Abstract
Adherence to antiretroviral therapy (ART) and second–line antituberculosis medications is essential to achieve successful outcomes among individuals co–infected with HIV and multi or extensively drug-resistant TB (M/XDR–TB). In 2012–13, we designed a qualitative study to explore barriers to adherence in KwaZulu–Natal, South Africa. We conducted six focus groups comprising 23 adults receiving treatment for either MDR-TB (n=2) or XDR-TB (n=21); 17 were on concurrent ART. Participants expressed a preference for ART over M/XDR–TB treatment as a result of greater tolerability, lower pill burden, and a commitment to ART. Treatment outcomes and the social morbidity associated with M/XDR-TB, characterised by public notification, stigma, and social isolation, were perceived to be worse than with HIV. Poor communication, low patient involvement, and provider supervision of treatment exacerbated participants’ negative experiences with TB care. To improve adherence, it is critical that new regimens for drug-resistant TB be developed with better efficacy, lower pill burden, and fewer adverse effects. For the first time, such improved regimens are on the horizon. In parallel and equally important is the implementation of a cohesive approach that promotes patient involvement, empowerment, and treatment literacy for HIV and for TB.
Keywords: adherence, HIV/AIDS, drug–resistant tuberculosis, co–infection, qualitative methods
Background
Drug resistance is one of the greatest challenges to mitigating the global tuberculosis (TB) epidemic. This includes multidrug–resistant tuberculosis or MDR–TB, that is resistant to first–line antituberculosis therapy, and extensively drug–resistant tuberculosis or XDR–TB, a subset of MDR–TB that is additionally resistant to second–line therapies (WHO, 2010).
In 2010, South Africa reported approximately 71% of XDR–TB cases worldwide (WHO, 2010). An estimated 80% of South African XDR–TB patients are HIV co–infected, resulting in poor treatment outcomes, low levels of TB culture conversion, and high mortality (Gandhi et al., 2012; O’Donnell et al., 2013). Antiretroviral therapy or ART may improve survival among co–infected individuals (Lawn, Kranzer, & Wood, 2009). However, ART does not appear to improve TB culture conversion (O’Donnell et al., 2013; Pietersen et al., 2014), and therefore may not prevent community transmission of drug-resistant TB. Adherence to ART and second-line TB medications is therefore critical to successful treatment outcomes among patients co–infected with HIV and drug-resistant TB (Brust, Gandhi, Carrara, Osburn, & Padayatchi, 2010; Gardner et al., 2008).
In 2009–11, we conducted a prospective observational study with XDR–TB/HIV co–infected patients in the high–burden province of KwaZulu—Natal. In serial monthly assessments via seven-day recall, we found significantly lower rates of self-reported adherence to XDR–TB treatment (67.7%) compared to ART (85%) (Padayatchi & O’Donnell, 2011). We assessed the implications of our findings in light of two major considerations. First, poor adherence to TB treatment is associated with the development of drug–resistance, and poor treatment outcomes (WHO, 2010). Second, while ART may prolong survival and transmission time among co–infected patients, it does not improve TB culture conversion (O’Donnell et al., 2013; Pietersen et al., 2014). Thus, adherence to ART without commensurate adherence to XDR–TB treatment has the potential to amplify TB drug–resistance. We conducted this qualitative study to further contextualise the barriers and facilitators to dual drug adherence, and to examine the issues underlying lower rates of adherence to second-line TB medications when compared to ART.
Methodology
The supplementary study was set at a specialist TB hospital in KwaZulu–Natal, where our foundational study had been based. Inpatients with M/XDR–TB receive treatment under daily nurse administration and monitoring, with intermittent direct observation of drug intake. HIV co–infected patients on ART receive monthly supplies from an onsite HIV clinic; however, there is no daily administration, monitoring or observation of ART intake.
