Abstract
Background
COPD is characterized by reduced airway lumen dimensions and fewer peripheral airways. Most studies of airway properties sample airways based upon lumen dimension or at random, which may bias comparisons given reduced airway lumen dimensions and number in COPD. We sought to compare central airway wall dimensions on computed tomography (CT) in COPD and controls using spatially matched airways, thereby avoiding selection bias of airways in the lung.
Methods
The MESA COPD Study and SPIROMICS recruited smokers with COPD and controls aged 50–79 years and 40–80 years, respectively. COPD was defined by current guidelines. Using CT image data, airway dimensions were measured for all central airway segments (generations 0–6) following 5 standardized paths into the lungs. Case-control airway comparisons were spatially matched by generation and adjusted for demographics, body size, smoking, CT dose, percent emphysema, airway length, and lung volume.
Results
Among 311 MESA COPD participants, airway wall areas at generations 3–6 were smaller in COPD compared to controls(all p<0.001). Among 1248 SPIROMICS participants, airway wall areas at generations 1–6 were smaller(all p<0.001), and this reduction was monotonic with increasing COPD severity(P<0.001). In both studies, sampling airways by lumen diameter or randomly resulted in a comparison of more proximal airways in COPD to more peripheral airways in controls(p<0.001) resulting in the appearance of thicker walls in COPD(p<0.02).
Conclusions
Airway walls are thinner in COPD when comparing spatially matched central airways. Other approaches to airway sampling result in comparisons of more proximal to more distal airways and potentially biased assessment of airway properties in COPD.
Keywords: Chronic obstructive pulmonary disease, computed tomography, airways, walls
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is defined by persistent airflow limitation and is a leading cause of morbidity and mortality in the US and globally.[1] Understanding the pathophysiology of COPD requires understanding of the relationship between airway structure and function. Airflow limitation is determined in part by the resistive properties of the tracheobronchial tree, which is a three-dimensional branching structure.[2] Weibel’s classic study of human lung morphometry demonstrated that airway dimensions vary according to the spatial location within the tracheobronchial tree.[3] Therefore, it is likely that the study of airway properties in COPD requires accurate anatomic localization and comparison of spatially equivalent airways in order to provide unbiased results.[4]
Studies accounting for spatial differences in airway dimensions on pathological section or computed tomography (CT) have consistently demonstrated reduced airway lumen dimensions and fewer peripheral airways in COPD.[5–12] Multiple histologic and CT studies have reported thicker airway walls in COPD.[10 12–17] However, these studies sampled airways either based upon lumen diameter or randomly within the identified airways in the lung. If COPD is characterized by reduced airway lumen size and fewer distal airways, such sampling is likely to lead to a comparison of more proximal airways in cases of COPD compared to controls. Such a comparison may introduce a selection bias that would yield erroneous conclusions of thickened airway walls in COPD.
In order to avoid selection bias in the study of airways in COPD, our objective was to compare central airway wall dimensions in COPD and controls that were matched spatially by generation number and anatomical name (e.g., lobar bronchi, segmental bronchi) in two multicenter case-controls studies of COPD, one of milder disease recruited predominantly from the general population and the other of more severe disease recruited predominantly from the subspecialist setting. In addition, we repeated the analyses of airway walls using potentially biased approaches, i.e., sampling airways by lumen diameter or randomly. Finally, we examined the implications of reduced airway lumen caliber and number in COPD for the validity of the Pi10, a derived measure commonly used to study wall thickness in COPD.[5 12 15–18]
Preliminary results were presented in abstract form.[19]
METHODS (See Web Supplement for Additional Details and References)
Study participants
The Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study recruited cases of COPD and controls predominantly from MESA, a population-based prospective cohort study of subclinical atherosclerosis, a non-overlapping lung cancer screening study, and the outpatient community at Columbia University Medical Center. Participants were 50–79 years of age with ≥10 pack-year smoking history. Exclusion criteria were clinical cardiovascular disease, stage IIIb–V chronic kidney disease, asthma prior to age 45 years, prior lung resection, contraindication to magnetic resonance imaging, and pregnancy.
The Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) is recruiting participants 40–80 years of age with >20 pack-year smoking history with COPD and controls with >20 pack-year smoking history, as well as never smokers.[20] Exclusion criteria include other chronic lung diseases except asthma (e.g., sarcoidosis, interstitial lung disease), body mass index (BMI) >40 kg/m2, prior lung resection, metal in the chest (e.g., pacemaker) and pregnancy. The present analysis was performed on the first 1278 current or former smokers completing the baseline evaluation.
Study protocols were approved by the institutional review board of participating institutions and by the National Heart, Lung, and Blood Institute. Written informed consent was obtained from all participants.
Chest computed tomography (CT) acquisition and analysis
All participants in both studies underwent full-lung thoracic CT on 64 or 128-slice helical scanners (120 kVp, 0.625–0.75 mm slice thickness, 0.5 sec. rotation time). Scans were acquired with milliamperes (mA) set by BMI to maintain a consistent volume CT dose index (6.1, 7.6, 11.4 mGy respectively). Images were obtained at suspended full inspiration. Airway dimensions were assessed at a single reading center for both studies blinded to other participant information.
The central airway tree was identified using Apollo Software (VIDA Diagnostics, Coralville, Iowa). Airways were labeled anatomically from trachea to subsegmental bronchi along five pre-specified paths: RB1, RB4, RB10, LB1, and LB10. Segmentation and labeling were visually verified by a dedicated image analyst and all labeled airways were assigned a generation number based upon the number of branch points from the trachea, which was assigned generation 0. Cross-sectional airway wall area and wall thickness, as well as lumen area, diameter, and perimeter were measured perpendicular to the local airway segment’s long axis using a subvoxel resolution algorithm in the Apollo Software, within an image plane, and measurements were averaged along the middle third of each labeled airway segment. Airway length was measured as the distance between branch points.
Percent wall area was calculated for each airway as the ratio of wall area to the sum of wall and lumen area, multiplied by 100. Pi10 was calculated by regressing the square-root wall area on internal perimeter of included airways to predict the square-root wall area of a single hypothetical airway with internal perimeter of 10 mm. A Pi10 was calculated for each participant using all measured airways, as well as using airways from each generation with five or more airways. Airway counts were determined by software summing all visually-confirmed airway segments detected along the five pre-specified paths and stratified by lumen diameter.. Intra-class correlation coefficients for reproducibility of airway measure in the MESA COPD Study were 0.79–0.99, 0.74–0.99 and 0.78–0.96 for wall area, lumen area, and airway count, respectively (Web Supplement Tables E1–E2).
