Abstract
Introduction
In an effort to improve prenatal screening for Trisomy 21, we evaluated pregnancy associated plasma protein-A2 (PAPP-A2) as a potential novel second trimester biomarker for Trisomy 21.
Methods
Trisomy 21 and normal control mid-trimester placental samples were subjected to quantitative rt PCR analysis of seven genes we had previously found to be differentially expressed in Trisomy 21 placentae. The localization and differential expression of PAPP-A2 in second trimester placentae from normal and Trisomy 21 pregnancies was determined by immunohistochemistry. PAPP-A2 maternal serum protein levels in ten Trisomy 21 and ten diploid pregnancies were compared by Western blotting. Maternal serum PAPP-A2 levels were measured in 30 Down syndrome cases and 142 normal controls, using ELISA. Regression analysis was used to determine the correlation of PAPP-A2 with other existing markers of Trisomy 21.
Results
PAPP-A2 (aka PLAC3) mRNA and protein expression were both increased in Down syndrome placentae as compared to diploid placentae. PAPP-A2 was also increased in maternal serum from Down syndrome pregnancies as compared to diploid pregnancies. PAPP-A2 expression correlated weakly with established markers.
Discussion
This work takes advantage of our previously performed systematic approach to the discovery of novel maternal serum biomarkers for Trisomy 21, using cDNA microarray analysis. Beginning with the validation of the microarray results, we have tracked PAPP-A2 overexpression in Down syndrome from placental mRNA to maternal serum protein.
Conclusion
PAPP-A2 could serve as an additional maternal serum marker in prenatal screening for Trisomy 21.
Keywords: Pregnancy associated plasma protein-A2, biomarker, Down syndrome, placenta, maternal serum, second trimester
1. INTRODUCTION
Prenatal screening has evolved significantly in the past two decades with the introduction of novel markers. Merkatz et al. was the first to identify an association between low maternal serum concentrations of alpha-fetoprotein (AFP) and fetal Trisomy 21 (1). Before this association, maternal age was the only known risk factor for an aneuploid pregnancy (2–4). Subsequently, maternal serum AFP concentration combined with maternal age was part of generally practiced prenatal screening protocol. Combining maternal age with AFP levels the detection rate achieved was about 40% (1, 5). The quadruple test, which significantly changed the landscape of prenatal screening comprises four maternal serum markers, namely human chorionic gonadotropin (hCG) , Inhibin A, AFP and and uE3. Levels of hCG and Inhibin A are elevated whereas levels of AFP and uE3 are decreased in Trisomy 21.(6–10).
At a 5% false positive rate, in combination with maternal age, maternal serum markers can detect approximately 70 to 80% of cases of Trisomy 21 and, by incorporating fetal ultrasound measurements, slightly improved detection rates can be achieved (11, 12). The issue of improved screening remains a priority due to the number of pregnant women affected, residual risk and false positive rates, and the serious ramifications with respect to reproductive health care (13–15). In the current study, we have evaluated the potential of pregnancy associated plasma protein-A2 (PAPP-A2) as a novel biomarker for screening pregnancies for Trisomy 21. PAPP-A2 is expressed abundantly in human placenta and in non pregnant mammary gland and to a lower extent in various other tissues, including the kidney, fetal brain and pancreas (16–18). It is known to cleave insulin growth factor binding proteins IGFBP-4 and IGFBP-5 and has been found to be up-regulated in hypertensive disorders associated with pregnancy, such as preeclampsia (19–21)
The current study builds on previous work that was based on a systematic approach to discover better maternal serum markers of Trisomy 21, using microarray analysis of Trisomy 21 and diploid placentae (22). Differential expression of placental proteins, because of the highly vascularized nature of the placenta and its proximity to maternal circulation, may be reflected in maternal serum and may be the basis of improved detection in prenatal screening for Trisomy 21.
2. METHODS
The current study was approved by the St. John’s University and Jacobi Medical Center Institutional Review Boards.
2.1. Sample collection
Serum samples from the second trimester of normal and Trisomy 21 pregnancies were used in the experiment. All the serum samples from normal pregnant women were collected by a phlebotomist at Jacobi Medical Center, New York with informed consent and double IRB approval (St. John’s University and Jacobi Medical Center IRBs) prior to amniocentesis or CVS and concurrently with quad screening. Diagnoses were ultimately confirmed by pregnancy outcomes. Serum samples from Trisomy 21 pregnancies were procured with patient informed consent from the clinical laboratory Endoclab, Porto, Portugal with permission obtained from Endoclab. Placental tissues were obtained from second trimester voluntary terminations or spontaneous abortions at Jacobi Medical Center with appropriate consent and IRB approval.
