Summary
Children and adolescents with Burkitt Lymphoma (BL) and combined central nervous system (CNS) and bone marrow involvement still have a poor prognosis with chemotherapy alone. We therefore investigated in children and adolescents with bone marrow (≥25% blasts) and/or CNS-positive Burkitt lymphoma the chemoimmunotherapy combination of rituximab (375 mg/m2) and the standard chemotherapy arm of our previously reported French-American-British (FAB) Lymphome Malins de Burkitt (LMB) 96 trial. Central pathological and cytogenetic characterization was also performed. There were 40 evaluable patients with Burkitt histology (25 with leukaemia and 15 with CNS disease ± leukaemia). The chemoimmunotherapy regimen was well tolerated. The incidence of grade III/IV mucositis during induction cycles with combined chemotherapy and rituximab was 31% and 26%, respectively. The 3-year event-free survival (EFS)/overall survival (OS) was 90% (95% confidence interval [CI], 76–96%) in the entire cohort and 93% (95% CI, 61–99%) in patients with CNS disease. Based on the results of this trial, an international randomized study of FAB/LMB 96 chemotherapy ± rituximab for high-risk patients is currently under investigation.
Keywords: children, adolescents, Burkitt Lymphoma, Burkitt Leukaemia, chemotherapy, Rituximab
Introduction
Approximately 1 in 4 children and adolescents with de novo mature and Burkitt lymphoma (BL) present with high-risk disease that is either mature B-cell leukaemia (bone marrow [BM] blasts ≥ 25%) or/and has central nervous system (CNS) involvement. Both the Berlin-Frankfurt-Münster (BFM) and French-American-British (FAB) international cooperative studies have unsuccessfully attempted to reduce the overall burden of chemotherapy in this high risk group of patients. In the FAB 96 study, a randomized attempt to reduce the dose of cytarabine during consolidation and eliminate three final cycles of maintenance was halted early due to inferior event-free survival (EFS) (Cairo, et al 2012, Cairo, et al 2007), while the BFM 95 study concluded that reducing the infusion duration of methotrexate from 24 to 4 h led to significantly inferior EFS in high risk (R3/R4) patients.(Woessmann, et al 2005) Subsets of children with BL, such as those with poor response to initial reduction, complex karyotypes and those with combined BM and CNS disease, have a significantly worse prognosis (Cairo, et al 2012, Cairo, et al 2007, Poirel, et al 2009).
Rituximab has been shown to improve EFS and overall survival (OS) when added to CHOP (cyclophosphamide, adriamycin,oncovin, prednisone)-based therapy in adults with diffuse large B-cell lymphoma (DLBCL) and also when combined with more aggressive therapy in adults with mature B-cell (Burkitt) lymphoma (BL) (Barnes, et al 2011, Coiffier, et al 2002, Corazzelli, et al 2012, Dunleavy, et al 2013, Pfreundschuh, et al 2006, Thomas, et al 2006). Unfortunately, all of these studies of rituximab and chemotherapy in adults with BL had few or no patients with BM and/or CNS involvement. Meinhardt et al. (2010) reported the safety and efficacy of one pre-dose of rituximab prior to reduction therapy in children and adolescents with mature de novo BL, including 19 evaluable patients with BM and/or CNS disease. We have previously reported the safety and efficacy of the combination of rituximab plus FAB/ Lymphome Malins de Burkitt (LMB) 96 Group B chemotherapy in children with Stage III/IV DLBCL/BL (Goldman, et al 2013). However, to date there has been no prospective study investigating the combination of rituximab with FAB/LMB 96 Group C chemotherapy in children, adolescents or young adults with BL and CNS disease and/or BM involvement. Importantly, our previous study in children with BL and CNS disease only had a 4-year EFS of 75% following standard systemic and intrathecal chemotherapy with FAB/LMB therapy (Cairo, et al 2007).
Patients and methods
General
The Children’s Oncology Group (COG) ANHL01P1 investigated the addition of rituximab to the FAB 96 C1 systemic and intrathecal chemotherapy backbone (Cairo, et al 2007). The trial was open to all COG centres in the United States, Canada, Australia and New Zealand and the protocol was approved by each respective institutional review board. Staging classification utilized the St. Jude Staging for non-Hodgkin lymphoma (NHL) (Murphy 1980). Parents or patients over 18 years of age signed an institutional review board-approved informed consent before study enrollment in accordance with the Declaration of Helsinki. Safety reports and interim analyses were reviewed every 6 months, then annually by the COG independent Data and Safety Monitoring Committee.
Eligibility and Evaluation
Patients under 30 years of age with newly diagnosed denovo mature B-cell lymphoma, classified according to the World Health Organization (WHO) criteria (Swerdlow, et al 2008) were eligible. CD20-positive immunohistochemistry was required for study eligibility. Group C risk was defined as patients with BM blasts ≥25% and/or CNS disease (Cairo, et al 2007). CNS disease was defined as any cerebrospinal fluid (CSF) blasts found on diagnostic lumbar puncture and/or isolated intracerebral mass, cranial nerve palsy, clinical spinal cord compression and parameningeal extension, as previously described (Cairo, et al 2007). Carriers of hepatitis B were eligible but carefully monitored for reactivation, as previously described (Goldman, et al 2013).
