Abstract
Although high density lipoprotein (HDL)-mediated reverse cholesterol transport is crucial to the prevention and reversal of atheroma, a recent meta-analysis makes evident that current pharmaceutical strategies for modulating HDL cholesterol levels lower cardiovascular risk only to the extent that they concurrently decrease low density lipoprotein (LDL) cholesterol. This corresponds well with findings of a recent Mendelian randomization analysis, in which genetic polymorphisms associated with HDL cholesterol but no other known cardiovascular risk factors failed to predict risk for myocardial infarction. Although it is still seems appropriate to search for therapies that could improve the efficiency with which HDL particles induce reverse cholesterol transport, targeting HDL cholesterol levels per se with current measures appears to be futile. It may therefore be more promising to promote reverse cholesterol transport with agents that directly target foam cells. Macrophage expression of the cholesterol transport proteins adenosine triphosphate binding cassette transporter A1, adenosine triphosphate binding cassette transporter G1, and scavenger receptor class B member 1 is transcriptionally up-regulated by activated liver X receptors (LXR), whereas nuclear factor (NF)-kappaB antagonizes their expression. Taurine, which inhibits atherogenesis in rodent studies, has just been discovered to act as a weak agonist for LXRalpha. Conversely, it may be possible to oppose NF-kappaB activation in macrophages with a range of measures. Induction of heme oxygenase-1, which can be attained with phase 2 inducer phytochemicals such as lipoic acid and green tea catechins, promotes reverse cholesterol transport in macrophages and inhibits atherogenesis in rodents, likely due to, in large part, NF-kappaB antagonism. Inhibition of macrophage nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity with the spirulina-derived bilirubin-mimetic phycocyanobilin may also oppose NF-kappaB activation, and salicylic acid similarly should be useful for this purpose. The 5' adenosine monophosphate-activated protein kinase activator berberine promotes macrophage reverse cholesterol transport in cell culture; metformin probably shares this property. Many of these measures could also be expected to promote plaque stability by suppressing foam cell production of inflammatory cytokines and matrix metalloproteinases, and to reduce intimal monocyte infiltration by anti-inflammatory effects on vascular endothelium. Direct targeting of foam cells with agents such as phase 2 inducers, spirulina, salicylate, taurine, and berberine or metformin, may hence have considerable potential for preventing and reversing atheroma, and for preventing the plaque rupture that triggers vascular thrombosis.
Keywords: Atherosclerosis, Cholesterol, Inflammation, Phytochemical, Nutraceutical, Atherogenesis, Plaque, Cytokine, Antioxidant
Core tip: Reverse cholesterol transport from foam cells is of key importance to prevention and control of atherosclerosis. This essay reviews the molecular biology of foam cell regulation, and proposes that certain agents may be capable of acting directly on foam cells to amplify reverse cholesterol transport while also promoting plaque stability by limiting foam cell production of inflammatory cytokines and matrix metalloproteinases. Phase 2 inducers such as lipoic acid and green tea catechins, spirulina, salicylate, taurine, and 5' adenosine monophosphate-activated protein kinase activators such as metformin or berberine, appear to have potential in this regard-while acting in additional ways to benefit vascular health.
PHARMACEUTICAL HIGH DENSITY LIPOPROTEIN MODULATION HAS PROVED DISAPPOINTING
Although reverse cholesterol transport from foam cells mediated by high density lipoprotein (HDL) particles clearly plays a key role in the prevention and control of atherosclerosis (Figure 1) and its complications[1-3], a recent meta-analysis strongly suggests that current pharmaceutical measures for increasing HDL cholesterol (e.g., niacin, fibrates, cholesterylester transfer protein inhibitors) do not enhance health outcomes in at-risk subjects-or rather, only do so to the extent that, like niacin, they favorably influence other determinants of atherogenesis such as low density lipoprotein (LDL) and apoB-bearing lipoproteins[4]. The failure of niacin in the AIM-HIGH trial-despite evidence of benefit in other studies[5,6]-might then be explained by the fact that patients in the control group received a higher dose of statin such that reductions of LDL cholesterol were equivalent in each group[7]. Analogously, a Mendelian randomization analysis has determined that genotypes associated with elevated HDL cholesterol (but no other known determinants of cardiovascular risk), are not associated with a decline in risk for myocardial infarction[8]. A similar analysis focusing on genetic determinants of LDL cholesterol provides striking confirmation of LDL’s pathogenicity[9]. The well-established epidemiological association of low HDL cholesterol with increased cardiovascular risk might therefore reflect the fact that low HDL cholesterol levels can serve as a marker for metabolic states-such as the metabolic syndrome-that are truly pathogenic; a similar analysis applies to moderately elevated homocysteine. There still may be scope for developing new drugs or procedures that improve the capacity of HDL particles to achieve reverse transport[10-13]-but available pharmaceutical agents capable of elevating HDL cholesterol do not seem to have that property. As the authors of the recent meta-analysis note: “Raising high density lipoprotein cholesterol without considering effects on high density lipoprotein function seem to have little promise for the prevention of cardiovascular events”[4].
Figure 1.
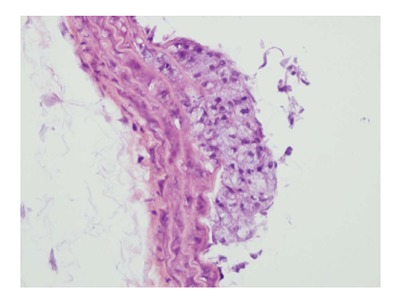
An atherosclerotic plaque at its early stage of development in the thoracic aorta of an apolipoprotein E-KO mouse is illustrated. The plaque is primarily composed of apparent foam cells. HE staining × 400.
It bears mentioning that the low HDL cholesterol levels seen in subjects carrying the Milano variant of apoA-1 are not associated with aggravated cardiovascular risk[14]; perhaps this reflects the efficiency with which Milano HDL delivers cholesterol to the liver for catabolism. Conversely, the elevation of HDL cholesterol associated with niacin therapy may reflect the fact that clinical concentrations of niacin impede the liver’s ability to catabolize holo-HDL particles[15]; while this increases the circulating apoA-1 pool, the amount of cholesterol per HDL particle also rises. Whether the increase in HDL associated with moderate alcohol consumption-likely attributable to enhanced hepatic synthesis of apoA-1[16]-is partially responsible for the decrease in cardiovascular risk observed in by prudent drinkers, is not yet clear; activation of 5’ adenosine monophosphate-activated protein kinase (AMPK) by ethanol-derived acetate may contribute to alcohol’s vascular benefits[17].
TARGETING FOAM CELLS DIRECTLY TO MODULATE FOAM CELL FORMATION AND BEHAVIOUR
Despite the seeming inutility of current efforts to modulate HDL, it may still be feasible to promote reverse cholesterol transport with agents that act directly on foam cells to enhance their capacity to export cholesterol. Moreover, some of these agents could be expected to decrease foam cell uptake of modified LDL particles, and to work in other ways to promote plaque stabilization.
Egress of cholesterol from macrophages and foam cells is mediated by several membrane transport proteins, namely adenosine triphosphate binding cassette transporter A1 (ABCA1), adenosine triphosphate binding cassette transporter G1 (ABCG1), and scavenger receptor class B member 1 (SRB-1); ABCA1 preferentially interacts with lipid-poor apoA-1, ABCG1 can transfer cholesterol to all HDL particles, and SRB-1 interacts with a wide range of lipoproteins[18]. The transcription of ABCA1 and ABCG1 is promoted by the liver X receptors (LXR) receptor, a transcription factor whose physiological activation is mediated by certain hydroxylated metabolites of cholesterol produced within macrophages which can function as ligands for LXR[19,20]. Increased intracellular cholesterol in macrophages also promotes increased expression of SRB-1, although this effect does not seem to be mediated via LXR[21]. In this way, increased cholesterol uptake by macrophages provokes a compensatory increase in cholesterol export induced by cholesterol metabolites. This LXR-mediated promotion of reverse cholesterol transport via HDL can be antagonized by a number of pro-inflammatory cytokines and agonists which have the common effect of activating nuclear factor (NF)-kappaB; concurrent suppression of NF-kappaB activity largely eliminates this inhibition of reverse cholesterol transport[22-27]. NF-kappaB activity somehow opposes the transcription of ABCA1, ABCG1, and SRB-1; how this occurs is still unclear. The balance between LXR and NF-kappaB activities is hence a key determinant of foam cell formation. NF-kappaB activation also is a mediator of inflammatory cytokine production by foam cells, and can promote plaque destabilization by inducing production of matrix metalloproteinases (MMP)[25,28]-whereas LXR suppresses production of MMP-9[29].
