Abstract
Indirect proton detection of 13C hyperpolarized contrast agents potentially enables greater sensitivity. Presented here is a study of sub-second projection imaging of hyperpolarized 13C contrast agent addressing the obstacle posed by water suppression for indirect detection in vivo. Sodium acetate phantoms were used to develop and test water suppression and sub-second imaging with frequency selective RF pulses using spectroscopic and imaging indirect proton detection. A 9.8 mM aqueous solution of 13C PHIP hyperpolarized 2-hydroxyethyl-13C-propionate-d2,3,3 (HEP), <P>~25% was used for demonstration of indirect proton sub-second imaging detection. Balanced 2D FSSFP (Fast Steady State Free Precession) allowed recording proton images with FOV = 64×64 mm2 and spatial resolution 2×2 mm2 with total acquisition time of less than 0.2 s. In thermally polarized sodium 1-13C-acetate, 13C to 1H polarization transfer efficiency of 45.1% of the theoretically predicted values was observed in imaging detection corresponding to an 11 fold of overall sensitivity improvement compared to direct 13C FSSFP imaging. 13C to 1H polarization transfer efficiency of 27% was observed in imaging detection corresponding to a 3.25 fold sensitivity improvement compared to direct 13C FSSFP imaging with hyperpolarized HEP. The range of potential applications and limitations of this sub-second and ultra-sensitive imaging approach are discussed.
Keywords: NMR, Hyperpolarization, Contrast Agent, sub-second imaging, 13C, indirect detection, parahydrogen, PHIP
1. Introduction
Hyperpolarization techniques increase MR sensitivity by several orders of magnitude (1). In principle, many nuclear spins can be hyperpolarized including 19F, 1H, 15N, and 13C. Abundant protons in biologically relevant molecules such as lactate have the largest magnetic moments and MR Larmor frequencies and therefore, should be superior from a detection perspective. However, biomedical use of hyperpolarized in vivo contrast agents require a significant time scale on the order of tens of seconds for contrast agent manipulation and in vivo delivery from the site of injection to the volume of interest (e.g. tumor) (2). The delay between synthesis of the hyperpolarized state and delivery in vivo is long compared to the typical lifetime of protons (~ 2 sec), therefore precluding direct detection of hyperpolarized protons. For example, if T1 is 2 s, polarization decreases by ~400 fold after 12 s. As a result, long-lived nuclear spin sites, e.g. carboxyl 13C, are prepared by dissolution Dynamic Nuclear Polarization (DNP) (3) directly. In contrast, in parahydrogen induced polarization (PHIP) (4), the initial singlet state of nascent parahydrogen protons is converted to magnetization on a longer lived nuclear spin (e.g. carboxyl 13C) (5). Regardless of preparation, this heteronuclear storage nucleus is subsequently detected directly by 13C NMR to interrogate metabolism in vivo (6). Examples include 1-13C-pyruvate (7), 1-13C-succinate (8), 1-13C-glutamine (9) and others.
Despite nearly perfect polarization P → 100% achieved by dissolution DNP and PASADENA (Para-Hydrogen and Synthesis Allow Dramatically Enhanced Nuclear Alignment) (10), sensitivity could be further improved by reading out heteronuclear magnetization on protons. Protons are inherently more sensitive under conditions of identical polarization and T2*, because of the other two gamma factors: greater resonant frequency and larger magnetic moment. This results in a combined enhancement of up to 15.7 fold, (γ1H/γ13C)2, not accounting for T2*, RF coil sensitivity, and subject noise.
Other, more practical factors, make 13C detection challenging. (γ1H/γ13C)2 more gradient power is required to achieve the same spatial resolution as in conventional MRI. In addition, all clinical and preclinical MRI scanners are equipped with 1H detection as a standard feature, while 13C requires specialized hardware and software. The latter can actually become a major impediment to the widespread use of hyperpolarized contrast agents.
Proton detection of 13C hyperpolarized contrast agents can solve the above challenges by the use of pulse sequences requiring only RF 13C excitation. The latter requires significantly less demanding hardware and can potentially provide more than an order of magnitude sensitivity gain. This additional sensitivity gain would be helpful even in hyperpolarized MR, because it can improve spatial resolution, decrease the dose of contrast agents, and therefore potentially allow for deeper penetration of metabolic fluxes.
Candidate polarization transfer sequences for reading out hyperpolarized heteronuclear magnetization can be found from high-resolution NMR, such as DEPT (Distortionless Enhancement by Polarization Transfer), INEPT (Insensitive Nuclei Enhanced by Polarization Transfer), and others. Here, conventional INEPT was chosen (11), and specifically a refocused INEPT sequence, which can theoretically provide detection sensitivity enhancements of up to (γ1H/γ13C)2, where γ1H and γ13C are gyromagnetic ratios of detection and polarization storage nuclear spin sites. The INEPT sequence was chosen due to its relative simplicity and known utility in clinical research (12).
The concept of a more sensitive, indirect proton detection was demonstrated for highly polarized 13C PHIP contrast agents (13) as well as DNP polarized contrast agents spectroscopically (14) and more recently using fast imaging sequences (15). However, the vast majority of these experiments were performed in D2O to suppress background signals from water and other protons. DNP coupled with polarization transfer techniques have been shown to effectively transfer polarization in a multidimensional framework (16–18) including a recent study with DNP hyperpolarized 13C-acetate in vivo (17). However, the demonstrated polarization transfer efficiency was rather low (17). It should be noted, however, that the choice of 1-13C-acetate in our in vitro studies was determined by the recent use of its hyperpolarized form for studying metabolism in vivo (19–21).
Translation of this approach in vivo requires sequence development for proton imaging of dilute millimolar proton spins in the presence of > 50 M water. The work presented here demonstrates sub-second proton imaging of 13C hyperpolarized contrast agent in water medium. A 25% polarized contrast agent at ~10 mM concentration was used to simulate in vivo conditions of highly polarized contrast agent with low concentration and decaying non-equilibrium hyperpolarization.
High polarization transfer efficiency and 2×2 mm2 in-plane resolution were achieved. The prospects of pre-clinical translation, method potential and limitations for high and low magnetic fields are discussed from the perspective of metabolic imaging of cancer. Other polarization transfer approaches (22,23) can be used to further maximize the use of nonrenewable hyperpolarization.