Treatment
Systemic and Intrathecal Chemotherapy
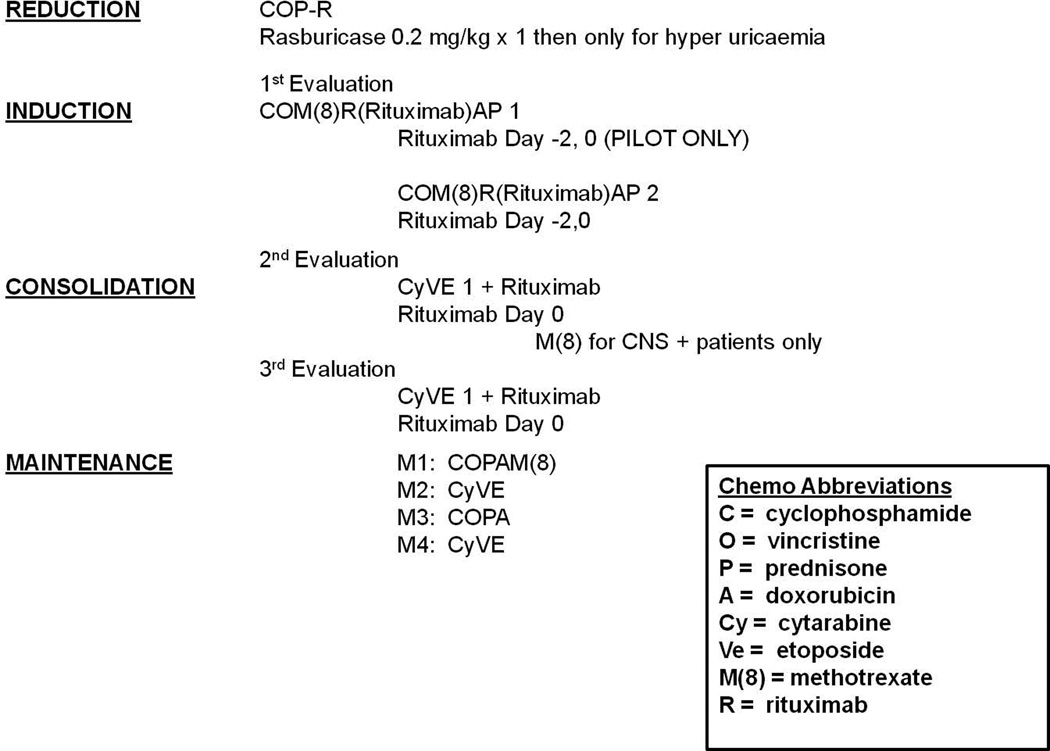
The systemic and intrathecal chemotherapy backbone for group C patients was similar to that reported in the FAB/LMB 96 study for the C1 arm (Cairo, et al 2007). The previous study (FAB/LMB 96) initially employed a 48-h infusion of doxorubicin during the two induction cycles and the study was amended midway for unacceptable rates of grade III/IV mucositis and the anthracycline infusion time was reduced to a 6-hours (Patte, et al 2007). The doxorubicin infusion time was reduced to 30–60 min in the current COG ANHL01P1 group C trial. Non-alkaline hydration and one or more doses of rasburicase 0.2 mg/kg IV, generously supplied by Sanofi (Bridgewater, NJ), was administered to all patients at least 4 h prior to initiating reduction therapy for prevention or treatment of tumour lysis syndrome (TLS). An initial reduction phase consisted of low-dose cyclophosphamide, Oncovin® (vincristine) and prednisone (COP). Induction courses consisted of COPADM1+2 (cyclophosphamide 1.5 g/m2/course 1 and 3 g/m2/course 2 - fractionated, Oncovin®, prednisone, doxorubicin, high dose methotrexate [HDMTX] [8 g/m2 in 4-h infusion]), as previously described (Cairo, et al 2007). Patients then received two identical consolidation courses, CYVE 1+2 (continuous infusion and high dose cytarabine and etoposide) (Fig 1). CNS-negative patients received 10 prophylactic intrathecal injections while CNS-positive patients received 13 intrathecal injections and an additional course of HDMTX between CYVE consolidation cycles. A disease evaluation was performed after completion of consolidation cycles with any biopsy-proven residual disease considered an event and the patient removed from study. Patients without disease after consolidation received four maintenance courses as previously described (Fig 1) (Cairo, et al 2007). No patients received cranial irradiation, including those presenting with CNS disease.
Figure 1.
Treatment Schema. Sub-pilot and Pilot Group C patients were treated with identical FAB/LMB96 C1 systemic and intrathecal chemotherapy backbones (Patte, et al 2007). Sub-pilot schema: rituximab 375 mg/m2/dose was administered on day -2 and day 0 of second induction cycle and day 0 of both consolidation cycles (4 total doses). Pilot schema: rituximab 375 mg/m2/dose was administered on day -2 and day 0 of both induction cycles and day 0 of both consolidation cycles (6 total doses). No rituximab was administered during maintenance.
Immunotherapy
All rituximab infusions were administered at the standard dose of 375 mg/m2. Patients were pre-medicated with a combination of acetaminophen and diphenhydramine. Rituximab, generously supplied by Genentech (San Francisco, CA) through the Cancer Therapy Evaluation Program (CTEP), National Cancer Institute, was diluted in normal saline at a concentration of 1 mg/ml. The monitoring and methods of administration of rituximab were identical to those described previously (Goldman, et al 2013). During induction cycles (COPADM), rituximab was administered 48 h prior (day –2) and repeated on the day of chemotherapy administration (day 0). During consolidation cycles (CYVE), rituximab was administered just prior to chemotherapy administration (day 0). In the initial sub-pilot, the rituximab administration began with the second induction cycle (4 total doses) (Fig 1). The reason for initiating rituximab in the second induction cycle in the small initial cohort was to avoid any potential overlapping toxicity with the other experimental agent in the study (rasburicase in reduction). After the fourth sub-pilot patient was entered, the study was temporarily closed (~ 5 months) to accrual until all sub-pilot patients completed consolidation chemoimunotherapy and were evaluated for toxicity. In the larger pilot study, rituximab was given beginning in the first induction cycle (6 total doses) (Fig 1).