We adopted a qualitative approach (Seale, Gobo, Gubrium, & Silverman, 2004). Data was collected via focus group discussions (FGDs) with inpatients aged 18 years or older who had initiated treatment for drug–resistant TB within the past 12 months. Each FGD comprised a purposively small number of participants, stratified by gender and inpatient ward, to facilitate an open exchange of viewpoints and capture greater thematic diversity than would have been possible through individual, didactic interviews. FGDs were audio–recorded and conducted in isiZulu by a facilitator and moderator trained in qualitative inquiry and the management of group dynamics. Findings from the broader study were kept confidential to mitigate interviewer and participant biases. Main questions, comprised within a guide, were open–ended and exploratory to gather participants’ subjective perspectives on treatment in a nonjudgmental manner. Follow–up probes, and the wording and sequence of questions changed per group based on participants’ individual and shared experiences. The facilitator led as little as possible to encourage a naturalistic collection of accounts, while emphasising respect for each participant’s privacy and confidentiality.
The study aimed to capture a range of issues that could affect adherence to XDR–TB and/or HIV treatment. With the aid of inpatient staff, the moderator visited the site bi–monthly to actively recruit a heterogeneous sample of inpatients based on their gender, age, ART status, duration on ART and drug–resistant TB treatment, period of hospitalisation, and ward. All patients approached agreed to participate in a group interview and provided their informed consent immediately prior to commencement of the FGD. The study received ethics approval from the University of KwaZulu—Natal, South Africa and Albert Einstein College of Medicine, USA.
FGDs were transcribed, anonymised, translated, and subjected to thematic analysis (Seale et al., 2004) by the study investigators in consultation with the facilitator and moderator. Substantive coding helped identify an initial set of broad codes, which were reapplied to each transcript to identify more selective, conceptual codes. This enabled the organic emergence of latent patterns and intersecting themes that were contextualised by participants’ social and clinical circumstances. Qualitative analysis was discursive and critically reflexive, as the data were considered to be a product of interactive dialogue between the researchers and researched.
Findings
Between November 2012 and February 2013, six FGDs of average duration 70 minutes (range, 49–109) were conducted with 23 participants, including 13 women and 10 men (Table 1). Participants’ median age was 36 years (range, 18–56). Twenty–one participants (91%) were receiving treatment for XDR–TB, and 2 for MDR–TB, for 1 to 10 months. Twenty–two participants (96%) had experienced at least one prior episode of pulmonary TB, including 12 (52%) with a history of MDR–TB. Eighteen participants (78%) were HIV co–infected, 17 (74%) of who were on ART for 1 month to 7 years. Most (91%) were unemployed. The sample was aptly heterogeneous and allowed for the capture and analysis of a broad range of treatment experiences.
Table 1.
Characteristics of focus group participants.
| Patient characteristics | Total | Female | Male |
|---|---|---|---|
|
| |||
| Sample | 23 | 13 (57%) | 10 (43%) |
|
| |||
| Age | |||
| Average (years) | 36 | 35.7 | 37.5 |
| Range (years) | 18–56 | 18–56 | 29–47 |
|
| |||
| Employment status | |||
| Employed | 2 (9%) | 1 (8%) | 1 (10%) |
| Unemployed1 | 21 (91%) | 12 (92%) | 9 (90%) |
|
| |||
| HIV status | |||
| Positive2 | 18 (78%) | 8 (62%) | 10 (100%) |
| Negative | 5 (22%) | 5 (38%) | 0 |
|
| |||
| TB status | |||
| MDR–TB | 2 (9%) | 1 (8%) | 1 (10%) |
| XDR–TB | 21 (91%) | 12 (92%) | 9 (90%) |
Includes 3 participants who became unemployed after being diagnosed with XDR–TB.
All HIV–positive participants, except 1, were receiving ART at the time of the study.
Of note, participants were not asked about their individual TB, HIV, or ART status during FGDs. However, they each shared personal stories about their TB diagnosis, HIV serostatus, and experiences with TB treatment and/or ART. Disclosure may have resulted from ensuing discussions about illness and treatment, familiarity with co–participants, and the facilitator’s rapport.
