Abstract
Background
Persons with pre-diabetes and diabetes are at high risk for cardiovascular events. However, the relationships of pre-diabetes and diabetes to development of subclinical myocardial damage are unclear.
Methods and Results
We measured cardiac troponin T with a highly sensitive assay (hs-cTnT) at two time points, 6 years apart, among 9,331 participants of the community-based Atherosclerosis Risk in Communities (ARIC) Study with no diabetes, pre-diabetes, or diabetes but without cardiovascular disease including silent MI by ECG. First, we examined incidence of elevated hs-cTnT (≥14 ng/L) at 6 years of follow-up. Second, we examined clinical outcomes during the subsequent ~14 years of follow-up among persons with and without incident elevated hs-cTnT. Cumulative probabilities of elevated hs-cTnT at 6 years among persons with no diabetes, pre-diabetes, and diabetes were 3.7%, 6.4%, and 10.8%, respectively. Compared to normoglycemic persons, the adjusted relative risks for incident elevated hs-cTnT were 1.38 (95%CI 1.07-1.77) for pre-diabetes and 2.46 (95%CI 1.77-3.42) for diabetes. Persons with diabetes and incident elevations in hs-cTnT were at a substantially higher risk of heart failure (HR 6.37, 95%CI 4.27-9.51), death (HR 4.36, 95%CI 3.14-6.07) and coronary heart disease (HR 3.84, 95%CI 2.52-5.84) compared to persons without diabetes and no incident elevation in hscTnT.
Conclusions
Pre-diabetes and diabetes were independently associated with development of subclinical myocardial damage, as assessed by hs-cTnT, and those persons with evidence of subclinical damage were at highest risk for clinical events. These results support a possible deleterious effect of hyperglycemia on the myocardium, possibly reflecting a microvascular etiology.
Keywords: high sensitivity cardiac troponin, cardiac biomarkers, subclinical cardiac damage, pre-diabetes, diabetes, epidemiology, ARIC
INTRODUCTION
Cardiovascular disease is the leading cause of death among persons with diabetes and there is evidence that cardiac damage is often present at the time of clinical diagnosis of diabetes 1, 2. In addition, persons with hyperglycemia even below the threshold for the diagnosis of diabetes are known to be at high risk for cardiovascular events 3-5. Elevated glucose levels are thought to induce hyperglycemia-mediated coronary microvascular dysfunction and result in myocardial injury 6-11. Previous studies have shown that persons with pre-diabetes or diabetes have an increased prevalence of atherosclerosis as measured by carotid intimal thickness or coronary artery calcium11-16. Much less is known about the relationships of pre-diabetes and diabetes with subclinical myocardial damage, particularly with respect to its progression over time.
Cardiac troponins are elevated in the setting of myocardial damage and are a standard measure used for diagnosis of myocardial infarction. There have been several generations of increasingly sensitive tests that reliably detect lower and lower levels of troponin in the blood. A novel high-sensitivity assay for cardiac troponin T (hs-cTnT) developed by Roche Diagnostics allows for the reliable measurement of troponin far below the conventional limit of detection 17. It has been suggested that cardiac troponin detected in asymptomatic persons with this novel assay may represent chronic subclinical myocardial injury, of a non-atherosclerotic origin 18, 19, with very strong associations with subsequent heart failure and death and only a moderate association with risk of coronary heart disease 20. Previous studies have shown cross-sectional associations of diabetes with hs-cTnT 21, 22. However, the associations of diabetes and pre-diabetes with progression of myocardial damage, as indicated by temporal changes in hs-cTnT concentrations in a community-based population, are unknown.
Our objective was to characterize the associations of diabetes and pre-diabetes with 6-year incidence of subclinical myocardial injury, as assessed by hs-cTnT, in a community-based population without clinically evident cardiovascular disease. We conducted secondary analyses to evaluate associations of the incident elevations in hs-cTnT with subsequent risk of coronary heart disease, heart failure, and all-cause mortality in persons with and without diabetes at baseline.
METHODS
Study Population
The Atherosclerosis Risk in Communities (ARIC) Study is a community-based prospective cohort of 15,792 participants sampled from four U.S. communities. The first clinic examinations (visit 1) took place from 1987 to 1989, with three follow-up visits approximately every three years 23. A fifth visit was completed in 2011 to 2013. Institutional review boards at each clinical site reviewed the study and informed consent was obtained from all participants. The second clinic examination (visit 2) took place from 1990 to 1992, was attended by 14,348 participants, and was the first ARIC visit with measurement of hs-cTnT. We excluded all persons who had coronary heart disease (including silent MI detected by ECG), stroke, or heart failure at or prior to visit 2 (n=1,542), were fasting less than eight hours, were non-white or non-black, were missing variables of interest (n=1,763), were missing hs-cTnT (n=41), had hs-cTnT ≥14 ng/L at visit 2 (n=376), or who were missing the follow-up hs-cTnT measurement at visit 4 in 1996 to 1998 (n=1295). Thus, there were 9,331 participants included in our main study population (Supplemental Figure 1). There were 926 incident cardiovascular events and 240 non-cardiovascular deaths that occurred between visits 2 and 4 and were accounted for in our analyses. In secondary analyses of incident coronary heart disease, heart failure, and all-cause mortality risk among persons with and without incident elevations in hs-cTnT, we further excluded 160 persons missing covariates of interest at visit 4 for a study population of 8,005 in our analyses of clinical events.
Definitions Diabetes and Pre-diabetes
Diabetes was defined as a self-reported physician diagnosis of diabetes, current use of glucose-lowering medications, or an HbA1c value greater than or equal to 6.5% at baseline. Among persons without diabetes, pre-diabetes was defined based on the clinical cut-points for HbA1c of 5.7 to 6.4% 24. In sensitivity analyses, we compared definitions of diabetes and pre-diabetes based on diagnostic cut-points for HbA1c and fasting glucose.
Measurement of Highly Sensitive Cardiac Troponin T (hs-cTnT)
Cardiac troponin T was measured at two time points, 6 years apart, using the same highly sensitive (pre-commercial) sandwich immunoassay method (Roche Elecsys T; Roche Diagnostics, Indianapolis, Indiana). We measured hs-cTnT in stored serum samples collected at visit 2 (1990-1992) using a Roche Elecys 2010 Analyzer (Roche Diagnostics) at the University of Minnesota in 2012-2013. We measured hs-cTnT in stored plasma samples collected at visit 4 (1996-1998) using a Cobas e411 analyzer (Roche Diagnostics) at Baylor College of Medicine. We conducted a formal calibration study (N=200 paired samples) to evaluate possible differences across specimen type and laboratory. No significant differences were observed and statistical correction was not indicated 25.