Heme oxygenase-1, phase 2 inducers, bilirubin, and spirulina
A number of studies reveal that induction of heme oxygenase-1 (HO-1) in foam cells promotes reverse cholesterol transport, induces increased expression of ABCA1, ABCG1, and SRB-1, and acts in other ways to suppress foam cell production of pro-inflammatory cytokines and plaque-destabilizing metalloproteinases[30-37]. Hence, HO-1 induction can aid prevention of plaque formation, promote plaque regression, and render plaque more stable. Suppression of NF-kappaB activation appears likely to underlie many of these protective effects, since HO-1 activity has been shown to impede NF-kappaB activation in a number of circumstances[38-47]. There appears to be no evidence that HO-1 could influence LXR function. Macrophage HO-1 induction can also oppose AP-1 activation, an effect which could be expected to reduce uptake of modified LDL by diminishing expression of the SR-A receptor[32,33]. The respective roles of HO-1 products carbon monoxide and biliverdin/bilirubin in favorable modulation of foam cell function have not yet been clarified. As HO-1 can be induced by phase 2-inductive phytochemicals via the Nrf2 transcription factor[48], such agents evidently have potential for promoting reverse cholesterol transport and aiding prevention, regression, and stabilization of plaque. Lipoic acid, a broad range of flavanoids (including notably green tea catechins), isothiocyanates from crucifera, and organosulfur compounds from garlic and onions, can serve as phase 2 inducers[49-57]-albeit what intakes of these might have a functionally significant impact on HO-1 in foam cells is unknown. Lipoic acid is of particular interest in this regard, inasmuch as well-defined dose schedules (600-1800 mg daily) exert protective effects in diabetic neuropathy, which seem likely to reflect phase 2 induction[58]. Not surprisingly, lipoic acid exerts anti-atherosclerotic activity in rodents[59-62].
The antioxidant effects of HO-1 are mediated largely by bilirubin, which functions physiologically to inhibit certain isoforms of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase[63-66]. The inverse correlation of serum bilirubin levels with cardiovascular risk observed in many epidemiological studies[67-69] may well reflect the antioxidant impact of free bilirubin on the vascular wall-endothelium, foam cells, and smooth muscle cells. A number of agonists which stimulate NF-kappaB activity in macrophages concurrently activate NADPH oxidase, which boosts NF-kappaB activation via oxidant mechanisms[70-79]. It is therefore reasonable to suspect that HO-1 induction promotes reverse cholesterol transport, in part, by suppressing the up-regulatory impact of NADPH oxidase on NF-kappaB activity. Consistent with this possibility, the ability of advanced glycation endproducts to suppress expression of ABCA1 and ABCG1 expression in macrophages is blocked by inhibitors of NADPH oxidase[80,81]. Macrophage NADPH oxidase activity could also be expected to promote foam cell formation by promoting oxidative modification of LDL.
Recent studies indicate that bilirubin’s antioxidant effect can be mimicked by phycocyanobilin (PhyCB), a prominent light-absorbing chromophore in cyanobacteria such as spirulina; PhyCB is a metabolite and close structural analog of biliverdin, the precursor of bilirubin[82,83]. Not surprisingly, the only study to date which has evaluated oral administration of spirulina or its PhyCB-bearing protein phycocyanin in a rodent model of atherogenesis (cholesterol-fed hamsters) observed a profound anti-atherosclerotic effect[84]. An anti-inflammatory impact on vascular endothelial cells, coupled with a suppressive impact on intimal foam cell formation, seems likely to account for this observation. The ability of bilirubin and of PhyCB to maintain reverse cholesterol transport in macrophages stimulated with various pro-inflammatory agonists that otherwise would inhibit it, should be assessed.
Salicylate suppresses NF-kappaB activity
Activation of NF-kappaB can often be suppressed more directly with salicylate, a direct inhibitor of inhibit the inhibitor of nuclear factor kappa-B kinase beta (IKK-beta), in clinical doses that do not entail important inhibition of cyclooxygenase, and hence are relatively safe[85-88]. In foam cells in vitro, aspirin (which shares salicylate’s capacity to inhibit IKK-beta) was found to suppress the transcriptional activity of NF-kappaB and-likely as a result - boost expression of ABCA1 and SRB-1 while suppressing that of matrix metalloproteinase-9 (a mediator of plaque instability)[25]. In doses of 3-4.5 g daily, salicylate (preferably as salsalate) has been shown to modestly aid glycemic control in diabetics, likely via its inhibition of IKK-beta[89-91]; it might be feasible to employ salicylate in comparable doses to promote reverse cholesterol transport and stabilize plaque in patients with atheroma.
Taurine as an LXR agonist
Pharmaceutical LXR agonists can promote reverse cholesterol transport in macrophages, and some of these are being evaluated as potential new drugs for control of atherosclerosis[92-94]. Unfortunately, most such agents also boost hepatic lipogenesis via LXR activity, an effect viewed as undesirable[94]. A particularly intriguing recent discovery is that the essential cofactor taurine can act as a weak agonist for LXRalpha; moreover, taurine can enhance the expression of ABCA1and ABCG1, and promote reverse cholesterol transport, in cultured macrophages[95]. Curiously, owing to a countervailing effect, taurine fails to promote hepatic lipogenesis, and is very well tolerated[95]. A number of studies have demonstrated that dietary taurine can impede atherogenesis in rodent models of this disorder[96-103]; this effect is stronger than could be predicted from the modest hypolipidemic effects of taurine in rodents, and it would be of interest to know whether a favorable impact on the function of intimal macrophages plays a role in taurine’s anti-atherosclerotic activity. If so, taurine-which appears to have minimal impact on serum lipids in humans-might have clinical utility for preventing and controlling atherosclerosis[104,105]. Of related interest is the possibility that taurine’s antioxidant activity may be helpful for preventing LDL modification mediated by hypochlorous acid, a myeloperoxidase product[106]. Moreover, rodent and limited clinical studies suggest that taurine supplementation has the potential to favorably influence platelet stability, blood pressure, and the failing heart[107]. The continuing neglect of this inexpensive and well tolerated nutrient by clinical researchers is mystifying.
AMPK activators
The anti-diabetic nutraceutical berberine, whose clinical efficacy resembles that of metformin in being contingent on activation of AMPK, has exerted anti-atherogenic effects in some but not all rodent models of this disorder[108-110]. The AMPK activator AICAR has been shown to promote reverse cholesterol transport in cultured macrophages by boosting expression of ABCG1[111]. Studies examining the impact of berberine on cultured macrophages report that it can exert a range of effects likely to antagonize foam cell formation and stabilize plaque-inhibiting activation of NADPH oxidase and NF-kappaB, inhibiting MMP-9 expression, and antagonizing cholesterol accumulation by inducing expression of ABCA1 or SRB-1, or suppressing expression of the LOX-1 LDL receptor for oxidized LDL[112-114]. On the other hand, one study found that berberine exposure increased macrophage uptake of modified LDL by increasing expression of the SRA-1 receptor[115]. The impact of metformin on foam cell function appears to have received little or no study. In vivo, berberine could also be expected to reduce foam cell formation by decreasing circulating LDL; it boosts hepatocyte expression of the LDL receptor by a mechanism that is complementary to that of statins[116].
