Abstract
Men who have sex with men (MSM) in Bangkok may experience multiple psychosocial health conditions, such as substance use, suicidality, and a history of sexual abuse. These factors may contribute to HIV vulnerability in a syndemic way. A syndemic is defined as a number of synergistically interacting health conditions producing excess disease in a population. The objective of this study is to examine whether psychosocial health conditions among MSM have a syndemic association with HIV prevalence and HIV incidence. To do this, we evaluated psychosocial health conditions and their associations with unprotected sex, HIV prevalence and HIV incidence in a cohort of Thai MSM (N = 1,292). There was a positive and significant association between the number of psychosocial health conditions and increased levels of unprotected sex and HIV prevalence at study baseline. The number of psychosocial health conditions at baseline was also associated with increased HIV incidence during follow-up (no conditions, HIV incidence = 15.3 %; one to three conditions, 23.7 %; four to five conditions, 33.2 %). The number of psychosocial health conditions was positively associated with HIV risk behavior and HIV prevalence and incidence. Prevention efforts among MSM need to address the existence of multiple psychosocial health conditions and their synergy to effectively decrease the spread of HIV infection.
Keywords: Syndemics, HIV/AIDS, Men who have sex with men, Thailand
Introduction
Since the beginning of the HIV/AIDS epidemic, researchers have identified and studied psychosocial health conditions that were hypothesized to be important to either HIV transmission or HIV disease progression. Several of these conditions, such as substance abuse, suicidality, and a history of sexual abuse (among others), are common among men who have sex with men (MSM) and are also associated with HIV infection [1–8]. The term syndemic, or “two or more epidemics, interacting synergistically and contributing, as a result of their interaction, to excess burden of disease in a population” [24], has been applied to characterize the unique, population-level associations between psychosocial health, behavior, and disease. First introduced in 1994 to explain a cluster of epidemics of substance abuse, violence, and HIV/AIDS that disproportionately afflicted those living in poverty in cities in the United States [9], the term syndemic has also been used to describe tuberculosis and HIV [10]; obesity, metabolic disorders, and diabetes [11]; syringes and infectious diseases [12]; intimate partner violence and HIV [13]; and others. In populations at high risk for HIV infection, epidemic-level psychosocial health conditions may be more than individual risk factors alone; instead, they may affect the occurrence of disease in a population in a syndemic way. Several scholars have studied the presence of syndemics and their effects in populations of MSM in the United States. It appeared that the presence of conglomerates of psychosocial health conditions in MSM was associated with higher levels of behavioral risk and HIV infection than one would expect in the presence of each of these conditions alone. A combination of psychosocial health conditions may therefore have contributed to the HIV epidemic among MSM in the United States, in excess of the sum of the effects of the individual conditions combined [8, 14–17].
The psychosocial health conditions explored in this analysis and others are not merely risk factors for HIV infection. Each condition represents another epidemic, with a unique set of risk factors. For example, while sexual abuse is associated with HIV infection [1], it is independently associated with various other mental and psychosocial health conditions [18–20]. Traditional mental and psychosocial health conditions as described by Stall and colleagues [21] included substance abuse, depression, intimate partner violence, and sexual risk behaviors. However, there have been recent syndemic analyses that have expanded beyond these variables to include sexual/HIV-related stigma, homelessness, suicidal attempts and sexual assault [15, 22, 23]. Considering these additional associations when describing the HIV epidemic is a key distinction of the syndemic concept. In comparison, the individual risk-disease model, which is frequently used to describe the HIV epidemic, primarily examines one-to-one risk-disease associations. The syndemic concept proposes that multiple epidemics form a condition that affects the population in a way that is distinct from individual epidemics [24]. Conditions arising in early adolescence may also play a role in HIV vulnerability [21, 25, 26]. A set of recent meta-analyses have shown that several psychosocial health conditions, including substance abuse [27], violence victimization [28], and having sex under the influence of drugs or alcohol [29], arise in adolescence or early adulthood among MSM primarily in North America. Studies in Thailand have shown higher health risks among young bisexual and homosexual adolescents [30] and a similar set of predictors for inconsistent condom use [31] and HIV infection [32]. These results suggest that the experience of marginalization and low self-esteem at a very early age, along with other conditions and developmental measures including health or economic disparities, may make young men vulnerable to psychosocial health conditions, which cluster to raise levels of risk for HIV infection.
In 1984, the first AIDS cases in Thailand were identified among young homosexual men [33]. Twenty-one years later, it is estimated that as many as one in five new HIV infections in Thailand were attributable to unprotected sex between men [34]. In Bangkok, the prevalence of HIV infection among MSM, measured in cross-sectional assessments using venue-day-time sampling, was 17.3 % in 2003, 28.3 % in 2005, 30.8 % in 2007 [35]. In response to the high HIV prevalence, the Thailand Ministry of Public Health—U.S. Centers for Disease Control and Prevention Collaboration began a cohort study of HIV infection, sexually transmitted infections, and preventive interventions among MSM in Bangkok in 2006. Although this study was not specifically designed to investigate syndemics, the data allowed a statistical analysis of the presence of possible syndemic effects of psychosocial health conditions on HIV risk and HIV prevalence and incidence. Recently, cross-sectional studies among MSM in China and Vietnam reported syndemic conditions that contributed to increased sexual risk behaviors [36, 37]. While these were among the first syndemic findings outside of North America, they were still limited to cross-sectional designs and self-reported outcomes. Here, we add to the syndemics literature by reporting results of our analysis of syndemic associations between psychosocial health conditions, unprotected sex and HIV prevalence and incidence among MSM in Bangkok. As far as known to us, all previous studies of the existence of syndemics among MSM concerned cross-sectional studies of HIV risk and prevalence and no analyses have been reported concerning HIV incidence.
