.03 | 0.94 ± 0.06 | 63 ± 3 |
| Chill | 0.24 ± 0.04 | 1.00 ± 0.02 | 60 ± 6 |
Extractable leaf activities are shown for plants maintained at 25°C (day)/19°C (night; control) or transferred to 10°C/8°C for 2 d (chill). All activities are expressed as nmol mg−1protein min−1 and are means ± se of three independent extracts from different plants.
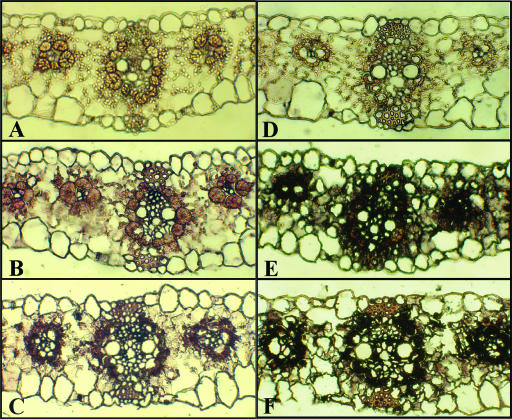
As indicated in “Materials and Methods”, the antibodies were raised against specific peptide sequences predicted from the cloned gene sequences. Each antibody recognized a single band at the corresponding predicted molecular mass. Immunoblots using extended incubation periods for immunocomplex detection with the anti-γ-ECS antibody showed no indication of cross-reactivity with other leaf polypeptides (data not shown). The lack of cross-reactivity enabled us to use these antibodies for immunolocalization studies of leaf sections (Fig. 4). Transverse sections of maize leaves were thus incubated with preimmune serum and specific antibodies against γ-ECS (Fig. 4, left) and GSH-S (Fig. 4, right). γ-ECS protein (Fig. 4, compare A and B) and GSH-S protein (Fig. 4, compare D and E) were clearly found in both M and BS cells of maize leaves under optimal growth conditions, though both were less apparent in the epidermal cells. Although the antibody was raised against a peptide fragment encoded by genes lacking known transit peptide sequences, Figure 4 demonstrates that staining in the chloroplasts was also apparent, in agreement with our recent data showing that the anti-γ-ECS antibody recognizes a single polypeptide of around 50 kD in purified tomato chloroplasts (Mittova et al., 2003). No evidence was found for an increase in GSH-S protein abundance in response to chilling in any of the cell types (Fig. 4, compare E and F). While there was a clear indication of increased staining for γ-ECS in the BS cells, no evidence was found for chilling-induced increases in γ-ECS in the M cells (Fig. 4, compare B and C). In fact, M γ-ECS appeared to decrease in chilled leaves.
Figure 4.
Immunohistochemistry of γ-ECS (A–C) and GSH-S (D–F) proteins in maize leaves. A to D, Plants grown at 25°C/19°C, sections treated with preimmune serum. B and E, Plants grown at 25°C/19°C; sections treated with γ–ECS or GSH-S antibodies. C and F, Plants transferred to 10°C/8°C for 2 d (chill); sections treated with γ-ECS or GSH-S antibodies. Staining with preimmune serum was not affected by chilling (data not shown).
Responses of Leaf Thiols to Chilling and Subsequent Recovery
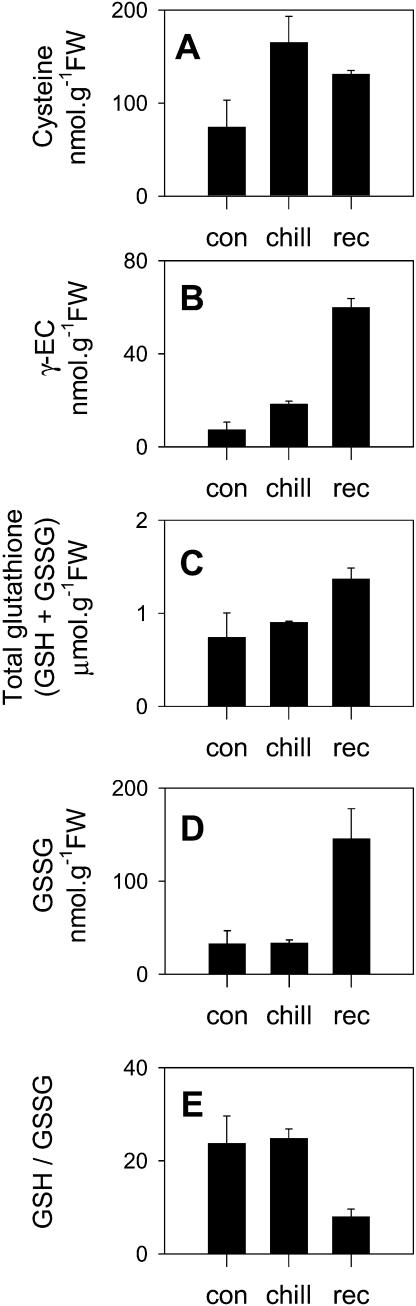
Figure 5 shows the effects of chilling and subsequent recovery on leaf glutathione and thiol precursors, as well as on the GSH to GSSG ratio. The leaf Cys pool of maize leaves after 2-d exposure to chilling was more than double that of leaves maintained at optimal growth temperatures (Fig. 5A). Moreover, leaf contents of the pathway intermediate, γ-glutamylcysteine (γ-EC), were increased more than 2-fold after 2 d at suboptimal growth temperatures (Fig. 5B). Despite this, the glutathione pool was not significantly enhanced during the chilling period: only after 1 d of recovery did the leaf glutathione pool increase (by about 70%: Fig. 5C). This delayed accumulation of glutathione was linked to a minor fall in Cys and a marked increase in γ-EC (Fig. 5, A–C). Leaf contents of GSSG did not change during the chill (Fig. 5D), and the GSH to GSSG ratio was not decreased from the high values recorded in the controls (Fig. 5E). Following the recovery period at optimal temperature and concomitant with the increase in total glutathione (Fig. 5C) and leaf chlorosis (Fig. 1), GSSG was increased about 5-fold, causing the GSH to GSSG ratio to decrease markedly (Fig. 5E).
