Abstract
Membrane potentials (Vm) and intracellular calcium variations were studied in Lima bean (Phaseolus lunatus) leaves when the Mediterranean climbing cutworm (Spodoptera littoralis) was attacking the plants. In addition to the effect of the feeding insect the impact of several N-acyl Glns (volicitin, N-palmitoyl-Gln, N-linolenoyl-Gln) from the larval oral secretion was studied. The results showed that the early events upon herbivore attack were: a) a strong Vm depolarization at the bite zone and an isotropic wave of Vm depolarization spreading throughout the entire attacked leaf; b) a Vm depolarization observed for the regurgitant but not with volicitin {N-(17-hydroxy-linolenoyl)-Gln} alone; c) an enhanced influx of Ca2+ at the very edge of the bite, which is halved, if the Ca2+ channel blocker Verapamil is used. Furthermore, the dose-dependence effects of N-acyl Gln conjugates-triggered influx of Ca2+ studied in transgenic aequorin-expressing soybean (Glycine max) cells, showed: a) a concentration-dependent influx of Ca2+; b) a configuration-independent effect concerning the stereochemistry of the amino acid moiety; c) a slightly reduced influx of Ca2+ after modification of the fatty acid backbone by functionalization with oxygen and; d) a comparable effect with the detergent SDS. Finally, the herbivore wounding causes a response in the plant cells that cannot be mimicked by mechanical wounding. The involvement of Ca2+ in signaling after herbivore wounding is discussed.
Several plant species, including Lima bean (Phaseolus lunatus), when attacked by herbivores emit volatiles that attract natural predators of the damaging insects. This signaling by the plant to higher trophic levels has been interpreted as the plant's cry for help (Dicke and Sabelis, 1992; Turlings et al., 1995; DeMoraes et al., 1998) and involves at least three different levels of trophic interaction (Agrawal, 2000). Thus, volatile plant compounds released in response to insect feeding are directly associated with the feeding herbivore, which allows the plant to differentiate between mechanical wounding and wounding caused by the chewing insect (Paré et al., 1998). In fact, upon herbivore attack, plants activate a series of genes indicating that feeding insects are able to elicit and up-regulate defense in plants (Thaler, 1999; Baldwin et al., 2001; Schittko et al., 2001; Hui et al., 2003). Furthermore, uninfested Lima bean leaves activate defense genes when exposed to volatiles from conspecific leaves infested with herbivores, e.g. spider mites (Tetranychus urticae), but not when exposed to volatiles from artificially wounded leaves (Arimura et al., 2000a). Expression of these genes very often requires some of the early events in the signal transduction cascade such as calcium influx and protein phosphorylation/dephosphorylation, jasmonate, ethylene, and salicylates (Blumwald et al., 1998; Poppy, 1999; Arimura et al., 2000a, 2002; Winz and Baldwin, 2001). The first events following leaf wounding and introduction of the herbivore elicitors are not well understood, but in several cases the activation of the octadecanoid-signaling cascade has been demonstrated (Koch et al., 1999, and references cited therein). However, damaging leaves also generates a first-line of cell reactions involving both electrical signals and production of reactive oxygen species.
Plants are continuously interacting with the external world. The coordination of internal processes and their balance with the environment are connected with the excitability of plant cells. The primary candidate for intercellular signaling in higher plants is the stimulus-induced change in plasma membrane potential (Vm; Labady et al., 2002). Vm are the result of an imbalance in the quantity of cations and anions across biological membranes. However, the binding of many plant natural products to membranes causes conformational changes in the ion channels and membrane-bound proteins as well as formation of new ion channels or pores, increasing or decreasing ion flow (Warber, 1998; Engelberth et al., 2001; Maffei et al., 2001). Recent electrophysiological studies allowed to identify the involvement of a rapid electrical signal in root to shoot communications in Sorghum bicolor (Mishra et al., 2001), whereas in soybean (Glycine max) phloem appears to participate in the transmission of fast root-to-shoot action potentials upon stress (Shvetsova et al., 2001).
The Vm of the plasma membrane, which lies in the range of −120 to −200 mV in plant cells, may be shifted either to more negative (hyperpolarization) or to more positive values (depolarization) in response to various biotic or abiotic stresses. In plant cells Ca2+ plays a key physiological role as intracellular second messenger. It is especially important for the maintenance of cellular homeostasis and signal transduction pathways (Evans et al., 1991; Piñeros and Tester, 1997; Roh et al., 1998). Among the Ca2+-permeable channels characterized in the plasma membrane of plant cells, voltage-activated Ca2+ channels may contribute to calcium signaling (Thuleau et al., 1998). Interestingly, the plant plasma membrane contains at least three distinct classes of voltage-activated Ca2+ channels stimulated by hyperpolarization (Gelli and Blumwald, 1997), depolarization (Piñeros and Tester, 1995) and voltage insensitive channels (White, 2000), respectively.
Recently, it has been demonstrated that when plants are wounded, jasmonate is synthesized and employed as a long-distance signal that activates the wound response program in unwounded leaves (Stratmann, 2003). Also the floral scent methyl jasmonate has been demonstrated to be involved in gene-activation control and systemic long-distance signaling (Cheong and Choi, 2003). Much less is known about the individual components from the salivary secretions of the feeding insects that lead to the up-regulation of the jasmonate signaling. Volicitin, N-(17-hydroxylinolenoyl)-Gln, a major component from the regurgitant of Spodoptera littoralis Boisd. larvae, has been shown to induce a systemic release of volatiles from maize (Zea mays) plants (Alborn et al., 1997) and N-acyl Glus, for example N-linolenoyl-Glu, from the regurgitant of the tobacco hornworm (Manduca sexta) elicit nicotine biosynthesis in tobacco (Nicotiana tabacum) leaves. Both compound types are highly surface active amphiphiles and were shown to act via the jasmonate pathway (Halitschke et al., 2001; Schmelz et al., 2003). Other molecules are also involved in wound responses and the most studied are H2O2 (Orozco-Cárdenas et al., 2001; Pellinen et al., 2002), salicylic acid (Shah, 2003), and ethylene (Winz and Baldwin, 2001).
So far, most of the work on plant-insect interaction has been done on gene activation and evaluation of the various elements of the signaling pathway in plant cells. To our knowledge almost nothing is known about the early signals upon herbivore attack at the membrane level and the connections between individual components of the salivary secretions of the insects and the subsequent up-regulation of the octadecanoid biosynthesis. In this work we present data on the early events during feeding of S. littoralis on Lima bean leaves and on the effect of individual, especially the surface active spit components, on changes in the Vm and their correlation with intracellular calcium variations.
RESULTS
Effect of Feeding S. littoralis on Lima Bean Leaf Vm
In plants unable to produce constitutive defenses such as glandular trichomes filled with irritating essential oils or morphological structures such as thorns and stinging hairs, the attack of herbivores is a devastating experience, leading to the destruction of fed tissues. However, some plants react to this event by producing volatile organic compounds able to reduce wounding by attracting predators of the attacking herbivore. This type of induced defense requires recognition of the attacking herbivore and the specific interactions of the plant physiology with the oral secretions. The primary candidate for intercellular signaling in higher plants is the stimulus-induced (herbivore wounding) change in Vm (Shvetsova et al., 2001).