Other Variables
Serum glucose was measured using the hexokinase method. HbA1c was measured in stored whole blood samples using high-performance liquid chromatography with instruments standardized to the Diabetes Control and Complications Trial assay (Tosoh A1c 2.2 and Tosoh G7)26. Plasma lipid concentrations 27-30, body mass index 31, and blood pressure 32 were measured as part of the original ARIC study protocol. C-reactive protein was measured in 2012-13 in stored serum samples (Roche Diagnostics). Hypertension was defined as the mean of the second and third readings at the visit (with cutoff for systolic blood pressure of 140 mm Hg or higher and a cutoff for diastolic blood pressure of 90 mm Hg or higher) or the use of hypertension medication. Participants reported their alcohol use and smoking status. Glomerular filtration rate was estimated from serum creatinine, age, sex, and race using the CKD-EPI 2009 equation33. Left ventricular hypertrophy was assessed using resting 12-lead electrocardiograms and defined by Cornell criteria 34.
Incident Coronary Heart Disease, Heart Failure, and All Cause Mortality
The ascertainment of deaths and classification and adjudication of cardiovascular events in ARIC have been previously published 35,36. Briefly, any hospitalization was reported annually by participants or their proxy and also identified through surveillance of hospitals in each community. Trained personnel abstracted hospital records for potential cardiovascular events. Coronary heart disease events were adjudicated by an endpoints committee and were defined here as a definite or probable myocardial infarction, death from coronary heart disease, or a cardiac procedure. Heart failure cases were identified from hospitalization diagnosis codes (ICD-9 code 428) and death surveillance (hospital discharge records for inpatient deaths and death certificates for deaths outside the hospital)37.
Statistical Analyses
Elevated hs-cTnT was defined as a concentration of 14 ng/L or higher, the previously reported 99th percentile for a healthy reference group of persons aged 20-70 years, as defined by the manufacturer of the assay 20, 38. We conducted prospective analyses to characterize the association of baseline diabetes and pre-diabetes status with progression of hs-cTnT from non-elevated (<14 ng/L) at baseline (1990-1992) to elevated (≥14 ng/L) at follow-up (1996-1998). We used multinomial logistic regression to estimate the relative risks of subclinical myocardial damage as defined by an incident elevation in hs-cTnT at the 6-year follow-up (visit 4) comparing persons with no diabetes, pre-diabetes, and diabetes at visit 2 and accounting for intervening cardiovascular events and deaths between visits 2 and 4. All multivariable models were adjusted for age (years), race-center (whites-Washington County; whites-Minneapolis; blacks-Jackson; blacks-Forsyth County, whites-Forsyth County), sex (male or female), body mass index (kg/m2), C-reactive protein (mg/L), smoking (current; former; never), mean systolic blood pressure (mm Hg), LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), triglycerides (mg/dL), current use of hypertension medication (yes or no), current use of cholesterol-lowering medication (yes or no); estimated glomerular filtration rate (mL/min/1.73m2); alcohol use (current; former; never); and left ventricular hypertrophy (yes or no). We conducted sensitivity analyses excluding participants with hs-cTnT >30 ng/L at follow-up, and stratified by race (black or white) or baseline category of hs-cTnT level (<5, 5 to 8, and 9-13 ng/L).
We conducted secondary analyses of the association of incident elevated hs-cTnT categories with subsequent risk of coronary heart disease, heart failure, and all-cause mortality comparing risk in persons with no diabetes, pre-diabetes, and diabetes. We used Cox proportional hazards models with visit 4 as baseline and with follow up to January 1, 2012 (median follow-up of approximately 14 years). Models were adjusted for all covariates as listed above but measured at visit 4. Model discrimination was assessed using Harrell's C-statistic 39 and we assessed improvement in the C-statistic for the addition of elevated hs-cTnT to models containing all other covariates overall and, separately, in persons with diabetes. We also conducted sensitivity analyses to account for incident cases of diabetes between visits 2 and 4.
RESULTS
In this study population of persons with no clinical cardiovascular disease and non-elevated hscTnT (<14 ng/L) at baseline, persons with pre-diabetes or diabetes were more likely to be older, black, and obese and more likely to have hypertension, high C-reactive protein, left ventricular hypertrophy, and a poorer lipid profile compared to persons without diabetes (Table 1). Diabetes status at baseline was also strongly associated with higher levels of hs-cTnT; the percentage of persons with hs-cTnT 9-13 ng/L was 15.7% in persons with diabetes compared to 6.2% among persons without diabetes.
Table 1.
