Abstract
Purpose
To retrospectively evaluate safety and effectiveness of CT-guided percutaneous microwave ablation (MWA) in 47 patients with medically inoperable stage I peripheral non-small cell lung cancer (NSCLC).
Methods
From February 2008 to October 2012, 47 patients with stage I medically inoperable NSCLC were treated in 47 MWA sessions. The clinical outcomes were evaluated. Complications after MWA were also summarized.
Results
At a median follow-up period of 30 months, the median time to the first recurrence was 45.5 months. The local control rates at 1, 3, 5 years after MWA were 96%, 64% and 48%, respectively. The median cancer-specific and median overall survivals were 47.4 months and 33.8 months. The overall survival rates at 1, 2, 3 and 5 years after MWA were 89%, 63%, 43%, and 16 %, respectively. Tumors ≤3.5 cm were associated with better survival than were tumors >3.5 cm. The complications after MWA included pneumothorax (63.8%), hemoptysis (31.9%), pleural effusion (34%), pulmonary infection (14.9%), and bronchopleural fistula (2.1%).
Conclusions
MWA is safe and effective for the treatment of medically inoperable stage I peripheral NSCLC.
Keywords: microwave ablation, non-small cell lung cancer, percutaneous, CT-guided
Introduction
Lung cancer is the leading cause of death among all types of cancers around the world. More than 1, 600, 000 people died worldwide every year [1]. Radical lobectomy is the preferred treatment for stage I non-small cell lung cancer (NSCLC) [2]. And for stages IA and IB NSCLC the 5-year survival rates of surgery has been reported to be 71–77% and 35–58% [3]. However, patients with poor medical conditions such as cardiopulmonary dysfunctions, diabetes, and elder (> 75 years old) are not suitable for surgery. In the last decade, many new local treatment methods, including thermal ablation and the stereotactic radiotherapy, have been developed to treat lung cancer patients who have limited benefit from traditional chemotherapy or radiotherapy [4]. CT-guided percutaneous thermal ablation, including radiofrequency ablation (RFA) and microwave ablation (MWA), has evolved as a minimally invasive treatment option for early stage inoperable lung cancer [5]. This study retrospectively evaluated the safety, effectiveness and complications of MWA as an alternative method for the treatment of stage I medically inoperable NSCLC.
Materials and Methods
Clinical data
From February 2008 to October 2012, 47 patients (29 men, 18 women; mean age 69.4 years, range 56–82 years) with stage IA or IB peripheral NSCLC underwent CT-guided percutaneous MWA in 47 sessions. Patient and tumor characteristics were listed in Table 1. The NSCLC clinical staging was confirmed by contrast CT scan within a mean of 10 days pre-ablation (range 5-15 days). CT-guided biopsy was taken before MWA to get the pathological diagnosis. All patients were evaluated by an interdisciplinary group consisting of radiation oncologist, thoracic surgeon, thoracic radiologist, and medical oncologist. Patients in this study met the following criteria: 1) stage IA or IB (T1-2N0M0), patients with poor lung function (FEV1<1L, FEV1%<50%, MVV<50%); 2) patients who were medically inoperable with renal or heart dysfunction and other comorbid medical conditions (such as severe diabetes); 3) patients who refused surgery; 4) patients who have had previous therapy to the treated lesion were excluded. Patients were informed in detail about the risks and benefits associated with MWA treatment and provided written informed consent for the ablation procedure. Ethics approval to conduct this study was obtained by the Institutional Review Board of Shandong Provincial Hospital Affiliated to Shandong University Hospital.
Table 1. Patient and tumor characteristics.
| Age (mean) 69.4 (56-82) years Follow up (median) 30 ms (7-70) |
No. | % |
|---|---|---|
| Gender | ||
| Male | 30 | 63.8 |
| Female | 17 | 36.2 |
| Pathology | ||
| Adenocarcinoma | 28 | 59.6 |
| Squamous | 13 | 27.7 |
| Other # | 6 | 12.7 |
| Location | ||
| Right Lung | 24 | 51.1 |
| Left Lung | 23 | 48.9 |
| Size | ||
| >3.5cm(3.6-5.0) | 24 | 51.1 |
| ≤3.5cm(2.4-3.5) | 23 | 48.9 |
| Complication | ||
| Pulmonary diseases | 19 | |
| Cardiovascular diseases | 39 | |
| Diabetes | 16 | |
| Renal insufficiency | 2 | |
| Refused surgery | 1 | |
| Local progression | ||
| Yes | 13 | 27.7 |
| No | 34 | 72.3 |
| Death or not | ||
| Yes | 26 | 55.3 |
| No | 21 | 44.7 |
3 patients with adeno-squamous carcinoma; 2 patients with large cell; 1 patient with sarcoma.