Lung volumes were quantified from segmented lung images. Percent emphysema-like lung was defined as the percentage of total voxels within the lung field <−950 Hounsfield units (percent emphysema−950HU).
Spirometry
Post-bronchodilator spirometry was performed following American Thoracic Society recommendations on a dry-rolling-sealed spirometer in MESA COPD and a pneumotachograph spirometer in SPIROMICS. Predicted spirometry values were calculated using Hankinson reference equations.[21] COPD was defined as post-bronchodilator ratio of forced expired volume in one second to forced vital capacity (FEV1/FVC) less than 0.7 and spirometric severity as mild (FEV1≥80% predicted), moderate (50%≤FEV1<80% predicted), severe (30%≤FEV1<50% predicted), and very severe (FEV1<30% predicted).[1] Controls had a post-bronchodilator FEV1/FVC>0.7 and an FVC above the lower limit of normal.
Anthropometry and other co-variates
Age, gender, and race-ethnicity were self-reported, and height and weight were measured following standardized protocols. Smoking history was assessed using standard questionnaire items; current smoking status was confirmed with urine or plasma cotinine levels in MESA COPD.
Statistical analysis
The MESA COPD and SPIROMICS data were examined separately because the former recruited predominantly from the general population with milder disease, and the latter recruited from the subspecialist setting with more severe disease. Dichotomous variables are presented as proportions and continuous variables as means with standard deviation unless otherwise indicated.
The primary analysis compared central airway wall areas among participants with COPD to controls stratified spatially by generation number. All airways in the pre-specified paths of a given generation were included in the analyses. Within-generation generalized estimating equations with exchangeable covariance matrix structure and robust standard errors were used to account for multiple airway measures per participant;[22] and linear regression to adjust for age, gender, height, BMI-determined CT dose, race-ethnicity, current smoking status, airway length, percent emphysema−950HU, and lung volume achieved at CT. Height and lung volume were included to normalize body size and to account for lung hyperinflation and depth of inspiration at CT, which may influence airway wall dimensions.[23] Sensitivity analyses modelled percent predicted FEV1 and FVC, and stratified by anatomic name as an alternate method of comparing spatially matched airways, and by COPD severity. Airway lumen areas and percent wall areas were also compared according to COPD status by generation number adjusting for the same covariates.
To assess the potential bias of alternative sampling methods, secondary analyses compared airway wall areas in COPD and controls selected based upon airway lumen diameter, as well as randomly sampled (n=15 airways) from each participant, and adjusting for the same covariates. Comparison of the spatial location of airways sampled by these methods according to COPD status was assessed using the χ2-test. The number of observed airways within lumen diameter strata was compared according to COPD status. Finally, Pi10 was calculated for each participant using all airways, as well as for airways from each generation, and compared with respect to COPD status. Calculation of Pi10 required 5 or more airway wall measures per participant; therefore, Pi10 was not calculated for generations 0 to 2.
All calculations were performed using SAS 9.3 (Cary, NC) with a hypothesis testing alpha level of 0.05.
RESULTS
Of 329 participants enrolled in the MESA COPD Study, 311 had visually confirmed spatial mapping of the tracheobronchial tree. Similarly, 1248 of the 1278 SPIROMICS participants had visually confirmed mapping of the tracheobronchial tree. Participants included in the analyses were similar to those with incomplete measures except for differences in severity of airflow obstruction (Web Supplement Table E3).
Clinical characteristics of included participants by COPD status are summarized in Table 1. The MESA COPD Study participants had a mean age of 68±7 years with 37±24 pack-years of smoking. Forty-seven percent of participants had COPD that was predominantly moderate in severity. The SPIROMICS sample had mean age of 65±9 years, 50±24 pack-years of smoking, and more severe COPD. In both studies, the prevalence of white race-ethnicity and number of pack-years of smoking were greater among participants with COPD compared to controls.
Table 1.
Characteristics of Participants included in Airway Dimensions Analysis.
| MESA COPD | SPIROMICS | |||
|---|---|---|---|---|
| No COPD N=166 |
COPD N=145 |
No COPD N=438 |
COPD N=810 |
|
| Age –year | 68±7 | 68±7 | 61±10 | 66±8 |
| Male – % | 54 | 66 | 46 | 59 |
| Race-ethnicity – % | ||||
| White | 45 | 62 | 70 | 84 |
| Black | 25 | 28 | 26 | 12 |
| Other | 30 | 10 | 5 | 5 |
| Height – cm | 167±9 | 171±9 | 170±10 | 171±10 |
| Weight – kg | 80±17 | 80±19 | 83±18 | 80±17 |
| Smoking status – % | ||||
| Former | 77 | 67 | 54 | 68 |
| Current | 23 | 33 | 46 | 32 |
| Pack-years | 32±19 | 44±32 | 43±21 | 54±25 |
| Percent predicted FEV1 | 100±16 | 74±19 | 95±14 | 62±23 |
| FEV1/FVC | 78±5 | 58±11 | 87±5 | 51±13 |
| COPD GOLD severity – % | ||||
| Mild (FEV1≥80% predicted) | - | 39 | - | 24 |
| Moderate (50% ≥ FEV1 < 80% predicted) | - | 47 | - | 44 |
| Severe (30% ≥ FEV1 < 50% predicted) | - | 12 | - | 23 |
| Very severe (FEV1<30% predicted) | - | 1 | - | 9 |
| Lung volume at CT – L | 4.2±1.1 | 4.8±1.2 | 5.3±1.2 | 6.3±1.4 |
| Percent emphysema−950 HU – median (IQR) | 1.2 (1.8) | 4.5 (7.7) | 0.9 (1.7) | 6.9 (14) |
| No. of airways per participant – median (1st, 3rd quartile) | ||||
| Lumen diameter > 11.5 mm | 2 (2, 3) | 2 (2, 3) | 2 (2, 3) | 2 (2, 3) |
| 10.0 mm < lumen diameter ≤ 11.5 mm | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | 1 (0, 2) |
| 8.5 mm < lumen diameter ≤ 10.0 mm | 2 (1, 2) | 2 (1, 2) | 2 (1, 3) | 2 (1, 2)* |
| 7.0 mm < lumen diameter ≤ 8.5 mm | 3 (2, 4) | 3 (2, 3)* | 3 (2, 4) | 3 (2, 4)* |
| 5.5 mm < lumen diameter ≤ 7.0 mm | 4 (3, 5) | 4 (2, 5)* | 3 (2, 5) | 3 (2, 4)* |
| 4.0 mm < lumen diameter ≤ 5.5 mm | 7 (6, 10) | 7 (5, 8)* | 8 (6, 10) | 6 (4, 8)* |
| 2.5 mm < lumen diameter ≤ 4.0 mm | 28 (20, 38) | 19 (14, 27)* | 34 (24, 44) | 20 (15, 28)* |
| Lumen diameter – mm | ||||
| Generation 0 | 16.0±2.3 | 16.5±2.6 | 16.7±2.4 | 16.4±2.5 |
| Generation 1 | 12.0±1.9 | 12.0±1.9 | 12.6±2.1 | 12.3±2.0 |
| Generation 2 | 8.5±1.6 | 8.4±1.6 | 8.8±1.5 | 8.6±1.6 |
| Generation 3 | 6.1±1.5 | 5.7±1.5* | 6.3±1.6 | 6.0±1.6* |
| Generation 4 | 4.4±1.4 | 4.1±1.3* | 4.7±1.7 | 4.3±1.5* |
| Generation 5 | 3.2±1.0 | 3.0±1.0* | 3.4±1.0 | 3.1±1.0* |
| Generation 6 | 2.6±0.9 | 2.5±1.0* | 2.7±0.9 | 2.5±0.8* |
Plus-minus values are means±SD.
