Abstract
In order to develop a model for fluorescence-guided surgery (FGS), 143B human osteosarcoma cells expressing red fluorescent protein (RFP) cells were injected into the intramedullary cavity of the tibia in nude mice. The fluorescent area of residual tumors after bright-light surgery (BLS) and FGS was 10.2 ± 2.4 mm2 and 0.1 ± 0.1 mm2, respectively (p < 0.001). The BLS-treated mice and BLS+cisplatinum (CDDP)-treated mice had significant recurrence. In contrast, the FGS mice and FGS+CDDP mice had very little recurring tumor growth. Disease-free survival (DFS) in the BLS-, BLS+CDDP-, FGS-, and FGS+CDDP-treated mice were 12.5%, 37.5%, 75.0%, and 87.5%, respectively. The FGS-treated mice had a significantly higher DFS rate than the BLS-treated mice (p = 0.021). The FGS+CDDP-treated mice had significantly higher DFS rate than the BLS+CDDP-treated mice (p = 0.043). Although chemotherapy significantly reduced multiple metastases (p = 0.033), there was no significant correlation between FGS and lung metastasis. FGS significantly reduced the recurrence of the tumor but did not reduce lung metastasis. The combination of FGS and adjuvant CDDP reduced tumor recurrence and prevented multiple metastases. FGS and adjuvant chemotherapy should be performed as early as possible in the disease to prevent both recurrence and metastatic development.
Keywords: osteosarcoma, RFP, fluorescence-guided surgery, orthotopic mouse model, chemotherapy, cisplatinum (CDDP)
Introduction
Accurate visualization of primary tumor margins and identification of metastatic nodules at the time of surgery are the main factors in determining the success of any cancer surgery. Fluorescence imaging enables cancer navigation and offers higher resolution and sensitivity compared to radiological imaging and to visual inspection and palpation during surgery.1
A variety of labeling compounds have been used for fluorescence-guided surgery in human subjects. For example, sentinel lymph nodes in breast cancer patients were labeled by the near-infrared (NIR) fluorescing dye indocyanine.2 However, indocyanine does not specifically label tumor cells. The hemoglobin precursor, 5-aminolevulinic acid (5-ALA) can drive the accumulation of porphyrins within malignant glioma. Porphyrin fluorescence in the brain can then be visualized.3 In patients given 5-ALA, 65% of 139 patients had complete removal of their tumors, but only 36% of 131 patients showed complete tumor resection with standard surgery. Furthermore, patients who underwent fluorescence-guided surgery had higher 6-month progression-free survival rates (41%) than did those who had surgery under white light (21%).4
Van Dam et al. 1 conjugated folate to fluorescein isothiocyanate (FITC) for labeling olate receptor–α (FR-α)—which is often overexpressed in ovarian cancers. Fluorescence-guided surgery enabled resectioned tumor deposits less than 1 mm. However, overexpression of FR-α varies greatly among different tumors types, which reduces the general applicability of this approach.
Urano et al. 5 topically sprayed tumors with a fluorescence-imaging probe, gammaglutamyl hydroxymethyl rhodamine green (γGlu-HMRG); the probe is activated in a tumor-specific manner by cleavage of the γGlu moiety from HMRG by an enzyme commonly found in some cancer cells, gammaglutamyltranspeptidase (GGT).
In another approach, monoclonal antibodies directed against one of two cancer-specific proteins—cancer antigen 19-9 (CA19-9) or carcino-embryonic antigen (CEA)—was conjugated to a green fluorophore and delivered intravenously into nude mice with orthotopic human pancreatic or colon tumors to make the tumors fluorescent.6 Tumors were resected under fluorescence guidance with significant improvement of outcome compared to BLS.6-12
Tumors can also be genetically labeled in situ with fluorescent proteins. For example, Fong et al. 13 used a herpes simplex virus (NV1066) vector that expresses the green fluorescent protein (gfp) gene to label metastatic lung tumor in the pleural cavity. The tumor foci became fluorescent because NV1066 selectively replicates in cancer cells and expresses GFP. Kishimoto et al.14 labeled tumors with GFP using a telomerase-dependent adenovirus (OBP-401) that expresses the gfp gene only in cancer cells. The labeled tumors could then be resected under fluorescence guidance. Tumors that recurred after FGS maintained GFP expression.15
Inadequate surgical margins were found to be associated with poor event-free survival in a study of 789 patients with extremity osteosarcomas.16 Residual tumor independently increased risk of death in another study including 1,702 cases of osteosarcomas.17
In the present study, we report the effectiveness of using FGS and FGS with adjuvant chemotherapy to improve outcomes in an orthotopic nude mouse model of human osteosarcoma including reducing residual tumor tissue and thereby decreasing recurrence of the tumor.
