Abstract
Head and neck squamous cell carcinomas (HNSCC) contain a small sub-population of stem cells endowed with unique capacity to generate tumors. These cancer stem cells (CSC) are localized in perivascular niches and rely on crosstalk with endothelial cells for survival and self-renewal, but the mechanisms involved are unknown. Here, we report that stromal interleukin (IL)-6 defines the tumorigenic capacity of CSC sorted from primary human HNSCC and transplanted into mice. In search for the cellular source of IL-6, we observed a direct correlation between IL-6 levels in tumor-associated endothelial cells and the tumorigenicity of CSC. In vitro, endothelial cell-IL-6 enhanced orosphere formation, p-STAT3 activation, survival and self-renewal of human CSC. Notably, a humanized anti-IL-6R antibody (tocilizumab) inhibited primary human CSC-mediated tumor initiation. Collectively, these data demonstrate that endothelial cell-secreted IL-6 defines the tumorigenic potential of CSC, and suggest that HNSCC patients might benefit from therapeutic inhibition of IL-6/IL-6R signaling.
Keywords: Perivascular niche, Self-renewal, Survival, Squamous cell carcinoma, Angiogenesis
Introduction
Head and neck squamous cell carcinomas (HNSCC) contain a small population of highly tumorigenic cells that exhibit self-renewal and multipotency, hallmarks of stem cells [1,2]. Emerging evidence from HNSCC and other tumor types suggests that cancer stem cells exhibit resistance to chemotherapy and radiotherapy and might be involved in the establishment of metastasis, which is a major cause of cancer-related deaths [3-5]. Such findings led to the hypothesis that improvements in the long-term outcome of patients with cancer will require ablation of cancer stem cells. Cancer stem cells and physiological stem cells share similar characteristics, such as differentiation and self-renewal [6,7]. Therefore, direct targeting of cancer stem cells might be accompanied by toxicities related to the unintended elimination of normal stem cells (e.g. hematopoietic stem cells). We recently demonstrated that cancer stem cells reside in perivascular niches in patients with HNSCC, and that endothelial cells contribute to the survival and self-renewal of cancer stem cells [8]. Importantly, cancer stem cells depend on crosstalk with tumor-associated endothelial cells for their survival and maintenance of an undifferentiated state [8], which may also contribute to tumor dormancy [9]. These discoveries raise the exciting possibility that cancer patients may benefit from the therapeutic blockade of the crosstalk between endothelial cells and cancer stem cells within the perivascular niche.
Both, physiological and cancer stem cells, depend on their microenvironment for survival and proliferation [6,7,10]. The protective function of the crosstalk among cells within the perivascular niche has been identified in neural stem cells [11] and neural tumors [12]. The observation that cancer stem cells reside in perivascular niches in HNSCC [8] suggest that potent anti-angiogenic drugs may have a therapeutic effect on both, the endothelial cells and the cancer stem cells. However, emerging evidence demonstrated that certain anti-angiogenic therapies might lead to the development of evasive resistance by enhancing the invasive phenotype of tumor cells [13-15]. These studies suggest that patients may benefit from a targeted approach that blocks signaling pathways initiated by endothelial cells and that contribute to cancer stem cell survival and self-renewal. However, the mechanisms involving the crosstalk between endothelial cells and cancer stem cells are unknown, leaving these targets unidentified.
Independent studies have shown a strong correlation between high serum Interleukin-6 (IL-6) levels and poor survival of patients with head and neck cancer [16,17]. These studies have proposed the use of serum IL-6 as a biomarker to predict tumor recurrence and patient survival. IL-6 activates its downstream target signal transducer and activator of transcription 3 (STAT3), which is constitutively active in several malignancies including those of the head and neck [18]. Indeed, therapeutic inhibition of STAT3 has been found to slow down tumor growth [19,20]. Interestingly, IL-6 plays a critical role in the biology of cancer stem cells in breast and brain tumors [21-23]. It is known that endothelial cells secrete high levels of IL-6, especially in response to inflammatory stimuli [24], which play a major role in the pathobiology of most epithelial cancers. It has also been shown that the IL-6 receptors (IL-6R, gp130) exhibit aberrant expression patterns in some cancer stem cells, such as gliomas [22]. However, the role of IL-6 signaling in the crosstalk between endothelial cells and cancer stem cells remains unknown.
Here, we unveiled a paracrine signaling pathway initiated by IL-6 secreted by endothelial cells that enhances the survival, self-renewal and tumorigenic potential of primary human head and neck cancer stem-like cells. Using laser capture microdissection (LCM), we determined that IL-6 expression is higher in the endothelial cells than in the tumor cells of primary human HNSCC, providing strong clinical rationale for this study. Notably, we observed that endothelial cell-secreted IL-6 enhances the tumorigenic potential and the survival of cancer stem-like cells using a series of complementary approaches that included transplantation of primary human head and neck cancer stem-like cells (ALDHHIGHCD44HIGH) into athymic IL-6−/− mice, generation of xenograft tumors with ALDHHIGHCD44HIGH cells that are vascularized with human endothelial cells stably transduced with shRNA-IL-6, and treatment of mice bearing tumors generated with ALDHHIGHCD44HIGH cells with an anti-IL-6R antibody (tocilizumab). Collectively, these data demonstrate that endothelial IL-6 contributes to the biology of cancer stem cells and suggest that therapeutic inhibition of this pathway would be beneficial for patients with head and neck cancer.
Materials and Methods
Head and neck cancer stem-like cell sorting
The use of primary human tumor specimens was done under protocols approved by the University of Michigan Institutional Review Board. Tumors were cut and minced with a sterile scalpel until they could pass through a 25 ml pipette tip. They were mixed in a 9:1 solution of DMEM-F12 (Hyclone) and Collagenase/Hyaluronidase (Stem Cell Technologies). This mixture was then incubated at 370C for 1 hour and passed through a 10-ml pipette every 15 minutes for mechanical dissociation. Cells were filtered through a 40-μm nylon sieve (BD Falcon), washed with low glucose DMEM and centrifuged at 800 rpm for 3 minutes. Single cell suspensions obtained from primary specimens (as well as from cell lines or xenografts) were washed, counted, and re-suspended at 1×106 cells/ml PBS. The Aldefluor kit (Stem Cell Technologies) was used to identify cells with high ALDH activity. Briefly, cells were suspended in activated Aldefluor substrate (BAA) or in a specific ALDH inhibitor (DEAB) for 45 minutes at 370C. Then, cells were exposed to anti-CD44 (clone G44-26BD; Pharmingen). A series of lineage markers (i.e. anti-CD2, CD3, CD10, CD16, CD18, CD31; BD Pharmingen) was used to eliminate lineage cells, mouse cells were eliminated using anti-H2Kd antibody (BD Biosciences), and 7-Aminoactinomycin (7-AAD; BD Pharmingen) was used to eliminate non-viable cells. Here and throughout this manuscript, in vitro studies were done in at least triplicate specimens per condition, and three independent experiments were performed to verify reproducibility of the data.
Orosphere assay
Non-adherent spheroids of head and neck cancer cells (named orospheres) were generated from FACS-sorted cells (5×103 cells/well) cultured in triplicate wells in ultra-low attachment plates (Corning), as we showed [25]. Briefly, cells were cultured in low glucose DMEM containing or not 24-hour conditioned medium (CM) from primary human dermal microvascular endothelial cells (HDMEC, Lonza) collected in serum-free endothelial basal medium (EBM; Lonza) at a ratio of 3:1. Alternatively, cells were treated with HDMEC CM containing 0.4 μg/ml anti-IL-6 antibody (R&D Systems), 10 μg/ml humanized anti-IL-6R antibody (Tocilizumab, Chugai Pharmaceuticals) or non-specific isotype-matched IgG. As a positive control, we used 20 ng/ml rhIL-6 (R&D Systems). After 3 days, orospheres (defined as colonies of ≥25 cells) were visualized and quantified under light microscopy.
SCID mouse model of human tumor angiogenesis
Xenograft tumors vascularized with functional human microvessels were generated in severe combined immunodeficient (SCID) mice (CB17 SCID; Taconic), as we described [26]. Briefly, 0-1,000 head and neck cancer stem-like cells (ALDHHIGHCD44HIGH) or 1,000 non-cancer stem cells (ALDHLOWCD44LOW) were seeded along with 5×105 HDMEC-shRNA-IL-6 or HDMEC-shRNA-C in poly-(L-lactic) acid biodegradable scaffolds. Bilateral scaffolds were implanted subcutaneously in the dorsum of each mouse. Alternatively, mice received scaffolds containing 5×105 HDMEC cells and 1×103 ALDHHIGHCD44HIGH or ALDHLOWCD44LOW cells. Starting on Day 1, mice received weekly intraperitoneal injections of either 10 mg/kg humanized anti-IL-6R antibody (Chugai Pharmaceuticals) or non-specific IgG, as described [27]. After 30 days, mice were euthanized and tumors were retrieved, measured, weighed and processed. The impact of host IL-6 on tumor initiation was evaluated in C57B6 IL-6−/− and IL-6+/+ mice [28] originally purchased from the Jackson Laboratories, and backcrossed onto an immunodeficient/athymic mouse background.