2. Materials and Methods
2.1. Phantom Preparation for Boltzmann polarization studies
Sodium acetate and sodium 1-13C-acetate (99% atom 13C) were obtained from Isotec-Sigma-Aldrich Corporation (Miamisburg, OH), and concentrated to 4.3 M in H2O and 99.8% D2O respectively. Spherical phantom plastic containers were fully filled for each sample to a total solution volume of 2.8 mL.
2.2. Phantom preparation for hyperpolarized studies
2-hydroxyethyl-1-13C-acrylate-d2,3,3 (HEA) was hydrogenated using a home-built PHIP polarizer with in situ detection capability operating at 5.9 mT. This device (Coffey, A. M. et al., unpublished results) is similar to our previously published polarizer operating at 47.5 mT using PASADENA (parahydrogen and synthesis allow dramatically enhanced nuclear alignment) (24,25). Production of PHIP hyperpolarized 2-hydroxyethyl-13C-propionate-d2,3,3 (HEP) has been previously described by us (5,24–26) and others (27). Briefly, parahydrogen gas was produced with a custom lab-built generator by pulsing ambient ortho-hydrogen into an iron oxide catalyst filled chamber at 14–20 K (26). This enriched parahydrogen gas was then added to unsaturated molecular precursors using water-soluble rhodium(I) based molecular catalysts (28,29) in a high pressure chemical reactor. 1,4-bis[(phenyl-3-propanesulfonate)phosphine]butane, disodium salt (717347, Sigma-Aldrich-Isotec, OH, USA) and bis (norbornadiene) rhodium (I) tetrafluoroborate (45–0230, Strem Chemicals, MA, USA) were used for hydrogenation catalyst preparation in water medium as described earlier (5). PHIP precursor, HEA, was added to the aqueous solution after catalyst preparation. The concentration for the hyperpolarized product, HEP, was 9.8 mM, with an average hyperpolarization level of 25% at the 13C site measured in situ. Approximately 4 mL of aqueous solution containing hyperpolarized HEP was produced and transferred to a plastic syringe in the fringe field of a 4.7 T MR scanner, where 13C T1 = 50 s (30). The hyperpolarized material was injected into a 2.8 mL hollow polypropylene sphere near the bore of the scanner and placed inside the RF coil, with a total delivery time of ~50 s.
2.3. Spectroscopy and Imaging
All spectroscopy and imaging experiments were performed on a Varian 4.7 T animal imaging system using the VNMRJ version 3.2 software suite. The experiments were conducted with a custom-built 38 mm ID two-channel RF coil, dual-tuned to 1H (200.25 MHz) and 13C (50.25 MHz). All the experiments used the shim gradient values obtained from shimming a 2.8 mL sample of deionized water in a plastic spherical phantom container, resulting in a half-height line width of 4 Hz. Once the magnetic field was shimmed, the shim sample was swapped with the experimental samples, with care taken to replicate the sample position in the RF coil and the RF coil position in the magnet as used for shimming so that the magnetic field optimizations were accurately reflected across all the samples. Phantom replacement was necessary for hyperpolarized experiments, because the lifetime of hyperpolarization prevented shimming on individual samples.
All 1H and 13C spectroscopic experiments consisted of a single acquisition with 1 s of acquisition time, 10 kHz spectral width, 10,000 complex points, and calibrated 90° excitation RF pulse. The INEPT delay for sodium acetate was τINEPT = 42 ms and the refocusing delay was τrefocus = 16 ms for 1H to 13C polarization transfer in the 13C-CH3 molecular framework with J2CH = 6.0 Hz (11). The INEPT delay for sodium acetate was τINEPT = 17 ms and the refocusing delay was τrefocus = 35 ms for one 13C to three protons polarization transfer in the 13C-CH3 molecular framework with J2CH = 6.0 Hz. For hyperpolarized HEP, the polarization transfer from 13C to two protons derived from parahydrogen in the 13C-CH-CH molecular framework used delays of τINEPT = 20 ms and τrefocus = 16 ms as determined previously (13) based on prior knowledge of the spin-spin couplings (27). In all imaging and spectroscopic experiments, the generic refocused INEPT sequence was augmented by τ-90y at the end of the second refocus delay as shown in Fig. 1a on the detection channel to prepare magnetization along the z-axis for subsequent detection with frequency selective RF pulses using spectroscopic or imaging acquisition.
Figure 1. 13C Spectroscopy and Imaging of sodium 1-13C-acetate.
(a) Refocused INEPT sequence. (b) 13C reference spectrum of a thermally polarized 2.8 mL phantom of 4.3 M sodium 1-13C-acetate in water using a single scan acquisition, and (c) FSSFP projection image. (d) 13C INEPT spectroscopy and (e) INEPT enhanced imaging of sodium 1-13C-acetate using the sequence shown in (a). All the images were acquired with TR = 2 ms, TE = 1 ms, a field of view (FOV) of 96×96 mm2, and an in-plane resolution of 3×3mm2, with the same contour scaling levels. The following INEPT delays were used τINEPT = 42 ms and τrefocus = 16 ms for polarization from 1H→13C.
All imaging sequences used Varian’s version of 2D FSSFP with single slice acquisition. Slice thickness was set to 60 mm to encompass the entire phantom. 90° acquisition flip angle and 50 kHz spectral width were used for 13C and 1H imaging. Other imaging parameters such as TR/TE varied depending on the frequency selective excitation pulses. All 13C FSSFP images were acquired with 96×96 mm2 FOV with 3×3 mm2 spatial resolution with τ90° = 52.5 μs at 51.9 W and TR = 2.00 ms, TE = 1.00 ms with total acquisition time of 64 ms. 1H τ90° was 57 μs and 1H τ180° was 114 μs at 51.9 W for all non-frequency selective RF pulses. 1H frequency selective RF pulses were 1H τ90° = 4.35 ms at 50.7 mW. Refocused INEPT polarization transfer blocks did not use frequency selective RF pulses. When 1H frequency selective RF pulses were used during imaging, TR/TE increased from 1.91/0.95 ms (no frequency selection) to 6.20/3.10 ms (with frequency selection) respectively. All 1H FSSFP images were acquired with 64×64 mm2 FOV with 2×2 mm2 spatial resolution with total imaging time of 61.1 ms (no frequency selection) and 198 ms (with frequency selection). Refocused INEPT polarization transfer imaging experiments utilized the same delays as described in the spectroscopy experiments.
