Abstract
We sought to examine which socioeconomic indicators are risk factors for virologic failure among HIV-1 infected patients receiving antiretroviral therapy in KwaZulu-Natal, South Africa. A case-control study of virologic failure was conducted among patients recruited from the outpatient clinic at McCord Hospital in Durban, South Africa between October 1, 2010 and June 30, 2012. Cases were those failing first-line antiretroviral therapy (ART), defined as viral load > 1000 copies/mL. Univariate logistic regression was performed on sociodemographic data for the outcome of virologic failure. Variables found significant (p<.05) were used in multivariate models and all models were stratified by gender. Of 158 cases and 300 controls, 35% were male and median age was 40 years. Gender stratification of models revealed automobile ownership was a risk factor among males, while variables of financial insecurity (unemployment, non-spouse family paying for care, staying with family) were risk factors for women. In this cohort, financial insecurity among women and automobile ownership among men were risk factors for virologic failure. Risk factor differences between genders demonstrate limitations of generalized risk factor analysis.
Keywords: HIV/AIDS, antiretroviral treatment, virologic failure, structural barriers, gender, South Africa
INTRODUCTION
Globally over 34.2 million people are living with HIV (1). Efforts to reduce morbidity and mortality from HIV via roll-out of antiretroviral therapy (ART) have increased HIV treatment access world-wide. Yet as access has expanded, HIV drug resistance (HIVDR) and virologic failure (VF) have risen, posing a threat to ART sustainability (2, 3). A 2012 World Health Organization (WHO) report on HIVDR based on surveys performed in 2010 estimates global HIVDR at around 6.8%, an increase from previous levels (4). In Southern Africa specifically, prevalence of any drug resistance mutation has increased by approximately 14% per year (5).
Starting in 2003, South Africa, with the largest population of individuals living with HIV globally and some areas with the highest prevalence of HIV in the world (1, 6, 7), began implementing a large-scale rollout of ART and comprehensive HIV care to address the HIV epidemic. Yet, in line with global trends, ART failure and HIVDR emerged as access to ART increased. First-line ART failure in South Africa is estimated at 4.9% to 19%, using varying viral load thresholds (8–11). KwaZulu-Natal province (KZN) has the highest prevalence of HIV in South Africa, estimated at up to 40%(12), and studies based in its largest city, Durban, and surrounding rural areas demonstrated ART failure rates of 8–20% in the first year of therapy (13, 14). Of those failing first ART, most (>83.5%) have at least one major resistance mutation and 64.3% have dual-class resistance mutations (13).
To tackle increasing HIVDR, the WHO urged countries to adopt strategies aimed at reducing VF and HIVDR. These strategies included monitoring program-level measurements of ART outcomes (termed Early Warning Indicators), such as clinic retention rate, on-time pill pickup, and drug supply continuity (15, 16). However, individual-level factors associated with virologic failure among those engaged in continuing HIV care are equally important to identify and understand in order to improve treatment outcomes.
In order to identify and explain individual-level factors, this study used a socio-ecological perspective (17) by drawing on the analytical concept of “structural factors.” Structural factors are the economic, institutional, political, sociocultural and other environmental factors that shape the context in which individuals contract, treat, and live with HIV and can act as facilitators or barriers to treatment (18–20). Structural factors always impact health outcomes, but are especially pertinent in resource-limited settings and among vulnerable populations (21–23). Socioeconomic factors (e.g. level of a community’s economic development, degree of unemployment, economic and health resources), a subset of structural factors, in particular have great impact when there are competing demands in the face of constrained resources, such as low income, food insecurity or inadequate public transportation (18, 24–32).
Socioeconomic factors often do not affect the entire population equally. Some of these factors may have a greater impact among specific subpopulations, defined by gender, age, or socioeconomic status (20). For example, women in traditionally patriarchal societies, who often lack access to personal financial or productive resources (33, 34), may be greatly impacted by financial insecurity (18, 35, 36). Conversely, men greatly affected by cultural expectations to be strong and provide for family may find employment a greater barrier to accessing care when they cannot get time away from work to attend clinic due to lost wages or fear of losing their job by revealing their HIV status (37–40). It is known that men in Sub-Saharan Africa seek ART less often or later and have poorer treatment outcomes than women (41, 42); however, the reasons for these discrepancies remain unknown. Analyzing these subpopulations to ascertain key individual factors affecting ART treatment outcomes could be vitally important for both identifying those at risk of VF and providing direction for improving ART outcomes through appropriate interventions.
In an effort to better characterize the key individual-level indicators of VF and HIVDR in South Africa, the Risk Factors for Virologic Failure (RFVF) study (43)examined a multitude of factors to find those most strongly associated with VF among a cohort of 458 people living in urban and peri-urban areas of the KZN province, South Africa. However, the RFVF analysis, focused on identifying the most significant risk factors for the entire cohort, did not intend to look closely at significant impacts of additional factors on specific subpopulations. An analysis that does not consider subpopulation factors can miss potentially remediable barriers, such as transportation constraints, that are overshadowed by statistically stronger, non-remediable risk factors, such as age. Finally, a better understanding of the relationships among socioeconomic risk factors can shed light on the mechanisms of VF.
In this analysis, we examine the impact of socioeconomic factors on ART outcomes and the differences in this impact between men and women living with HIV and receiving care at an outpatient clinic of McCord Hospital in Durban, South Africa. In doing so, we attempt to contribute to the construction of a mechanistic model for VF. We believe this model will be critical in developing effective strategies to prevent VF and HIVDR.
METHODS
Study Setting and Population
Data for this study was collected from the Sinikithemba Clinic (STC), an outpatient HIV clinic at McCord Hospital (MCH). MCH is a semi-private hospital serving the city of Durban and surrounding townships, an area with a high level of poverty: 44% lives in poverty and unemployment is estimated at 30–40% and increasing (44, 45). The STC, supported by the South African government and the President’s Emergency Plan For AIDS Relief (PEPFAR), provided outpatient HIV care to around 6000 patients per year, referred from both MCH and local providers. Clinic attendees paid a fixed clinic fee of around $15 USD per visit, which covered all clinical care and medication (46). Monitoring at STC followed the National Antiretroviral Treatment Guidelines set by the South African Department of Health, including HIV-1 viral load and CD4 cell count monitoring every 6 months or more frequently if indicated (47).
Study Design
The present analysis is a sub-study of data from the RFVF study, which utilized a case-control design and is described elsewhere (43). Patients eligible for the RFVF parent study were ≥18 years old, HIV positive, receiving care at MCH or affiliated clinics, and on ART for ≥5 months. Cases were those with VF, defined as having a single viral load (15)> 1000 copies/mL obtained through routine clinical care (47). Two unmatched controls, defined as having a VL ≤ 1000 on routine labwork, were selected from clinic weekly for every case enrolled.
