Abstract
Introduction
The liver plays a central role in transforming and clearing foreign substances. The continuous exposure of the liver to xenobiotics sometimes leads to impaired liver function, referred to as drug-induced liver injury (DILI). The pregnane X receptor (PXR) tightly regulates the expression of genes in the hepatic drug-clearance system and its undesired activation plays a role in DILI.
Areas covered
This review focuses on the recent progress in understanding PXR-mediated DILI and highlights the efforts made to assess and manage PXR-mediated DILI during drug development.
Expert opinion
Future efforts are needed to further elucidate the mechanisms of PXR-mediated liver injury, including the epigenetic regulation and polymorphisms of PXR. Novel in vitro models containing functional PXR could improve our ability to predict and assess DILI during drug development. PXR inhibitors may provide chemical tools to validate the potential of PXR as a therapetic target and to develop drugs to be used in the clinic to manage PXR-mediated DILI.
Keywords: pregnane X receptor, xenobiotic detoxification, drug-induced liver injury, xenobiotic receptor, adverse drug reaction
1. Introduction
The liver plays a central role in transforming and clearing foreign substances (also known as xenobiotics) through the detoxification system in order to protect the human body from possible toxicity. Continuous exposure of the liver to xenobiotics during drug treatment makes this organ susceptible to toxicity. Drug-induced liver injury (DILI) is, therefore, defined as injury to the liver associated with impaired liver function by drug treatment [1], which is the leading cause of acute liver failure and one of the severe adverse drug reactions (ADRs) [2, 3]. The overall incidence of DILI in the general population is largely unknown due to under-reporting, but a crude incidence rate is estimated to be 14 per 100,000 inhabitants per year [4]. The incidence of DILI of an individual drug is also largely unknown but is figured to be between 1 in 10,000 and 1 in 100,000 patients per year for most clinically used drugs [5]. DILI is uncommon, but it is hard to effectively prevent and treat, and it can be life-threatening for patients, who may have to be referred for liver transplantation [6].
DILI is a leading cause for previously approved drugs to be withdrawn from the market and for the discontinuation of drugs in development [7]. Although this complication may not occur in clinical trials with limited numbers of participants because of its low incidence rate, rare cases of DILI may arise after a drug is approved for clinical use because of the larger number of patients. If a drug is found to cause even rare hepatotoxicity, it may be withdrawn from the clinic, regardless of its use by millions [1]. DILI, therefore, is considered to be a liability risk and may lead to financial losses during drug development [8–10].
The hepatic drug-clearance system, which includes drug-metabolizing enzymes (DMEs) and transporters, tightly controls the detoxification and elimination of drugs in the liver. Phase I enzymes such as cytochrome P450 enzymes (CYPs) are mainly responsible for the first step of the detoxification of organic compounds, which involves catalyzing hydroxylation or oxidation reactions to convert lipophilic drugs into more soluble derivatives that are suitable for excretion from the body [11–13]. Phase II conjugation reactions add additional polar functional groups onto xenobiotics to produce water-soluble, inactive metabolites facilitating biliary and urinary excretion, which is catalyzed by a large group of transferases such as sulfotransferase (SULT), glutathione S-transferase (GST), and UDP-glucuronosyltransferase (UGT) [14]. The excretion process is regulated by members of the ATP-binding cassette (ABC) transporter family and the solute carrier family [15, 16].
The pregnane X receptor (PXR), predominantly expressed in the liver, regulates the expression of a large number of genes in the hepatic drug-clearance system, such as the human CYP3A4 isoenzyme, UDP-glucuronosyltransferase 1 family polypeptide A1 (UGT1A1), and multidrug resistance protein 1 [MDR1, also known as P-glycoprotein (P-gp) or ABCB1] [17, 18]. In the absence of xenobiotics, genes in the hepatic drug-clearance system are expressed at the minimal basal levels. PXR is regarded as a master xenobiotic sensor, which can bind to various structurally-diverse chemicals to rapidly induce the expression of DMEs and transporters, ultimately leading to the detoxification of xenobiotics [19]. PXR displays structural features commonly found in other nuclear receptors [20]. The N-terminal DNA-binding domain (DBD) interacts with hormone response elements (HREs) via two zinc-finger motifs. The ligand-binding domain (LBD) of PXR is depicted as a flexible and large cavity that can accommodate a wide range of compounds of differing size and chemical structure, which accounts for the promiscuous nature of PXR.
A variety of mechanisms contribute to DILI, such as the generation of reactive metabolites, the activation of stress signaling, mitochondrial dysfunction, and immunological response [21]. CYP-dependent formation of reactive metabolites is one of the most common causes of DILI [10]. The reactive metabolites are either hepatotoxic by themselves or can covalently bind to hepatic proteins to alter their function and become hepatotoxic [22]. Other potential mechanisms of DILI include inhibition of mitochondrial functions via accumulation of reactive oxygen species, inhibition of drug metabolism pathways, inhibition of bile acid transport, and immune responses [23, 24]. A recent correlation study showed that drugs significantly metabolized by the liver (>50% amount of the compound metabolized in the liver) are more likely to be associated with hepatotoxicity [25], suggesting a critical role for the hepatic drug-clearance system in DILI. PXR predominantly functions in the liver and tightly regulates the expression of genes in the hepatic drug-clearance system. During drug treatment, PXR can contribute to significant ADRs, including drug-drug interactions (DDIs) and DILI, leading to severe clinical ramifications [26, 27]. This review focuses on the recent advances in understanding PXR-mediated DILI and highlights the efforts made to evaluate and manage PXR-mediated DILI during drug development.
2. Clinical classification of DILI
Hepatotoxicity is classified into three major categories based on its clinical presentation: hepatocellular injury (caused by specific injury to hepatocytes), cholestatic injury (a result of specific toxicity to biliary epithelial cells and/or bile pumps), or mixed pattern of injury [1]. In general, the clinical significance of liver injury correlates to the levels of serum alanine aminotransferase (ALT), alkaline phosphatase (ALP), or total bilirubin (TBL) measured with clinical chemistry assays indicating liver biochemical and functional profiles [1]. Acute hepatocellular injury is defined as an ALT greater than twice the upper limit of the normal range and/or an ALT/ALP ratio greater than or equal to 5 [1]. Acute cholestatic liver injury is defined as a serum ALP level greater than twice the upper limit of the normal range and/or an ALT/ALP ratio less than 2 [1]. Mixed injury is the designation if the ALT/ALP ratio is higher than 2 but less than 5 [1].
Each pattern of DILI can be attributed to the action of the drug causing it and is associated with a different mechanism of injury. A predominantly hepatocellular pattern of damage is usually observed when the stress of xenobiotics is mediated via mechanisms that directly damage the hepatocyte. Acute hepatocellular injury is the most common form of DILI, accounting for approximately 90% of cases [28], and is associated with mortality rates of 10%–50% [29, 30]. For example, CYPs mediate high-energy reactions that lead to covalent binding of drugs and/or metabolites to extracellular proteins, eliciting the adaptive immune response and contributing to the apoptosis of hepatic cells (also known as “hapten hypothesis”) [31, 32]. In addition, the reactive metabolites produced by CYPs cause oxidative stress and the depletion of glutathione (GSH) and are associated with hepatocellular injury [22].
A cholestatic pattern of liver injury is mainly associated with injury of biliary epithelium or impairment of bile transport rather than hepatocellular cell death. Cholestasis is characterized by accumulation of bile acids in liver because of impaired bile flow. Drug-induced cholestasis can be caused by the inhibition of the uptake and efflux systems for hepatobiliary bilirubin or bile acid [33]. For example, drugs or their metabolites that directly or indirectly inhibit the bile salt efflux pump (BSEP) can result in cholestasis because of the dysfunction of hepatocytes and/or biliary epithelial cells on bile formation and excretion; one such drug is cyclosporin A [34]. However, a mixed pattern of DILI may occur in such cases because the injury patterns are not mutually exclusive.
3. PXR-mediated hepatotoxicity and its underlying mechanisms
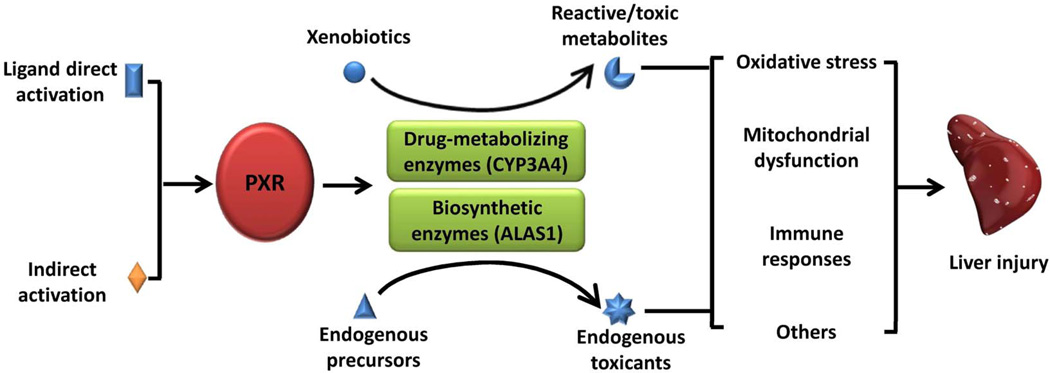
A growing body of evidence indicates that some PXR agonists used in the clinic can lead to different clinicopathological subtypes of hepatotoxicity, suggesting that PXR plays a role in DILI. At least two types of mechanisms could explain PXR-mediated DILI during therapy (Figure 1): 1) PXR agonist activates PXR-regulated expression of DMEs and transporters, contributing to the formation of toxic metabolites; and/or 2) PXR agonists activate the expression of critical liver enzymes in major metabolic pathways that may alter the balance of endobiotic formation and clearance, leading to accumulation of endogenous toxicants.