Pathology
Central pathology review consisted of review of morphological, immunophenotypical and genetic data from the original diagnostic biopsy. In addition, CD20 expression of the tumour was confirmed by immunohistochemistry. Sections (4-µm thick) underwent immunoperoxidase staining for CD20 (L-26 clone, DAKO Cytomation, Carpinteria, CA) by standard methods following heat-induced epitope retrieval in citrate buffer (pH 6.0) on an automated stainer (ES, Ventana Medical Systems, Tucson, AZ). All steps were performed at 40°C. A case was scored as positive if >80% of the tumour cells stained for CD20.
Cytogenetics and fluorescence in situ hybridization (FISH) analysis
Cytogenetic analysis was performed, as previously described (Poirel, et al 2009), with nomenclature according to the International System for Human Chromosome Nomenclature (Shaffer and Tommerup 2005). Interphase FISH analysis for MYC rearrangement was performed on a portion of the specimen submitted for cytogenetic analysis. A dual-colour MYC/IGH translocation probe, designed to detect t(8;14)(q24.1;q32), or a dual-colour MYC break-apart probe, designed to detect rearrangements of the MYC gene region at 8q24.1 with various partner chromosomes,was utilized according to the COG reference laboratories standard protocols. Slides were counterstained with 4,6-diamidino-2-phenylindole (DAPI) in Antifade solution and the nuclei were visualized and captured using a fluorescence microscope equipped with appropriate filters and imaging software.
Statistics
The objective of this study was to estimate the toxicity of and response rate to chemoimmunotherapy in Group C patients. Stevens-Johnson Syndrome (SJS), toxic epidermal necrosis (TEN), the frequency of Grade ≥ 4 stomatitis (Grade ≥3 stomatitis for the pilot portion), delayed recovery beyond day +42 of the induction phase and toxic deaths were considered events in the sub-pilot and pilot studies.
Statistical considerations for the sub-pilot study for Group C patients
If a single toxic death occurred during the period from the second cycle of induction to the end of the second maintenance course among the 4 sub-pilot patients, the Study Committee would review the events and determine if the study would continue, be modified or permanently stopped.
Statistical considerations for assessing toxicity in the pilot
A 2-stage stopping rule was used to terminate this study if too many Group C patients experienced Grade ≥3 stomatitis during either cycle of COPADM + rituximab therapy (Induction). A rate greater than 70%, the observed rate in FAB LMB 96, was considered too high. The pilot study also monitored for the occurrence of SJS and toxic epidermal necrosis with any incidence of either during induction leading to temporary closure and review of the event. A 2-stage stopping rule was used to close the study for toxic deaths (> 1.2%) from after the reduction phase until completion of second maintenance course of therapy. If any stopping rule was triggered, it was followed by a review by the Study Committee and COG Data and Safety Monitoring Committee.
EFS was defined as the time from enrollment to the first occurrence of disease progression, relapse after response or death from any cause. OS was defined as the time from enrollment to death from any cause. Time to event for patients not experiencing an event was censored at their time of last follow-up. Estimates of the EFS and OS distributions were calculated using the Kaplan and Meier method. Ninety-five percent confidence intervals (95% CI) for the Kaplan-Meier estimates of EFS and OS were calculated using standard errors according to Greenwood (1926).
Results
Patient demographics
Four sub-pilot and 42 pilot patients were enrolled, however, four pilot patients were determined to be ineligible (all prior to receiving rituximab) for the following reasons: chemotherapy started prior to enrollment (2 patients) and major informed consent deficiencies (2 patients). In addition, 2 initially eligible patients were excluded for incorrect staging in one patient and parental withdrawal of consent prior to study therapy. Thus, 36 pilot and 4 sub-pilot patients were included in the final analysis making a total of 40 patients that were evaluated for the planned endpoints. The results of the pharmacokinetics of rituximab and the incidence and grade of TLS have been previously been reported in this patient population, respectively (Barth, et al 2013, Galardy, et al 2013).
The mean age at study entry was 11 years (range 3–23). Eighty percent of patients were between 4 and 15 years. The male to female ratio was 4:1. Sixty-three percent of patients had isolated BM involvement (≥25% blasts); 18% (7/40) had CNS disease with BL (< 25% BM blasts) and 20% (8/40) had combined CNS and BM disease. Seventy-eight percent of patients had elevated lactate dehydrogenase ≥2× the upper institutional limit of normal at diagnosis (Table I).
Table I.