Characteristics of study participants without clinical cardiovascular disease and highly sensitive cardiac troponin T (hs-cTnT) <14 ng/L at baseline according to pre-diabetes and diabetes status (visit 2, 1990-1992), the Atherosclerosis Risk in Communities (ARIC) Study
| Overall, N=9,331 | No diabetes (HbA1c <5.7%), N=6,333 | Pre-diabetes (HbA1c 5.7-6.4%), N=2,172 | Diabetes (diagnosis or HbA1c ≥6.5%), N=826 | |
|---|---|---|---|---|
| Age (years), mean (SD) | 56.6 (5.7) | 56.2 (5.6) | 57.5 (5.7) | 57.6 (5.7) |
| Male | 41.7 | 41.1 | 44.4 | 38.6 |
| Black | 20.9 | 12.8 | 36.1 | 42.6 |
| Current smoker | 20.4 | 18.1 | 27.3 | 19.4 |
| Current drinker | 59.2 | 64.3 | 52.0 | 38.6 |
| Hypertension | 30.7 | 25.0 | 39.0 | 53.1 |
| Body mass index (kg/m2) categories | ||||
| Normal weight (BMI <25) | 32.3 | 38.5 | 22.4 | 11.1 |
| Overweight (BMI 25-30) | 40.7 | 41.6 | 40.7 | 33.7 |
| Obese (BMI ≥30) | 27.0 | 20.0 | 36.8 | 55.2 |
| C-reactive protein (mg/L) | ||||
| <1 | 25.1 | 30.2 | 16.1 | 9.4 |
| 1 to <3 | 38.3 | 39.4 | 38.9 | 28.7 |
| ≥3 | 36.6 | 30.4 | 45.0 | 61.9 |
| LDL-cholesterol (mg/dl) | ||||
| <100 (Optimal) | 16.8 | 18.1 | 13.4 | 15.4 |
| 100–129 (Near optimal) | 32.1 | 33.7 | 30.1 | 25.8 |
| 130–159 (Borderline high) | 30.0 | 29.6 | 30.2 | 32.3 |
| 160–189 (High) | 15.1 | 13.7 | 18.3 | 17.3 |
| ≥190 (Very high) | 6.0 | 4.9 | 8.0 | 9.2 |
| High triglycerides (≥150 mg/dl) | 27.7 | 24.6 | 30.6 | 44.2 |
| Low HDL-cholesterol (< 40 mg/dl) | 26.6 | 23.5 | 31.1 | 38.1 |
| Low eGFR-creatinine (<60 mL/min/1.73m2) | 1.0 | 0.9 | 1.3 | 1.2 |
| Left ventricular hypertrophy | 1.8 | 1.2 | 3.0 | 3.5 |
| Hs-cTnT (ng/L) categories | ||||
| <5 | 69.0 | 72.8 | 64.0 | 52.8 |
| 5-8 | 23.1 | 20.9 | 26.2 | 31.5 |
| 9-13 | 7.9 | 6.2 | 9.9 | 15.7 |
Estimates are percentages unless otherwise indicated. Abbreviations: eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HDL, high density lipoprotein; Hs-cTnT, cardiac troponin T measured with a high sensitivity assay; LDL, low density lipoprotein
At the follow-up visit 6 years after baseline among persons who did not develop cardiovascular disease, 4.8% (n=395) of the study population had incident elevated hs-cTnT (≥14 ng/L); of these, 43.6% (n=172) had hs-cTnT 9-13 ng/L at baseline, 36.7% (n=145) had hs-cTnT 5-8 ng/L at baseline, and 19.7% (n=78) were undetectable (hs-cTnT <5 ng/L) at baseline 6 years prior (Supplemental Table 1). Among those persons with incident hs-cTnT ≥14 ng/L at the 6-year follow-up visit, the median [25th percentile, 75th percentile] hs-cTnT was 17 ng/dL [15, 21]. Among person who remained free of cardiovascular disease over the follow-up period, persons with diabetes (7.4% of the population) and pre-diabetes (22.1% of the population) at visit 2 had a higher crude incidence of hs-cTnT ≥14 ng/L at visit 4 compared to persons without diabetes (Figure 1, Panel A). In multinomial regression models accounting for the competing risk of cardiovascular events or death between the two visits, diabetes and pre-diabetes were significantly associated with incident subclinical myocardial damage (incident hs-cTnT ≥14 ng/L) even after adjustment for cardiovascular risk factors (Figure 1, Panel B). The relative risks (RRs) and 95% confidence intervals (CIs) comparing persons with pre-diabetes or diabetes defined by clinical categories of HbA1c to persons with no diabetes at visit 2 were 1.38 (95%CI 1.07, 1.77) and 2.46 (95%CI 1.77, 3.42), respectively (Figure 1, Panel B and Supplemental Table 2).
Figure 1.
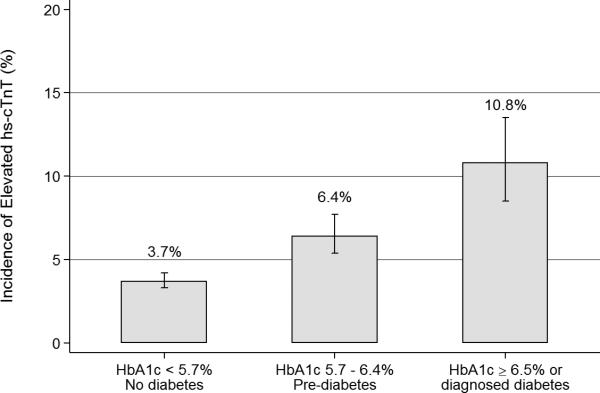
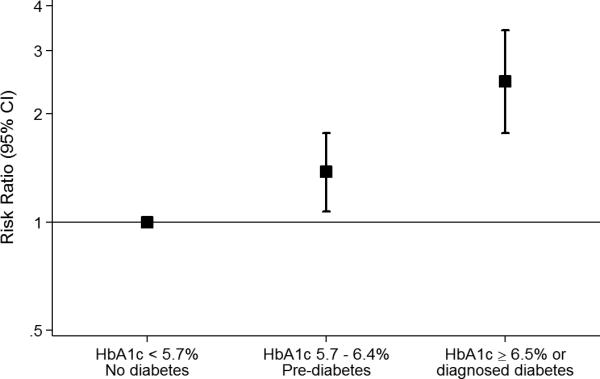
A. Cumulative incidence of elevated (≥ 14 ng/L) highly sensitive cardiac troponin T (hs-cTnT) at follow-up (visit4, 1996-1998) according to categories of pre-diabetes and diabetes at baseline (visit 2, 1990-1992) among persons who remained free of cardiovascular disease during the follow-up period, N = 8,165. B. Adjusted risk ratios (95% confidence intervals) for the association of pre-diabetes and diabetes with 6-year incident elevated (≥14 ng/L) according to pre-diabetes and diabetes at baseline (visit 2, 1990-1992), N = 9,331.
Our results were not appreciably altered when persons with hs-cTnT values greater than 30 ng/L (n=33) at the follow-up visit were excluded (data not shown). When diabetes and pre-diabetes were defined using fasting glucose criteria, a similar pattern but weaker associations with incident elevations in hs-cTnT were observed (Supplemental Table 2). Interaction by race was not statistically significant (p-value-for-interaction=0.271) and race-stratified analyses showed similar patterns of association in black and white adults (Supplemental Table 3). Analyses stratified by baseline category of hs-cTnT revealed that the strongest associations were observed among those persons with hs-cTnT levels ≤8 ng/L at baseline (Table 2).
Table 2.