Materials and Methods
RFP Vector Production
The RFP (DsRed-2) gene (BD Biosciences Clontech, Palo Alto, CA) was inserted in the retroviral-based mammalian expression vector pLNCX (BD Bioscience Clontech) to form the pLNCX DsRed-2 vector. Production of retrovirus resulted from transfection of pLNCX DsRed-2 into PT67 packaging cells, which produce retroviral supernatants containing the DsRed-2 gene. Briefly, PT67 cells were grown as monolayers in DMEM supplemented with 10% FCS (Gemini Biological Products, Calabasas, CA). Exponentially-growing cells (in 10-cm dishes) were transfected with 10 μg expression vector using LipofectAMINE Plus (Life Technologies, Grand Island, NY). Transfected cells were replated 48 hours after transfection and 100 μg/mL G418 was added 7 hours later. Two days later, the concentration of G418 was increased to 200 μg/mL G418. After 25 days of drug selection, surviving colonies were visualized under fluorescence microscopy and RFP-positive colonies were isolated. Several clones were selected and expanded into sub-cell lines after virus tittering on the 3T3 cell line.18-20
RFP Gene Transduction of Osteosarcoma Cells
143B osteosarcoma cells were incubated with a 1:1 precipitated mixture of retroviral supernatants of PT67-RFP cells and RPMI 1640 (Irvine Scientific) containing 10% fetal bovine serum for 72 hours. Fresh medium was replenished at this time. Cells were harvested with trypsin/EDTA 72 hours post-transduction and subcultured at a ratio of 1:15 into selective medium, which contained 200 μg/mL G418. The level of G418 was increased stepwise up to 800 μg/mL. RFP-expressing cancer cells were isolated with cloning cylinders (Bel-Art Products, Pequannock, NJ) using trypsin/EDTA and amplified by conventional culture methods.18-20
Mice
Athymic NCR female nude mice (nu/nu) (AntiCancer Inc., San Diego, CA) were used in this study. Mice were maintained in a barrier facility on high efficiency particulate air (HEPA)-filtered racks. The animals were fed with autoclaved laboratory rodent diet. Animal experiments were performed in accordance with the Guide-lines for the Care and Use of Laboratory Animals under National Institutes of Health assurance number A3873-01.
Orthotopic Tumor Implantation
Eight-week-old female mice were anesthetized by a ketamine mixture (10 μL ketamine HCL, 7.6 μL xylazine, 2.4 μL acepromazine maleate and 10 μL H2O) via s.c. injection. The front left leg was sterilized with alcohol and an approximately 5 mm midline skin incision was made just below the knee joint in order to expose the tibial tuberosity.21 143B-RFP cells (5 × 105) in Matrigel (5 μL) (BD Bioscience, San Jose, CA) per mouse were injected into the intramedullary cavity of the tibia with a 0.5 mL 28 G latex-free insulin syringe (TYCO Health Group LP, Mansfield, MA). The skin was closed with a 6-0 suture. Female mice were used since 143B cells originated in a female patient (ATCC catalog CRL-8303, www.atcc.org).