p<0.05 for comparison between COPD and controls of airway lumen diameter (Student t-test) or number of airways per participant (Pearson χ2-test).
Abbreviations: MESA denotes Multi-Ethnic Study of Atherosclerosis, SPIROMICS Subpopulations and Intermediate Outcome Measures in COPD Study, COPD chronic obstructive pulmonary disease, FEV1 forced expired volume in the first second, FVC forced vital capacity, GOLD Global Initiative for chronic Obstructive Lung Disease, CT computed tomography, HU Hounsfield units, IQR inter-quartile range, and SD standard deviation.
The number of detectable airways with lumen diameter between 2.5 and 4.0 mm was reduced in COPD compared to controls in both studies (Table 1), and this difference was independent of age, gender, height, BMI-determined CT dose, race-ethnicity, smoking status, percent emphysema−950HU, and lung volume (p<0.001).
Central airway lumen size was significantly smaller in COPD compared to controls in both cohorts, and this was independent of covariates (Web Supplement Table E4). Consistent observations were made for percent predicted FEV1 and FVC (Web Supplement Table E5).
Airway wall areas in COPD: spatially matched central airways
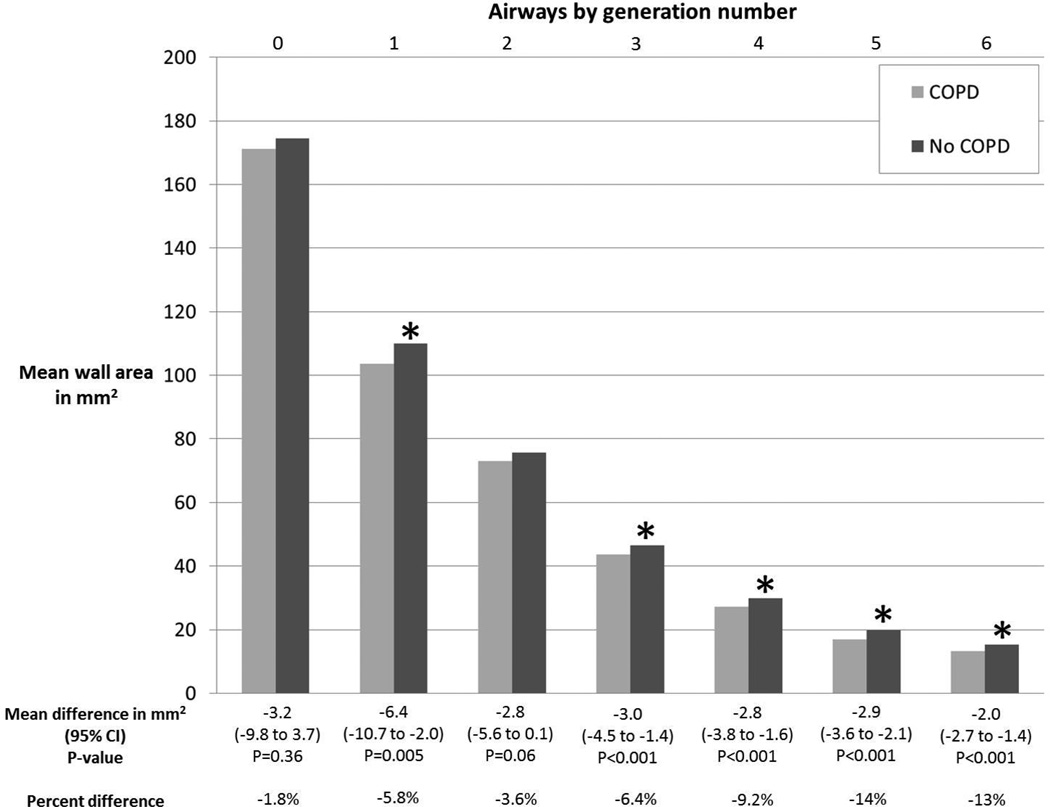
Table 2 summarizes mean airway wall areas according to airway generation number and differences between COPD and controls. In the MESA COPD Study, generation 4 to 6 airway wall areas were significantly smaller in COPD compared to controls in crude comparisons (p≤0.01 for all). In adjusted comparisons (Figure 1), these differences remained significant (p<0.001 for all), and extended to generations 1 and 3 (p≤0.005 for both). Similar associations between airway wall area and COPD status were obtained when matching by anatomic name (Web Supplement Table E6), or using airway wall thickness instead of wall area (Web Supplement Table E7).
Table 2.