Upon enrollment in the RFVF study, a survey administered by ART counselors covered demographic, socioeconomic, and psychosocial information as well as medical and HIV history. The data collected in the enrollment survey were used for this secondary analysis. Demographic and socioeconomic questions targeted basic demographic information, education, relationship status, living situation, housing type and material, transportation to clinic, funding for clinic visits, food security, employment, and asset ownership, including data used by USAID and the Demographic and Health Surveys (DHS)to create wealth indices (48). The survey, designed with attention to local context, language, and culture, was administered in person in English or isiZulu, and was not included in the patient medical record.
Statistical Analysis
Univariate logistic regression was used to evaluate the marginal associations of VF with demographic and socioeconomic variables recorded in the enrollment survey. VF was selected as the most clinically relevant statistical endpoint, given the current in accuracies in measuring adherence. Free response answers for type of employment were categorized into basic labor, skilled labor, and professional labor based on contextual research, including interviews with ART counselors (Supplemental Table I). Age was analyzed both as a continuous variable and as a dichotomous variable with a cutoff at 40 years of age, which is the mean and median population age. Education was categorized as ≤8 years, 9–11 years, ≥12 years and as dichotomous variables with cutoffs at 8 years and 12 years, based on the education system in South Africa. Number of cohabitants and dependents had similar distributions and were both analyzed by quartile categories and as dichotomous variables with cutoffs at 0, 1, 3, 5, and 7 people. Living situation was separated into categories of staying with friends or family (dependent situation), living independently in a house or apartment, or living with employer. A wealth index was created using Principal Component Analysis on proxies of wealth, namely assets and utilities (Supplemental Table II), and the first extraction was used to assess association with VF and to adjust for wealth in models.
Those variables involving demographic and socioeconomic status found statistically significant (p<=0.05)were further evaluated by multivariate logistic regression. Variables that trended toward significant and were meaningful to a socioeconomic analysis, such as level of education, were forced in the multivariate models. Multiple multivariate models were run assessing the effects of variables of financial insecurity. Collinearity and multicollinearity were assessed using the Condition Index (CI) with CI<10 indicating mild/little collinearity, CI=10–30 indicating modest collinearity, and CI>100 indicating significant collinearity. Models were run with and without variables showing modest collinearity by CI. The largest CI included in the final models was 16; all others were <10. Variables that were closely related or demonstrated collinearity were examined both individually and in combinations in models to assess effect; ultimately the variable most significantly associated with VF was selected for multivariate models. Association between employment type and VF was analyzed separately from the multivariate models due to non-convergence of the model caused by overlap with unemployment and income variables. All multivariate models were adjusted for age, gender, and treatment length, which have established association with VF (14, 49–53).
These univariate and multivariate models and adjustments were then repeated after stratifying by genderto determine differences in risk factors between gender groups. Additionally “having non-spouse family pay for care” and “automobile ownership” were each analyzed individually by univariate analysis to further define these populations (Supplemental Tables III, IV, VIII). SAS 9.1 was used for all analysis and the REDCap electronic database for storage of all data (54).
RESULTS
Of the 458 patients enrolled in the study (158 cases and 300 unmatched controls), ages ranged from 18–74 with the mean age at enrollment approximately 39.6 years (SD=9.0). Thirty five percent (35.4%) were men, 98.9% black South African, and 53% had ≥12 years of education. 19% were unemployed (12% of men and 23% of women) and 17% owned an automobile (30% of men and 9.1% of women). Among cases, the median HIV-1 viral load was 95,221 copies/mL and median CD4 count was 300.5 cells/uL (IQR 183–447) with 88 people >500 cells/uL and 126 people <200 cells/uL. The mean treatment time was 2.54 years (SD=2.06) and median treatment time was 2.05 years (Table I).
Table I.
Baseline Characteristics, Univariate (UV), and Multivariate (MV) analysis of entire cohort
| Characteristic | UNIVARIATE | MULTIVARIATEi | ||||
|---|---|---|---|---|---|---|
| Total (n=458) | Case (n=158) | Control (n =300) | p | OR (95% CI) | p | |
| Age mean years (SD) | 39.6 (9.0) | 37.1 (8.4) | 40.9 (9.1) | <.0001 | 0.95 (0.93–0.98) | 0.0007 |
| Male gender % (SD) | 35 (2.2) | 47 (4.0) | 29 (2.6) | <.0001 | 2.20 (1.41–3.45) | 0.0006 |
| Years on ARTii, mean (SD) | 2.54 (2.1) | 2.11 (2.1) | 2.77 (2.0) | 0.0012 | 0.88 (0.79–0.98) | 0.024 |
| ≥12 years education % (SD) | 53 (2.3) | 59 (3.9) | 50 (2.9) | 0.0767 | 1.11 (0.71–1.75) | 0.64 |
| CD4 Count Categories% (SD) | ||||||
| >500 | 19 (1.8) | 11 (2.5) | 23 (2.4) | |||
| 350–500 | 23 (2.0) | 11 (2.5) | 29 (2.6) | |||
| 200–350 | 30 (2.1) | 30 (3.6) | 30 (2.6) | |||
| <200 | 28 (2.1) | 47 (4.0) | 17 (2.2) | |||
|
| ||||||
| No incomeiii % (SD) | 21 (1.9) | 28 (3.6) | 18 (2.2) | 0.015 | 0.68 (0.20–2.33) | 0.53 |