3. Results
3.1. Heteronuclear Polarization From Three Methyl Protons to 13C in Isolated Methyl Group, Sodium 1-13C-Acetate Phantom in D2O: Imaging and Spectroscopy
The experimental polarization enhancement εexp can be calculated as the ratio of SINEPT (see Supporting Information for details) and Sthermal, the signal intensities of the corresponding refocused INEPT and thermal experiments:
| (2) |
Spectroscopic detection resulted in εexp = 3.23 for INEPT transfer from 1H→13C. This translates to 70% polarization transfer efficiency, η = εexp/εtheory, for the refocused INEPT sequence when compared to the theoretical enhancement value, 4.59 (Supporting Information).
When refocused INEPT was coupled with the FSSFP fast imaging sequence at 4.7 T, Fig. 2, the magnitude of the INEPT enhanced image was increased with εexp = 3.99, corresponding to η = 87%, Figs. 1d and 1e. Imaging values of εexp and η were different from those of spectroscopic acquisition due to the relatively low SNR of 13C images acquired with thermal levels of 13C polarization.
Figure 2. INEPT Enhanced 1H FSSFP sequence diagrams.
(a) The RINEPT-FSSFP RF pulse sequence contains a 2 s presaturation pulse (Presat), a refocused INEPT block (RINEPT), and FSSFP imaging block using a low power frequency selective Gaussian pulse for detection. τINEPT represents the INEPT delay, while τrefocus is the refocusing delay, (b) Double RINEPT-FSSFP RF pulse sequence with extra RINEPT block for prepolarization of 13C from more polarized protons of thermally polarized phantoms in sodium 1-13C-acetate.
3.2. 1H Background Suppression Using Multiband RF Excitation Pulses and 1H Pre-saturation: Imaging and Spectroscopy
The approach of using water presaturation along with frequency selective Gaussian RF pulses was adopted to test water suppression in the phantom sample containing 2.8 mL of water, Fig. S1 (see Supporting Information for details). A 2 s presaturation pulse block (square RF pulse block at 1.6 W) coupled with a 50 mW Gaussian detection pulse was applied with an offset frequency of 750 Hz upfield from the water frequency. Spectroscopically, this approach resulted in 99.88% reduction of the background water signal for the water phantom when compared with a baseline single pulse MR spectrum (Figs. S1a and S1b), as determined through signal integration. Additionally, when the water suppression scheme was coupled to FSSFP, the resulting MR images produced the same result (Figs. S1c and S1d). The intensity of the water image was diminished from 101.5 a.u. without water suppression to 1.36 a.u. with suppression.
3.3. Proton Indirect Detection in Sodium Acetate Phantoms: Imaging and Spectroscopy
To examine the ability to indirectly detect protons from stored 13C magnetization, a series of experiments were conducted using refocused INEPT coupled with the frequency selective water suppression method discussed above. A single scan MR spectrum and MR image of a 1-13C-acetate phantom in water provided a baseline for examining 13C polarization transfer efficiency and water suppression. With a peak integral value of nearly 1.19•108 a.u., the water peak from the baseline spectrum (Fig. 3a) overshadowed the acetate signal, 1.26•107 a.u., and resulted in an image where the voxel intensities were dominated by water proton magnetization. Next, 1H signal from acetate methyl protons was detected after polarization transfer from pre-polarized 13C1 (see above). The sequence (Fig. 2) started with multiband proton presaturation followed by the refocused INEPT sequence block (13C1→1H), and ended with either spectroscopic or imaging 1H detection using a frequency selective Gaussian shaped RF pulse as described above. The multiband proton suppression block was different from the one used in Fig. 2 in its use of a series of 50 ms-long RF pulses of identical amplitude but frequency alternating between the resonant frequencies of water and the methyl peaks of 1-13C-acetate. The resulting spectrum (Fig. 3c) showed nearly full suppression, 99.83%, of the water peak, while maintaining 40% of the baseline acetate signal integral value. This trend was also confirmed in a follow-up study of a 1-13C-acetate phantom in D2O (Fig. 3e). Indirect detection of 13C magnetization using proton MRI images with multiband 1H presaturation and Gaussian RF excitation pulse is shown in Figs. 3d and 3f with SNR of 144.7 and 197.2 for 1-13C-acetate in H2O and 1-13C-acetate in D2O respectively. Additionally identical spectroscopy and imaging studies were conducted on a sample of sodium acetate with natural abundance enrichment level of 13C and the same concentration and volume as used in both the experiments involving 13C sodium acetate in water and in D2O. By testing a natural abundance sample with the INEPT enhanced pulse sequence, the resulting spectrum and image (Figs. 3g and 3h) with little to no signal assured that the sequence is performing properly and that the resulting images (Figs. 3d and 3f) were not from background water protons. The natural abundance sample contains vanishing quantity of 13C spin labels, ~1/91th of that in Figs. 3c–f, which is likely responsible for a small signal seen in Fig. 3g.
Figure 3. Indirect 1H Spectroscopic and Imaging Detection Using INEPT Enhancement.
Single scan 1H detection of a 2.8 mL phantom of 4.3 M sodium 1-13C-acetate in water (a and b) without background multiband 1H suppression (Off) or INEPT enhancement (Off). Indirect 1H detection enhanced by INEPT for spectroscopy, and INEPT enhanced FSSFP for imaging for 2.8 mL phantoms of 4.3 M 13C-sodium acetate in water (c and d) and in D2O (e and f), and also 4.3 M natural abundance sodium acetate in D2O (g and h). A small amount of thermal naturally abundant acetate signal is detected even with background 1H suppression and can be observed in the inset of (g) and faintly in the image (h). All the images were acquired using TR = 6.20 ms, TE = 3.10 ms, FOV = 64×64 mm2, and an in-plane resolution of 2×2 mm2.