Preferential adherence to ART
Participants complained about their high pill burden. They reported ingesting between 7 and 32 pills every day, the majority of which were for XDR–TB. Co–infected patients attributed common side effects including dizziness, nausea, vomiting, diarrhoea and confusion to XDR–TB treatment as opposed to ART, which they had taken without issue for several months prior.
‘ARVs have never ever given me any problems since I started taking them but when I was taking them with TB treatment, I started to have problems.’ (female1)
In all FGDs, several patients voiced a preference for taking ART over XDR–TB treatment. They admitted that they as well as their peers would find ways to ‘dodge’ XDR–TB treatment to escape the associated side effects and ‘enjoy’ the day. Patients hid the tablets dispensed by inpatient staff beneath their hospital sheets.
‘If I can dodge, I can dodge TB. I can take for HIV, even if I can take them my whole life I don’t have a problem. But for TB, no, I can dodge them if I can be given a chance… there are many injections, lots of tablets, they are bitter, they are sour. It’s all mixed here, it’s something you don’t know. But HIV, you just get three, maybe once a day.’ (female)
The greater perceived tolerability and lower pill burden of ART, as suggested in these excerpts, funnelled patients’ adherence toward ART and against XDR–TB treatment. Another driver appeared to be patients’ personal commitment to adhere to ART. They had attended education and counselling sessions prior to initiating HIV treatment. They had been taught that antiretrovirals were ‘strict with time’ and felt accountable for taking the prescribed doses. Adherence to XDR–TB treatment, in contrast, was considered to be the responsibility of the administering nurse, who controlled when and how patients received their daily dose.
‘You can’t take ARVs late because you have to take them in time. But these for TB don’t have time the way they do.’ (male)
The stringency of adhering to TB treatment had not been inculcated in the same way as it had been for ART. Patients consequently did not retain a commensurate level of commitment to XDR–TB treatment. The excerpt below, from an interview with women, succinctly illustrates the complex pathways underlying their shared preference to adhere to ART.
P1: ‘I can choose to take ARVs.’
P2: ‘Because you can see it’s work.’
P1: ‘You can see… you recover from point A to point B. I was like this, I am like this. [TB treatment] has got so many side effects… Sometimes you wake up with nausea and you will feel like inducing vomiting…’
P2: ‘Other one develops itchiness in the body.’
P1: ‘Sometimes you feel like you are bleeding… the time you were not taking TB treatment, you don’t feel anything.’
P2: ‘Then the problem starts when you start TB treatment…’
P1: ‘[ARVs], I never dodge them’
P2: ‘It never happened’
P3: ‘No I don’t default those. This is our free way.’
P1: ‘It’s because before you take ARVs, they tell us tablets might do this and do this, if you don’t take it on time this is what is happening, you know. They even tell us that if you start taking them, they might do this.’
Participants’ accounts exposed the negative impact of adverse TB drug effects on adherence, and the potential ways in which patient education, awareness, and treatment responsibility could trigger a hierarchy of differential adherence in favour of ART.
Greater social morbidity of drug–resistant TB
The social morbidity imposed by patients’ protracted stay within a centralised setting, on account of being diagnosed with drug–resistant TB, emerged during all FGDs. Men, in particular, expressed feeling isolated and trapped as inpatients, away from their families who typically resided in more remote areas. They perceived little need to remain hospitalised once their acute symptoms had abated.
Women felt they had been notified of their diagnosis with XDR–TB in publicly incriminating ways, which instilled fear within their communities. Several patients had been alienated from relatives who became afraid to visit or share physical spaces with those identified as having a deadly and ‘dangerous disease’.