The results of the measurement of Vm after mechanical wounding and herbivore attack indicate a specific response of the leaf tissue. Lima bean leaf Vm varies according to the cell type. Preliminary tests on intact leaves allowed evaluating the average Vm of epidermal, guard cell, palisade, and spongy parenchyma cells. Epidermal cells have an average Vm of −50 mV (±5.7 mV), guard cells have an average Vm of −200 mV (±12.2 mV), palisade cells have an average Vm of −140 mV (±9.8 mV), and spongy parenchyma cells have an average Vm of −100 mV (±10.5 mV). Different trials demonstrated that Lima bean palisade cells are the most responsive cells when leaf tissues are attacked by larvae of S. littoralis.
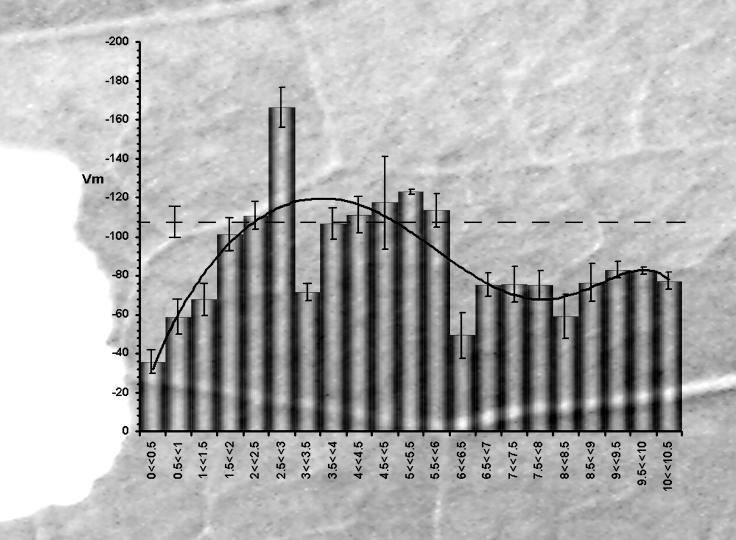
To study the early effects at the bite zone and subsequent signal spreading, Vm was evaluated at increasing distances from the site of damage. The response was a strong Vm depolarization in the bite zone, followed by a transient Vm hyperpolarization and, finally, a constant Vm depolarization throughout the rest of the attacked leaf. Figure 1 shows the Vm variations superimposed on the wounded Lima bean leaf tissue. The ordinates represent Vm expressed in mV, while in the abscissa the bands (and the corresponding histogram bars) represent different distances (and the corresponding Vm values) from the bite zone. The Vm of the mechanically wounded leaf (control) is represented by the dashed line. Exponential interpolation shows the trend of Vm variation. A strong Vm depolarization was found up to about 1.5 mm from the bite zone, whereas a Vm hyperpolarization was found at about 2.5 to 3 mm from the bite zone, immediately followed by a second strong Vm depolarization. Vm differences from control in the zone from 3.5 to about 6 mm from the bite zone were not significant, but Vm displayed depolarized values from 6 mm throughout all the attacked leaf (Fig. 1).
Figure 1.
Lima bean leaf Vm values as a function of distance from the bite zone 15 min after herbivore damage. The histogram superimposed on Lima bean leaf wounded by a larva of S. littoralis represents Vm values (and sd) measured at increasing distances from the bite zone. The dotted line (and its sd) represents the average Vm value from a mechanically wounded Lima bean leaf. In the close vicinity of the bite zone (up to 1.5 mm) there is a strong drop in the Vm (depolarization), whereas at about 2.5 to 3 mm from the bite zone an increase of Vm is observed (hyperpolarization). About 6 mm from the bite zone throughout all leaf there is a constant Vm depolarization.
The trend of the Vm variation prompted a series of experiments aimed to better understand the nature and the reasons for this effect. The first attempt was to probe whether the feeding activity of the herbivore was perceived as a Vm variation even at considerable distances from the bite zone in the same leaf. An intact leaf from a potted plant was fixed to the Vm apparatus and the Vm determined. When Vm reached a constant value S. littoralis was allowed to start its feeding activity. Figure 2 depicts Vm variations as a function of time and distance from mechanically wounded (MW) Lima bean leaf tissue, starting with a potential of about −137 mV, and Vm from a leaf under attack by S. littoralis. It is evident that feeding activity starts a series of Vm variations eventually leading to Vm depolarization within the first 15 min after the onset of the feeding activity. In particular, when Vm was taken from palisade cells at an average distance of 5 mm a strong and transient hyperpolarization occurred within 5 min after the herbivore bite, followed by a constant depolarization. The same pattern was observed when Vm palisade cell was measured at a distance of 30 mm from the bite zone, but depolarization was higher than in cells at 5 mm distance. Finally, in palisade cells that were 60 mm distant from the bite zone Vm depolarization occurred within 2 to 3 min from the bite event and no hyperpolarization was observed (Fig. 2). From Figure 2 it is evident that the recognition of the bite activity of S. littoralis is quickly perceived in the same leaf at increasing distances from the bite area. However, the attempt to find variations in neighboring leaves (OL) resulted in no obvious variations as did mechanical wounding (MW) on the same leaf (Fig. 2).
Figure 2.
Time-course of the Vm variations in palisade cells distant 5, 30, and 60 mm from the bite activity of S. littoralis. The feeding larva induces a series of Vm variations leading to Vm depolarization after about 15 min from the onset of the feeding activity. In particular, when Vm was taken at an average distance of 5 mm, a strong and transient hyperpolarization occurred within 5 min after the herbivore bite, followed by a constant depolarization. The same pattern was observed when Vm was measured at a distance of 30 mm from the bite zone, but depolarization was higher than in cells at 5 mm distance. In cells 60 mm distant from the bite zone, Vm depolarization occurred within 2 to 3 min after the bite, and no hyperpolarization was observed. MW = Vm value after mechanical wounding; OL = Vm value of the opposite leaf.
Effect of S. littoralis Regurgitate and Regurgitate Components on Lima Bean Leaf Vm
In order to evaluate which molecule may be responsible of Vm variations a series of experiments was carried out using regurgitate (R) collected from larvae previously feeding on Lima bean leaves for 24 h.
Perfusion with R caused a Vm depolarization; however, the effect was found not to be linearly linked to concentration. In fact, perfusion with 100 μg mL−1 R depolarized Vm more than perfusion with 250 μg mL−1, but less than perfusion with 500 μg mL−1. Interestingly, when R was washed out with fresh buffer, palisade Vm experienced a hyperpolarization for all concentrations, with an opposite trend as observed during depolarization (Fig. 3).
Figure 3.
Effect of S. littoralis oral secretions and regurgitants (R) on the Vm of Lima bean palisade cells. At the lowest concentration(100 μg mL−1) R caused an intermediate Vm depolarization when compared to concentrations of 250 and 300 μg mL−1. After washing the tissues with fresh buffer (double arrow) Vm was hyperpolarized at all concentrations, and once again R at 100 μg mL−1 had an intermediate value. The single arrow indicates start of perfusion after Vm stabilization. Metric bars = sd.