From a theoretical perspective, for 1-13C-acetate polarization transfer from 13C→1H can be expressed with the equation:
| (3) |
For 13C-sodium acetate, the J-coupling between 13C1 and 1H is 6 Hz, and when τINEPT =17 ms and τrefocus =35 ms, P1H reaches the maximum value of 0.372•P13C. The value of P13C was boosted by a refocused INEPT 1H → 13C pre-polarization of 13C as described above. This improves P13C, which without pre-polarization would be 0.251 that of P1H at equilibrium.
The experimental polarization transfer enhancement for indirect 1H detection can be calculated similarly to Eq. 2 with modification accounting for artificially boosted 13C polarization above the thermal level used in our experiments:
| (4) |
where P13C is nuclear spin polarization of 13C immediately before the refocused INEPT transfer procedure and is experimentally detected 1H polarization calculated as follows:
| (5) |
where is imaging or spectroscopic signal detected after INEPT transfer, is the equilibrium thermal polarization of 1H spins, and is corresponding imaging or spectroscopic signal detected using identical acquisition protocol as . For the refocused INEPT transfer from 13C → 1H between the 13C1 and three methyl protons of acetate, εexp is 0.367 for spectroscopic acquisition, 0.320 for imaging detection in D2O and 0.167 for imaging detection in H2O corresponding to polarization efficiency η values of 98.6%, 85.9% and 45.1% respectively.
3.4. 13C and Indirect 1H Detection of Aqueous 13C Hyperpolarized Contrast Agent: Imaging and Spectroscopy
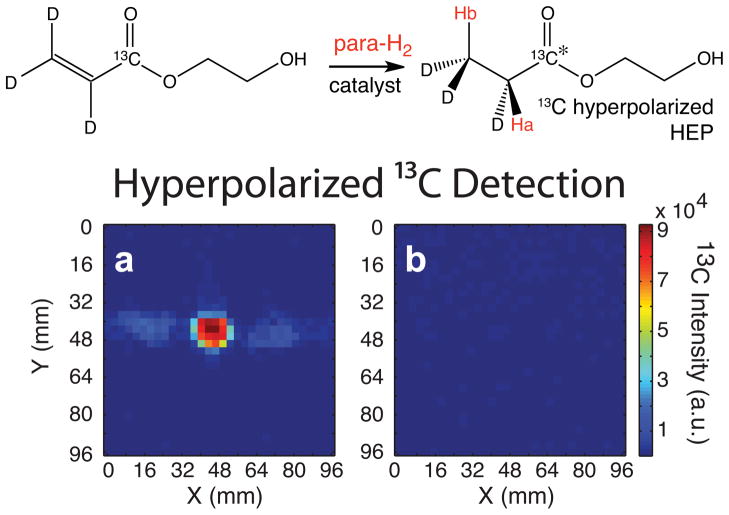
Single scan FSSFP 13C imaging was performed to determine the hyperpolarization levels for 13C HEP (Fig. 4a). The 13C hyperpolarization level was calculated to be %P = 5.3% at 4.7 T (Fig. 4a) after ~50–60 seconds of delivery time from the polarizer to the MRI scanner using Eq. 6, which compares FSSFP images of hyperpolarized 13C contrast agent (Fig. 4a) and reference compound (Fig. 1e) with known values of polarization using the same imaging parameters according to:
| (6) |
where is the nuclear polarization at equilibrium at 298 K and 4.7 T, and the enhancement ε is determined using the second part of this equation with measured signal, SREF (reference) and SX (hyperpolarized), and quantity information from reference compound χREF and the hyperpolarized agent χX of the same X nucleus, where X = 1H or 13C. With B0 = 4.7 T and T = 298 K, is 4.055*10−6 and is 1.61*10−5. The 5.3% 13C hyperpolarization of HEP detected at 4.7 T correlates to an enhancement of 13,200 fold when compared to equilibrium of 1-13C-acetate at 4.7 T. The hyperpolarized 13C image of HEP had maximum SNR of 177. To demonstrate the relatively short lifetime of 13C HEP hyperpolarization, a 13C single slice FSSFP experiment (Fig. 4b) was followed by an identical imaging acquisition 90 s after the first scan, resulting in no detectable signal in the image, signifying a full decay of 13C hyperpolarization in HEP. 90 s delay corresponds to approximately 2xT1.
Figure 4. Hyperpolarized 13C FSFFP Imaging.
(a) 13C detection of 13C hyperpolarized HEP using sub-second FSSFP, (b) FSFFP image of the “cold” contrast agent 90 s after acquisition shown in (a). Hyperpolarized 13C images were acquired with TR = 2 ms, TE = 1 ms, a field of view (FOV) of 96×96 mm2, and an in-plane resolution of 3×3 mm2. No extrapolation or zero filling was applied to images or spectra, and images are shown in their raw resolution.
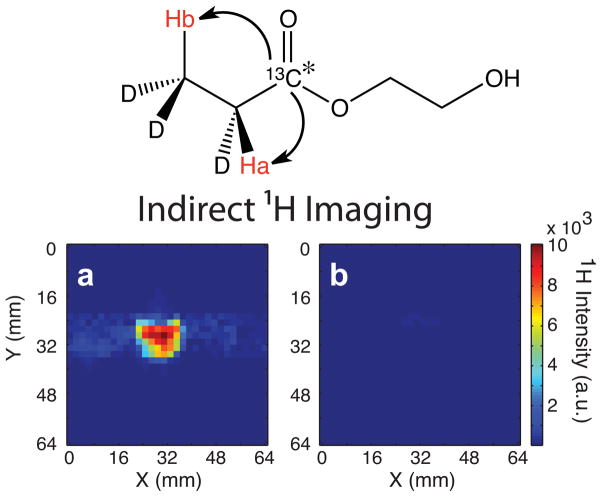
For indirect 1H imaging of 13C hyperpolarized HEP upon delivery to the 4.7 T scanner magnet, polarization was transferred from 13C to the nascent protons via refocused INEPT using the same imaging sequence with proton multiband presaturation and selective RF excitation pulses as was used in the acetate studies above. A 2×2 mm2 in-plane resolution (Fig. 5a) was obtained for hyperpolarized HEP using indirect proton detection. Total experimental time for proton imaging was on the order of 3 s, consisting largely of the multiband proton presaturation period of 2 s. The 1H hyperpolarized image had an SNR of 256 corresponding to 1.41% (corresponding to εexp = 900) hyperpolarization of total polarized 1H intensity per 0.0098 mM effective concentration, similar to previous studies (13). As with the 13C direct detection experiments using 13C hyperpolarized HEP, the lifetime of the hyperpolarization was short and hyperpolarization decayed after 90 s to undetectable levels in proton indirect imaging (Fig. 5b).