Figure 5.
Changes in leaf GSH and GSSG and their precursor thiols during chilling and subsequent recovery. A, Cys. B, γ-EC. C, total glutathione (GSH plus GSSG). D, GSSG. E, GSH to GSSG ratios. Data are means ± se of three independent extracts from different plants.
DISCUSSION
The aim of the this study was to establish how the enzymes that synthesize glutathione are distributed between the different cell types in maize leaves and how this distribution responds to brief chilling, a stress similar to that which young maize plants often experience in the field. To assess the importance of such changes for leaf redox state, glutathione content and redox state were also determined in parallel. Strong evidence for a relationship between tissue glutathione and chilling tolerance in maize has come from several studies by Kocsy and co-workers. In particular, these authors have shown that chilling tolerance is accompanied by increased γ-ECS activity and GSH accumulation (Kocsy et al., 1996, 2000a, 2000b, 2001b). Hence, we cloned maize genes encoding γ-ECS and GSH-S. The sequences show that the cloned genes are structurally related to those from C3 dicotyledonous species. The absence of obvious plastidial transit peptide sequences may suggest that the encoded enzymes are directed to the cytosol. However, literature data show the two enzyme activities are found both inside and outside the plastid (Noctor et al., 2002) and may also be targeted to organelles such as mitochondria (Schäfer et al., 1998). The relative intracellular distribution of the enzyme activities in maize leaves is not known, but plastids isolated from very young maize roots were reported to contain about half the total γ-ECS activity and a small proportion of the root GSH-S activity (Ruegsegger and Brunold, 1993). It may be that the genes we have cloned encode proteins that are at least partly directed to the chloroplast through as yet unknown recognition sequences. Although we cannot exclude the possibility that other genes encoding plastid isoforms remain to be identified in maize, it is noteworthy that our screening of a bacterial artificial chromosome library of maize genomic DNA did not detect other putative γ-ECS or GSH-S sequences. The results presented in this article allow us to draw the following conclusions.
γ-ECS Transcripts Are Up-Regulated by Chilling with No Concomitant Increase in GSH-S Transcripts
By northern-blot and reverse transcription (RT)-PCR analysis, we identified the chilling conditions that caused maximal induction of γ-ECS (2 d at 10°C/8°C). These conditions also led to extensive leaf bleaching after return to optimal temperature. Although it is possible that our probe did not detect all forms of γ-ECS, it is clear that the gene encoding this enzyme is up-regulated in response to suboptimal temperature. The in situ hybridizations indicated that the increase in leaf γ-ECS transcripts during chilling was predominantly due to changes in the BS, whereas γ-ECS transcripts decreased in the M cells. Unlike γ-ECS, GSH-S transcripts were insensitive to chilling. There have been very few previous studies on the response of γ-ECS and GSH-S transcripts to stress. Exposure of Arabidopsis to heavy metals was shown to increase transcripts for both γ-ECS and GSH-S, but the addition of H2O2 was required to bring about GSH accumulation (Xiang and Oliver, 1998; Xiang and Bertrand, 2000). However, previous studies have not determined whether stress-induced accumulation of γ-ECS transcripts results in an increase in protein abundance and enzyme activity.
Although Global Leaf γ-ECS Total Protein and Activity Are Not Increased by Chilling, the Relative Intercellular Distribution of Protein Is Modified
Our data for global enzyme activities agree with previous studies suggesting that, of the two enzymes that synthesize GSH from amino acids, γ-ECS is the more limiting; the relative extractable activities were 4-fold higher for GSH-S (Table I). The literature contains very little information on the intercellular distribution of the enzymes that catalyze glutathione synthesis. A study using in situ labeling techniques in Arabidopsis showed that trichomes have much higher contents of and flux to GSH than the surrounding epidermal and M cells (Gutierrez-Alcala et al., 2000). With regard to synthesis within the principal maize leaf photosynthetic tissues, i.e. the M and BS cells, a previous study using rapid tissue fractionation techniques showed that GSH-S activity was enriched in tissues other than the BS, though no data was reported for γ-ECS (Burgener et al., 1998). Our results suggest that γ-ECS and GSH-S proteins are found in both cell types, as well as in the epidermal cells (Fig. 4).