Since previous studies have demonstrated that R of S. littoralis contains several surface active, amphiphilic compounds, especially N-acyl Gln conjugates (Alborn et al., 1997; Pohnert et al., 1999a; Spiteller and Boland, 2003a, 2003b) these compounds were used to study Vm variations.
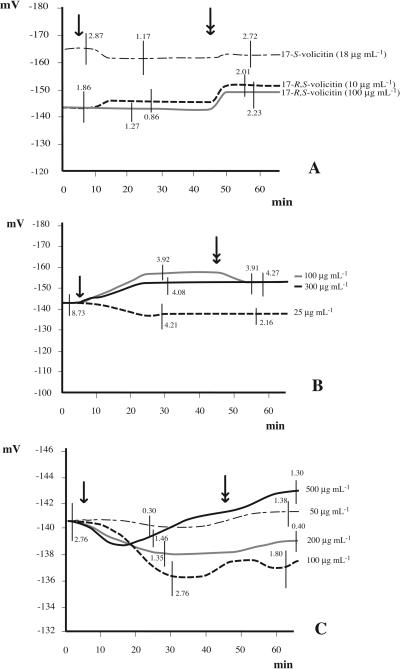
The effect of racemic volicitin (Alborn et al., 1997, 2000) and that of the naturally occurring (17S)-N-(17-hydroxylinoleoyl)-l-Gln (Spiteller et al., 2001) on Vm was low (Fig. 4A). In order to assess whether fatty acid chain length and degree of saturation have an impact on Vm, leaves were perfused with N-palmitoleoyl-l-Gln and N-linolenoyl-l-Gln. Perfusing cells with N-palmitoleoyl-l-Gln caused a Vm depolarization at the lowest concentration used (25 μg mL−1), but a Vm hyperpolarization when higher concentrations (100–300 μg mL−1) were applied (Fig. 4B). Removal of conjugates with fresh buffer had no effect on 25 and 300 μg mL−1 concentrations. A Vm depolarization was, however, observed in perfusion with 100 μg mL−1 (Fig. 4B). Perfusion with N-linolenoyl-l-Gln caused no Vm variation when used at 50 and 500 μg mL−1, whereas the strongest Vm depolarization was observed at 100 μg mL−1 (Fig. 4C).
Figure 4.
A, Vm changes after perfusion of Lima bean leaves with 17-R,S-volicitin (= N-[17R,S-hydroxylinoleoyl]-l-Gln) and the naturally occurring 17S-volicitin (= N-[17S-hydroxylinoleoyl]-l-Gln). At all concentrations, both the racemic mixture and the natural volicitin caused no variation of Vm. B, Effect on the Vm of different N-palmitoleoyl-l-Gln concentrations. The highest concentration (300 μg mL−1) had lower Vm hyperpolarization effects than 100 μg mL−1, whereas at 25 μg mL−1 N-palmitoleoyl-l-Gln caused a constant Vm depolarization even after washing with fresh buffer (double arrow). C, Effect of different N-linolenoyl-l-Gln concentrations on Vm. Clear effects were only caused by concentrations at 100 μg mL−1. A single arrow indicates start of perfusion after Vm stabilization. The double arrow indicates washing with fresh buffer. Metric bars = sd.
To study the impact of the fatty acid and amino acid building blocks of the conjugates, Lima bean leaves were individually treated with linolenic acid and Gln. Linolenic acid caused no obvious effect on Vm at low concentrations (10 and 50 μg mL−1), while a weak Vm depolarization was observed when leaf tissues were perfused with l-Gln (data not shown).
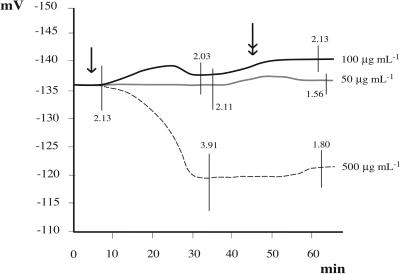
Owing to their molecular architecture N-acyl Glns are amphiphilic compounds with a pronounced ability to form micelles (Asselineau, 1991), similar to known detergents such as SDS. In order to test whether a detergent has an effect on Vm, increasing concentrations of SDS were applied to Lima bean leaves. Low concentrations had no effect, whereas at high concentration (500 mm) a clear Vm depolarization was observed, even after washing with fresh buffer (Fig. 5).
Figure 5.
Effect of increasing concentrations of the detergent SDS on Vm. Lower concentrations (from 50–100 μg mL−1) had no effect, whereas at higher concentration (500 μg mL−1) a considerable Vm depolarization was observed, even after washing with fresh buffer (double arrow). The single arrow indicates start of perfusion after Vm stabilization. Metric bars = sd.
Effect of Feeding S. littoralis and Individual R Components on Lima Bean Leaf Cytosolic Calcium Concentration [Ca2+]c
Intracellular calcium variations may depend on both the entry of Ca2+ in the cytoplasm upon release from cell organelles and the entry from the apoplasm. Since plant cells respond to extracellular stimuli with changes in cytosolic calcium concentration that ultimately controls many integrated physiological processes (Bush, 1995), the impact of feeding on cytosolic calcium concentration, [Ca2+]c, was investigated. Using the membrane-permeant Ca2+-selective fluorescent dye, Fluo-3 AM, the [Ca2+]c were determined by confocal laser scanning microscopy in mechanically- and herbivore-wounded leaf tissue, as well as in tissues treated with linolenoyl-l-Gln. Verapamil, a calcium channel blocker, was additionally used in all experiments. [Ca2+]c was expressed as the percentage of calcium variation in image analysis where the lowest value (0%) refers to zero fluorescence (value 0 on the 0–256 gray level scale) and the highest values (100%) refer to the maximum fluorescence (value 256 on the 0–256 gray level scale).
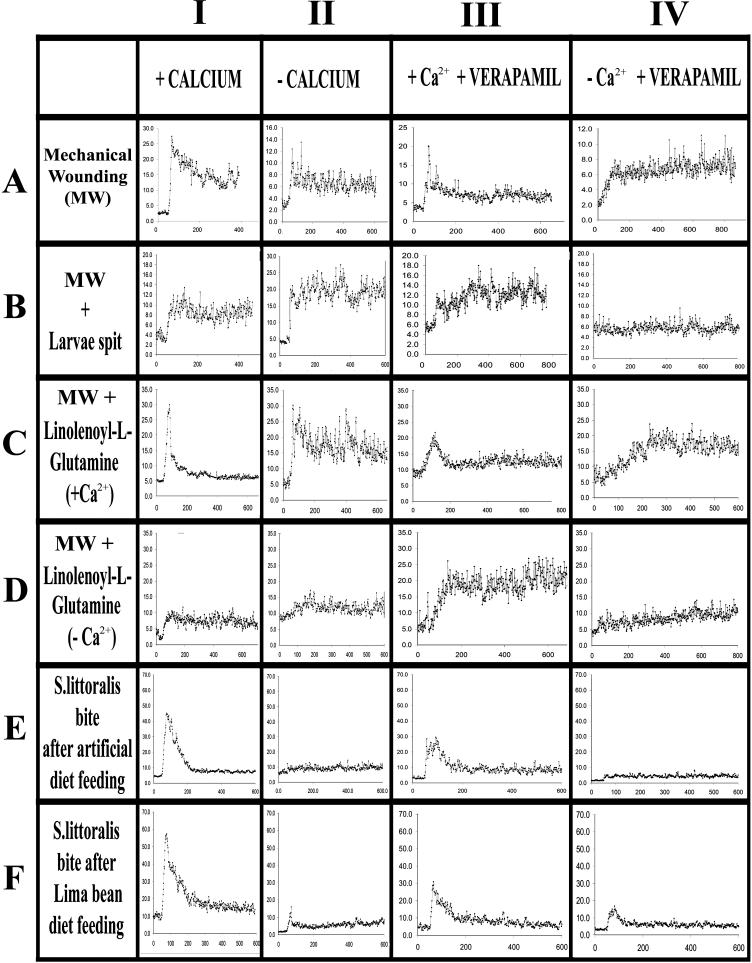
Figures 6 and 7 show the results of the experiments performed incubating Lima bean leaves with 5 mm Fluo-3 AM in 50 mm MES buffer both in the presence of 0.5 mm calcium and without calcium in the incubation medium and in the presence or absence of 100 μm of Verapamil. As a general consideration, in all experiments performed in the presence of Ca2+ a clear peak of Fluo-3 AM fluorescence was observed between 30 and 200 μm from the bite zone, with a sharp decrease at distances greater than 230 μm (Figs. 6 and 7).