Figure 5. 1H Imaging of 13C Hyperpolarization.
(a) Indirect FSSFP 1H imaging of polarization transferred from 13C nuclei of 13C hyperpolarized HEP to neighboring protons using INEPT, (b) 1H image of “cold” state, no detectable signal is observed in the indirect 1H FSFFP image. Indirect proton images were acquired using TR = 6.35 ms, TE = 3.17 ms, FOV = 64×64 mm2, and an in-plane resolution of 2×2 mm2. No extrapolation or zero filling was applied to images, and images are shown in their raw resolution.
Examining the theoretical polarization transfer efficiency η in HEP from 13C to 1H was not pursued because in addition to the methyl protons of interest, HEP contains a methylene proton as well. However, we note that 1.41% 1H polarization was achieved after polarization transfer from 5.3% polarized 13C in HEP corresponding to 27% polarization transfer efficiency η calculated as P1H/P13C.
4. Discussion
4.1. Polarization transfer efficiency
When comparing polarization transfer efficiency from 1H to 13C in 1-13C-acetate, η differed in the spectroscopic and imaging detection approaches. The difference between 70% and 87% respectively was likely a manifestation of large error in η of imaging, because of the relatively low SNR of the reference thermal 13C image, Fig. 1c. Therefore, the η value derived from the H→13C spectroscopic study was used in further calculations of 13C→H polarization εexp and η.
13C→H polarization transfer efficiency η was lower in the imaging studies of 1-13C-acetate/D2O phantom compared to corresponding spectroscopic studies, 85.7% vs. 98.6%. Moreover, η decreased further, down to 45.1% in the case of the 1-13C-acetate/H2O phantom. A likely explanation for such significant decreases in the imaging studies and especially for the case of 1-13C-acetate/H2O is the T2 effect, because RF pulses are applied every 6 ms and non-equilibrium 1H polarization transferred from 13C is mixed with thermal polarization of protons with relatively short T2. A potential improvement can be achieved through imaging acceleration using faster acquisition. However, frequency selective RF pulses as implemented here represented the largest fraction of total acquisition time. Therefore, more optimal design and implementation of RF excitation pulses with spectral selectivity would potentially improve the overall SNR of indirect proton imaging detection. The latter can be achieved through a variety of solutions. (i) Higher field MRI systems such as ≥9.4 T increase chemical shift dispersion leading to shorter RF pulses with identical frequency selectivity. (ii) Sinc RF pulses or other waveforms can also potentially lead to shorter RF excitation pulses with similar or better frequency selectivity. (iii) Low field MRI indirect detection potentially allows for significantly lower water background and could potentially eliminate the need for frequency selective RF pulses. For example, it has been shown that high detection sensitivity can be achieved at 0.0475 T, a field that has 100 times less polarized water (25). In that case, simple proton presaturation would perform better and may be sufficient. Regardless of the approach to improve the apparent η of 13C→H for indirect proton imaging, it will significantly increase the detection sensitivity presented here by several fold.
A similar trend in η for 13C→H polarization transfer in hyperpolarized HEP was observed in corresponding imaging experiments, Table. 1. η of the imaging study was only 27% and clearly has room for potential improvement.
Table 1.
Summary of the results. Note the error is ±1% unless otherwise noted.
| Transfer | Spatial Resolution | SNR | 13C Polarization | 1H Polarization | εexp | εtheory | η | J (Hz) |
|---|---|---|---|---|---|---|---|---|
| Sodium 1-13C-acetate/D2O | ||||||||
| 13C→H | Spectroscopy | 6209 | 1.30*10−5 | 4.75*10−6 | 0.367 | 0.372 | 98.6% | 6.0 |
| H→13C | Spectroscopy | 960 | 1.30*10−5 | 1.61*10−5 | 3.23 | 4.59 | 70.3% | 6.0 |
| 13C→H | 2×2 mm2 | 197 | 1.30*10−5 | 4.14*10−6 | 0.319 | 0.372 | 85.7% | 6.0 |
| H→13C | 3×3 mm2 | 29.8 | (1.61±0.3)*10−6 | 1.61*10−5 | 3.99±0.6 | 4.59 | 87±15% | 6.0 |
| Sodium 1-13C-acetate/H2O | ||||||||
| 13C→H | 2×2 mm2 | 145 | 1.30*10−5 | 2.17*10−5 | 0.167 | 0.372 | 45.1% | 6.0 |
| 13C hyperpolarized HEP | ||||||||
| 13C→Hb | 2×2 mm2 | 256 | (5.3±0.5)*10−2 | (1.41*±0.14)*10−2 | 900±90 | - | - | 5.6, 7.3 |
| 13C | 3×3 mm2 | 177 | (5.3±0.5)*10−2 | - | 13,200±1,300 | - | - | - |
4.2. Spatial resolution improvement
Despite reduced apparent η values in the 13C→H imaging study with 1-13C-acetate in water, proton imaging SNR was 145 for a 2×2 mm2 in-plane resolution. This correlates with a SNR of 29.8 and 3×3 mm2 in-plane resolution where 13C was imaged with the same level of 13C polarization. We note that the RF coil was not particularly optimized for 1H detection. Nevertheless, indirect proton detection yielded 11.0 times the SNR per unit of volume. This proves the underlying hypothesis that indirect proton detection of hyperpolarized compounds is a more sensitive means of imaging. To a certain extent, this rather high value of sensitivity enhancement is enabled by three protons rather than one 13C carbon, even under conditions of relatively low Eff, and εexp ≪ 1. It should also be pointed out that the overall experimentally observed sensitivity enhancement factor of 11.0 described above also reflects 1H sensitivity loss due to the implemented RF excitation pulses. Approximately 60% signal loss was observed in the implementation of frequency selective pulses during 1H imaging compared to square RF pulses with τ90° = 57 μs. This shortcoming should be addressed in future studies. However, if properly addressed, SNR gains in excess of 20 fold are potentially achievable.