Patient Demographics of Evaluable Children and Adolescents with Advanced De Novo Mature B-Cell Leukaemia/Lymphoma Treated on COG ANHL01P1
| Total N (%) |
||
|---|---|---|
| N | 40 | |
| Gender | Male | 32 (80%) |
| Age, years | Mean ± SD | 11±5.5 |
| Pathological diagnosis | Burkitt | 40 (100%) |
| Sites of Disease | BM+/CNS− | 25 (62.5%) |
| BM+/CNS+ | 8 (20%) | |
| BM−/CNS+ | 7 (17.5%) | |
| LDH | ≥ 2 times ULN | 31 (77.5%) |
BM +: Bone marrow with ≥ 25 % blasts by morphology; CNS+: Central nervous system involvement (See text for details); LDH, lactate dehydrogenase; ULN, upper limit of normal; SD, standard deviation; COG, Children’s Oncology Group.
Safety
There were two toxic deaths among the 40 evaluable patients. The first death occurred in a teenager whom had been ill for several weeks prior to arrival at the COG centre for therapy of Burkitt leukaemia. This patient died of pre-existing pulmonary aspergillosis soon after reduction therapy. The second death on study was secondary to grade V typhlitis and infection, which occurred in a patient during the second induction cycle. The institutional investigator graded the typhlitis as probably related to rituximab. Although the study required leucovorin rescue and alkaline hydration until the serum methotrexate level was less than 0.1 µmol/l, the patient was discharged from the hospital with a methotrexate level of 0.12 µmol/l. This second toxic death triggered a pre-specified study suspension. Enhanced methotrexate guidelines, as well added recommendations for aneorbic treatment of suspected typhlitis and mandatory notification of study chair of infectious complications, were amended to the study. After a ten-month suspension, the study was reopened after approval of the data safety monitoring board without any subsequent toxic deaths.
Table II details the most common recurrent non-haematological grade III/IV toxicities by cycle of therapy with combined chemotherapy and rituximab. There were 54 reports of serious adverse events (SAE) in 13 patients (33%). Of these, only 1 SAE, of grade 3 infusion reaction, was definitely attributed to rituximab (total of 227 rituximab infusions). Of the remaining 53 SAE, only 1 had a probable attribution to rituximab (grade V typhlitis in the previously mentioned patient). The specified stopping rules for mucositis/stomatitis and SJS/TEN were not met during the conduct of the study. The focus on SJS/TEN was based on the rare but highly morbid incidence reported in adult lymphoma and concern about combining with other skin and mucus membrane toxic agents, such as HDMTX (Foran, et al 2000). The incidence of grade III/IV mucositis during induction cycles with combined chemotherapy and rituximab was 31% and 26% in COPADM1 and COPADM2, respectively.
Table II.
Grade III–IV Non-Haematological Toxicities Stratified by Cycle During Group C Therapy (with Combined Chemotherapy and Rituximab) on Children’s Oncology Group ANHL01P1
| Induction 1 % |
Induction 2 % |
Consolidation 1 % |
Consolidation 2 % |
|
|---|---|---|---|---|
| Infection | 63 | 45 | 49 | 39 |
| Mucositis | 31 | 26 | 8.1 | 11 |
| Anorexia | 23 | 11 | 16 | 8.3 |
| Pain | 20 | 13 | 11 | 8.3 |
| Transaminitis | 31 | 7.9 | 22 | 11 |
| Hyperglycaemia | 11 | 13 | 2.7 | 2.8 |
Haematopathology
Adequate tissue for centrally reviewed histology was available in 73% (29/40) of patients enrolled. All of the patients had classical BL (WHO) or Burkitt leukaemia (as defined by >25% BM involvement) (Swerdlow, et al 2008). The remaining 11 cases had classical BL by report of the local pathologist. CD20 was strongly and uniformly expressed on all centrally reviewed cases.
FISH analysis
Conventional cytogenetic studies were attempted on 36 cases and resulted in abnormal findings in 26 (72%). Normal karyotypes were observed in 2 cases (5.5%) and 8 cases (22%) failed to yield metaphase cells for analysis. Cytogenetically, 25/26 (96%) abnormal karyotypes exhibited a MYC rearrangement. The various MYC rearrangements detected included, a t(2;8)(p11.2;q24.1) in 1/25 cases (4%), a t(8;22)(q24.1;q11.2) in 3/25 cases (12%) and t(8;14)(q24.1;q32) in 21/25 cases (84%). Secondary cytogenetic abnormalities were observed in 58% of the cases, of which the most frequent was gain of 1q (8/26, 31%). Other recurrent secondary changes included loss of 13q (5/26, 19%), loss of 6q (5/26, 19%), gain of 7q (4/26, 15%) and loss of 17p (3/26, 12%). All ten cases that had a normal karotype or failed in culture were positive for MYC rearrangement by FISH.