CT-guided MWA
Instrumentation
MTC-3C microwave ablation system (Nanjing Qi Ya Research Institute of Microwave Electric, China. Registration standard: YZB/country 1408-2003. NO: SFDA (III) 20073251059) was used for MWA treatment. The microwave emission frequency is 2450±50MHz, output power: 0∼100W. Microwave antenna has effective length of 100-180 mm and 14G--20G outside diameter, with a 15-mm active tip, using water circulation cooling system to reduce the surface temperature of the antenna. CT (GE-lightspeed 64V spiral, USA) was used to guide the MWA and assess the outcomes.
Anesthesia
Local anesthesia and preemptive analgesia were used [6]. Before anesthesia, patients had a preoperative fasting for 12 hours. Patients were intramuscularly injected 10 mg morphine and 10 mg diazepam to achieve anesthesia 30 minutes before the procedure. Patients then had preemptive analgesia through intravenous injection of 50 mg of flurbiprofen axetil 15 minutes before procedure. Another 50 mg of flurbiprofen axetil was injected 8 hours after procedure. The local anesthesia to the location of tumor was conducted by injection of 2% lidocaine.
Procedure
Preoperative localization was confirmed by the observation of CT images and movement of different positions of patients (supine position, lateral position, prone position, etc.). Treatment plan was designed through CT images in which the location-coordinate scale of CT was adhered to the body surface of the tumor area longitudinally. The treatment plan included: 1) to determine the location, size, shape and relation to the nearby organs of lesion; 2) to position the punctured points on body surface; 3) to determine the best entry route from the punctured point to the deepest margin of the lesion (“target skin distance”). In addition, the treatment plan includes selecting the number of microwave antenna (single antenna was used for tumors ≤3.5cm, double antennae were used for tumors 3.6-5.0 cm [7-10]. In this study, 19 tumors used single antenna and 28 tumors used double antennae) and presetting the power and duration for ablation.
After achieving the satisfactory anesthesia, procedure was performed by positioning the antenna into the deepest margin of the lesion according to the preoperative-planned route. CT scan was used to confirm whether the antenna was positioned properly. MWA could be carried out after connected the cold circulating pipes and circulating pumps, linked the MWA antenna and MWA machine with a cable, turned on the power for ablation in accordance with the preset ablation power and duration (generally selected 60-80W, 6-8 min.). The microwave ablation antenna was extracted after the “needle track” was ablated. Then local disinfection was carried out and bandage was used to seal the wound. CT scan was used immediately after the ablation to observe the size, shape and relation to nearby organs of the lesion, as well as to determine if there were any signs of pneumothorax or bleeding, etc. The patients could return back to their wards if they had normal blood pressure, heart rate and oxygen saturation, and no symptoms of hemoptysis, shortness of breath, difficulty in breathing, etc. The patients were given oxygen inhalation and continuously monitored throughout the procedure with electro-cardiography and pulse oximetry. Blood pressure was measured and recorded at 5-minute intervals.
Follow-up imaging and outcome evaluate
All patients received a non-contrast chest CT scan 24-48hs after the ablation to detect the potential complications and ground-glass opacity (GGO). Patients were requested to have serial repeat contrast-enhanced CT (CECT) scans at 3-, 6-, 9- and 12-month intervals. A modified solid tumor evaluation criteria (modified RECIST) was used to evaluate the therapeutic outcome of MWA[11-13]. Local progression was referred to as the contrast-enhancement by CT scans in the site of ablation.
MWA complication assessment
Complication assessment was guided by standards which were set by the International Working Group on Imagine-Guided Tumor Ablation in 2005[14]. 1) Major complications: If clinical symptoms during or after the image-guided ablation were not treated, there would be possibilities for life-threatening safety or leading to substantial damage and dysfunction. Major complications could be also considered as occurred in patients who need hospitalization or prolonged the duration of hospitalization. 2) Minor complications: Those clinical symptoms could be self-limiting, without sequelae, required only a short period of hospitalization for observation or minor-treatments. 3) Side effects were minor complications such as pain, post ablation syndrome, or small asymptomatic hemorrhage or effusion which could be seen on the images.