Figure 1.
A proposed mechanistic mode for PXR-mediated DILI.
3.1 Rifampicin
Rifampicin (Rif) is a prototypical hPXR agonist (but not an agonist of mouse PXR [mPXR]) and is used as a first-line drug to treat tuberculosis (TB). Rif-induced severe hepatotoxicity is rare, with an incidence of less than 1.1% when used alone; however, in combination with isoniazid (another first-line anti-TB drug), the hepatotoxicity incidence significantly increases to 5% – 8%, occurring more frequently and earlier than it does with either medication alone [35]. The toxic metabolites of isoniazid, acetylhydrazine and hydrazine, were previously thought to be associated with its induced hepatotoxicity [35]. However, a recent study showed that co-administration of Rif and isoniazid increased the amount of protoporphyrin IX (PPIX, a heme precursor) in the bile through an hPXR-mediated mechanism, that leads to higher levels of ALT, ALP, and bile plugs in humanized PXR mice containing hPXR than in wild type mice containing mPXR or Pxr-null mice containing neither hPXR nor mPXR [36]. A previous study has shown that the nuclear receptors, PXR and constitutive androstane receptor, can activate the expression of aminolevulinic acid synthase 1 (ALAS1), a rate-limiting enzyme for PPIX production [37]. Thus, PXR-mediated increase of ALAS1 expression could be an underlying mechanism for the Rif-and isoniazid–induced hepatotoxicity.
3.2 Acetaminophen
Acetaminophen (APAP) overdose accounts for 50% of cases of drug-induced acute liver failure in the clinic and represents an example of dose-dependent hepatotoxin [38]. The mechanism of APAP-induced hepatotoxicity is well-characterized and involves the formation of a highly reactive intermediate metabolite, N-acetyl-p-benzoquinoneimine (NAPQ1), by CYP3A4, CYP2E1, or CYP1A2 [39]. At high doses of APAP, increased levels of NAPQ1 in the hepatocytes can result in depletion of GSH, induction of oxidative stress reactions, dysfunctions of mitochondria, and DNA damage, eventually leading to cell damage. Early studies showed that co-administration of phenobarbital and dexamethasone (both are PXR activators) with APAP increases the level of hepatotoxicity in mice, suggesting that PXR may play a role in APAP-induced hepatotoxicity [40, 41]. Another study demonstrated that pregnenolone 16α-carbonitrile (an mPXR agonist) markedly augments APAP-induced liver injury in wild-type mice but not in Pxr-null mice [42]. Further mechanistic studies revealed that the induction of CYP3A11 or CYP1A2 by ligand-activated PXR increases the formation of toxic metabolite NAPQ1 in mouse liver, leading to hepatotoxicity [42, 43]. In a humanized PXR and CYP3A4 mouse model (TgCYP3A4/hPXR), rifampicin (an hPXR agonist) can activate hPXR to enhance APAP-induced hepatotoxicity via CYP3A4 induction, suggesting that the activity and level of hPXR might be a contributing factor in APAP-induced liver injury [44].
3.3 Flucloxacillin
Flucloxacillin is an antibiotic associated with cholestatic hepatotoxicity and is also a reported PXR agonist that can induce CYP3A4 expression at pharmacologically relevant concentrations [45, 46]. The CYP3A4-generated 5’hydroxymethyl metabolite of flucloxacillin is selectively toxic to biliary epithelial cells but not to hepatocytes [47], suggesting that the activation of PXR by this compound could contribute to the cholestatic pattern of liver injury. However, a human PXR polymorphisms study showed that the polymorphism C-25385T (rs3814055) is associated with an increased risk of flucloxacillin-induced hepatotoxicity in humans [46]. Yet, patients with homozygous expression of C-25385T have lower expression of PXR and decreased induction of CYP3A4 expression, suggesting that higher levels of the parental compound are present in liver. Because flucloxacillin can form adducts that elicit an immune response to induce the apoptosis of hepatic cells [48], the reduced amount of PXR in those with the C-25385T substitution could lead to hepatocellular injury. These studies also indicate that PXR may have a dichotomous role in flucloxacillin-induced hepatotoxicity.
3.4 Troglitazone
Troglitazone, a peroxisome proliferator–activated receptor gamma (PPARγ) agonist, was approved for the treatment of type 2 diabetes in 1997 and was withdrawn from the market in 2000 due to its severe hepatotoxicity [49]. The exact mechanism of troglitazone-induced hepatotoxicity is still unclear; however, the reactive metabolites produced during troglitazone metabolism could be involved in the liver injury. The results of in vitro biochemical and cellular assays indicate that troglitazone can be metabolized by CYP3A4 at the thiazolidinedione moiety to form reactive intermediates [50]. The reactive metabolites, quinone and 0-quinone methide, can covalently bind to liver microsomal proteins and GSH, leading to severe hepatocellular damage [50]. Intriguingly, troglitazone can not only activate PPARγ but is also a prototypical PXR agonist [51] and can strongly activate PXR-mediated CYP3A4 expression [52, 53]. Thus, troglitazone-induced PXR activation might be an underlying mechanism for its hepatotoxicity and merits further investigation.
3.5 Phenytoin
Phenytoin is an anticonvulsant widely used for epilepsy and is associated with liver injury [54]. Phenytoin metabolism is associated with the production of reactive oxygen species and depletion of hepatic glutathione, leading to the damage of mitochondria in hepatic cells [55]. The formation of reactive metabolites could contribute to the hepatotoxicity of phenytoin. The CYP2C9-generated reactive metabolite of phenytoin, 5-(p-hydroxyphenyl),5-phenylhydantoin (HPPH), is further oxidized to generate catechol, which then forms protein adducts in the liver to elicit immune responses [56]. PXR can activate CYP2C9 expression [57, 58], and phenytoin can moderately activate PXR target gene expression, including CYP3A4 and CYP2C9 [56, 59–61]. Therefore, PXR-mediated increase of CYP2C9 could be an underling mechanism for phenytoin-induced hepatotoxicity during either phenytoin monotherapy or phenytoin combination therapy with PXR agonists.
4. In vitro and in vivo models to predict PXR-mediated hepatotoxicity
Because PXR plays a contributing role in DILI, in vitro models with PXR-mediated induction of DMEs and transporters, can be used to predict in vivo PXR-mediated hepatotoxicity. A number of cell-based models stably expressing hPXR have been developed for assessing xenobiotic-induced PXR activation [62, 63]. In such cellular systems, the expression of reporter gene driven by the PXR responsive element can indicate the transcriptional activity of PXR. Traditionally, liver-related in vitro models are used for the prediction of in vivo DILI, including liver microsomes, hepatic cell lines, primary human hepatocytes (PHHs), and liver slices [64]. However, there are very limited examples using hepatic cell lines stably expressing hPXR to successfully evaluate PXR-mediated DILI, partially because PXR in these cell lines induces to a lower degree phases I and II DMEs than does PXR in PHHs or intact human liver [64]; such low levels of phases I and II DMEs may not generate sufficient levels of toxic metabolite to induce liver injury in certain treatment period. PHHs have been used as the in vitro “gold standard” for predicting DILI, and the prediction correlates to in vivo hepatotoxicity [65, 66], because PHHs retain high levels of hPXR-induced DMEs and transporters with functional activities. For example, a high content screening (HCS) approach improved significantly the ability of in vitro system to predict in vivo DILI [67, 68]. More recently, a quantitative HTS method has been developed in a 1536-well-plate format to successfully assess DILI risk using cryopreserved human hepatocytes by evaluating cell viability [69]. However, several disadvantages of PHHs limit its use to predict DILI in vitro, including short-term viability, limited availability, batch-to-batch variability among different donors, and dedifferentiation leading to the lack of relevant gene expression. Therefore, new in vitro models with the following features are needed to evaluate hPXR-mediated DILI: 1) retention of major liver functions and high metabolic CYP activities induced by PXR; 2) suitability for long-term and repeated compound exposures; 3) high availability and easy management. Three-dimensional (3D) cell culture systems using hepatic cell lines and induced pluripotent stem (iPS) cells may be promising in vitro systems to assess PXR-mediated DILI [70–72].