Results
Stromal IL-6 enhances the survival of cancer stem-like cells
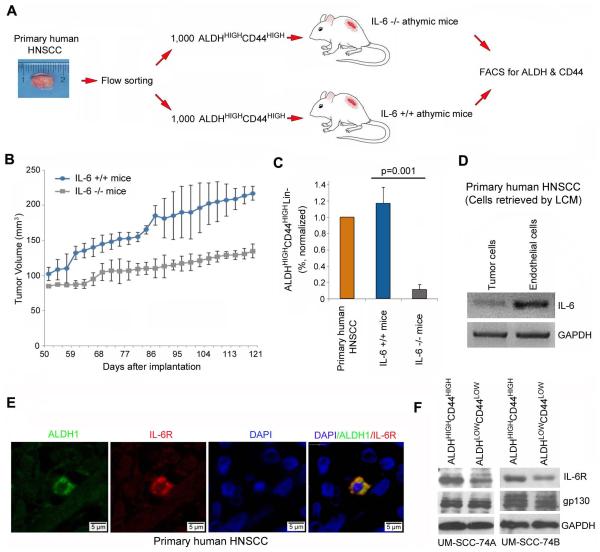
Serum IL-6 has been identified as an independent predictor of poor survival in head and neck squamous cell carcinoma patients [17]. It is known that IL-6 is a potent inflammatory cytokine secreted by stromal cells, and that IL-6 enhances stem cell properties in gliomas [22]. However, the mechanisms underlying the effect of endothelial cell-secreted IL-6 on the biology of HNSCC are unknown. To begin to understand the impact of stromal IL-6 on the survival of cancer stem cells, we generated tumor xenografts by transplanting primary human cancer stem-like cells in IL-6−/− athymic mice (Fig. 1A). For this, immediately after surgical removal of the primary tumor from patients with HNSCC (Supplementary Fig. S1), cancer stem-like cells (i.e. ALDHHIGHCD44HIGH) were sorted and transplanted in the IL-6+/+ mice. 1,000 ALDHHIGHCD44HIGH cells transplanted in the IL-6+/+ mice generated more and larger tumors than transplantation of 1,000 ALDHHIGHCD44HIGH into IL-6−/− deficient littermates (Fig. 1B). Importantly, the fraction of ALDHHIGHCD44HIGH cells in the tumor xenografts generated in the IL-6−/− mice was approximately 10-fold smaller than the fraction of ALDHHIGHCD44HIGH cells in control mice (p=0.001) (Fig. 1C). These data demonstrate that stromal IL-6 plays a critical role in the head and neck tumor microenvironment, and contributes cancer stem cell survival, tumor initiation and growth.
Figure 1.
Stromal IL-6 supports the survival of cancer stem-like cells. (A) Schematic representation of the approach used for testing the role of stromal IL-6 on the tumorigenic potential of cancer stem-like cells (ALDHHIGHCD44HIGH) in primary tumors. ALDHHIGHCD44HIGH cells were isolated from primary human HNSCC and implanted in IL-6−/− or IL-6+/+ immunodeficient mice. At the termination of the experiment, tumors were retrieved, single cell suspensions prepared, and the proportion of ALDHHIGHCD44HIGH cells determined by FACS. (B) Graph represents the volume of tumors arising from primary human ALDHHIGHCD44HIGH cells transplanted into IL-6−/− or IL-6+/+ immunodeficient mice. (C) Graph depicting the percentage of cancer stem-like cells in tumor xenografts, as compared to the primary tumor. (D) RT-PCR showing the mRNA expression of IL-6 in human endothelial cells as compared to the tumor cells. Cells were retrieved by LCM from paraffin-embedded sections from 4 different patients with primary HNSCC. (E) Confocal microscopy of a primary human HNSCC immunostained for ALDH1 (green) and IL-6R (red). DAPI (blue) identified nuclei (Patient 19). (F) Western Blot for IL-6R and gp130 in head and neck cancer stem-like cells (ALDHHIGHCD44HIGH) and control cells (ALDHLOWCD44LOW) sorted from two HNSCC cell lines (UM-SCC-74A, UM-SCC-74B).
Considering our recent observation that cancer stem cells reside in the perivascular niche in HNSCC [8], and the demonstration that endothelial cells challenged by mediators of inflammation secrete high levels of IL-6 [24], we explored the possibility that tumor-associated endothelial cells constitute an important source of IL-6 within the perivascular cancer stem cell niche. To quantify the relative expression of IL-6 in different microenvironmental compartments in human HNSCC, we performed laser capture microdissection of endothelial cells or carcinoma cells from several head and neck cancer patients [29] and observed that IL-6 expression was significantly higher in the endothelial cells than in the tumor cells (Fig. 1D). These data were corroborated by the observation that human endothelial cells secrete more IL-6 than HNSCC cell lines (p<0.05) (Supplementary Fig. S2A, S2B). Confocal microscopy from human HNSCC tissue sections revealed co-localization of ALDH and IL-6R (Fig. 1E). Western blots showed that ALDHHIGHCD44HIGH cells express higher levels of IL-6R than control ALDHLOWCD44LOW cells, while the expression of gp130 was similar (Fig. 1F). Collectively, these data demonstrated that the elements required for an IL-6-mediated crosstalk within the perivascular stem cell niche are in place in human head and neck tumors.
Endothelial cell-secreted IL-6 enhances the survival of cancer stem-like cells and promotes tumor initiation
To evaluate the impact of endothelial cell-secreted IL-6 on the biology of cancer stem-like cells, we silenced its expression in primary human dermal microvascular endothelial cells (HDMEC) and used these cells to generate tumor xenografts with a humanized vasculature, as we described [26]. In attempt to verify the specificity of lentiviral-mediated IL-6 silencing in primary endothelial cells, we observed a significant inhibition of IL-6 expression, but no significant change in expression of IL-8 (Supplementary Fig. S2C). Using this approach, we have shown that the vast majority of the blood vessels within the xenograft tumors are lined with the human endothelial cells that were transplanted in the scaffold [26]. We observed that xenograft tumors vascularized with IL-6-silenced endothelial cells exhibited similar microvessel density as those vascularized with endothelial cells transduced with empty lentiviral vectors (p=0.117). The tumor cells used here were primary human cancer stem-like (ALDHHIGHCD44HIGH) cells sorted from 3 different patients with HNSCC (Fig. 2A-2D). Time to palpability was used as a determinant of the tumorigenic potential of the cell combinations tested here. We observed that transplantation of 1,000 ALDHHIGHCD44HIGH cells generated tumors in 100% of the mice (n=26) within 38 days (Fig. 2B). However, co-transplantation of HDMEC-shRNA-IL-6 with ALDHHIGHCD44HIGH significantly delayed (p=0.0016) the time to tumor palpability as compared to co-transplantation of scrambled sequence control endothelial cells and ALDHHIGHCD44HIGH (Fig. 2B). When individual tumors are tracked, one observes an overall trend for earlier initiation and larger volumes of tumors vascularized with HDMEC-shRNA-C than in tumors vascularized with HDMEC-shRNA-IL-6 cells (Fig. 2C). Notably, the average volume was smaller when endothelial cells stably transduced with shRNA-IL-6 were used to vascularize the xenograft tumors generated with cancer stem-like cells sorted from three different patients, demonstrating that this effect was not patient-specific (Fig. 2D).
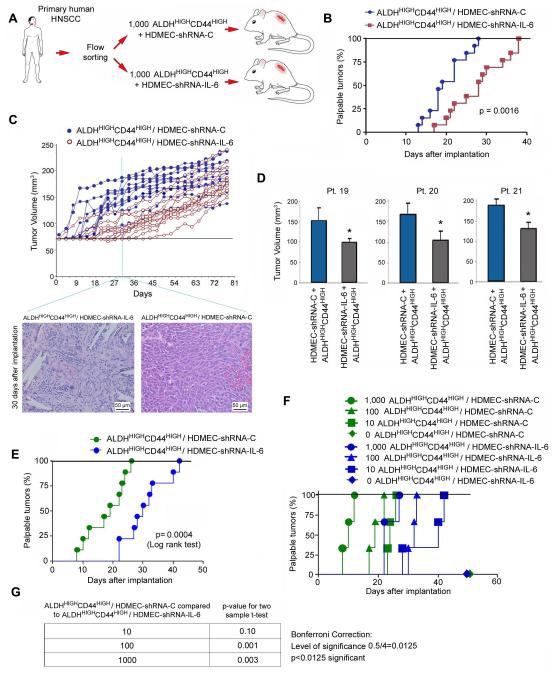
Figure 2.