2.2. Quantitative real time reverse transcription polymerase chain reaction (RT-PCR)
Quantitative RT-PCR was performed to compare the expression levels of PAPPA2, ALDH7, KISS1, PTTG1P, KRT8 and EST transcripts (id433020, id611475) in Trisomy 21 and normal placental tissue, all previously found to be differentially expressed in microarray analysis of Trisomy 21 placentae (22). Total RNA was extracted from the chorionic villi of human placental tissues using an RNAeasy kit (Qiagen, Valencia, CA), after which quantitation and purification assessment was performed using NanoDrop spectrophotometer (Thermo scientific, USA) and Agilent 2100 Bioanalyzer (Agilent Technologies). The relative expression levels of the above mentioned seven genes were assessed using quantitative RT-PCR with an ABI PRISM 1500 Sequence Detection System (Perkin–Elmer, Foster City, CA, USA). Equimolar amounts of total RNA from 7 Trisomy 21 and 7 diploid placentae were reverse transcribed and amplified using a one step RT-PCR kit (Qiagen), according to the manufacturer’s instructions. The GenBank access numbers of the genes are listed in Table 1. The expression of the housekeeping gene GAPDH was also measured. Quantitative RT-PCR was performed using 1 cycle for 10 min at 95° C then 40 cycles at 95°C for 30 sec, 60°C for 1 min and finally at 72°C for 30 sec. A standard curve was obtained for all 7 genes using various dilutions and samples were measured in triplicate.
Table 1.
Chromosomal location of various placental genes looked at using RT-PCR*
| GenBank Access # | N31645 | AA598517 | H51848 | AA001841 | N93686 | AA464595 | AA156461 |
|---|---|---|---|---|---|---|---|
| Gene | EST 433020 | KRT8 | PAPPA2 | EST 611475 | ALDH7 | KISS1 | PTTG1IP |
| Chromosome | 21q22 | 12q13 | 1q23–25 | 21 | 11q31 | 1q31 | 21 |
Genes named in table were previously found to be differentially expressed in Trisomy 21 placentae by one of us (SJG), using cDNA microarraly analysis.
2.3. Immunohistochemistry
Placental tissue sections from 7 Trisomy 21 placentae and 7 diploid placentae were deparaffinized with 100 per cent xylene (BDH-VWR) for 4 min, rehydrated with serial alcohol solutions (100 to 70%) and washed in distilled water. The deparaffinized sections were treated with 3%H2O2 for 30 min to quench endogenous peroxidase activity. Sections were rinsed in distilled water and then microwaved in Antigen Retrieval Solution (8.2 mM sodium citrate, 1.8 mM citric acid-pH 6.0, containing 0.01 per cent Triton X-100). After subsequent washing with TBS-T (Tris-buffered saline with 0.1% Tween-20, pH 7.8), sections were incubated in a humidified chamber and a 1:15 dilution of the primary monoclonal anti-PAPP-A2 antibody (Sigma-Aldrich Corp. St. Louis, MO, USA) in TBS containing 1%bovine serum albumin and 0.2%Tween 20 overnight at 4° C. In negative controls, serial sections were incubated with 1% BSA without the primary antibody. After overnight incubation, the sections were washed in TBS containing 0.2%Tween 20 and immunostaining was achieved using an avidin–biotin complex developer kit (Dako, Carpinteria, CA, USA) and 3, 3-diaminobenzidine as a substrate. Sections were counterstained with Mayer’s haematoxylin. The grading of immunohistochemistry staining intensity was performed by three blinded observers by choosing the three most intensely staining regions of chorionic villi, following a pre-defined scoring scheme established by a practicing placental pathologist (SER). The inter-observer variability was tested among the three observers and was found to be insignificant. .
These were graded based on a scale of 1 – 4 with 1 being the lightest staining and 4 being the most darkly staining villi seen by each observer.
2.4. Western blotting
Protein concentrations of 10 Trisomy 21 maternal serum samples and 10 normal control maternal serum samples were determined by performing a Bradford assay, using bovine serum albumin as the standard. SDS sample buffer 4X (Invitrogen) was added to the samples, which were denatured at 95°C for 4 min. Gel electrophoresis was carried out and the proteins were separated using NuPAGE® Novex 3–8% Tris-Acetate Gels, 1.5 mm, 10 welled (Invitrogen, Carlsbad, CA). –Pure PAPP-A2 protein (R&D systems) was used as a positive control. The proteins were then transferred to polyvinylidene difluoride membranes (Invitrogen). The membranes were wetted with TBS-T (Tris-buffered saline with 0.1% Tween-20, pH 7.8) for 10 min, and were blocked with Western blocker solution (Sigma) for 120 minutes. The membranes were then incubated with anti-PAPP-A2 primary antibody (R&D systems),diluted to 0.66 µg/ ml (1:1500 dilution) in TBS-T. The membranes were washed with TBS-T several times and incubated with secondary antibody, anti-rabbit IgG, horseradish peroxidase-linked whole antibody (GE Healthcare, Buckinghamshire UK), at a 1:2500 dilution in western blocker solution at room temperature for 120 min. The membranes were washed again with TBS-T three times for 10 min and treated with the ECL Plus Western blotting detection system (GE Healthcare) and chemifluorescence was detected by exposure to X-ray film (Kodak). Densitometric quantification of the Western blot signal intensity of the bands was performed by densitometric analysis program image J (from the NIH). The density values of albumin were used for normalization. For immunoblotting of albumin, the membranes were washed again with TBS-T three times for 10 min and were stripped by incubating in stripping buffer (Thermo Scientific) at 50° C for 30 minutes. The Western blotting was carried out using albumin primary antibody (Sigma, 1:1000 dilution) according to the procedure described above.