Statistical analysis
Data analysis was performed by using SPSS 13.0 statistical software. Survival curves for overall was constructed with the Kaplan–Meier method and compared with the log rank test. Comparison between groups was performed by the χ2 test. Statistical significance was set at P <0.05.
Results
Clinical outcome
The median follow-up post-ablation was 30 months (range 7-70 months) with CECT (Figure 1-4). Local progression was identified following 13/47 (27.7%) ablation sessions. There were 9 cases of tumor diameter greater than 3.5 cm appeared in the local progression, and for tumor diameter ≤ 3.5 cm, only 4 had local progression (Table 2). The median time to the first recurrence was 45.5 months (95% CI: 28.8-61.8months). The local control rates at 1,3, 5 years after MWA were 96%, 64% and 48%, respectively. The overall 1-, 2-, 3 and 5-year survivals (Figure 5) were 89%, 63%, 43%, and 16 %, respectively. Median overall survival was 33.8 months (95% CI: 31.9-35.7 months) (Figure 5).The overall 1-, 2-, 3 and 5-year survivals for tumors ≤3.5 cm were 91%, 72%, 59% and 36%, respectively. Tumors ≤3.5 cm were associated with better survival than were tumors >3.5 cm (P=0.016, Figure 6). Seventeen patients died of tumor and the median cancer-specific survival was 47.4 months (95% CI: 25.7-69.1 months) (Figure 7).
Figure 1.
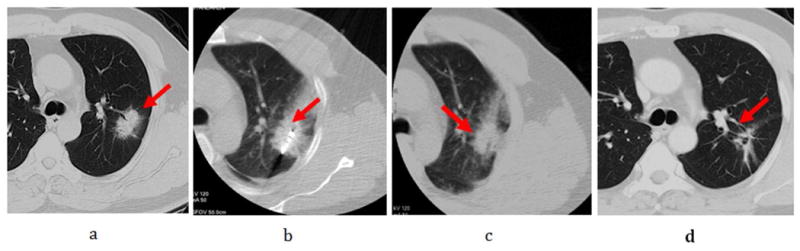
Male, 72-year-old patient with 3.4× 2.0cm left lung cancer (adenocarcinoma) complete response from microwave ablation (MWA) based on modified response evaluation criteria in solid tumors (RECIST) criteria. a. Tumor lesion (arrow) seen on CT immediately prior to MWA; b. The microwave antenna was punctured into lesion (arrow); c. Ablated lesion with surrounding ground-glass opacity seen on repeat CT scan immediately post-MWA. d. Follow-up CT scan at 12 months shows a fibrous scar at the site of the ablated lesion (arrow).
Figure 4.
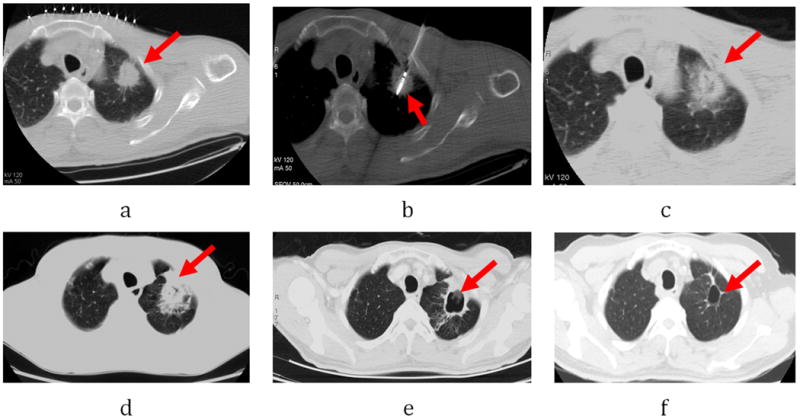
Male, 76-year-old patient with 3.5× 3.0cm left lobe NSCLC (adenocarcinoma) who had complete response from microwave ablation (MWA) based on modified RECIST criteria. (a) Tumor lesion (arrow) seen on CT immediately prior to MWA. b. Microwave antenna puncture into the lesion (arrow). (b) Ablated lesion (arrow) with surrounding ground-glass opacity seen on repeat CT scan 3 h post-MWA. (c) Follow-up CT scan at 1 month shows a cavitating structure developing at the site of the ablated lesion (arrow). (d) CT scan at 6 months shows a cystic structure at the ablation site (arrow). (e) CT scan at 24 months shows the cystic structure shrinking (arrow).