While total γ-ECS transcripts were clearly enhanced by chilling (Fig. 2), no increase in total leaf γ-ECS protein or activity was observed (Fig. 4; Table I). The change in the intercellular distribution pattern of γ-ECS transcripts during chilling (Fig. 3) was, however, in broad agreement with the modified distribution of protein, which also appeared to favor the BS cells at the expense of the M cells (Fig. 4). Our tissue blots suggest, therefore, that during chilling, γ-ECS protein increased in the BS relative to the M cells. Taken together, these observations suggest that chilling induces a shift in the intercellular distribution of glutathione synthesis, from the M cells toward the BS cells.
The disparity between changes in total γ-ECS transcript abundance and protein may be explained either by a restriction on translation or by enhanced γ-ECS protein turnover, or both. It is clear that control of glutathione metabolism is complex and occurs at multiple levels. We have previously shown that GR transcripts cannot be translated in the BS cells, such that GR protein and activity are restricted to the mesophyll cells in maize at both optimal and low growth temperatures (Pastori et al., 2000a). Translation of γ-ECS in Arabidopsis seems to be controlled by factors linked to redox state, such as H2O2 and/or the GSH to GSSG ratio (Xiang and Oliver, 1998). Long-term growth of maize at low temperature increases total leaf H2O2, but much of this is localized in the M cells (Pastori et al., 2000b). However, the modified distribution of γ-ECS during chilling suggests that H2O2 concentrations in the BS cells do not strongly impede γ-ECS translation. Although in our study there was a close relationship between overall leaf γ-ECS protein and enzyme activity (i.e. neither changed in response to chilling), it should be noted that other workers have found discrepancies, which they have explained by interactions with a hypothetical regulatory protein (May et al., 1998b).
Cys Availability Plays a Key Role in the Maize Chilling Response
Previous studies have shown that the most important factors controlling flux through the GSH synthetic pathway are the availability of Cys and the synthesis and activity of γ-ECS (Noctor et al., 1998b, 2002). In maize, Cys is produced in the BS cells (Burgener et al., 1998, and references therein). Our data show that during chilling, Cys and γ-EC were increased 2-fold, with no increase in glutathione (Fig. 5). These data suggest that chilling enhances sulfur assimilation or decreases utilization of Cys. After return to optimal temperatures, the dramatic increase in γ-EC was accompanied by a significant increase in glutathione (to 1.7-fold the values in control plants). This delayed accumulation of γ-EC and glutathione could reflect increased Cys for γ-ECS activity, increased γ-ECS capacity, or reversal of extensive complexing of glutathione at low temperature. Enhanced Cys availability per se has been shown to promote appreciable increases in tissue glutathione (e.g. Strohm et al., 1995; Noctor et al., 1996). It may therefore be that only part of the Cys accumulated during chilling is available for γ-ECS activity or that the temperature is simply too low to allow γ-ECS to convert the increased Cys into γ-EC. Thus, temperature restrictions, either on Cys transport to the M cells or on γ-ECS activity throughout the leaf, means that Cys accumulation during the chill does not fully feed through to γ-EC and free glutathione. The return to optimal temperature allows a burst of γ-ECS activity as these limitations are mitigated, leading to accumulation of γ-EC and glutathione.
Chilling-Induced Perturbation of the Glutathione Pool Only Occurs Subsequently to the Stress and Is Correlated with Leaf Chlorosis
In animal cells, substantial evidence implicates redox potential as an important factor determining cell fate, and the glutathione redox couple is considered the key player (Schafer and Buettner, 2001). Like the total glutathione pool, the GSH to GSSG ratio did not change during the chill (Fig. 5). Thus, the leaf was able to maintain an unchanged glutathione redox potential at low temperature. The data of Figure 5 show that GSSG increased from 34 (control leaves) to 146 (recovery) nmol g−1 fresh weight, causing a 3-fold decrease in the GSH to GSSG ratio. Thus, the redox potential becomes more positive. However, it can be calculated that the overall increase in glutathione acts in a compensatory manner, so that the considerable increase in GSSG causes a significantly smaller shift in the redox potential than would occur if the overall pool (GSH plus GSSG) remains constant. This illustrates the importance of glutathione synthesis (and accumulation) in counterbalancing stress that favors oxidizing conditions.
Two pertinent questions arise from this analysis. The first concerns the nature of the link between redox state perturbation and enhanced glutathione accumulation. Current literature data suggest at least two links. First, the activity of adenylylsulfate reductase, a key enzyme in sulfate assimilation, may be activated by decreases in the GSH to GSSG ratio (Bick et al., 2001). Second, as noted above, H2O2 or GSH to GSSG ratios dominate control of γ-ECS translation (Xiang and Oliver, 1998).