Adjusted* risk ratios (95% confidence intervals) for the association of pre-diabetes and diabetes with 6-year incident elevated (≥ 14 ng/L) highly sensitive cardiac troponin T (hs-cTnT) at visit 4 (1996-1998) according to diagnostic categories of pre-diabetes and diabetes defined by hemoglobin A1c (HbA1c) and stratified by baseline category of hs-cTnT at visit 2 (1990-1992)
| Baseline (visit 2, 1990-1992) Troponin Group (N = 9,331) | ||||||
|---|---|---|---|---|---|---|
| < 5 ng/L (N = 6,438) | 5 – 8 ng/L (N = 2,155) | 9 – 13 ng/L (N = 738) | ||||
| n cases /Total N | RR (95%CI) | n/N | RR (95%CI) | n/N | RR (95%CI) | |
| HbA1c diagnostic criteria | ||||||
| < 5.7 % (no diabetes) | 42/4613 | 1 (reference) | 78/1326 | 1 (reference) | 94/394 | 1 (reference) |
| 5.7 – 6.4% (pre-diabetes) | 25/1389 | 2.00 (1.17-3.43) | 40/569 | 1.18 (0.77-1.80) | 51/214 | 0.98 (0.62-1.55) |
| Diabetes (≥ 6.5% or diagnosis) | 11/436 | 3.34 (1.60-6.98) | 27/260 | 2.31 (1.36-3.93) | 27/130 | 1.42 (0.78-2.60) |
| p-value for trend† | <0.001 | 0.006 | 0.375 | |||
Adjusted for age (years), race-center (whites-Washington County; whites-Minneapolis; blacks-Jackson; blacks-Forsyth County, whites-Forsyth County), sex (male or female), body mass index (kg/m2), C-reactive protein (mg/L), smoking (current; former; never), mean systolic blood pressure (mm Hg), LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), triglycerides (mg/dL), estimated glomerular filtration rate (mL/min/1.73m2), current use of hypertension medication (yes or no), current lipid-lowering medication use (yes or no), alcohol use (current; former; never) and left ventricular hypertrophy (yes or no).
p-values for linear trend were obtained by numbering the categories 1 through 3 and including this categorical variable as a linear term in the model.
In secondary analyses, we found that incident elevations in hs-cTnT were significantly associated with incident coronary heart disease, heart failure, and all-cause mortality (Figure 2 and Supplemental Table 4). For heart failure and mortality, there were robust and monotonic associations across groups defined by diabetes status within persons with and without incident elevated hs-cTnT. Those persons who developed subclinical myocardial damage as assessed by incident elevations in hs-cTnT had higher risks of heart failure and all-cause mortality even across diabetes categories compared to those persons who did not develop subclinical myocardial damage. Incident elevations in hs-cTnT were less strongly associated with coronary heart disease and the overall pattern of association was somewhat less robust. Nonetheless, persons with incident elevated hs-cTnT and diabetes had substantially increased risks of heart failure (HR 6.37, 95%CI 4.27 to 9.51), all-cause mortality (HR 4.36, 95%CI 3.14 to 6.07), and coronary heart disease (HR 3.84, 95%CI 2.52 to 5.84) compared to persons with no incident elevations in hs-cTnT and without diabetes. These observed patterns with clinical events were similar in black and white adults (Supplemental Table 5). In sensitivity analyses accounting for incident cases of diabetes that occurred in persons with no diabetes and pre-diabetes at visit 2, persons who developed diabetes during the follow-up period were at higher risk of events compared persons who remained nondiabetic (Supplemental Table 6). And persons who remained in the pre-diabetes group over the 6-year period were at higher risk, particularly if hs-cTnT was elevated. When added to models with diabetes and all other covariates already included, elevations in hscTnT significantly improved model discrimination for heart failure (p<0.001) and death (p=0.005), but the improvement in prediction for coronary heart disease was of only borderline significance (p=0.085) (Table 3). Similar patterns were observed when the analyses were limited to persons with diabetes, although our power was correspondingly lower with only N=569 subjects in this group. The improvement in the C-statistic for coronary heart disease was no longer statistically significant (p=0.173).
Figure 2.
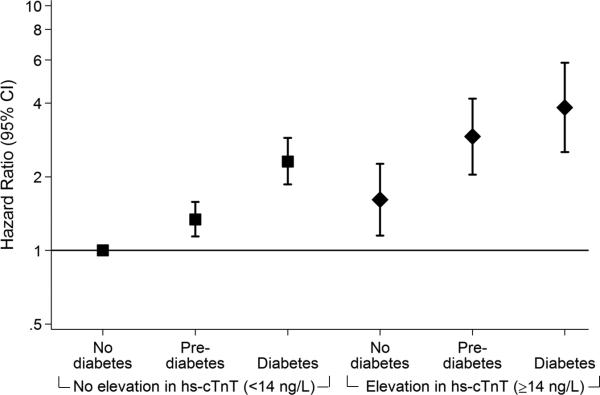
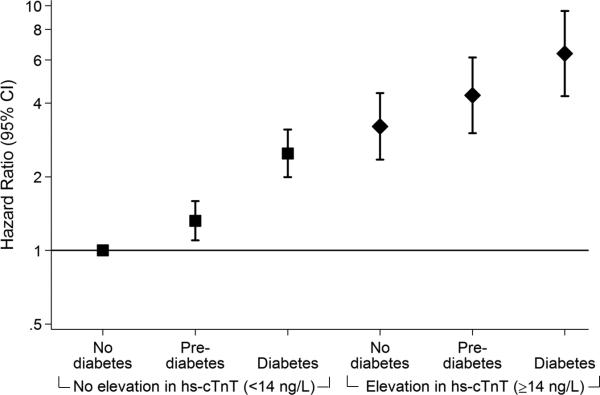
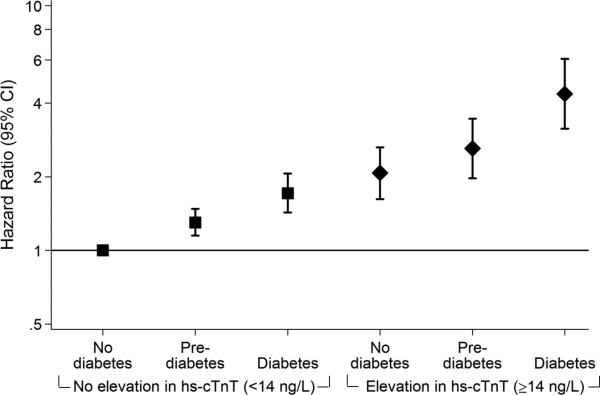
Adjusted hazard ratios (95% confidence intervals) for the association of diabetes status with incident coronary heart disease (A), heart failure (B) and all-cause mortality (C) among persons with and without subsequent progression of myocardial damage as assessed by 6-year incident elevation (≥14 ng/L) in highly sensitive cardiac troponin T (hs-cTnT), N=8,005. Panel A. Coronary heart disease. Panel B. Heart failure. Panel C. All-cause mortality.