Methods
Study Population
Details of the Bangkok MSM Cohort Study has been described elsewhere [32]. Briefly, participants needed to be at least 18 years old, Thai national, male at birth, resident of the Bangkok metropolitan area, had penetrative oral or anal sex with another man in the past 6 months, and were available for four-monthly follow-up visits for a minimum of 3 and a maximum of 5 years. Between April 2006 and January 2008, 1,487 men were screened for enrollment, 1,311 (88.2 %) were eligible, of whom 1,292 enrolled in the study (enrollment rate 98.6 %) [32]. Men were recruited from a variety of sources, including HIV voluntary counseling and testing services provided at the study clinic, venues where MSM congregate for socializing and seeking sexual partners, the Internet, and by word of mouth. On every visit, men received pretest and posttest HIV and risk behavior counseling. Men who tested HIV positive were referred to antiretroviral treatment according to Thai national guidelines [38], and men who presented with active sexually transmitted infections were treated free of charge. The study location was a clinic in Central Bangkok.
Measures
Men were tested for HIV infection at baseline and every 4 months thereafter using a saliva-based test, OraQuick (OraSure Technologies Inc., Beaverton, Oregon, USA). If reactive, three additional rapid blood tests confirmed the result according to Thai national guidelines (DetermineTM HIV 1/2, Abbott Japan, Minato-Ku, Tokyo, Japan; DoubleCheckTM II HIV 1&2, Organics Ltd., Iverness Medical, Israel; CappilusTM HIV-1/HIV-2, Trinity Biotech, Jamestown, NY, USA [after 11/2008 replaced by CoreTM HIV1/2, Birmingham, United Kingdom]). If all three blood tests were positive for HIV infection, the OraQuick test then confirmed as HIV positive.
Other measures, including demographics, psychosocial health conditions, and unprotected sex, were collected through audio-computer-assisted self-interviewing (ACA-SI) at baseline and follow-up. Demographic variables included age, education (primary school, secondary/vocational, university or higher), and living situation (alone, with partner, roommate or family [parents, brother, sister or other relative]). Unprotected sex was defined as not always having used a condom from the start to finish during anal or vaginal sexual intercourse with steady, casual, or commercial male, female and transgender partners in the past 4 months. Psychosocial health conditions included history of forced sex [1, 39], social isolation [40], suicidal thoughts or actions [5, 6, 16], ‘club’ drug use [8, 41, 42], alcohol intoxication [5, 15, 42], and selling sex [4, 43]. These measures were adapted from the CDC’s Young Men’s Survey [44] and locally tested and validated for social and cultural contexts of Thailand and for Thai MSM [45]. The conditions were defined as follows:
History of forced sex: ever having been forced to have sexual intercourse against his will (yes/no).
Social isolation: currently not having either a close friend or family member to talk to in case of personal problems (at least one negative response to two yes/no questions).
Suicidal thoughts or actions: having ever seriously thought about committing suicide or having tried to commit suicide (at least one positive response to two yes/no questions).
Club drug use: using recreational drugs (ecstasy, meth-amphetamine, ketamine, nitrite inhalants, cocaine, gamma-hydroxybutyrate [GHB]) one or more times in the past 4 months (at least one positive response to six yes/no questions).
Alcohol intoxication: becoming drunk two to three times per week or more in the past 4 months (affirmative response to “two to three times per week” or “almost everyday” or “everyday”).
Selling sex: having received money, gifts, or valuables in exchange for sex in the past 4 months (positive response to yes/no question).
Data Analysis
Based on a methodology developed by Stall et al. [17], we evaluated syndemic associations between psychosocial health conditions, unprotected sex, and HIV prevalence and incidence. We calculated bivariate odds ratios (OR) and 95 % confidence intervals (CI) between pairs of psychosocial health conditions assessed at baseline to see if they were associated. Only those conditions that were significantly associated with two or more other conditions were included in further analyses. Social isolation was associated with one other condition (Table 1) and was excluded. From the remaining, mutually associated psychosocial health conditions, we created a syndemic count variable by counting the number of psychosocial health conditions each participant reported, yielding a number ranging from 0 to 5.
Table 1.