CONCLUSION
It should not go unnoted that many of the agents discussed here-notably phase 2 inducers[117-122], PhyCB[123-125], salsalate[126-128], and berberine or metformin[129-134]-have the potential to impede foam cell formation by exerting anti-inflammatory effects on endothelial cells that would be expected to impede monocyte migration across the endothelial barrier into arterial intima. Each of these agents can work in various ways to inhibit endothelial NF-kappaB activity, which promotes the adhesion of monocytes to the endothelial surface and their subsequent transmigration (Figure 2)[135-137].
Figure 2.
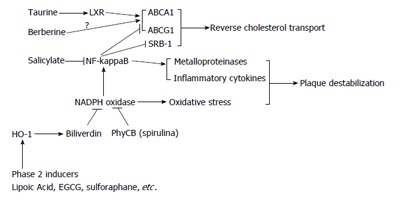
Nutraceutical/drug regulation of foam cell cholesterol transport and plaque stability. LXR: Liver X receptors; HO-1: Heme oxygenase-1; NF-kappaB: Nuclear factor-kappaB; ABCA1: Adenosine triphosphate binding cassette transporter A1; ABCG1: Adenosine triphosphate binding cassette transporter G1; SRB-1: Scavenger receptor class B member 1; NADPH oxidase:: Nicotinamide adenine dinucleotide phosphate oxidase:; EGCG: Epigallocatechin gallate; HO-1: Heme oxygenase-1; PhyCB: Phycocyanobilin.
In summation-whereas current pharmaceutical strategies for increasing HDL cholesterol appear to have little clinical utility (aside from those which concurrently lower LDL levels), other clinically feasible measures which directly influence intimal macrophages have the potential to promote reverse cholesterol transport, and hence achieve the primary purpose intended for HDL elevation. These measures include administration of phase 2-inducing nutraceuticals (such as lipoic acid, green tea catechins, and cruciferous isothiocyanates), spirulina or PhyCB, salsalate, taurine, and berberine. These effects are mediated primarily by inhibition of NF-kappaB activation or by LXRalpha agonism. Moreover, most of these agents might be expected to impact foam cell function in other complementary ways that would be clinically useful-suppressing macrophage uptake of modified LDL, and inhibiting macrophage production of inflammatory cytokines and matrix metalloproteinases that could destabilize plaque. And most of them, via direct anti-inflammatory effects on vascular endothelium, should also impede foam cell formation by suppressing transendothelial migration of monocytes. These agents evidently merit further evaluation, both in animal models and ultimately clinical trials, as measures for preventing, reversing, and stabilizing arterial plaque. And the fact that most of these agents are nutraceuticals suggests that they might be especially feasible for use in primary prevention.
Footnotes
P- Reviewer: Mihaila RG, Nasu K, Yang H S- Editor: Wen LL L- Editor: A E- Editor: Liu SQ
References
- 1.Poglitsch CL, Thompson NL. Interaction of antibodies with Fc receptors in substrate-supported planar membranes measured by total internal reflection fluorescence microscopy. Biochemistry. 1990;29:248–254. doi: 10.1021/bi00453a033. [DOI] [PubMed] [Google Scholar]
- 2.Oram JF. Tangier disease and ABCA1. Biochim Biophys Acta. 2000;1529:321–330. doi: 10.1016/s1388-1981(00)00157-8. [DOI] [PubMed] [Google Scholar]
- 3.Kolovou GD, Mikhailidis DP, Anagnostopoulou KK, Daskalopoulou SS, Cokkinos DV. Tangier disease four decades of research: a reflection of the importance of HDL. Curr Med Chem. 2006;13:771–782. doi: 10.2174/092986706776055580. [DOI] [PubMed] [Google Scholar]
- 4.Briel M, Ferreira-Gonzalez I, You JJ, Karanicolas PJ, Akl EA, Wu P, Blechacz B, Bassler D, Wei X, Sharman A, et al. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. BMJ. 2009;338:b92. doi: 10.1136/bmj.b92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruckert E, Labreuche J, Amarenco P. Meta-analysis of the effect of nicotinic acid alone or in combination on cardiovascular events and atherosclerosis. Atherosclerosis. 2010;210:353–361. doi: 10.1016/j.atherosclerosis.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 6.Phan BA, Muñoz L, Shadzi P, Isquith D, Triller M, Brown BG, Zhao XQ. Effects of niacin on glucose levels, coronary stenosis progression, and clinical events in subjects with normal baseline glucose levels (& lt; 100 mg/dl): a combined analysis of the Familial Atherosclerosis Treatment Study (FATS), HDL-Atherosclerosis Treatment Study (HATS), Armed Forces Regression Study (AFREGS), and Carotid Plaque Composition by MRI during lipid-lowering (CPC) study. Am J Cardiol. 2013;111:352–355. doi: 10.1016/j.amjcard.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 8.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Hólm H, Ding EL, Johnson T, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ference BA, Yoo W, Alesh I, Mahajan N, Mirowska KK, Mewada A, Kahn J, Afonso L, Williams KA, Flack JM. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a Mendelian randomization analysis. J Am Coll Cardiol. 2012;60:2631–2639. doi: 10.1016/j.jacc.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 12.Waksman R, Torguson R, Kent KM, Pichard AD, Suddath WO, Satler LF, Martin BD, Perlman TJ, Maltais JA, Weissman NJ, et al. A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J Am Coll Cardiol. 2010;55:2727–2735. doi: 10.1016/j.jacc.2009.12.067. [DOI] [PubMed] [Google Scholar]
- 13.Roberts CK, Ng C, Hama S, Eliseo AJ, Barnard RJ. Effect of a short-term diet and exercise intervention on inflammatory/anti-inflammatory properties of HDL in overweight/obese men with cardiovascular risk factors. J Appl Physiol (1985) 2006;101:1727–1732. doi: 10.1152/japplphysiol.00345.2006. [DOI] [PubMed] [Google Scholar]
- 14.Sirtori CR, Calabresi L, Franceschini G, Baldassarre D, Amato M, Johansson J, Salvetti M, Monteduro C, Zulli R, Muiesan ML, et al. Cardiovascular status of carriers of the apolipoprotein A-I(Milano) mutant: the Limone sul Garda study. Circulation. 2001;103:1949–1954. doi: 10.1161/01.cir.103.15.1949. [DOI] [PubMed] [Google Scholar]
- 15.Zhang LH, Kamanna VS, Zhang MC, Kashyap ML. Niacin inhibits surface expression of ATP synthase beta chain in HepG2 cells: implications for raising HDL. J Lipid Res. 2008;49:1195–1201. doi: 10.1194/jlr.M700426-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Amarasuriya RN, Gupta AK, Civen M, Horng YC, Maeda T, Kashyap ML. Ethanol stimulates apolipoprotein A-I secretion by human hepatocytes: implications for a mechanism for atherosclerosis protection. Metabolism. 1992;41:827–832. doi: 10.1016/0026-0495(92)90162-4. [DOI] [PubMed] [Google Scholar]
- 17.Sakakibara S, Murakami R, Takahashi M, Fushimi T, Murohara T, Kishi M, Kajimoto Y, Kitakaze M, Kaga T. Vinegar intake enhances flow-mediated vasodilatation via upregulation of endothelial nitric oxide synthase activity. Biosci Biotechnol Biochem. 2010;74:1055–1061. doi: 10.1271/bbb.90953. [DOI] [PubMed] [Google Scholar]