EFS and OS in total cohort of patients
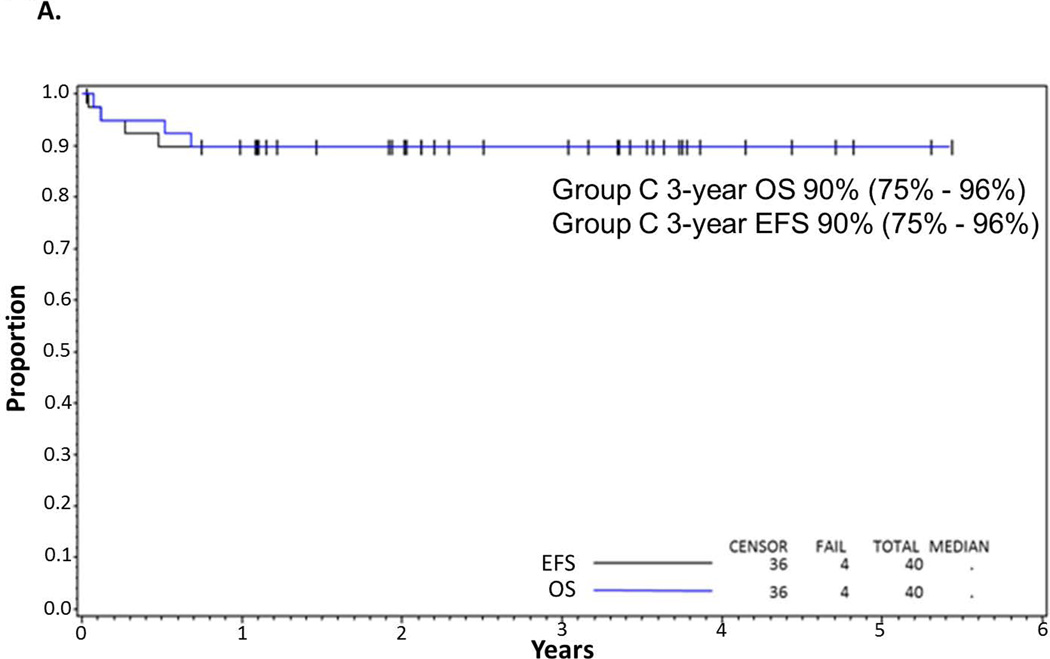
The outcome of the COP reduction therapy with rasburicase prophylaxis and treatment of TLS has been previously reported (Galardy, et al 2013). All evaluable patients were able to receive chemoimmunotherapy after reduction and control of tumour lysis. Of the 40 evaluable patients there were 4 deaths, two toxic and two due to recurrent disease at 6 months and 8 months post-study enrollment, respectively. Both patients who developed recurrent disease initially achieved complete responses to chemoimmunotherapy. The median follow-up for the 36 surviving patients was 3.6 years, range (1–6). The 3-year EFS/OS for all evaluable patients was 90% (95% CI: 76–96%) (Fig 2A).
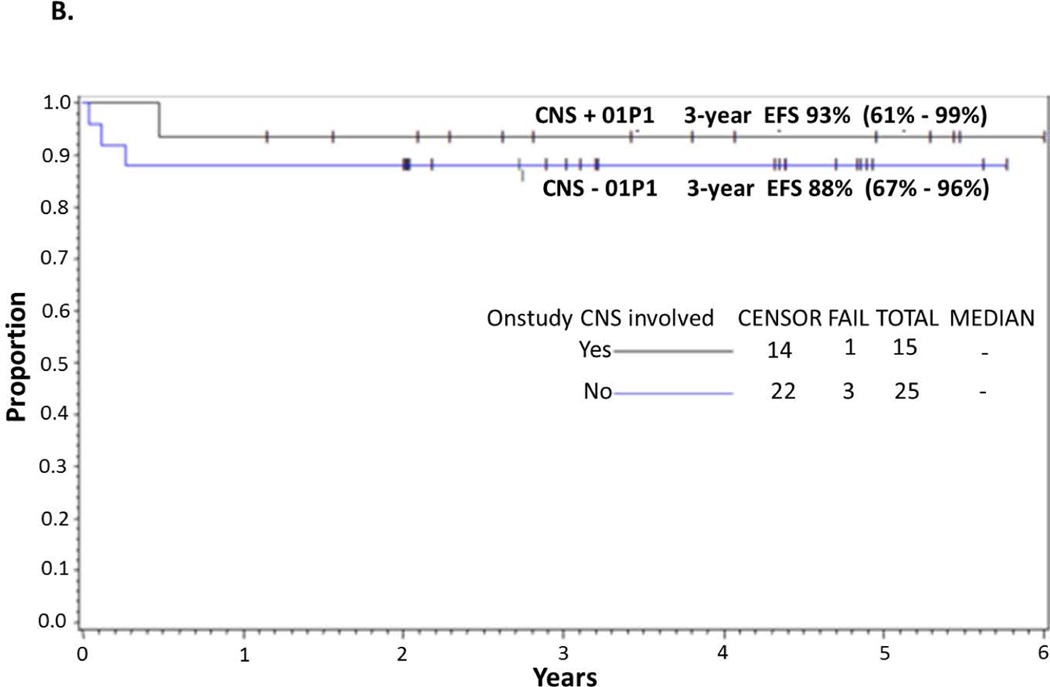
Figure 2.
(A) EFS and OS in all patients. Probability of EFS and OS in children and adolescents with BM and/or CNS disease with de novo mature B-cell non-Hodgkin lymphoma (B-NHL) treated with Rituximab and FAB Group C1 Chemotherapy Pilot on COG-ANHL01P1 as determined by Kaplan-Meier method. (B) EFS in CNS-positive and CNS-negative patients. Probability of EFS in children and adolescents with BM and/or CNS-positive and -negative disease with advanced de-novo mature B-NHL treated with Rituximab and FAB Group C1 chemotherapy stratified by CNS+ vs. CNS− on COG-ANHL01P1 as determined by Kaplan-Meier method.
EFS, event-free survival; OS, overall survival; BM, bone marrow; CNS, central nervous system; 01P1, ANHL01P1 protocol.