The second question relates to the potential role of glutathione in determining leaf fate, e.g. in response to chilling and other stresses. Glutathione has long been considered to have roles in plant growth and development, but these remain poorly defined. High leaf glutathione contents were found to stimulate early flowering in Arabidopsis (Ogawa et al., 2002). Moreover, enhanced leaf glutathione promoted bolting in Eustoma grandiflorum with or without vernalization (Yanagida et al., 2004). Chilled maize leaves undergo a process that ultimately leads to leaf glutathione accumulation and leaf bleaching upon return to optimal growth temperatures. We may ask what factors contribute to this process. Oxidative stress is well known to trigger cell death, both in animals and plants, and this inherently involves a change in redox potential and an increase in oxidative markers such as GSSG. As discussed above, loss of chlorophyll is associated with a modest change in glutathione redox potential. More marked, however, is the extent of GSSG accumulation, which we may speculate is perceived by the cell and is one initiator of death. This may be involved in the response to both abiotic and biotic stress. It is interesting to note that in the hypersensitive response to powdery mildew, an accumulation of glutathione and a decreased GSH to GSSG ratio accompany cell death (Vanacker et al., 2000; Noctor et al., 2002). Although it has been known for some years that glutathione elicits marked changes in gene expression, these effects have not to date been fully characterized. Moreover, GSSG may participate in protein thiolation reactions to form mixed disulfides that are known to modify protein function, perhaps including proteins involved in signal transduction (Klatt and Lamas, 2000). Among other leaf proteins, triose phosphate isomerase and aldolase undergo glutathionylation (Ito et al., 2003), showing that this process participates in the regulation of leaf carbon metabolism.
MATERIALS AND METHODS
Isolation and Sequence Characterization of Maize γ-ECS and GSH-S cDNA
Poly(A+) RNA was isolated directly from 0.5 g of maize (Zea mays) leaves tissue using QuickPrep micro mRNA purification kit (Amersham Pharmacia Biotech, Uppsala). On the basis of published genes, degenerated primers were designed to conserved γ-ECS and GSH-S cDNA sequences of Arabidopsis, Brassica juncea, Medicago truncatula, and tomato (Lycopersicon esculentum). Degenerated primers used to amplify partial GSH-S cDNA were: sense, 5′-GC(A/T)AC(A/G)GC(T/A)(C/A)T(A/T)T TTGC(G/C/A/T)A ATTC-3′; and antisense, 5′-CC(T/A)CCATCAGC(G/A)CCTCTCAT (T/C)T-3′. Degenerated primers used to amplify partial GSH-S cDNA were: sense, 5′-GC(T/C) GA(T/C/A/G)GC(A/C)(T/A)TGGCTAAAGCTTGG-3′; and antisense, 5′-GTT (T/A)CCT CC(A/G)CCTTCTC(T/G)(C/T)TG-3′. A total of 0.1 μg of mRNA in the presence of 5 pmol of the corresponding antisense primer was used to synthesize the first strand of cDNA with Superscript II RT (Gibco-BRL, Cleveland). After RNAse treatment (RNAse H; Gibco-BRL), a 40-cycle PCR reaction was carried out using the cDNA as template, with annealing temperatures of 53°C and 56°C for the γ-ECS and GSH-S primers, respectively. The PCR products were cloned in pGEM-T Easy (Promega) and sequenced using an ABI Prism Dye Terminator Cycle Sequencing Ready Reaction kit and 310 Genetic Analyzer capillary sequencer (Perkin Elmer Applied Biosystems, Foster City, CA).
To complete the full-length cDNA, the 3′ ends of both cDNAs were isolated by 3¢ RACE System (Gibco-BRL). A total of 0.1 μg of mRNA was reverse transcribed using Superscript II (Gibco-BRL) and the adapter primer provided with the kit, according to the manufacturer's instructions. Sense gene-specific primers (γ-ECS, 5′-CTGGAATGACCTTCAGGGAC-3′; nested primer, 5′-CTTGGCAGGAAAGCTTCCTTG-3′; GSH-S, 5′-GCCTAATGTGCTTGAAAGGTTCC-3′; and nested primer, 5′-GCTGGCTTATGGAGTTTGGATG-3′) were designed based in the partial clones obtained with the RT-PCR. Asymmetric PCR was performed adding the abridged universal amplification primer provided with the kit, after 10 cycles of amplification only with the gene-specific primer. PCR products were cloned in pGEM-T Easy vector (Promega) and sequenced as described above.
5′ RACE system (Gibco-BRL) was used to obtain the 5′ cDNA ends. The manufacturer's instructions were modified in order to avoid truncated products due to rich G:C regions in the cDNA. Antisense gene-specific primers (γ-ECS, 5′-GGGGACTTCTAATGCATAGTCC-3′; nested primer, 5′-GGGAGCATCCCTGCACGATTA-3′; GSH-S, 5′-CTGGCCACCTACAAGAAGAG-3′; and nested primer, 5′-GAGAACCTGGCCTTCCGCTTC-3′) were designed based on the 5′ region of the partial cDNA clones obtained by the RT-PCR. Thermoscript RT (Gibco-BRL) was used to synthesize cDNA from 0.1 μg of mRNA. After denaturing the mRNA for 10 min at 70°C, the first strand synthesis was carried out at 62.5°C for 1 h. The 5′ end of the cDNA corresponding to γ-ECS was polyadenylated, while GSH-S cDNA was polycytosine tailed. PCR and nested PCR were carried out using gene-specific primers and the primers provided with the 5′ system, and the amplification products were cloned and sequenced as described above. Once fully sequenced, sense and antisense primers were designed to amplify the complete cDNA, and the amplification products from this RT-PCR approach were cloned in pGEM-T Easy vector (Promega) and resequenced. Database searches, sequence alignments, and further analysis were performed using the tools provided by the European Bioinformatics Institute Web site.