2.5. Enzyme linked immunosorbent assay (ELISA)
PAPP-A2 concentrations were measured by a sandwich enzyme-linked immunosorbent assay (ELISA) using a kit (Ansh labs) according to the manufacturer’s protocol.
2.6. Statistical analysis
Differences of the parameters between the Trisomy 21 and diploid groups were analyzed by two tailed Student’s t test. P<0.05 was considered as statistically significant. For PAPP-A2, the median level at each completed week of gestation was calculated from the results of the analyses in the control women’s serum samples. PAPP-A2 multiple of the median (MoM) is obtained by measuring PAPP-A2 in maternal serum and comparing this value to the median PAPP-A2 value at the same gestational age. Correlations of PAPP-A2 with markers of the quadruple test were evaluated with linear straight-line regression analysis using the Pearson correlation coefficient, P<0.05 was considered statistically significant. The intra-class correlation coefficient analysis was used to measure the reliability of immunohistochemistry grading data by blinded observers.
3. RESULTS
3.1. Differential expression of various genes measured by quantitative RT-PCR
Gross et al. previously performed gene expression profiling of placental tissue derived from Trisomy 21 and normal pregnancies using whole-genome oligonucleotide microarrays (22). Among several genes that were found to be differentially expressed in that study, we specifically chose seven genes (Table 1) that had prominent differential expression and performed quantitative RT-PCR analysis to compare the mRNA levels of these genes in Trisomy 21 and normal placentae (n=7) . Relative levels of gene expression were quantified based on standard curves after normalization to GAPDH. The association between gene expression and Trisomy 21 (cases vs. controls) for each gene was calculated using the Student’s t-test (p<0.05) .Based on the quantitative RT-PCR finding of 2.4-fold up-regulation of PAPPA2 (aka PLAC 3) in Trisomy 21 placentae, we went on to evaluate the potential of PAPP-A2 as a biomarker for prenatal screening. (Fig. 1)
Fig. 1.
Differential expression of various genes in Trisomy 21 placentae (n=7) . Results are expressed as fold changes in Trisomy 21 placentae compared to normal placentae. mRNA levels are normalized to GAPDH. Error bars refer to standard deviations. All samples were run in triplicate.
3.2. Expression and localization of PAPP-A2 in normal and Trisomy 21 human placentae
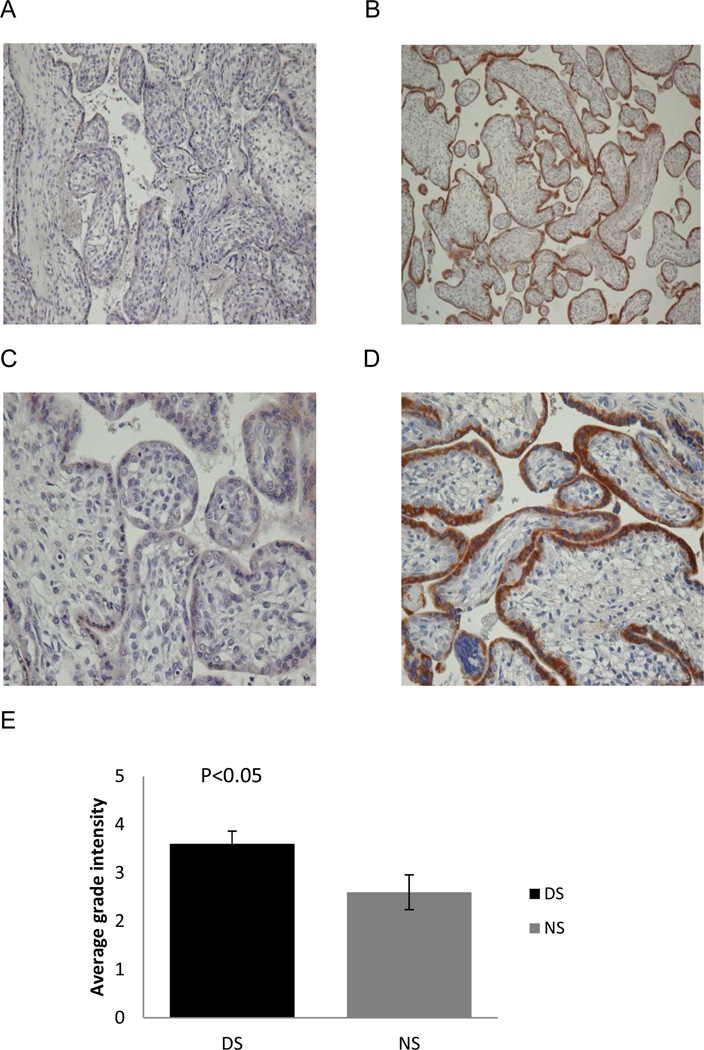
The localization and expression pattern of PAPP-A2 in second trimester placentae from normal and Trisomy 21 pregnancies was determined by immunohistochemistry. The maternal characteristics associated with the placentas are shown in Table 2. Trisomy 21 and normal human placental tissue sections were immunostained with anti-PAPP-A2 antibody (Fig.2A–D). PAPP-A2 positive signals were detected in the cytoplasm of syncytiotrophoblasts in the chorionic villi of both Trisomy 21 and normal pregnancies. This observation suggests that the syncytiotrophoblasts are the prominent source of PAPP-A2 production in both Trisomy 21 and normal placentae. This finding regarding the localization of PAPP-A2 in normal placenta is coherent with a previous report by Nishizawa et al. (19).