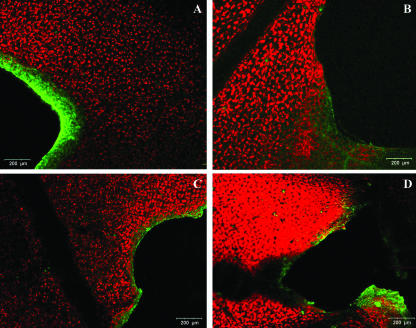
Figure 6.
False-color image analysis reconstructions from confocal laser scanning microscope observations and fluochemical intracellular Ca2+ determination. The green fluorescence refers to binding of Fluo-3 AM with Ca2+, whereas the chloroplasts are evidenced by a bright red color caused by chlorophyll fluorescence. A, Portion of a Lima bean leaf incubated with 5 mm Fluo-3 AM in 50 mm MES buffer in the presence of 0.5 mm Ca2+ attacked by the herbivore S. littoralis. B, Portion of a Lima bean leaf bite zone after incubation with 5 mm Fluo-3 AM in 50 mm MES buffer deprived of Ca2+. The green fluorescence is considerably lower than in A. C, Portion of a Lima bean leaf bite zone incubated with 5 mm Fluo-3 AM in 50 mm MES buffer in the presence of both 0.5 mm Ca2+ and 100 μm Verapamil. The fluorescence is decreased in the presence of the channel blocker Verapamil, with respect to A. D, Portion of a Lima bean leaf mechanically wounded after incubation with 5 mm Fluo-3 AM in 50 mm MES buffer in the presence of 0.5 mm Ca2+. Metric bars are indicated on pictures.
Figure 7.
Percentage of Fluo-3 AM fluorescence (y axis) as a function of the distance (in μm; x axis) from the bite zone. The experiment was designed in order to evaluate the effect of the presence or absence of exogenous Ca2+ in the incubation media and the presence or absence of the Ca2+ channel blocker Verapamil. Mechanical wounding was inferred with a razor blade. Values are the mean of at least five repetitions (see text for description).
Mechanical wounding caused an influx of Ca2+ (Figs. 6D and 7, AI). When Ca2+ was absent from the incubation medium (Fig. 7, AII) or when Verapamil was present (Fig. 7, AIII and AIV) a reduced Ca2+ influx was observed. The average percentage of fluorescence in mechanically wounded leaves was 25% near the damage in the presence of Ca2+ and 5% to 10% without Ca2+. An average of 10% to 15% was recorded in the presence of Ca2+ at increasing distances from the bite zone, whereas only 5% to 10% was found without Ca2+. When mechanically wounded leaves were treated with the larval R, an increase in Fluo-3 AM fluorescence was found in the absence of Ca2+ (Fig. 7, BII) whereas in the presence of Verapamil the occurrence of Ca2+ increased Fluo-3 AM fluorescence (Fig. 7, BIII). When N-linolenoyl-Gln (100 μg mL−1) was given as a 0.5 mm Ca2+-containing solution to mechanically wounded leaves, no differences were observed between tissues treated with (Fig. 7, CI) or without (Fig. 7, CII) Ca2+ in the medium, and the same was observed for Verapamil (Fig. 7, CIII and CIV). Almost the same results were found when N-linolenoyl-Gln was applied as a Ca2+ free solution (Fig. 7, DI–IV). On the other hand, when larvae were allowed to feed on tissues incubated with Fluo-3 AM, a strong Fluo-3 AM fluorescence was observed. In the presence of Ca2+, in both larvae reared on artificial diet (Fig. 7, EI) and larvae reared on Lima bean leaves (Figs. 6A and 7, FI), a sharp and consistent peak of fluorescence was observed up to 100 μm from the bite zone. The absence of Ca2+ dramatically decreased Fluo-3 AM fluorescence in both feeding experiments (Figs. 6B, 7EII, and 7FII). When Verapamil was added in the presence of Ca2+ (Figs. 6C, 7EIII, and 7FIII) it almost halved Fluo-3 AM fluorescence, whereas the absence of Ca2+ in Verapamil treated tissues (Fig. 7, EIV and FIV) showed a decreased Fluo-3 AM fluorescence.
Quantification of Ca2+-Release by N-acyl Glns in Suspension Cultures of Soybean
Loading of Ca2+-sensitive fluorescent probes into plant cells is an essential step to measuring activities of cytoplasmic free Ca2+ ions with a fluorescent imaging technique. However, barriers to the loading of the test compounds or the Ca2+-sensitive fluorescent dyes could be represented by a low permeability of the cell wall as well as by a massive cuticle. This would allow the penetration of only a limited number of cell layers probably near the infection zone. Thus, in order to study dose dependent effects of N-acyl-Gln-triggered Ca2+ concentration changes in the plant cytosol, transgenic soybean suspension cells expressing the Ca2+ sensitive aequorin system were used for further experiments (Mithöfer et al., 1999; Mithöfer and Mazars, 2002). Although among independent experiments the maximum values of the [Ca2+]c varied to some extent, in a single set of measurements using the same population of suspension cells the same day, the relative activities of the effectors analyzed have always been comparable. The Ca2+ response was determined in a concentration-dependent fashion for the most common N-acyl conjugate N-linolenoyl-Gln. For this compound the transiently accumulating [Ca2+]c appeared to be linearly correlated with the amount of effector applied (Fig. 8A). Moreover, it is interesting to note that the configuration of the amino acid moiety had no effect on the Ca2+-response. Both conjugates, containing either l-Gln or d-Gln joined to linolenic acid were able to trigger the Ca2+ response (Fig. 8B).
Figure 8.