Table 2. 47 cases of patients with local recurrence rate.
| n | Recurrent cases | Recurrence rate % | |
|---|---|---|---|
| Total | (47) | 13 | 27.7 (13/47) |
| 3.6-5.0cm | (24) | 9 | 37.5 (9/24) |
| ≤ 3.5 cm | (23) | 4 | 17.4 (4/23) |
Note: recurrent rate: tumor diameter greater than 3.5 cm group compared with ≤ 3.5 cm, p = 0.037.
Figure 5.
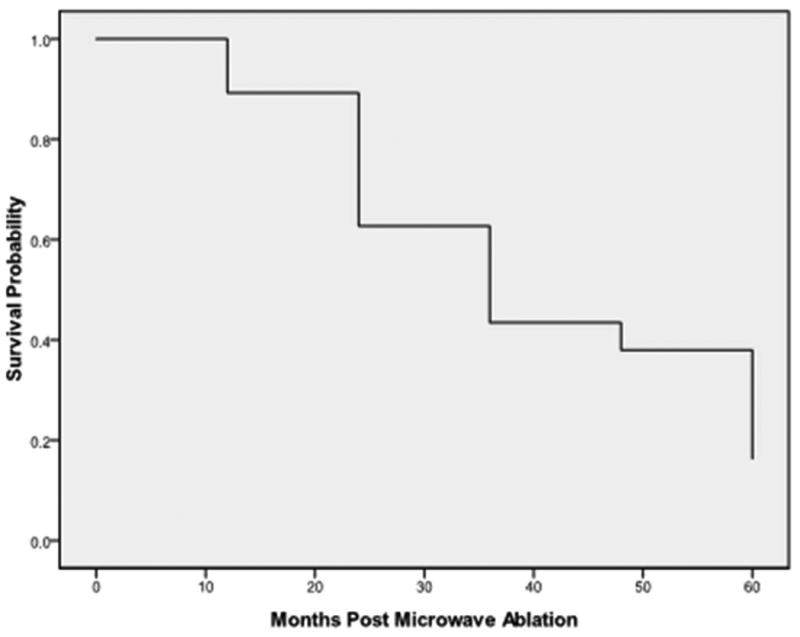
Overall survival in the cohort of 47 patients was 89% at 1 year, 63% at 2 years, 43% at 3 years, and 16 % at 5 years; median survival was 33.8 months.
Figure 6.
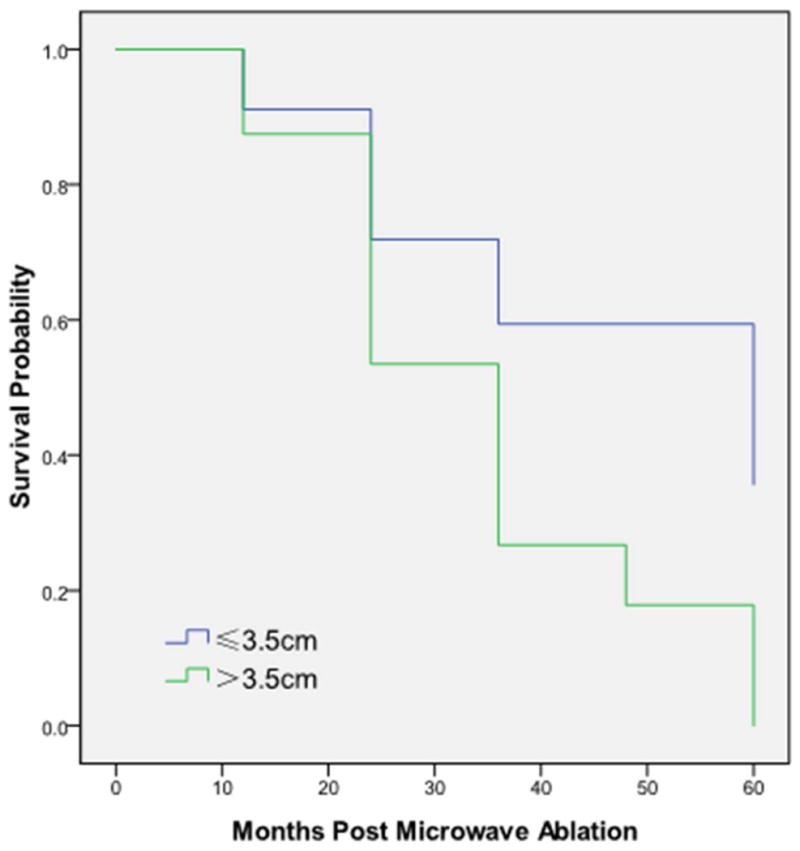
Overall 1-, 2-, 3 and 5-year survivals, stratified by tumor size, were 91%, 72%, 59% and 36% for tumors ≤3.5 cm, and 88%, 53%, 27% and 0% for tumors >3.5cm. (P=0.016).