Endothelial cell-secreted IL-6 enhances the tumorigenic potential of cancer stem-like cells. (A) Schematic representation of the approach used for testing the role of endothelial cell-secreted IL-6 on the tumorigenic potential of primary human ALDHHIGHCD44HIGH cells. ALDHHIGHCD44HIGH cells were sorted from primary HNSCC and implanted in immunodeficient mice to generate xenograft tumors vascularized with HDMEC-shRNA-IL-6 or control HDMEC-shRNA-C. (B) Graph showing the percentage of palpable tumors over time in ALDHHIGHCD44HIGH tumors vascularized with HDMEC-shRNA-IL-6 or HDMEC-shRNA-C. (C) Graph showing individual tumor volume over time (n=13). Photomicrographs (200×) of HE-stained tissue sections of scaffolds containing 1,000 primary human ALDHHIGHCD44HIGH cells and vascularized with HDMEC-shRNA-IL-6 or HDMEC-shRNA-C, 30 days after implantation in SCID mice. Few (if any) tumor cells are visible in the implants vascularized with HDMEC-shRNA-IL-6, while the entire microscopy field is populated with tumor cells in the implants vascularized with HDMEC-shRNA-C. The PLLA scaffold (white areas) is still visible in the implants containing ALDHHIGHCD44HIGH/HDMEC-shRNA-IL-6, while in the ALDHHIGHCD44HIGH/HDMEC-shRNA-C group the scaffold has already been completely resorbed by the enzymatic activity of the tumor cells. (D) Graph showing the average tumor volume of tumor xenografts generated by cells sorted from HNSCC from 3 patients at Day 45 (p<0.05). (E-G) ALDHHIGHCD44HIGH cells were isolated from a primary HNSCC (Patient 26) and implanted in serial dilutions of 1000, 100, 10 or 0 cells along with 500,000 HDMEC-shRNA-IL-6 or HDMEC-shRNA-C cells in immunodeficient mice. (E) Graph showing the overall trend for time to palpability. In this case, the zero ALDHHIGHCD44HIGH cell group was not included, as no tumors were observed in this experimental condition. (F) Graph showing the percentage of palpable tumors over time for each individual experimental condition. (G) Table showing the pairwise statistics for the experimental conditions that generated tumors, as determined using the Bonferroni correction at p<0.0125.
To more rigorously study the effect of endothelial cell-secreted IL-6 on tumor initiation, we performed a serial dilution experiment. We implanted 1,000, 100, 10, or 0 ALDHHIGHCD44HIGH cells sorted from a primary human HNSCC along with either HDMEC-shRNA-C or HDMEC-shRNA-IL-6 cells. We observed that co-transplantation of HDMEC-shRNA-IL-6 with ALDHHIGHCD44HIGH significantly delayed (p=0.0004) tumor initiation as compared to co-transplantation of control endothelial cells and ALDHHIGHCD44HIGH (Fig. 2E), confirming the data discussed above (Fig. 2B). Notably, all implants containing at least 10 ALDHHIGHCD44HIGH cells generated tumors (Fig. 2F). However, the larger the number of cancer stem-like cells, the shorter the time to palpability (Fig. 2F). A pair-wise statistical comparison between different ALDHHIGHCD44HIGH cell densities demonstrated that the greater the initial number of cancer stem-like cells, the greater the effect of endothelial cell-secreted IL-6 in promoting tumors (Fig. 2G). Together, these studies demonstrate that endothelial cell-secreted IL-6 enhances the tumorigenic potential of cancer stem-like cells in vivo.
The density (fraction) of cancer stem cells in a tumor is considered indicative of poor prognosis for cancer patients [30]. To evaluate the effects of endothelial cell-secreted IL-6 on the fraction of cancer stem-like cells in vivo, we evaluated the percentage of ALDHHIGHCD44HIGH cells in xenograft tumors generated with cancer stem-like cells retrieved from primary human HNSCC (Fig. 2A). Ablation of endothelial cell-secreted IL-6 within the tumor microenvironment resulted in a significant decrease in the proportion of cancer stem-like cells (p=0.041), as compared to xenografts vascularized with control endothelial cells from cancer stem-like cells from primary patients (Fig. 3A). To confirm the studies performed with cells sorted from primary human HNSCC, we repeated these analyses with cells sorted from a well-characterized human HNSCC cell line (UM-SCC-74A). We observed that the fraction of cancer stem-like cells was significantly reduced (p=0.010) and the tumor initiation in xenografts was delayed (p=0.0008) when xenografts were vascularized with IL-6-silenced human endothelial cells (Fig. 3B, 3C). Notably, the tumor microvascular density was not affected by the silencing of IL-6 in the endothelial cells, as determined by sorting these xenograft tumors for the endothelial marker CD31 (Supplementary Fig. S2D).
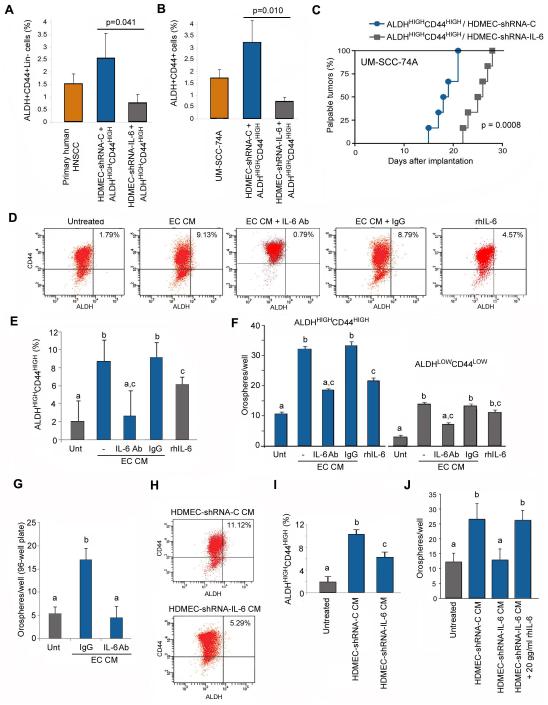
Figure 3.
Endothelial cell-secreted IL-6 promotes the survival and self-renewal of cancer stem-like cells. (A) ALDHHIGHCD44HIGH cells were isolated from a primary human HNSCC (Patient 22) and transplanted into SCID mice along with HDMEC-shRNA-IL-6 or control HDMEC-shRNA-C. Tumors were retrieved after 30 days. Graph showing the percentage of ALDHHIGHCD44HIGH cells as determined by FACS, p=0.041. (B) ALDHHIGHCD44HIGH cells were isolated from a HNSCC cell line (UM-SCC-74A) and transplanted into immunodeficient mice along with HDMEC-shRNA-IL-6 or control HDMEC-shRNA-C. Graph showing the percentage of ALDHHIGHCD44HIGH cells after 30 days, as determined by FACS, p=0.010. (C) Graph showing the percentage of palpable tumors over time (n=6), p=0.0008. (D) Representative FACS analysis of UM-SCC-74A cells treated with endothelial cell CM with or without neutralizing antibody against IL-6 for 48 hours. Number in the top right quadrant depicts the percentage of ALDHHIGHCD44HIGH cells in the sample. (E) Graph depicting the quantification and statistical analyses of the specimens showed in panel D. (F) Graph depicting the number of orospheres arising from ALDHHIGHCD44HIGH and ALDHLOWCD44LOW cells cultured under ultra-low attachment conditions and treated with CM from endothelial cells (HDMEC) in presence of anti-IL-6 neutralizing antibody or IgG control. (G) Graph depicting the number of orospheres arising from ALDHHIGHCD44HIGH cells sorted from UM-SCC-74A, cultured in 96-well ultra-low attachment plates and treated with CM from endothelial cells (HDMEC) in presence of anti-IL-6 antibody or IgG. In this case, 1 cell was seeded per well, to eliminate the possibility of cell aggregation. (H) Representative FACS analysis of UM-SCC-74A cells treated with CM from HDMEC-shRNA-IL-6 or control HDMEC-shRNA-C for 48 hours and (I) is the quantification and statistical analysis of these data. (J) Graph depicting the number of orospheres arising from ALDHHIGHCD44HIGH cells (UM-SCC-74A) cultured in ultra-low attachment plates and treated with CM from HDMEC-shRNA-C, HDMEC-shRNA-IL-6 +/− 20 ng/ml rhIL-6, or unconditioned medium. Different lower case letters represent p<0.05.