Table 2.
Maternal characteristics of the study population (placental specimens)
| Maternal Characteristic | DS (n=7) | NS (n=7) |
|---|---|---|
| Age range (Years) Median Age |
22–40 39 |
19–35 29 |
| Mean Gestational age (Weeks) | 18± 0.81 | 19.7± 2.21 |
| Ethnic origin | 85.72% White 14.28% African American |
42.85% Hispanic 42.85% African American 14.28% White |
Fig. 2.
PAPP-A2 immunohistochemical staining in diploid and Trisomy 21 placentae. Representative immunohistochemistcal staining of PAPP-A2 in second trimester normal (n=7, A, C) and diploid placentae (n=7) at 200× (A, B) and 400× (C, D). Prominent staining is apparent in the cytoplasm of syncytiotrophoblasts. Signal intensity is much stronger in Trisomy 21 placentae (B, D) as compared to normal placentae (left). Significantly increased PAPP-A2 expression (p<0.05) was seen in the cytoplasm of syncytiotrophoblasts of Trisomy 21 placentae (n=7) relative to normal placenta (n=7) in the second trimester (E).
However the PAPP-A2 signal intensity (brown DAB stain) was clearly much stronger in the syncytiotrophoblasts of Trisomy 21 placentae compared to normal placentae (Fig. 2A–D). The specificity of PAPP-A2 was confirmed with use of negative controls and no staining was observed (negative control not shown). PAPP-A2 expression was significantly increased in the cytoplasm of syncytiotrophoblasts of Trisomy 21 placentae relative to normal placentae (p<0.05, Fig. 2E).
3.3. Elevated levels of PAPP-A2 protein in maternal sera of Trisomy 21 pregnancies
After confirming increased PAPP-A2 expression in Trisomy 21 placenta with the immunohistochemistry study we next looked at the levels of PAPP-A2 in the second trimester maternal sera of Trisomy 21 and normal pregnancies using Western blotting (Fig. 3A). Serum levels of PAPP-A2 were significantly higher in Trisomy 21 pregnancies compared to uncomplicated pregnancies (mean immunoblot band density on 0 to 1 scale of 0.58 for Trisomy 21 samples vs. 0.34 for normal control samples, p<0.05) (Fig. 3B). All the sera used in this experiment were collected from second trimester pregnancies. The band intensities were measured using the image J software from the NIH and the relative band intensity, i.e. the ratio of PAPP-A2 band intensity to albumin band intensity was taken into consideration for statistical analysis.
Fig. 3.
Western blot analysis of PAPP-A2 in maternal sera from second trimester Trisomy 21 (DS, n=10) and normal (NS, n=10) pregnancies (A). The band intensities were measured using image J software from the NIH and normalized to albumin. Serum levels of PAPP-A2 were significantly elevated in Trisomy 21 pregnancies compared to uncomplicated pregnancies (B) (p<0.05).
3.4. Concentration of PAPP-A2 in Trisomy 21 and normal pregnancies at various weeks of gestation
We next determined PAPP-A2 protein levels in maternal sera from women with Trisomy 21 pregnancies (n=30) and normal pregnancies (n=142) at various weeks of gestation. A sandwich ELISA system was used for the evaluation of the serum concentrations of PAPP-A2. The maternal characteristics of the study population are provided in Table 3. The levels of PAPP-A2 increased with gestational age both in normal (NS) and Trisomy 21 (DS) maternal serum samples (Fig. 4A). Only mid-trimester samples were used for comparisons between DS and NS. The mean PAPP-A2 was 1.53 MoM in Trisomy 21 samples as compared to normal samples between 15 and 20 weeks of gestation (Fig. 4B). The mean concentration of PAPP-A2 between 15 and 20 weeks was 82.70±12.13 ng/ml in aneuplioid pregnancies compared to 56.35±3.63 ng/ml in normal pregnancies. This difference between Trisomy 21 and diploid pregnancies was statistically significant (p<0.05).
Table 3.