Monitoring of cytosolic Ca2+ concentration ([Ca2+]c) changes in soybean cell suspension cultures expressing the Ca2+ sensing aequorin system. A, Enhancement of [Ca2+]c determined upon treatment with increasing concentrations (2, 10, 50, and 200 μg mL−1)of N-linolenoyl-Gln. B, Comparison of the amino acid configurations (l- or d-Gln and l- or d-Glu, respectively) of the linolenic acid conjugates (50 μg mL−1) on their [Ca2+]c increasing activity. C, Effect of various N-acyl Glns (50 μg mL−1) on the [Ca2+]c increase: N-linolenoyl-Gln, N-(15,16-epoxyoctadeca-9,12-dienoyl)-l-Gln, (15,16-epoxy-Gln), 17-hydroxylinolenoyl-l-Gln (volicitin). D, Effect of the detergent SDS (25 μg mL−1) on the [Ca2+]c increase in soybean cell cultures.
Similar results were observed with the corresponding N-acyl Glus, which are typical constituents of the regurgitant of the tobacco horn worm Manduca sexta (Halitschke et al., 2001). Again, both conjugates of linolenic acid with either d- or l-Glu proved to be active (Fig. 8B). A direct comparison among volicitin, N-linolenoyl Gln, and the recently identified 15,16-epoxyoctadeca-9,12-dienyl-Gln (Spiteller and Boland, 2003a, 2003b) at the same concentration, showed that the nonfunctionalized N-linolenoyl-Gln is the most effective compound (Fig. 8C); however, differences were small. For comparison, we also tested the detergent SDS (25 μg mL−1), which showed a highly similar pattern of Ca2+-influx (Fig. 8D) demonstrating that the influx of Ca2+ is a rather unspecific effect linked to the intrinsic molecular properties of amphiphilic compounds.
DISCUSSION
Plant responses to herbivore attack are complex and involve an array of signals, leading to activation of multiple defenses. Feeding herbivores cause extensive and irreversible wounding along with an introduction of salivary secretions. Both wounding and components from the insects' secretions have an obvious, but clearly different impact on the plants' response (Schittko et al., 2001, and references cited therein). In the model system Nicotiana attenuata and its specialist herbivore Manduca sexta, feeding elicits a jasmonate burst, a large transcriptional reorganization of the plant host and, after hours, a systemic release of terpenoids (Halitschke et al., 2001, 2003). Principally the same sequence is passed through in the interaction between Lima bean and spider mites (Arimura et al., 2000a, 2000b, 2002), and in the interaction of maize (Zea mays) plants with the beet armyworm (Spodoptera exigua; Schmelz et al., 2003; for review, see Gatehouse, 2002).
The results of the present work add novel facets to the previously known sequence and demonstrates that herbivore attack onto a Lima bean leaf is associated with (1) a strong Vm depolarization at the bite zone causing a wave of Vm depolarization spreading throughout the entire attacked leaf; and (2) a consistent influx of Ca2+ at the very edge of the bite, which is halved by application of the Ca2+ channel blocker Verapamil. Although none of the amphiphilic N-acyl Glns had a visible impact on the influx of Ca2+ when monitored by fluorescence microscopy in the presence of Fluo-3 AM, the more sensitive assay with the soybean suspension cultures expressing aequorin revealed that these compounds, at least to a certain extent, may be also involved in the Ca2+-signaling. The quantitative study of the dose dependence effects of N-acyl Gln conjugates upon Ca2+-influx revealed (1) a concentration-dependent influx of Ca2+; (2) a configuration-independent effect concerning the stereochemistry of the amino acid (Gln); (3) a slightly reduced influx of Ca2+ after modification of the fatty acid backbone by functionalization with oxygen; and (4) a comparable effect with the detergent SDS.
In general, Vm variations depend on unbalanced ion distribution across the plasma membrane and depolarization occurs when cations (such as K+ and Ca2+) are allowed to enter the cell or upon anion efflux. On the other hand, hyperpolarization mainly depends on the activity of the plasma membrane H+-ATPase or when inward anion channels (or outward cation channels) are opened. The primary candidate for intercellular signaling in higher plants is the stimulus-induced change in Vm (Shvetsova et al., 2001). According to Volkov and coworkers (Volkov, 2000; Shvetsova et al., 2001, and references cited therein) insects induce electrical signals that can influence both biophysical and biochemical processes in remote tissues. Moreover, excitation waves transmit information from one part of the plant to another with a speed of propagation of the action potential that in soybean can reach 40 m s−1 (Shvetsova et al., 2001).
Since ion fluxes through channels directly influence Vm, it seems reasonable to assume that molecules able to act on channel activity might be considered as important factors inducing electrical signals. Among the various channels, calcium channels are predominantly involved in cell signaling and the specificity of the cytosolic calcium concentration signal in triggering a response depends on amplitude, temporal, and spatial changes (White, 2000). In fact, the longer-lasting influxes are expected to penetrate farther into the cytoplasm and therefore encounter more centrally located Ca2+-dependent enzymes (Trewavas, 2000). Recent studies have raised the challenging idea that depolarization-activated calcium channels can be a sensor for stimuli (such as touch) and might be an exclusive signaling pathway element (Miedema et al., 2001).
Oral secretions and some of its components such as the fatty acid-amino acid conjugates of, for example, Manduca sexta have been shown to be necessary and sufficient to elicit a set of herbivore-specific responses in tobacco (Halitschke et al., 2003). Our results demonstrate that regurgitants and N-acyl-amino acid conjugates interact with the plasma membrane and alter Vm. R from Lima bean reared larvae altered Vm in a concentration-independent fashion and its effect is clearly different from that observed in Vm studies with the individual compounds. Especially, the nonlinear response of Vm to the concentration of R and R-factors remains to be clarified. Possibly the effects are related to different modes of membrane Vm depolarization by either micellar transport of ions or pore-formations by the conjugates and other components of R (Abramson and Shamoo, 1979). The most common fatty acid conjugate, namely N-linolenoyl-Gln, exhibits Vm depolarization up to a concentration of 200 μg mL−1, representing the natural concentration in lepidopteran larvae R (Pohnert et al., 1999a) and this reflects the magnitude of Vm depolarization observed in R. A typical minor component of lepidopteran R is N-palmitoleoyl-Gln. At low concentration (25 μg mL−1), corresponding to a typical R concentration, this compound shows Vm depolarization comparable to N-linolenoyl-Gln and to R. Application of the fatty acid or amino acid components of the conjugates shows virtually no effect for linolenic acid but a clear Vm depolarization for Gln. The latter effect could play a role during larval feeding after enzymatic cleavage of the conjugates and may rely on transport processes (e.g. symport) of the amino acid (Delrot et al., 2001) and/or interaction of free Gln with receptors. However, as yet, nothing is known about the stability of N-acyl amino acids in the plant cells.
The time-course and distance-dependence spreading of the Vm depolarization upon herbivore attack in intact leaves (Fig. 2) is probably associated with a molecule able to disperse within tissues at a relatively high speed. Preliminary results perfusing leaves with H2O2 and Ethephon (the ethylene releasing agent) indicate a Vm depolarizing effect of these molecules (unpublished data).