Airway Wall Area According to COPD Status Stratified by Generation Number in the MESA COPD Study and SPIROMICS.
| MESA COPD | Airway generation number | ||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Unadjusted mean airway wall area in mm2 | |||||||
| COPD | 179.1 | 109.3 | 75.7 | 45.1 | 27.9 | 17.3 | 13.7 |
| No COPD | 168.0 | 106.9 | 73.3 | 45.4 | 29.4 | 19.1 | 14.9 |
| Difference (95% CI) P-value |
11.1 (2.3 to 20.3) 0.01 |
2.4 (−3.2 to 8.4) 0.41 |
2.3 (−1.1 to 5.9) 0.19 |
−0.4 (−2.1 to 1.4) 0.68 |
−1.6 (−2.7 to −0.4) 0.01 |
−1.7 (−2.5 to −0.9) <0.001 |
−1.2 (−1.7 to −0.6) <0.001 |
| Mean airway wall area in mm2 adjusted for age, gender, height, race-ethnicity, smoking status, airway length, percent emphysema−950HU, BMI-determined CT dose, and lung volume at CT | |||||||
| COPD | 171.3 | 103.6 | 73.0 | 43.5 | 27.2 | 16.9 | 13.2 |
| No COPD | 174.5 | 110.0 | 75.8 | 46.5 | 30.0 | 19.8 | 15.3 |
| Difference (95% CI) P-value |
−3.2 (−9.8 to 3.7) p=0.36 |
−6.4 (−10.7 to −2.0) p=0.005 |
−2.8 (−5.6 to 0.1) p=0.06 |
−3.0 (−4.5 to −1.4) p<0.001 |
−2.8 (−3.8 to −1.6) p<0.001 |
−2.9 (−3.6 to −2.1) p<0.001 |
−2.0 (−2.7 to −1.4) p<0.001 |
| SPIROMICS | Airway generation number | ||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Unadjusted mean airway wall area in mm2 | |||||||
| COPD | 181.3 | 113.5 | 75.7 | 44.9 | 29.0 | 17.7 | 13.3 |
| No COPD | 173.5 | 112.0 | 76.7 | 46.8 | 31.3 | 20.0 | 14.9 |
| Difference (95% CI) P-value |
7.8 (3.5 to 12.2) <0.001 |
1.5 (−1.9 to 5.0) 0.40 |
−1.0 (−3.0 to 1.1) 0.36 |
−1.9 (−2.9 to −0.9) <0.001 |
−2.3 (−2.9 to −1.6) <0.001 |
−2.4 (−2.8 to −2.0) <0.001 |
−1.6 (−1.9 to −1.4) <0.001 |
| Mean airway wall area in mm2 adjusted for age, gender, height, race-ethnicity, smoking status, airway length, percent emphysema−950HU, BMI-determined CT dose, and lung volume at CT | |||||||
| COPD | 177.0 | 97.4 | 67.4 | 40.8 | 25.6 | 16.5 | 14.0 |
| No COPD | 180.6 | 102.4 | 72.3 | 43.9 | 28.1 | 18.7 | 16.1 |
| Difference (95% CI) P-value |
−3.6 (−7.2 to 0) p=0.049 |
−5.1 (−7.8 to −2.2) p<0.001 |
−4.9 (−6.6 to −3.2) p<0.001 |
−3.1 (−3.9 to −2.3) p<0.001 |
−2.5 (−3.1 to −1.9) p<0.001 |
−2.2 (−2.6 to −1.8) p<0.001 |
−2.1 (−2.4 to −1.8) p<0.001 |
Mean values and differences, along with 95% CI and p-values were estimated using linear regression with generalized estimating equations.
Abbreviations: COPD denotes chronic obstructive pulmonary disease, MESA Multi-Ethnic Study of Atherosclerosis, SPIROMICS Subpopulations and Intermediate Outcome Measures in COPD Study, HU Hounsfield units, BMI body mass index, CT computed tomography, and CI confidence interval.
Figure 1. Airway Wall Area According to COPD Status Stratified by Generation Number in the MESA COPD Study.
*p<0.05 for within-generation comparison of mean wall area between participants with no COPD to those with COPD. Mean values and differences adjusted for age, gender, height, race-ethnicity, smoking status, airway length, percent emphysema−950HU, BMI-determined CT dose, and lung volume at CT.
Abbreviations: COPD denotes chronic obstructive pulmonary disease, MESA Multi-Ethnic Study of Atherosclerosis, CI confidence interval, HU Hounsfield units, BMI body mass index, and CT computed tomography.
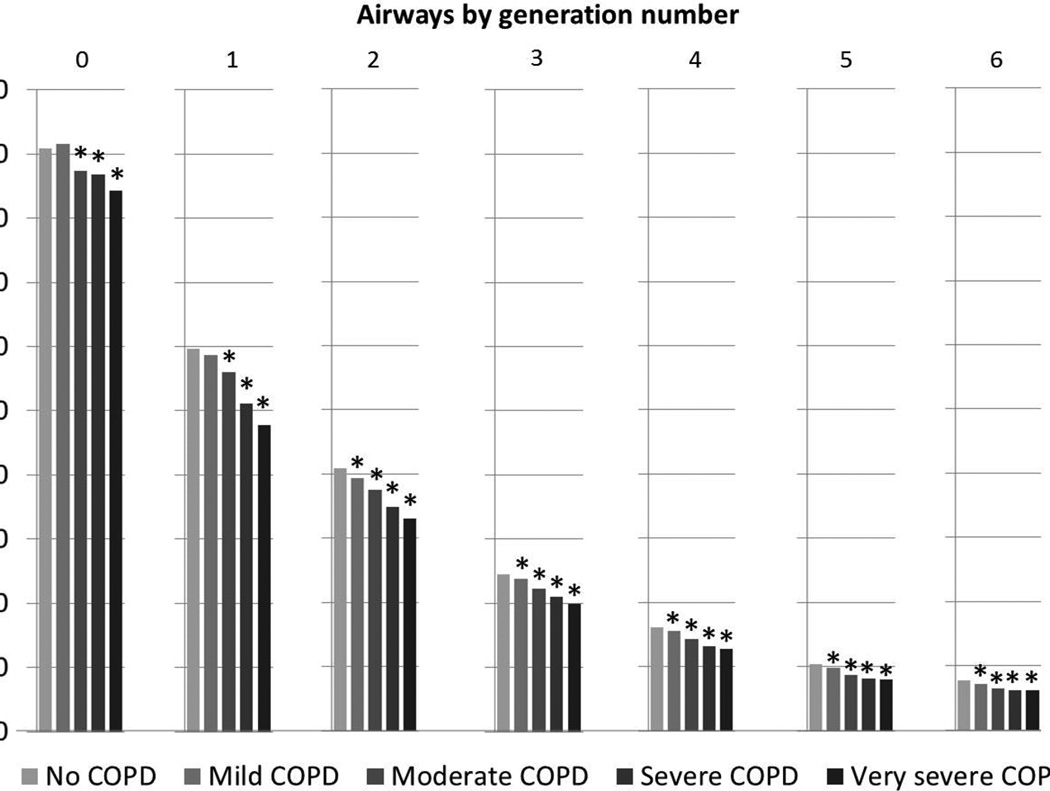
In SPIROMICS, generation 4 to 6 airway wall areas were significantly smaller in COPD compared to controls (Table 2). In adjusted comparisons, these differences were observed at generations 1 through 6 (Table 2), and remained significant with matching by anatomic name (Web Supplement Table E6), or using airway wall thickness (Web Supplement E7). Compared to controls, greater COPD severity was associated with monotonically thinner airway wall areas from generation 0 to 6 in SPIROMICS (Figure 2).