“Yellow car came to my house and ask the surname. She came out and put the gloves on and it was clear that this is bad. When she came inside the house, every thing was special and urgent… My child said, ‘We learned about this at school. It’s better when it’s MDR. When it’s X, it’s the last stage. That is when you are about to die’… I am sick of this vehicle because people knows about it… They were standing on the road, putting these things [i.e., masks] and writing names on the bottles… They don’t even come in the house. They ask while they are outside… [My son] just ran away. He doesn’t want to sleep there. It’s because they told me about XDR in his presence.” (female)
Patients felt further isolated when health care workers avoided coming into direct contact, much less communicating, with them once they were diagnosed with XDR–TB. This instilled provider mistrust and, among some patients, an unwillingness to comply with treatment recommendations.
‘In another hospital I was staying alone. I was given water to wipe myself. When it was time for food, it was a closed house and scary and you can see that nobody comes in here. If she gives you food, she gives it while she is at the door… I felt so bad because I was isolated from other people.’ (female)
‘Why are they confiscating people here… Why they keep us here? They are not giving information. You know if you are sympathetic towards a patient, you are healing her spiritually. But if you come and talk anyhow, I don’t see a need to take tablets.’ (female)
Both men and women expressed how their communities were less aware and consequently less accepting of XDR–TB as compared to HIV. XDR–TB, commonly referred to as ‘big TB’, was perceived to be an ‘incurable’ and ‘killing’ disease. HIV, on the other hand, was understood to ‘attack anyone’. It had become ‘everybody’s food’.
‘You have a disease which can’t be treated, you see yourself at the last stage. It’s [i.e., XDR–TB is] worse than HIV. I’m scared of it more than HIV. To me, HIV is not a problem.’ (female)
Those who had developed XDR–TB subsequent to experiencing an episode of drug–susceptible TB spoke about the difficulty in sharing their new diagnosis with close confidantes.
‘I can choose to talk about HIV. I now prefer to than TB… It’s better if they say I am HIV than this XDR. I had that hope that I am well, I am alive, I don’t have a problem, [but] this XDR came like when we first know about HIV.’ (female)
As a result of the poor perceived outcomes associated with XDR–TB and the greater perceived acceptability towards HIV, co–infected patients felt more comfortable disclosing they had HIV over XDR–TB.
Lack of education and support for XDR–TB
Patients with XDR–TB had spent several months, if not years, being treated for drug–susceptible and/or MDR–TB within a range of health care facilities. Aside from being progressively isolated, many of them did not understand how and when they had progressed to ‘big TB’. Several patients felt demoralised and expressed a desire to be encouraged about their prognosis by health care workers.
‘It’s like we are in a waiting list of dying. It’s wrong… you need to encourage me and console me, show me that this is not the end of the day. I am still going to live, I am going to see the light.’ (male)
The paucity of information about XDR–TB, particularly at the point of case–notification, appeared to exacerbate some patients’ experiences with discrimination and neglect.
‘You find out, as my [co–participant] is saying for [her] not being accepted at home, it’s because if the person from department of health will explain very well to the family, okay this is what’s happening. If you do 1, 2, 3, there won’t be any problem. Don’t just come to old people and tell them there is big TB. Once you talk about big TB, they will start to neglect her. They will do all things they are not supposed to. They will start to notice which cup she is using… There is lack of transparency from government, lack of communication from government, and level of education.’ (female)
Several patients expressed wanting to be educated about XDR–TB, and be given the responsibility to take TB treatment in the same way that they had been counselled for ART.
‘What I can say is that it would be better if they explain about tablets, as you take so many, maybe this is doing this and this is doing this, or just give the information about XDR that it affects you here… no one tells us what is going on with it for that period of two years, is it treatable or not.’ (male)
This intersected with participants’ desire to return home to their families, self–administer medication and be held accountable for their treatment outcomes, as is suggested in this final excerpt between two men.
P1: ‘These XDR tablets we use is the same as ARVs, even if you can stay a month… But if you are sharp, you can learn about these tablets…’
P2: ‘…There is a need for them to give you your pill and take it at home, and if you miss that it means you are throwing your life away.’