- 18.Berrougui H, Khalil A. Age-associated decrease of high-density lipoprotein-mediated reverse cholesterol transport activity. Rejuvenation Res. 2009;12:117–126. doi: 10.1089/rej.2009.0840. [DOI] [PubMed] [Google Scholar]
- 19.Tontonoz P, Mangelsdorf DJ. Liver X receptor signaling pathways in cardiovascular disease. Mol Endocrinol. 2003;17:985–993. doi: 10.1210/me.2003-0061. [DOI] [PubMed] [Google Scholar]
- 20.Beyea MM, Heslop CL, Sawyez CG, Edwards JY, Markle JG, Hegele RA, Huff MW. Selective up-regulation of LXR-regulated genes ABCA1, ABCG1, and APOE in macrophages through increased endogenous synthesis of 24(S),25-epoxycholesterol. J Biol Chem. 2007;282:5207–5216. doi: 10.1074/jbc.M611063200. [DOI] [PubMed] [Google Scholar]
- 21.Yu L, Cao G, Repa J, Stangl H. Sterol regulation of scavenger receptor class B type I in macrophages. J Lipid Res. 2004;45:889–899. doi: 10.1194/jlr.M300461-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Baranova I, Vishnyakova T, Bocharov A, Chen Z, Remaley AT, Stonik J, Eggerman TL, Patterson AP. Lipopolysaccharide down regulates both scavenger receptor B1 and ATP binding cassette transporter A1 in RAW cells. Infect Immun. 2002;70:2995–3003. doi: 10.1128/IAI.70.6.2995-3003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira V, van Dijk KW, Groen AK, Vos RM, van der Kaa J, Gijbels MJ, Havekes LM, Pannekoek H. Macrophage-specific inhibition of NF-kappaB activation reduces foam-cell formation. Atherosclerosis. 2007;192:283–290. doi: 10.1016/j.atherosclerosis.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 24.Chen M, Li W, Wang N, Zhu Y, Wang X. ROS and NF-kappaB but not LXR mediate IL-1beta signaling for the downregulation of ATP-binding cassette transporter A1. Am J Physiol Cell Physiol. 2007;292:C1493–C1501. doi: 10.1152/ajpcell.00016.2006. [DOI] [PubMed] [Google Scholar]
- 25.Lu L, Liu H, Peng J, Gan L, Shen L, Zhang Q, Li L, Zhang L, Su C, Jiang Y. Regulations of the key mediators in inflammation and atherosclerosis by aspirin in human macrophages. Lipids Health Dis. 2010;9:16. doi: 10.1186/1476-511X-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang J, Mo ZC, Yin K, Zhao GJ, Lv YC, Ouyang XP, Jiang ZS, Fu Y, Tang CK. Epigallocatechin-3-gallate prevents TNF-α-induced NF-κB activation thereby upregulating ABCA1 via the Nrf2/Keap1 pathway in macrophage foam cells. Int J Mol Med. 2012;29:946–956. doi: 10.3892/ijmm.2012.924. [DOI] [PubMed] [Google Scholar]
- 27.Yu XH, Jiang HL, Chen WJ, Yin K, Zhao GJ, Mo ZC, Ouyang XP, Lv YC, Jiang ZS, Zhang DW, et al. Interleukin-18 and interleukin-12 together downregulate ATP-binding cassette transporter A1 expression through the interleukin-18R/nuclear factor-κB signaling pathway in THP-1 macrophage-derived foam cells. Circ J. 2012;76:1780–1791. doi: 10.1253/circj.cj-11-1338. [DOI] [PubMed] [Google Scholar]
- 28.Chase AJ, Bond M, Crook MF, Newby AC. Role of nuclear factor-kappa B activation in metalloproteinase-1, -3, and -9 secretion by human macrophages in vitro and rabbit foam cells produced in vivo. Arterioscler Thromb Vasc Biol. 2002;22:765–771. doi: 10.1161/01.atv.0000015078.09208.92. [DOI] [PubMed] [Google Scholar]
- 29.Castrillo A, Joseph SB, Marathe C, Mangelsdorf DJ, Tontonoz P. Liver X receptor-dependent repression of matrix metalloproteinase-9 expression in macrophages. J Biol Chem. 2003;278:10443–10449. doi: 10.1074/jbc.M213071200. [DOI] [PubMed] [Google Scholar]
- 30.Ishikawa K, Sugawara D, Wang Xp K, Itabe H, Maruyama Y, Lusis AJ. Heme oxygenase-1 inhibits atherosclerotic lesion formation in ldl-receptor knockout mice. Circ Res. 2001;88:506–512. doi: 10.1161/01.res.88.5.506. [DOI] [PubMed] [Google Scholar]
- 31.Ishikawa K, Sugawara D, Goto J, Watanabe Y, Kawamura K, Shiomi M, Itabe H, Maruyama Y. Heme oxygenase-1 inhibits atherogenesis in Watanabe heritable hyperlipidemic rabbits. Circulation. 2001;104:1831–1836. doi: 10.1161/hc3901.095897. [DOI] [PubMed] [Google Scholar]
- 32.Ma JL, Yang PY, Rui YC, Lu L, Kang H, Zhang J. Hemin modulates cytokine expressions in macrophage-derived foam cells via heme oxygenase-1 induction. J Pharmacol Sci. 2007;103:261–266. doi: 10.1254/jphs.fp0060270. [DOI] [PubMed] [Google Scholar]
- 33.Orozco LD, Kapturczak MH, Barajas B, Wang X, Weinstein MM, Wong J, Deshane J, Bolisetty S, Shaposhnik Z, Shih DM, et al. Heme oxygenase-1 expression in macrophages plays a beneficial role in atherosclerosis. Circ Res. 2007;100:1703–1711. doi: 10.1161/CIRCRESAHA.107.151720. [DOI] [PubMed] [Google Scholar]
- 34.Tsai JY, Su KH, Shyue SK, Kou YR, Yu YB, Hsiao SH, Chiang AN, Wu YL, Ching LC, Lee TS. EGb761 ameliorates the formation of foam cells by regulating the expression of SR-A and ABCA1: role of haem oxygenase-1. Cardiovasc Res. 2010;88:415–423. doi: 10.1093/cvr/cvq226. [DOI] [PubMed] [Google Scholar]
- 35.Kuhn AM, Tzieply N, Schmidt MV, von Knethen A, Namgaladze D, Yamamoto M, Brüne B. Antioxidant signaling via Nrf2 counteracts lipopolysaccharide-mediated inflammatory responses in foam cell macrophages. Free Radic Biol Med. 2011;50:1382–1391. doi: 10.1016/j.freeradbiomed.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 36.Araujo JA, Zhang M, Yin F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front Pharmacol. 2012;3:119. doi: 10.3389/fphar.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li XY, Kong LX, Li J, He HX, Zhou YD. Kaempferol suppresses lipid accumulation in macrophages through the downregulation of cluster of differentiation 36 and the upregulation of scavenger receptor class B type I and ATP-binding cassette transporters A1 and G1. Int J Mol Med. 2013;31:331–338. doi: 10.3892/ijmm.2012.1204. [DOI] [PubMed] [Google Scholar]
- 38.Sasaki T, Takahashi T, Maeshima K, Shimizu H, Toda Y, Morimatsu H, Takeuchi M, Yokoyama M, Akagi R, Morita K. Heme arginate pretreatment attenuates pulmonary NF-kappaB and AP-1 activation induced by hemorrhagic shock via heme oxygenase-1 induction. Med Chem. 2006;2:271–274. doi: 10.2174/157340606776930781. [DOI] [PubMed] [Google Scholar]
- 39.Zabalgoitia M, Colston JT, Reddy SV, Holt JW, Regan RF, Stec DE, Rimoldi JM, Valente AJ, Chandrasekar B. Carbon monoxide donors or heme oxygenase-1 (HO-1) overexpression blocks interleukin-18-mediated NF-kappaB-PTEN-dependent human cardiac endothelial cell death. Free Radic Biol Med. 2008;44:284–298. doi: 10.1016/j.freeradbiomed.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaea HJ, Kim HR, Kang YJ, Hyun KC, Kim HJ, Seo HG, Lee JH, Yun-Choi HS, Chang KC. Heme oxygenase-1 induction by (S)-enantiomer of YS-51 (YS-51S), a synthetic isoquinoline alkaloid, inhibits nitric oxide production and nuclear factor-kappaB translocation in ROS 17/2.8 cells activated with inflammatory stimulants. Int Immunopharmacol. 2007;7:1559–1568. doi: 10.1016/j.intimp.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 41.Jadhav A, Torlakovic E, Ndisang JF. Interaction among heme oxygenase, nuclear factor-kappaB, and transcription activating factors in cardiac hypertrophy in hypertension. Hypertension. 2008;52:910–917. doi: 10.1161/HYPERTENSIONAHA.108.114801. [DOI] [PubMed] [Google Scholar]