Treatment Protocol and Tumor Resection
A total of 32 mice were used (Fig. 1): 16 mice underwent FGS, and the other 16 mice underwent bright-light surgery (BLS). Two weeks after the implantation of the cancer cells, tumor-bearing mice were randomly assigned to the BLS or FGS group. Before resection of the osteosarcoma, mice were anesthetized by ketamine mixture, and their limbs were sterilized with alcohol. An approximately 1.5 cm incision was made above the tumor. Resection of the primary osteosarcoma was performed using the MVX-10 long-working distance, high-numerical aperature microscope (Olympus Corp, Tokyo, Japan) under bright-light illumination for the BLS-treated mice and under fluorescence illumination through an RFP filter (excitation HQ 545/30x; emission 620/60m) for the FGS-treated mice. For osteosarcoma resection, intralesional and marginal tumor excision was performed in all the mice. Postoperatively, the surgical resection bed was imaged with the Olympus OV-100 Small Animal Imaging System (Olympus Corp) under both standard bright field and fluorescence illumination to assess the completeness of surgical resection.
Figure 1. Schematic diagram of study timeline.
Two weeks after orthotopic tumor implantation in 32 mice, the tumor-bearing mice were randomly assigned to either BLS or FGS groups. Resection of the tumor was performed using an MVX-10 long-working distance microscope under bright-light illumination for BLS and under fluorescence illumination for FGS. After BLS or FGS, half of the mice received chemotherapy using cisplatinum (CDDP) (5 mg/kg ip) weekly.
Postoperative Chemotherapy
Half of the BLS and FGS mice (8 mice in each group) were randomly selected to undergo adjuvant chemotherapy using cisplatinum (CDDP) (Fig. 1). Starting on postoperative day 1, mice received CDDP (5 mg/kg) weekly ip for 5 weeks.
Noninvasive Imaging of Tumor Recurrence and Progression
To assess for recurrence and to follow tumor progression postoperatively, weekly noninvasive whole-body imaging of the mice was performed using the iBOX Scientia Small Animal Imaging System (UVP LLC, Upland, CA, USA). RFP-expressing areas were recorded every week.
Lung Metastases
Five weeks after surgery, all lungs of the study mice were resected and evaluated for metastases with the OV100. Lung images were classified into three groups; multiple metastases, solitary metastasis, and no metastasis. The correlation between occurrence of lung metastases (total, solitary, and multiple metastases) and treatment including chemotherapy and FGS was determined.
Statistical Analysis
Data showing comparisons between two groups were assessed using the Student's t-test or χ2 test. Comparisons among more than two groups were assessed using analysis of variance (ANOVA). Disease-free survival (DFS), which was defined as the time from the surgical resection to local recurrence, was calculated using the Kaplan-Meier method and log-rank test. Differences were considered significant when P < 0.05. Data are expressed as mean ± SE. Statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University).