Several mouse models that were developed to study the in vivo function of hPXR are also suitable for the in vivo evaluation of hPXR-mediated DILI. Ligand selectivity between hPXR and mPXR occurs because of the significant differences in amino acid sequences of the receptors’ ligand-binding domains (LBDs) [73]. For instance, rifampicin strongly activates hPXR but not mPXR, whereas 5-pregnen-3β-ol-20-one-16α-carbonitrile (PCN) is a potent mPXR agonist but activates hPXR to a lower degree [74]. Thus, the humanized PXR mouse models can be used for in vivo investigation of hPXR-mediated DILI. In the first generation of hPXR mouse model, the hPXR gene was randomly integrated into the mouse genome, with the mPXR gene deleted and the hPXR gene under the control of either the liver-specific albumin promoter [75] or the rat fatty acid–binding protein promoter [76]. Likewise, in the second-generation hPXR mouse model, a genomic fragment containing the entire hPXR gene and its promoter was randomly integrated into the mouse genome in a Pxr-null background [77]. In order to further improve this mouse model, a double transgenic mouse model expressing hPXR and CYP3A4 was generated by using bacterial artificial chromosome transgenesis in Pxr-null mice. In this double transgenic mouse model, rifampicin treatment robustly induces CYP3A4, mimicking the human response to rifampicin [78]. The latest hPXR mouse model was developed through knock-in strategies by simultaneously replacing the mPXR gene with the hPXR gene under the control of the endogenous mouse promoter [79]. These new humanized PXR mouse models express hPXR at a physiological level and respond well to PXR agonists. By using the second-generation hPXR mouse model and the hPXR-CYP3A4 double transgenic mouse model, researchers recently showed that hPXR plays a role in both acetaminophen- and RIF-induced hepatotoxicity, highlighting the potential of these mice as in vivo models for evaluating hPXR-mediated hepatotoxicity during drug development [36, 44].
5. PXR as a potential target to manage drug-induced liver injury
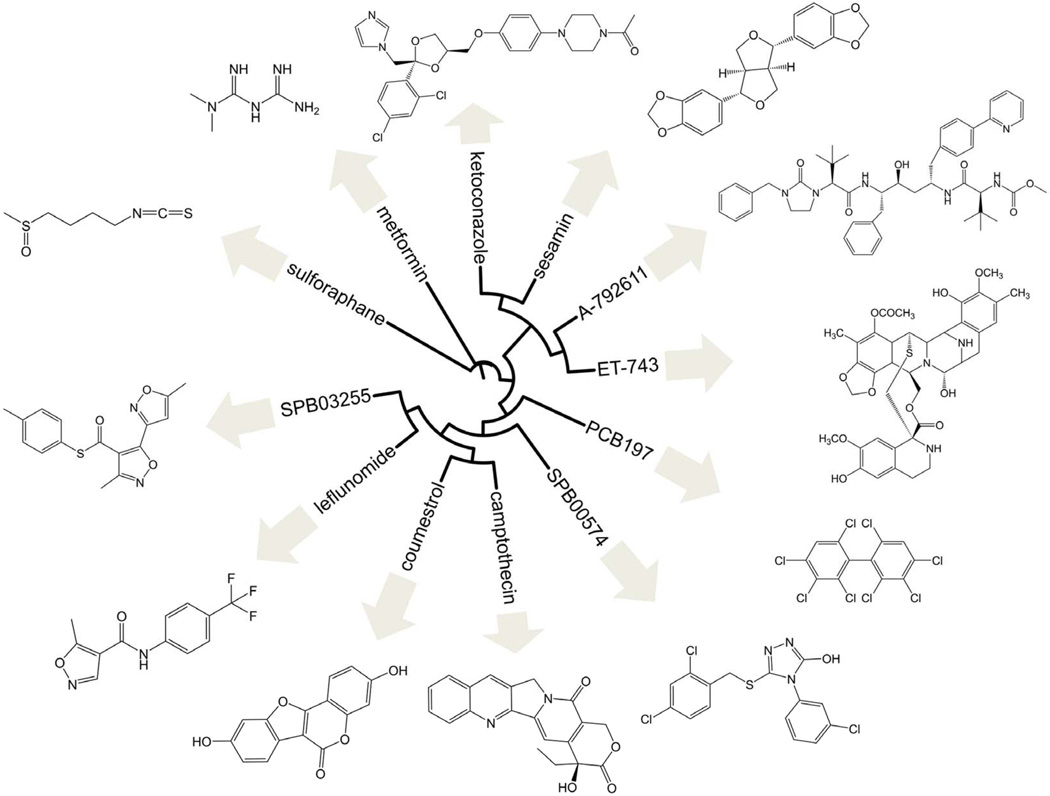
The finding that undesirable activation of PXR by xenobiotics contributes to DILI provides a rationale for developing therapeutics that counteract PXR activation to prevent DILI, therefore, future preclinical or clinical experiments are needed to determine how pharmacologically counteracting activated-PXR can prevent or ameliorate DILI. The activity of a nuclear receptor can be repressed by a number of mechanisms. An inhibitor is generally regarded as any entity that blocks the response generated by an agonist, regardless of whether it competes with the activating ligand for receptor binding. In contrast, an antagonist displaces the binding of an agonist to the ligand binding pocket of the receptor. The antagonist by itself has little or no effect on the receptor. Although these definitions are loosely defined in the literature, the designations used in this manuscript correspond to those as reported in the original sources when possible. It is noteworthy to mention that there are several other ways to abrogate the function of a receptor. For example, a compound can bind to the outer surface of the receptor distinct from the ligand binding pocket, blocking (either directly or allosterically) the recruitment of partner proteins such as co-activators or retinoid X receptor (RXR) or heightening the interactions with a co-repressor. Alternatively, it is plausible to modulate upstream events such as posttranslational modifications of the receptor that eventually lead to a reduction of its target genes. Based on the relatively large numbers of PXR inhibitors reported, it is evident that there is great emphasis in the development of PXR modulators that can suppress its activity. These compounds display structural diversity as illustrated in Figure 2, which summarizes the PXR inhibitors in hierarchical clustering of structures based on similarity using ChemMine [80] and Interactive Tree of Life [81].
Figure 2.
Reported PXR inhibitors. Hierarchical clustering of structures based on similarity using ChemMine [80] and Interactive Tree of Life [81].
Ecteinascidin 743 (ET 743) was the first reported compound to inhibit PXR transactivation, displaying high potency with an EC50 value of 3 nM [82]. This natural product derived from the marine-sourced Caribbean tunicate Ecteinascidia turbinata and belonging to the tetrahydroisoquinoline chemical class can repress the induction of CYP3A4 and MDR1 by the PXR agonist SR12813. However, ET 743’s practical use as a PXR inhibitor is very limited because of its potent cytotoxic properties: its antitumor activity was ascribed to its binding to the minor groove of DNA, perturbation of the cell cycle, ability to cause microtubule disorganization, and interference with DNA repair pathways [83].
Among all the compounds that inhibit PXR transactivation reported up to now, the most-investigated are the azole class of chemicals, including ketoconazole and its derivatives [84]. The antifungal ketoconazole disrupts co-activator and co-repressor recruitment without affecting the interactions of PXR with ligands, DNA, or its heterodimeric partner RXR [85]. PXR remained functional with the double mutations T248E/K277Q, even though those residues are important for interactions with the activation function 2 (AF-2) helix, and the activity of PXR was not altered by ketoconazole. Ketoconazole analogs, notably FLB-12, have been synthesized to have reduced toxicity, CYP3A4 inhibition, and effects on other nuclear receptors, while retaining similar PXR-inhibition potencies, which normally lie in the 10–20 µM range [86, 87].
Polychlorinated biphenyls (PCBs) are ubiquitous organic compounds that tend to accumulate in tissues because of their lipophilic nature [88]. It was noticed that highly chlorinated PCBs could activate mPXR but not the human ortholog. Some of these molecules antagonize PXR at the submicromolar range in cell-based assays, with PCB 197 being among the most potent and having a Ki of 0.6 µM. They were also shown to displace a radiolabeled PXR agonist in purified protein fractions, suggesting that they bind directly to PXR. Structure-activity studies indicate that distinct chlorine arrangements in the biphenyl backbone strikingly differentiate antagonists from inactive congeners [88]. Although PCBs exhibit adverse health effects, further development on this class of PXR inhibitors may improve their safety with the potential for therapeutic purposes.
Pharmacophore studies of PXR antagonists indicate that potential inhibitors can bind the AF-2 surface, taking advantage of both hydrophobic and hydrogen bond–acceptor interactions in a small pocket, where relatively small compounds are predicted to bind [89]. Based on this finding, a group of novel PXR inhibitors were discovered as what the authors claimed to be the first such strategy involving docking approaches [90]. SPB03255 and SPB00574 were among the most notable inhibitors, with cell-based assays indicating IC50 values of 6.3 and 24.8 µM, respectively. Leflunomide, an FDA-approved drug used as an antirheumatic agent to treat rheumatoid arthritis and psoriatic arthritis, has a substructure similar to SPB03255 and SPB00574 and abrogates PXR activity with an IC50 value of 6.8 µM. The study’s authors hailed it to be the first of such FDA-approved compounds to be repurposed for PXR inhibition [90].