Maternal characteristics of the study population (serum specimens)*
| Maternal Characteristic | DS (n=30) | NS (n=142) |
|---|---|---|
| Age range (years) | 22–40 (median 31, p>0.05 compared to NS) |
17–43 (median 30) |
| Weight range (lbs) | 93–307 | 101–309 |
| Gestational age (weeks) | 13.65 ± 2.69 (Overall study) (p>0.05 compared to NS) 16.45±1.61 (15–20 weeks) (p>0.05 compared to NS) |
16.69 ± 2.04 (Overall study) 17.25±1.38 (15–20 weeks) |
| Ethnic origin | 100% Portuguese | 49.29% Hispanic 34.5% African American 15.74% Pacific Island 3.52% White 1.4% American Indian 2.81% Other |
There were no significant differences in maternal age, weight or gestational age between the two study populations. However the ethnic origins between the groups were different, as discussed in text.
Fig. 4.
Scatter plot of concentration of PAPP-A2 (ng/ml) versus weeks of gestation in normal (NS) and Trisomy 21 (DS) maternal serum (A). Box plot representing concentrations of PAPP-A2 (ng/ml) between 15 and 20 weeks of gestation in normal (NS) and Trisomy 21 (DS) maternal serum (B).
3.5. Correlation of PAPP-A2 with markers of quadruple test
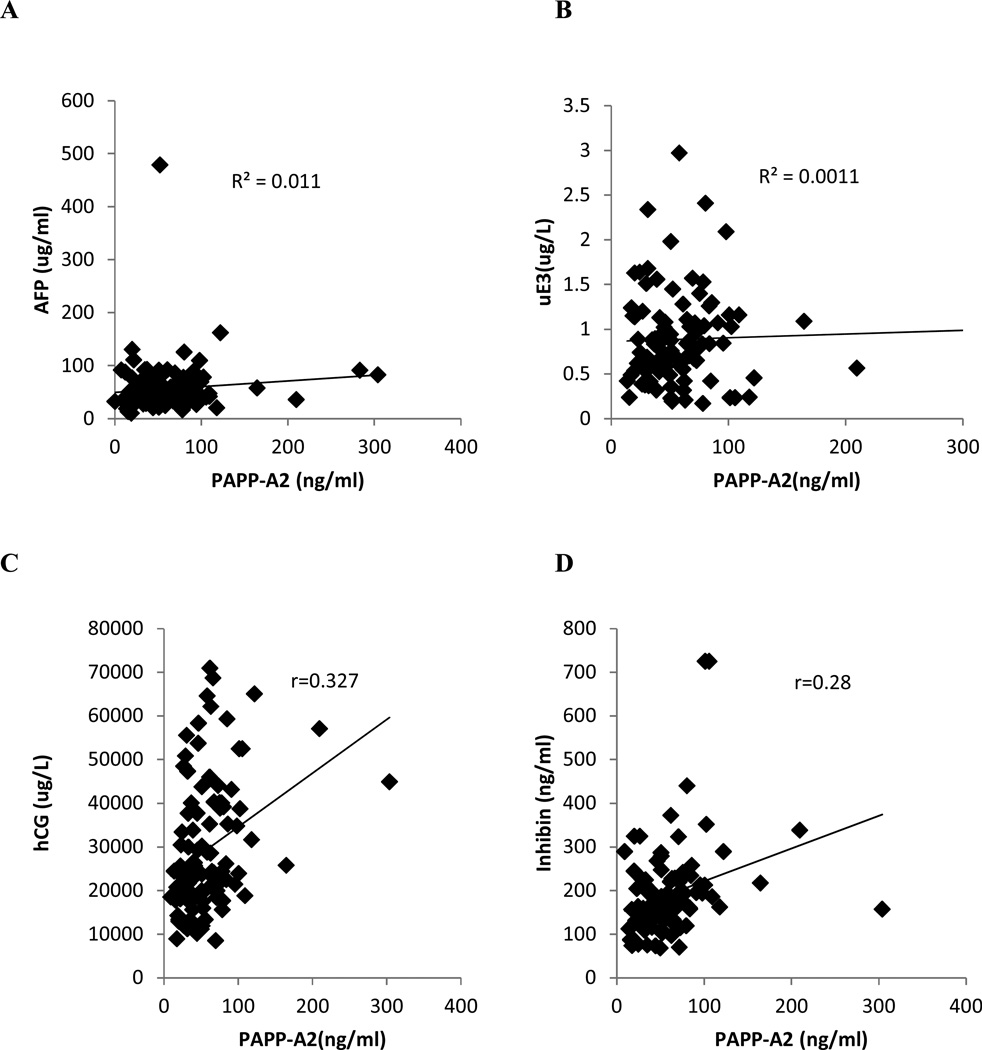
Regression analysis was used to determine if there was any association of PAPP-A2 with other markers of Trisomy 21 in second trimester normal maternal serum samples. Levels of the Trisomy 21 markers AFP, uE3, hCG, and inhibin-A were obtained from the results of the quadruple test that pregnant women undergo in the second trimester. The Pearson correlation coefficient (r) value was 0.104 for AFP (Fig. 5A), 0.032 for uE3 (Fig. 5B), 0.327 for hCG (Fig. 5C), 0.289 for inhibin (Fig. 5D). The findings of this correlation study demonstrate that at 15–20 weeks of gestation, the maternal serum PAPP-A2 levels in normal pregnancies are not significantly correlated with the levels of AFP and uE3 (P>0.05) . However there is significant correlation of PAPP-A2 with hCG and Inhibin (P<0.05).