Endothelial cell-secreted IL-6 promotes cancer stem cell properties in vitro
To evaluate the functional role of endothelial cell secreted IL-6 on cancer stem-like cells in vitro, we exposed unsorted head and neck cancer cells (UM-SCC-74A) to endothelial cell conditioned medium (CM) in presence of a neutralizing antibody to IL-6 and measured the percentage of ALDHHIGHCD44HIGH by flow cytometry (Fig. 3D). Endothelial cell CM increased the fraction of cancer stem-like cells (Fig. 3D, 3E). However, treatment of the CM with anti-IL-6 antibody reduced the percentage of cancer stem-like cells back to baseline levels (Fig. 3E), without a significant effect in overall cell survival (data not shown). To evaluate the effect of endothelial cell-secreted IL-6 on the survival and self-renewal of cancer cells, we plated ALDHHIGHCD44HIGH or ALDHLOWCD44LOW cells in ultra-low attachment plates and evaluated the formation of non-adherent colonies of cells (named orospheres). As expected, more orospheres were generated by ALDHHIGHCD44HIGH cells than from ALDHLOWCD44LOW cells, and the number of orospheres was increased upon treatment with endothelial cell CM (Fig. 3F; Supplementary Fig. S3), confirming our previous report (8). Notably, neutralizing anti-IL-6 antibody inhibited the inductive effect of endothelial cell conditioned medium, while rhIL-6 increased the number of orospheres (but not to the same extent as the conditioned medium). To ascertain that the orospheres were not formed simply by cell aggregation, we also performed single-cell colony formation assays in ultra-low attachment plates (Fig. 3G). We observed that endothelial cell conditioned medium increased the number of orospheres as compared to controls, and that an anti-IL-6 neutralizing antibody blocked this effect.
To verify the data obtained with anti-IL-6 antibody, we used a second approach consisted of treating unsorted tumor cells with CM from the HDMEC-shRNA-IL-6 or HDMEC-shRNA-C cells (Fig. 3H). A more potent induction of the cancer stem cell fraction upon treatment with CM from HDMEC-shRNA-C than with HDMEC-shRNA-IL-6 CM was observed (Fig. 3H, 3I). Importantly, silencing of IL-6 in the endothelial cells abrogated endothelial cell conditioned medium-induced orosphere formation (Fig. 3J). And adding recombinant human IL-6 was sufficient to rescue the effect of IL-6-depleted conditioned-media on orosphere formation (Fig. 3J).
It has been postulated that cancer stem cells exhibit an invasive phenotype, which could be involved in the generation of secondary tumors [31]. Considering the fact that human HNSCC are highly invasive, we evaluated the effects of endothelial cell-secreted IL-6 on the invasive phenotype of ALDHHIGHCD44HIGH cells in vitro. We observed that CM from endothelial cells significantly enhanced the invasive potential of ALDHHIGHCD44HIGH cells (Supplementary Fig. S4A, S4B). Notably, treatment with neutralizing antibody against IL-6, or exposure to HDMEC-shRNA-IL-6 CM, abrogated the inductive effect of the endothelial cell CM (Supplementary Fig. S4). Taken together, these findings demonstrate that endothelial cells constitute a critical source of the IL-6 that promotes the survival, self-renewal, and invasive potential of head and neck cancer stem-like cells in vitro.
Endothelial cell-secreted IL-6 induces STAT3 phosphorylation in cancer stem cells
The canonical IL-6 signal transduction pathway involves binding to IL-6R and to the common signal transducing receptor gp130, signaling through the Janus kinases and activation of STAT3. Activation of STAT3 signaling plays an important role in the self-renewal and stemness of embryonic stem cells, intestinal stem cells, and glioblastoma stem cells [23,32-34]. Therefore, phosphorylation of STAT3 might be considered indicative of stemness. Immunohistochemical examination of primary HNSCC tissues showed strong expression of p-STAT3 around tumor blood vessels (Fig. 4A), where most human HNSCC cancer stem-like cells are located. To evaluate the expression pattern of p-STAT3 in cancer stem-like cells, we sorted cells from a primary HNSCC and observed that ALDHHIGHCD44HIGH cells exhibit constitutive phosphorylation of STAT3 while the non-stem cell population (ALDHLOWCD44LOW) did not (Fig. 4B). It is known that the activation of the ERK-MAPK pathway correlates with the loss of stemness and acquisition of a more differentiated state (35,36). Here, we observed that cancer stem-like cells (ALDHHIGHCD44HIGH) showed low expression of p-ERK, while ALDHLOWCD44LOW cells exhibited constitutive activation of this pathway (Fig. 4B). Parallel experiments with a head and neck cancer cell line (UM-SCC-74A) also confirmed there was significant expression of P-STAT3 with cells sorted from primary HNSCC (Fig. 4B).
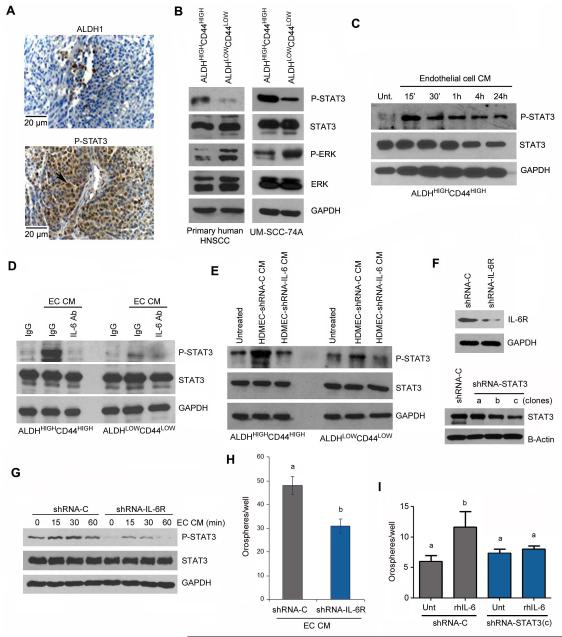
Figure 4.
Endothelial cell-secreted IL-6 activates the STAT3 pathway in head and neck cancer stem-like cells (A) Immunohistochemical localization of ALDH1-positive and p-STAT3-positive cells in tumor xenografts generated with ALDHHIGHCD44HIGH cells sorted from primary human HNSCC. (B) Western blot showing baseline phosphorylation of STAT3 and ERK in head and neck cancer stem-like cells (ALDHHIGHCD44HIGH) compared to control cells (ALDHLOWCD44LOW) sorted from a primary HNSCC (Patient 28) or from a head and neck cancer cell line (UM-SCC-74A). (C) Western blot for phosphorylated and total STAT3 in ALDHHIGHCD44HIGH cells cultured in ultra-low attachment plates and treated with endothelial cell conditioned medium (CM) for 0-24 hours. (D) Western blots for phosphorylated and total STAT3 in ALDHHIGHCD44HIGH or ALDHLOWCD44LOW cells treated with endothelial cell CM in presence of anti-IL-6 antibody or IgG for 15 minutes. (E) Western blots for phosphorylated and total STAT3 in ALDHHIGHCD44HIGH or ALDHLOWCD44LOW cells treated with CM collected from HDMEC-shRNA-IL-6 or control HDMEC-shRNA-C for 30 minutes. (F) Western blot to determine the effectiveness of IL-6R knockdown in UM-SCC-74A cells (shRNA-IL-6R); and STAT3 knockdown in UM-SCC-74B cells (shRNA-STAT3). We used 3 different shRNA sequences and determined that sequence (c) was the most effective to downregulate STAT3 expression. As controls, we transduced cells with a scrambled sequence (shRNA-C). (G) Western blots for phosphorylated and total STAT3 in UM-SCC-74A cells transduced with shRNA-IL-6R (or control shRNA-C) and exposed to endothelial cell conditioned medium for 0-60 minutes. (H) Graph depicting the number of orospheres arising from UM-SCC-74A cells stably transduced with shRNA-IL-6R or shRNA-C and cultured in ultra-low attachment plates. All cells were cultured in presence of 24-hour endothelial cell conditioned medium (EC CM). The untreated control for these experiments was serum-free endothelial basal (EBM) medium. (I) Graph depicting the number of orospheres arising from UM-SCC-74B cells stably transduced with shRNA-STAT3(c) or shRNA-C and cultured in ultra-low attachment plates. Cells were cultured in presence (or absence) of 20 ng/ml rhIL-6. The untreated control for this experiment was serum-free EBM supplemented with PBS (vehicle for rhIL-6). Different lower case letters represent p<0.05.