Results and Discussion
Fluorescence Imaging Before and After Tumor Resection
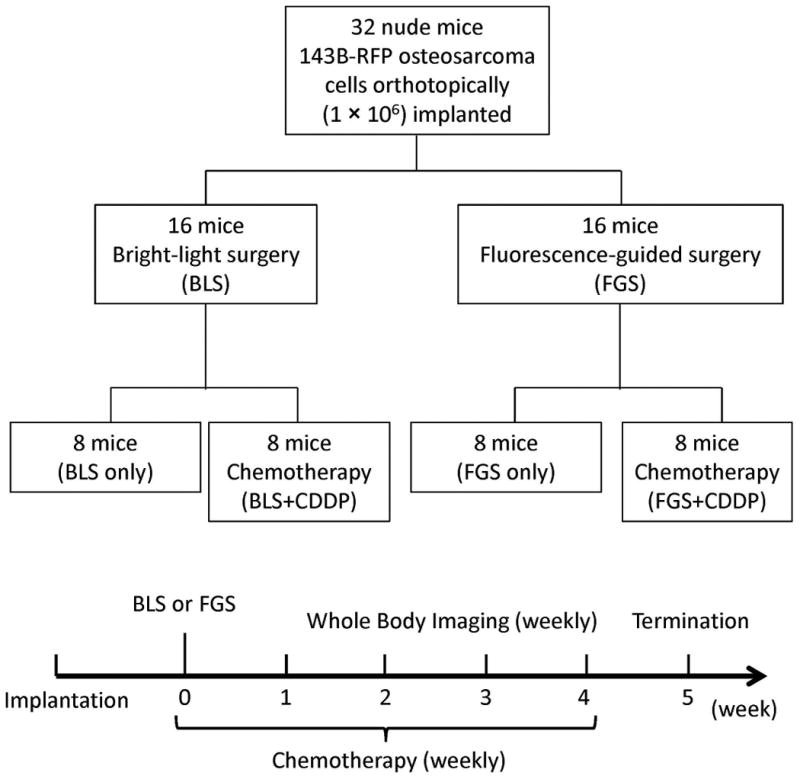
Orthotopic mouse models of human osteosarcoma using brightly red-fluorescent 143B-RFP osteosarcoma cells were established in 32 mice (Fig. 2a). Two weeks after orthotopic implantation, the mice were randomly divided into the BLS or FGS groups (16 mice in each group). Before the surgical resection, the fluorescent area of the mice in the BLS and FGS groups were 44.4 ± 2.6 mm2 and 45.8 ± 1.6 mm2, respectively (Fig. 2b). There was no significant difference in preoperative tumor burden between BLS and FGS mice.
Figure 2. Preoperative images in orthotopic models of osteosarcoma.
a. Orthotopic osteosarcoma mouse model
b. Fluorescent areas before surgery. Before BLS or FGS, there was no significant difference in fluorescent areas between BLS and FGS mice. The experimental data are expressed mean ± SE. Statistical analysis was performed using the Student's t-test.
Significant improvement in visualization of primary osteosarcoma lesions enhanced distinction of tumor from surrounding normal bone and soft tissues due to RFP expression by the tumor (Fig. 3a). FGS resulted in a more complete resection of tumor, demonstrated by a significant decrease in the fluorescent area of residual tumor compared to BLS. Fluorescent areas of the residual tumors after BLS and FGS were 10.2 ± 2.4 mm2 and 0.1 ± 0.1 mm2, respectively (Fig. 3b, P < 0.001).
Figure 3. Pre- and post-operative images in the orthotopic osteosarcoma model.

a. Pre- and post-operative images of mice in the BLS and FGS groups. After BLS, fluorescence imaging visualized residual tumor in the surgical field. The enhanced ability to visualize and identify tumor margins under fluorescence guidance permitted more complete resection.
b. Fluorescent area of residual tumor after BLS and FGS. Fluorescent areas of residual tumors in BLS and FGS mice were 10.2 ± 2.4 mm2 and 0.1 ± 0.1 mm2, respectively (P < 0.001). The experimental data are expressed mean ± SE. Statistical analysis was performed using the Student's t-test.
Growth of Residual Tumor After BLS and FGS
Time-lapse imaging visualized rapid growth of the fluorescent areas in the BLS-treated mice after surgery (Fig. 4). There was minimal growth of the residual tumor in the FGS-treated mice (Fig. 4). Fluorescent areas of the mice treated with BLS only and mice treated with BLS+CDDP mice increased, whereas mice treated with FGS and mice treated with FGS+CDDP had minimal tumor recurrence (Fig. 5). Five weeks after surgery, the fluorescent area of BLS-, BLS+CDDP-, FGS-, and FGS+CDDP-treated mice were 183.6 ± 53.0; 44.8 ± 31.1; 10.6 ± 9.5; and 1.3 ± 1.3 mm2 (Fig. 5). There were significant differences between the BLS-treated mice and the other mice (P < 0.05).
Figure 4. Time lapse imaging of the mice after BLS and FGS.
Fluorescence imaging after BLS demonstrated growth of the residual tumor. In contrast, imaging after FGS showed little recurrent tumor growth.
Figure 5. Fluorescent areas after BLS or FGS.