Sequences used for the analysis (GenBank accession nos. in parentheses) are as follows: for the gsh1 gene (γ-ECS), Pisum sativum (AF128455), M. truncatula (AF041340), Phaseolus vulgaris (AF128454), B. juncea (Y10848), Arabidopsis (Z29490), and tomato (AF017983); and for the gsh2 gene (GSH-S), P. sativum (AF231137), B. juncea (Y10984), Arabidopsis (U22359), and tomato (AF017984).
Plants Growth Conditions and Chilling Treatment
Maize (H99 line) was grown in a controlled-environment chamber (Sanyo 970, Sanyo, Osaka) with a 16-h photoperiod at 1,000 μmol m−2 s−1 at 25°C (day)/19°C (night) and at 70% (v/v; day)/80% (v/v; night) relative humidity. Chilling treatment was conducted on 11-d-old seedlings for up to 3 d. Chilled plants were then returned to optimal temperatures, and changes in given parameters were followed at the times indicated in the figure legends.
Northern-Blot Analysis
Total RNA was extracted from plant material (0.5 g) at a ratio 1:6 (grams of tissue:milliliters of Trizol reagent; Gibco-BRL). Northern-blot analysis was performed using 25 μg of total RNA as described previously (Church and Gilbert, 1984). Probes were generated from partial cDNA fragments corresponding to γ-ECS and GSH-S and radiolabeled with [α-32P]dCTP using the Rediprime II random prime system (Amersham Bioscience, Chalfont St. Giles, UK). Overnight hybridization and stringent washes were carried out at 65°C. Kodak MS films (Kokak, London) were developed for 72 h.
In Situ Hybridization
Leaf tissue (second leaf) were fixed in 4% (v/v) paraformaldehyde dissolved in 50% ethanol, 5% acetic acid, and RNAse-free phosphate-buffered saline at 4°C. Tissue was vacuum-infiltrated six times every hour for 1 min (<0.4 bar), and the fixative was changed each time the pressure was released. The fixed tissue was embedded in paraplast (Sigma, St. Louis), and 10-μm sections were transferred onto Suprafrost microscope slides (BDH, Poole, Dorset, UK). The entire procedure was carried out under RNAse-free conditions.
The in situ hybridization for γ-ECS and GSH-S transcripts was performed according to Jackson (1991). RNA probes were labeled with digoxigenin (DIG) using the Boehringer nucleic acid labeling kit (Roche Diagnostics, Basel). pGEM-T Easy vector (Promega) containing a cDNA fragment of 750 bp corresponding to γ-ECS or a 500-bp fragment for GSH-S was linearized with restriction enzymes, leaving the T7 or SP6 RNA polymerase promoter sequences contiguous to the inserted fragment, to archive the synthesis of antisense and sense probes. One microgram of the linearized vector was used as a template for RNA synthesis in the presence of the corresponding polymerase. The resulting DIG-labeled RNA was subjected to alkaline hydrolysis by incubating in 100 mm carbonate buffer (pH 10.2) at 60°C to a final probe size of 250 bp. After ethanol/acetic acid precipitation, the labeled probes were resuspended in 50% (v/v) formamide.
After a prehybridization procedure consisting of a pronase treatment (125 μg/mL) and acetylation (0.5% (v/v) acetic anhydride; 10 min) of the tissue sections, slides were incubated overnight at 50°C with 10 ng/kb of probe per microliter of mixture. The hybridization mixture was 50% (v/v) formamide, 10% (w/v) dextran sulfate, 2.5× Denhart's solution, 100 μg/mL denatured herring sperm DNA (Gibco-BRL), 100 μg/mL tRNA (Gibco-BRL), 5 mm dithiothreitol, and 40 units/mL RNAse inhibitor (Roche Diagnostics, Manheim, Germany). Slides were washed several times in 2× SSC at 50°C and treated with RNAse A (20 μg/mL) in 0.5 m NaCl, 10 mm Tris-HCl (pH 8.0), and 1 mm EDTA for 30 min at 37°C. Finally, slides were washed in 0.2× SSC several times at 50°C.