To evaluate the impact of endothelial cell-secreted factors on the activation of STAT3, we exposed ALDHHIGHCD44HIGH cells to endothelial cell CM and analyzed the phosphorylation levels of STAT3 over time. We found that endothelial cell CM enhanced STAT3 phosphorylation in ALDHHIGHCD44HIGH cells in a time-dependent manner (Fig. 4C). Blockade of IL-6 with neutralizing antibodies abrogated the inductive effect of endothelial cells on STAT3 phosphorylation (Fig. 4D). Likewise, exposure of cancer stem-like cells to CM from IL-6-silenced endothelial cells did not induce STAT3 phosphorylation beyond baseline levels (Fig. 4E). Notably, knockdown STAT3 in ALDHHIGHCD44HIGH cells (Fig. 4F) prevented rhIL-6-induced increase in the number of orospheres (Fig. 4I). And silencing of IL-6R (Fig. 4F) inhibited baseline levels of STAT3 phosphorylation, and partially inhibited endothelial cell CM-induced P-STAT3 (Fig. 4G). The number of orospheres induced by endothelial cell CM was also smaller in IL-6R-silenced tumor cells, as compared to controls (Fig. 4H). Collectively, these data demonstrated that IL-6 secreted by endothelial cells signals through IL-6R to activate STAT3 signaling, which functions as a critical regulator of the self-renewal and survival of head and neck cancer stem cells.
Humanized anti-IL-6R antibody inhibits the survival of cancer stem-like cells and delays tumor initiation
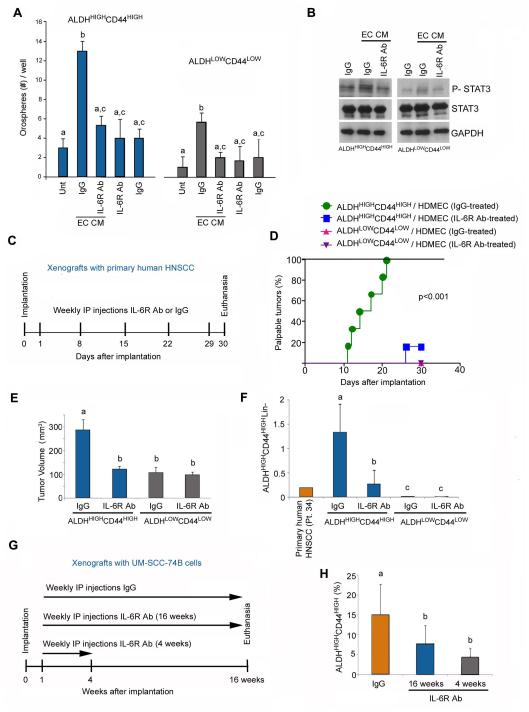
To enhance the translational significance of this work, we performed a series of experiments with the humanized anti-human IL-6R antibody tocilizumab developed by Chugai/Roche [27]. This antibody was approved by the FDA for the treatment of rheumatoid arthritis in 2010. Tocilizumab blocked the effect of endothelial cell CM on the generation of orospheres with cells sorted from primary HNSCC (Fig. 5A) and inhibited endothelial cell-induced phosphorylation of STAT3 in vitro (Fig. 5B). To test the efficacy of inhibition of IL-6R signaling on tumor initiation, we implanted ALDHHIGHCD44HIGH or ALDHLOWCD44LOW cells sorted from a primary HNSCC and treated the mice with tocilizumab (or IgG controls) for 4 weeks (Fig. 5C). No significant weight reduction was observed in mice treated with tocilizumab during the course of this experiment, suggesting that this drug was well tolerated (Supplementary Fig. S5). While all mice transplanted with 1,000 ALDHHIGHCD44HIGH cells developed tumors in the IgG control group (6/6), only one tumor (1/6) was created in the tocilizumab group (Fig. 5D). As expected, none of the mice (0/12) transplanted with control ALDHLOWCD44LOW cells developed tumors within the experimental time (Fig. 5D). The average tumor volume of the mice in the IgG group was significantly higher than in the tocilizumab-treated group (Fig. 5E). Importantly, we observed a significant reduction in the proportion of cancer stem cells (ALDHHIGHCD44HIGH cells) in mice treated with tocilizumab, as compared to mice from the IgG control group (Fig. 5F).
Figure 5.
A humanized anti-human IL-6R antibody (tocilizumab) inhibits self-renewal and tumor initiation of human head and neck cancer stem-like cells transplanted in mice. (A) Graph represents the relative number of orospheres arising from ALDHHIGHCD44HIGH and ALDHLOWCD44LOW cells cultured in ultra-low attachment plates with endothelial cell CM in presence of 0 or 10 μg/ml anti-IL-6R antibody. (B) Western blot showing the phosphorylation of STAT3 in ALDHHIGHCD44HIGH and ALDHLOWCD44LOW cells cultured in ultra-low attachment plates with endothelial cell CM in presence of 0 or 10 μg/ml anti-IL-6R antibody. (C) Schematic representation of the experimental design. Tumor xenografts were generated by the implantation of 1,000 ALDHHIGHCD44HIGH or ALDHLOWCD44LOW cells sorted from a primary human HNSCC (Patient 34) along with 5×105 primary endothelial cells (HDMEC). Mice received weekly intraperitoneal injections of 10 mg/kg anti-IL-6R antibody or IgG for 4 weeks. (D,E) Kaplan-Meier analysis showing the percentage of palpable tumors over time (D). Palpable tumor is defined as tissue overgrowth beyond the size of the scaffold used for cell transplantation. Tumors were only observed upon transplantation of ALDHHIGHCD44HIGH cells, i.e. 6 tumors out of 6 transplants in the IgG-treated group, and 1 tumor out of 6 transplants in the IL-6R antibody-treated group. None of the scaffolds transplanted with ALDHLOWCD44LOW cells generated a tumor. (E) Graph showing the tumor volume 30 days after transplantation. Please note that the volume of the scaffold by itself (without palpable tumor) measured through the skin is typically 70-100 mm3. (F) Graph showing the percentage of ALDHHIGHCD44HIGH cells as determined by FACS. These data is derived from the xenografts treated (or not) with the anti-IL-6R antibody and compared to the percentage of ALDHHIGHCD44HIGH cells found in the primary human HNSCC. (G) Schematic representation of the experimental design. Tumor xenografts (n=18) were generated by the implantation of ALDHHIGHCD44HIGH cells sorted from UM-SCC-74B cells along with primary human endothelial cells (HDMEC). Mice received weekly intraperitoneal injections of 10 mg/kg anti-IL-6R antibody or IgG for 16 weeks. Alternatively, treatment with anti-IL-6R antibody was stopped after 4 weeks and mice were followed-up for additional 12 weeks without treatment. (H) Graph depicting the percentage of ALDHHIGHCD44HIGH cells at the end of the experimental period (16 weeks). Different lower case letters represent p<0.05.
To more fully understand the effect of the anti-IL-6R antibody tocilizumab on the fraction of cancer stem cells, we performed a long-term in vivo of 4 months (Fig. 5G). In this case, we generated xenograft tumors with ALDHHIGHCD44HIGH cells sorted from UM-SCC-74B and administered weekly anti-IL-6R antibody for 16 weeks or for 4 weeks followed by 12 weeks without antibody. We observed that treatment with the anti-IL-6R antibody resulted in a smaller fraction of ALDHHIGHCD44HIGH cells in this tumor model. Surprisingly, this effect was maintained in the group without antibody for the last 12 weeks, suggesting that the effect of IL-6 signaling blockade on head and neck cancer stem cells is long lasting.
Discussion
Acquired resistance to therapy is a major challenge in the management of patients with head and neck cancer. Platinum-based drugs have substantially improved the control of local-regional disease [37]. However, with improved local control of the disease with enhanced radiation therapy, concurrent chemotherapy and advanced surgical techniques, distant metastasis has become a larger percentage of failures in patients with oral cancer [38]. As a consequence, the survival of patients with HNSCC remained largely unchanged over the last 35 years [39]. The poor survival and high recurrence/metastasis rates in patients with HNSCC can possibly be explained by the cancer stem cell hypothesis where the regeneration of a tumor (or the establishment of metastatic foci) requires at least one cancer stem cell. Notably, cancer stem cells are believed to be resistant to conventional chemotherapeutic drugs, exhibit an invasive phenotype and resist anoikis or death by anchorage independence. These characteristics make cancer stem cells prime candidates to drive the process of recurrence and metastasis frequently observed in patients with head and neck cancer. Here, we performed a series of complementary studies to understand mechanisms employed by primary human head and neck cancer stem cells to survive while retaining an undifferentiated and highly tumorigenic phenotype.