The typical response pattern to different Ca2+ concentrations lie in the ability of cells to generate specific cytosolic Ca2+ concentration signatures. They may be unique, in terms of spatio-temporal characteristics and in response to an individual stimulus (McAinsh and Hetherington, 1998). In the bite zone, there is a dramatic Ca2+ influx limited to few cell layers (Figs. 6A, 7EI, and 7FI). Usually stimulus-induced increases of Ca2+ concentration occur in the form of oscillations or in the form of spikes (McAinsh and Hetherington, 1998). In the case of larvae feeding on Lima bean leaves a spike is observed and depends on Ca2+ channel activity, since the response can be reduced by Verapamil. The involvement of N-acyl-Glns in Ca2+ influx is independently confirmed with the test system of aequorin-transformed soybean cells. As previously shown for the established elicitors cryptogenin, oligogalacturonides (Lecourieux et al., 2002), and β-(1,3)-(1,6)-glucans (Mithöfer et al., 1999), the Ca2+ influx increases with increasing N-linolenoyl-Gln concentration. Moreover, in our assay, no difference is found between d- and l-amino acid building blocks of N-linolenoyl conjugate, and, thus, the effect of induced Ca2+ influx is, in the first instance, linked to the overall physico-chemical properties of the amphiphilic compounds, as confirmed by the action of SDS. It should be not ignored, however, that only the conjugate with l-Gln has been shown to induce specific defense responses in maize (Alborn et al., 1997), demonstrating that, besides triggering the Ca2+ influx, additional interactions between N-acyl-Glns and the plant are involved in the elicitation process of maize plants. Overall, our findings are in agreement with previous work demonstrating that in Lima bean the signaling pathway(s) mediating expression of defense genes in the receiver cells requires the calcium influx into the cells (Arimura et al., 2000a).
Signals induced by herbivore attack rapidly spread over the leaf leading to a strong Ca2+-dependent Vm depolarization in the bite zone followed by a transient Vm hyperpolarization in the close vicinity and a constant depolarization in distances greater than 6 to 7 mm. At the long distance (6–7 cm) the overall process takes not more than 5 to 6 min which requires for signal molecules traveling with the same speed (approximately 1 cm min−1).
Another interesting target is the analysis of the early events in the interaction of volatiles (including ethylene, H2O2, and NO) emitted from wounded plants and/or perceived by neighbored healthy plants. Preliminary results already indicate compound-specific variations in Vm. Studies are under way and will be reported soon.
MATERIALS AND METHODS
Plant Material
Feeding experiments were carried out using the Lima bean Phaseolus lunatus (cv Ferry Morse var Jackson Wonder Bush). Individual plants were grown from seed in a plastic pot with sterilized potting soil at 23°C and 60% humidity using daylight fluorescent tubes at approximately 270 μE m−2 s−1 with a photophase of 16 h. Experiments were conducted with 12- to 16-d-old seedlings showing two fully developed primary leaves, which were found to be the most responsive leaves.
Animal Material
Larvae of Spodoptera littoralis (Boisd.; Lepidoptera, Noctuidae) were grown in petri dishes at long photoperiod (14–16 h photophase) and 22°C to 24°C. They were fed an artificial diet consisting of 300 g L−1 agar, 400 g L−1 bean flour, 3 g sodium ascorbate, 3 g ethyl p-hydroxybenzoate, and 1 g formaldehyde (Bergomaz and Boppré, 1986). Small cubes of the diet were placed into rearing dishes on pieces of aluminum foil. Alternatively, larvae were fed with Lima bean leaves. The dishes were lined with filter paper to reduce humidity and sawdust was provided for pupation when the larvae terminated feeding. Adults were allowed to emerge in glass containers supplied with water and honey solution. Eggs were deposited on strips of filter paper.
Vm
Vm was determined in leaf segments. The Vm was determined with glass micropipettes with a tip resistance of 4 to 10 MΩ and filled with 3 m KCl. Micropipettes were used as micro-salt bridges to Ag/AgCl electrodes obtained with a Narishighe PE-21 puller and inserted vertically in the tissue by means of a micromanipulator (Maffei et al., 2001). Leaf segments were always equilibrated for 60 to 120 min in 5 mm MES-NaOH (pH 6.0). Perfusion of solutions was granted by a multichannel Ismatec Reglo peristaltic pump (flow rate 1 mL min−1). Vm variations were recorded both on a pen recorder and through a digital port of a PC using a data logger. Measurements were performed at increasing distances from the leaf wounding caused by herbivore feeding and the data were plotted with respect to controls. Measurements were also performed after perfusion with the compounds listed below as well as during larvae feeding on intact plants connected to the apparatus.
All chemicals were dissolved in 1% methanol, which was present in the control solutions, and perfused in a 50 mm MES buffered solution (pH 6.0) containing 0.5 mm calcium sulfate and 2.5 μm 3-(3,4-dichlorophenyl)-1,1-dimethylurea, used to poison photosynthetic electron transfer. After a period of Vm stabilization, saturation of the well where leaf tissues have been placed occurred in 7 min, after which perfusion was carried out for a variable time (until stabilization of the Vm), washing of the well was done by perfusing with fresh buffer. Saturation with fresh buffer took 20 to 25 min and then the solution was allowed to perfuse until Vm reached a constant value.
Oral Secretions and Regurgitant Collection
Five-day-old larvae (approximately 2–3 cm long) were grown on artificial diet and reared on Lima bean leaves for 24 h prior to collection of R. Oral secretions were collected into glass capillaries connected to an evacuated sterile vial (peristaltic pump) by gently squeezing the larva with a forceps behind the head which caused immediate regurgitation. Secretions were stored at (−20°C) until perfusion.
Intracellular Calcium Variation Determination
Fluo-3 AM (acetoxy-methyl ester of Fluo-3, more permeant for cells) purchased in vials containing the molecule as a stock solution in dimethyl sulfoxide (Fluka, Milwaukee, WI), was diluted in 50 mm MES buffer, pH 6.0, with the addition of 0.5 mm calcium sulfate, 2.5 μm 3-(3,4-dichlorophenyl)-1,1-dimethylurea to reach the concentration of 5 μm. This resulting solution was used for an initial treatment of Lima bean leaves not separated from the plant; the leaf was gently fixed over a glass slide, and a drop (about 20 μL) of 5 μm Fluo-3 AM solution was applied and covered with another glass slide. Thirty minutes after treatment with Fluo-3 AM, the leaf was fixed on an Olympus (Tokyo) FLUOview confocal scanning laser microscope stative without separating the leaf from the plant. Measurements were taken in intact leaves, with leaves wounded mechanically and after herbivore feeding, both in the presence and absence of exogenous calcium. In addition the leaves were perfused with undiluted R and N-linolenoyl-Gln (at 100 μg mL−1), as well as with the calcium channel blocker Verapamil (100 μm; White, 2000). The microscope is operated with a Krypton/Argon laser at 488 nm and 568 nm wavelengths; the first wavelength excitates the Fluo-3 dye, resulting in emission of green light and the second mostly excitates chloroplasts, which emit red fluorescence. Images generated by the FluoView software were analyzed using the public domain NIH Image program (developed at the United States National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image).