Odds ratios (ORs) and 95 % confidence intervals (95 % CIs) of psychosocial health conditions, unprotected sex, and HIV prevalence among men in the Bangkok MSM Cohort Study (N = 1,292)
| Predictors | Unadjusted OR (95 % CI) |
|||||
|---|---|---|---|---|---|---|
| Suicidal | Social isolation | Alcohol | Club drugs | Selling sex | Forced sex | |
| Suicidal | ||||||
| Social isolation | 1.05 (0.82–1.34) | |||||
| Alcohol | 1.39 (0.98–1.97)^ | 1.02 (0.73–1.42) | ||||
| Club drugs | 2.05 (1.50–2.81)* | 0.71 (0.53–0.97)* | 3.06 (2.11–4.45)* | |||
| Selling sex | 1.56 (1.16–2.09)* | 0.89 (0.68–1.17) | 2.82 (1.98–4.01)* | 3.58 (2.58–4.97)* | ||
| Forced sex | 2.15 (1.61–2.88)* | 0.91 (0.69–1.21) | 1.83 (1.26–2.66)* | 1.49 (1.04–2.14)* | 2.06 (1.50–2.82)* | |
| Unprotected sex | 1.31 (1.02–1.68)* | 1.03 (0.83–1.29) | 1.46 (1.04–2.05)* | 1.34 (0.98–1.83)^ | 1.44 (1.09–1.91)* | 1.30 (0.97–1.72)^ |
| HIV prevalence | 1.07 (0.80–1.43) | 0.93 (0.71–1.21) | 1.22 (0.84–1.78) | 2.09 (1.51–2.91)* | 1.39 (1.02–1.90)* | 1.55 (1.14–2.13) |
P < 0.05,
P <0.10, all P values are 2-tailed
HIV prevalence was calculated by dividing the number of HIV positive men at baseline by the total number of men enrolled in the study multiplied by 100. To evaluate the possible presence of syndemic effects, we evaluated the association between syndemic count, unprotected sex and HIV prevalence at baseline by computing Pearson χ2 for trend, OR’s and 95 % CI’s. No reports of conditions were used as the referent, as we hypothesized that the group with no conditions would be least affected by any syndemic effect. Cumulative HIV incidence was estimated using Kaplan–Meier analysis. The date of HIV seroconversion was estimated as the midpoint between the date of the last HIV negative test and the first HIV positive test. Curve and differences in incidence by level of the syndemic count were evaluated using log-rank tests. (SPSS 17.0 for Windows; SPSS Inc., Chicago, Illinois, USA).
Human Subjects Review
The protocol of this study was reviewed and approved by the Ethical Review Committee of the Thailand Ministry of Public Health and an Institutional Review Board of the U.S. Centers for Disease Control and Prevention. Written informed consent was obtained from all study participants.
Results
Characteristics
The mean age of participants was 27 years (median: 25 years, standard deviation: 6 years). Almost half reported having a university education (42.8 % [n = 553]), 17.7 % (n = 228) reported completing technical or vocational school, and 39.6 % (n = 551) completed secondary school or less. Most participants (61.7 % [n = 797]) lived away from the family, while 38.3 % lived with family.
In participants’ history, 57.6 % (n = 744) reported social isolation, 27.6 % (n = 356) reported suicidal thoughts or actions, and 18.9 % (n = 244) reported forced sex; in the past 4 months, 19.9 % (n = 257) reported selling sex, 15.2 % (n = 196) reported using ‘club’ drugs, and 12.7 % (n = 164) reported alcohol intoxication. At baseline, 56.8 % (n = 734) reported unprotected sex in the past 4 months and 22.4 % (n = 290) were HIV-positive.
Syndemic Count and Unprotected Sex
Suicidal thoughts/actions, alcohol intoxication, and selling sex were significantly associated with unprotected sex at baseline (Table 1). A higher syndemic count at baseline was significantly and positively associated with higher levels of unprotected sex (no conditions, 51.0 %; one condition, 59.2 %; two conditions, 60.7 %; three conditions, 70.1 %; four to five conditions, 66.7 %; χ2, P < 0.01) and higher ORs for unprotected sex at baseline (Table 2).
Table 2.
Odds ratios (ORs) and 95 % confidence intervals (95 % CIs) to evaluate the associations between syndemic count, levels of unprotected sex, and HIV prevalence among men in the Bangkok MSM Cohort Study (N = 1,292)
| Syndemic count |
Unprotected sex |
HIV prevalence |
|||
|---|---|---|---|---|---|
| n (%) | n (%) | OR (95 % CI) | n (%) | OR (95 % CI) | |
| 0 | 551 (42.6) | 281 (51.0) | 1 | 106 (19.2) | 1 |
| 1 | 422 (32.7) | 250 (59.2) | 1.40 (1.08–1.80)* | 91 (21.6) | 1.15 (0.84–1.58) |
| 2 | 206 (15.9) | 125 (60.7) | 1.48 (1.07–2.05)* | 55 (26.7) | 1.53 (1.05–2.22)* |
| 3 | 77 (6.0) | 54 (70.1) | 2.26 (1.35–3.78)* | 24 (31.2) | 1.90 (1.12–3.22)* |
| 4–5 | 36 (2.8) | 24 (66.7) | 1.92 (0.94–3.92) | 14 (38.9) | 2.67 (1.32–5.40)* |
P <0.05, all p values are 2-tailed
OR odds ratio, CI confidence interval
Syndemic Count and HIV Prevalence
Use of ‘club’ drugs, selling sex, and forced sex were significantly associated with HIV prevalence (Table 1). A higher syndemic count at baseline was significantly and positively associated with higher baseline HIV prevalence (no conditions, 19.2 %; one condition, 21.6 %; two conditions, 26.7 %; three conditions, 31.2 %; four to five conditions, 38.9 %; χ2, P < 0.01).