Figure 7.
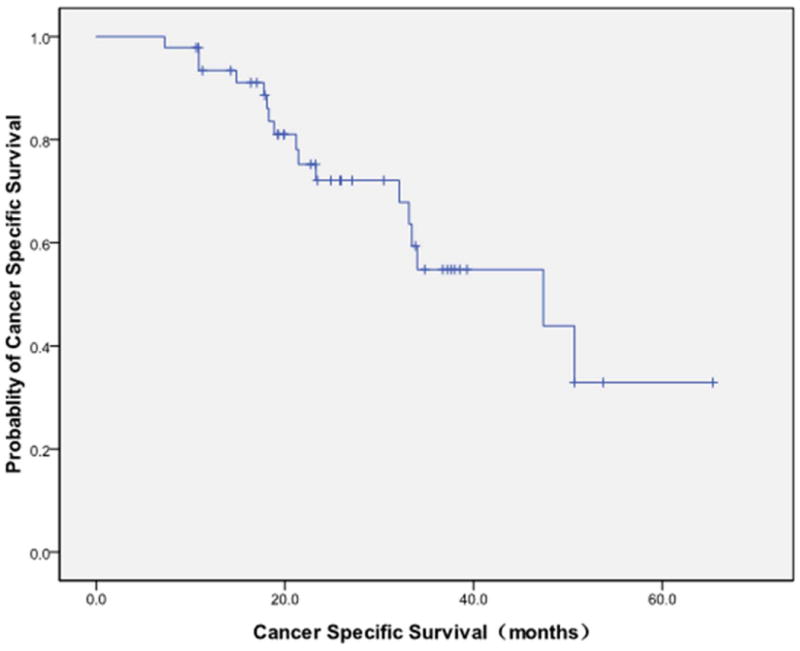
17 patients died of tumor. The median cancer-specific survival was 47.4 months (95% CI: 25.7-69.1 months).
Side effects and complications of MWA treatment
Treatment was completed and well tolerated in all cases. No patient died during the procedure or in 30 days after MWA.
Pain
Pain was the common side effect under the local anesthesia conditions during the procedure. In 47 sessions of 47 patients treated by MWA, 10 sessions had moderate to severe pain, of which 3 sessions were severe. Tumors of these 3 cases were less than 1.5cm close to the chest wall and the procedure was stopped when there was severe pain, following subcutaneous injection of morphine. At the same time, adequate amount of sedatives such as midazolam were intravenously injected. Thirteen patients suffered moderate pain after WMA, but no severe post-ablation pain occurred.
Post-ablation syndrome
The main symptoms were fever (under 38.5°C), fatigue, general malaise, nausea and vomiting etc. Fifteen patients showed these post ablation syndromes.
Complications
Pneumothorax was the most complication. There were a total of 30/47 (63.8%) pneumothorax with 5 patients (13.5%) requiring chest tube drainage. Among 30 cases with pneumothorax, 14 cases were combined with subcutaneous emphysema. Hemoptysis occurred in 15 cases (31.9%) and the conventional application of hemostatic agents including snake venom thrombin, glucocorticoids, could effectively stop bleeding. In 15 cases with hemoptysis, 7 cases occurred in the process of ablation (due to the fact that ablation itself can cause blood coagulation, hemoptysis during ablation process would gradually stop with no special treatment required). There were 16 cases (34%) of pleural effusion (of which 3 cases underwent chest tube insertion). Seven patients (14.9%) suffered from pneumonia after the procedure which could be controlled by effective antibiotics according to the sputum or blood culture. There was 1 case (2.1%) of bronchopleural fistula and the air leak resolved with pleural tube drainage for two months. The average length of hospital stay was 4.7 days (3-19 days). The grade of complications was illustrated in Table 3.