Table 3.
C-statistics and differences in C-statistics for clinical events (coronary heart disease, heart failure, and all-cause mortality) from models with and without incident elevated hs-cTnT in the overall population and among persons with diabetes
| Overall Population (N=8005) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Coronary heart disease | Heart failure | All-cause mortality | |||||||
| Model | C-statistic | Difference | p-value | C-statistic | Difference | p-value | C-statistic | Difference | p-value |
| Base model* | 0.7093 | Ref. | - | 0.7447 | Ref. | - | 0.7199 | Ref. | - |
| + elevated hs-cTnT | 0.7118 | 0.0025 | 0.085 | 0.7541 | 0.0094 | <0.001 | 0.7237 | 0.0038 | 0.005 |
| Persons with diabetes (N=569) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Coronary heart disease | Heart failure | All-cause mortality | |||||||
| Model | C-statistic | Difference | p-value | C-statistic | Difference | p-value | C-statistic | Difference | p-value |
| Base model* | 0.6519 | Ref. | - | 0.6860 | Ref. | - | 0.6871 | Ref. | - |
| + elevated hs-cTnT | 0.6551 | 0.0033 | 0.173 | 0.6998 | 0.0139 | 0.001 | 0.6941 | 0.0070 | 0.002 |
Base model includes: age (years), race-center (whites-Washington County; whites-Minneapolis; blacks-Jackson; blacks-Forsyth County, whites-Forsyth County), sex (male or female), diabetes status (HbA1c <5.7%, HbA1c 5.7% to 6.4%, diabetes diagnosis or HbA1c ≥6.5%), body mass index (kg/m2), C-reactive protein (mg/L), smoking (current; former; never), mean systolic blood pressure (mm Hg), LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), triglycerides (mg/dL), estimated glomerular filtration rate (mL/min/1.73m ), current use of hypertension medication (yes or no), current lipid-lowering medication use (yes or no), alcohol use (current; former; never) and left ventricular hypertrophy (yes or no). In the analysis limited to persons with diabetes, diabetes status was not included in the model.
DISCUSSION
In this community based population without clinical cardiovascular disease, we found that pre-diabetes and diabetes were significantly associated with the 6-year incidence of subclinical myocardial damage as assessed by elevation of cardiac troponin T detected with a novel highly sensitive assay. We further observed that these incident elevations in hs-cTnT were associated with future clinical outcomes, particularly heart failure and death.
In 2010, HbA1c was added to the tests recommended for use in the diagnosis of diabetes and identification of persons at risk for diabetes 40. Our results suggest that persons with pre-diabetes, when defined by HbA1c criteria, are at risk for not only the subsequent development of diabetes3 but also for the progression of subclinical cardiac damage and ensuing cardiovascular events. There has been controversy regarding the categories used to define pre-diabetes, particularly the discordance between those persons identified using the recommended clinical cut-points of 5.7-6.4% for HbA1c and 100-125 mg/dL for fasting glucose (impaired fasting glucose). Pre-diabetes, particularly when defined by HbA1c criteria, was associated with incident elevations of hs-cTnT and clinical outcomes, demonstrating that while the pre-diabetes category of HbA1c identifies fewer people 41, persons with HbA1c 5.7-6.4% are at higher risk of progression of myocardial damage compared to persons with impaired fasting glucose. Because there were 435 deaths between the two visits excluded from study, our estimates of the magnitude of the association of diabetes and pre-diabetes with progression of myocardial damage may be conservative. The 99th percentile of hs-cTnT was 22 ng/L at end of the 6-year follow-up period in this study population of participants who remained free of cardiovascular disease. This is higher than 14 ng/L, the 99th percentile of the healthy reference population defined by the manufacturer. There is currently much debate regarding approaches to defining the reference ranges for highly sensitive cardiac troponin assays in the general population 42, 43; “normal” values for these assays in the population have not yet been established.
Given the controversy regarding the clinical implications of racial differences in HbA1c 44, 45, the lack of an interaction by race in our study is reassuring. We found that HbA1c diagnostic categories were associated with progression of myocardial damage and clinical outcomes in both blacks and whites, consistent with other studies suggesting no racial disparity in the prognostic value of HbA1c as a risk marker of microvascular or macrovascular outcomes 46-48. Persons with diabetes have a substantially elevated risk of cardiovascular events and death compared to persons without diabetes, even after accounting for major cardiovascular risk factors 4, 49. Previous studies have also established robust associations between cardiac troponin T measured by the same assay as studied here and the incident development of heart failure, stroke, coronary heart disease and all-cause mortality in community-based populations 20, 50-52. A previous study has also shown that increases in hs-cTnT over 2-3 years are associated with subsequent risk of heart failure and cardiovascular death 50. We have previously shown a cross-sectional association between HbA1c and hs-cTnT in the ARIC cohort 21.
Our results extend these previous findings and support a possible deleterious effect of hyperglycemic states on the myocardium. We also found that persons with prediabetes or diabetes who subsequently developed subclinical myocardial damage—as indicated by an incident elevation of hs-cTnT at the 6-year follow-up visit—were at highest risk of clinical events, particularly heart failure and mortality. Furthermore, those persons without diabetes but who had incident elevated hs-cTnT, were at similar or higher risk of heart failure and mortality compared to persons with diabetes but no incident elevation in hs-cTnT. Indeed, hs-cTnT significantly improved risk stratification for heart failure and death in the overall population and among persons with diabetes.