- 42.Kim KM, Pae HO, Zhung M, Ha HY, Ha YA, Chai KY, Cheong YK, Kim JM, Chung HT. Involvement of anti-inflammatory heme oxygenase-1 in the inhibitory effect of curcumin on the expression of pro-inflammatory inducible nitric oxide synthase in RAW264.7 macrophages. Biomed Pharmacother. 2008;62:630–636. doi: 10.1016/j.biopha.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 43.Yeh CH, Chen TP, Wang YC, Lin YM, Lin PJ. HO-1 activation can attenuate cardiomyocytic apoptosis via inhibition of NF-kappaB and AP-1 translocation following cardiac global ischemia and reperfusion. J Surg Res. 2009;155:147–156. doi: 10.1016/j.jss.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 44.Park SY, Lee SW, Baek SH, Lee SJ, Lee WS, Rhim BY, Hong KW, Kim CD. Induction of heme oxygenase-1 expression by cilostazol contributes to its anti-inflammatory effects in J774 murine macrophages. Immunol Lett. 2011;136:138–145. doi: 10.1016/j.imlet.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Leung PO, Wang SH, Lu SH, Chou WH, Shiau CY, Chou TC. Simvastatin inhibits pro-inflammatory mediators through induction of heme oxygenase-1 expression in lipopolysaccharide-stimulated RAW264.7 macrophages. Toxicol Lett. 2011;207:159–166. doi: 10.1016/j.toxlet.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Park SY, Park da J, Kim YH, Kim Y, Choi YW, Lee SJ. Schisandra chinensis α-iso-cubebenol induces heme oxygenase-1 expression through PI3K/Akt and Nrf2 signaling and has anti-inflammatory activity in Porphyromonas gingivalis lipopolysaccharide-stimulated macrophages. Int Immunopharmacol. 2011;11:1907–1915. doi: 10.1016/j.intimp.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 47.Kim CK, Cho DH, Lee KS, Lee DK, Park CW, Kim WG, Lee SJ, Ha KS, Goo Taeg O, Kwon YG, et al. Ginseng Berry Extract Prevents Atherogenesis via Anti-Inflammatory Action by Upregulating Phase II Gene Expression. Evid Based Complement Alternat Med. 2012;2012:490301. doi: 10.1155/2012/490301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alam J, Cook JL. Transcriptional regulation of the heme oxygenase-1 gene via the stress response element pathway. Curr Pharm Des. 2003;9:2499–2511. doi: 10.2174/1381612033453730. [DOI] [PubMed] [Google Scholar]
- 49.Flier J, Van Muiswinkel FL, Jongenelen CA, Drukarch B. The neuroprotective antioxidant alpha-lipoic acid induces detoxication enzymes in cultured astroglial cells. Free Radic Res. 2002;36:695–699. doi: 10.1080/10715760290029155. [DOI] [PubMed] [Google Scholar]
- 50.Cao Z, Tsang M, Zhao H, Li Y. Induction of endogenous antioxidants and phase 2 enzymes by alpha-lipoic acid in rat cardiac H9C2 cells: protection against oxidative injury. Biochem Biophys Res Commun. 2003;310:979–985. doi: 10.1016/j.bbrc.2003.09.110. [DOI] [PubMed] [Google Scholar]
- 51.Jia Z, Hallur S, Zhu H, Li Y, Misra HP. Potent upregulation of glutathione and NAD(P)H: quinone oxidoreductase 1 by alpha-lipoic acid in human neuroblastoma SH-SY5Y cells: protection against neurotoxicant-elicited cytotoxicity. Neurochem Res. 2008;33:790–800. doi: 10.1007/s11064-007-9496-5. [DOI] [PubMed] [Google Scholar]
- 52.Na HK, Surh YJ. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem Toxicol. 2008;46:1271–1278. doi: 10.1016/j.fct.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 53.Romeo L, Intrieri M, D’Agata V, Mangano NG, Oriani G, Ontario ML, Scapagnini G. The major green tea polyphenol, (-)-epigallocatechin-3-gallate, induces heme oxygenase in rat neurons and acts as an effective neuroprotective agent against oxidative stress. J Am Coll Nutr. 2009;28 Suppl:492S–499S. doi: 10.1080/07315724.2009.10718116. [DOI] [PubMed] [Google Scholar]
- 54.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci USA. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riedl MA, Saxon A, Diaz-Sanchez D. Oral sulforaphane increases Phase II antioxidant enzymes in the human upper airway. Clin Immunol. 2009;130:244–251. doi: 10.1016/j.clim.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kensler TW, Curphey TJ, Maxiutenko Y, Roebuck BD. Chemoprotection by organosulfur inducers of phase 2 enzymes: dithiolethiones and dithiins. Drug Metabol Drug Interact. 2000;17:3–22. doi: 10.1515/dmdi.2000.17.1-4.3. [DOI] [PubMed] [Google Scholar]
- 57.Chen C, Pung D, Leong V, Hebbar V, Shen G, Nair S, Li W, Kong AN. Induction of detoxifying enzymes by garlic organosulfur compounds through transcription factor Nrf2: effect of chemical structure and stress signals. Free Radic Biol Med. 2004;37:1578–1590. doi: 10.1016/j.freeradbiomed.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 58.Ziegler D, Ametov A, Barinov A, Dyck PJ, Gurieva I, Low PA, Munzel U, Yakhno N, Raz I, Novosadova M, et al. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care. 2006;29:2365–2370. doi: 10.2337/dc06-1216. [DOI] [PubMed] [Google Scholar]
- 59.Yi X, Maeda N. alpha-Lipoic acid prevents the increase in atherosclerosis induced by diabetes in apolipoprotein E-deficient mice fed high-fat/low-cholesterol diet. Diabetes. 2006;55:2238–2244. doi: 10.2337/db06-0251. [DOI] [PubMed] [Google Scholar]
- 60.Zulkhairi A, Zaiton Z, Jamaluddin M, Sharida F, Mohd TH, Hasnah B, Nazmi HM, Khairul O, Zanariyah A. Alpha lipoic acid possess dual antioxidant and lipid lowering properties in atherosclerotic-induced New Zealand White rabbit. Biomed Pharmacother. 2008;62:716–722. doi: 10.1016/j.biopha.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 61.Ying Z, Kherada N, Farrar B, Kampfrath T, Chung Y, Simonetti O, Deiuliis J, Desikan R, Khan B, Villamena F, et al. Lipoic acid effects on established atherosclerosis. Life Sci. 2010;86:95–102. doi: 10.1016/j.lfs.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee WR, Kim A, Kim KS, Park YY, Park JH, Kim KH, Kim SJ, Park KK. Alpha-lipoic acid attenuates atherosclerotic lesions and inhibits proliferation of vascular smooth muscle cells through targeting of the Ras/MEK/ERK signaling pathway. Mol Biol Rep. 2012;39:6857–6866. doi: 10.1007/s11033-012-1511-5. [DOI] [PubMed] [Google Scholar]
- 63.Lanone S, Bloc S, Foresti R, Almolki A, Taillé C, Callebert J, Conti M, Goven D, Aubier M, Dureuil B, et al. Bilirubin decreases nos2 expression via inhibition of NAD(P)H oxidase: implications for protection against endotoxic shock in rats. FASEB J. 2005;19:1890–1892. doi: 10.1096/fj.04-2368fje. [DOI] [PubMed] [Google Scholar]