| Unemployed %iv (SD) | 19 (1.8) | 25 (3.4) | 16 (2.1) | 0.025 | 1.50 (0.49–4.63) | 0.48 |
| Full time employed% (SD) | 52 (2.3) | 47 (4.0) | 54 (2.9) | 0.13 | ||
| Self-employed% (SD) | 13 (1.6) | 15 (2.8) | 11 (1.8) | 0.24 | ||
| Basic labor% (SD) | 68 (2.2) | 70 (3.6) | 67 (2.7) | 0.56 | ||
| Skilled labor% (SD) | 23 (2.0) | 16 (2.9) | 26 (2.5) | 0.051 | ||
| Professional labor% (SD) | 9.3 (1.4) | 14 (2.8) | 7.0 (1.5) | 0.060 | ||
| Employment (categ.) v% (SD) | 0.19 | |||||
|
| ||||||
| Self pays for care % (SD) | 79 (1.9) | 73 (3.5) | 81 (2.3) | 0.051 | ||
| Family pays for care vi% (SD) | 14 (1.6) | 22 (3.3) | 11 (1.8) | 0.001 | 2.71 (1.04–7.04) | 0.041 |
| Spouse pays for care % (SD) | 4.4 (1.0) | 3.2 (1.4) | 5.0 (1.3) | 0.37 | ||
| Lives in house% (SD) | 92 (1.3) | 91 (2.3) | 93 (1.5) | 0.48 | ||
| Living (categ.)vii | -- | -- | -- | 0.59 | ||
| Owns own home% (SD) | 36 (2.2) | 32 (3.7) | 38 (2.8) | 0.20 | ||
| Owns or rents home % (SD) | 50 (2.3) | |||||
| Dependent livingviii% (SD) | 50 (2.3) | 52 (4.0) | 41 (2.8) | 0.031 | 1.00 (0.61–1.63) | 1.00 |
| Married% (SD) | 21 (1.9) | 17 (3.0) | 22 (2.4) | 0.25 | ||
| In a relationshipix% (SD) | 64 (2.2) | 73 (3.5) | 60 (2.8) | 0.005 | ||
|
| ||||||
| Any cohabitants% (SD) | 96 (1.0) | 95 (1.7) | 97 (1.0) | 0.37 | ||
| >2 cohabitants% (SD) | 73 (2.1) | 72 (3.6) | 74 (2.5) | 0.67 | ||
| >5 cohabitants% (SD) | 36 (2.2) | 38 (3.9) | 35 (2.8) | 0.58 | ||
| Any dependents % (SD) | 94 (1.1) | 94 (1.9) | 93 (1.5) | 0.69 | ||
| >2 dependents % (SD) | 54 (2.3) | 51 (4.0) | 56 (2.9) | 0.27 | ||
| >5 dependents % (SD) | 28 (2.1) | 28 (3.6) | 29 (2.6) | 0.85 | ||
|
| ||||||
| Patient owns a car % (SD) | 17 (1.8) | 25 (3.4) | 13 (1.9) | 0.0008 | 2.35 (1.35–4.11) | 0.0027 |
| Own car to clinicx % (SD) | 13 (1.8) | 20 (3.2) | 9.7 (1.7) | 0.0032 | ||
| Any car to clinicxi % (SD) | 14 (1.6) | 21 (3.2) | 11 (1.8) | 0.0034 | ||
| Bus/minibus to clinic % (SD) | 84 (1.7) | 78 (3.3) | 87 (1.9) | 0.014 | ||
The multivariate model includes variables found significant (p<0.05) in univariate analysis, excluding variables with significant colinearity. Education was forced in multivariate models despite borderline significance
for each increasing year
”No Income” refers to the patient having no income from any source
“Unemployed” does not include those who are students or are retired
Employment categorical variable includes basic, skilled, and professional labor categories
Family (non-spouse) pays for care: dichotomous variable consisting of family pays for care vs all other payers
Living situation categorical variable includes: owns home, rents home, stays with friends, stays with family, stays with employer
”dependent living” indicates a dependent living situation, meaning living with family or friends as opposed to other living situations such as owning or renting a home
”In a relationship” includes both married and non-married couples who self-identified as having a partner
“Own car to clinic” indicates the subject uses his or her own car to get to clinic
Univariate analyses of all variables for the entire cohort were reported elsewhere (43), but analysis of demographic and socioeconomic variables can be found in Table I. Socioeconomic variables found significant by univariate analyses among the entire cohort were lack of income (OR=1.76, p=0.015), unemployment (OR=1.72, p=0.025), dependent living situation, indicated by staying with family or friends (OR=1.53, p=0.031), driving an automobile to clinic (OR=2.21, p=0.0034), owning an automobile (OR=2.35, p=0.0026), and family (non-spouse) paying for care (OR=2.38, p=0.0013). Professional labor (OR=2.04, p=0.060) and 12 years or more of education (OR=1.43, p=0.071) showed borderline significance. Skilled labor showed border line inverse association with VF (OR=0.56, p=0.051).
In a multivariable model including all socioeconomic variables found significant in univariate analyses (Table I), other than age < 40 years and male gender, only owning an automobile (OR=2.35, p=0.0027) and family paying for care (OR=2.71, p=0.041)were significantly associated with VF (Table I). Skilled employment and taking an automobile to clinic were excluded from the multivariable analysis due non-convergence for the former and collinearity with automobile ownership for the latter.
Univariate analysis of all socioeconomic variables stratified by gender revealed a stronger association for women (Table II) in the majority of variables found to be significant overall (p<0.05). Strong risk factors for VF among women included lack of income (OR=2.01 p=0.011), unemployment (OR=1.91 p=0.027), dependent living situation (OR=1.98 p=0.010), and non-spouse family member paying for care (OR=2.97 p=0.0006). An additional risk factor for women not found significant in the entire cohort was professional employment (OR=2.85, p=0.024), however only 22 women were in this category (Supplemental Table I). In multivariable models for women, family paying for medication (p=0.040) remained significantly associated with VF when including other indicators of financial dependency (lacking income, staying with family). Among men, univariate analyses revealed the main significant risk factors for VF were younger age and owning an automobile (OR=2.36, p=0.013) while transport to clinic via minibus (OR=0.42, p=0.013) and skilled labor (OR=0.42, p=0.04) were inversely associated with VF. In a multivariate analysis among men, besides younger age, only owning an automobile remained significantly associated with VF (OR=3.10, p=0.0031) (Table II).
Table II.
Univariate and multivariate analysis of demographic and socioeconomicrisk factors stratified by gender.