4.3. Limitations and future perspectives, and subject RF noise
The 13C→H imaging study with 13C hyperpolarized HEP in water also yielded relatively low η of 27%. This compares to η = 45.1% in the 13C→H imaging study with 1-13C-acetate in water. The comparison of imaging SNR of direct 13C and indirect 1H studies, Table 1, shows that indirect detection yielded 3.25 times more SNR. The significant discrepancy with the factor of 11.0 described above can be explained by lower η value and three acetate protons per molecule compared to the one proton per chemical group in perdeuterated PASADENA addition product of HEA (HEP). Moreover, polarization transfer delays could likely be further optimized to improve experimental transfer yields.
It should also be pointed out that proton detection is conducted at significantly higher frequency, which is one of the contributing factors in the (γ1H/γ13C)2 expression, not accounting for T2, RF coil sensitivity and subject RF noise. While RF coil sensitivity can be optimized for the detection frequency of interest, T2 (31) and subject RF noise (32) are fundamentally unavoidable, but have frequency dependence. Therefore, it is likely that the efficiency of improved proton detection can decrease with transition to in vivo studies, larger sample and subject sizes and higher resonance frequencies. While the increased body noise at greater proton frequencies is a clear limitation of this method (33), we point out that it can be significantly alleviated in the low-field MRI regime, where RF losses and body noise on the clinical subjects is negligible (34). As a result, low-field MRI of hyperpolarized contrast agents in humans is an attractive approach from sensitivity perspective.
Specific absorption rate (SAR) is undesirable, because it leads to heat deposition from RF pulses. The pulse sequence described here is indeed RF pulse intense. However, the polarization transfer block uses only a few high power RF pulses. The remainder of RF pulses, corresponding to excitation RF pulses of FSSFP, are extremely low power (~50 mW). Moreover, the sequence is played only once in less than 1 second. Therefore, SAR is not expected to be an impediment for pre-clinical translation of the presented work at magnetic fields of 4.7 T and lower. However, the clinical translation and high-field preclinical translation will likely result in increased RF pulse power, which could raise SAR concerns. This will likely be exacerbated by 3D applications, where additional RF pulses are needed for 3D encoding. However, it should be stressed that the sequence is ultra-short and power deposition will be limited to ~ 1 second. In case if SAR will exceed the safe limits, a potential solution is to replace FSSFP imaging block by less RF-pulse power intense gradient echo sequences, where a small tipping angle RF pulse is used, i.e. only a small fraction (2–5%) of a full 90° RF pulse is utilized.
The RF pulse width used in frequency selective FSSFP component was > 4 ms, which is sufficiently long for potential clinical translation of this sequence. Because the polarization transfer delays are long due to relatively small heteronuclear spin-spin coupling of isolated 13C sites, the RF pulses of polarization transfer block can also be potentially increased as needed (up to the width of frequency selective RF pulses of > 4 ms) for clinical translation.
While the body noise has a negligible contribution to overall NMR noise in low magnetic field (34), the body noise dominates the overall noise at higher frequencies. Because there is more body noise at high frequencies as compared to in vitro phantoms, there will be more noise in proton MR compared to 13C MR at the same magnetic field. Therefore, the observed SNR gains for indirect proton detection will be reduced in vivo at high magnetic fields and frequencies. However, hyperpolarized MRI sensitivity does not gain from higher fields in general and therefore, translation of this approach will be more beneficial at lower fields, i.e. < 4.7 T.
13C→H imaging studies used proton background suppression, taking advantage of multiband pre-saturation RF pulses and frequency selective RF excitation pulses, which resulted in an overall increase of experimental time and more importantly imaging time. For example, imaging time increased from 64 ms (no frequency selective pulse) to 192 ms (with frequency selective pulses). As mentioned above, most of the imaging time was spent on RF excitation pulses. While the latter can be potentially decreased by smarter RF pulse design and implementation, the alternative solution is to increase spatial resolution while decreasing the RF pulse width and maintaining the same background level. For example, the relatively high SNR of 256 with 2×2 mm2 in-plane resolution can be traded for improved spatial resolution with 1×1 mm2, which is also desirable from a cancer imaging perspective, while maintaining relatively high and certainly acceptable levels of SNR.
We anticipate that this work can be readily translated to in vivo imaging of hyperpolarized contrast agents. Indirect imaging of hyperpolarized 1-13C-lactate as a metabolic product of injected hyperpolarized 1-13C-pyruvate is particularly attractive using the demonstrated strategy. Because of larger chemical shift separation in the 13C dimension between 1-13C-pyruvate and 1-13C-lactate > 10 ppm, 1-13C resonance of lactate can be selectively excited (35) for polarization transfer to its three methyl protons, while retaining hyperpolarized magnetization of 1-13C-pyruvate. Additionally, lactate similarly to the acetate presented here, has three methyl protons and similarly large SNR gains are expected, as much as an order of magnitude as presented here. This can be translated to significantly improved spatial resolution or improved sensitivity of hyperpolarized MR, which are still lacking and can benefit from the improvements described here.
Extension of this method to 3D hyperpolarized imaging (36) can be potentially realized, but challenges need to be addressed including the required short imaging time due to imposed T2 limitations of protons. These limitations can be addressed by decreasing the acquisition time necessary to scan each line of k-space as well as implementing compressed sensing.
A single proton image was recorded for 13C hyperpolarized contrast agent here, while multiple acquisitions may be desired for kinetics studies of metabolism in vivo. The latter can be realized by the use of partial polarization transfer from 13C to 1H (23), where only a fraction of long-lived 13C hyperpolarized is consumed for a single 2D or potentially 3D image, and multiple polarization transfer can enable acquisition of multiple proton images of 13C hyperpolarized contrast media.
5. Conclusion
We have used frequency selective FSSFP method for indirect proton imaging of the aqueous 13C hyperpolarized contrast agent HEP. Indirect proton imaging allowed for notable sensitivity improvement. Frequency selective RF pulses combined with proton multiband pre-saturation significantly decreased water and other proton background signals to an acceptable level for quantitative imaging, demonstrating that 13C hyperpolarized MR can significantly benefit from indirect proton sub-second imaging.