Syndemic Count and HIV Incidence
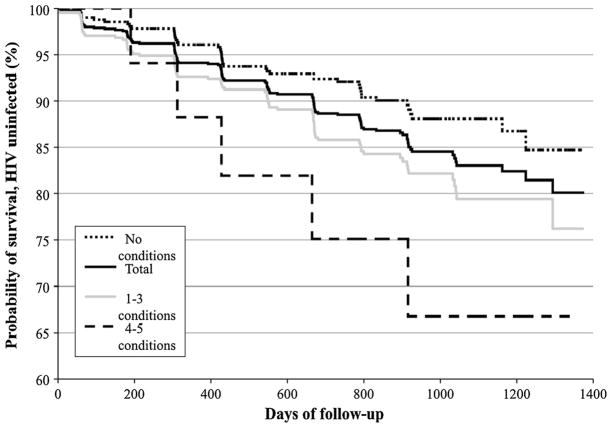
From baseline until February 25, 2010, 125 HIV seroconversions occurred, accounting for an overall cumulative HIV incidence of 19.9 %. The Kaplan–Meier survival curves for syndemic count are shown in Fig. 1. The longest follow-up period was 3.8 years. Among participants reporting no conditions at baseline, the cumulative HIV incidence was 15.3 %; among those reporting one to three conditions, 23.7 %; and among those reporting four to five conditions, 33.2 % (P < 0.01) (Fig. 1).
Fig. 1.
HIV incidence (Kaplan–Meier analysis) in the HIV-negative fraction of the cohort (N = 1,002). The total estimated HIV incidence was 19.9 % (dark solid line). Among participants reporting no conditions, HIV incidence was 15.3 % (dashed dotted line); among participants reporting 1–3 conditions, 23.7 % (grey solid line); and among participants reporting 4–5 conditions, 33.2 % (dashed line) (log rank test, P < 0.01)
Discussion
In this study, we found that having a higher number of psychosocial health conditions was significantly associated with [1] a higher level of unprotected sex, [2] a higher HIV prevalence and [3] a higher HIV incidence among MSM in Bangkok. These findings support the notion of possible syndemic effects of mutually associated psychosocial health conditions on the spread of HIV among MSM in Bangkok. Because our results also show associations with incident HIV infection, these findings therefore provide the first longitudinal evidence to support syndemic theory. Our data from Bangkok also provides evidence that syndemic effects may act independently of the cultural setting in which they occur. Although the results of our analysis underscore the importance of psychosocial health conditions in the study of disease burden in different populations, there are currently no methods available to quantify syndemic effects or that of its components.
Merrill Singer, who coined the term ‘syndemic,’ [9] suggested that syndemic conditions are perpetuated “because of harmful social conditions and injurious social connections” [24]. Stall and colleagues [21, 26] expanded on this hypothesis, proposing a model for a developmental pathway of syndemics among American MSM. In addition, they proposed several methods that may improve gay men’s health, including strengthening social networks, integrating and tailoring public health services, and initiating improved research into the health issues of racial and ethnic minorities [21, 26, 46]. These models, as well as our results, suggest that combinations of psychosocial health conditions may have supplemental effects on other health conditions, such as HIV infection, further down the causal pathway.
This causal pathway must also include unprotected anal sex, as this behavior is the main biological mechanism of HIV transmission among MSM. In our analysis, the levels of unprotected sex seem to plateau at the highest number of conditions. However, this could be a result of the smaller sample size or indicative of a syndemic ceiling effect. On the other hand, the risk of unprotected sex is not equal in all situations, as it largely depends on the background HIV prevalence, community HIV viral load and the number of persons in acute HIV infection. These factors may modulate the levels of risk in unprotected sex.
Other factors, such as depression [47], may have biological mechanisms that increase vulnerability to HIV and may help explain residual variance in the analysis of HIV infection. While there has been limited attention in international research to mental health, the World Health Organization and the Lancet have recently highlighted this discrepancy and have initiated efforts to focus more research on mental health in developing countries [48, 49]. While this study did not assess depression, suicidal thoughts or actions may be seen as another mental health variable with a relationship to HIV infection, as it was associated with other syndemic conditions and with unprotected sex. However, suicidal thoughts or actions were not associated with HIV prevalence, suggesting a more nuanced interpretation of this mental health variable with HIV infection. In a recent syndemics analysis, suicidal attempts was used as the primary outcome variable [22], suggesting a syndemic relationship between psychosocial conditions and suicide attempts.