It is noteworthy that hs-cTnT was strongly associated with microvascular risk factors (e.g. hypertension, diabetes) and only weakly associated with traditional atherosclerotic risk factors (e.g. LDL-cholesterol). Our previous work is also consistent with this finding that hs-cTnT reflects cardiac damage occurring via non-atherosclerotic mechanisms 21, 53. The robust association between hyperglycemia and hs-cTnT may be mediated through micro-ischemia due to the insufficiency of small intra-myocardial arterioles (or possibly capillaries), reflecting primary small vessel disease. Or it is possible that this may reflect myocardial hypertrophy, the out-stripping the capacity of the microvasculature to supply nutrients to the myocardium. Indeed, hyperglycemia-induced injury to the myocardium may be an important contributor to the growing epidemic of heart failure associated with diabetes and obesity 54.
Recent, large randomized clinical trials of interventions to reduce cardiovascular risk in persons with diabetes or pre-diabetes have been disappointing55-59. Major advances in the medical management of lipids and blood pressure over the past several decades and evidence of possible adverse effects of glucose-lowering drugs have complicated the interpretation of contemporary trials designed to further lower cardiovascular risk in persons with diabetes. A concern is that these interventions have been “too little, too late,” focusing on reducing risk in high-risk persons often with a history of cardiovascular disease and/or long duration of diabetes who are already being aggressively managed. Recent trials have focused of the relatively narrow end point of combined hard cardiovascular events, typically incorporating fatal and non-fatal myocardial infarction, stroke, and coronary heart disease. By contrast, the STOP-NIDDM trial demonstrated a significant reduction in cardiovascular risk (an a priori secondary outcome) in a high risk pre-diabetes population treated with acarbose compared to placebo, although the total number of cardiovascular events was small (n=15 in the treatment arm and n=32 in the placebo arm) 60. Nonetheless, there are scant data on the effect of glucose-lowering interventions in persons with pre-diabetes or diabetes with no clinical cardiovascular disease at the outset.
The current study had several limitations. We only had two measurements of hs-cTnT, 6 years apart, to characterize progression of myocardial damage. Adjustment for LVH was limited to assessment using ECG data only and despite rigorous adjustment for potential confounding factors including demographics, blood pressure, lipids, adiposity, kidney function, and medication use, we cannot eliminate the possibility of residual confounding in this observational setting. Strengths of this study include the large, community-based sample, rigorous measurement of traditional cardiovascular risk factors, and the availability of HbA1c and fasting glucose measurements to characterize pre-diabetes. The long-term follow-up of the ARIC cohort and active surveillance for cardiovascular events and deaths allowed us to conduct secondary analyses of incident cardiovascular outcomes.
In summary, this study provides evidence for a deleterious effect of hyperglycemia on the myocardium, even below the threshold for a diagnosis of diabetes. Furthermore, those persons with evidence of incident subclinical myocardial damage were at high risk for future mortality and cardiovascular events, particularly heart failure. With the growing dual epidemics of obesity and diabetes, these results underscore the importance preventing progression to early hyperglycemic states and development of diabetes. Our results suggest that primary and secondary prevention of atherosclerotic disease in diabetes, for example via statin therapy, may not be sufficient to fully address the cardiac risk associated with hyperglycemic states.
Supplementary Material
Acknowledgements
The authors thank the staff and participants of the ARIC Study for their important contributions. Reagents for the high sensitivity cardiac troponin and C-reactive protein assays were donated by Roche Diagnostics.
Funding Sources: This research was supported by NIH/NIDDK grant R01 DK089174 to Dr. Selvin. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Footnotes
Conflict of Interest Disclosures: Drs. Hoogeveen and Ballantyne have received grant support from Roche Diagnostics and are co-investigators on a provisional patent filed by Roche for use of biomarkers in heart failure prediction. The other authors declare no commercial conflicts of interest.
References
- 1.Davis TM, Coleman RL, Holman RR, Group U. Prognostic significance of silent myocardial infarction in newly diagnosed type 2 diabetes mellitus: United kingdom prospective diabetes study (ukpds) 79. Circulation. 2013;127:980–987. doi: 10.1161/CIRCULATIONAHA.112.000908. [DOI] [PubMed] [Google Scholar]
- 2.Hu FB, Stampfer MJ, Haffner SM, Solomon CG, Willett WC, Manson JE. Elevated risk of cardiovascular disease prior to clinical diagnosis of type 2 diabetes. Diabetes Care. 2002;25:1129–1134. doi: 10.2337/diacare.25.7.1129. [DOI] [PubMed] [Google Scholar]
- 3.Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, Coresh J, Brancati FL. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362:800–811. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emerging Risk Factors C, Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njolstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarwar N, Aspelund T, Eiriksdottir G, Gobin R, Seshasai SRK, Forouhi NG, Sigurdsson G, Danesh J, Gudnason V. Markers of dysglycaemia and risk of coronary heart disease in people without diabetes: Reykjavik prospective study and systematic review. PLoS Med. 2010;7:e1000278. doi: 10.1371/journal.pmed.1000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitkanen OP, Nuutila P, Raitakari OT, Ronnemaa T, Koskinen PJ, Iida H, Lehtimaki TJ, Laine HK, Takala T, Viikari JS, Knuuti J. Coronary flow reserve is reduced in young men with iddm. Diabetes. 1998;47:248–254. doi: 10.2337/diab.47.2.248. [DOI] [PubMed] [Google Scholar]
- 7.Yokoyama I, Momomura S, Ohtake T, Yonekura K, Nishikawa J, Sasaki Y, Omata M. Reduced myocardial flow reserve in non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1997;30:1472–1477. doi: 10.1016/s0735-1097(97)00327-6. [DOI] [PubMed] [Google Scholar]