| MALE | FEMALE | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| UNIVARIATE | MULTIVARIATE8 | UNIVARIATE | MULTIVARIATE | |||||
| Characteristic9 | OR | p | OR (95% CI) | p | OR | p | OR (95% CI) | p |
| Age | 0.96 | 0.028 | 0.95 (0.9–1.0) | 0.042 | 0.94 | <.0001 | 0.95 (0.9–1.0) | 0.0038 |
| Years on ART | 0.91 | 0.247 | 0.98 (0.8–1.2) | 0.80 | 0.82 | 0.0049 | 0.83 (0.7–1.0) | 0.012 |
| ≥ 12 yrs education | 1.62 | 0.13 | 1.20 (0.6–2.4) | 0.61 | 1.53 | 0.11 | 1.10 (0.6–2.0) | 0.75 |
| No income | 2.27 | 0.085 | 0.98 (0.05–20.6) | 0.99 | 2.01 | 0.011 | 0.55 (0.1–2.3) | 0.41 |
| Unemployed | 2.40 | 0.080 | 2.14 (0.1–44.5) | 0.62 | 1.91 | 0.027 | 1.47 (0.4–5.1) | 0.54 |
| Basic Labor | 1.83 | 0.12 | 0.79 | 0.50 | ||||
| Skilled Labor | 0.42 | 0.040 | 0.64 | 0.31 | ||||
| Professional Labor | 1.59 | 0.50 | 2.85 | 0.024 | ||||
| Self pay for care | 0.53 | 0.19 | 0.51 | 0.018 | ||||
| Family pay for care | 2.52 | 0.11 | 1.53 (0.2–11.0) | 0.68 | 2.97 | 0.001 | 3.30 (1.1–10.3) | 0.040 |
| Dependent living | 1.41 | 0.29 | 0.87 (0.4–1.9) | 0.74 | 1.98 | 0.010 | 0.99 (0.5–1.9) | 0.98 |
| Owns a car | 2.36 | 0.013 | 3.10 (1.5–6.5) | 0.0031 | 1.32 | 0.52 | 1.61 (0.7–4.0) | 0.30 |
| Travel via Minibus | 0.68 | 0.26 | 0.71 | 0.46 | ||||
Multivariate models are those used in Table 1, utilizing variables found significant (p<0.05) in univariate analysis, excluding variables of significant colinearity. Education was forced despite borderline significance in univariate models (p=0.08 for entire cohort)
Categories are as stated in Table I
DISCUSSION
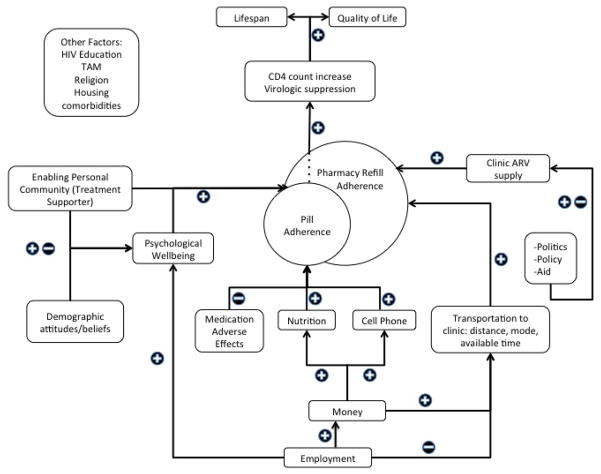
We analyzed individual socioeconomic characteristics among a cohort of 458 individuals being treated for HIV in KZN, South Africa to identify socioeconomic risk factors for VF. Socioeconomic risk factors are an important subset of structural risk factors for VF. They can help identify those at risk and provide direction for discrete interventions in order to improve ART outcomes (18, 29, 55). However, the impact of such entrenched and interrelated influences can be difficult to concretely define (18, 29). A directed acyclic graph (Figure 1) illustrates some possible interconnections between structural factors at play in this context and their impact on ART outcomes. The impact of each of these factors can vary significantly by population subgroup, such as gender, as seen in this analysis (18).
Figure 1.
Directed acyclic graph of structural factors and their potential interconnections and impact on ART outcomes
This diagram proposes a mechanistic pathway to successful ART treatment, specifically viral suppression. A failure in one of these pathways or a negative effect of one of these inputs may lead to virologic failure instead. This diagram highlights the role of socioeconomic factors in ART success. In this diagram, we see the effect of employment as potentially positive or negative. Employment provides income and access to personal funds to pay for treatment, adherence aids (cell phone), and food [36], transport to clinic. Employment also potentially precludes available time for clinic visits and introduces the barrier of stigma in the workplace[50]. We see the dual nature of an “enabling personal community”, which includes the effect of financial dependence (as demonstrated in the present analysis), the positive effects of treatment supporter programs [69–71], and the potentially negative impact of community “support” depending on pervasive stigma [36]. We also see the effect of “demographic attitudes/beliefs”, which can include stigma, gender roles, religion, and Traditional African Medicine (TAM in diagram)[72].
Analyses among women in this cohort showed that socioeconomic risk factors for VF were related to financial dependency and financial insecurity:unemployment, lack of income, having a non-spouse family member pay for medical care, and staying with family or friends instead of owning or renting a home. Existing literature has demonstrated that financial insecurity is a barrier to care and is associated with poor adherence and VF, yet no studies have assessed differences in this association between genders (25, 26, 56–58). The association between financial dependency indicators and VF among women but not men in this cohort may indicate that personal financial insecurity is a risk factor for VF more specific to women in this context, as has been suggested by some findings in countries where women are often dependent upon men (16, 59, 60).
Moreover, our results show a difference in treatment outcomes depending on who pays for care among female clinic attendees, which raises the question of whether payer identity affects ART outcomes. Studies noting higher VF or loss to follow-up when a fee was required for care demonstrated a financial barrier to ART, but did not address payer identity (25, 26, 56, 57). From our analysis, among women clinic attendees, women dependent upon non-spouse family to pay for care are more likely to experience VF. Similar results from a study in Uganda assessing quality of life among HIV+ patients found that the only clinically significant sociodemographic predictor of poorer quality of life was financial dependence on another individual (61). In a separate analysis, women in this cohort whose family paid for care were more likely to be <40 years old, unmarried, staying with family, and unemployed.
While we focus on the structural barriers generated by, for example, financial insecurity, it is clear that this socioeconomic factor can have psychological effects as well, such as arousing feelings of shame for asking for money or symptoms of depression or poor self-esteem. Financial insecurity can also generate psycho-social pressures such as being a perceived burden on others (62). However, psychological effects and psycho-social processes are difficult to assess quantitatively, while direct impacts of financial insecurity are quite obvious structural barriers: inability to pay for medication copays, clinic visits, or transportation. While the mechanistic pathways are not fully characterized, our results indicate that financial insecurity, namely financially depending on a non-spouse family member, unemployment, and staying with family or friends, are significant risk factors for VF among women in this cohort.
Among males the main significant socioeconomic risk factor for VF in both univariate and multivariate analysis was automobile ownership. Automobile ownership was not found to be significant among women, though fewer women owned cars. While not likely a direct cause of ART failure, automobile ownership is correlated with unmeasured factors that could be directly related to VF. First, automobile ownership requires money to pay for the automobile, gasoline and maintenance, which can compete with other financial needs. Consequently, automobile ownership becomes a visual symbol of financial success and status (63–65). Automobile ownership can also come with family and social responsibility to help others who do not own an automobile, as suggested by those patients who did not own an automobile but used one for travel to clinic visits. Finally, automobiles could distract from HIV care when owners are “Too busy to take ARVs,” which may indicate prioritization of responsibility to family, work, or community over HIV treatment (Supplemental Table VIII).
To examine possible underlying risk factors for VF that are correlated with automobile ownership, we further characterized the automobile owner subpopulation. In a separate univariate analysis of data from this cohort, automobile ownership among men was associated with marriage, having income, being self-employed, owning a home, and being educated beyond 9 years (Supplemental Table VIII). These characteristics fit conceptually with both a higher level of wealth and with the South African ideal of masculinity. Adjusting for wealth (using a wealth index) or income did not offset or dampen the strong association between car ownership and VF, as would be expected were these characteristics directly linked to individual wealth. Automobile ownership, as a mark of status and a means of providing for family, may be part of the ideal of masculinity in South Africa (66), as has been noted in other cultures (40, 63, 67, 68), and may impact HIV care (40, 41, 69).