Notably, many participants reported no conditions, comparable to other studies of syndemics among MSM [15, 17]. Because those reporting no conditions had significantly lower HIV prevalence and incidence, this sub-population deserves further attention and research to explore potential resiliencies existing amidst a high-risk population [50]. While research among MSM (and other populations) tends to focus on those with disease, some have explored resiliency, finding high levels among MSM. A study among young MSM in New York City found that more than half who experienced childhood adversity had signs of resiliency [51], while Mutchler et al. [52] described potential pathways of resiliency among young MSM. Additional research on resiliency among high-risk populations, such as MSM, may reveal improved methods of HIV prevention. However, it is important to note that, in this study, even participants reporting no conditions were subject to relatively high levels of HIV prevalence and incidence. These participants may still be affected by syndemic effects, as they potentially interact with or form a community with those affected by numerous conditions. Additionally, conditions such as drug abuse and sex work are stigmatized and may be subject to underreporting. It is important to note that during the time when the cohort study began, Thailand was undergoing a government-sponsored social order campaign to decrease the supply and demand of illicit drugs and to restructure Thai society into moral order [53]. As a result, venues where local MSM usually congregate, such as bars and saunas, were raided for drugs and closed down. This type of moral policing by the Thai government may therefore further stigmatize MSM and limit their self-reports of illicit drug use. However, in our study we used ACASI for the reporting of behavioral histories, which has been shown to generate higher response rates of sensitive behaviors and increased concordance with biological measures of tabooed behavior such as drug use and smoking [54].
Several limitations should be considered when interpreting our results. First, the method of using syndemic count does not account for the possibility that psychosocial health conditions differ in magnitude. Similarly, a count variable does not consider potential interactions between its components. While these interactions may be particularly relevant on the individual level, in this analysis we are most interested in exploring the possible existence of synergistic effects of a number of psychosocial health conditions on unprotected sex and HIV prevalence and incidence. In addition, the variables selected were not comprehensive assessments of each condition, in that each condition was typically assessed with one to two questions. Moreover, the population of MSM in this study was homogenous, primarily consisting of relatively young, well-educated men in urban Bangkok. This may therefore limit the generalizability of findings. Finally, since the main objective of this cohort was not to investigate syndemic effects on HIV prevalence and incidence, we thus did not assess psychosocial health conditions at each follow up visit, limiting our analysis to baseline conditions. Further research into syndemics should include additional analysis of the conditions that may lead to a syndemic effect over time.
Conclusion
The results reported in this analysis show that the presence of multiple psychosocial health conditions might influence vulnerability to HIV infection. While current prevention approaches tend to focus on one health issue, integration and simultaneous implementation of prevention programs for multiple conditions needs serious consideration [46]. This premise also points at the importance of finding ways to move the study of syndemics forward as part of the overall public health research agenda. While syndemic conditions may not exist in all high-risk populations, demonstrating the interplay between various health conditions may spur increased interest in creating and implementing holistic programs to address psychosocial health conditions and HIV infection simultaneously. Such holistic programs could include sustained behavioral change initiatives, combination prevention methods, and tailored approaches to particular populations that address all syndemic conditions and their interconnections [46, 55]. In addition to further study of syndemic conditions and their trends over time, studying potential protective or resilience factors is warranted: participants reporting no syndemic conditions were less likely to engage in unprotected sex and had lower HIV prevalence and HIV incidence, despite high community HIV levels.
Increasing efforts to address the psychosocial health conditions described in this paper may be effective in reducing HIV risk in this population. Further study of how syndemics and resilency emerge in marginalized populations such as MSM, and how they might be disentangled, is likely to yield important public health outcome.
Acknowledgments
The authors would like to thank the participants in the Bangkok MSM Cohort Study, the staff of the Silom Community Clinic, the HIV STD Research Program and the Thailand Ministry of Public Health—U.S. Centers for Disease Control and Prevention Collaboration for their contributions to the study. We would like to also acknowledge the leadership of the Thai Red Cross AIDS Research Center for granting the time needed to complete the study and to finalize the manuscript for publication. K. McCarthy was supported by the ASPPH/CDC Allan Rosenfield Global Health Fellowship and TE Guadamuz was supported by a Mentored Research Scientist Development Award from the U.S. National Institute of Mental Health (K01MH085567). The study was funded by the Division of HIV/AIDS Prevention, U.S. Centers for Disease Control and Prevention. The findings and conclusions in this manuscript are those of the authors and do not necessary represent the views of the U.S. Centers for Disease Control and Prevention.