SNR gains of up to 11 fold from proton imaging versus direct 13C imaging were demonstrated with a sodium 1-13C-acetate phantom in water, where three methyl protons were used for detection. SNR gain of 3.25 for hyperpolarized HEP was somewhat lower. The demonstrated method can be applied to a number of other hyperpolarized contrast agents including hyperpolarized 1-13C-pyruvate and 1-13C-lactate, 5-13C-glutamine, succinate and others. The demonstrated SNR gains can be further improved significantly by (i) implementation of more suitable frequency selective RF pulses and better chemical shift dispersion at high field or (ii) use of low field MR imaging. We speculate that SNR can be improved by a factor of 2 to 5.
The demonstrated method can potentially be extended to 3D and in vivo application. Moreover, this method of indirect proton detection of hyperpolarized contrast agents solves an important challenge of 13C hyperpolarized MR by eliminating the need for 13C detection. Conventional proton detection is universally available on clinical MRI scanners and can be utilized for detection of hyperpolarized contrast agents while requiring only 13C RF excitation during the polarization transfer sequence. Furthermore, 1H imaging is significantly less demanding from the perspective of gradient power requirements.
Supplementary Material
Figure 6.
Molecular structures and polarization transfers.
Acknowledgments
Funding Sources
We gratefully acknowledge the financial support from NIH/NCI 5R00 CA134749-03, ICMIC 5P50 CA128323-03, R25 CA136440, 3R00CA134749-02S1, NIH EB001628-10, Prevent Cancer Foundation, and Department of Defense CDMRP Era of Hope Breast Cancer Award W81XWH-12-1-0159/BC112431.
ABBREVIATIONS
- PHIP
parahydrogen induced polarization
- HEP
2-hydroxyethyl 1-13C-propionate-d2,3,3
- HEA
2-hydroxyethyl 1-13C-acrylate-d2,3,3
- INEPT
insensitive nulcei enhanced by polarization transfer
- RINEPT
refocused insensitive nuclei enhanced by polarization transfer
- DEPT
distortionless enhancement by polarization transfer
- APT
attached proton test
- FSSFP
fast stead-state free precession
- FOV
field of view
- η
efficiency
- PASADENA
Para-Hydrogen and Synthesis Allow Dramatically Enhanced Nuclear Alignment
- DNP
dynamic nuclear polarization
References
- 1.Abragam A, Goldman M. Principles of Dynamic Nuclear Polarization. Rep Prog Phys. 1978;41(3):395–467. [Google Scholar]
- 2.Day SE, Kettunen MI, Gallagher FA, Hu DE, Lerche M, Wolber J, Golman K, Ardenkjaer-Larsen JH, Brindle KM. Detecting tumor response to treatment using hyperpolarized C-13 magnetic resonance imaging and spectroscopy. Nat Med. 2007;13(11):1382–1387. doi: 10.1038/nm1650. [DOI] [PubMed] [Google Scholar]
- 3.Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A. 2003;100(18):10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisenschmid TC, Kirss RU, Deutsch PP, Hommeltoft SI, Eisenberg R, Bargon J, Lawler RG, Balch AL. Para Hydrogen Induced Polarization In Hydrogenation Reactions. J Am Chem Soc. 1987;109(26):8089–8091. [Google Scholar]
- 5.Cai C, Coffey AM, Shchepin RV, Chekmenev EY, Waddell KW. Efficient transformation of parahydrogen spin order into heteronuclear magnetization. J Phys Chem B. 2013;117(5):1219–1224. doi: 10.1021/jp3089462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurhanewicz J, Vigneron DB, Brindle K, Chekmenev EY, Comment A, Cunningham CH, DeBerardinis RJ, Green GG, Leach MO, Rajan SS, Rizi RR, Ross BD, Warren WS, Malloy CR. Analysis of Cancer Metabolism by Imaging Hyperpolarized Nuclei: Prospects for Translation to Clinical Research Neoplasia. 2011;13(2):81–97. doi: 10.1593/neo.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golman K, in’t Zandt R, Thaning M. Real-time metabolic imaging. Proc Natl Acad Sci U S A. 2006;103(30):11270–11275. doi: 10.1073/pnas.0601319103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chekmenev EY, Hovener J, Norton VA, Harris K, Batchelder LS, Bhattacharya P, Ross BD, Weitekamp DP. PASADENA hyperpolarization of succinic acid for MRI and NMR spectroscopy. J Am Chem Soc. 2008;130(13):4212–4213. doi: 10.1021/ja7101218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallagher FA, Kettunen MI, Day SE, Lerche M, Brindle KM. C-13 MR spectroscopy measurements of glutaminase activity in human hepatocellular carcinoma cells using hyperpolarized C-13-labeled glutamine. Magn Reson Med. 2008;60(2):253–257. doi: 10.1002/mrm.21650. [DOI] [PubMed] [Google Scholar]
- 10.Bowers CR, Weitekamp DP. Para-Hydrogen and Synthesis Allow Dramatically Enhanced Nuclear Alignment. J Am Chem Soc. 1987;109(18):5541–5542. [Google Scholar]
- 11.Morris GA, Freeman R. Enhancement of Nuclear Magnetic-Resonance Signals by Polarization Transfer. J Am Chem Soc. 1979;101(3):760–762. [Google Scholar]
- 12.Klomp DWJ, Wijnen JP, Scheenen TWJ, Heerschap A. Efficient H-1 to P-31 Polarization Transfer on a Clinical 3T MR System. Magn Reson Med. 2008;60(6):1298–1305. doi: 10.1002/mrm.21733. [DOI] [PubMed] [Google Scholar]
- 13.Chekmenev EY, Norton VA, Weitekamp DP, Bhattacharya P. Hyperpolarized (1)H NMR Employing Low gamma Nucleus for Spin Polarization Storage. J Am Chem Soc. 2009;131(9):3164–3165. doi: 10.1021/ja809634u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarkar R, Comment A, Vasos PR, Jannin S, Gruetter R, Bodenhausen G, Hall H, Kirik D, Denisov VP. Proton NMR of N-15-Choline Metabolites Enhanced by Dynamic Nuclear Polarization. J Am Chem Soc. 2009;131(44):16014. doi: 10.1021/ja9021304. [DOI] [PubMed] [Google Scholar]