We considered the hegemonic masculinity of both Zulu culture, given that 92% of patients in this cohort are of Zulu ethnicity, as well as that of South Africa as a whole. The hegemonic masculinity in Zulu culture is one of strength, invulnerability, and power (34, 70, 71). In the contemporary and traditional, patriarchal Zulu home life, the man’s role is to marry, work and provide for his wife (or wives and/or girl friends) and family (72, 73). Qualitative research has shown that a similar masculine ideal of the strong provider pervades the whole of South Africa (34, 37, 39, 74).
Although gender roles are changing in South Africa, traditional ideas of manhood persist (75), and can be a barrier to men seeking and adhering to treatment (38, 39, 41, 70, 72, 74, 76). Recent focus on men and access to HIV care in South Africa has yielded some insight into the impact of pervasive ideas of masculinity on men’s actions and motivations regarding HIV treatment (66, 69, 71, 74, 76). Admitting vulnerability to HIV, as well as periods of illness or unemployment because of HIV, are in conflict with the South African ideal of manhood (38, 70, 71, 74, 77), potentially leading to compensatory responses or public denial of HIV infection (38, 70). Additionally, although association between “Too busy to take ARVs” and automobile ownership could not be adequately assessed with this sample size (Supplemental Table VIII), literature suggests that family, employment, and other societal expectations and responsibilities can be a barrier to ART (38, 58, 74, 78).
If automobile ownership is part of subscribing to the prevailing ideal of masculinity and societal expectations for men, these men may be susceptible to other aspects of this masculinity that make it difficult to fully subscribe to treatment needs (38, 70, 73, 74). The association between automobile ownership and VF allows identification of males at higher risk of VF, but also may indicate a need for interventions such as support groups or treatment supporters, which have been successful in similar contexts (79–81), or even male-centered HIV clinics (41)to help men manage the conflict between culturally determined gender roles and HIV care.
Several limitations of the present study should be noted. While baseline characteristics of this study population mirror the larger population engaged in HIV treatment in KZN (45, 82), the study population exhibits a lower unemployment rate (20%) as compared to the general population (33%)(45)and the greater HIV population as found in other studies (82). Though the magnitude of this difference is unlikely to either preclude generalizability or change the association found between VF and unemployment given that unemployment rates are lower in the study population, it is a notable difference. Additionally, while the sample size was powered appropriately for analysis of the entire cohort, stratification by gender and age decreased sample size and thus power. With only 35% of patients in the cohort being male, it is possible that the lack of significant association among men in variables shown to be risk factors for VF among women is a result of insignificant power to detect those associations. Along these lines, it is much less common for women to own automobiles than men, and as such, had more women owned automobiles there may have been an association evident between automobile ownership and VF among women as well. Further limitations were described in the RFVF study. Of note as pertaining to both this analysis and the larger RFVF study, we performed many statistical tests but only report the positive results and do not adjust for multiple comparisons. It is possible that some positive findings from this study are spurious and would not be reproducible given another iteration of the same study.
CONCLUSION
Key factors to successful expansion of ART in South Africa, as in much of the world, include improving ART program effectiveness, efficiency and sustainability. Identifying risk factors for virologic failure is a key piece in addressing these three concerns. While algorithms to identify higher risk patients are useful, easy, and definitely have a place in treatment centers that are under staffed and over-crowded, there is also a need for deeper understanding of risk factors specific to the sub-groups of the patients being treated. Our data suggests that some risk factors are more specific to select population groups and can collectively paint a clearer picture of structural factors associated with or contributing to VF and potential ways to mitigate these risk factors. From this analysis we postulate that certain individuals might benefit from additional assistance. Women who do not have employment or personal income and depend on extended family to pay for their clinic visits may benefit from employment counseling, vocational training or microfinance initiatives to increase employment, independence, and self-empowerment (36). Men who feel pressure to fulfill perceived expectations and ideals of masculinity may benefit from treatment supporters, support groups, motivational interviewing or male-centered clinics to address the sociocultural expectations they experience and develop some solidarity and emotional support. While these associations are still generalizations to be used cautiously, this analysis highlights that socioeconomic risk factors can differ significantly among a population, particularly along gender lines. These differences should be considered in future exploration of structural risk factors and their solutions.
Supplementary Material
Acknowledgments
Foremost, we express our deepest admiration and appreciation for the patients who participated in the RFVF study for their great courage and support of this research; for the counselors, nurses, medical officers and staff of Sinikithemba for their tireless commitment to patient care; and to the entire RFVF team. Funding for this research came from Emory University Center for AIDS Research (CFAR) (P30AI050409), Emory University School of Medicine Division of Infectious Diseases, NIH (R01 AI098558-01A1), Elizabeth Glaser Pediatric AIDS Foundation as part of Project HEART, the Gilead Foundation and Research and Health Sciences IT Division grant support (UL1RR025008).