EFS and OS in CNS-positive patients
Details of the presentation features of CNS-positive patients are shown in Tables IIIA and IIIB. Among CNS-positive BL patients (n=15) the 3-year EFS is 93% (95% CI: 61–99%) (Fig 2B). Eight CNS-positive BL cases had CSF blasts [WBC median 35 (range 1–1104)]. Of the 7 CNS-positive patients without BM disease, 100% had no evidence of disease (mean of 50 months [range 22–66]). In the 8 CNS+ Burkitt leukaemia cases, 7 (88%) had no evidence of disease with a mean of 41 months (range, 14–72 months). One CSF-positive+ patient with initial cranial nerve palsy and BM involvement recurred with both systemic and CNS disease.
| A. Frequency of different CNS+ presentations | |
|---|---|
| CNS Disease | Patients, n (%) |
| Isolated CSF+ | 5 (33%) |
| Isolated PME+ | 4 (27%) |
| CNP+ & CSF+ | 3 (20%) |
| CNP+ & ICM+ | 1 (7%) |
| Isolated CNP+ | 2 (13%) |
| B. Coincidence of bone marrow and CSF positivity with other CNS findings | |
|---|---|
| Disease Involvement | Patients, n (%) |
| BM+ & CNS+ | 8 (53%) |
| BM+ & CSF+ (± CNP) | 5 (33%) |
| BM+ & isolated CSF+ | 3 (20%) |
| BM+, CSF+, & CNP+ | 2 (13%) |
| BM+ & CSF− (+ CNP, ICM, and/or PME) | 3 (20%) |
| BM+, CSF−, & isolated CNP+ | 1 (7%) |
| BM+, CSF−, CNP+, & ICM+ | 1 (7%) |
| BM+, CSF−, & isolated PME+ | 1 (7%) |
| BM− & CNS+ | 7 (47%) |
| BM−, CSF+ (± CNP) | 3 (20%) |
| BM− & isolated CSF+ | 2 (13%) |
| BM−, CSF+, & CNP+ | 1 (7%) |
| BM−, CSF−, & isolated PME+ BM−, CSF−, & isolated CNP+ |
3 (20%) 1 (7%) |
BM, bone marrow; CNS, central nervous system; CSF, cerebrospinal fluid; CNP, cranial nerve palsy; ICM, intra-cranial mass; PME, parameningeal extension.
Discussion
This study demonstrates for the first time that rituximab can be combined safely with FAB C1 systemic and intrathecal chemotherapy. There were, however, two toxic deaths on study. The first patient death, due to pulmonary aspergillosis and multi-organ failure, was most probably related to pre-existing aspergillosis. The second death, which occurred during the recovery phase after the second induction cycle, was due to severe mucositis/typhlitis and sepsis and considered possibly related to rituximab therapy. Although rituximab may have contributed to additional mucosal breakdown and resultant sepsis, the patient did not receive the required leucovorin rescue following HDMTX (8 g/m2) clearance. The rate of toxic death, in 2 of 40 patients (5%), is nearly identical to the rates reported in the same population of patients treated with FAB96 C1 without rituximab (Cairo, et al 2007, Woessmann, et al 2005). In the previous FAB/LMB 96 group C trial, there were 11 protocol deaths not directly related to disease progression (5 infectious, 3 haemorrhage, 1 thrombosis and 2 other). The incidence of grade III/IV mucositis in the previous FAB 96 high-risk study was 68% and 52% during the two induction cycles, respectively. That latter study utilized a doxorubicin infusion time of 6–48 h (Cairo, et al 2007). For this reason, we empirically reduced the doxorubicin infusion time to 30–60 min in the current study and, with the incorporation of rituximab, carefully monitored for excess rates of grade III/IV mucositis. The decrease in anthracycline infusion time appeared to have a dramatic effect on the incidence of grade III/IV mucositis with an almost 50% reduction in the current trial despite the addition of targeted immunotherapy.
The 3-year EFS of 93% in CNS-positive patients (without cranial radiation) is encouraging and at least as efficacious as previous reports in paediatric CNS-positive mature B cell leukemia/lymphoma. This pilot study was not powered to compare outcomes to the previous FAB 96 trial (Cairo, et al 2012, Cairo, et al 2007, Corazzelli, et al 2012, Meinhardt, et al 2010, Thomas, et al 2006, Woessmann, et al 2005). Among CNS-positive patients who relapsed on the previous FAB 96 trial, 44% had isolated systemic recurrence and 11% had combined CNS and systemic disease at relapse. The remaining 45% had recurrent disease in the CNS only (Cairo et al 2007). Rituximab has poor (~ 0.1%) CNS penetration when given intravenously, but may have reduced the incidence of systemic relapse (Cairo, et al 2007, Rubenstein, et al 2003). Our results in CNS-positive Burkitt patients compares favourably to the results of combined aggressive chemotherapy and rituximab in adults published by multiple adult groups. Three recent reports of combination aggressive chemotherapy (including infusional HDMTX and intrathecal therapy) with the addition of rituximab reported improved efficacy of therapy with the addition of rituximab compared to past historical series in adult BL and Burkitt leukaemia. This included the MD Anderson Cancer Center hyper-CVAD regimen (hyper-fractionated etoposide, prednisone, vincristine (Oncovin®), doxorubicin), a regimen of CODOX-M/ IVAC (ifosfamide, etoposide, Ara-C), and a German short intensive chemotherapy regimen (Barnes, et al 2011, Intermesoli, et al 2013, Pfreundschuh, et al 2006). Rituximab led to improved EFS in these three adult series for the entire patient cohorts. However, the outcomes for CNS-positive adult patients with BL (n= 24; all three series combined) were poor, with 3-year EFS in the three reports of 50%, 50% and 40%, respectively (Barnes, et al 2011, Intermesoli, et al 2013, Pfreundschuh, et al 2006). A recent uncontrolled prospective series of low-intensity EPOCH-R (etoposide, prednisone, vincristine (Oncovin®), adriamycin and rituximab), which does not include any infusional HDMTX, reported excellent efficacy in BL, but, of note, only one patient with CNS disease was included in this report (Dunleavy, et al 2013). Other potential CNS-directed therapy, such as longer acting intrathecal therapy (e.g. liposomal Ara-C) or direct administration of immune therapy into the CNS may need to be studied to improve CNS outcomes in adults with BL (Corazzelli, et al 2012, Rubenstein, et al 2003).