Table 3. Grade of complications during and post MWA.
| Grade | Complications | Number |
|---|---|---|
|
| ||
| Major complications | pneumothorax | 5 |
| pleural effusion | 3 | |
| Bronchopleural fistula | 1 | |
|
| ||
| Minor complications | pneumothorax | 15 |
| pleural effusion | 7 | |
| hemoptysis | 10 | |
| pneumonia | 7 | |
|
| ||
| Adverse events | pain | 20 |
| post-ablation syndrome | 15 | |
| pneumothorax | 10 | |
| pleural effusion | 6 | |
| hemoptysis | 5 | |
Discussion
Recently more and more NSCLC in early stage was diagnosed with the highly developed radiographic techniques. However elder patients with severe cardiopulmonary dysfunction and other concomitant diseases were medically inoperable. The image-guided thermo-ablation including RFA and MWA has been used for these patients. RFA has been indicated to be effective, feasible and minimally invasive in the treatment of early stage NSCLC [15-16]. But there was very few clinical study about the effect and safety of MWA in early stage treatment of NSCLC.
MWA has several advantages over RFA including larger volumes of necrosis in shorter procedure time, less “heat sink” effect for better treatment of perivascular tissue, and maximizing the ablation zone size by positioning multiple MWA antenna into larger lesion simultaneously [17-20]. Based on the substantial advantages for MWA, more patients with pulmonary malignancies were given the MWA treatment as an alternative option.
Wolf et al [21] found that index tumors treated with MWA were larger (mean diameter, 3.5cm±1.6), with a mean size twice that of those tumors treated with RFA (all tumors <4cm; mean diameter, 1.7cm±1.0). In this study, we observed 27.7% local tumor progression at the median follow-up of 30 months after MWA. Liu et al reported the local progression of 15 tumors in 16 MWA sessions for patients with medically inoperable stage I NSCLC. At a median follow-up period of 1 year, the local progression was 31% (5/16) [22]. These results indicated that MWA had good local control for the treatment of stage I NSCLC. Tumors with diameter ≤ 3.5 cm had better local control and overall survival outcome than tumors >3.5cm after MWA. Possible reasons for poor local control to larger tumors include: 1) the irregular tumor shapes were difficult for the ablation antenna to optimize the entry route to completely kill tumor cells, which resulted in the tumor residues; 2) Tumor was too close to large blood vessels, which resulted in tumor residues around the vessel due to the “heat sink” effect. For larger tumors, other treatment methods should be combined with MWA, such as radiotherapy [23].
From the follow-up result of this study, the median overall survival was 33.8 months and the median cancer-specific survival was 47.4 months. The overall 1-, 2-, 3 and 5-year survivals were 89%, 63%, 43%, and 16 %, respectively. Dupuy et al [21] reported a 50-patients clinical study about inoperable lung malignancies with MWA. The 1,2,3-year survivals were 65%, 55% and 45% respectively. These results suggested that MWA was effective in improving the survival of patients with inoperable lung malignancies.
Pneumothorax is the most common complication for MWA treatment. The incidence of pneumothorax for this study was 63.8% (30/47). Although the occurrence rate of pneumothorax was high, only 5 cases required chest tube insertion. Other complications were haemoptysis 31.9%, pleural effusion 34%, pulmonary infection 14.9%, bronchopleural fistula 2.1%, and post-ablation syndrome 31.9%. These side effects and complications mentioned above could be well controlled through observation or proper treatments. There was no 30-day mortality in our patient series. This study suggested that MWA was feasible and safe for the treatment of inoperable early stage NSCLC.
Conclusions
In conclusion, for early stage NSCLC, MWA is safe and effective. It would become an alternative method for the treatment of medically inoperable early stage peripheral NSCLC. There are still many problems to be addressed, such as whether it can replace surgery, how to increase the efficiency by combining MWA technique with other treatment methods, etc. Therefore, more pilot studies with properly designed randomized, prospective and multi-center clinical trials are needed to further confirm these results.
Figure 2.