Metformin is another marketed drug that is reported to inhibit PXR activity, although very high concentrations of up to 2 mM were used to observe clear effects [91]. This biguanide compound is used as an antihyperglycemic agent in the treatment of diabetes. The authors stipulate that metformin does not suppress PXR activity due to the induction of small heterodimer partners (SHPs) or the involvement of AMP-activated protein kinase (AMPK) activation, biological processes that were previously described to be affected by metformin. Instead, the compound was hypothesized to disrupt co-activator recruitment, as shown by the results of experiments in two-hybrid systems using wild-type PXR and the constitutively active S247W/C284W PXR mutant. Metformin also represses CYP3A4 upregulation by other nuclear receptors, including constitutive androstane receptor (CAR).
The HIV protease inhibitor A-792611 represses PXR activation, with an IC50 value of approximately 2 µM in cell-based transactivation assays [92]. It seemed to be fairly selective for PXR, as it did not significantly induce nor antagonize farnesoid X receptor (FXR), CAR, vitamin D receptor (VDR), or PPARα. According to microarray studies, there was downregulation of CYP3A4, CYP2B6, CYP2C8, CYP2C9 and MDR1. Oddly, A-792611 is metabolized and also inhibits CYP3A4, and it is part of a class of HIV protease inhibitors that are known for inducing PXR activity.
The phytochemical isothiocyanate sulforaphane, which is abundant in certain cruciferous vegetables, was the first naturally occurring PXR antagonist to be reported that is present in a modern diet. In cell-based assays, this compound has IC50 values of 12–14 µM, showing minimal ligand-dependent activation of mouse or rat PXR or of human CAR, VDR, PPARα, and PPARγ. Sulforaphane was inferred to bind to the PXR LBD on the basis of scintillation proximity assays yielding a Ki of 16 µM, which would result in the disruption of co-activator recruitment as observed in the mammalian two-hybrid assay [93]. Computational results suggest that the molecule may bind the AF-2 domain, albeit very weakly [90]. Interestingly, sulforaphane and other analogs activate phase II detoxification enzymes through the Nrf2/Keap1 pathway [94]. As expected, this compound exhibits low toxicity, making it suitable as a drug candidate. In a human clinical study, the in vivo antagonistic efficacy of sulforaphane was refuted; the authors indicated dosing and pharmacokinetics as being potential causes for the discrepancies between in vitro and in vivo studies [95]. However, sulforaphane is still believed to hold promise in vivo because reduced basal CYP3A4 activity was noticed in a population subset.
Coumestrol, a phytoestrogen prevalent in legumes and soy beans, is another natural-sourced chemical that affects PXR transcriptional regulation [96]. In competitive ligand binding assays of radiolabeled agonists, coumestrol competes for binding to the PXR LBD, having a Ki of 13 µM for this domain and 54 µM for the CAR LBD. In cell-based reporter assays, the phytochemical antagonizes PXR, having an IC50 of 12 µM, and it displayed CAR inverse agonistic profile, having an IC50 of 30 µM. However, mutagenesis studies show that the compound binds to the outer surface of the PXR LBD. This alternative mode of PXR antagonism was shown in mutants having ligand binding pockets that had been blocked via replacement of the wild-type residues with bulkier ones, conferring ligand-independent constitutive activity. Furthermore, coumestrol prevents binding of the co-activator peptide SRC-1 to PXR LBD irrespective of the agonist concentration. These findings contradict earlier reports that coumestrol is a PXR agonist instead [97]. These discrepancies could be due to the liable nature of the compound, as coumestrol analogs were found to be weak agonists [96].
Sesamin, a lignan present in sesame seeds, attenuated PXR activity in a dose-dependent manner in cell-based assays, and it was shown to interfere with the binding of the co-activator SRC-1 and hepatocyte nuclear factor 4α (HNF4α) [98]. Although a concentration in the tens of micromolar range was required to achieve noticeable effects in mouse studies, it was speculated that desirable plasma concentrations of sesamin might be achieved in humans through oral administration as determined from studies with mice. However, sesamin seemed to be able to upregulate UGT1A1 expression through a non-PXR–mediated pathway.
Camptothecin is a quinoline alkaloid isolated from the plant Camptotheca acuminata [99] On the basis of reporter assays, the compound was reported to be a PXR inhibitor having submicromolar potency (IC50 of 0.58 µM). The results of mechanistic studies indicate that camptothecin is unlikely to affect heterodimerization with RXRα or binding to responsive elements, but instead, camptothecin likely disrupts PXR’s recruitment of SRC-1. In a biochemical assay, the compound did not show any significant effect on ligand binding to PXR at concentrations of up to 10 µM, suggesting that it binds to a site distinct from the ligand binding pocket. In primary hepatocytes, camptothecin was able to diminish rifampicin-induced metabolism of nifedipine, which is a substrate of CYP3A4. Camptothecin displays cell toxicity and is used as an antineoplastic agent, belonging to a class of topoisomerase inhibitors. This natural product is unstable at physiological conditions because of its lactone E-ring, being poorly soluble in aqueous solutions and with limited pharmacokinetic half-time [100]. Vast efforts have been made to develop analogs that address these drawbacks. Some of these analogs activate PXR, including irinotecan [101] and topotecan [102], which is in line with the fact that the ligand cavity is very flexible and may be able to accommodate structurally similar molecules in various orientations [103]. As a matter of fact, the agonist SR12813 occupies the ligand pocket in multiple orientations [104], but it is fixed in a single distinct position upon co-activator binding [105]. Hence, it is deemed extremely challenging to discover antagonists that displace the receptor ligand.
6. Conclusion
Although DILI is infrequent, it is a severe adverse drug reaction and can be life-threatening, posing a great concern to patients, physicians, regulatory agencies, and the pharmaceutical industry. A great deal of progress has been made in understanding, evaluating, and managing DILI. Recently, a growing body of evidence suggests that PXR plays a role in DILI, and PXR agonists have been reported to induce liver injury. Although the mechanisms responsible for PXR-mediated liver injury need further investigation, studies have demonstrated that activation of PXR by agonists can increase the expression of PXR target genes, including those encoding liver enzymes, transporters, and other enzymes involved in biosynthetic pathways, leading to the accumulation of toxic metabolites or intermediate endogenous substances in the liver (Figure 1). Thus, information about the effect of drugs on PXR would be useful for predicting and evaluating their potential hepatotoxicity in vivo. Despite the development and use of several in vitro models for HTS evaluations of the effect of compounds on PXR, there are no reports of systematic examinations of the correlation between drug-induced PXR activation and the occurrence of DILI in the clinic because of the lack of integrated in vitro models that can simultaneously evaluate the extent of drug-induced PXR activity and DILI. Therefore, future efforts are needed to develop such in vitro models to assess DILI. These new models may improve the accuracy of using in vitro models to predict the potential for in vivo DILI during drug development. In addition, observations of PXR-mediated DILI implicate PXR as a potential target for developing drugs to manage liver injury. Although several classes of chemicals have been reported to attenuate PXR activity, many of them have major drawbacks that include cytotoxicity, lack of selectivity, low potency, poor pharmacokinetic/pharmacodynamic properties, and poor in vivo activity. Most of the known PXR inhibitors seem to bind to a region other than the ligand binding pocket. Although some compounds are suggested to competitively displace the agonist, more-thorough investigations are still needed to generate useful PXR antagonists with in vivo activity. Additionally, the efforts to find clinically useful inhibitors are hampered by the promiscuous nature of PXR, as the receptor can be activated by a number of distinct molecules with diverse chemical properties. Advances in developing PXR antagonists may provide useful chemical tools with which to further investigate the mechanisms of DILI, validate PXR as a therapeutic target for DILI, and develop therapeutics to manage DILI.
7. Expert opinion
The transcriptional activity of PXR is regulated directly by ligand binding and indirectly through other mechanisms, including transcriptional, post-transcriptional, and post-translational regulation, affecting either the levels or activities of this receptor [106, 107]. Previous studies indicate that ligand-regulated transcriptional activity of this receptor, which controls the expression of target genes such as DMEs, transporters, and biosynthetic enzymes (e.g. ALAS1), could be the mechanism responsible for PXR-mediated DILI. Compounds that do not bind directly to PXR but are able to elevate PXR activity and induce its target genes could also lead to PXR-mediated DILI, a possibiliy that can not be ignored.
At the transcriptional level, the expression of PXR can be regulated by other NRs, such as HNF-4α [108], glucocorticoid receptor (GR) [109], and FXR [110], and can also be controlled by methylation of its promoter [111, 112]. At the post-transcriptional level, the 3′-untranslated region (3′-UTR) of PXR mRNA can be targeted by miRNA-148a, leading to downregulation of PXR [113]. Intriguingly, miRNA-148a has recently been identified as a potential circulating biomarker for DILI [114]. Therefore, the functional relationship between PXR and miRNA-148a and its implications in DILI merit further investigation.