Figure 2. Airway Wall Areas According to COPD Severity Stratified by Generation Number in SPIROMICS.
*p<0.05 for within-generation comparison of airway wall area between participants with no COPD to those with the COPD severity indicated. Mean values and differences adjusted for age, gender, height, race-ethnicity, smoking status, airway length, percent emphysema−950HU, BMI-determined CT dose, and lung volume at CT.
Abbreviations: COPD denotes chronic obstructive pulmonary disease, SPIROMICS Subpopulations and Intermediate Outcome Measures in COPD Study, HU Hounsfield units, BMI body mass index, and CT computed tomography.
Similar associations were observed between airway wall area and percent predicted FEV1 and FVC in both cohorts (Web Supplement Table E8).
Percent wall area was significantly greater in COPD compared to controls in both cohorts, and independent of covariates (Web Supplement Table E9). Consistent observations were made using percent predicted FEV1 and FVC (Web Supplement Table E10). These associations, when combined with the above observations of smaller airway wall and lumen dimensions in COPD, imply proportionally smaller lumen area compared to wall area.
Airway wall areas in COPD assessed with alternative approaches to airway sampling
When airways were selected according to lumen diameter in the MESA COPD Study, a significantly greater proportion of proximal airways in COPD compared to controls was observed for airways 2.5 to 4.0 mm in diameter (Global χ2: p<0.001). Similar results were observed for airways of lumen diameter 4.0 to 5.5 mm, and 5.5 to 7 mm (Global χ2: p≤0.01 for both) in the MESA COPD Study. In SPIROMICS, a greater proportion of proximal airways in COPD compared to controls were observed for airways of lumen diameter 2.5 to 4.0 mm, 4.0 to 5.5 mm, 5.5 to 7.0 mm, 7.0 to 8.5 mm, and 8.5 to 10.0 mm (Global χ2: p<0.001 for all).
In both MESA COPD and SPIROMICS, airways selected based upon lumen size yielded associations of greater wall area in COPD in unadjusted and adjusted comparisons for airways of lumen diameter 2.5 to 4.0 mm, 4.0 to 5.5 mm, and 5.5 to 7.0 mm (p<0.001 for all; Table 3).
Table 3.
Airway Wall Area According to COPD Status Stratified by Lumen Diameter Strata in SPIROMICS and the MESA COPD Study.
| MESA COPD | Lumen diameter strata in mm | ||||||
| >11.5 | 10.0 to 11.5 | >8.5 to 10.0 | >7.0 to 8.5 | >5.5 to 7.0 | >4.0 to 5.5 | >2.5 to 4.0 | |
| Unadjusted mean airway wall area in mm2 | |||||||
| COPD | 149.7 | 96.4 | 81.1 | 65.5 | 50.9 | 35.1 | 20.4 |
| No COPD | 144.4 | 92.6 | 78.5 | 62.0 | 46.8 | 32.7 | 19.7 |
| Difference (95% CI) P-value |
5.3 (−0.2 to 10.9) 0.06 |
3.8 (−0.6 to 8.4) 0.09 |
2.6 (−0.1 to 5.5) 0.06 |
3.5 (1.6 to 5.5) <0.001 |
4.1 (2.9 to 5.4) <0.001 |
2.4 (1.6 to 3.2) <0.001 |
0.7 (0.4 to 1.1) <0.001 |
| Mean airway wall area in mm2 adjusted for age, gender, height, race-ethnicity, smoking status, airway length, percent emphysema−950HU, BMI-determined CT dose, and lung volume at CT | |||||||
| COPD | 141.9 | 93.6 | 78.2 | 64.9 | 50.6 | 34.9 | 22.0 |
| No COPD | 147.1 | 93.8 | 78.1 | 62.5 | 46.8 | 32.7 | 21.6 |
| Difference (95% CI) P-value |
−5.2 (−10.1 to −0.2) p=0.04 |
−0.2 (−4.4 to 4.3) p=0.9426 |
0.1 (−2.7 to 3.0) p=0.96 |
2.3 (0.5 to 4.2) p=0.01 |
3.8 (2.5 to 5.2) p<0.001 |
2.2 (1.3 to 3.0) p<0.001 |
0.4 (0.1 to 0.7) p=0.003 |
| SPIROMICS | Lumen diameter strata in mm | ||||||
| >11.5 | 10.0 to 11.5 | >8.5 to 10.0 | >7.0 to 8.5 | >5.5 to 7.0 | >4.0 to 5.5 | >2.5 to 4.0 | |
| Unadjusted mean airway wall area in mm2 | |||||||
| COPD | 150.8 | 96.3 | 78.8 | 63.1 | 48.7 | 33.9 | 18.8 |
| No COPD | 145.6 | 94.1 | 77.6 | 61.6 | 46.2 | 32.1 | 18.6 |
| Difference (95% CI) P-value |
5.2 (2.5 to 8.0) <0.001 |
2.2 (−0.3 to 4.6) 0.08 |
1.2 (−0.2 to 2.7) 0.09 |
1.5 (0.5 to 2.4) 0.003 |
2.5 (1.8 to 3.2) <0.001 |
1.7 (1.4 to 2.1) <0.001 |
0.3 (0.1 to 0.5) <0.001 |
| Mean airway wall area in mm2 adjusted for age, gender, height, race-ethnicity, smoking status, airway length, percent emphysema−950HU, BMI-determined CT dose, and lung volume at CT | |||||||
| COPD | 145.6 | 95.0 | 78.0 | 62.8 | 48.7 | 33.6 | 18.9 |
| No COPD | 146.1 | 94.5 | 78.2 | 62.8 | 47.0 | 32.2 | 18.6 |
| Difference (95% CI) P-value |
−0.5 (−3.5 to 2.5) p=0.74 |
0.6 (−2.1 to 3.3) p=0.68 |
−0.2 (−1.8 to 1.3) p=0.77 |
0.0 (−1.1 to 1.0) p=0.97 |
1.7 (0.9 to 2.5) p<0.001 |
1.4 (1.0 to 1.8) p<0.001 |
0.2 (0.1 to 0.4) p=0.01 |
Mean values and differences, along with 95% CI and p-values were estimated using linear regression with generalized estimating equations.