We have previously demonstrated that the combination of high ALDH activity and high CD44 expression discriminates a small sub-population of highly tumorigenic cells that have the features of cancer stem cells in HNSCC [8]. We found that these cells exist in perivascular niches and that endothelial cell-secreted factors enhanced the survival of the cancer stem cells, but the critical mediators of this crosstalk were unclear. The importance of stromal IL-6 on tumor initiation and tumor growth was demonstrated in the present study by experiments with xenografts generated with stem-like cells sorted from primary human HNSCC and implanted in immunodeficient IL-6−/− or IL-6+/+ mice. We observed a 10-fold decrease in the proportion of cancer stem-like cells in tumors generated in the IL-6−/− mice. These results demonstrated that host-derived IL-6 plays an important role in the survival/self-renewal of cancer stem cells. Ongoing studies attempt to determine whether this effect was mediated by a direct interaction of the host IL-6 with the cancer stem-like cells, or indirectly by modulating the inflammatory response. Here, our focus was directed to the endothelial cells as a source of the stromal IL-6 for the following reasons: A) We observed that endothelial cells express high IL-6 levels in human HNSCC; B) IL-6 is upregulated under inflammatory conditions [24], which are typically observed in HNSCC; and C) Cancer stem cells exhibit perivascular localization in the head and neck tumor microenvironment.
We observed that endothelial-secreted IL-6 enhances the survival and the stem cell properties of head and neck cancer cells. Interestingly, IL-6 is secreted from thymic blood vessels in response to therapy and promotes the survival of doxorubicin-resistant cells in Burkitt’s lymphoma [40]. One could speculate that these resistant cells exhibit stem-like properties. Furthermore, work in lung carcinomas recently showed that stromal endothelial cells contribute to tumor growth [41]. We observed here that IL-6 secreted by endothelial cells is a potent inducer of the tumor-initiating capacity of head and cancer stem-like cells. These data do not exclude the possibility that other cell types (e.g. myeloid cells) contribute to the overall levels of IL-6 in the tumor microenvironment. However, the experiments in which complementary xenograft tumor models are vascularized with IL-6-silenced endothelial cells (or controls) provide strong evidence that IL-6 secreted by the vascular endothelium is a key determinant of the fraction of cancer stem cells and the time-to-palpability of tumors. Taken together, these studies identify endothelial cell-initiated signaling events as important mediators of the tumorigenicity of cancer stem cells and suggest that such events may contribute to the development of acquired resistance to therapy.
While the concept of perivascular niche for stem cells has been described several years ago [11,12], the understanding of the complex cell-cell interactions within the niche is still emerging. It is known that stem cells reside nearby blood vessels in many tissues, including the bone marrow, brain, and heart [42]. It is also known that endothelial cells in steady state secrete IL-6, and that IL-6 expression can be upregulated by inflammatory cytokines such as IL-1β [43]. These studies suggest that endothelial cell-secreted IL-6 may play a role in the maintenance of the stem cell pool within physiological conditions, and perhaps also during inflammation. In head and neck tumors, we observed that IL-6 secreted by endothelial cells induces survival and self-renewal of ALDHHIGHCD44HIGH cancer stem-like cells. Though we observed an increase in the number of orospheres with rhIL-6 treatment, it was not as potent as the induction mediated by complete endothelial cell conditioned medium. This suggests that other endothelial cell-secreted factors might contribute to the biology and behavior of cancer stem cells. For example, interleukin-8 (CXCL8) is a chemokine that signals through CXCR1 and CXCR2, is expressed in head and neck cancer, and has been implicated in processes that regulate the biology of cancer stem cells [44-46]. We have also recently observed that endothelial cell-secreted EGF induces epithelial to mesenchymal transition (EMT) in head and neck cancer cells, enhancing their migratory and invasive phenotype [47]. It is certainly possible that complex signaling networks are activated by multiple cytokines and chemokines secreted by endothelial cells, which would explain the preferential localization of cancer stem cells in perivascular areas.
Our in vitro results demonstrated that the invasive potential of head and neck cancer stem cells is enhanced by the growth factor milieu secreted by endothelial cells in an IL-6-dependent manner. Such findings suggest that IL-6 secreted by endothelial cells could facilitate the initiation of the metastatic spread by generating a chemotactic gradient that attracts tumor cells towards blood vessels. In fact, a recent study showed that targeting STAT3 with Cucurbitacin I inhibited tumorigenicity and distant metastasis in head and neck tumor models [20]. This suggests a role of IL-6/STAT3 signaling in EMT of HNSCC, as seen in breast cancers [48]. Likewise, local secretion of IL-6 at sites of endothelial cell inflammation or injury might facilitate the homing of circulating tumor progenitor cells to specific sites or organs, and contribute to the development of distant metastases. Although the mechanisms underlying the effect of stem cells on the biology of HNSCC are still unclear, our data suggest that a crosstalk initiated by endothelial cells contributes to the progression of head and neck tumors and that interference with this crosstalk may constitute a viable conceptual target for treatment of these tumors.
Collectively, the understanding of the physiological role of IL-6 and the mechanistic studies performed here, provided the rationale for the therapeutic targeting of the IL-6 signaling pathway in HNSCC. It has been observed that the most common adverse effects of therapeutic inhibition of IL-6R in clinical studies are respiratory tract infections, headaches and hypertension [49], which might be related to biological roles of endothelial cell-derived IL-6 in the recruitment of immune cells and regulation of vascular physiology. Further, IL-6 inhibitors are currently being tested in clinical trials for cancer apart from their use in chronic inflammatory conditions. For example, the anti-IL-6 monoclonal antibody CNTO 328 (siltuximab), is in clinical trials for refractory multiple myelomas and metastatic renal cell cancer [50,51]. Blockade of IL-6R signaling with tocilizumab inhibited xenograft tumor angiogenesis [27]. Here, we observed that tocilizumab dramatically inhibited the tumor-initiating capacity of primary human cancer stem-like cells. Notably, the fraction of cancer stem-like cells was significantly decreased in the mice treated with tocilizumab suggesting that inhibition of IL-6R signaling was sufficient to reduce the survival and/or self-renewal of head and neck cancer stem-like cells in vivo.
Conclusion
We have recently demonstrated that silencing of IL-6 in endothelial cells is sufficient to slowdown tumor growth in proof-of-principle experiments using a cervical adenocarcinoma model [52]. Here, we demonstrated that endothelial cell secreted-IL-6 signaling through IL-6R promotes self-renewal and survival of human primary head and neck cancer stem-like cells (Fig. 6). Blockade of this signaling pathway inhibited tumor initiation and reduced the fraction of cancer stem-like cells in existing tumors. This work establishes the crosstalk between endothelial cells and putative cancer stem cells as a critical mechanism of tumor initiation, and singles out IL-6 as an important signaling molecule in this process. Considering the fact that endothelial cells are readily accessible to systemic therapies, and the recent discovery of potent and specific inhibitors, therapeutic targeting of the IL-6 signaling pathway is emerging as a promising therapeutic strategy for patients with head and neck squamous cell carcinoma.
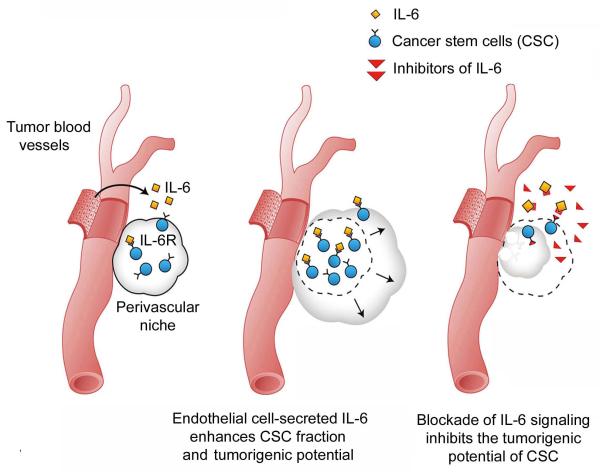
Figure 6.