PXR is also subject to post-transcriptional regulation, including phosphorylation, ubiquitination, SUMOylation, and acetylation. Several kinases can modulate PXR activity via phosphorylation, including cAMP-dependent protein kinase (PKA), protein kinase C (PKC), glycogen synthase kinase 3 (GSK3), casein kinase II (CK2), cyclin-dependent kinase 5 (CDK5), 70-kDa ribosomal S6 kinase (p70 S6K), and cyclin-dependent kinase 2 (CDK2) [115–118]. In addition, the stability and subsequent transcriptional activity of PXR can be modulated by components of the proteasome pathway, including the UBR5–DYRK2 complex, SUG, RBCK1, and GAL1 [119, 120]. Moreover, PXR can be modified by other post-translational mechanisms, such as SUMOylation and lysine acetylation. For example, ligand-bound, SUMOylated PXR that contains SUMO 2/3 chains is found in the inflammatory liver [121], and SIRT1 plays a role in the acetylation of PXR [122]. Although not yet investigated, compounds that affect these post-translational modifications of PXR might contribute to PXR-mediated DILI (Figure 1).
The genetic polymorphisms of hPXR in the human population can be contributing factors to DILI. For example, studies have shown that liver injury induced by the hPXR agonist flucloxacillin is associated with an hPXR polymorphism C-25385T [46]. The hPXR polymorphism rs2461823 (A/G) is signicifantly associated with intrahepatic cholestasis of pregnancy, and a positive correlation was observed between this polymorphism and elevated serum ALT and AST levels [123]. The polymorphisms rs2461823 (A/G) and re7643645 (A/G) are associated with increased liver ALT levels and with severity in nonalcoholic fatty liver disease [124]. To date, more than one hundred hPXR singlenucleotide polymorphisms have been detected [125]. A number of nonsynonymous hPXR polymorphisms in the coding region of PXR functional domain could affect its function (e.g. the ability of DNA binding and ligand binding), while polymorphisms in the 5′ or 3′ UTR of hPXR could affect its expression level and, subsequently, its transcriptional activity [126, 127]. Therefore, the role of hPXR polymorphisms in hPXR-mediated DILI merits further investigation.
Article highlights.
Transcriptional activation of PXR plays a role in drug-induced liver injury (DILI).
High-throughput screening (HTS) using primary hepatocytes or hepatic cells in 2D- or 3D-culture systems provides a way to assess and predict in vivo DILI.
Humanized PXR mouse models are useful in vivo tools for evaluating human PXR (hPXR)-mediated hepatotoxicity during drug development.
PXR inhibitors are useful for validating PXR as a target to develop drugs for the management of PXR-mediated DILI.
Transcriptional, post-transcriptional, and post-translational regulation and polymorphisms of PXR could also be contributing factors in DILI.
Acknowledgements
This work was supported by the American Lebanese Syrian Associated Charities (ALSAC), National Institutes of Health National Institute of General Medical Sciences [Grants GM086415 & GM110034], and National Cancer Institute [Grant P30-CA21765]. The authors thank members of the Chen group for their valuable discussions and Cherise Guess PhD, ELS, for editing the manuscript.
References
- 1.Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006;354:731–739. doi: 10.1056/NEJMra052270. [DOI] [PubMed] [Google Scholar]
- 2.Lee WM. Acute liver failure in the United States. Semin Liver Dis. 2003;23:217–226. doi: 10.1055/s-2003-42641. [DOI] [PubMed] [Google Scholar]
- 3.Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–1934. 1934. doi: 10.1053/j.gastro.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sgro C, Clinard F, Ouazir K, et al. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology. 2002;36:451–455. doi: 10.1053/jhep.2002.34857. [DOI] [PubMed] [Google Scholar]
- 5.Larrey D. Epidemiology and individual susceptibility to adverse drug reactions affecting the liver. Semin Liver Dis. 2002;22:145–155. doi: 10.1055/s-2002-30105. [DOI] [PubMed] [Google Scholar]
- 6.Stine JG, Lewis JH. Drug-induced liver injury: a summary of recent advances. Expert Opin Drug Metab Toxicol. 2011;7:875–890. doi: 10.1517/17425255.2011.577415. [DOI] [PubMed] [Google Scholar]
- 7.Temple R. Hy's law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf. 2006;15:241–243. doi: 10.1002/pds.1211. [DOI] [PubMed] [Google Scholar]
- 8.Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov. 2005;4:489–499. doi: 10.1038/nrd1750. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Cortes M, Stephens C, Lucena MI, et al. Causality assessment methods in drug induced liver injury: strengths and weaknesses. J Hepatol. 2011;55:683–691. doi: 10.1016/j.jhep.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Corsini A, Bortolini M. Drug-induced liver injury: the role of drug metabolism and transport. J Clin Pharmacol. 2013;53:463–474. doi: 10.1002/jcph.23. [DOI] [PubMed] [Google Scholar]
- 11.Guengerich FP. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem Res Toxicol. 2001;14:611–650. doi: 10.1021/tx0002583. [DOI] [PubMed] [Google Scholar]
- 12.Nebert DW, Gonzalez FJ. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- 13.Willson TM, Kliewer SA. PXR, CAR and drug metabolism. Nat Rev Drug Discov. 2002;1:259–266. doi: 10.1038/nrd753. [DOI] [PubMed] [Google Scholar]
- 14.McCarver DG, Hines RN. The ontogeny of human drug-metabolizing enzymes: phase II conjugation enzymes and regulatory mechanisms. J Pharmacol Exp Ther. 2002;300:361–366. doi: 10.1124/jpet.300.2.361. [DOI] [PubMed] [Google Scholar]
- 15.Ayrton A, Morgan P. Role of transport proteins in drug discovery and development: a pharmaceutical perspective. Xenobiotica. 2008;38:676–708. doi: 10.1080/00498250801923855. [DOI] [PubMed] [Google Scholar]
- 16.El-Sheikh AA, Masereeuw R, Russel FG. Mechanisms of renal anionic drug transport. Eur J Pharmacol. 2008;585:245–255. doi: 10.1016/j.ejphar.2008.02.085. [DOI] [PubMed] [Google Scholar]
- 17.Tolson AH, Wang H. Regulation of drug-metabolizing enzymes by xenobiotic receptors: PXR and CAR. Adv Drug Deliv Rev. 2010;62:1238–1249. doi: 10.1016/j.addr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang YM, Lin W, Chai SC, et al. Piperine activates human pregnane X receptor to induce the expression of cytochrome P450 3A4 and multidrug resistance protein 1. Toxicol Appl Pharmacol. 2013;272:96–107. doi: 10.1016/j.taap.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang YM, Ong SS, Chai SC, et al. Role of CAR and PXR in xenobiotic sensing and metabolism. Expert Opin Drug Metab Toxicol. 2012;8:803–817. doi: 10.1517/17425255.2012.685237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong Su Sien, Wang Yue-Ming, Chai Sergio C, Chen aosheng. Pregnane X Receptor in Drug Development. Drug Development - A Case Study Based Insight into Modern Strategies. InTech. 2011 [Google Scholar]
- 21.Yuan L, Kaplowitz N. Mechanisms of drug-induced liver injury. Clin Liver Dis. 2013;17:507–518. vii. doi: 10.1016/j.cld.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han D, Dara L, Win S, et al. Regulation of drug-induced liver injury by signal transduction pathways: critical role of mitochondria. Trends Pharmacol Sci. 2013;34:243–253. doi: 10.1016/j.tips.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner M, Zollner G, Trauner M. Nuclear receptors in liver disease. Hepatology. 2011;53:1023–1034. doi: 10.1002/hep.24148. [DOI] [PubMed] [Google Scholar]
- 24.Roberts RA, Ganey PE, Ju C, et al. Role of the Kupffer cell in mediating hepatic toxicity and carcinogenesis. Toxicol Sci. 2007;96:2–15. doi: 10.1093/toxsci/kfl173. [DOI] [PubMed] [Google Scholar]
- 25.Lammert C, Bjornsson E, Niklasson A, et al. Oral medications with significant hepatic metabolism at higher risk for hepatic adverse events. Hepatology. 2010;51:615–620. doi: 10.1002/hep.23317. [DOI] [PubMed] [Google Scholar]
- 26.Ma X, Idle JR, Gonzalez FJ. The pregnane X receptor: from bench to bedside. Expert Opin Drug Metab Toxicol. 2008;4:895–908. doi: 10.1517/17425255.4.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinz MW. Evaluation of pregnane X receptor (PXR)-mediated CYP3A4 drug-drug interactions in drug development. Drug Metab Rev. 2013;45:3–14. doi: 10.3109/03602532.2012.743560. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Ouyang J, Thung SN. Histopathologic manifestations of drug-induced hepatotoxicity. Clin Liver Dis. 2013;17:547-viii. doi: 10.1016/j.cld.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Zimmerman HJ. Drug-induced liver disease. Clin Liver Dis. 2000;4:73–96. vi. doi: 10.1016/s1089-3261(05)70097-0. [DOI] [PubMed] [Google Scholar]