Detection of the DIG-labeled probes was carried out using a DIG-nucleic acid detection kit (Roche). Sections were incubated for 1 h in 1% (w/v) blocking reagent dissolved in Tris-buffered saline (TBS), dried at room temperature, and incubated for 2 h with the antibody-conjugate (1.75 units/mL of alkaline phosphatase) dissolved in blocking solution. After washing with TBS, color was developed by incubating overnight with 150 μL of the nitroblue tetrazolium/5-bromo-4-chloro-3-indoyl phosphate toluidinium solution provided with the detection kit, dissolved in 10 mL of 100 mm NaCl, 50 mm MgCl2, 6% polyvinyl alcohol, and 100 mm Tris-HCl (pH 9.2). Slides were mounted in 50% glycerol and photographed.
Preparation of Antibodies and Western-Blot Analysis
Polyclonal antibodies against γ-ECS and GSH-S were raised in rabbits immunized with synthetic peptides conjugated to keyhole limpet hemocyanin. The peptides were designed according to amino acid sequences derived from the maize cDNA cloned for these enzymes. The complete amino acid sequences were evaluated to select peptides according to their surface exposure (hydrophilicity), presence of β-turns, and difficulty of synthesis. The selected peptides (KNGLERRGYKEVGFLREV for γ-ECS and VGTKKIQQELAKPNVLERFL for GSH-S) were conjugated to keyhole limpet hemocyanin and used as antigens to raise the antibodies. The computer analysis was carried out using the service provided by the Bioinformatics Unit, Weizmann Institute of Science, Israel.
For western blotting, 25 μg of protein extracted from the second leaf was separated by SDS-PAGE and transferred to Hybond-C extra (Amersham-Pharmacia, Chalfont St. Giles, UK. After transfer, membranes were blocked in TBS containing 5% (w/v) bovine serum albumin (BSA) for 24 h at 4°C and subsequently incubated in TBS containing 1% BSA and a 1:10,000 dilution of the antibody. The membranes were then washed three times in TBS before incubating in TBS containing 1% BSA (w/v) and a 1:50,000 dilution of goat anti-rabbit horseradish peroxidase-conjugated secondary antibody. After washing with TBS, the signal generated by secondary antibody was visualized using the ECL detection kit (Amersham-Pharmacia) according to the manufacturer's instructions.
Histoimmunolocalization of γ-ECS and GSH-S Proteins
Transverse sections (10 μm) of leaf tissue were cut from paraffin-embedded tissue, as described for in situ hybridization studies. After dehydration sections were blocked in TBS containing 1% dimethyl sulfoxide and 3% BSA for 1 h at room temperature. Sections on slides were then incubated overnight with the primary antibody (1:5,000 dilution in blocking reagent). The slides were washed eight times in TBS, incubated in secondary antibody conjugated with alkaline phosphatase (goat anti-rabbit diluted 1:10,000 [Sigma]), and subsequently washed six times in TBS. Detection of the alkaline phosphatase signal was performed as described for the in situ hybridization.
Enzyme Activities and Thiol Measurements
GR, γ-ECS, and GSH-S activities were measured as in Noctor et al. (2002). Thiols were determined by HPLC separation of monobromobimane-labeled compounds (Noctor and Foyer, 1998b). The GSH to GSSG ratio was determined spectrophotometrically in the same extracts, as the GR-dependent increase in reduction of 5,5′-dithio-bis(-2-nitrobenzoic acid) prior to (total glutathione) or subsequent to (GSSG) the formation of complex with 2-vinylpyridine. The ratio between total glutathione measured spectrophotometrically and total glutathione determined by HPLC was 1.06 ± 0.10 (n = 15).
This work was supported by the National Research Council, Argentina (Consejo Nacional de Investigaciones Científicas y Técnicas; fellowship to L.D.G.) and the Biotechnology and Biological Sciences Research Council, UK.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.033027.