P1: ‘Because if you go home, they give you a bottle to spit in. If you do wrong things, your results will tell if you were taking your treatment properly, and if you are not doing well with your treatment, they take you in… because if you don’t take your treatment properly, it will then be you who is asking to go back inside.’
Discussion
To our knowledge, this is the first study to investigate issues underlying differential adherence to concurrent treatment for HIV and drug–resistant TB from the perspective of co–infected patients. We found that the barriers and facilitators to HIV and TB treatment adherence may be unique and warrant distinct examination. While those co–infected with HIV and drug–resistant TB may practice non–adherent behaviour, their behaviour is not always replicated across regimens. In other words, patients who are non–adherent to treatment for drug–resistant TB are not necessarily non–adherent individuals overall. Interventions should consequently target the specific challenges associated with adherence to each regimen, and harness their facilitators, so as to have maximum relevance and impact.
Studies conducted with individuals affected by multiple chronic co–morbidities show that differential adherence to particular medications may be influenced by their perceived side effects and perceived contribution to their overall wellbeing (Mishra, Gioia, Childress, Barnet, & Webster, 2011; Stack, Elliott, Noyce, & Bundy, 2008; Williams & Manias, 2014). Accounts from this study suggest that challenges to adherence for HIV and drug–resistant TB treatment are likely more complex. Together with regimen complexity, pill burden and adverse effects, non–adherence to MDR– and XDR–TB treatment was additionally tied to poor perceived treatment outcomes, social isolation, stigmatisation, and inadequate attention to patient education and support. Indeed, in a recent study comparing treatment experiences of patients receiving treatment for either TB or HIV, patients on TB treatment reported lower scores related to quality of life, social belonging, social support, and symptom control (Corless et al., 2009).
Low adherence to second-line TB medications is not surprising. Patients with XDR–TB take on average 6 antimycobacterial medications (in addition to ART) with numerous significant adverse effects including nausea, skin discoloration, neuropsychiatric effects, renal toxicity, ocular and ototoxicity (Gandhi et al., 2012; O’Donnell et al., 2013). In retrospective studies, between 41 and 58% of patients experience serious side effects (Brust et al., 2013; O’Donnell et al., 2013). In addition, these regimens need to be taken for greater than 18 months (MDR–TB) and greater than 24 months (XDR–TB) with a low probability of successful outcome, particularly in resource-poor settings. Better regimens for drug-resistant TB are therefore urgently needed. For the first time, with the regulatory approval of medications such as bedaquiline and delaminid, such improved drug-resistant TB regimens are on the horizon (Dooley, Nuermberger, & Diacon, 2013).
Our findings urge review of the structural norms that guide treatment provision and monitoring for HIV and TB infections, particularly M/XDR–TB, and their potential to impact clinical outcomes over the long term (Figure 1). The greater attention to patient education and treatment literacy within HIV programs may help explain their high rates of adherence to ART (Abdool Karim, Churchyard, Abdool Karim, & Lawn, 2009), as was seen in our broader study (Padayatchi & O’Donnell, 2011). A lack of patient empowerment within TB programs, perpetuated by treatment supervision, may help explain low rates of patient retention within many TB programs (Abdool Karim et al., 2009; Macq, Torfoss, & Getahun, 2007). A balance between these approaches is likely needed when responding to the compounded threat of co–infection. Our study reinforces the imperative to promote a cohesive model of social support for HIV and TB co–infection, which facilitates patient involvement and ownership, treatment literacy, and instils a commitment to adhere despite the clinical hardships of dual treatment. This is crucial to mitigate the burden of M/XDR–TB in particular, where burgeoning rates of drug resistance are fuelled by non–adherence.
Figure 1.