- 64.Matsumoto H, Ishikawa K, Itabe H, Maruyama Y. Carbon monoxide and bilirubin from heme oxygenase-1 suppresses reactive oxygen species generation and plasminogen activator inhibitor-1 induction. Mol Cell Biochem. 2006;291:21–28. doi: 10.1007/s11010-006-9190-y. [DOI] [PubMed] [Google Scholar]
- 65.Jiang F, Roberts SJ, Datla Sr, Dusting GJ. NO modulates NADPH oxidase function via heme oxygenase-1 in human endothelial cells. Hypertension. 2006;48:950–957. doi: 10.1161/01.HYP.0000242336.58387.1f. [DOI] [PubMed] [Google Scholar]
- 66.Datla SR, Dusting GJ, Mori TA, Taylor CJ, Croft KD, Jiang F. Induction of heme oxygenase-1 in vivo suppresses NADPH oxidase derived oxidative stress. Hypertension. 2007;50:636–642. doi: 10.1161/HYPERTENSIONAHA.107.092296. [DOI] [PubMed] [Google Scholar]
- 67.Schwertner HA, Vítek L. Gilbert syndrome, UGT1A1*28 allele, and cardiovascular disease risk: possible protective effects and therapeutic applications of bilirubin. Atherosclerosis. 2008;198:1–11. doi: 10.1016/j.atherosclerosis.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 68.Lin JP, Vitek L, Schwertner HA. Serum bilirubin and genes controlling bilirubin concentrations as biomarkers for cardiovascular disease. Clin Chem. 2010;56:1535–1543. doi: 10.1373/clinchem.2010.151043. [DOI] [PubMed] [Google Scholar]
- 69.Horsfall LJ, Nazareth I, Petersen I. Cardiovascular events as a function of serum bilirubin levels in a large, statin-treated cohort. Circulation. 2012;126:2556–2564. doi: 10.1161/CIRCULATIONAHA.112.114066. [DOI] [PubMed] [Google Scholar]
- 70.Bonizzi G, Piette J, Schoonbroodt S, Greimers R, Havard L, Merville MP, Bours V. Reactive oxygen intermediate-dependent NF-kappaB activation by interleukin-1beta requires 5-lipoxygenase or NADPH oxidase activity. Mol Cell Biol. 1999;19:1950–1960. doi: 10.1128/mcb.19.3.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kono H, Rusyn I, Yin M, Gäbele E, Yamashina S, Dikalova A, Kadiiska MB, Connor HD, Mason RP, Segal BH, et al. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106:867–872. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoshida M, Korfhagen TR, Whitsett JA. Surfactant protein D regulates NF-kappa B and matrix metalloproteinase production in alveolar macrophages via oxidant-sensitive pathways. J Immunol. 2001;166:7514–7519 [PMI: 11390505]. doi: 10.4049/jimmunol.166.12.7514. [DOI] [PubMed] [Google Scholar]
- 73.Sadikot RT, Zeng H, Yull FE, Li B, Cheng DS, Kernodle DS, Jansen ED, Contag CH, Segal BH, Holland SM, et al. p47phox deficiency impairs NF-kappa B activation and host defense in Pseudomonas pneumonia. J Immunol. 2004;172:1801–1808. doi: 10.4049/jimmunol.172.3.1801. [DOI] [PubMed] [Google Scholar]
- 74.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 75.Yamashina S, Takei Y, Ikejima K, Enomoto N, Kitamura T, Sato N. Ethanol-induced sensitization to endotoxin in Kupffer cells is dependent upon oxidative stress. Alcohol Clin Exp Res. 2005;29:246S–250S. doi: 10.1097/01.alc.0000191128.54871.40. [DOI] [PubMed] [Google Scholar]
- 76.Au-Yeung KK, Yip JC, Siow YL, O K. Folic acid inhibits homocysteine-induced superoxide anion production and nuclear factor kappa B activation in macrophages. Can J Physiol Pharmacol. 2006;84:141–147. doi: 10.1139/Y05-136. [DOI] [PubMed] [Google Scholar]
- 77.Higai K, Sano R, Satake M, Azuma Y, Matsumoto K. Glycated human serum albumin induces interleukin 8 mRNA expression through reactive oxygen species and NADPH oxidase-dependent pathway in monocyte-derived U937 cells. Biol Pharm Bull. 2007;30:1833–1837. doi: 10.1248/bpb.30.1833. [DOI] [PubMed] [Google Scholar]
- 78.San José G, Bidegain J, Robador PA, Díez J, Fortuño A, Zalba G. Insulin-induced NADPH oxidase activation promotes proliferation and matrix metalloproteinase activation in monocytes/macrophages. Free Radic Biol Med. 2009;46:1058–1067. doi: 10.1016/j.freeradbiomed.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 79.Kim SY, Moon KA, Jo HY, Jeong S, Seon SH, Jung E, Cho YS, Chun E, Lee KY. Anti-inflammatory effects of apocynin, an inhibitor of NADPH oxidase, in airway inflammation. Immunol Cell Biol. 2012;90:441–448. doi: 10.1038/icb.2011.60. [DOI] [PubMed] [Google Scholar]
- 80.Mo ZC, Xiao J, Liu XH, Hu YW, Li XX, Yi GH, Wang Z, Tang YL, Liao DF, Tang CK. AOPPs inhibits cholesterol efflux by down-regulating ABCA1 expression in a JAK/STAT signaling pathway-dependent manner. J Atheroscler Thromb. 2011;18:796–807. doi: 10.5551/jat.6569. [DOI] [PubMed] [Google Scholar]
- 81.Ishibashi Y, Matsui T, Takeuchi M, Yamagishi S. Rosuvastatin blocks advanced glycation end products-elicited reduction of macrophage cholesterol efflux by suppressing NADPH oxidase activity via inhibition of geranylgeranylation of Rac-1. Horm Metab Res. 2011;43:619–624. doi: 10.1055/s-0031-1283148. [DOI] [PubMed] [Google Scholar]
- 82.McCarty MF. Clinical potential of Spirulina as a source of phycocyanobilin. J Med Food. 2007;10:566–570. doi: 10.1089/jmf.2007.621. [DOI] [PubMed] [Google Scholar]
- 83.Zheng J, Inoguchi T, Sasaki S, Maeda Y, McCarty MF, Fujii M, Ikeda N, Kobayashi K, Sonoda N, Takayanagi R. Phycocyanin and phycocyanobilin from Spirulina platensis protect against diabetic nephropathy by inhibiting oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2013;304:R110–R120. doi: 10.1152/ajpregu.00648.2011. [DOI] [PubMed] [Google Scholar]
- 84.Riss J, Décordé K, Sutra T, Delage M, Baccou JC, Jouy N, Brune JP, Oréal H, Cristol JP, Rouanet JM. Phycobiliprotein C-phycocyanin from Spirulina platensis is powerfully responsible for reducing oxidative stress and NADPH oxidase expression induced by an atherogenic diet in hamsters. J Agric Food Chem. 2007;55:7962–7967. doi: 10.1021/jf070529g. [DOI] [PubMed] [Google Scholar]
- 85.Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 86.Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-{kappa}B activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation. 2009;119:1284–1292. doi: 10.1161/CIRCULATIONAHA.108.804294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morris HG, Sherman NA, McQuain C, Goldlust MB, Chang SF, Harrison LI. Effects of salsalate (nonacetylated salicylate) and aspirin on serum prostaglandins in humans. Ther Drug Monit. 1985;7:435–438. doi: 10.1097/00007691-198512000-00012. [DOI] [PubMed] [Google Scholar]