- 30.Marschall HU, Wagner M, Zollner G, et al. Clinical hepatotoxicity. Regulation and treatment with inducers of transport and cofactors. Mol Pharm. 2007;4:895–910. doi: 10.1021/mp060133c. [DOI] [PubMed] [Google Scholar]
- 31.Tujios S, Fontana RJ. Mechanisms of drug-induced liver injury: from bedside to bench. Nat Rev Gastroenterol Hepatol. 2011;8:202–211. doi: 10.1038/nrgastro.2011.22. [DOI] [PubMed] [Google Scholar]
- 32.Uetrecht J. Immunoallergic drug-induced liver injury in humans. Semin Liver Dis. 2009;29:383–392. doi: 10.1055/s-0029-1240007. [DOI] [PubMed] [Google Scholar]
- 33.Meier Y, Pauli-Magnus C, Zanger UM, et al. Interindividual variability of canalicular ATP-binding-cassette (ABC)-transporter expression in human liver. Hepatology. 2006;44:62–74. doi: 10.1002/hep.21214. [DOI] [PubMed] [Google Scholar]
- 34.Jemnitz K, Veres Z, Vereczkey L. Contribution of high basolateral bile salt efflux to the lack of hepatotoxicity in rat in response to drugs inducing cholestasis in human. Toxicol Sci. 2010;115:80–88. doi: 10.1093/toxsci/kfq044. [DOI] [PubMed] [Google Scholar]
- 35.Yew WW, Leung CC. Antituberculosis drugs and hepatotoxicity. Respirology. 2006;11:699–707. doi: 10.1111/j.1440-1843.2006.00941.x. [DOI] [PubMed] [Google Scholar]
- 36.Li F, Lu J, Cheng J, et al. Human PXR modulates hepatotoxicity associated with rifampicin and isoniazid co-therapy. Nat Med. 2013;19:418. doi: 10.1038/nm.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fraser DJ, Zumsteg A, Meyer UA. Nuclear receptors constitutive androstane receptor and pregnane X receptor activate a drug-responsive enhancer of the murine 5-aminolevulinic acid synthase gene. J Biol Chem. 2003;278:39392–39401. doi: 10.1074/jbc.M306148200. [DOI] [PubMed] [Google Scholar]
- 38.Lee WM. Acetaminophen and the U.S. Acute Liver Failure Study Group: lowering the risks of hepatic failure. Hepatology. 2004;40:6–9. doi: 10.1002/hep.20293. [DOI] [PubMed] [Google Scholar]
- 39.James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 2003;31:1499–1506. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- 40.Kalhorn TF, Lee CA, Slattery JT, et al. Effect of methylxanthines on acetaminophen hepatotoxicity in various induction states. J Pharmacol Exp Ther. 1990;252:112–116. [PubMed] [Google Scholar]
- 41.Madhu C, Maziasz T, Klaassen CD. Effect of pregnenolone-16 alpha-carbonitrile and dexamethasone on acetaminophen-induced hepatotoxicity in mice. Toxicol Appl Pharmacol. 1992;115:191–198. doi: 10.1016/0041-008x(92)90323-k. [DOI] [PubMed] [Google Scholar]
- 42.Guo GL, Moffit JS, Nicol CJ, et al. Enhanced acetaminophen toxicity by activation of the pregnane X receptor. Toxicol Sci. 2004;82:374–380. doi: 10.1093/toxsci/kfh286. [DOI] [PubMed] [Google Scholar]
- 43.Wolf KK, Wood SG, Hunt JA, et al. Role of the nuclear receptor pregnane X receptor in acetaminophen hepatotoxicity. Drug Metab Dispos. 2005;33:1827–1836. doi: 10.1124/dmd.105.005256. [DOI] [PubMed] [Google Scholar]
- 44.Cheng J, Ma X, Krausz KW, et al. Rifampicin-activated human pregnane X receptor and CYP3A4 induction enhance acetaminophen-induced toxicity. Drug Metab Dispos. 2009;37:1611–1621. doi: 10.1124/dmd.109.027565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huwyler J, Wright MB, Gutmann H, et al. Induction of cytochrome P450 3A4 and P-glycoprotein by the isoxazolyl-penicillin antibiotic flucloxacillin. Curr Drug Metab. 2006;7:119–126. doi: 10.2174/138920006775541534. [DOI] [PubMed] [Google Scholar]
- 46. Andrews E, Armstrong M, Tugwood J, et al. A role for the pregnane X receptor in flucloxacillin-induced liver injury. Hepatology. 2010;51:1656–1664. doi: 10.1002/hep.23549. •• This is the first report of the association between PXR polymorphisms and DILI.
- 47.Lakehal F, Dansette PM, Becquemont L, et al. Indirect cytotoxicity of flucloxacillin toward human biliary epithelium via metabolite formation in hepatocytes. Chem Res Toxicol. 2001;14:694–701. doi: 10.1021/tx0002435. [DOI] [PubMed] [Google Scholar]
- 48.Carey MA, van Pelt FN. Immunochemical detection of flucloxacillin adduct formation in livers of treated rats. Toxicology. 2005;216:41–48. doi: 10.1016/j.tox.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 49.Jaeschke H. Troglitazone hepatotoxicity: are we getting closer to understanding idiosyncratic liver injury? Toxicol Sci. 2007;97:1–3. doi: 10.1093/toxsci/kfm021. [DOI] [PubMed] [Google Scholar]
- 50.He K, Talaat RE, Pool WF, et al. Metabolic activation of troglitazone: identification of a reactive metabolite and mechanisms involved. Drug Metab Dispos. 2004;32:639–646. doi: 10.1124/dmd.32.6.639. [DOI] [PubMed] [Google Scholar]
- 51.Hartley DP, Dai X, Yabut J, et al. Identification of potential pharmacological and toxicological targets differentiating structural analogs by a combination of transcriptional profiling and promoter analysis in LS-180 and Caco-2 adenocarcinoma cell lines. Pharmacogenet Genomics. 2006;16:579–599. doi: 10.1097/01.fpc.0000220561.59972.7a. [DOI] [PubMed] [Google Scholar]
- 52.Ekins S, Erickson JA. A pharmacophore for human pregnane X receptor ligands. Drug Metab Dispos. 2002;30:96–99. doi: 10.1124/dmd.30.1.96. [DOI] [PubMed] [Google Scholar]
- 53.Trubetskoy O, Marks B, Zielinski T, et al. A simultaneous assessment of CYP3A4 metabolism and induction in the DPX-2 cell line. AAPS J. 2005;7:E6–E13. doi: 10.1208/aapsj070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Devarbhavi H, Andrade RJ. Drug-induced liver injury due to antimicrobials, central nervous system agents, and nonsteroidal anti-inflammatory drugs. Semin Liver Dis. 2014;34:145–161. doi: 10.1055/s-0034-1375956. [DOI] [PubMed] [Google Scholar]
- 55.Eghbal MA, Taziki S, Sattari MR. Mechanisms of phenytoin-induced toxicity in freshly isolated rat hepatocytes and the protective effects of taurine and/or melatonin. J Biochem Mol Toxicol. 2014;28:111–118. doi: 10.1002/jbt.21542. [DOI] [PubMed] [Google Scholar]
- 56.Sasaki E, Matsuo K, Iida A, et al. A novel mouse model for phenytoin-induced liver injury: involvement of immune-related factors and P450-mediated metabolism. Toxicol Sci. 2013;136:250–263. doi: 10.1093/toxsci/kft184. [DOI] [PubMed] [Google Scholar]
- 57.Chen Y, Ferguson SS, Negishi M, et al. Induction of human CYP2C9 by rifampicin, hyperforin, and phenobarbital is mediated by the pregnane X receptor. J Pharmacol Exp Ther. 2004;308:495–501. doi: 10.1124/jpet.103.058818. [DOI] [PubMed] [Google Scholar]
- 58.Al-Dosari MS, Knapp JE, Liu D. Activation of human CYP2C9 promoter and regulation by CAR and PXR in mouse liver. Mol Pharm. 2006;3:322–328. doi: 10.1021/mp0500824. [DOI] [PubMed] [Google Scholar]
- 59.Chaudhry AS, Urban TJ, Lamba JK, et al. CYP2C9*1B promoter polymorphisms, in linkage with CYP2C19*2, affect phenytoin autoinduction of clearance and maintenance dose. J Pharmacol Exp Ther. 2010;332:599–611. doi: 10.1124/jpet.109.161026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luo G, Cunningham M, Kim S, et al. CYP3A4 induction by drugs: correlation between a pregnane X receptor reporter gene assay and CYP3A4 expression in human hepatocytes. Drug Metab Dispos. 2002;30:795–804. doi: 10.1124/dmd.30.7.795. [DOI] [PubMed] [Google Scholar]
- 61.Raucy JL. Regulation of CYP3A4 expression in human hepatocytes by pharmaceuticals and natural products. Drug Metab Dispos. 2003;31:533–539. doi: 10.1124/dmd.31.5.533. [DOI] [PubMed] [Google Scholar]
- 62.Sinz MW. Evaluation of pregnane X receptor (PXR)-mediated CYP3A4 drug-drug interactions in drug development. Drug Metab Rev. 2013;45:3–14. doi: 10.3109/03602532.2012.743560. [DOI] [PubMed] [Google Scholar]
- 63.Raucy JL, Lasker JM. Cell-based systems to assess nuclear receptor activation and their use in drug development. Drug Metab Rev. 2013;45:101–109. doi: 10.3109/03602532.2012.737333. [DOI] [PubMed] [Google Scholar]
- 64. Godoy P, Hewitt NJ, Albrecht U, et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch Toxicol. 2013;87:1315–1530. doi: 10.1007/s00204-013-1078-5. • A comprehensive review on recent advances in in vitro systems for the evaluation of hepatotoxicity.