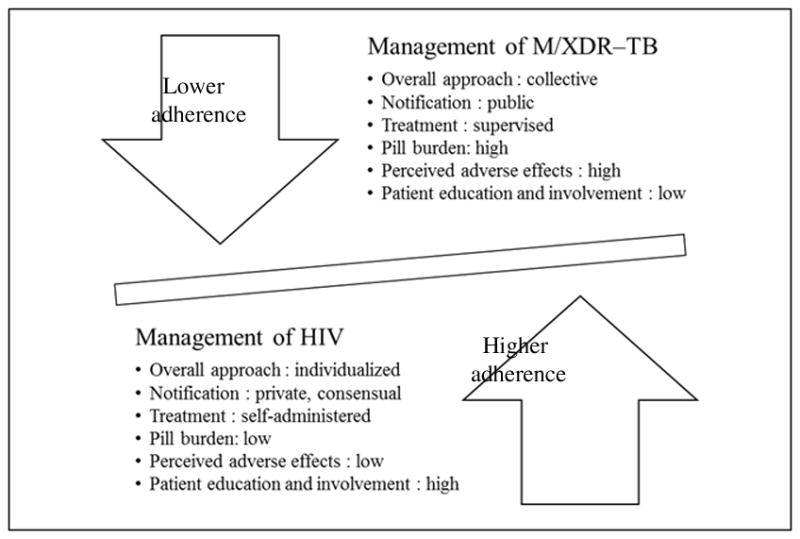
Delineating differential adherence to treatment for HIV and M/XDR–TB
The findings of this supplementary study are bound by our qualitative approach. The small cross–sectional sample and data subjectivity preclude drawing direct associations between participant characteristics and adherence outcomes. The findings may not be generalizable to all persons co–infected with HIV and drug–resistant TB, including those with primary drug–susceptible or latent infection. Our methodology nonetheless allowed for greater patient engagement, and enabled a more comprehensive understanding of treatment related behaviours and decisions that are seldom captured in traditional adherence studies. Emergent themes may inform the distinct approaches that are needed to improve adherence for HIV, TB, and other HIV–related co–morbidities in similar high–burden settings.
Acknowledgments
The authors are grateful to the study participants, study site, Ms. Z. Gwamanda, Ms. N. Depargo, Centre for the AIDS Programme of Research (CAPRISA) at University of KwaZulu–Natal, and Canadian Institutes of Health Research Social Research Centre for HIV Prevention at University of Toronto, for their research contributions and support.
Funding
AD was supported by the Canadian Institutes of Health Research [ZNF-107572]. NP was supported by the Columbia University–Southern African Fogarty AIDS International Training and Research Program [D43TW000231–16S1]. CAPRISA was established as part of the Comprehensive International Program of Research on AIDS [CIPRA; grant no. AI51794] from the U.S. National Institutes of Health (NIH). MO was supported by the NIH National Institute of Allergy and Infectious Disease [NIAID; 5K23AI098479], Albert Einstein Center for Global Health & Clinical and Translational Research Institute, and Stony–Wold Herbert Fund.
Footnotes
Quotes are linked to participants’ gender. Additional characteristics have been withheld to maintain confidentiality and anonymity, given our small sample and narrow time frame.
