Figure 1.
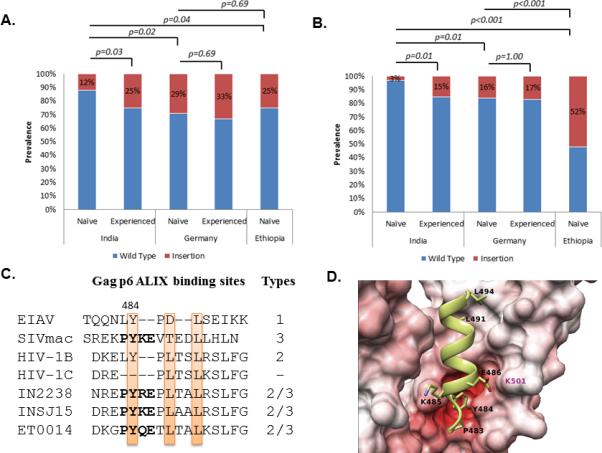
Prevalence of (A) duplications in the TSG101 binding PTAPP motif and (B) insertions in the ALIX-binding LYPxnLxxL motif. Statistically significant differences are marked. (C) Different types of ALIX-binding sites in lentiviral Gag-p6 region as describe by Zhai et al 2011 [20]. Representative clinical isolates from the cohort are presented which had PYxE insertion. All the strains had the key residues conserved (highlighted). The 3D-molecular models of the Gag-p6-ALIX complex (D) The ALIX is shown in surface representation, interacting with the Gag-p6 protein PYKE shown in ribbon and stick (side-chain only) representation. The residues that belong to the ALIX binding motif and the insertions are all labelled in black. The residues in ALIX that is crucial to mediate the interaction with Gag-p6 are labelled in magenta. The surface of the ALIX is coloured according to the electrostatic potential with blue indicating positively charged regions and red indicating negatively charged regions. The intensity of the colour reflects charge intensity. The models also establish that the Y484 residue in the insertions play a role similar to the Tyr residue in the wild type HIV-1B/SIV Gag-p6 protein by forming a specific hydrogen bond with the ALIX. Similar results were observed with PYRE and PYQE insertions (data not shown).