- 65.LeCluyse EL. Human hepatocyte culture systems for the in vitro evaluation of cytochrome P450 expression and regulation. Eur J Pharm Sci. 2001;13:343–368. doi: 10.1016/s0928-0987(01)00135-x. [DOI] [PubMed] [Google Scholar]
- 66.Gomez-Lechon MJ, Lahoz A, Gombau L, et al. In vitro evaluation of potential hepatotoxicity induced by drugs. Curr Pharm Des. 2010;16:1963–1977. doi: 10.2174/138161210791208910. [DOI] [PubMed] [Google Scholar]
- 67.Garside H, Marcoe KF, Chesnut-Speelman J, et al. Evaluation of the use of imaging parameters for the detection of compound-induced hepatotoxicity in 384-well cultures of HepG2 cells and cryopreserved primary human hepatocytes. Toxicol In Vitro. 2014;28:171–181. doi: 10.1016/j.tiv.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 68.Xu JJ, Henstock PV, Dunn MC, et al. Cellular imaging predictions of clinical drug-induced liver injury. Toxicol Sci. 2008;105:97–105. doi: 10.1093/toxsci/kfn109. [DOI] [PubMed] [Google Scholar]
- 69.Moeller TA, Shukla SJ, Xia M. Assessment of compound hepatotoxicity using human plateable cryopreserved hepatocytes in a 1536-well-plate format. Assay Drug Dev Technol. 2012;10:78–87. doi: 10.1089/adt.2010.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gunness P, Mueller D, Shevchenko V, et al. 3D organotypic cultures of human HepaRG cells: a tool for in vitro toxicity studies. Toxicol Sci. 2013;133:67–78. doi: 10.1093/toxsci/kft021. [DOI] [PubMed] [Google Scholar]
- 71.Takayama K, Kawabata K, Nagamoto Y, et al. 3D spheroid culture of hESC/hiPSC-derived hepatocyte-like cells for drug toxicity testing. Biomaterials. 2013;34:1781–1789. doi: 10.1016/j.biomaterials.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 72.Kostadinova R, Boess F, Applegate D, et al. A long-term three dimensional liver co-culture system for improved prediction of clinically relevant drug-induced hepatotoxicity. Toxicol Appl Pharmacol. 2013;268:1–16. doi: 10.1016/j.taap.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 73.Ekins S, Mirny L, Schuetz EG. A ligand-based approach to understanding selectivity of nuclear hormone receptors PXR, CAR, FXR, LXRalpha, and LXRbeta. Pharm Res. 2002;19:1788–1800. doi: 10.1023/a:1021429105173. [DOI] [PubMed] [Google Scholar]
- 74.Kliewer SA, Moore JT, Wade L, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 75. Xie W, Barwick JL, Downes M, et al. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature. 2000;406:435–439. doi: 10.1038/35019116. •• This is the first report of the humanized PXR mouse model.
- 76.Gong H, Singh SV, Singh SP, et al. Orphan nuclear receptor pregnane X receptor sensitizes oxidative stress responses in transgenic mice and cancerous cells. Mol Endocrinol. 2006;20:279–290. doi: 10.1210/me.2005-0205. [DOI] [PubMed] [Google Scholar]
- 77.Ma X, Shah Y, Cheung C, et al. The PREgnane X receptor gene-humanized mouse: a model for investigating drug-drug interactions mediated by cytochromes P450 3A. Drug Metab Dispos. 2007;35:194–200. doi: 10.1124/dmd.106.012831. [DOI] [PubMed] [Google Scholar]
- 78.Ma X, Cheung C, Krausz KW, et al. A double transgenic mouse model expressing human pregnane X receptor and cytochrome P450 3A4. Drug Metab Dispos. 2008;36:2506–2512. doi: 10.1124/dmd.108.022723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scheer N, Ross J, Rode A, et al. A novel panel of mouse models to evaluate the role of human pregnane X receptor and constitutive androstane receptor in drug response. J Clin Invest. 2008;118:3228–3239. doi: 10.1172/JCI35483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Backman TWH, Cao YQ, Girke T. ChemMine tools: an online service for analyzing and clustering small molecules. Nucleic Acids Res. 2011;39:W486–W491. doi: 10.1093/nar/gkr320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Letunic I, Bork P. Interactive Tree Of Life v2: online annotation and display of phylogenetic trees made easy. Nucleic Acids Res. 2011;39:W475–W478. doi: 10.1093/nar/gkr201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Synold TW, Dussault I, Forman BM. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med. 2001;7:584–590. doi: 10.1038/87912. [DOI] [PubMed] [Google Scholar]
- 83.van Kesteren C, de Vooght MMM, Lopez-Lazaro L, et al. Yondelis (R) (trabectedin, ET-743): the development of an anticancer agent of marine origin. Anti-Cancer Drug. 2003;14:487–502. doi: 10.1097/00001813-200308000-00001. [DOI] [PubMed] [Google Scholar]
- 84. Mani S, Dou W, Redinbo MR. PXR antagonists and implication in drug metabolism. Drug Metab Rev. 2013;45:60–72. doi: 10.3109/03602532.2012.746363. •• A comprehensive review on recent advances in PXR inhibitors development.