Fig. 5.
Correlation of [PAPP-A2] with other maternal serum markers. The Pearson correlation coefficient (R) value was 0.104 for AFP (A), 0.032 for uE3 (B), 0.327 for hCG (C), 0.289 for inhibin (D) in normal maternal serum samples at 15–20 weeks of gestation.
4. DISCUSSION
Prenatal screening using maternal serum markers has been in practice for over two decades now and despite current international research efforts the detection rate of Trisomy 21 pregnancies using the quadruple test remains close to only 75%. This detection rate could be improved significantly with additional biomarkers with high sensitivity and specificity (3, 24). In the current study we have evaluated in a small scale the potential of PAPP-A2 as a novel biomarker for screening pregnancies for Trisomy 21. Our study is based on a previous microarray study by Gross et al. on Trisomy 21 and normal placentae that revealed differential expression of certain genes (22). Among these differentially expressed genes we chose seven that had prominent differential expression and validated the microarray findings using quantitative RT-PCR. Our preliminary RT-PCR study findings substantially support the results from the microarray study. The mRNA levels of PAPPA2 were strikingly higher in Trisomy 21 pregnancies as compared to normal pregnancies. Although there was another expressed sequence tag that showed prominent up-regulation of mRNA by about 2.5 fold, we were particularly interested in looking at PAPP-A2, due to its functional and structural similarity to PAPP-A, an existing biomarker for Down syndrome (25).
After confirming the microarray results with RT-PCR, we performed immunohistochemical analysis of second trimester Trisomy 21 and normal human placentae. In the second trimester of pregnancy it was observed that PAPP-A2 is expressed in the synctiotrophoblast layer of the placental villi (Fig. 2A–D) in coherence with previous studies on PAPP-A2 localization in the first trimester (23). In our immunohistochemistry study we, for the first time, have found that PAPP-A2 is prominently up regulated in Trisomy 21 placentae when compared to normal placentae (Fig. 2E). Our immunohistochemical results substantiate our RT-PCR findings.
Although we have shown differential up regulation of PAPP-A2 at the mRNA level and at the protein level in the syncytiotrophoblasts of placental tissue, it was vital to show that levels of PAPP-A2 were elevated in the maternal sera from Trisomy 21 pregnancies as compared to uncomplicated pregnancies, if the potential of PAPP-A2 as a maternal serum marker was to be considered. We therefore performed Western blot analysis on second trimester maternal serum samples and showed that PAPP-A2 levels were significantly elevated in Trisomy 21 pregnancies. Furthermore, our investigation of maternal serum levels of PAPP-A2 in second trimester Trisomy 21 and normal pregnancies with ELISA further support our hypothesis that the increase in expression of placental PAPP-A2 protein is reflected in maternal serum (Fig. 4B–C).
Multiple factors can contribute to inter individual variation of PAPP-A2 levels. In our study, the gestational age of the subjects was determined according to the last menstrual period and in most cases was confirmed by ultrasound. However, in cases that were not confirmed by ultrasound there is the chance of a subject providing an incorrect date of her last menstrual period at the time of sample collection. Also it needs to be pointed out that the storage time for the Trisomy 21 serum samples was longer than that of the normal samples. Consequently, small differences in levels of PAPP-A2 found in Trisomy 21 serum samples may at least in part reflect the longer storage time for those samples. There is a possibility that the increase in PAPP-A2 levels in Trisomy 21 samples might be more pronounced if fresh samples were used. One of the major limitations of our study is that the adjustment for PAPP-A2 levels was only made for gestational age but not ethnicity or maternal weight. Multiple regression analysis with larger samples containing various ethnicities at different weeks of gestation can be done to define the contribution of maternal variables that influence the measured concentration of PAPP-A2. Studies are also needed to determine the interactions among these covariates. In addition, we also found that in normal pregnancies, there is no or weak association between levels of PAPP-A2 and levels of currently used maternal serum markers. These correlation studies support the hypothesis that PAPP-A2, as a fifth marker, may substantially improve upon the currently used quadruple test.
In the current work, we have demonstrated increased levels of PAPP-A2 in Trisomy 21 pregnancies compared to uncomplicated pregnancies in the mid-trimester. Future larger scale studies are needed to establish the sensitivity and specificity of this novel biomarker. Such studies would also measure the incremental gain in detection using a “quint” test that includes PAPP-A2 in comparison to the existing quadruple test. In recent years, prenatal screening has been dominated by first trimester screening and, very recently, by non-invasive prenatal testing (NIPT). Although we focused on expression of PAPP-A2 in the mid-trimester in the current work, a future direction for this research would be to evaluate PAPP-A2 as a potential first trimester biomarker for aneuploidy. An analysis of costs and outcomes of NIPT for Trisomy 21 using cell free fetal DNA in the UK National Health Service concluded that, as first line testing, although NIPT was likely to be more sensitive than conventional prenatal screening, it would be more costly. Further research was needed to see how it would fare in “real world” conditions (26). Despite the recent advances in the field, mid-trimester prenatal screening will continue to add value, especially in cases of borderline first trimester screening results, in multiple gestations and in parts of the world where NIPT remains unaffordable.