In summary, we have demonstrated that rituximab can be safely added to the modified FAB group C1 chemotherapy backbone in children and adolescents with advanced denovo mature B-cell BL/Burkitt leukaemia. The 3-year EFS/OS of 90% is encouraging given the patient population including ultra-high risk subgroups, such as patients with combined BM and CNS disease and complex cytogenetics. Based on the results of this trial, an international randomized study of FAB/LMB 96 chemotherapy ± rituximab for high risk patients is currently under investigation.
Supplementary Material
Acknowledgements
Supported by the Division of Cancer Treatment, National Cancer Institute (NCI), and National Institutes of Health, Department of Health and Human Services (COG) (CA98543-09 and CA98413-09) and the Pediatric Cancer Research Foundation. NCI provided support for data collection and analysis but no role in data interpretation, writing of the manuscript or the decision on journal submission.
Footnotes
Authorship and Disclosures.
SG designed and performed the research, analysed the results and wrote the paper; LS and JRA analysed the data and wrote the paper; SLP analysed the data and critically reviewed the paper; PG analysed the data and critically reviewed the paper; JKF analysed the data and critically reviewed the paper; BS analysed the data and critically reviewed the paper; TGG analysed the data and critically reviewed the paper; WS analysed the data and critically reviewed the paper; HW analysed the data and critically reviewed the paper; LH performed the research, analysed the data and wrote the paper; MB analysed the data and critically reviewed the paper; and MSC designed and performed the research, analysed the results and wrote the paper. T. G. is on the Scientific Advisory Boards for Genentech/Roche & Boehringer Ingelheim Pharma GmbH & Co. M.S.C. is a consultant and on the Speakers Bureau for Sanofi and on a Scientific Advisory Board for Genentech/Roche.
Presented in part at the American Society of Hematology, December 2011, San Diego, CA and the American Society of Clinical Oncology, June 2012, Chicago, IL.
REFERENCES
- Barnes JA, Lacasce AS, Feng Y, Toomey CE, Neuberg D, Michaelson JS, Hochberg EP, Abramson JS. Evaluation of the addition of rituximab to CODOX-M/IVAC for Burkitt's lymphoma: a retrospective analysis. Ann Oncol. 2011;22:1859–1864. doi: 10.1093/annonc/mdq677. [DOI] [PubMed] [Google Scholar]
- Barth MJ, Goldman S, Smith L, Perkins S, Shiramizu B, Gross TG, Harrison L, Sanger W, Geyer MB, Giulino-Roth L, Cairo MS. Rituximab pharmacokinetics in children and adolescents with de novo intermediate and advanced mature B-cell lymphoma/leukaemia: a Children's Oncology Group report. Br J Haematol. 2013;162(5):678–683. doi: 10.1111/bjh.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairo M, Sposto R, Gerrard M, Auperin A, Goldman S, Harrison L, Pinkerton CR, Raphael M, McCarthy K, Perkins S, Patte C. Advanced stage, elevated LDH and primary site, but not adolescent age (≥15 years), are associated with an increased risk of failure in children and adolescents with mature B-NHL: results of the FAB/LMB 96 study. J Clin Oncol. 2012;30:387–393. doi: 10.1200/JCO.2010.33.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairo MS, Gerrard M, Sposto R, Auperin A, Pinkerton CR, Michon J, Weston C, Perkins SL, Raphael M, McCarthy K, Patte C. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood. 2007;109:2736–2743. doi: 10.1182/blood-2006-07-036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- Corazzelli G, Frigeri F, Russo F, Frairia C, Arcamone M, Esposito G, De Chiara A, Morelli E, Capobianco G, Becchimanzi C, Volzone F, Saggese M, Marcacci G, De Filippi R, Vitolo U, Pinto A. RD-CODOX-M/IVAC with rituximab and intrathecal liposomal cytarabine in adult Burkitt lymphoma and 'unclassifiable' highly aggressive B-cell lymphoma. Br J Haematol. 2012;156:234–244. doi: 10.1111/j.1365-2141.2011.08947.x. [DOI] [PubMed] [Google Scholar]
- Dunleavy K, Pittaluga S, Shovlin M, Steinberg SM, Cole D, Grant C, Widemann B, Staudt LM, Jaffe ES, Little RF, Wilson WH. Low-intensity therapy in adults with Burkitt's lymphoma. N Engl J Med. 2013;369:1915–1925. doi: 10.1056/NEJMoa1308392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foran JM, Gupta RK, Cunningham D, Popescu RA, Goldstone AH, Sweetenham JW, Pettengell R, Johnson PW, Bessell E, Hancock B, Summers K, Hughes J, Rohatiner AZ, Lister TA. A UK multicentre phase II study of rituximab (chimaeric anti-CD20 monoclonal antibody) in patients with follicular lymphoma, with PCR monitoring of molecular response. Br J Haematol. 2000;109:81–88. doi: 10.1046/j.1365-2141.2000.01965.x. [DOI] [PubMed] [Google Scholar]