Abbreviations: COPD denotes chronic obstructive pulmonary disease, MESA Multi-Ethnic Study of Atherosclerosis, SPIROMICS Subpopulations and Intermediate Outcome Measures in COPD Study, HU Hounsfield units, BMI body mass index, CT computed tomography, and CI confidence interval.
When fifteen airways were selected randomly from the observed airways for each participant, a significantly greater proportion of proximal airways were selected in COPD cases compared to controls in MESA COPD and SPIROMICS (Global χ2: p≤0.01 for both). Analyses using these airways sampled randomly from observed airways also resulted in larger wall areas in COPD compared to controls (Table 4).
Table 4.
Airway Wall Areas According to COPD Status from 15 Randomly Selected Airways per Participant.
| MESA COPD | Fifteen randomly selected airways per participant |
| Unadjusted mean airway wall area in mm2 | |
| COPD | 19.9 |
| No COPD | 18.2 |
| Difference (95% CI) P-value |
1.7 (0.6 to 2.7) 0.001 |
| Mean airway wall area in mm2 adjusted for age, gender, height, race-ethnicity, smoking status, airway length, percent emphysema−950HU, BMI-determined CT dose, and lung volume at CT | |
| COPD | 18.8 |
| No COPD | 17.7 |
| Difference (95% CI) P-value |
1.2 (0.1 to 2.2) 0.02 |
| SPIROMICS | Fifteen randomly selected airways per participant |
| Unadjusted mean airway wall area in mm2 | |
| COPD | 17.7 |
| No COPD | 17.0 |
| Difference (95% CI) P-value |
0.7 (0.2 to 1.2) 0.003 |
| Mean airway wall area in mm2 adjusted for age, gender, height, race-ethnicity, smoking status, airway length, percent emphysema−950HU, BMI-determined CT dose, and lung volume at CT | |
| COPD | 17.7 |
| No COPD | 17.1 |
| Difference (95% CI) P-value |
0.5 (0.1 to 1.0) 0.02 |
Mean values and differences, along with 95% CI and p-values were estimated using linear regression with generalized estimating equations.
Abbreviations: COPD denotes chronic obstructive pulmonary disease, MESA Multi-Ethnic Study of Atherosclerosis, SPIROMICS Subpopulations and Intermediate Outcome Measures in COPD Study, HU Hounsfield units, BMI body mass index, CT computed tomography, and CI confidence interval.
Pi10 in COPD
Achieving an unbiased comparison of Pi10 when the spatial distribution of sampled airways differs requires that the ratio of square-root wall area to lumen perimeter is similar across the sampled range of generations. In both MESA COPD and SPIROMICS, however, significant differences in this ratio were observed (Kruskal-Wallis: p<0.001 for both).
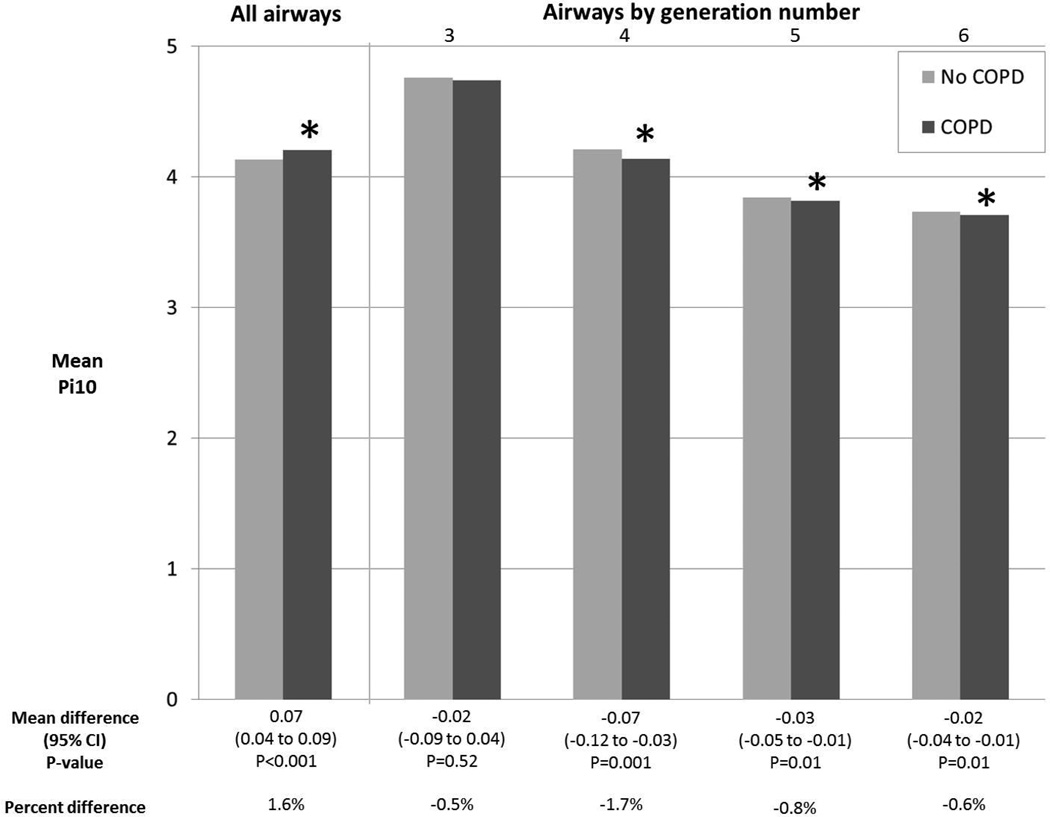
Hence, calculation of Pi10 among spatially matched airways should yield an unbiased estimate of the Pi10. Indeed, spatial matching by generation number resulted in significantly smaller Pi10 in COPD compared to controls for generations 4 to 6 in MESA COPD (p<0.03 for all) and SPIROMICS (p≤0.01 for all; Figure 3).