Aequorin Dependent Luminescence Measurements
Transgenic soybean 6.6.12 cell lines carrying the stably integrated plasmid pGNAequ/neo2 and expressing apoaequorin (Mithöfer et al., 1999) were used to reconstitute aequorin in vivo with 10 μm synthetic coelenterazine (Calbiochem, Bad Soden, Germany) on a shaker (125 rpm) in the dark for 24 h. The Ca2+-specific luminescence (470 nm) was measured in a final volume of 200 μL using a digital luminometer (Bio-Orbit 1250, Turku, Finland) as described (Mithöfer et al., 1999; Mithöfer and Mazars, 2002). Treatments with various compounds were performed by adding 1 to 10 μL of different concentrations of aqueous stock solutions to the cell suspension culture. Mixing time for the addition of any compound was 5 to 7 s. In each experiment, the concentration of reconstituted aequorin was not limiting under any of the experimental conditions, with a maximal consumption not exceeding 10%. The residual aequorin was completely discharged by adding 200 μL of 20% ethanol containing 2 m CaCl2 (final concentration 10% and 1 m, respectively). The resulting luminescence was used to estimate the total amount of aequorin present in various experiments in order to determine the rate of aequorin consumption for the calculation of the cytosolic Ca2+ concentrations according to Allen et al. (1977).
Chemicals Used for Perfusion
Natural volicitin, 17S-(17-hydoxylinolenoyl)-l-Gln was synthesized as described (Pohnert et al., 1999b). N-linolenoyl-l-Gln, N-linolenoyl-d-Gln, the corresponding Glus and N-palmitoleoyl-l-Gln were available according to the protocol of Pohnert et al. (1999a). N-(15,16-epoxy-linoleoyl)-Gln and N-(15,16-dihydroxylinoleoyl)-Gln were synthesized as described by Spiteller and Boland (2003a). Linolenic acid, SDS, and l-Gln were purchased from Sigma-Aldrich (St. Louis).
Statistics
At least five repetitions were used for the statistical treatment of the data. More than five repetitions contributed to the mean values given in Figures 3, 4A to C, and 5. The data are expressed as mean values; metric bars indicate the sd. To evaluate the difference significance of the control and the treatments at the given concentrations, variance analysis (ANOVA) was performed for all data using the Tukey test.
Acknowledgments
M.M. and S.B. are thankful to the graduated students who gave their technical support (particularly D. Salvetto). We are thankful to Dr. A. Elbert (Bayer AG, D-40789 Monheim) for supplying us with egg clutches of lepidopteran larvae, and to Angelika Berg for caterpillar rearing. We thank Dr. G. Pohnert for synthesis of 17S-volicitin and proofreading of the manuscript.
This work was supported in part by the Department of Plant Biology (Turin) and by the MIUR-ITALY (grant ex 60%). Financial support by the Fonds der Chemischen Industrie, Frankfurt a.M., is gratefully acknowledged.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.034165.
References
- Abramson JJ, Shamoo AE (1979) Anionic detergents as divalent-cation ionophores across black lipid-membranes. J Membr Biol 50: 241–255 [DOI] [PubMed] [Google Scholar]
- Agrawal AA (2000) Mechanisms, ecological consequences and agricultural implications of tri-trophic interactions. Curr Opin Plant Biol 3: 329–335 [DOI] [PubMed] [Google Scholar]
- Alborn HT, Jones TH, Stenhagen GS, Tumlinson JH (2000) Identification and synthesis of volicitin and related components from beet armyworm oral secretions. J Chem Ecol 26: 203–220 [Google Scholar]
- Alborn HT, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH (1997) An elicitor of plant volatiles from beet armyworm oral secretion. Science 276: 873–949 [Google Scholar]
- Allen DG, Blinks JR, Prendergast FG (1977) Aequorin luminescence: relation of light emission to calcium concentration – a calcium-independent component. Science 195: 996–998 [DOI] [PubMed] [Google Scholar]
- Arimura G-I, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J (2000. a) Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 406: 512–515 [DOI] [PubMed] [Google Scholar]
- Arimura G-I, Tashiro K, Kuhara S, Nishioka T, Ozawa R, Takabayashi J (2000. b) Gene responses in bean leaves induced by herbivory and by herbivore-induced volatiles. Biochem Biophys Res Commun 277: 305–310 [DOI] [PubMed] [Google Scholar]
- Arimura G-I, Ozawa R, Nishioka T, Boland W, Koch T, Kühnemann F, Takabayashi J (2002) Herbivore-induced volatiles induce the emission of ethylene in neighboring lima bean plants. Plant J 29: 87–98 [DOI] [PubMed] [Google Scholar]
- Asselineau J (1991) Bacterial lipids containing amino acids or peptides linked by amide bonds. In W Herz, H Grisebach, GW Kirby, C Thamm, eds, Progress in the Chemistry of Organic Natural Products. Springer-Verlag, Vienna, New York, pp 1–85 [DOI] [PubMed]
- Baldwin IT, Halitschke R, Kessler A, Schittko U (2001) Merging molecular and ecological approaches in plant-insect interactions. Curr Opin Plant Biol 4: 351–358 [DOI] [PubMed] [Google Scholar]
- Bergomaz B, Boppré M (1986) A simple insect diet for rearing Arctiidae and other moths. Journal of Lepidopterists' Society 40: 131–137 [Google Scholar]
- Blumwald E, Aharon GS, Lam BC-H (1998) Early signal transduction pathways in plant-pathogen interactions. Trends Plant Sci 3: 342–346 [Google Scholar]
- Bush DS (1995) Calcium regulation in plant cells and its role in signaling. Annu Rev Plant Physiol Plant Mol Biol 46: 95–122 [Google Scholar]
- Cheong J-J, Choi YD (2003) Methyl jasmonate as a vital substance in plants. Trends Genet 19: 409–413 [DOI] [PubMed] [Google Scholar]
- Delrot S, Atanassova R, Gomès E, Coutos-Thévenot P (2001) Plasma membrane transporters: a machinery for uptake of organic solutes and stress resistance. Plant Sci 161: 391–404 [Google Scholar]
- DeMoraes CM, Lewis WJ, Paré PW, Alborn HT, Tumlinson JH (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393: 570–573 [Google Scholar]
- Dicke M, Sabelis MW (1992) Costs and benefits of chemical information conveyance: proximate and ultimate factors. In BD Roitberg, MB Isman, eds, Insect Chemical Ecology: An Evolutionary Approach. Chapman and Hall, New York, pp 122–155
- Engelberth J, Koch T, Schüler G, Bachmann N, Rechtenbach J, Boland W (2001) Ion channel-forming alamethicin is a potent elicitor of volatile biosynthesis and tendril coiling. Cross talk between jasmonate and salicylate signaling in Lima bean. Plant Physiol 125: 369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE, Briars SA, Williams LE (1991) Active Ca2+ transport by plant cell membranes. J Exp Bot 42: 285–303 [Google Scholar]
- Gatehouse JA (2002) Plant resistance towards insects herbivores: a dynamic interaction. New Phytol 156: 145–169 [DOI] [PubMed] [Google Scholar]
- Gelli A, Blumwald E (1997) Hyperpolarization-activated Ca2+-permeable channels in the plasma membrane of tomato cells. J Membr Biol 155: 35–45 [DOI] [PubMed] [Google Scholar]