References
- Abdool Karim SS, Churchyard GJ, Abdool Karim Q, Lawn SD. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. The Lancet. 2009;374(9693):921–933. doi: 10.1016/S0140-6736(09)60916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust JC, Gandhi NR, Carrara H, Osburn G, Padayatchi N. High treatment failure and default rates for patients with multidrug-resistant tuberculosis in KwaZulu-Natal, South Africa, 2000–2003. The International Journal of Tuberculosis and Lung Disease. 2010;14(4):413–419. [PMC free article] [PubMed] [Google Scholar]
- Brust JC, Shah NS, van der Merwe TL, Bamber S, Ning Y, Heo M, Gandhi NR. Adverse events in an integrated home-based treatment program for MDR-TB and HIV in KwaZulu-Natal, South Africa. Journal of Acquired Immune Deficiency Syndromes. 2013;62(4):436–440. doi: 10.1097/QAI.0b013e31828175ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corless IB, Wantland D, Bhengu B, McInerney P, Ncama B, Nicholas PK, Davis SM. HIV and tuberculosis in Durban, South Africa: adherence to two medication regimens. AIDS Care. 2009;21(9):1106–1113. doi: 10.1080/09540120902729932. [DOI] [PubMed] [Google Scholar]
- Dooley KE, Nuermberger EL, Diacon AH. Pipeline of drugs for related diseases: tuberculosis. Current Opinion in HIV and AIDS. 2013;8(6):579–585. doi: 10.1097/COH.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi NR, Andrews JR, Brust JC, Montreuil R, Weissman D, Heo M, Shah NS. Risk factors for mortality among MDR- and XDR-TB patients in a high HIV prevalence setting. The International Journal of Tuberculosis and Lung Disease. 2012;16(1):90–97. doi: 10.5588/ijtld.11.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EM, Sharma S, Peng G, Hullsiek KH, Burman WJ, Macarthur RD, Mannheimer SB. Differential adherence to combination antiretroviral therapy is associated with virological failure with resistance. AIDS. 2008;22(1):75–82. doi: 10.1097/QAD.0b013e3282f366ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn SD, Kranzer K, Wood R. Antiretroviral therapy for control of the HIV-associated tuberculosis epidemic in resource-limited settings. Clinics in Chest Medicine. 2009;30(4):685–699. doi: 10.1016/j.ccm.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macq J, Torfoss T, Getahun H. Patient empowerment in tuberculosis control: reflecting on past documented experiences. Tropical Medicine & International Health. 2007;12(7):873–885. doi: 10.1111/j.1365-3156.2007.01858.x. [DOI] [PubMed] [Google Scholar]
- Mishra SI, Gioia D, Childress S, Barnet B, Webster RL. Adherence to medication regimens among low-income patients with multiple comorbid chronic conditions. Health & Social Work. 2011;36(4):249–258. doi: 10.1093/hsw/36.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell MR, Padayatchi N, Kvasnovsky C, Werner L, Master I, Horsburgh CR., Jr Treatment outcomes for extensively drug-resistant tuberculosis and HIV co-infection. Emerging Infectious Diseases. 2013;19(3):416–424. doi: 10.3201/eid1903.120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padayatchi N, O’Donnell M. Knowledge, attitudes and beliefs and adherence among patients treated for XDR-TB and HIV. 42nd Union World Conference on Lung Health; Lille, France. 2011. (OP-696-28) [Google Scholar]
- Pietersen E, Ignatius E, Streicher EM, Mastrapa B, Padanilam X, Pooran A, Dheda K. Long-term outcomes of patients with extensively drug-resistant tuberculosis in South Africa: a cohort study. The Lancet. 2014;383(9924):1230–1239. doi: 10.1016/S0140-6736(13)62675-6. [DOI] [PubMed] [Google Scholar]
- Seale C, Gobo G, Gubrium JF, Silverman D. Qualitative Research Practice. Thousand Oaks: Sage; 2004. [Google Scholar]
- Stack RJ, Elliott RA, Noyce PR, Bundy C. A qualitative exploration of multiple medicines beliefs in co-morbid diabetes and cardiovascular disease. Diabetic Medicine. 2008;25(10):1204–1210. doi: 10.1111/j.1464-5491.2008.02561.x. [DOI] [PubMed] [Google Scholar]
- WHO. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. Geneva: World Health Organization; 2010. [Google Scholar]
- Williams A, Manias E. Exploring motivation and confidence in taking prescribed medicines in coexisting diseases: a qualitative study. Journal of Clinical Nursing. 2014;23(3–4):471–481. doi: 10.1111/jocn.12171. [DOI] [PubMed] [Google Scholar]