References
- Bick JA, Setterdahl AT, Knaff DB, Chen Y, Pitcher LH, Zilinskas BA, Leustek T (2001) Regulation of the plant-type 5′-adenylylsulfate reductase by oxidative stress. Biochemistry 40: 9040–9048 [DOI] [PubMed] [Google Scholar]
- Burgener M, Suter M, Jones S, Brunold C (1998) Cyst(e)ine is the transport metabolite of assimilated sulfur from bundle-sheath to mesophyll cells in maize leaves. Plant Physiol 116: 1315–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church GM, Gilbert W (1984) Genomic sequencing. Proc Natl Acad Sci USA 81: 1991–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doulis AG, Debian N, Kingston-Smith AH, Foyer CH (1997) Differential localization of antioxidants in maize leaves. Plant Physiol 114: 1031–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Harbinson J (1994) Oxygen metabolism and the regulation of photosynthetic electron transport. In CH Foyer, P Mullineaux, eds, Causes of Photooxidative Stresses and Amelioration of Defence Systems in Plants. CRC Press, Boca Raton, FL, pp 1–42
- Foyer CH, Noctor G (2000) Oxygen processing in photosynthesis: regulation and signalling. New Phytol 146: 359–388 [Google Scholar]
- Fryer MJ, Andrews JR, Oxborough K, Blowers DA, Baker NR (1998) Relationship between CO2 assimilation, photosynthetic electron transport, and active O2 metabolism in leaves of maize in the field during periods of low temperature. Plant Physiol 116: 571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Alcala G, Gotor C, Meyer AJ, Fricker M, Vega JM, Romero LC (2000) Glutathione biosynthesis in Arabidopsis trichome cells. Proc Natl Acad Sci USA 97: 11108–11113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell R, Bergmann L (1988) Glutathione synthetase in tobacco suspension cultures; catalytic properties and localisation. Physiol Plant 72: 70–76 [Google Scholar]
- Hell R, Bergmann L (1990) γ-Glutamylcysteine synthetase in higher plants: catalytic properties and subcellular localisation. Planta 180: 603–612 [DOI] [PubMed] [Google Scholar]
- Hughes MA, Dunn MA (1990) The effect of temperature on plant growth and development. Biotechnol Genet Eng Rev 8: 161–188 [Google Scholar]
- Ito H, Iwabuchi M, Ogawa K (2003) The sugar-metabolic enzymes aldolase and triose-phosphate isomerase are targets of glutathionylation in Arabidopsis thaliana: detection using biotinylated glutathione. Plant Cell Physiol 44: 655–660 [DOI] [PubMed] [Google Scholar]
- Jackson DP (1991) In situ hybridization in plants. In DJ Bowles, SJ Gurr, M McPhereson, eds, Molecular Plant Pathology: A Practical Approach. Oxford University Press, Oxford, pp 163–174
- Kingston-Smith AH, Foyer CH (2000) Bundle sheath proteins are more sensitive to oxidative damage than those of the mesophyll in maize leaves exposed to paraquat or low temperatures. J Exp Bot 51: 123–130 [PubMed] [Google Scholar]
- Kingston-Smith AH, Harbinson J, Foyer CH (1999) Acclimation of photosynthesis, H2O2 content and antioxidants in maize (Zea mays) grown at sub-optimal temperatures. Plant Cell Environ 22: 1071–1083 [Google Scholar]
- Klatt P, Lamas S (2000) Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem 267: 4928–4944 [DOI] [PubMed] [Google Scholar]
- Kocsy G, Brunner M, Rüegsegger A, Stamp P, Brunold C (1996) Glutathione synthesis in maize genotypes with different sensitivity to chilling. Planta 198: 365–370 [Google Scholar]
- Kocsy G, Galiba G, Brunold C (2001. a) Role of glutathione in adaptation and signalling during chilling and cold acclimation in plants. Physiol Plant 113: 158–164 [DOI] [PubMed] [Google Scholar]
- Kocsy G, Szalai G, Vágújfalvi A, Stéhli L, Orosz G, Galiba G (2000. a) Genetic study of glutathione accumulation during cold hardening in wheat. Planta 210: 295–301 [DOI] [PubMed] [Google Scholar]
- Kocsy G, von Ballmoos P, Rüegsegger A, Szalai G, Galiba G, Brunold C (2001. b) Increasing the glutathione content in a chilling-sensitive maize genotype using safeners increased protection against chilling-induced injury. Plant Physiol 127: 1147–1156 [PMC free article] [PubMed] [Google Scholar]
- Kocsy G, von Ballmoos P, Suter M, Rüegsegger A, Galli U, Szalai G, Galiba G, Brunold C (2000. b) Inhibition of glutathione synthesis reduces chilling tolerance in maize. Planta 211: 528–536 [DOI] [PubMed] [Google Scholar]
- Kunert KJ, Foyer CH (1993) Thiol/disulphide exchange in plants. In LJ De Kok, I Stulen, H Rennenberg, C Brunold, W Rauser, eds, Sulfur Nutrition and Assimilation in Higher Plants. Regulatory Agricultural and Environmental Aspects. SPB Academic Publishers, The Hague, The Netherlands, pp 139–151
- Leegood RC (1985) The intercellular compartmentation of metabolites in leaves of Zea mays L. Planta 164: 163–171 [DOI] [PubMed] [Google Scholar]
- Levitt J (1962) A sulphydryl-disulfide hypothesis of frost injury and resistance in plants. J Theor Biol 3: 355–391 [Google Scholar]
- May MJ, Vernoux T, Leaver C, van Montagu M, Inzé D (1998. a) Glutathione homeostasis in plants: implications for environmental sensing and plant development. J Exp Bot 49: 649–667 [Google Scholar]
- May MJ, Vernoux T, Sanchez-Fernandez R, Van Montagu M, Inzé D (1998. b) Evidence for post-transcriptional activation of γ-glutamylcysteine synthetase during plant stress responses. Proc Natl Acad Sci USA 95: 12049–12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A (1988) Glutathione metabolism and its selective modification. J Biol Chem 263: 17205–17208 [PubMed] [Google Scholar]