- 88.McCarty MF. Salsalate may have broad utility in the prevention and treatment of vascular disorders and the metabolic syndrome. Med Hypotheses. 2010;75:276–281. doi: 10.1016/j.mehy.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 89.Goldfine AB, Silver R, Aldhahi W, Cai D, Tatro E, Lee J, Shoelson SE. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin Transl Sci. 2008;1:36–43. doi: 10.1111/j.1752-8062.2008.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goldfine AB, Fonseca V, Jablonski KA, Pyle L, Staten MA, Shoelson SE. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2010;152:346–357. doi: 10.1059/0003-4819-152-6-201003160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Desouza CV. An overview of salsalate as a potential antidiabetic therapy. Drugs Today (Barc) 2010;46:847–853. doi: 10.1358/dot.2010.46.11.1534820. [DOI] [PubMed] [Google Scholar]
- 92.Calkin AC, Tontonoz P. Liver x receptor signaling pathways and atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:1513–1518. doi: 10.1161/ATVBAHA.109.191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li X, Yeh V, Molteni V. Liver X receptor modulators: a review of recently patented compounds (2007 - 2009) Expert Opin Ther Pat. 2010;20:535–562. doi: 10.1517/13543771003621269. [DOI] [PubMed] [Google Scholar]
- 94.Zhu Y, Li Y. Liver X receptors as potential therapeutic targets in atherosclerosis. Clin Invest Med. 2009;32:E383–E394. doi: 10.25011/cim.v32i5.6927. [DOI] [PubMed] [Google Scholar]
- 95.Hoang MH, Jia Y, Jun HJ, Lee JH, Hwang KY, Choi DW, Um SJ, Lee BY, You SG, Lee SJ. Taurine is a liver X receptor-α ligand and activates transcription of key genes in the reverse cholesterol transport without inducing hepatic lipogenesis. Mol Nutr Food Res. 2012;56:900–911. doi: 10.1002/mnfr.201100611. [DOI] [PubMed] [Google Scholar]
- 96.Petty MA, Kintz J, DiFrancesco GF. The effects of taurine on atherosclerosis development in cholesterol-fed rabbits. Eur J Pharmacol. 1990;180:119–127. doi: 10.1016/0014-2999(90)90599-2. [DOI] [PubMed] [Google Scholar]
- 97.Kondo Y, Toda Y, Kitajima H, Oda H, Nagate T, Kameo K, Murakami S. Taurine inhibits development of atherosclerotic lesions in apolipoprotein E-deficient mice. Clin Exp Pharmacol Physiol. 2001;28:809–815. doi: 10.1046/j.1440-1681.2001.03527.x. [DOI] [PubMed] [Google Scholar]
- 98.Murakami S, Kondo Y, Nagate T. Effects of long-term treatment with taurine in mice fed a high-fat diet: improvement in cholesterol metabolism and vascular lipid accumulation by taurine. Adv Exp Med Biol. 2000;483:177–186. doi: 10.1007/0-306-46838-7_19. [DOI] [PubMed] [Google Scholar]
- 99.Murakami S, Kondo Y, Sakurai T, Kitajima H, Nagate T. Taurine suppresses development of atherosclerosis in Watanabe heritable hyperlipidemic (WHHL) rabbits. Atherosclerosis. 2002;163:79–87. doi: 10.1016/s0021-9150(01)00764-x. [DOI] [PubMed] [Google Scholar]
- 100.Balkan J, Kanbağli O, Hatipoğlu A, Kücük M, Cevikbaş U, Aykaç-Toker G, Uysal M. Improving effect of dietary taurine supplementation on the oxidative stress and lipid levels in the plasma, liver and aorta of rabbits fed on a high-cholesterol diet. Biosci Biotechnol Biochem. 2002;66:1755–1758. doi: 10.1271/bbb.66.1755. [DOI] [PubMed] [Google Scholar]
- 101.Matsushima Y, Sekine T, Kondo Y, Sakurai T, Kameo K, Tachibana M, Murakami S. Effects of taurine on serum cholesterol levels and development of atherosclerosis in spontaneously hyperlipidaemic mice. Clin Exp Pharmacol Physiol. 2003;30:295–299. doi: 10.1046/j.1440-1681.2003.03828.x. [DOI] [PubMed] [Google Scholar]
- 102.Zulli A, Lau E, Wijaya BP, Jin X, Sutarga K, Schwartz GD, Learmont J, Wookey PJ, Zinellu A, Carru C, et al. High dietary taurine reduces apoptosis and atherosclerosis in the left main coronary artery: association with reduced CCAAT/enhancer binding protein homologous protein and total plasma homocysteine but not lipidemia. Hypertension. 2009;53:1017–1022. doi: 10.1161/HYPERTENSIONAHA.109.129924. [DOI] [PubMed] [Google Scholar]
- 103.Murakami S. Taurine and atherosclerosis. Amino Acids. 2014;46:73–80. doi: 10.1007/s00726-012-1432-6. [DOI] [PubMed] [Google Scholar]
- 104.Wójcik OP, Koenig KL, Zeleniuch-Jacquotte A, Costa M, Chen Y. The potential protective effects of taurine on coronary heart disease. Atherosclerosis. 2010;208:19–25. doi: 10.1016/j.atherosclerosis.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yamori Y, Taguchi T, Hamada A, Kunimasa K, Mori H, Mori M. Taurine in health and diseases: consistent evidence from experimental and epidemiological studies. J Biomed Sci. 2010;17 Suppl 1:S6. doi: 10.1186/1423-0127-17-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jerlich A, Fritz G, Kharrazi H, Hammel M, Tschabuschnig S, Glatter O, Schaur RJ. Comparison of HOCl traps with myeloperoxidase inhibitors in prevention of low density lipoprotein oxidation. Biochim Biophys Acta. 2000;1481:109–118. doi: 10.1016/s0167-4838(00)00112-6. [DOI] [PubMed] [Google Scholar]
- 107.McCarty MF. Rationale for a novel nutraceutical complex ‘K-water’: potassium taurine bicarbonate (PTB) Med Hypotheses. 2006;67:65–70. doi: 10.1016/j.mehy.2005.09.056. [DOI] [PubMed] [Google Scholar]
- 108.Lee S, Lim HJ, Park HY, Lee KS, Park JH, Jang Y. Berberine inhibits rat vascular smooth muscle cell proliferation and migration in vitro and improves neointima formation after balloon injury in vivo. Berberine improves neointima formation in a rat model. Atherosclerosis. 2006;186:29–37. doi: 10.1016/j.atherosclerosis.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 109.Wang Q, Zhang M, Liang B, Shirwany N, Zhu Y, Zou MH. Activation of AMP-activated protein kinase is required for berberine-induced reduction of atherosclerosis in mice: the role of uncoupling protein 2. PLoS One. 2011;6:e25436. doi: 10.1371/journal.pone.0025436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li K, Yao W, Zheng X, Liao K. Berberine promotes the development of atherosclerosis and foam cell formation by inducing scavenger receptor A expression in macrophage. Cell Res. 2009;19:1006–1017. doi: 10.1038/cr.2009.76. [DOI] [PubMed] [Google Scholar]
- 111.Li D, Wang D, Wang Y, Ling W, Feng X, Xia M. Adenosine monophosphate-activated protein kinase induces cholesterol efflux from macrophage-derived foam cells and alleviates atherosclerosis in apolipoprotein E-deficient mice. J Biol Chem. 2010;285:33499–33509. doi: 10.1074/jbc.M110.159772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee TS, Pan CC, Peng CC, Kou YR, Chen CY, Ching LC, Tsai TH, Chen SF, Lyu PC, Shyue SK. Anti-atherogenic effect of berberine on LXRalpha-ABCA1-dependent cholesterol efflux in macrophages. J Cell Biochem. 2010;111:104–110. doi: 10.1002/jcb.22667. [DOI] [PubMed] [Google Scholar]
- 113.Guan S, Wang B, Li W, Guan J, Fang X. Effects of berberine on expression of LOX-1 and SRB-1 in human macrophage-derived foam cells induced by ox-LDL. Am J Chin Med. 2010;38:1161–1169. doi: 10.1142/S0192415X10008548. [DOI] [PubMed] [Google Scholar]