- 85.Huang H, Wang H, Sinz M, et al. Inhibition of drug metabolism by blocking the activation of nuclear receptors by ketoconazole. Oncogene. 2007;26:258–268. doi: 10.1038/sj.onc.1209788. [DOI] [PubMed] [Google Scholar]
- 86.Das BC, Madhukumar AV, Anguiano J, et al. Synthesis of novel ketoconazole derivatives as inhibitors of the human Pregnane X Receptor (PXR; NR1I2; also termed SXR, PAR) Bioorg Med Chem Lett. 2008;18:3974–3977. doi: 10.1016/j.bmcl.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 87.Venkatesh M, Wang HW, Cayer J, et al. In Vivo and In Vitro Characterization of a First-in-Class Novel Azole Analog That Targets Pregnane X Receptor Activation. Mol Pharmacol. 2011;80:124–135. doi: 10.1124/mol.111.071787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tabb MM, Kholodovych V, Grun F, et al. Highly chlorinated PCBs inhibit the human xenobiotic response mediated by the steroid and xenobiotic receptor (SXR) Environ Health Persp. 2004;112:163–169. doi: 10.1289/ehp.6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ekins S, Chang C, Mani S, et al. Human pregnane X receptor antagonists and Agonists define molecular requirements for different binding sites. Mol Pharmacol. 2007;72:592–603. doi: 10.1124/mol.107.038398. [DOI] [PubMed] [Google Scholar]
- 90.Ekins S, Kholodovych V, Ai N, et al. Computational discovery of novel low micromolar human pregnane X receptor antagonists. Mol Pharmacol. 2008;74:662–672. doi: 10.1124/mol.108.049437. [DOI] [PubMed] [Google Scholar]
- 91.Krausova L, Stejskaova L, Wang HW, et al. Metformin suppresses pregnane X receptor (PXR)-regulated transactivation of CYP3A4 gene. Biochem Pharmacol. 2011;82:1771–1780. doi: 10.1016/j.bcp.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Healan-Greenberg C, Waring JF, Kempf DJ, et al. A human immunodeficiency virus protease inhibitor is a novel functional inhibitor of human pregnane x receptor. Drug Metab Dispos. 2008;36:500–507. doi: 10.1124/dmd.107.019547. [DOI] [PubMed] [Google Scholar]
- 93.Zhou CC, Poulton EJ, Grun F, et al. The dietary isothiocyanate sulforaphane is an antagonist of the human steroid and xenobiotic nuclear receptor. Mol Pharmacol. 2007;71:220–229. doi: 10.1124/mol.106.029264. [DOI] [PubMed] [Google Scholar]
- 94.Morimitsu Y, Nakagawa Y, Hayashi K, et al. A sulforaphane analogue that potently activates the Nrf2-dependent detoxification pathway. J Biol Chem. 2002;277:3456–3463. doi: 10.1074/jbc.M110244200. [DOI] [PubMed] [Google Scholar]
- 95.Poulton EJ, Levy L, Lampe JW, et al. Sulforaphane is not an effective antagonist of the human pregnane X-receptor in vivo. Toxicol Appl Pharm. 2013;266:122–131. doi: 10.1016/j.taap.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang HW, Li H, Moore LB, et al. The phytoestrogen Coumestrol is a naturally occurring antagonist of the human pregnane x receptor. Mol Endocrinol. 2008;22:838–857. doi: 10.1210/me.2007-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Blumberg B, Sabbagh W, Juguilon H, et al. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Gene Dev. 1998;12:3195–3205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lim YP, Ma CY, Liu CL, et al. Sesamin: A Naturally Occurring Lignan Inhibits CYP3A4 by Antagonizing the Pregnane X Receptor Activation. Evid-Based Compl Alt. 2012 doi: 10.1155/2012/242810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wall ME, Wani MC, Cook CE, et al. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from camptotheca acuminata1, 2. 1966;88:3888–3890. [Google Scholar]
- 100.Huang QQ, Wang L, Lu W. Evolution in medicinal chemistry of E-ring-modified Camptothecin analogs as anticancer agents. Eur J Med Chem. 2013;63:746–757. doi: 10.1016/j.ejmech.2013.01.058. [DOI] [PubMed] [Google Scholar]
- 101.Chen YK, Tang Y, Robbins GT, et al. Camptothecin Attenuates Cytochrome P450 3A4 Induction by Blocking the Activation of Human Pregnane X Receptor. J Pharmacol Exp Ther. 2010;334:999–1008. doi: 10.1124/jpet.110.168294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schuetz E, Lan LB, Yasuda K, et al. Development of a real-time in vivo transcription assay: Application reveals pregnane X receptor-mediated induction of CYP3A4 by cancer chemotherapeutic agents. Mol Pharmacol. 2002;62:439–445. doi: 10.1124/mol.62.3.439. [DOI] [PubMed] [Google Scholar]
- 103.Xue Y, Chao E, Zuercher WJ, et al. Crystal structure of the PXR-T1317 complex provides a scaffold to examine the potential for receptor antagonism. Bioorgan Med Chem. 2007;15:2156–2166. doi: 10.1016/j.bmc.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Watkins RE, Wisely GB, Moore LB, et al. The human nuclear xenobiotic receptor PXR: Structural determinants of directed promiscuity. Science. 2001;292:2329–2333. doi: 10.1126/science.1060762. [DOI] [PubMed] [Google Scholar]
- 105.Watkins RE, Davis-Searles PR, Lambert MH, et al. Coactivator binding promotes the specific interaction between ligand and the pregnane X receptor. J Mol Biol. 2003;331:815–828. doi: 10.1016/s0022-2836(03)00795-2. [DOI] [PubMed] [Google Scholar]
- 106.Smutny T, Mani S, Pavek P. Post-translational and post-transcriptional modifications of pregnane X receptor (PXR) in regulation of the cytochrome P450 superfamily. Curr Drug Metab. 2013;14:1059–1069. doi: 10.2174/1389200214666131211153307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tian Y. Epigenetic regulation of pregnane X receptor activity. Drug Metab Rev. 2013;45:166–172. doi: 10.3109/03602532.2012.756012. [DOI] [PubMed] [Google Scholar]
- 108.Tirona RG, Lee W, Leake BF, et al. The orphan nuclear receptor HNF4alpha determines PXR- and CAR-mediated xenobiotic induction of CYP3A4. Nat Med. 2003;9:220–224. doi: 10.1038/nm815. [DOI] [PubMed] [Google Scholar]
- 109.Shi D, Yang D, Yan B. Dexamethasone transcriptionally increases the expression of the pregnane X receptor and synergistically enhances pyrethroid esfenvalerate in the induction of cytochrome P450 3A23. Biochem Pharmacol. 2010;80:1274–1283. doi: 10.1016/j.bcp.2010.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jung D, Mangelsdorf DJ, Meyer UA. Pregnane X receptor is a target of farnesoid X receptor. J Biol Chem. 2006;281:19081–19091. doi: 10.1074/jbc.M600116200. [DOI] [PubMed] [Google Scholar]
- 111.Misawa A, Inoue J, Sugino Y, et al. Methylation-associated silencing of the nuclear receptor 1I2 gene in advanced-type neuroblastomas, identified by bacterial artificial chromosome array-based methylated CpG island amplification. Cancer Res. 2005;65:10233–10242. doi: 10.1158/0008-5472.CAN-05-1073. [DOI] [PubMed] [Google Scholar]
- 112.Habano W, Gamo T, Terashima J, et al. Involvement of promoter methylation in the regulation of Pregnane X receptor in colon cancer cells. BMC Cancer. 2011;11:81. doi: 10.1186/1471-2407-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Takagi S, Nakajima M, Mohri T, et al. Post-transcriptional regulation of human pregnane X receptor by micro-RNA affects the expression of cytochrome P450 3A4. J Biol Chem. 2008;283:9674–9680. doi: 10.1074/jbc.M709382200. [DOI] [PubMed] [Google Scholar]
- 114.Wang K, Zhang S, Marzolf B, et al. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci U S A. 2009;106:4402–4407. doi: 10.1073/pnas.0813371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lin W, Wu J, Dong H, et al. Cyclin-dependent kinase 2 negatively regulates human pregnane X receptor-mediated CYP3A4 gene expression in HepG2 liver carcinoma cells. J Biol Chem. 2008;283:30650–30657. doi: 10.1074/jbc.M806132200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lichti-Kaiser K, Brobst D, Xu C, et al. A systematic analysis of predicted phosphorylation sites within the human pregnane X receptor protein. J Pharmacol Exp Ther. 2009;331:65–76. doi: 10.1124/jpet.109.157180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pondugula SR, Brimer-Cline C, Wu J, et al. A phosphomimetic mutation at threonine-57 abolishes transactivation activity and alters nuclear localization pattern of human pregnane x receptor. Drug Metab Dispos. 2009;37:719–730. doi: 10.1124/dmd.108.024695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Elias A, High AA, Mishra A, et al. Identification and characterization of phosphorylation sites within the pregnane X receptor protein. Biochem Pharmacol. 2014;87:360–370. doi: 10.1016/j.bcp.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ong SS, Goktug AN, Elias A, et al. Stability of the human pregnane X receptor is regulated by E3 ligase UBR5 and serine/threonine kinase DYRK2. Biochem J. 2014;459:193–203. doi: 10.1042/BJ20130558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rana R, Coulter S, Kinyamu H, et al. RBCK1, an E3 ubiquitin ligase, interacts with and ubiquinates the human pregnane X receptor. Drug Metab Dispos. 2013;41:398–405. doi: 10.1124/dmd.112.048728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Staudinger JL, Xu C, Biswas A, et al. Post-translational modification o f pregnane x receptor. Pharmacol Res. 2011;64:4–10. doi: 10.1016/j.phrs.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Biswas A, Pasquel D, Tyagi RK, et al. Acetylation of pregnane X receptor protein determines selective function independent of ligand activation. Biochem Biophys Res Commun. 2011;406:371–376. doi: 10.1016/j.bbrc.2011.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Castano G, Burgueno A, Fernandez GT, et al. The influence of common gene variants of the xenobiotic receptor (PXR) in genetic susceptibility to intrahepatic cholestasis of pregnancy. Aliment Pharmacol Ther. 2010;31:583–592. doi: 10.1111/j.1365-2036.2009.04210.x. [DOI] [PubMed] [Google Scholar]
- 124.Sookoian S, Castano GO, Burgueno AL, et al. The nuclear receptor PXR gene variants are associated with liver injury in nonalcoholic fatty liver disease. Pharmacogenet Genomics. 2010;20:1–8. doi: 10.1097/FPC.0b013e328333a1dd. [DOI] [PubMed] [Google Scholar]
- 125.Li T, Yu RT, Atkins AR, et al. Targeting the pregnane X receptor in liver injury. Expert Opin Ther Targets. 2012;16:1075–1083. doi: 10.1517/14728222.2012.715634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Zhang B, Xie W, Krasowski MD. PXR: a xenobiotic receptor of diverse function implicated in pharmacogenetics. Pharmacogenomics. 2008;9:1695–1709. doi: 10.2217/14622416.9.11.1695. • A comprehensive review concerning PXR polymorphisms in relation to disease risk and treatment response.
- 127. Kotta-Loizou I, Patsouris E, Theocharis S. Pregnane X receptor polymorphisms associated with human diseases. Expert Opin Ther Targets. 2013;17:1167–1177. doi: 10.1517/14728222.2013.823403. • A comprehensive review concerning PXR polymorphisms in relation to disease risk and treatment response.