References
- 1.UNAIDS. Report on the Global AIDS Epidemic 2012. United Nations Programme on HIV/AIDS. 2012 [Google Scholar]
- 2.Hong SNJ, Kelley K, Bertagnolio S, Marconi V, Jordan M. The Global Status of HIV Drug Resistance: Clinical and Public Health Approaches for Detection, Treatment and Prevention. Infect Disord Drug Targets. 2011;11(2):124–33. doi: 10.2174/187152611795589744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nachega JB, Marconi VC, van Zyl GU, et al. HIV Treatment Adherence, Drug Resistance, Virologic Failure: Evolving Concepts. Infectious Disorders -- Drug Targets. 2011;11:167–74. doi: 10.2174/187152611795589663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong SY, Nachega JB, Kelley K, Bertagnolio S, Marconi VC, Jordan MR. The global status of HIV drug resistance: clinical and public-health approaches for detection, treatment and prevention. Infect Disord Drug Targets. 2011 Apr;11(2):124–33. doi: 10.2174/187152611795589744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta RK, Jordan MR, Sultan BJ, et al. Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: a global collaborative study and meta-regression analysis. Lancet. 2012 Oct 6;380(9849):1250–8. doi: 10.1016/S0140-6736(12)61038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Data on the size of the HIV/AIDS epidemic. World Health Organization; 2012. [Google Scholar]
- 7.Gloabl AIDS Response Progress Report 2012. South Africa: 2012. [Google Scholar]
- 8.Barth RE, van der Loeff MF, Schuurman R, Hoepelman AI, Wensing AM. Virological follow-up of adult patients in antiretroviral treatment programmes in sub-Saharan Africa: a systematic review. The Lancet infectious diseases. 2010 Mar;10(3):155–66. doi: 10.1016/S1473-3099(09)70328-7. [DOI] [PubMed] [Google Scholar]
- 9.Boulle A. Antiretroviral therapy and early mortality in South Africa. Bulletin of the World Health Organization. 2008;86(9):678–87. doi: 10.2471/BLT.07.045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox MP, Cutsem GV, Giddy J, et al. Rates and predictors of failure of first-line antiretroviral therapy and switch to second-line ART in South Africa. Journal of acquired immune deficiency syndromes (1999) 2012 Aug 1;60(4):428–37. doi: 10.1097/QAI.0b013e3182557785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Khatib Z, Katzenstein D, Marrone G, et al. Adherence to drug-refill is a useful early warning indicator of virologic and immunologic failure among HIV patients on first-line ART in South Africa. PloS one. 2011;6(3):e17518. doi: 10.1371/journal.pone.0017518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice BD, Batzing-Feigenbaum J, Hosegood V, et al. Population and antenatal-based HIV prevalence estimates in a high contracepting female population in rural South Africa. BMC public health. 2007;7:160. doi: 10.1186/1471-2458-7-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marconi VC, Sunpath H, Lu Z, et al. Prevalence of HIV-1 drug resistance after failure of a first highly active antiretroviral therapy regimen in KwaZulu Natal, South Africa. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008 May 15;46(10):1589–97. doi: 10.1086/587109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mutevedzi PC, Lessells RJ, Heller T, Barnighausen T, Cooke GS, Newell ML. Scale-up of a decentralized HIV treatment programme in rural KwaZulu-Natal, South Africa: does rapid expansion affect patient outcomes? Bull World Health Organ. 2010 Aug 1;88(8):593–600. doi: 10.2471/BLT.09.069419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jordan MR, Bennett DE, Wainberg MA, et al. Update on World Health Organization HIV drug resistance prevention and assessment strategy: 2004–2011. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012 May;54(Suppl 4):S245–9. doi: 10.1093/cid/cis206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett DE, Jordan MR, Bertagnolio S, et al. HIV drug resistance early warning indicators in cohorts of individuals starting antiretroviral therapy between 2004 and 2009: World Health Organization global report from 50 countries. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012 May;54(Suppl 4):S280–9. doi: 10.1093/cid/cis207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baral S, Logie CH, Grosso A, Wirtz AL, Beyrer C. Modified social ecological model: a tool to guide the assessment of the risks and risk contexts of HIV epidemics. BMC public health. 2013;13:482. doi: 10.1186/1471-2458-13-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kagee A, Remien R, Berkman A, Hoffman S, Campos L, Swartz L. Structural barriers to ART adherence in Southern Africa: Challenges and potential ways forward. Global public health. 2011;6(1):83–97. doi: 10.1080/17441691003796387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sumartojo E. Structural factors in HIV prevention: concepts, examples, and implications for research. Aids. 2000 Jun;14(Suppl 1):S3–10. doi: 10.1097/00002030-200006001-00002. [DOI] [PubMed] [Google Scholar]
- 20.Denton M, Prus S, Walters V. Gender differences in health: a Canadian study of the psychosocial, structural and behavioural determinants of health. Soc Sci Med. 2004 Jun;58(12):2585–600. doi: 10.1016/j.socscimed.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Parker RG, Easton D, Klein CH. Structural barriers and facilitators in HIV prevention: a review of international research. Aids. 2000 Jun;14(Suppl 1):S22–32. doi: 10.1097/00002030-200006001-00004. [DOI] [PubMed] [Google Scholar]
- 22.Rebolledo P, Kourbatova E, Rothenberg R, Del Rio C. Factors associated with utilization of HAART amongst hard-to-reach HIV-infected individuals in Atlanta, Georgia. J AIDS HIV Res. 2011 Mar;3(3):63–70. [PMC free article] [PubMed] [Google Scholar]
- 23.Gruskin S, Ferguson L, Alfven T, Rugg D, Peersman G. Identifying structural barriers to an effective HIV response: using the National Composite Policy Index data to evaluate the human rights, legal and policy environment. Journal of the International AIDS Society. 2013;16(1) doi: 10.7448/IAS.16.1.18000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rachlis BS, Mills EJ, Cole DC. Livelihood security and adherence to antiretroviral therapy in low and middle income settings: a systematic review. PloS one. 2011;6(5):e18948. doi: 10.1371/journal.pone.0018948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Posse M, Meheus F, van Asten H, van der Ven A, Baltussen R. Barriers to access to antiretroviral treatment in developing countries: a review. Tropical medicine & international health : TM & IH. 2008 Jul;13(7):904–13. doi: 10.1111/j.1365-3156.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- 26.Byakika-Tusiime J, Oyugi JH, Tumwikirize WA, Katabira ET, Mugyenyi PN, Bangsberg DR. Adherence to HIV antiretroviral therapy in HIV+ Ugandan patients purchasing therapy. International Journal of STD & AIDS. 2005;16(1):38–41. doi: 10.1258/0956462052932548. [DOI] [PubMed] [Google Scholar]