Highlights.
Expression of PAPPA2 and the protein it encodes are elevated in Down syndrome placentae.
Up-regulation of PAPP-A2 in Down syndrome placentae is reflected in maternal serum.
PAPP-A2 correlates weakly with two existing markers for aneuploidy.
Elevated PAPP-A2 is a potential novel biomarker for Down syndrome.
Acknowledgements
This work was supported by NIH grants 1K08HD1209 and 1R01NS069577 (to SER) and by St. John’s University, Queens, NY. We are very grateful to Dr. Stephen Apfelroth for his intellectual contribution, Dr. Ivan Ngai for his expert technical assistance with immunoblottting and to Ms. Ernestine Middleton for preparing the slides for immunohistochemistry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have nothing to disclose and no conflict of interest.
References
- 1.Merkatz IR, Nitowsky HM, Macri JN, Johnson WE. An association between low maternal serum alpha-fetoprotein and fetal chromosomal abnormalities. Obstet Gynecol. 1984 Apr 1;148(7):886–894. doi: 10.1016/0002-9378(84)90530-1. 1984. [DOI] [PubMed] [Google Scholar]
- 2.Wald NJ, Bestwick JP, George LM, Huttly WJ. Antenatal Screening for Down Syndrome Using Serum Placental Growth Factor with the Combined, Quadruple, Serum Integrated and Integrated Tests. PLoS One. 2012 Oct;7(10) doi: 10.1371/journal.pone.0046955. 2012 n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wald NJ, Huttly WJ, Hackshaw AK. Antenatal screening for Down's syndrome with the quadruple test. The Lancet. 2003;361(9360):835–836. doi: 10.1016/S0140-6736(03)12680-3. 3/8. [DOI] [PubMed] [Google Scholar]
- 4.Cuckle HS, Wald NJ, Lindenbaum RH. Maternal serum alpha-fetoprotein measurement: a screening test for Down syndrome. Lancet. 1984 Apr 28;1(8383):926–929. doi: 10.1016/s0140-6736(84)92389-4. 1984. [DOI] [PubMed] [Google Scholar]
- 5.Haddow JE, Kloza EM, Smith DE, Knight GJ. Data from an alpha-fetoprotein pilot screening program in Maine. Obstet Gynecol. 1983 Nov;62(5):556–560. [PubMed] [Google Scholar]
- 6.Aitken DA, PhD, Wallace EM, MRCOG, Crossley JA, PhD, Swanston IA, BSc, van Pareren Y, MSc, van Maarle M, MSc, et al. Dimeric inhibin A as a marker for Down's syndrome in early pregnancy. N Engl J Med. 1996 May 9;334(19):1231–1236. doi: 10.1056/NEJM199605093341904. 1996. [DOI] [PubMed] [Google Scholar]
- 7.Wenstrom KD, Owen J, Chu DC, Boots L. Elevated second-trimester dimeric inhibin A levels identify Down syndrome pregnancies. Obstet Gynecol. 1997 Nov;177(5):992–996. doi: 10.1016/s0002-9378(97)70002-4. 1997. [DOI] [PubMed] [Google Scholar]
- 8.Benn PA. Advances in prenatal screening for Down syndrome: I. general principles and second trimester testing. Clin Chim Acta. 2002 Sep;323(1–2):1–16. doi: 10.1016/s0009-8981(02)00186-9. 2002. [DOI] [PubMed] [Google Scholar]
- 9.Bogart MH, Pandian MR, Jones OW. Abnormal maternal serum chorionic gonadotropin levels in pregnancies with fetal chromosome abnormalities. Prenat Diagn. 1987 Nov;7(9):623–630. doi: 10.1002/pd.1970070904. 1987. [DOI] [PubMed] [Google Scholar]
- 10.Canick JA, Knight GJ, Palomaki GE, Haddow JE, Cuckle HS, Wald NJ. Low second trimester maternal serum unconjugated oestriol in pregnancies with Down's syndrome. Br J Obstet Gynaecol. 1988 Apr;95(4):330–333. doi: 10.1111/j.1471-0528.1988.tb06601.x. 1988. [DOI] [PubMed] [Google Scholar]
- 11.Wilson KL, Czerwinski JL, Hoskovec JM, Noblin SJ, Sullivan CM, Harbison A, et al. NSGC Practice Guideline: Prenatal Screening and Diagnostic Testing Options for Chromosome Aneuploidy. Journal of Genetic Counseling. 2013 Feb;22(1):4–15. doi: 10.1007/s10897-012-9545-3. 2013. [DOI] [PubMed] [Google Scholar]
- 12.Driggers RW, Seibert DC. Prenatal Screening: New Guidelines, New Challenges. The Journal for Nurse Practitioners. 2008 May;4(5):351–356. 2008. [Google Scholar]