Contributor Information
T. E. Guadamuz, Email: tguadamu@hotmail.com, Department of Society and Health, Faculty of Social Sciences and Humanities, Mahidol University, 25/25 Buddhamonthon 4 Road Salaya, Nakorn Pathom 73170, Thailand. Department of Behavioral and Community Health Sciences, University of Pittsburgh Graduate School of Public Health, Pittsburgh, PA, USA
K. McCarthy, Thailand Ministry of Public Health—U.S. Centers for Disease Control and Prevention Collaboration, Nonthaburi, Thailand
W. Wimonsate, Thailand Ministry of Public Health—U.S. Centers for Disease Control and Prevention Collaboration, Nonthaburi, Thailand
W. Thienkrua, Thailand Ministry of Public Health—U.S. Centers for Disease Control and Prevention Collaboration, Nonthaburi, Thailand
A. Varangrat, Thailand Ministry of Public Health—U.S. Centers for Disease Control and Prevention Collaboration, Nonthaburi, Thailand
S. Chaikummao, Thailand Ministry of Public Health—U.S. Centers for Disease Control and Prevention Collaboration, Nonthaburi, Thailand
A. Sangiamkittikul, Thailand Ministry of Public Health—U.S. Centers for Disease Control and Prevention Collaboration, Nonthaburi, Thailand
R. D. Stall, Department of Behavioral and Community Health Sciences, University of Pittsburgh Graduate School of Public Health, Pittsburgh, PA, USA
F. van Griensven, Thai Red Cross AIDS Research Center and HIV Netherlands Australia Thailand Research Collaboration, Bangkok, Thailand. Division of Preventive Medicine and Public Health, University of California at San Francisco, San Francisco, CA, USA
References
- 1.Bartholow BN, Doll LS, Joy D, et al. Emotional, behavioral, and HIV risks associated with sexual abuse among adult homosexual and bisexual men. Child Abuse Negl. 1994;18:747–61. doi: 10.1016/0145-2134(94)00042-5. [DOI] [PubMed] [Google Scholar]
- 2.Chesney MA, Barrett DC, Stall R. Histories of substance use and risk behavior: precursors to HIV seroconversion in homosexual men. Am J Public Health. 1998;88:113–6. doi: 10.2105/ajph.88.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cochran SD, Mays VM. Lifetime prevalence of suicide symptoms and affective disorders among men reporting same-sex sexual partners: results from NHANES III. Am J Public Health. 2000;90:573–8. doi: 10.2105/ajph.90.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He Q, Wang Y, Lin P, et al. High prevalence of risk behaviour concurrent with links to other high-risk populations: a potentially explosive HIV epidemic among men who have sex with men in Guangzhou. China Sex Transm Infect. 2009;85:383–90. doi: 10.1136/sti.2009.035808. [DOI] [PubMed] [Google Scholar]
- 5.Koblin BA, Husnik MJ, Colfax G, et al. Risk factors for HIV infection among men who have sex with men. AIDS. 2006;20:731–9. doi: 10.1097/01.aids.0000216374.61442.55. [DOI] [PubMed] [Google Scholar]
- 6.Remafedi G. Suicidality in a venue-based sample of young men who have sex with men. J Adolesc Health. 2002;31:305–10. doi: 10.1016/s1054-139x(02)00405-6. [DOI] [PubMed] [Google Scholar]
- 7.Van Tieu H, Koblin BA. HIV, alcohol, and noninjection drug use. Curr Opin HIV AIDS. 2009;4:314–8. doi: 10.1097/COH.0b013e32832aa902. [DOI] [PubMed] [Google Scholar]
- 8.Walkup J, Blank MB, Gonzales JS, et al. The impact of mental health and substance abuse factors on HIV prevention and treatment. J Acquir Immun Defic Syndr. 2008;47(Suppl 1):S15–9. doi: 10.1097/QAI.0b013e3181605b26. [DOI] [PubMed] [Google Scholar]
- 9.Singer M. AIDS and the health crisis of the U.S. urban poor: the perspective of critical medical anthropology. Soc Sci Med. 1994;39:931–48. doi: 10.1016/0277-9536(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 10.Kwan CK, Ernst JD. HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev. 2011;24:351–76. doi: 10.1128/CMR.00042-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Candib LM. Obesity and diabetes in vulnerable populations: reflection on proximal and distal causes. Ann Fam Med. 2007;5:547–56. doi: 10.1370/afm.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bulled N, Singer M. Syringe-mediated syndemics. AIDS Behav. 2011;15:1539–45. doi: 10.1007/s10461-009-9631-1. [DOI] [PubMed] [Google Scholar]
- 13.Gielen AC, Burke JG, Mahoney P, et al. HIV/AIDS and intimate partner violence: intersecting women’s health issues in the United States. Trauma Violence Abuse. 2007;8:178–98. doi: 10.1177/1524838007301476. [DOI] [PubMed] [Google Scholar]
- 14.Kurtz SP. Arrest histories of high-risk gay and bisexual men in Miami: unexpected additional evidence for syndemic theory. J Psychoact Drugs. 2008;40:513–21. doi: 10.1080/02791072.2008.10400657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mustanski B, Garofolo R, Herrick A, Donenberg G. Psychosocial health problems increase risk for HIV among urban young men who have sex with men: preliminary evidence of a syndemic in need of attention. Ann Behav Med. 2007;34:37–45. doi: 10.1080/08836610701495268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salomon EA, Mimiaga MJ, Husnik MJ, et al. Depressive symptoms, utilization of mental health care, substance use and sexual risk among young men who have sex with men in EXPLORE: implications for age-specific interventions. AIDS Behav. 2009;13:811–21. doi: 10.1007/s10461-008-9439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stall R, Mills TC, Williamson J, et al. Association of co-occurring psychosocial health problems and increased vulnerability to HIV/AIDS among urban men who have sex with men. Am J Public Health. 2003;93:939–42. doi: 10.2105/ajph.93.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braitstein P, Asselin JJ, Schilder A, et al. Sexual violence among two populations of men at high risk of HIV infection. AIDS Care. 2006;18:681–9. doi: 10.1080/13548500500294385. [DOI] [PubMed] [Google Scholar]