Schematic representation of the effect of endothelial IL-6-initiated signaling in head and neck cancer. Tumor associated endothelial cells secrete factors including IL-6 that binds to IL-6R and enhances self-renewal and survival of cancer stem-like cells within the perivascular niche. Therapeutic inhibition of IL-6 signaling blocks the crosstalk between endothelial cells and cancer stem-like cells. As a result, the fraction of cancer stem cells is decreased and tumor growth is delayed or prevented.
Supplementary Material
Supplementary Figure S1. Table containing the demographics, tumor type, site, and TNM status of the patients that donated the primary head and neck tumors used in this work.
Supplementary Figure S2. (A) RT-PCR to evaluate the relative expression of IL-6 in a human head and neck squamous cell carcinoma cell line (UM-SCC-74A) and in primary human endothelial cells (HDMEC). (B) Graph showing IL-6 protein levels (ELISA) in primary endothelial cells compared to three different human head and neck squamous cell carcinoma cell lines, i.e. UM-SCC-74A, UM-SCC-74B, UM-SCC-17B. (C) ELISA for IL-6 and IL-8 expression in HDMEC stably transduced with shRNA-C or shRNA-IL-6. Data were normalized by cell number. Different letters represent p<0.001. (D) Graph depicting the fraction of CD31-positive endothelial cells in xenograft tumors generated by ALDHHIGHCD44HIGH cells that were isolated from a HNSCC cell line (UM-SCC-74A) and transplanted into immunodeficient mice along with HDMEC-shRNA-IL-6 or control HDMEC-shRNA-C. Different lower case letters represent p<0.05.
Supplementary Figure S3. Photomicrographs of representative orospheres arising from ALDHHIGHCD44HIGH or ALDHLOWCD44LOW cells cultured in ultra-low attachment plates. Cells were treated with endothelial cell CM containing neutralizing antibody against IL-6 or non-specific isotype-matched IgG. Orospheres were defined as non-adherent colonies consisting of at least 25 cells.
Supplementary Figure S4. (A) Graph depicting the relative number of cancer stem-like cells (ALDHHIGHCD44HIGH) that invaded through Matrigel-coated Transwells. Invasion was induced by treatment with endothelial cell CM containing anti-IL-6 neutralizing antibody or non-specific isotype-matched IgG. Unconditioned medium (untreated) was used as control. (B) ALDHHIGHCD44HIGH cell invasion was evaluated in presence of CM from HDMEC-shRNA-C, HDMEC-shRNA-IL-6 or unconditioned medium (untreated). Invasion was evaluated by staining with Cell Tracker Green followed by quantification in a spectrophotometer. Different lower case letters represent p<0.05.
Supplementary Figure S5. Graph showing the weight of mice that received weekly injections of the humanized anti-IL-6R antibody (tocizilumab) or non-specific isotype matched IgG.
Acknowledgments
We thank Amy Koh-Paige for her help with the IL-6−/− mice, Renata Licks for tissues specimens used for LCM, Chris Strayhorn for assistance with the histology, and Chris Jung for his expertise in medical illustration. We also thank the University of Michigan Flow Cytometry Core and the Imaging Core for their expertise. We thank Chugai Pharmaceutical Co. and Genentech for providing us with the humanized anti-IL-6R antibody (tocilizumab). Support for this project was provided by Weathermax foundation, University of Michigan Comprehensive Cancer Center; grant P50-CA-97248 (University of Michigan Head and Neck SPORE) and P01-CA093900 from the NIH/NCI; and grants R21-DE19279 and R01-DE21139 from the NIH/NIDCR.
Financial support: Support for this project was provided by Weathermax foundation, University of Michigan Comprehensive Cancer Center; grant P50-CA-97248 (University of Michigan Head and Neck SPORE) and P01-CA093900 from the NIH/NCI; and grants R21-DE19279, R01-DE21139 from the NIH/NIDCR.
Footnotes
Conflict of interest: The authors have the following conflict of interest to declare: Max Wicha is an advisor for and has equity in OncoMed Pharmaceuticals. Jacques Nör is the Co-PI of a research grant from MedImmune.
References
- 1.Prince ME, Sivanandan R, Kaczorowski A, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. PROC NATL ACAD SCI USA. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hermann PC, Bhaskar S, Cioffi M, et al. Cancer stem cells in solid tumors. SEMIN CANCER BIOL. 2010;20:77–84. doi: 10.1016/j.semcancer.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Al-Swiahb JN, Chen CH, Chuang HC, et al. Clinical, pathological and molecular determinants in squamous cell carcinoma of the oral cavity. FUTURE ONCOL. 2010;6:837–850. doi: 10.2217/fon.10.35. [DOI] [PubMed] [Google Scholar]
- 4.Chen YC, Chang CJ, Hsu HS, et al. Inhibition of tumorigenicity and enhancement of radiochemosensitivity in head and neck squamous cell cancer-derived ALDH1-positive cells by knockdown of Bmi-1. ORAL ONCOL. 2010;46:158–165. doi: 10.1016/j.oraloncology.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Kakarala M, Wicha MS. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J CLIN ONCOL. 2008;26:2813–2820. doi: 10.1200/JCO.2008.16.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. NAT REV CANCER. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 7.Lobo NA, Shimono Y, Qian D, et al. The biology of cancer stem cells. ANNU REV CELL DEV BIOL. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 8.Krishnamurthy S, Dong Z, Vodopyanov D, et al. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. CANCER RES. 2010;70:9969–9978. doi: 10.1158/0008-5472.CAN-10-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghajar CM, Peinado H, Mori H, et al. The perivascular niche regulates breast tumour dormancy. NAT CELL BIOL. 2013;15:807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parmar A, Marz S, Rushton S, et al. Stromal niche cells protect early leukemic FLT3-ITD+ progenitor cells against First-generation FLT3 Tyrosine kinase inhibitors. CANCER RES. 2011;71:4696–4706. doi: 10.1158/0008-5472.CAN-10-4136. [DOI] [PubMed] [Google Scholar]
- 11.Shen Q, Goderie SK, Jin L, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. SCIENCE. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 12.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. CANCER CELL. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 13.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. NAT REV CANCER. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keunen O, Johansson M, Oudin A, et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. PROC NATL ACAD SCI USA. 2011;108:3749–3754. doi: 10.1073/pnas.1014480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. CANCER CELL. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riedel F, Zaiss I, Herzog D, et al. Serum levels of interleukin-6 in patients with primary head and neck squamous cell carcinoma. ANTICANCER RES. 2005;25:2761–2765. [PubMed] [Google Scholar]
- 17.Duffy SA, Taylor JM, Terrell JE, et al. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. CANCER. 2008;113:750–757. doi: 10.1002/cncr.23615. [DOI] [PubMed] [Google Scholar]
- 18.Grandis JR, Drenning SD, Zeng Q, et al. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. PROC NATL ACAD SCI USA. 2000;97:4227–4232. doi: 10.1073/pnas.97.8.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leong PL, Andrews GA, Johnson DE, et al. Targeted inhibition of Stat3 with a decoy oligonucleotide abrogates head and neck cancer cell growth. PROC NATL ACAD SCI USA. 2003;100:4138–4143. doi: 10.1073/pnas.0534764100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen YW, Chen KH, Huang PI, et al. Cucurbitacin I suppressed stem-like property and enhanced radiation-induced apoptosis in head and neck squamous carcinoma-derived CD44(+)ALDH1(+)cells. MOL CANCER THER. 2010;11:2879–2892. doi: 10.1158/1535-7163.MCT-10-0504. [DOI] [PubMed] [Google Scholar]
- 21.Sansone P, Storci G, Tavolari S, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J CLIN INVEST. 2007;117:3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Lathia JD, Wu Q, et al. Targeting interleukin-6 signaling suppresses glioma stem cell survival and tumor growth. STEM CELLS. 2009;27:2393–2404. doi: 10.1002/stem.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marotta LL, Almendro V, Marusyk A, et al. The JAK2/STAT3 signaling pathway is required for growth of CD44+CD24- stem cell-like breast cancer cells in human tumors. J CLIN INVEST. 2011;121:2723–2735. doi: 10.1172/JCI44745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makó V, Czúcz J, Weiszhár Z, et al. Proinflammatory activation pattern of human umbilical vein endothelial cells induced by IL-1β, TNF-α, and LPS. CYTOMETRY A. 2010;77:962–970. doi: 10.1002/cyto.a.20952. [DOI] [PubMed] [Google Scholar]