- Halitschke R, Gase K, Hui D, Schmidt DD, Baldwin IT (2003) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. VI. Microarray analysis reveals that most herbivore-specific transcriptional changes are mediated by fatty acid-amino acid conjugates. Plant Physiol 131: 1894–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT (2001) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol 125: 711–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D, Iqbal J, Lehmann K, Gase K, Saluz HP, Baldwin IT (2003) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata: V. Microarray analysis and further characterization of large-scale changes in herbivore-induced mRNAs. Plant Physiol 131: 1877–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch T, Krumm T, Jung V, Engelberth J, Boland W (1999) Differential induction of plant volatile biosynthesis in the Lima bean by early and late intermediates of the octadecanoid-signaling pathway. Plant Physiol 121: 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labady A, Thomas DJ, Shvetsova T, Volkov AG (2002) Plant bioelectrochemistry: effects of CCCP on electrical signaling in soybean. Bioelectrochemistry 57: 47–53 [DOI] [PubMed] [Google Scholar]
- Lecourieux D, Mazars C, Pauly N, Ranjeva R, Pugin A (2002) Analysis and effects of cytosolic free calcium increases in response to elicitors in Nicotiana plumbaginifolia cells. Plant Cell 11: 2627–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei M, Camusso W, Sacco S (2001) Effect of Mentha x piperita essential oil and monoterpenes on cucumber root membrane potential. Phytochemistry 58: 703–707 [DOI] [PubMed] [Google Scholar]
- McAinsh MR, Hetherington AM (1998) Encoding specificity in Ca2+ signalling system. Trends Plant Sci 3: 32–36 [Google Scholar]
- Miedema H, Bothwell JHF, Brownlee C, Davies JM (2001) Calcium uptake by plant cells: channels and pumps acting in concert. Trends Plant Sci 6: 514–519 [DOI] [PubMed] [Google Scholar]
- Mishra NS, Mallik BN, Sopory SK (2001) Electrical signal from root to shoot in Sorghum bicolor: induction of leaf opening and evidence for fast extracellular propagation. Plant Sci 160: 237–245 [DOI] [PubMed] [Google Scholar]
- Mithöfer A, Ebel J, Bhagwat AA, Boller T, Neuhaus-Url G (1999) Transgenic aequorin monitors cytosolic calcium transients in soybean cells challenged with β-glucan or chitin elicitors. Planta 207: 566–574 [Google Scholar]
- Mithöfer A, Mazars C (2002) Aequorin-based measurements of intracellular Ca2+-signatures in plant cells. Biol Proced Online 4: 105–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cárdenas M, Narváez-Vásquez J, Ryan CA (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13: 179–181 [PMC free article] [PubMed] [Google Scholar]
- Paré PW, Alborn HT, Tumlinson JH (1998) Conterted biosynthesis of an insect elicitor of plant volatiles. Proc Natl Acad Sci USA 95: 13971–13975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellinen RI, Minna-Sisko K, Tauriainen AA, Palva ET, Kangasjärvi J (2002) Hydrogen peroxide activates cell death and defense gene expression in birch. Plant Physiol 130: 549–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñeros M, Tester M (1995) Characterization of a voltage-dependent Ca2+ selective channel from wheat roots. Planta 195: 478–488 [Google Scholar]
- Piñeros M, Tester M (1997) Calcium channels in higher plant cells: selectivity, regulation and pharmacology. J Exp Bot 48: 551–577 [DOI] [PubMed] [Google Scholar]
- Pohnert G, Jung V, Haukioja E, Lemoa K, Boland W (1999. a) New fatty acid amides from caterpillar (Noctuide, Geometridae) regurgitants. Tetrahedron 55: 11275–11280 [Google Scholar]
- Pohnert G, Koch T, Boland W (1999. b) Synthesis of volicitin: stereoselective one pot formation of 17-hydroxylinolenic acid. Chem Commun 12: 1087–1088 [Google Scholar]
- Poppy GM (1999) The raison d'être of secondary plant chemicals? Trends Plant Sci 4: 82–83 [Google Scholar]
- Roh MH, Shingles R, Cleveland MJ, McCarty RE (1998) Direct measurement of calcium transport across chloroplast inner-envelope vesicles. Plant Physiol 118: 1447–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schittko U, Hermsmeier D, Baldwin IT (2001) Molecular interactions between the specialist herbivore Manduca sexta (lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. II. Accumulation of plant mRNAs in response to insect-derived cues. Plant Physiol 125: 701–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz EA, Alborn HT, Tumlinson JH (2003) Synergistic interactions between volicitin, jasmonic acid and ethylene mediate insect induced volatile emission in Zea mays. Physiol Plant 117: 403–412 [DOI] [PubMed] [Google Scholar]
- Shah J (2003) The salicylic acid loop in plant defense. Curr Opin Plant Biol 6: 365–371 [DOI] [PubMed] [Google Scholar]
- Shvetsova T, Mwesigwa J, Volkov AG (2001) Plant electrophysiology: FCCP induces action potential and excitation waves in soybean. Plant Sci 161: 901–909 [Google Scholar]
- Spiteller D, Boland W (2003. a) N-(15,16-dihydroxylinoleoyl)-glutamine and N-(15,16-epoxylinoleoyl)-glutamine isolated from oral secretions of lepidopteran larvae. Tetrahedron 59: 135–139 [Google Scholar]
- Spiteller D, Boland W (2003. b) Identification and synthesis of N-(17-acyloxyacyl)-glutamine conjugates from oral secretion of lepidopteran larvae. J Org Chem 68: 8743–8749 [DOI] [PubMed] [Google Scholar]
- Spiteller D, Pohnert G, Boland W (2001) Absolute configuration of volicitin; an elicitor of plant volatile biosynthesis from Lepidopteran larvae. Tetrahedron Lett 42: 1483–1485 [Google Scholar]
- Stratmann JW (2003) Long distance run in the wound response – jasmonate acid is pulling ahead. Trends Plant Sci 8: 247–250 [DOI] [PubMed] [Google Scholar]
- Thaler JS (1999) Jasmonate-inducible plant defences cause increased parasitism of herbivores. Nature 399: 686–688 [Google Scholar]
- Thuleau P, Schroeder JI, Ranjeva R (1998) Recent advances in the regulation of plant calcium channels: evidence for regulation byG-proteins, the cytoskeleton and second messengers. Curr Opin Plant Biol 1: 424–427 [DOI] [PubMed] [Google Scholar]
- Trewavas A (2000) Signal perception and transduction. In Biochemistry and Molecular Biology of Plants. B Buchanan, W Gruissem, R Jones, eds. American Society of Plant Physiologists. pp 930–987
- Turlings TCJ, Loughrin JH, McCall PJ, Röse USR, Lewis WJ, Tumlinson JH (1995) How caterpillar-wounded plants protect themselves by attracting parasitic wasps. Proc Natl Acad Sci USA 92: 4169–4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov AG (2000) Green plants: electrochemical interfaces. J Electroanal Chem 483: 150–156 [Google Scholar]
- Warber S (1998) Modes of action at target sites. In PB Kaufman, JC Leland, S Warber, JA Duke, HL Brielmann, eds, Natural Products from Plants. CRC Press, Boca Raton, FL, pp 157–182
- White PJ (2000) Calcium channels in higher plants. Biochim Biophys Acta 1465: 171–189 [DOI] [PubMed] [Google Scholar]
- Winz RA, Baldwin IT (2001) Molecular interactions between the specialist herbivore Manduca sexta (lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. IV. Insect-induced ethylene reduces jasmonate-induced nicotine accumulation by regulating putrescine N-methyltransferase transcripts. Plant Physiol 125: 2189–2202 [DOI] [PMC free article] [PubMed] [Google Scholar]