Fluorescent areas of BLS- and BLS+CDDP–treated mice demonstrated growth of recurrent tumors. FGS- and FGS+CDDP-treated mice had only minimal growth of recurrent tumors. Five weeks after surgery, the fluorescent area of BLS-, BLS+CDDP-, FGS-, and FGS+CDDP-treated mice were 183.6 ± 53.0, 44.8 ± 31.1, 10.6 ± 9.5, and 1.3 ± 1.3 mm2. The experimental data are expressed mean ± SE. Statistical analysis was performed using ANOVA. *p < 0.05, compared with BLS group.
Disease-Free Survival (DFS) After FGS and BLS
Five-week DFS rates of BLS-, BLS+CDDP-, FGS-, and FGS+CDDP-treated mice were 12.5%, 37.5%, 75.0%, and 87.5%, respectively (Fig. 6a). Mice treated with FGS had a significantly higher survival rate than mice treated with BLS (p = 0.021). Mice treated with FGS+CDDP had a significantly higher survival rate than mice treated with BLS+CDDP (P = 0.043). BLS+CDDP–treated mice and FGS+CDDP-treated mice had higher disease-free survival rates than BLS mice and FGS mice, respectively, but there were no significant differences (P = 0.370, P = 0.564, respectively). There was no significant association between CDDP treatment and DFS. FGS mice had a significantly higher DFS than the BLS mice (Fig. 6c; P < 0.001).
Figure 6. Kaplan-Meier curve for disease-free survival (DFS).

a. Kaplan-Meier curve of DFS for each group
b. Correlation between chemotherapy and DFS
c. Correlation between FGS and DFS
Influence of Chemotherapy and FGS on Lung Metastases
Five weeks after surgery, the lungs of the mice were resected and imaged with the OV100 in order to visualize lung metastases. Images of the lungs were classified into three groups: multiple metastases, solitary metastasis, and no metastasis (Fig. 7). Lung metastases were observed in 4 mice with BLS, 1 mouse with BLS+CDDP, 2 mice with FGS, and 2 mice with FGS+CDDP (Table 1). Multiple metastases were observed in 3 mice with BLS and 1 mouse with FGS. Multiple metastases were not found in the BLS+CDDP and FGS+CDDP mice (Table 2). The data showed that chemotherapy significantly reduced multiple metastases (p = 0.033). Solitary metastasis were found in 1 mouse with BLS, 1 mouse of BLS+CDDP, 1 mouse with FGS, and 2 mice with FGS+CDDP. These data showed that there is no significant correlation between FGS and lung metastasis.
Figure 7. Lung metastases after surgery and chemotherapy.
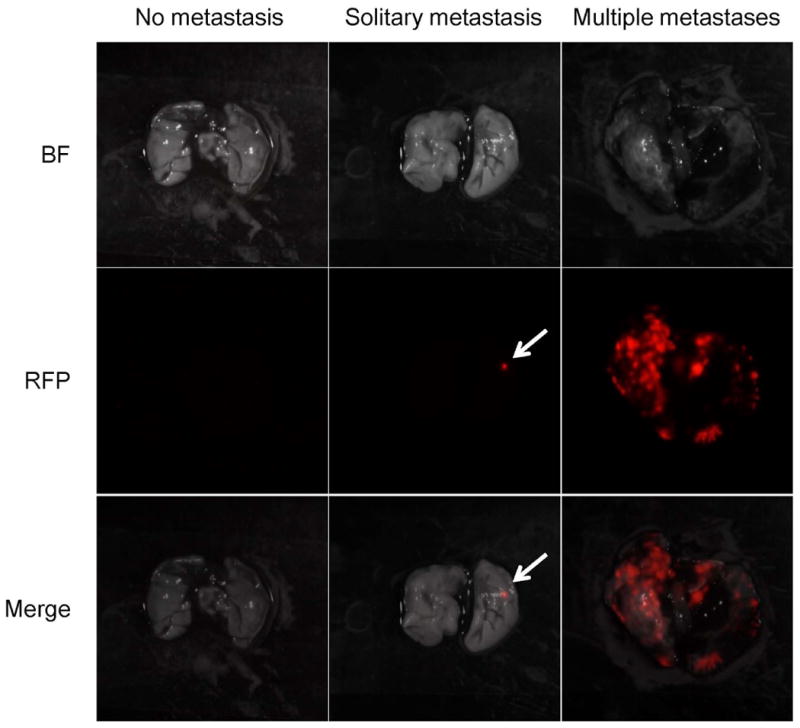
Fluorescent imaging using RFP-expressing cells enabled to identify the lesions of lung metastases. To distinguish the lung metastases as curative or not curative, the images of the lungs were classified into three groups; multiple metastases, solitary metastasis, and no metastasis.