- 8.Nitenberg A, Valensi P, Sachs R, Dali M, Aptecar E, Attali JR. Impairment of coronary vascular reserve and ach-induced coronary vasodilation in diabetic patients with angiographically normal coronary arteries and normal left ventricular systolic function. Diabetes. 1993;42:1017–1025. doi: 10.2337/diab.42.7.1017. [DOI] [PubMed] [Google Scholar]
- 9.Wallace TW, Abdullah SM, Drazner MH, Das SR, Khera A, McGuire DK, Wians F, Sabatine MS, Morrow DA, de Lemos JA. Prevalence and determinants of troponin t elevation in the general population. Circulation. 2006;113:1958–1965. doi: 10.1161/CIRCULATIONAHA.105.609974. [DOI] [PubMed] [Google Scholar]
- 10.Di Carli MF, Janisse J, Grunberger G, Ager J. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J Am Coll Cardiol. 2003;41:1387–1393. doi: 10.1016/s0735-1097(03)00166-9. [DOI] [PubMed] [Google Scholar]
- 11.Gerstein HC, Werstuck GH. Dysglycaemia, vasculopenia, and the chronic consequences of diabetes. The Lancet Diabetes Endocrinol. 2013;1:71–78. doi: 10.1016/S2213-8587(13)70025-1. [DOI] [PubMed] [Google Scholar]
- 12.Meigs JB, Larson MG, D'Agostino RB, Levy D, Clouse ME, Nathan DM, Wilson PW, O'Donnell CJ. Coronary artery calcification in type 2 diabetes and insulin resistance: The framingham offspring study. Diabetes care. 2002;25:1313–1319. doi: 10.2337/diacare.25.8.1313. [DOI] [PubMed] [Google Scholar]
- 13.Moebus S, Stang A, Mohlenkamp S, Dragano N, Schmermund A, Slomiany U, Hoffmann B, Bauer M, Broecker-Preuss M, Mann K, Siegrist J, Erbel R, Jockel KH. Association of impaired fasting glucose and coronary artery calcification as a marker of subclinical atherosclerosis in a population-based cohort--results of the heinz nixdorf recall study. Diabetologia. 2009;52:81–89. doi: 10.1007/s00125-008-1173-y. [DOI] [PubMed] [Google Scholar]
- 14.Lee KK, Fortmann SP, Fair JM, Iribarren C, Rubin GD, Varady A, Go AS, Quertermous T, Hlatky MA. Insulin resistance independently predicts the progression of coronary artery calcification. Am Heart J. 2009;157:939–945. doi: 10.1016/j.ahj.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Folsom AR, Eckfeldt JH, Weitzman S, Ma J, Chambless LE, Barnes RW, Cram KB, Hutchinson RG. Relation of carotid artery wall thickness to diabetes mellitus, fasting glucose and insulin, body size, and physical activity. Atherosclerosis risk in communities (aric) study investigators. Stroke. 1994;25:66–73. doi: 10.1161/01.str.25.1.66. [DOI] [PubMed] [Google Scholar]
- 16.McNeely MJ, McClelland RL, Bild DE, Jacobs DR, Jr., Tracy RP, Cushman M, Goff DC, Jr., Astor BC, Shea S, Siscovick DS. The association between a1c and subclinical cardiovascular disease: The multi-ethnic study of atherosclerosis. Diabetes Care. 2009;32:1727–1733. doi: 10.2337/dc09-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin t assay. Clin Chem. 2010;56:254–261. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 18.Morrow DA, Antman EM. Evaluation of high-sensitivity assays for cardiac troponin. Clin Chem. 2009;55:5–8. doi: 10.1373/clinchem.2008.117218. [DOI] [PubMed] [Google Scholar]
- 19.Sabatine MS, Morrow DA, de Lemos JA, Jarolim P, Braunwald E. Detection of acute changes in circulating troponin in the setting of transient stress test-induced myocardial ischaemia using an ultrasensitive assay: Results from timi 35. Eur Heart J. 2009;30:162–169. doi: 10.1093/eurheartj/ehn504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, Folsom AR, Heiss G, Coresh J, Ballantyne CM. Cardiac troponin t measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the atherosclerosis risk in communities study. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubin J, Matsushita K, Ballantyne CM, Hoogeveen R, Coresh J, Selvin E. Chronic hyperglycemia and subclinical myocardial injury. J Am Coll Cardiol. 2012;59:484–489. doi: 10.1016/j.jacc.2011.10.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng J, Ye P, Luo L, Xiao W, Xu R, Wu H. Association between blood glucose levels and high-sensitivity cardiac troponin t in an overt cardiovascular disease-free community-based study. Diabetes Res Clin Pract. 2012;97:139–145. doi: 10.1016/j.diabres.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 23.The atherosclerosis risk in communities (aric) study: Design and objectives. The aric investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 24.American Diabetes A. Standards of medical care in diabetes--2013. Diabetes Care. 2013;36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parrinello CM, Grams ME, Couper D, Ballantyne CM, Hoogeveen RC, Eckfeldt JH, Selvin E, Coresh J. Calibration of analytes over twenty-five years in the atherosclerosis risk in communities study. American Heart Association Epidemiology and Prevention and Nutrition, Physical Activity and Metabolism 2014 Scientific Sessions. 2014 [Google Scholar]
- 26.Selvin E, Coresh J, Zhu H, Folsom A, Steffes MW. Measurement of hba1c from stored whole blood samples in the atherosclerosis risk in communities study. J Diabetes. 2010;2:118–124. doi: 10.1111/j.1753-0407.2010.00070.x. PMID:20923494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siedel J, Hagele EO, Ziegenhorn J, Wahlefeld AW. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clin Chem. 1983;29:1075–1080. [PubMed] [Google Scholar]
- 28.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 29.Nagele U, Hagele EO, Sauer G, Wiedemann E, Lehmann P, Wahlefeld AW, Gruber W. Reagent for the enzymatic determination of serum total triglycerides with improved lipolytic efficiency. J Clin Chem Clin Biochem. 1984;22:165–174. doi: 10.1515/cclm.1984.22.2.165. [DOI] [PubMed] [Google Scholar]
- 30.Operations manual no. 10: Clinical chemistry determinations, version 1.0. ARIC Coordinating Center, School of Public Health, University of North Carolina; Chapel Hill, NC: 1987. [Google Scholar]
- 31.Operations manual no. 2: Cohort component procedures, version 1.0. ARIC Coordinating Center, School of Public Health, University of North Carolina; Chapel Hill, NC: 1987. [Google Scholar]
- 32.Operations manual no. 11: Sitting blood pressure, version 1.0. ARIC Coordinating Center, School of Public Health, University of North Carolina; Chapel Hill, NC: 1987. [Google Scholar]
- 33.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casale PN, Devereux RB, Alonso DR, Campo E, Kligfield P. Improved sex-specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: Validation with autopsy findings. Circulation. 1987;75:565–572. doi: 10.1161/01.cir.75.3.565. [DOI] [PubMed] [Google Scholar]