- Mittova V, Theodoulou FL, Kiddle G, Gomez L, Volokita M, Tal M, Foyer CH, Guy M (2003) Co-ordinate induction of glutathione biosynthesis and glutathione-metabolizing enzymes is correlated with salt tolerance in tomato. FEBS Lett 554: 417–421 [DOI] [PubMed] [Google Scholar]
- Noctor G, Arisi ACM, Jouanin L, Foyer CH (1998. a) Manipulation of glutathione and amino acid biosynthesis in the chloroplast. Plant Physiol 118: 471–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Arisi ACM, Jouanin L, Kunert KJ, Rennenberg H, Foyer CH (1998. b) Glutathione biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J Exp Bot 49: 623–647 [Google Scholar]
- Noctor G, Foyer CH (1998. a) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49: 249–279 [DOI] [PubMed] [Google Scholar]
- Noctor G, Foyer CH (1998. b) Simultaneous measurement of foliar glutathione, γ-glutamylcysteine and amino acids by high-performance liquid chromatography: comparison with two other assay methods for glutathione. Anal Biochem 264: 98–110 [DOI] [PubMed] [Google Scholar]
- Noctor G, Gomez L, Vanacker H, Foyer CH (2002) Glutathione homeostasis and signalling. The influence of biosynthesis, compartmentation and transport. J Exp Bot 53: 1283–1304 [DOI] [PubMed] [Google Scholar]
- Noctor G, Strohm M, Jouanin L, Kunert KJ, Foyer CH, Rennenberg H (1996) Synthesis of glutathione in leaves of transgenic poplar (Populus tremula x P. alba) overexpressing γ-glutamylcysteine synthetase. Plant Physiol 112: 1071–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K, Tasaka Y, Mino M, Tanaka Y, Iwabuchi M (2002) Association of glutathione with flowering in Arabidopsis thaliana. Plant Cell Physiol 42: 524–530 [DOI] [PubMed] [Google Scholar]
- Pastori GM, Foyer CH (2002) Common components, networks, and pathways of cross-tolerance to stress. The central role of “redox” and abscisic acid-mediated controls. Plant Physiol 129: 460–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastori G, Foyer CH, Mullineaux P (2000. a) Low temperature induces changes in the distribution of H2O2 and antioxidants between the bundle sheath and mesophyll cells of maize leaves. J Exp Bot 51: 107–113 [PubMed] [Google Scholar]
- Pastori G, Mullineaux P, Foyer CH (2000. b) Post-transcriptional regulation prevents accumulation of glutathione reductase protein and activity in the bundle sheath cells of maize. Plant Physiol 122: 667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennenberg H (1997) Molecular approaches to glutathione biosynthesis. In WJ Cram, LJ DeKok, I Stulen, C Brunold, H Rennenberg, eds, Sulphur Metabolism in Higher Plants. Backhuys Publishers, Leiden, The Netherlands, pp 59–70
- Ripoll PJ, O'Sullivan DM, Edwards KJ, Rodgers M (2000) Technique for cloning and sequencing the ends of bacterial artificial chromosome inserts. Biotechniques 29: 271–276 [DOI] [PubMed] [Google Scholar]
- Ruegsegger A, Brunold C (1993) Localization of γ-glutamylcysteine synthetase and glutathione synthetase activity in maize seedlings. Plant Physiol 101: 561–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GH (2001) Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 30: 1191–1212 [DOI] [PubMed] [Google Scholar]
- Schäfer HJ, Haag-Kerwer A, Rausch T (1998) cDNA cloning and expression analysis of genes encoding GSH synthesis in roots of the heavy-metal accumulator Brassica juncea L.: evidence for Cd-induction of a putative mitochondrial γ-glutamylcysteine synthetase isoform. Plant Mol Biol 37: 87–97 [DOI] [PubMed] [Google Scholar]
- Strohm M, Jouanin L, Kunert KJ, Pruvost C, Polle A, Foyer CH, Rennenberg H (1995) Regulation of glutathione synthesis in leaves of transgenic poplar (Populus tremula x P.alba) overexpressing glutathione synthetase. Plant J 7: 141–145 [Google Scholar]
- Vanacker H, Carver TLW, Foyer CH (2000) Early H2O2 accumulation in mesophyll cells leads to induction of glutathione during the hypersensitive response in the barley-powdery mildew interaction. Plant Physiol 123: 1289–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breusegem F (1998) Engineering oxidative stress tolerance in maize. PhD thesis. Ghent University, Ghent, Belgium
- Van Heyne G (1983) Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem 133: 17–21 [DOI] [PubMed] [Google Scholar]
- Xiang C, Bertrand D (2000) Glutathione synthesis in Arabidopsis: multilevel controls coordinate responses to stress. In C Brunold, H Rennenberg, LJ De Kok, I Stulen, JC Davidian, eds, Sulfur Nutrition and Sulphur Assimilation in Higher Plants. Paul Haupt, Bern, Switzerland, pp 409–412
- Xiang C, Oliver DJ (1998) Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell 10: 1539–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang C, Werner BL, Christensen EM, Oliver DJ (2001) The biological functions of glutathione revisited in Arabidopsis transgenic plants with altered glutathione levels. Plant Physiol 126: 564–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida M, Mino M, Iwabuchi M, Ogawa K (2004) Reduced glutathione is a novel regulator of vernalization-induced bolting in the rosette plant Eustoma grandiflorum. Plant Cell Physiol 45: 129–137 [DOI] [PubMed] [Google Scholar]