- Galardy P, Hochberg J, Perkins S, Harrison L, Goldman S, Cairo MS. Rasburicase in the prevention of laboratory/clinical tumour lysis syndrome in children with advanced mature B-NHL: A Children’s Oncology Group Report. Br J Haematol. 2013;163:365–372. doi: 10.1111/bjh.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S, Smith L, Anderson JR, Perkins S, Harrison L, Geyer MB, Gross TG, Weinstein H, Bergeron S, Shiramizu B, Sanger W, Barth M, Zhi J, Cairo MS. Rituximab and FAB/LMB 96 chemotherapy in children with Stage III/IV B-cell non-Hodgkin lymphoma: a Children's Oncology Group report. Leukemia. 2013;27:1174–1177. doi: 10.1038/leu.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood M. The natural duration of cancer. Reports on Public Health and Medical Subjects. 1926;33:1–26. [Google Scholar]
- Intermesoli T, Rambaldi A, Rossi G, Delaini F, Romani C, Pogliani EM, Pagani C, Angelucci E, Terruzzi E, Levis A, Cassibba V, Mattei D, Gianfaldoni G, Scattolin AM, Di Bona E, Oldani E, Parolini M, Gokbuget N, Bassan R. High cure rates in Burkitt lymphoma and leukemia: a Northern Italy Leukemia Group study of the German short intensive rituximab-chemotherapy program. Haematologica. 2013;98:1718–1725. doi: 10.3324/haematol.2013.086827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt A, Burkhardt B, Zimmermann M, Borkhardt A, Kontny U, Klingebiel T, Berthold F, Janka-Schaub G, Klein C, Kabickova E, Klapper W, Attarbaschi A, Schrappe M, Reiter A. Phase II window study on rituximab in newly diagnosed pediatric mature B-cell non-Hodgkin's lymphoma and Burkitt leukemia. J Clin Oncol. 2010;28:3115–3121. doi: 10.1200/JCO.2009.26.6791. [DOI] [PubMed] [Google Scholar]
- Murphy SB. Classification, staging and end results of treatment of childhood non-Hodgkin's lymphomas: dissimilarities from lymphomas in adults. Semin Oncol. 1980;7:332–339. [PubMed] [Google Scholar]
- Patte C, Auperin A, Gerrard M, Michon J, Pinkerton R, Sposto R, Weston C, Raphael M, Perkins SL, McCarthy K, Cairo MS. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood. 2007;109:2773–2780. doi: 10.1182/blood-2006-07-036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, Ma D, Gill D, Walewski J, Zinzani PL, Stahel R, Kvaloy S, Shpilberg O, Jaeger U, Hansen M, Lehtinen T, Lopez-Guillermo A, Corrado C, Scheliga A, Milpied N, Mendila M, Rashford M, Kuhnt E, Loeffler M. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- Poirel HA, Cairo MS, Heerema NA, Swansbury J, Auperin A, Launay E, Sanger WG, Talley P, Perkins SL, Raphael M, McCarthy K, Sposto R, Gerrard M, Bernheim A, Patte C. Specific cytogenetic abnormalities are associated with a significantly inferior outcome in children and adolescents with mature B-cell non-Hodgkin's lymphoma: results of the FAB/LMB 96 international study. Leukemia. 2009;23:323–331. doi: 10.1038/leu.2008.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Combs D, Rosenberg J, Levy A, McDermott M, Damon L, Ignoffo R, Aldape K, Shen A, Lee D, Grillo-Lopez A, Shuman MA. Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood. 2003;101:466–468. doi: 10.1182/blood-2002-06-1636. [DOI] [PubMed] [Google Scholar]
- Shaffer LG, Tommerup N, editors. ISCN 2005: An International System for Human Cytogenetic Nomenclature. Basel, Switzerland: S. Karger; 2005. [Google Scholar]
- Shiramizu B, Goldman S, Kusao I, Agsalda M, Lynch J, Smith L, Harrison L, Morris E, Gross TG, Sanger W, Perkins S, Cairo MS. Minimal disease assessment in the treatment of children and adolescents with intermediate-risk (Stage III/IV) B-cell non-Hodgkin lymphoma: a children's oncology group report. Br J Haematol. 2011;153:758–763. doi: 10.1111/j.1365-2141.2011.08681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IRAC Press; 2008. [Google Scholar]
- Thomas DA, Faderl S, O'Brien S, Bueso-Ramos C, Cortes J, Garcia-Manero G, Giles FJ, Verstovsek S, Wierda WG, Pierce SA, Shan J, Brandt M, Hagemeister FB, Keating MJ, Cabanillas F, Kantarjian H. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106:1569–1580. doi: 10.1002/cncr.21776. [DOI] [PubMed] [Google Scholar]
- Woessmann W, Seidemann K, Mann G, Zimmermann M, Burkhardt B, Oschlies I, Ludwig WD, Klingebiel T, Graf N, Gruhn B, Juergens H, Niggli F, Parwaresch R, Gadner H, Riehm H, Schrappe M, Reiter A. The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with B-cell neoplasms: a report of the BFM Group Study NHL-BFM95. Blood. 2005;105:948–958. doi: 10.1182/blood-2004-03-0973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.