- 15.Kadlecek S, Vahdat V, Nakayama T, Ng D, Emami K, Rizi R. A simple and low-cost device for generating hyperpolarized contrast agents using parahydrogen. NMR Biomed. 2011;24(8):933–942. doi: 10.1002/nbm.1757. [DOI] [PubMed] [Google Scholar]
- 16.Frydman L, Blazina D. Ultrafast two-dimensional nuclear magnetic resonance spectroscopy of hyperpolarized solutions. Nat Phys. 2007;3(6):415–419. [Google Scholar]
- 17.Mishkovsky M, Cheng T, Comment A, Gruetter R. Localized in vivo hyperpolarization transfer sequences. Magn Reson Med. 2012;68(2):349–352. doi: 10.1002/mrm.23231. [DOI] [PubMed] [Google Scholar]
- 18.Zeng H, Bowen S, Hilty C. Sequentially acquired two-dimensional NMR spectra from hyperpolarized sample. J Magn Reson. 2009;199(2):159–165. doi: 10.1016/j.jmr.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Bastiaansen JAM, Cheng T, Mishkovsky M, Duarte JMN, Comment A, Gruetter R. In vivo enzymatic activity of acetylCoA synthetase in skeletal muscle revealed by C-13 turnover from hyperpolarized 1-C-13 acetate to 1-C-13 acetylcarnitine. Biochim Biophys Acta-Gen Subj. 2013;1830(8):4171–4178. doi: 10.1016/j.bbagen.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 20.Jensen PR, Peitersen T, Karlsson M, in’t Zandt R, Gisselsson A, Hansson G, Meier S, Lerche MH. Tissue-specific Short Chain Fatty Acid Metabolism and Slow Metabolic Recovery after Ischemia from Hyperpolarized NMR in Vivo. J Biol Chem. 2009;284(52):36077–36082. doi: 10.1074/jbc.M109.066407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishkovsky M, Comment A, Gruetter R. In vivo detection of brain Krebs cycle intermediate by hyperpolarized magnetic resonance. J Cereb Blood Flow Metab. 2012;32(12):2108–2113. doi: 10.1038/jcbfm.2012.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfeilsticker JA, Ollerenshaw JE, Norton VA, Weitekamp DP. A selective N-15-to-H-1 polarization transfer sequence for more sensitive detection of N-15-choline. J Magn Reson. 2010;205(1):125–129. doi: 10.1016/j.jmr.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Norton VA, Weitekamp DP. Communication: Partial polarization transfer for single-scan spectroscopy and imaging. J Chem Phys. 2011;135(14) doi: 10.1063/1.3652965. [DOI] [PubMed] [Google Scholar]
- 24.Waddell KW, Coffey AM, Chekmenev EY. In situ Detection of PHIP at 48 mT: Demonstration using a Centrally Controlled Polarizer. J Am Chem Soc. 2011;133(1):97–101. doi: 10.1021/ja108529m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coffey AM, Shchepin RV, Wilkens K, Waddell KW, Chekmenev EY. A Large Volume Double Channel 1H-X RF Probe for Hyperpolarized Magnetic Resonance at 0. 0475 Tesla. J Magn Reson. 2012;220:94–101. doi: 10.1016/j.jmr.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng B, Coffey AM, Colon RD, Chekmenev EY, Waddell KW. A pulsed injection parahydrogen generator and techniques for quantifying enrichment. J Magn Reson. 2012;214(0):258–262. doi: 10.1016/j.jmr.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldman M, Johannesson H. Conversion of a proton pair para order into C-13 polarization by rf irradiation, for use in MRI. C R Physique. 2005;6(4–5):575–581. [Google Scholar]
- 28.Gridnev ID, Higashi N, Asakura K, Imamoto T. Mechanism of asymmetric hydrogenation catalyzed by a rhodium complex of (S,S)-1,2-bis(tert-butylmethylphosphino)ethane. Dihydride mechanism of asymmetric hydrogenation. J Am Chem Soc. 2000;122(30):7183–7194. [Google Scholar]
- 29.Gridnev ID, Imamoto T. On the mechanism of stereoselection in Rh-catalyzed asymmetric hydrogenation: A general approach for predicting the sense of enantioselectivity. Acc Chem Res. 2004;37(9):633–644. doi: 10.1021/ar030156e. [DOI] [PubMed] [Google Scholar]
- 30.Hövener J-B, Chekmenev E, Harris K, Perman W, Tran T, Ross B, Bhattacharya P. Quality assurance of PASADENA hyperpolarization for 13C biomolecules. Magn Reson Mat Phys Biol Med. 2009;22:123–134. doi: 10.1007/s10334-008-0154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheffler K, Hennig J. Is TrueFISP a gradient-echo or a spin-echo sequence? Magn Reson Med. 2003;49(2):395–397. doi: 10.1002/mrm.10351. [DOI] [PubMed] [Google Scholar]
- 32.Darrasse L, Ginefri JC. Perspectives with cryogenic RF probes in biomedical MRI. Biochimie. 2003;85(9):915–937. doi: 10.1016/j.biochi.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Golman K, Petersson JS. Metabolic imaging and other applications of hyperpolarized C-13. Acad Radiol. 2006;13(8):932–942. doi: 10.1016/j.acra.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Hayden ME, Bidinosti CP, Chapple EM. Specific absorption rates and signal-to-noise ratio limitations for MRI in very-low magnetic fields. Concept Magnetic Res A. 2012;40A(6):281–294. [Google Scholar]
- 35.von Morze C, Sukumar S, Reed GD, Larson PEZ, Bok RA, Kurhanewicz J, Vigneron DB. Frequency-specific SSFP for hyperpolarized C-13 metabolic imaging at 14. 1 T. Magn Reson Imaging. 2013;31(2):163–170. doi: 10.1016/j.mri.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larson PEZ, Hu S, Lustig M, Kerr AB, Nelson SJ, Kurhanewicz J, Pauly JM, Vigneron DB. Fast Dynamic 3D MR Spectroscopic Imaging With Compressed Sensing and Multiband Excitation Pulses for Hyperpolarized C-13 Studies. Magn Reson Med. 2011;65(3):610–619. doi: 10.1002/mrm.22650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.