- 114.Huang Z, Dong F, Li S, Chu M, Zhou H, Lu Z, Huang W. Berberine-induced inhibition of adipocyte enhancer-binding protein 1 attenuates oxidized low-density lipoprotein accumulation and foam cell formation in phorbol 12-myristate 13-acetate-induced macrophages. Eur J Pharmacol. 2012;690:164–169. doi: 10.1016/j.ejphar.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 115.Liu M, Wu H, Liu T, Li Y, Wang F, Wan H, Li X, Tang H. Regulation of the cell cycle gene, BTG2, by miR-21 in human laryngeal carcinoma. Cell Res. 2009;19:828–837. doi: 10.1038/cr.2009.72. [DOI] [PubMed] [Google Scholar]
- 116.Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, Wang Y, Wang Z, Si S, Pan H, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–1351. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- 117.Wu L, Noyan Ashraf MH, Facci M, Wang R, Paterson PG, Ferrie A, Juurlink BH. Dietary approach to attenuate oxidative stress, hypertension, and inflammation in the cardiovascular system. Proc Natl Acad Sci USA. 2004;101:7094–7099. doi: 10.1073/pnas.0402004101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim JH, Choi YK, Lee KS, Cho DH, Baek YY, Lee DK, Ha KS, Choe J, Won MH, Jeoung D, et al. Functional dissection of Nrf2-dependent phase II genes in vascular inflammation and endotoxic injury using Keap1 siRNA. Free Radic Biol Med. 2012;53:629–640. doi: 10.1016/j.freeradbiomed.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 119.Chen XL, Dodd G, Kunsch C. Sulforaphane inhibits TNF-alpha-induced activation of p38 MAP kinase and VCAM-1 and MCP-1 expression in endothelial cells. Inflamm Res. 2009;58:513–521. doi: 10.1007/s00011-009-0017-7. [DOI] [PubMed] [Google Scholar]
- 120.Liao BC, Hsieh CW, Liu YC, Tzeng TT, Sun YW, Wung BS. Cinnamaldehyde inhibits the tumor necrosis factor-alpha-induced expression of cell adhesion molecules in endothelial cells by suppressing NF-kappaB activation: effects upon IkappaB and Nrf2. Toxicol Appl Pharmacol. 2008;229:161–171. doi: 10.1016/j.taap.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 121.Chen XL, Dodd G, Thomas S, Zhang X, Wasserman MA, Rovin BH, Kunsch C. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am J Physiol Heart Circ Physiol. 2006;290:H1862–H1870. doi: 10.1152/ajpheart.00651.2005. [DOI] [PubMed] [Google Scholar]
- 122.Zhang WJ, Frei B. Alpha-lipoic acid inhibits TNF-alpha-induced NF-kappaB activation and adhesion molecule expression in human aortic endothelial cells. FASEB J. 2001;15:2423–2432. doi: 10.1096/fj.01-0260com. [DOI] [PubMed] [Google Scholar]
- 123.Soares MP, Seldon MP, Gregoire IP, Vassilevskaia T, Berberat PO, Yu J, Tsui TY, Bach FH. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol. 2004;172:3553–3563. doi: 10.4049/jimmunol.172.6.3553. [DOI] [PubMed] [Google Scholar]
- 124.Bellner L, Martinelli L, Halilovic A, Patil K, Puri N, Dunn MW, Regan RF, Schwartzman ML. Heme oxygenase-2 deletion causes endothelial cell activation marked by oxidative stress, inflammation, and angiogenesis. J Pharmacol Exp Ther. 2009;331:925–932. doi: 10.1124/jpet.109.158352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mazzone GL, Rigato I, Ostrow JD, Tiribelli C. Bilirubin effect on endothelial adhesion molecules expression is mediated by the NF-kappaB signaling pathway. Biosci Trends. 2009;3:151–157. [PubMed] [Google Scholar]
- 126.Pierce JW, Read MA, Ding H, Luscinskas FW, Collins T. Salicylates inhibit I kappa B-alpha phosphorylation, endothelial-leukocyte adhesion molecule expression, and neutrophil transmigration. J Immunol. 1996;156:3961–3969. [PubMed] [Google Scholar]
- 127.Zünd G, Dzus AL, Prêtre R, Niederhäuser U, Vogt P, Turina M. Endothelial cell injury in cardiac surgery: salicylate may be protective by reducing expression of endothelial adhesion molecules. Eur J Cardiothorac Surg. 1998;13:293–297. doi: 10.1016/s1010-7940(97)00318-7. [DOI] [PubMed] [Google Scholar]
- 128.Lesniewski LA, Durrant JR, Connell ML, Folian BJ, Donato AJ, Seals DR. Salicylate treatment improves age-associated vascular endothelial dysfunction: potential role of nuclear factor kappaB and forkhead Box O phosphorylation. J Gerontol A Biol Sci Med Sci. 2011;66:409–418. doi: 10.1093/gerona/glq233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Isoda K, Young JL, Zirlik A, MacFarlane LA, Tsuboi N, Gerdes N, Schönbeck U, Libby P. Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler Thromb Vasc Biol. 2006;26:611–617. doi: 10.1161/01.ATV.0000201938.78044.75. [DOI] [PubMed] [Google Scholar]
- 130.Cacicedo JM, Yagihashi N, Keaney JF, Ruderman NB, Ido Y. AMPK inhibits fatty acid-induced increases in NF-kappaB transactivation in cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2004;324:1204–1209. doi: 10.1016/j.bbrc.2004.09.177. [DOI] [PubMed] [Google Scholar]
- 131.Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension. 2006;47:1183–1188. doi: 10.1161/01.HYP.0000221429.94591.72. [DOI] [PubMed] [Google Scholar]
- 132.Huang NL, Chiang SH, Hsueh CH, Liang YJ, Chen YJ, Lai LP. Metformin inhibits TNF-alpha-induced IkappaB kinase phosphorylation, IkappaB-alpha degradation and IL-6 production in endothelial cells through PI3K-dependent AMPK phosphorylation. Int J Cardiol. 2009;134:169–175. doi: 10.1016/j.ijcard.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 133.Wang Y, Huang Y, Lam KS, Li Y, Wong WT, Ye H, Lau CW, Vanhoutte PM, Xu A. Berberine prevents hyperglycemia-induced endothelial injury and enhances vasodilatation via adenosine monophosphate-activated protein kinase and endothelial nitric oxide synthase. Cardiovasc Res. 2009;82:484–492. doi: 10.1093/cvr/cvp078. [DOI] [PubMed] [Google Scholar]
- 134.Wu YH, Chuang SY, Hong WC, Lai YJ, Chang GJ, Pang JH. Berberine reduces leukocyte adhesion to LPS-stimulated endothelial cells and VCAM-1 expression both in vivo and in vitro. Int J Immunopathol Pharmacol. 2012;25:741–750. doi: 10.1177/039463201202500320. [DOI] [PubMed] [Google Scholar]
- 135.Weber KS, Draude G, Erl W, de Martin R, Weber C. Monocyte arrest and transmigration on inflamed endothelium in shear flow is inhibited by adenovirus-mediated gene transfer of IkappaB-alpha. Blood. 1999;93:3685–3693. [PubMed] [Google Scholar]
- 136.Devaraj S, Davis B, Simon SI, Jialal I. CRP promotes monocyte-endothelial cell adhesion via Fcgamma receptors in human aortic endothelial cells under static and shear flow conditions. Am J Physiol Heart Circ Physiol. 2006;291:H1170–H1176. doi: 10.1152/ajpheart.00150.2006. [DOI] [PubMed] [Google Scholar]
- 137.Piga R, Naito Y, Kokura S, Handa O, Yoshikawa T. Short-term high glucose exposure induces monocyte-endothelial cells adhesion and transmigration by increasing VCAM-1 and MCP-1 expression in human aortic endothelial cells. Atherosclerosis. 2007;193:328–334. doi: 10.1016/j.atherosclerosis.2006.09.016. [DOI] [PubMed] [Google Scholar]