Table 1. Total lung metastases.
| BLS only (n = 8) |
BLS+CDDP (n = 8) |
FGS only (n = 8) |
FGS+CDDP (n = 8) |
|
|---|---|---|---|---|
| Multiple | 3 | 0 | 1 | 0 |
| Solitary | 1 | 1 | 1 | 2 |
| Total | 4 | 1 | 2 | 2 |
Table 2. Multiple lung metastases.
| CDDP (-) | CDDP (+) | |
|---|---|---|
| Multiple metastases (+) | 4 | 0 |
P = 0.033
The current standard first line protocol for multi-agent chemotherapy consists of doxorubicin, cisplatinum and high-dose methotrexate with leukovorin-rescue and/or ifosfamide.22 Cisplatinum was chosen for adjuvant chemotherapy for our study as a representative drug for the purpose of not introducing multiple variables as would be the case if a combination were used. Although, the incidence of lung metastasis was still high in the BLS and FGS groups treated with cisplatinum, multiple metastasis did not occur in the BLS and FGS groups when either was combined with cisplatinum. These results suggest possible activity of cisplatinum against the occurrence of at least multiple metastasis. Future experiments will use adjuvant combination chemotherapy which includes cisplatinum with both FGS and BLS and earlier time points of treatment to determine conditions for total prevention of lung metastasis in this osteosarcoma model.
In the present study, we utilized FGS to achieve complete resection in orthotopic osteosarcoma models. Our study showed that FGS significantly reduced the residual tumor and improved DFS. This procedure can be beneficial for patients with osteosarcoma to conserve limb function and prevent recurrence of the tumor, since fluorescence guidance can be used to distinguish between normal tissue and tumor tissue.
In the present study, there is no significant correlation between surgical procedure (BLS or FGS) and lung metastases. On the other hand, chemotherapy significantly reduced multiple metastases. These results indicate that lung metastases had already existed before the surgery, and that chemotherapy inhibited growth of the micrometastases. To improve the prognosis of the patients with osteosarcoma, the patients should receive chemotherapy because micrometastases may be present from the early stage of the disease. The combination of FGS and adjuvant chemotherapy should be tested in the clinic to reduce tumor recurrence and multiple metastases in osteosarcoma patients.
Acknowledgments
This study was supported in part by National Cancer Institute grant CA132971 and CA142669.
Footnotes
Competing Interests: The authors declare that they have no competing interests.
Dedication: This paper is dedicated to the memory of A.R. Moossa, M.D.