- 27.Kagee A, Delport T. Barriers to adherence to antiretroviral treatment: the perspectives of patient advocates. Journal of health psychology. 2010 Oct;15(7):1001–11. doi: 10.1177/1359105310378180. [DOI] [PubMed] [Google Scholar]
- 28.Obiako OR, Muktar HM. Challenges of HIV treatment in resource-poor countries: a review. Nigerian journal of medicine : journal of the National Association of Resident Doctors of Nigeria. 2010 Oct-Dec;19(4):361–8. doi: 10.4314/njm.v19i4.69785. [DOI] [PubMed] [Google Scholar]
- 29.Coetzee B, Kagee A, Vermeulen N. Structural barriers to adherence to antiretroviral therapy in a resource-constrained setting: the perspectives of health care providers. AIDS care. 2011 Feb 01;23(2):146–51. doi: 10.1080/09540121.2010.498874. [DOI] [PubMed] [Google Scholar]
- 30.Siedner MJ, Lankowski A, Tsai AC, et al. GPS-measured distance to clinic, but not self-reported transportation factors, are associated with missed HIV clinic visits in rural Uganda. Aids. 2013 Feb 21; doi: 10.1097/QAD.0b013e32835fd873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosen S, Ketlhapile M, Sanne I, deSilva MB. Cost to patients of obtaining treatment for HIV/AIDS in South Africa. 2007 [PubMed] [Google Scholar]
- 32.Hardon AP, Akurut D, Comoro C, et al. Hunger, waiting time and transport costs: time to confront challenges to ART adherence in Africa. AIDS Care. 2007 May;19(5):658–65. doi: 10.1080/09540120701244943. [DOI] [PubMed] [Google Scholar]
- 33.Women’s Property Rights, HIV and AIDS, and Violence in South Africa and Uganda: Preliminary Findings. Vol. 2007. Washington, DC, USA: International Center for Research on Women (ICRW); 2007. Report No. [Google Scholar]
- 34.Jewkes R, Morrell R. Gender and sexuality: emerging perspectives from the heterosexual epidemic in South Africa and implications for HIV risk and prevention. Journal of the International AIDS Society. 2010;13:6. doi: 10.1186/1758-2652-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dworkin SL, Grabe S, Lu T, et al. Property rights violations as a structural driver of women’s HIV risks: a qualitative study in Nyanza and Western Provinces, Kenya. Archives of sexual behavior. 2013 Jul;42(5):703–13. doi: 10.1007/s10508-012-0024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JC, Watts CH. Gaining a foothold: tackling poverty, gender inequality, and HIV in Africa. BMJ (Clinical research ed) 2005 Oct 1;331(7519):769–72. doi: 10.1136/bmj.331.7519.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beck D. Men and ARVs: How does being a man affect access to antiretroviral therapy in South Africa? An Investigation among Xhosa-speaking men in Khayelitsha. 2004 [Google Scholar]
- 38.Dageid W, Govender K, Gordon SF. Masculinity and HIV disclosure among heterosexual South African men: implications for HIV/AIDS intervention. Culture, health & sexuality. 2012;14(8):925–40. doi: 10.1080/13691058.2012.710337. [DOI] [PubMed] [Google Scholar]
- 39.Richter L, Morrell R. In: Baba: Men and Fatherhood in South Africa. Richter L, Morrell R, editors. Cape Town, South Africa: Human Sciences Research Council; 2006. [Google Scholar]
- 40.Skovdal M, Campbell C, Madanhire C, Mupambireyi Z, Nyamukapa C, Gregson S. Masculinity as a barrier to men’s use of HIV services in Zimbabwe. Globalization and health. 2011;7:13. doi: 10.1186/1744-8603-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barnighausen T, editor. Unequal Benefits from ART: A Growing Male Disadvantage in Life Expectancy in Rural South Africa; Conference on Retroviruses and Opportunistic Infections; 2014 March 6, 2014; Boston, Massachusettes, USA. [Google Scholar]
- 42.Druyts E, Dybul M, Kanters S, et al. Male sex and the risk of mortality among individuals enrolled in antiretroviral therapy programs in Africa: a systematic review and meta-analysis. Aids. 2013 Jan 28;27(3):417–25. doi: 10.1097/QAD.0b013e328359b89b. [DOI] [PubMed] [Google Scholar]
- 43.Marconi VC, Wu B, Hampton J, et al. Early warning indicators for first-line virologic failure independent of adherence measures in a South African urban clinic. AIDS patient care and STDs. 2013 Dec;27(12):657–68. doi: 10.1089/apc.2013.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marx C, Charlton S. The Case of Durban, South Africa. UN-Habitat and University College; London, Unit DP: 2003. [Google Scholar]
- 45.Lehohla P. Census 2011 Municipal report. KwaZulu-Natal; Pretoria, South Africa: 2012. p. 03-01-53. [Google Scholar]
- 46.Ramirez-Avila L, Regan S, Giddy J, et al. Depressive symptoms and their impact on health-seeking behaviors in newly-diagnosed HIV-infected patients in Durban, South Africa. AIDS and behavior. 2012 Nov;16(8):2226–35. doi: 10.1007/s10461-012-0160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Department of Health SA, editor. National Antiretroviral Treatment Guideline. South Africa: South African Department of Health; 2013. [Google Scholar]
- 48.Rutstein S. Working Paper. Washington, D.C., USA: United States Agency for International Devleopment, Research DaH; 2008. The DHS Wealth Index: Approaches for Rural and Urban Areas. Report No.: 60 Contract No.: 60. [Google Scholar]
- 49.Weintrob AC, Fieberg AM, Agan BK, et al. Increasing age at HIV seroconversion from 18 to 40 years is associated with favorable virologic and immunologic responses to HAART. Journal of acquired immune deficiency syndromes (1999) 2008 Sep 1;49(1):40–7. doi: 10.1097/QAI.0b013e31817bec05. [DOI] [PubMed] [Google Scholar]
- 50.Greenbaum AH, Wilson LE, Keruly JC, Moore RD, Gebo KA. Effect of age and HAART regimen on clinical response in an urban cohort of HIV-infected individuals. Aids. 2008 Nov 12;22(17):2331–9. doi: 10.1097/QAD.0b013e32831883f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhat VG, Ramburuth M, Singh M, et al. Factors associated with poor adherence to anti-retroviral therapy in patients attending a rural health centre in South Africa. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2010 Aug;29(8):947–53. doi: 10.1007/s10096-010-0949-4. [DOI] [PubMed] [Google Scholar]
- 52.Hawkins C, Chalamilla G, Okuma J, et al. Sex differences in antiretroviral treatment outcomes among HIV-infected adults in an urban Tanzanian setting. Aids. 2011 Jun 1;25(9):1189–97. doi: 10.1097/QAD.0b013e3283471deb. [DOI] [PubMed] [Google Scholar]
- 53.Lima VD, Bangsberg DR, Harrigan PR, et al. Risk of viral failure declines with duration of suppression on highly active antiretroviral therapy irrespective of adherence level. Journal of acquired immune deficiency syndromes (1999) 2010 Dec;55(4):460–5. doi: 10.1097/QAI.0b013e3181f2ac87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paul A, Harris RT, Thielke Robert, Payne Jonathon, Gonzalez Nathaniel, Conde Jose G. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta GR, Parkhurst JO, Ogden JA, Aggleton P, Mahal A. Structural approaches to HIV prevention. The Lancet. 2008;372(9640):764–75. doi: 10.1016/S0140-6736(08)60887-9. [DOI] [PubMed] [Google Scholar]