- 19.Houston E, McKirnan DJ. Intimate partner abuse among gay and bisexual men: risk correlates and health outcomes. J Urb Health. 2007;84:681–90. doi: 10.1007/s11524-007-9188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratner PA, Johnson JL, Shoveller JA, et al. Non-consensual sex experienced by men who have sex with men: prevalence and association with mental health. Patient Educ Couns. 2003;49:67–74. doi: 10.1016/s0738-3991(02)00055-1. [DOI] [PubMed] [Google Scholar]
- 21.Stall R, Friedman MS, Catania JA. Interacting epidemics and gay men’s health: a theory of syndemic production among urban gay men. In: Wolitski RJ, Stall R, Valdiserri RO, editors. Unequal opportunity: health disparities affecting gay and bisexual men in the United States. Oxford: Oxford University Press; 2008. pp. 251–74. [Google Scholar]
- 22.Mustanski B, Andrews R, Herrick A, Stall R, Schnarrs PW. A syndemic of psychosocial health disparities and associations with risk for attempting suicide among young sexual minority men. Am J Public Health. 2014;104:287–94. doi: 10.2105/AJPH.2013.301744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santos GM, Do T, Beck J, Makofane K, Arreola S, Pyun T, Hebert P, Wilson PA, Ayala G. Syndemic conditions associated with increased HIV risk in a global sample of men who have sex with men. Sex Transm Infect. 2014;90:250–3. doi: 10.1136/sextrans-2013-051318. [DOI] [PubMed] [Google Scholar]
- 24.Singer M, Clair S. Syndemics and public health: reconceptualizing disease in bio-social context. Med Anthropol Q. 2003;17:423–41. doi: 10.1525/maq.2003.17.4.423. [DOI] [PubMed] [Google Scholar]
- 25.Dyer TP, Shoptaw S, Guadamuz TE, Plankey M, Kao U, Ostrow D, Chmiel JS, Herrick A, Stall R. Application of syndemic theory to black men who have sex with men in the Multicenter AIDS Cohort Study. J Urb Health. 2012;89:697–708. doi: 10.1007/s11524-012-9674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guadamuz TE, Friedman, Marshal MP, Herrick AL, Lim SH, Wei C, Stall R. Health, sexual health, and syndemics: toward a better approach to STI and HIV preventive interventions for men who have sex with men (MSM) in the United States. In: Aral SO, Fenton KA, Lipshutz JA, editors. The new public health and STI/HIV prevention: personal, public, and health systems approaches. New York: Springer Science+Business Media, LLC; 2013. [Google Scholar]
- 27.Marshal MP, Friedman MS, Stall R, et al. Sexual orientation and adolescent substance use: a meta-analysis and methodological review. Addiction. 2008;103:546–56. doi: 10.1111/j.1360-0443.2008.02149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman MS, Marshal MP, Guadamuz TE, et al. A meta-analysis of disparities in childhood sexual abuse, parental physical abuse, and peer victimization among sexual minority and sexual non-minority individuals. Am J Public Health. 2011;101:1481–94. doi: 10.2105/AJPH.2009.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herrick AL, Marshal MP, Smith HA, Sucato G, Stall RD. Sex while intoxicated: a meta-analysis comparing heterosexual and sexual minority youth. J Adolesc Health. 2011;48:306–9. doi: 10.1016/j.jadohealth.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Griensven F, Kilmarx PH, Jeeyapant S, et al. The prevalence of bisexual and homosexual orientation and related health risks among adolescents in northern Thailand. Arch Sex Behav. 2004;33:137–47. doi: 10.1023/b:aseb.0000014328.49070.8c. [DOI] [PubMed] [Google Scholar]
- 31.Chemnasiri T, Netwong T, Visarutratana S, et al. Inconsistent condom use among young men who have sex with men, male sex workers, and transgenders in Thailand. AIDS Educ Prev. 2010;22:100–9. doi: 10.1521/aeap.2010.22.2.100. [DOI] [PubMed] [Google Scholar]
- 32.van Griensven F, Thienkrua W, McNicholl J, et al. Evidence of an explosive epidemic of HIV infection in a cohort of men who have sex with men in Thailand. AIDS. 2013;27:825–32. doi: 10.1097/QAD.0b013e32835c546e. [DOI] [PubMed] [Google Scholar]
- 33.Wangroongsarb Y, Weniger BG, Wasi C, et al. Prevalence of HTLV-III/LAV antibody in selected populations in Thailand. Southeast Asian J Trop Med Public Health. 1985;16:517–20. [PubMed] [Google Scholar]
- 34.Gouws E, White PJ, Stover J, Brown T. Short term estimates of adult HIV incidence by mode of transmission: Kenya and Thailand as examples. Sex Transm Infect. 2006;82:51–5. doi: 10.1136/sti.2006.020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Griensven F, Varangrat A, Wimonsate W, et al. Trends in HIV prevalence, estimated HIV incidence, and risk behavior among men who have sex with men in Bangkok, Thailand, 2003–2007. J Acquir Immune Defic Syndr. 2010;53:234–9. doi: 10.1097/QAI.0b013e3181c2fc86. [DOI] [PubMed] [Google Scholar]