Figure 3. Pi10 According to COPD Status in SPIROMICS.
*p<0.05 for comparison of mean Pi10 between participants with no COPD to those with COPD. Calculation of Pi10 required 5 or more airways per participant; therefore, Pi10 was not computed for generations 0 to 2. Mean values and differences adjusted for age, gender, height, race-ethnicity, smoking status, airway length, percent emphysema−950HU, BMI-determined CT dose, and lung volume at CT.
Abbreviations: COPD denotes chronic obstructive pulmonary disease, SPIROMICS Subpopulations and Intermediate Outcome Measures in COPD Study, CI confidence interval, HU Hounsfield units, BMI body mass index, and CT computed tomography.
In contrast, calculating Pi10 from all measured airways yielded results suggesting increased wall dimensions in COPD compared to controls in both MESA COPD and SPIROMICS (p<0.001 for both; Figure 3).
DISCUSSION
In two independent studies of smokers, COPD was associated, on average, with significantly smaller airway wall areas on CT compared to controls when central airways were matched spatially based on generation number or anatomic name. Analysis of airways sampled on lumen diameter or sampled randomly from observed airways resulted in a comparison of more proximal airways in COPD compared to controls, thus introducing a selection bias and suggesting larger wall areas in COPD. Results for the Pi10 were similar. In addition to the observed reduction of airway wall thickness, these results suggest that studies of airway wall morphology, histology and genomics should compare spatially matched airways in COPD cases compared to controls.
The present study is the first to compare commonly used airway sampling techniques to study airway wall dimensions in COPD. Consistent with our observation, the COPDGene Study also observed significantly smaller central airway wall areas when comparing anatomically matched airway segments.[24] In contrast, several studies have suggested thicker walls in COPD.[10 12–17 25] We suspect these associations may have been biased, however, due to sampling from different locations in the tracheobronchial tree depending on disease status, a bias that we replicate in the current study. Airway wall and lumen dimensions, as well as the ratio of square-root wall area to lumen perimeter, differ significantly by generation number,[3] which, we found, results in a differential spatial distribution of airways by COPD status when airways are sampled by lumen diameter or randomly. Applying these biased sampling techniques in the present study yielded results suggesting thicker airway walls in COPD.
Wall-lumen ratio measures (e.g., percent wall area) have been reported to be increased in COPD,[6–8 23] which has been interpreted by some as evidence that airway wall thickening encroaches upon and narrows airway lumens in COPD. Without comparing absolute airway dimensions, this inference assumes that total airway calibers are similar in COPD and controls. However, consistent with the COPDGene cohort,[24] we show that both wall and lumen areas are reduced in COPD compared to controls, although the difference is greater in lumen size, a finding which is likely of greater physiologic importance to airflow limitation.
The mechanism underlying the observed smaller wall areas in COPD was not the primary focus of this paper. However, differences in lung volume due either to COPD-related hyperinflation or submaximal inspiration at the time of CT do not appear to explain our findings.[23] Smaller wall areas in COPD were consistently observed with adjustments for lung volume achieved at CT, as well as airway-specific length, suggesting that the airways were not merely stretched and therefore thinner.
Other potential mechanisms include regression of airway smooth muscle resulting from reduced wall tension, apoptosis or replacement fibrosis resulting from chronic airways inflammation, or reduced bronchial vascular volume.[26 27] We did not assess airway wall histology in the present study. Therefore, some components of the airway wall may be increased in COPD.[5 25] Finally, we present differences in means, which suggests that most people with COPD have thinner airways but does not rule out the possibility of a subset having thicker airway walls.
Our analyses did not include many airways less than 2mm in lumen diameter, a threshold below which many believe the excess airways resistance arises in COPD.[28 29] This was due in part to CT resolution[9], as well as the technical demands of visually-confirmed spatial mapping of the tracheobronchial tree to the sixth generation in large studies. However, the classic studies[28 29] that describe airways less than 2mm as the predominant site of resistance in COPD may have been subject to the same bias described here: comparison of peripheral and central resistance when a fixed-diameter catheter may have wedged more proximally in COPD could bias inferences related to the site of airways obstruction. Central airways likely also contribute to airways resistance in COPD, as demonstrated by Yanai[29] and Macklem.[30] Nevertheless, histologic confirmation of fewer and thinner central airways, as well as a method of spatially matching peripheral airways is needed.
Alternative approaches to matching airways in COPD based on histologic characteristics (e.g., membranous,[18] cartilaginous,[5] or terminal bronchioles[9]) were not addressed in this paper. However, these histologically defined airways span multiple generations,[3] thus bias resulting from sampling of more proximal airways with similar histologic characteristics in COPD cannot be excluded.
Airway segments occluded by mucous may have gone undetected by the imaging software. We do not believe such a sampling bias contributed to our findings, however, given the association between increasing COPD severity and thinner walls was also observed in proximal airways (e.g., mainstem and lobar bronchi) where complete mucous occlusion is unlikely and spatial mapping reproducibility was excellent.[31]
In summary, in two independent studies of smokers, COPD was associated with significantly less airway wall thicknesses throughout most of the central tracheobronchial tree when comparing spatially matched airways. Sampling airways by lumen diameter or randomly resulted in differential spatial distributions by COPD status and introduced selection bias in the study of airway wall properties, as did the use of the Pi10. Studies of airway morphometry, histology and genomics in COPD should spatially match airways to avoid potentially large selection bias due to comparison of proximal-to-peripheral airways.
Supplementary Material
What is the key question? Are airway walls thicker or thinner in COPD?
What is the bottom line? Airway walls are thinner in COPD when comparing spatially matched central airways.
Why read on? We demonstrate that techniques commonly used to study airway wall properties in COPD, such as sampling airways based upon lumen diameter or at random, results in a biased comparison of more proximal airways in COPD to more peripheral airways in controls.
Acknowledgments
FUNDING: NIH/NHLBI R01-HL093081, R01-HL077612, R01-HL075476, N01-HC95159-HC95169, HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN2682009000019C, and HHSN268200900020C; Fonds de la 20echerche en santé Québec.
Footnotes
COMPETING INTERESTS: Authors have completed the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest.