References
- 1.Van Dam GM, Themelis G, Crane LM, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First inhuman results. Nat Med. 2011;17:1315–1319. doi: 10.1038/nm.2472. [DOI] [PubMed] [Google Scholar]
- 2.Troyan SL, Kianzad V, Gibbs-Strauss SL, et al. The FLARE intraoperative near-infrared fluorescence imaging system: A first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol. 2009;16:2943–2952. doi: 10.1245/s10434-009-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 4.Bouvet M, Hoffman RM. Glowing tumors make for better detection and resection. Sci Transl Med. 2011;3:110sf–10. doi: 10.1126/scitranslmed.3003375. [DOI] [PubMed] [Google Scholar]
- 5.Urano Y, Sakabe M, Kosaka N, et al. Rapid cancer detection by topically spraying a Y-glutamyltranspeptidase–activated, fluorescent probe. Sci Transl Med. 2011;3:110ra–119. doi: 10.1126/scitranslmed.3002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McElroy M, Kaushal S, Luiken GA, et al. Imaging of primary and metastatic pancreatic cancer using a fluorophore-conjugated anti-CA19-9 antibody for surgical navigation. World J Surg. 2008;32:1057–1066. doi: 10.1007/s00268-007-9452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metildi CA, Kaushal S, Lee C, et al. An LED light source and novel fluorophore combinations improve fluorescence laparoscopic detection of metastatic pancreatic cancer in orthotopic mouse models. J Am Coll Surg. 2012;214:997–1007. doi: 10.1016/j.jamcollsurg.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metildi CA, Kaushal S, Hardamon CR, et al. Fluorescence-Guided Surgery Allows for More Complete Resection of Pancreatic Cancer, Resulting in Longer Disease-Free Survival Compared with Standard Surgery in Orthotopic Mouse Models. J Am Coll Surg. 2012;215:126–136. doi: 10.1016/j.jamcollsurg.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran Cao HS, Kaushal S, Metildi CA, et al. Tumor-specific fluorescence antibody imaging enables accurate staging laparoscopy in an orthotopic model of pancreatic cancer. Hepatogastroenterology. 2012;59(118):1994–1999. doi: 10.5754/hge11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouvet M, Metildi CA, Hoffman RM. Reply re: Fluorescence-guided surgery allows for more complete resection of pancreatic cancer, resulting in longer disease-free survival compared with standard surgery in orthotopic mouse models. J Am Coll Surg. 2012;215:592–593. doi: 10.1016/j.jamcollsurg.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metildi CA, Kaushal S, Snyder CS, et al. Fluorescence-guided surgery of human colon cancer increases complete resection resulting in cures in an orthotopic nude mouse model. J Surg Res. 2013;179:87–93. doi: 10.1016/j.jss.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metildi CA, Hoffman RM, Bouvet M. Fluorescence-guided surgery and fluorescence laporascopy for gastrointestinal cancers in clinically-relevant mouse models. Gastroenterol Res Pract. 2013;2013 doi: 10.1155/2013/290634. 290634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stiles BM, Adusumilli PS, Bhargava A, et al. Minimally invasive localization of oncolytic herpes simplex viral therapy of metastatic pleural cancer. Cancer Gene Ther. 2006;13:53–64. doi: 10.1038/sj.cgt.7700860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kishimoto H, Zhao M, Hayashi K, et al. In vivo internal tumor illumination by telomerase-dependent adenoviral GFP for precise surgical navigation. Proc Natl Acad Sci USA. 2009;106:14514–14517. doi: 10.1073/pnas.0906388106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kishimoto H, Aki R, Urata Y, et al. Tumor-selective, adenoviral-mediated GFP genetic labeling of human cancer in the live mouse reports future recurrence after resection. Cell Cycle. 2011;10:2737–2741. doi: 10.4161/cc.10.16.16756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bacci G, Longhi A, Versari M, et al. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy. Cancer. 2006;106:1154–1161. doi: 10.1002/cncr.21724. [DOI] [PubMed] [Google Scholar]
- 17.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman RM, Yang M. Subcellular imaging in the live mouse. Nat Protoc. 2006;1:775–782. doi: 10.1038/nprot.2006.109. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman RM, Yang M. Color-coded fluorescence imaging of tumor-host interactions. Nat Protoc. 2006;1:928–935. doi: 10.1038/nprot.2006.119. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman RM, Yang M. Whole-body imaging with fluorescent proteins. Nat Protoc. 2006;1:1429–1438. doi: 10.1038/nprot.2006.223. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi K, Zhao M, Yamauchi K, et al. Systemic targeting of primary bone tumor and lung metastasis of high-grade osteosarcoma in nude mice with a tumor-selective strain of Salmonella typhimurium. Cell Cycle. 2009;8:870–875. doi: 10.4161/cc.8.6.7891. [DOI] [PubMed] [Google Scholar]
- 22.Bielack S, Carrle D, Casali P. Osteosarcoma: Esmo clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20:137–139. doi: 10.1093/annonc/mdp154. [DOI] [PubMed] [Google Scholar]