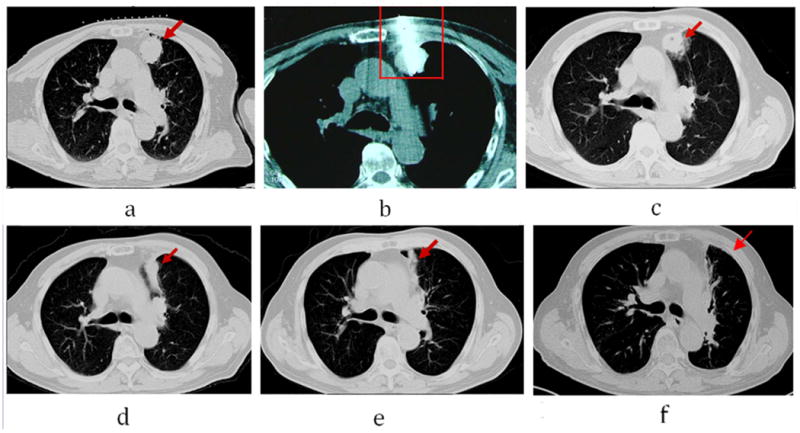
Male, 72-year-old patient with 3.0× 2.5cm left lung cancer (adenocarcinoma) complete response from microwave ablation (MWA) based on modified response evaluation RECIST criteria. a. Tumor lesion (arrow) seen on CT immediately prior to MWA; b. The microwave antenna was punctured into lesion (arrow); c. 6 months after ablation, lesion slightly shrinking (arrow); d: 12 months after ablation, lesions significantly shrinking (arrow); Surrounding ground-glass opacity seen on repeat CT scan immediately post-MWA. d. Follow-up CT scan at 12 months shows a fibrous scar at the site of the ablated lesion (arrow). e. 36 months after ablation, lesion become fiber cord (arrow); f. 60 months after ablation, the lesion disappeared (arrow).
Figure 3.
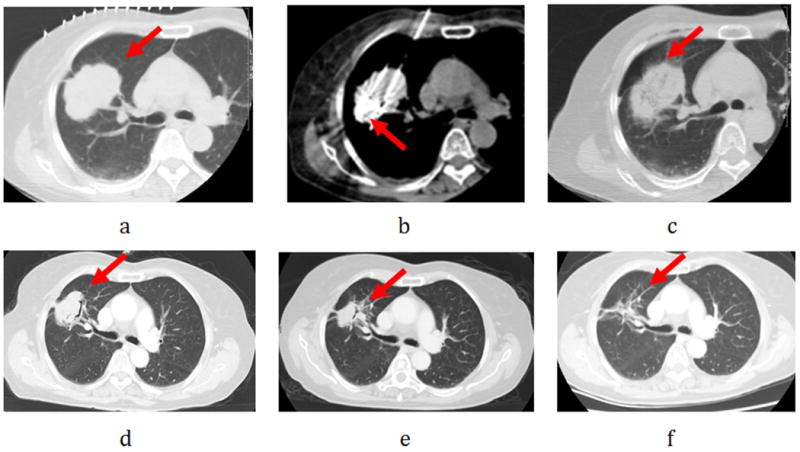
Female, 70-year-old patient with 5.0× 4.5cm right lung cancer (squamous) complete response from microwave ablation (MWA) based on modified response evaluation RECIST criteria. a. Tumor lesion (arrow) seen on CT immediately prior to MWA.; b. Two microwave antenna puncture into the lesion (arrow); c. Ablated lesion with surrounding ground-glass opacity seen on repeat CT scan immediately post-MWA.d.6 months after ablation, lesion slightly shrinking (arrow); e: 12 months after ablation, lesions significantly shrinking (arrow); f: Follow-up CT scan at 24 months shows a fibrous scar at the site of the ablated lesion (arrow).
Synopsis.
The clinical study retrospectively evaluated the safety and effectiveness of CT-guided percutaneous microwave ablation (MWA) in 47 patients with medically inoperable stage I peripheral non-small cell lung cancer (NSCLC). At a median follow-up period of 30 months, the median time to the first recurrence, the local control rates, the median overall survivals and the complications were summarized. It was concluded that MWA was safe and effective for the treatment of medically inoperable stage I peripheral NSCLC.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Das M, Abdelmaksoud MHK, Loo BW, et al. Alternatives to surgery for early stage non-small cell lung cancer-ready for prime time? Curr Treat Options Oncol. 2010;11:24–35. doi: 10.1007/s11864-010-0119-z. [DOI] [PubMed] [Google Scholar]
- 3.Eradat J, Abtin F, Gutierrez A, et al. Evaluation of treatment response after nonoperative therapy for early-stage non-small cell lung carcinoma. Cancer J. 2011;17:38–48. doi: 10.1097/PPO.0b013e31820a0948. [DOI] [PubMed] [Google Scholar]
- 4.Vogl TJ, Naguib NN, Lehnert T, et al. Radiofrequency, microwave and laser ablation of pulmonary neoplasms: Clinical studies and technical considerations-Review article. Eur J Radiol. 2011;77:346–57. doi: 10.1016/j.ejrad.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 5.Dupuy DE. Image-guided thermal ablation of lung malignancies. Radiology. 2011;260:633–55. doi: 10.1148/radiol.11091126. [DOI] [PubMed] [Google Scholar]
- 6.Cliff, Philipp, Robin, et al. The Efficacy of Preemptive Analgesia for Acute Postoperative Pain Management: A Meta-Analysis. Anesth Analg. 2005;100:757–73. doi: 10.1213/01.ANE.0000144428.98767.0E. [DOI] [PubMed] [Google Scholar]
- 7.Crocetti L, Bozzi E, Faviana P, et al. Thermal ablation of lung tissue: in vivo experimental comparison of microwave and radiofrequency. Cardiovasc Intervent Radiol. 2010;33:818–27. doi: 10.1007/s00270-010-9869-z. 9. [DOI] [PubMed] [Google Scholar]