- 35.Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle-aged adults : 9-year follow-up of the atherosclerosis risk in communities (aric) cohort. Stroke. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 36.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the atherosclerosis risk in communities (aric) study: Methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 37.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (aric) study: A comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agarwal SK, Avery CL, Ballantyne CM, Catellier D, Nambi V, Saunders J, Sharrett AR, Coresh J, Heiss G, Hoogeveen RC. Sources of variability in measurements of cardiac troponin t in a community-based sample: The atherosclerosis risk in communities study. Clin Chem. 2011;57:891–897. doi: 10.1373/clinchem.2010.159350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrell FE, Jr., Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 40.Association AD. Standards of medical care in diabetes—2010. Diabetes Care. 2010;33:S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the united states, 1988-1994 and 1999-2010. Ann Intern Med. 2014;160:517–525. doi: 10.7326/M13-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandoval Y, Apple FS. The global need to define normality: The 99th percentile value of cardiac troponin. Clin Chem. 2014;60:455–62. doi: 10.1373/clinchem.2013.211706. doi: 10.1373/clinchem.2013.211706. Epub 2013 Oct 10. [DOI] [PubMed] [Google Scholar]
- 43.Gore MO, Seliger SL, Defilippi CR, Nambi V, Christenson RH, Hashim IA, Hoogeveen RC, Ayers CR, Sun W, McGuire DK, Ballantyne CM, de Lemos JA. Age- and sex-dependent upper reference limits for the high-sensitivity cardiac troponin t assay. J Am Coll Cardiol. 2014;63:1441–1448. doi: 10.1016/j.jacc.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Selvin E, Steffes MW, Ballantyne CM, Hoogeveen RC, Coresh J, Brancati FL. Racial differences in glycemic markers: A cross-sectional analysis of community-based data. Ann Intern Med. 2011;154:303–309. doi: 10.1059/0003-4819-154-5-201103010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Selvin E, Brancati FL. A conundrum addressed: The prognostic value of hba1c. Nat Rev Endocrinol. 2011;7:c1. doi: 10.1038/nrendo.2010.126-c1. author reply c2. doi: 10.1038/nrendo.2010.126-c1. [DOI] [PubMed] [Google Scholar]
- 46.Selvin E, Rawlings AM, Bergenstal RM, Coresh J, Brancati FL. No racial differences in the association of glycated hemoglobin with kidney disease and cardiovascular outcomes. Diabetes Care. 2013;36:2995–3001. doi: 10.2337/dc12-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao W, Katzmarzyk PT, Horswell R, Wang Y, Johnson J, Hu G. Hba1c and heart failure risk among diabetic patients. J Clin Endocrinol Metab. 2014;99:E263–267. doi: 10.1210/jc.2013-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bower JK, Brancati FL, Selvin E. No ethnic differences in the association of glycated hemoglobin with retinopathy: The national health and nutrition examination survey 2005-2008. Diabetes Care. 2013;36:569–73. doi: 10.2337/dc12-0404. doi: 10.2337/dc12-0404. Epub 2012 Oct 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emerging Risk Factors C, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of serial measures of cardiac troponin t using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–2502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. Association of troponin t detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Folsom AR, Nambi V, Bell EJ, Oluleye OW, Gottesman RF, Lutsey PL, Huxley RR, Ballantyne CM. Troponin t, n-terminal pro-b-type natriuretic peptide, and incidence of stroke: The atherosclerosis risk in communities study. Stroke. 2013;44:961–967. doi: 10.1161/STROKEAHA.111.000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rubin J, Nambi V, Chambless LE, Steffes MW, Juraschek SP, Coresh J, Sharrett AR, Selvin E. Hyperglycemia and arterial stiffness: The atherosclerosis risk in the communities study. Atherosclerosis. 2012;225:246–251. doi: 10.1016/j.atherosclerosis.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horwich TB, Fonarow GC. Glucose, obesity, metabolic syndrome, and diabetesrelevance to incidence of heart failure. J Am Coll Cardiol. 2010;55:283–293. doi: 10.1016/j.jacc.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Selvin E, Bolen S, Yeh HC, Wiley C, Wilson LM, Marinopoulos SS, Feldman L, Vassy J, Wilson R, Bass EB, Brancati FL. Cardiovascular outcomes in trials of oral diabetes medications: A systematic review. Arch Intern Med. 2008;168:2070–2080. doi: 10.1001/archinte.168.19.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassaï B, Erpeldinger S, Wright JM, Gueyffier F, Cornu C. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: Meta-analysis of randomised controlled trials. BMJ. 2011:343. doi: 10.1136/bmj.d4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Look ARG, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Harrison B, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montez MG, Murillo A, Nathan DM, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Reboussin D, Regensteiner JG, Rickman AD, Ryan DH, Safford M, Wadden TA, Wagenknecht LE, West DS, Williamson DF, Yanovski SZ. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Investigators OT, Gerstein HC, Bosch J, Dagenais GR, Diaz R, Jung H, Maggioni AP, Pogue J, Probstfield J, Ramachandran A, Riddle MC, Ryden LE, Yusuf S. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319–328. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 59.Group NS, Holman RR, Haffner SM, McMurray JJ, Bethel MA, Holzhauer B, Hua TA, Belenkov Y, Boolell M, Buse JB, Buckley BM, Chacra AR, Chiang FT, Charbonnel B, Chow CC, Davies MJ, Deedwania P, Diem P, Einhorn D, Fonseca V, Fulcher GR, Gaciong Z, Gaztambide S, Giles T, Horton E, Ilkova H, Jenssen T, Kahn SE, Krum H, Laakso M, Leiter LA, Levitt NS, Mareev V, Martinez F, Masson C, Mazzone T, Meaney E, Nesto R, Pan C, Prager R, Raptis SA, Rutten GE, Sandstroem H, Schaper F, Scheen A, Schmitz O, Sinay I, Soska V, Stender S, Tamas G, Tognoni G, Tuomilehto J, Villamil AS, Vozar J, Califf RM. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362:1463–1476. doi: 10.1056/NEJMoa1001122. [DOI] [PubMed] [Google Scholar]
- 60.Chiasson J, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. STOP-NIDDM Trial Research Group. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: The stop-niddm trial. JAMA. 2003;290:486–494. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.