- 13.Dunstan FD, Gray JC, Nix AB, Reynolds T. Detection rates and false positive rates for Down's syndrome screening: how precisely can they be estimated and what factors influence their value? Stat Med. 1997 Jul 15;16(13):1481–1495. doi: 10.1002/(sici)1097-0258(19970715)16:13<1481::aid-sim575>3.0.co;2-0. 1997. [DOI] [PubMed] [Google Scholar]
- 14.Gagnon A, Audibert F. Prenatal screening and diagnosis of aneuploidy in multiple pregnancies. Best Practice & Research Clinical Obstetrics & Gynaecology. 2014;28(2):285–294. doi: 10.1016/j.bpobgyn.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Grant SS. Options for Down Syndrome Screening: What Will Women Choose? J Midwifery Womens Health. 2005;50(3):211–218. doi: 10.1016/j.jmwh.2005.01.008. 0. [DOI] [PubMed] [Google Scholar]
- 16.Page NM, Butlin DJ, Lomthaisong K, Lowry PJ. The characterization of pregnancy associated plasma protein-E and the identification of an alternative splice variant. Placenta. 2001;22(8–9):681–687. doi: 10.1053/plac.2001.0709. [DOI] [PubMed] [Google Scholar]
- 17.Conover CA, Boldt HB, Bale LK, Clifton KB, Grell JA, Mader JR, et al. Pregnancy-associated plasma protein-A2 (PAPP-A2): tissue expression and biological consequences of gene knockout in mice. Endocrinology. 2011 Jul;152(7):2837–2844. doi: 10.1210/en.2011-0036. 2011. [DOI] [PubMed] [Google Scholar]
- 18.Overgaard MT, Boldt HB, Laursen LS, Sottrup-Jensen L, Conover CA, Oxvig C. Pregnancy-associated plasma protein-A2 (PAPP-A2), a novel insulin-like growth factor-binding protein-5 proteinase. The Journal of biological chemistry. 2001 Jun 15;276(24):21849–21853. doi: 10.1074/jbc.M102191200. 2001. [DOI] [PubMed] [Google Scholar]
- 19.Nishizawa H, Pryor-koishi K, Suzuki M, Kato T, Kogo H, Sekiya T, et al. Increased levels of pregnancy-associated plasma protein-A2 in the serum of pre-eclamptic patients. Mol Hum Reprod. 2008 Oct;14(10):595–602. doi: 10.1093/molehr/gan054. 2008. [DOI] [PubMed] [Google Scholar]
- 20.Crosley E, Durland U, Seethram K, MacRae S, Gruslin A, Christians J. First trimester maternal circulating levels of pregnancy-associated plasma protein A2 (PAPP-A2) are elevated in pregnancies that subsequently develop preeclampsia. Placenta. 2013;34(9):A53. doi: 10.1177/1933719113512532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan X, Baxter RC, Firth SM. Involvement of pregnancy-associated plasma protein-A2 in insulin-like growth factor (IGF) binding protein-5 proteolysis during pregnancy: a potential mechanism for increasing IGF bioavailability. J Clin Endocrinol Metab. 2010 Mar;95(3):1412–1420. doi: 10.1210/jc.2009-2277. 2010. [DOI] [PubMed] [Google Scholar]
- 22.Gross SJ, Ferreira JC, Morrow B, Dar P, Funke B, Khabele D, et al. Gene expression profile of trisomy-21 placentas: a potential approach for designing noninvasive techniques of prenatal diagnosis. Obstet Gynecol. 2002 Aug;187(2):457–462. doi: 10.1067/mob.2002.123542. 2002. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Qiu Q, Haider M, Bell M, Gruslin A, Christians JK. Expression of pregnancy-associated plasma protein A2 during pregnancy in human and mouse. J Endocrinol. 2009 Sep;202(3):337–345. doi: 10.1677/JOE-09-0136. 2009. [DOI] [PubMed] [Google Scholar]
- 24.Wald NJ, Kennard A, Hackshaw AK. First trimester serum screening for Down's syndrome. Prenat Diagn. 1995 Dec;15(13):1227–1240. doi: 10.1002/pd.1970151305. 1995. [DOI] [PubMed] [Google Scholar]
- 25.Christiansen M, Jaliashvili I. Total pregnancy-associated plasma protein A--a first trimester maternal serum marker for Down's syndrome: clinical and technical assessment of a poly-monoclonal enzyme immunoassay. Scand J Clin Lab Invest. 2003;63(6):407–415. doi: 10.1080/00365510310002248. [DOI] [PubMed] [Google Scholar]
- 26.Morris S, Karisen S, Chung N, Hill M, Chitty LS. Model-based analysis of costs and outcomes of non-invasive prenatal testing for Down’s syndrome using cell free DNA in the UK National Health Service. PLoS One. 2014;9(4):e93559. doi: 10.1371/journal.pone.0093559. [DOI] [PMC free article] [PubMed] [Google Scholar]