- 36.Yu F, Nehl EJ, Zheng T, He N, Berg CJ, Lemieux AF, Lin L, Tran A, Sullivan PS, Wong FY. A syndemic including cigarette smoking and sexual risk behaviors among a sample of MSM in Shanghai. China Drug Alcohol Depend. 2013;132:265–70. doi: 10.1016/j.drugalcdep.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biello KB, Colby D, Closson E, Mimiaga MJ. The syndemic condition of psychosocial problems and HIV risk among male sex workers in Ho Chi Minh City, Vietnam. AIDS Behav. 2013 doi: 10.1007/s10461-013-0632-8. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sungkanuparph S, Techasathit W, Utaipiboon C, Chasombat S, Bhakeecheep S, Leechawengwongs M, Ruxrungtham K, Phanuphak P. Thai national guidelines for antiretroviral therapy in HIV-1 infected adults and adolescents. Asian Biomed. 2010;4:515–28. [Google Scholar]
- 39.Guadamuz TE, Wimonsate W, Varangrat A, et al. Correlates of forced sex among populations of men who have sex with men in Thailand. Arch Sex Behav. 2011;40:259–66. doi: 10.1007/s10508-009-9557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dowshen N, Binns HJ, Garofalo R. Experiences of HIV-related stigma among young men who have sex with men. AIDS Patient Care STDS. 2009;23:371–6. doi: 10.1089/apc.2008.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colfax G, Vittinghoff E, Husnik MJ, et al. Substance use and sexual risk: a participant- and episode-level analysis among a cohort of men who have sex with men. Am J Epidemiol. 2004;159:1002–12. doi: 10.1093/aje/kwh135. [DOI] [PubMed] [Google Scholar]
- 42.Stall R, Paul JP, Greenwood G, et al. Alcohol use, drug use and alcohol-related problems among men who have sex with men: the urban men’s health Study. Addiction. 2001;96:1589–601. doi: 10.1046/j.1360-0443.2001.961115896.x. [DOI] [PubMed] [Google Scholar]
- 43.Aynalem G, Smith L, Bemis C, et al. Commercial sex venues: a closer look at their impact on the syphilis and HIV epidemics among men who have sex with men. Sex Transm Infect. 2006;82:439–43. doi: 10.1136/sti.2006.020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacKellar D, Valleroy L, Karon J, Lemp G, Janssen R. The Young Men’s Survey: methods for estimating HIV seroprevalence and risk factors among young men who have sex with men. Public Health Rep. 1996;111:138–44. [PMC free article] [PubMed] [Google Scholar]
- 45.Mansergh G, Naorat S, Jommaroeng R, Jenkins RA, Jeeyapant S, Kanggarnrua K, Phanuphak P, Tappero JW, van Griensven F. Adaptation of venue-day-time sampling in Southeast Asia to access men who have sex with men for HIV assessment in Bangkok. Field Methods. 2006;18:135–52. [Google Scholar]
- 46.Stall R, Herrick A, Guadamuz TE, Friedman MS. Updating HIV prevention with gay men: Current challenges and opportunities to advance health among gay men. In: Mayer KH, Pizer H, editors. HIV prevention: a comprehensive approach. London: Academic Press; 2009. pp. 267–80. [Google Scholar]
- 47.Blume J, Douglas SD, Evans DL. Immune suppression and immune activation in depression. Brain Behav Immun. 2011;25:221–9. doi: 10.1016/j.bbi.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharan P, Levav I, Olifson S, de Francisco A, Saxena S, editors. Research capacity for mental health in low- and middle-income countries: results of a mapping project. Geneva: World Health Organization & Global Forum for Health Research; 2007. [Google Scholar]
- 49.Horton R. Launching a new movement for mental health. Lancet. 2007;370:806. doi: 10.1016/S0140-6736(07)61243-4. [DOI] [PubMed] [Google Scholar]
- 50.Herrick AL, Lim SH, Wei C, et al. Resilience as an untapped resource in behavioral intervention design for gay men. AIDS Behav. 2011;15:S25–9. doi: 10.1007/s10461-011-9895-0. [DOI] [PubMed] [Google Scholar]
- 51.Gwadz MV, Clatts MC, Yi H, et al. Resilience among young men who have sex with men in New York City. Sex Res Social Policy. 2006;3:13–21. doi: 10.1525/srsp.2006.3.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mutchler MG, Ayala G, Neith KL. Safer sex stories told by young gay men: building on resiliency through gay-boy talk. J Gay Lesb Issues Educ. 2005;2:37–50. [Google Scholar]
- 53.Vongchak T, Kawichai S, Sherman S, Celentano DD, Sirisanthana T, Latkin C, Wiboonnatakul K, Srirak N, Jittiwutikarn J, Aramrattana A. The influence of Thailand’s 2003 ‘war on drugs’ policy on self-reported drug use among injection drug users in Chiang Mai, Thailand. Int J Drug Policy. 2005;16:115–21. [Google Scholar]
- 54.van Griensven F, Naorat S, Kilmarx PH, Jeeyapant S, Manopaiboon C, Chaikummao S, Jenkins RA, Uthaivoravit W, Wasinrapee P, Mock PA, Tappero JW. Palmtop-assisted self-interviewing for the collection of sensitive behavioral data: randomized trial with drug use urine testing. Am J Epidemiol. 2006;163:271–8. doi: 10.1093/aje/kwj038. [DOI] [PubMed] [Google Scholar]
- 55.Coates TJ, Richter L, Caceres C. Behavioural strategies to reduce HIV transmission: how to make them work better. Lancet. 2008;372:669–84. doi: 10.1016/S0140-6736(08)60886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]