REFERENCE LIST
- 1.Vestbo J, Hurd SS, Agusti AG, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease GOLD Executive Summary. American journal of respiratory and critical care medicine. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 2.Fishman AP, Macklem PT, Mead J, et al. Handbook of physiology. 2v. Bethesda, Md: American Physiological Society; 1986. Mechanics of breathing; p. xxv, 784. [Google Scholar]
- 3.Weibel ER. Morphometry of the human lung. Berlin: Springer; 1963. [Google Scholar]
- 4.Hsia CC, Hyde DM, Ochs M, et al. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. American journal of respiratory and critical care medicine. 2010;181(4):394–418. doi: 10.1164/rccm.200809-1522ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tiddens HA, Pare PD, Hogg JC, et al. Cartilaginous airway dimensions and airflow obstruction in human lungs. American journal of respiratory and critical care medicine. 1995;152(1):260–266. doi: 10.1164/ajrccm.152.1.7599833. [DOI] [PubMed] [Google Scholar]
- 6.Nakano Y, Muro S, Sakai H, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. American journal of respiratory and critical care medicine. 2000;162((3 Pt 1)):1102–1108. doi: 10.1164/ajrccm.162.3.9907120. [DOI] [PubMed] [Google Scholar]
- 7.Berger P, Perot V, Desbarats P, et al. Airway wall thickness in cigarette smokers: quantitative thin-section CT assessment. Radiology. 2005;235(3):1055–1064. doi: 10.1148/radiol.2353040121. [DOI] [PubMed] [Google Scholar]
- 8.Hasegawa M, Nasuhara Y, Onodera Y, et al. Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2006;173(12):1309–1315. doi: 10.1164/rccm.200601-037OC. [DOI] [PubMed] [Google Scholar]
- 9.McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365(17):1567–1575. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuoka S, Kurihara Y, Yagihashi K, et al. Airway dimensions at inspiratory and expiratory multisection CT in chronic obstructive pulmonary disease: correlation with airflow limitation. Radiology. 2008;248(3):1042–1049. doi: 10.1148/radiol.2491071650. [DOI] [PubMed] [Google Scholar]
- 11.Diaz AA, Valim C, Yamashiro T, et al. Airway count and emphysema assessed by chest CT imaging predicts clinical outcome in smokers. Chest. 2010;138(4):880–887. doi: 10.1378/chest.10-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosken CH, Wiggs BR, Pare PD, et al. Small airway dimensions in smokers with obstruction to airflow. Am Rev Respir Dis. 1990;142(3):563–570. doi: 10.1164/ajrccm/142.3.563. [DOI] [PubMed] [Google Scholar]
- 13.Kim WD, Ling SH, Coxson HO, et al. The association between small airway obstruction and emphysema phenotypes in COPD. Chest. 2007;131(5):1372–1378. doi: 10.1378/chest.06-2194. [DOI] [PubMed] [Google Scholar]
- 14.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 15.Kim WJ, Silverman EK, Hoffman E, et al. CT metrics of airway disease and emphysema in severe COPD. Chest. 2009;136(2):396–404. doi: 10.1378/chest.08-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel BD, Coxson HO, Pillai SG, et al. Airway wall thickening and emphysema show independent familial aggregation in chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2008;178(5):500–505. doi: 10.1164/rccm.200801-059OC. [DOI] [PubMed] [Google Scholar]
- 17.Grydeland TB, Dirksen A, Coxson HO, et al. Quantitative computed tomography measures of emphysema and airway wall thickness are related to respiratory symptoms. American journal of respiratory and critical care medicine. 2010;181(4):353–359. doi: 10.1164/rccm.200907-1008OC. [DOI] [PubMed] [Google Scholar]
- 18.Kuwano K, Bosken CH, Pare PD, et al. Small airways dimensions in asthma and in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1993;148(5):1220–1225. doi: 10.1164/ajrccm/148.5.1220. [DOI] [PubMed] [Google Scholar]
- 19.The MESA COPD Study. European Respiratory Society Congress. Barcelona, Spain: 2013. [September 8, 2013]. Airway wall thinness and COPD: analysis of spatially comparable airways. [Google Scholar]
- 20.Couper D, Lavange LM, Han M, et al. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2013 doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. American journal of respiratory and critical care medicine. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 22.Liang KY, Zeger SL. Regression analysis for correlated data. Annual review of public health. 1993;14:43–68. doi: 10.1146/annurev.pu.14.050193.000355. [DOI] [PubMed] [Google Scholar]
- 23.Diaz AA, Bartholmai B, San Jose Estepar R, et al. Relationship of emphysema and airway disease assessed by CT to exercise capacity in COPD. Respiratory medicine. 2010;104(8):1145–1151. doi: 10.1016/j.rmed.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Washko GR, Diaz A, Kim V, et al. Computed tomographic measures of airway morphology in smokers and never-smoking normals. Journal of applied physiology 2014. 2014 Jan 16; doi: 10.1152/japplphysiol.00004.2013. Published online before print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiddens HA, Bogaard JM, de Jongste JC, et al. Physiological and morphological determinants of maximal expiratory flow in chronic obstructive lung disease. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 1996;9(9):1785–1794. doi: 10.1183/09031936.96.09091785. [DOI] [PubMed] [Google Scholar]
- 26.Cosio M, Ghezzo H, Hogg JC, et al. The relations between structural changes in small airways and pulmonary-function tests. N Engl J Med. 1978;298(23):1277–1281. doi: 10.1056/NEJM197806082982303. [DOI] [PubMed] [Google Scholar]
- 27.Thurlbeck WM, Pun R, Toth J, et al. Bronchial cartilage in chronic obstructive lung disease. Am Rev Respir Dis. 1974;109(1):73–80. doi: 10.1164/arrd.1974.109.1.73. [DOI] [PubMed] [Google Scholar]
- 28.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med. 1968;278(25):1355–1360. doi: 10.1056/NEJM196806202782501. [DOI] [PubMed] [Google Scholar]
- 29.Yanai M, Sekizawa K, Ohrui T, et al. Site of airway obstruction in pulmonary disease: direct measurement of intrabronchial pressure. J Appl Physiol(1985) 1992;72(3):1016–1023. doi: 10.1152/jappl.1992.72.3.1016. [DOI] [PubMed] [Google Scholar]
- 30.Macklem PT, Fraser RG, Brown WG. Bronchial Pressure Measurements in Emphysema and Bronchitis. J Clin Invest. 1965;44:897–905. doi: 10.1172/JCI105206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montaudon M, Berger P, de Dietrich G, et al. Assessment of airways with three-dimensional quantitative thin-section CT: in vitro and in vivo validation. Radiology. 2007;242(2):563–572. doi: 10.1148/radiol.2422060029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.