- 56.Ramadhani HO, Thielman NM, Landman KZ, et al. Predictors of incomplete adherence, virologic failure, and antiviral drug resistance among HIV-infected adults receiving antiretroviral therapy in Tanzania. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007 Dec 1;45(11):1492–8. doi: 10.1086/522991. [DOI] [PubMed] [Google Scholar]
- 57.Dabis F. Eearly loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bulletin of the World Health Organization. 2008;86(7):559–67. doi: 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller CM, Ketlhapile M, Rybasack-Smith H, Rosen S. Why are antiretroviral treatment patients lost to follow-up? A qualitative study from South Africa. Tropical medicine & international health : TM & IH. 2010 Jun;15(Suppl 1):48–54. doi: 10.1111/j.1365-3156.2010.02514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Topp SM, Li MS, Chipukuma JM, et al. Does provider-initiated counselling and testing (PITC) strengthen early diagnosis and treatment initiation? Results from an analysis of an urban cohort of HIV-positive patients in Lusaka, Zambia. Journal of the International AIDS Society. 2012;15(2) doi: 10.7448/IAS.15.2.17352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kiula ES, Damian DJ, Msuya SE. Predictors of HIV serostatus disclosure to partners among HIV-positive pregnant women in Morogoro, Tanzania. BMC public health. 2013 May 3;13(1):433. doi: 10.1186/1471-2458-13-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stangl AL, Wamai N, Mermin J, Awor AC, Bunnell RE. Trends and predictors of quality of life among HIV-infected adults taking highly active antiretroviral therapy in rural Uganda. AIDS care. 2007 May;19(5):626–36. doi: 10.1080/09540120701203915. [DOI] [PubMed] [Google Scholar]
- 62.Martikainen P, Bartley M, Lahelma E. Psychosocial determinants of health in social epidemiology. International journal of epidemiology. 2002 Dec;31(6):1091–3. doi: 10.1093/ije/31.6.1091. [DOI] [PubMed] [Google Scholar]
- 63.Hiscock R, Macintyre S, Kearns A, Ellaway A. Means of transport and ontological security: Do cars provide psycho-social benefits to their users? Transportation Research Part D: Transport and Environment. 2002 Mar;7(2):119–35. [Google Scholar]
- 64.Hargreaves JR, Morison LA, Gear JSS, et al. Hearing the Voices of the Poor”: Assigning Poverty Lines on the Basis of Local Perceptions of Poverty. A Quantitative Analysis of Qualitative Data from Participatory Wealth Ranking in Rural South Africa. World Development. 2007 Feb;35(2):212–29. [Google Scholar]
- 65.Kaus W. Conspicuous consumption and “race”: Evidence from South Africa. Journal of Development Economics. 2013 Jan;100(1):63–73. [Google Scholar]
- 66.Ragnarsson A, Townsend L, Ekstrom AM, Chopra M, Thorson A. The construction of an idealised urban masculinity among men with concurrent sexual partners in a South African township. Global health action. 2010;3 doi: 10.3402/gha.v3i0.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ellaway A, Macintyre S, Hiscock R, Kearns A. In the driving seat: psychosocial benefits from private motor vehicle transport compared to public transport. Transportation Research Part F: Traffic Psychology and Behaviour. 2003 Sep;6(3):217–31. [Google Scholar]
- 68.Vick M. Danger on Roads: Masculinity, the Car, and Safety. Youth Studies Australia. 2003 Mar;22(1):32–7. [Google Scholar]
- 69.Siu GE, Wight D, Seeley J. How a masculine work ethic and economic circumstances affect uptake of HIV treatment: experiences of men from an artisanal gold mining community in rural eastern Uganda. Journal of the International AIDS Society. 2012;15(Suppl 1):1–9. doi: 10.7448/IAS.15.3.17368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lynch I, Brouard PW, Visser MJ. Constructions of masculinity among a group of South African men living with HIV/AIDS: reflections on resistance and change. Culture, health & sexuality. 2010 Jan;12(1):15–27. doi: 10.1080/13691050903082461. [DOI] [PubMed] [Google Scholar]
- 71.Mfecane S. Narratives of HIV disclosure and masculinity in a South African village. Culture, health & sexuality. 2012;14(Suppl 1):S109–21. doi: 10.1080/13691058.2011.647081. [DOI] [PubMed] [Google Scholar]
- 72.Hadebe L. Zulu Masculinity: Culture, Faith and the Constitution in the South African Context. University of KwaZulu-Natal; Pietermaritzburg: 2010. [Google Scholar]
- 73.Mfecane S. Living with HIV as a man: Implications for masculinity. Psychology in Society. 2008;36:45–59. [Google Scholar]
- 74.Fitzgerald M, Collumbien M, Hosegood V. “No one can ask me ‘Why do you take that stuff?’”: men’s experiences of antiretroviral treatment in South Africa. AIDS care. 2010 Mar;22(3):355–60. doi: 10.1080/09540120903111536. [DOI] [PubMed] [Google Scholar]
- 75.Mantell JE, Needham SL, Smit JA, et al. Gender norms in South Africa: implications for HIV and pregnancy prevention among African and Indian women students at a South African tertiary institution. Culture, health & sexuality. 2009 Feb;11(2):139–57. doi: 10.1080/13691050802521155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harrison A, O’Sullivan LF, Hoffman S, Dolezal C, Morrell R. Gender role and relationship norms among young adults in South Africa: measuring the context of masculinity and HIV risk. Journal of urban health : bulletin of the New York Academy of Medicine. 2006 Jul;83(4):709–22. doi: 10.1007/s11524-006-9077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barker G, Ricardo C. Young Men and the Construction of Masculinity in Sub-Saharan Africa: Implications for HIV/AIDS, Conflict, and Violence. The World Bank; Jun, 2005. Report No.: 32712. [Google Scholar]
- 78.Cornell M, McIntyre J, Myer L. Men and antiretroviral therapy in Africa: our blind spot. Tropical medicine & international health : TM & IH. 2011 Jul;16(7):828–9. doi: 10.1111/j.1365-3156.2011.02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kunutsor S, Walley J, Katabira E, et al. Improving clinic attendance and adherence to antiretroviral therapy through a treatment supporter intervention in Uganda: a randomized controlled trial. AIDS and behavior. 2011 Nov;15(8):1795–802. doi: 10.1007/s10461-011-9927-9. [DOI] [PubMed] [Google Scholar]
- 80.Igumbor JO, Scheepers E, Ebrahim R, Jason A, Grimwood A. An evaluation of the impact of a community-based adherence support programme on ART outcomes in selected government HIV treatment sites in South Africa. AIDS care. 2011 Feb;23(2):231–6. doi: 10.1080/09540121.2010.498909. [DOI] [PubMed] [Google Scholar]
- 81.Kabore I, Bloem J, Etheredge G, et al. The effect of community-based support services on clinical efficacy and health-related quality of life in HIV/AIDS patients in resource-limited settings in sub-Saharan Africa. AIDS patient care and STDs. 2010 Sep;24(9):581–94. doi: 10.1089/apc.2009.0307. [DOI] [PubMed] [Google Scholar]
- 82.Peltzer K. HIV-related symptoms and management in HIV and antiretroviral therapy patients in KwaZulu-Natal, South Africa: a longitudinal study. SAHARA J : journal of Social Aspects of HIV/AIDS Research Alliance / SAHARA, Human Sciences Research Council. 2013;10(2):96–104. doi: 10.1080/17290376.2013.870119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.