- 25.Krishnamurthy S, Nör JE. Orosphere assay: a method for propagation of head and neck cancer stem cells. HEAD NECK. 2013;35:1015–1021. doi: 10.1002/hed.23076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nör JE, Peters MC, Christensen JB, et al. Engineering and characterization of functional human microvessels in immunodeficient mice. LAB INVEST. 2001;81:453–463. doi: 10.1038/labinvest.3780253. [DOI] [PubMed] [Google Scholar]
- 27.Shinriki S, Jono H, Ota K, et al. Humanized anti-interleukin-6 receptor antibody suppresses tumor angiogenesis and in vivo growth of human oral squamous cell carcinoma. CLIN CANCER RES. 2009;15:5426–5434. doi: 10.1158/1078-0432.CCR-09-0287. [DOI] [PubMed] [Google Scholar]
- 28.Dai J, Lin D, Zhang J, et al. Chronic alcohol ingestion induces osteoclastogenesis and bone loss through IL-6 in mice. J CLIN INVEST. 2000;106:887–895. doi: 10.1172/JCI10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaneko T, Okiji T, Kaneko R, et al. Laser-Capture Microdissection for Factor VIII-Expressing Endothelial Cells in Cancer Tissues. METHODS MOL BIOL. 2011;755:395–403. doi: 10.1007/978-1-61779-163-5_33. [DOI] [PubMed] [Google Scholar]
- 30.Neumeister V, Agarwal S, Bordeaux J, et al. In situ identification of putative stem cells by multiplexing ALDH1, CD44 and cytokeratin identifies breast cancer patients with poor prognosis. AM J PATHOL. 2010;176:2131–2138. doi: 10.2353/ajpath.2010.090712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biddle A, Liang X, Gammon L, et al. Cancer stem cells in squamous cell carcinoma switch between two distinct phenotypes that are preferentially migratory or proliferative. CANCER RES. 2011;71:5317–5326. doi: 10.1158/0008-5472.CAN-11-1059. [DOI] [PubMed] [Google Scholar]
- 32.Niwa H, Burdon T, Chambers I, et al. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. GENES DEV. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matthews JR, Sansom OJ, Clarke AR. Absolute requirement for STAT3 function in small-intestine crypt stem cell survival. CELL DEATH DIFFER. 2011;18:1934–1943. doi: 10.1038/cdd.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guryanova OA, Wu Q, Cheng L, et al. Nonreceptor tyrosine kinase BMX maintains self-renewal and tumorigenic potential of glioblastoma stem cells by activating STAT3. CANCER CELL. 2011;19:498–511. doi: 10.1016/j.ccr.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin HY, Tsai CC, Chen LL, et al. Fibronectin and laminin promote differentiation of human mesenchymal stem cells into insulin producing cells through activating Akt and ERK. J BIOMED SCI. 2010;17:56. doi: 10.1186/1423-0127-17-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim EK, Lim S, Park JM, et al. Human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by AMP-activated protein kinase. J CELL PHYSIOL. 2012;227:1680–1687. doi: 10.1002/jcp.22892. [DOI] [PubMed] [Google Scholar]
- 37.Forastiere AA. Chemotherapy in the treatment of locally advanced head and neck cancer. J SURG ONCOL. 2008;97:701–707. doi: 10.1002/jso.21012. [DOI] [PubMed] [Google Scholar]
- 38.Sano D, Myers JN. Metastasis of squamous cell carcinoma of the oral tongue. CANCER METASTASIS REV. 2007;26:645–662. doi: 10.1007/s10555-007-9082-y. [DOI] [PubMed] [Google Scholar]
- 39.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA CANCER J CLIN. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert LA, Hemann MT. DNA-damage-mediated induction of a chemoresistant niche. CELL. 2010;143:355–366. doi: 10.1016/j.cell.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franses JW, Baker AB, Chitalia VC, et al. Stromal endothelial cells directly influence cancer progression. SCI TRANSL MED. 2011;3:66ra5. doi: 10.1126/scitranslmed.3001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gómez-Gaviro MV, Lovell-Badge R, Fernández-Avilés F, et al. The vascular stem cell niche. J CARDIOVAS TRANSL RES. 2012;5:618–630. doi: 10.1007/s12265-012-9371-x. [DOI] [PubMed] [Google Scholar]
- 43.Makó V, Czúcz J, Weiszhár Z, et al. Proinflammatory activation pattern of human umbilical vein endothelial cells induced by IL-1β, TNF-α, and LPS. CYTOMETRY A. 2010;77:962–970. doi: 10.1002/cyto.a.20952. [DOI] [PubMed] [Google Scholar]
- 44.Neiva KG, Zhang Z, Miyazawa M, et al. Crosstalk initiated by endothelial cells enhances migration and inhibits anoikis of squamous cell carcinoma cells through STAT3/Akt/ERK signaling. NEOPLASIA. 2009;11:583–593. doi: 10.1593/neo.09266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ginestier C, Liu S, Diebel ME, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J CLIN INVEST. 2010;120:485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hwang WL, Yang MH, Tsai ML, et al. SNAIL regulates Interleukin-8 expression, stem cell-like activity, and tumorigenicity of human colorectal carcinoma cells. GASTROENTEROLOGY. 2011;141:279–291. doi: 10.1053/j.gastro.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Z, Dong Z, Lauxen IS, et al. Endothelial Cell-Secreted EGF Induces Epithelial to Mesenchymal Transition and Endows Head and Neck Cancer Cells with Stem-like Phenotype. CANCER RES. 2014;74:2869–2881. doi: 10.1158/0008-5472.CAN-13-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sullivan NJ, Sasser AK, Axel AE, et al. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. ONCOGENE. 2009;28:2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanaka T, Ogata A, Narazaki M. Tocilizumab for the treatment of rheumatoid arthritis. EXPERT REV CLIN IMMUNOL. 2010;6:843–854. doi: 10.1586/eci.10.70. [DOI] [PubMed] [Google Scholar]
- 50.Rossi JF, Négrier S, James ND, et al. A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer. BR J CANCER. 2010;103:1154–1162. doi: 10.1038/sj.bjc.6605872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Voorhees PM, Chen Q, Small GW, et al. Targeted inhibition of interleukin-6 with CNTO 328 sensitizes pre-clinical models of multiple myeloma to dexamethasone-mediated cell death. BR J HAEMATOL. 2009;145:481–490. doi: 10.1111/j.1365-2141.2009.07647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neiva KG, Warner KA, Campos MS, et al. Endothelial cell-derived interleukin-6 regulates tumor growth. BMC CANCER. 2014;14:99. doi: 10.1186/1471-2407-14-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Table containing the demographics, tumor type, site, and TNM status of the patients that donated the primary head and neck tumors used in this work.
Supplementary Figure S2. (A) RT-PCR to evaluate the relative expression of IL-6 in a human head and neck squamous cell carcinoma cell line (UM-SCC-74A) and in primary human endothelial cells (HDMEC). (B) Graph showing IL-6 protein levels (ELISA) in primary endothelial cells compared to three different human head and neck squamous cell carcinoma cell lines, i.e. UM-SCC-74A, UM-SCC-74B, UM-SCC-17B. (C) ELISA for IL-6 and IL-8 expression in HDMEC stably transduced with shRNA-C or shRNA-IL-6. Data were normalized by cell number. Different letters represent p<0.001. (D) Graph depicting the fraction of CD31-positive endothelial cells in xenograft tumors generated by ALDHHIGHCD44HIGH cells that were isolated from a HNSCC cell line (UM-SCC-74A) and transplanted into immunodeficient mice along with HDMEC-shRNA-IL-6 or control HDMEC-shRNA-C. Different lower case letters represent p<0.05.
Supplementary Figure S3. Photomicrographs of representative orospheres arising from ALDHHIGHCD44HIGH or ALDHLOWCD44LOW cells cultured in ultra-low attachment plates. Cells were treated with endothelial cell CM containing neutralizing antibody against IL-6 or non-specific isotype-matched IgG. Orospheres were defined as non-adherent colonies consisting of at least 25 cells.
Supplementary Figure S4. (A) Graph depicting the relative number of cancer stem-like cells (ALDHHIGHCD44HIGH) that invaded through Matrigel-coated Transwells. Invasion was induced by treatment with endothelial cell CM containing anti-IL-6 neutralizing antibody or non-specific isotype-matched IgG. Unconditioned medium (untreated) was used as control. (B) ALDHHIGHCD44HIGH cell invasion was evaluated in presence of CM from HDMEC-shRNA-C, HDMEC-shRNA-IL-6 or unconditioned medium (untreated). Invasion was evaluated by staining with Cell Tracker Green followed by quantification in a spectrophotometer. Different lower case letters represent p<0.05.
Supplementary Figure S5. Graph showing the weight of mice that received weekly injections of the humanized anti-IL-6R antibody (tocizilumab) or non-specific isotype matched IgG.