- 8.Dupuy DE. Microwave ablation compared with radiofrequency ablation in lung tissue is microwave not just for popcorn anymore? Radiology. 2009;251:617–8. doi: 10.1148/radiol.2513090129. 10. [DOI] [PubMed] [Google Scholar]
- 9.Lubner MG, Brace CL, Hinshaw JL, et al. Microwave tumor ablation: mechanism of action, clinical results and devices. J Vasc Interv Radiol. 2010;21:S192–203. doi: 10.1016/j.jvir.2010.04.007. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbas G, Pennathur A, Landreneau RJ, et al. Radiofrequency and microwave ablation of lung tumors. J Surg Oncol. 2009;100:645–50. doi: 10.1002/jso.21334. [DOI] [PubMed] [Google Scholar]
- 11.Herrera LJ, Fernando HC, Perry Y, et al. Radiofrequency ablation of pulmonary malignant tumors in nonsurgical candidates. J Thorac Cardiovasc Surg. 2003;125:929–37. doi: 10.1067/mtc.2003.18. [DOI] [PubMed] [Google Scholar]
- 12.Pennathur A, Abbas G, Landren∞u RJ, et al. Radio frequeucy ablation for the treatment of stage I non-small cell lung neoplasm. Semin Thorac Cardiovasc Surg. 2008;20:279–284. doi: 10.1053/j.semtcvs.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Casal RF, Tam AL, Eapen GA, et al. Radiofrequency ablation of lung tumors. Clin Chest Med. 2010;31:151–163. doi: 10.1016/j.ccm.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg N, Cardella G, Cardella JF, et al. Image-guided Tumor Ablation Standardization of Terminology and Reporting Criteria1. Radiology. 2005;235:728–739. doi: 10.1148/radiol.2353042205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics. 2005;25:S69–83. doi: 10.1148/rg.25si055501. [DOI] [PubMed] [Google Scholar]
- 16.Dupuy DE. Image-guided thermal ablation of lung malignancies. Radiology. 2011;260:633–55. doi: 10.1148/radiol.11091126. [DOI] [PubMed] [Google Scholar]
- 17.Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol. 2009;38:135–43. doi: 10.1067/j.cpradiol.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbas G, Schuchert MJ, Pennathur A, et al. Ablative treatments for lung tumors:radiofrequency ablation, stereotactic radiosurgery, and microwave ablation. Thorac Surg Clin. 2007;17:261–71. doi: 10.1016/j.thorsurg.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Wolf FJ, Aswad B, Dupuy DE, et al. Intraoperative microwave ablation of pulmonary malignancies with tumor permittivity feedback control: ablation and resection study in 10 consecutive patients. Radiology. 2012;262:353–60. doi: 10.1148/radiol.11110015. [DOI] [PubMed] [Google Scholar]
- 20.Sonntag PD, Hinshaw JL, Lubner MG, et al. Thermal ablation of lung tumors. Surg Oncol Clin NAm. 2011;20:369–87. doi: 10.1016/j.soc.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf FJ, Grand DJ, Machan JT, et al. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology. 2008;247(3):871–879. doi: 10.1148/radiol.2473070996. [DOI] [PubMed] [Google Scholar]
- 22.Liu H, Steinke K. High-powered percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: a preliminary study. J Med Imaging Radiat Oncol. 2013 Aug;57(4):466–74. doi: 10.1111/1754-9485.12068. [DOI] [PubMed] [Google Scholar]
- 23.Dupuy DE, DiPetrillo T, Gandhi S, et al. Radiofrequency ablation followed by conventional radiotherapy for medically inoperable stage I non-small cell lung cancer. Chest. 2006;129:738–745. doi: 10.1378/chest.129.3.738. [DOI] [PubMed] [Google Scholar]


