Abstract
The mechanism by which addictive drugs such as morphine regulate adult neurogenesis remains elusive. We now demonstrate that morphine can regulate neurogenesis by control of miR-181a and subsequent hippocampal neural progenitor cell (hNPC) lineages. In the presence of morphine, hNPCs preferentially differentiated into astrocytes, an effect blocked by the specific μ-opioid receptor antagonist, Cys2-Tyr3-Orn5-Pen7-amide. This effect was mediated by the Prox1/Notch1 pathway as demonstrated by an increase in Notch1 level in the morphine- but not fentanyl-treated hNPCs, and blocked by over-expression of Notch1 siRNA. Over-expression of Prox1 siRNA up-regulated Notch1 level and potentiated the morphine-induced lineage changes. Prox1 transcript level was regulated by direct interaction between miR-181a and its 3′UTR sequence. In vitro and in vivo treatment with morphine resulted in an increase in miR-181a level in hNPCs and mouse hippocampi, respectively. Over-expression of miR-181a mimics reduced Prox1 levels, increased Notch1 levels and enhanced hNPCs differentiation into astrocytes. Meanwhile, over-expression of the miR-181a inhibitor raised Prox1 levels, decreased Notch1 levels and subsequently blocked the morphine-induced lineage changes. Thus, by modulating Prox1/Notch1 activities via miR-181a, morphine influences the fate of differentiating hNPCs differentiation and therefore the ultimate quantities of mature neurons and astrocytes.
Keywords: progenitor cells, neural differentiation, astrocytes, miRNA, neural stem cell, Notch, adult stem cells
Introduction
Neural progenitor cells (NPCs) are a subset of precursors that retain the capacity of self-renewal and differentiation to neurons, astrocytes and/or oligodendrocytes [1]. In adult neurogenesis, hippocampal NPCs (hNPCs) and their offspring in the subgranular zone (SGZ) give rise to mature granular neurons and glial cells through asymmetric cell division [2, 3]. A variety of factors, including several common drugs of abuse, have been demonstrated to regulate adult neurogenesis [3, 4]. For example, down-regulation of cell proliferation was observed in the hippocampal granule cell layer of adult rats after chronic administration of morphine or heroin [5]. The psychomotor stimulants methamphetamine [6] and cocaine [7] have been shown to negatively influence neurogenesis in the dentate gyrus (DG). Other addictive drugs, such as alcohol [8] and cannabinoids [9] down-regulate adult neurogenesis by either inhibiting the proliferation or enhancing the apoptosis of hippocampal granule cells. A drug-induced reduction of adult neurogenesis may contribute to the observed transient anterograde and retrograde memory impairments in patients taking immediate-release morphine, which may negatively impact patients’ everyday functioning [10].
Previously, we have reported that morphine and fentanyl differentially modulate the activity of the transcriptional factor neurogenic differentiation 1 (NeuroD1) via their regulation of miR-190 [11]. This differential regulation subsequently results in divergent effects on neurogenesis and drug memory retention (memory reconstruction), in that morphine attenuates neuronal differentiation and memory retention, while fentanyl does not [12]. Because NeuroD1 is one of the basic helix-loop-helix transcription factors essential for the differentiation and maturation of newborn neurons [13], it is probable that morphine can regulate various stages of adult neurogenesis, from the birth of NPCs to the eventual synapse formation of the new neurons [14]. In addition to the observed decrease in cell proliferation and NeuroD1-dependent differentiation [5, 11], morphine might also modulate the overall number of matured newborn neurons by inducing lineage-preferential differentiation of the NPCs.
Notch is a well-known master regulator of NPCs and neural development. It functions as a receptor with four subtypes, among which Notch1 is expressed mainly in the nervous system [15]. It has been demonstrated that Notch1 is one of the putative downstream targets of NeuroD1 [16]. Meanwhile, as its most well known role, Notch1 maintains NPCs in the undifferentiated state by inhibiting neuronal differentiation. It was reported that Notch1 inhibits neurogenesis by turning CSL-SKIP into an activator and stimulating the transcription of Hes1 and Hes5 [17]. Moreover, transient activation of Notch1 and Notch3 was able to induce an irreversible switch from NPCs to astrocytes in a way independently of STAT3 [18]. This ability to promote astrogliogenesis was further shown to depend on CSL-mediated glial gene activation [19].
Prospero homeobox protein 1 (Prox1) is a transcription factor necessary for progenitor cell proliferation and cell fate determination in embryonic tissue. Recent studies have shown that Prox1 is exclusively expressed in the dentate granule cell lineage, and is required for the differentiation and survival of newly generated granule cells in the adult hippocampus [20]. Prox1 expression occurs initially in type 2 proliferating neuronal progenitors, and remains high throughout all maturational phases. Hence, Prox1 specifies neuronal linage in hNPCs [20], with functional evidence indicating its implication in Notch1 gene suppression during neurogenesis. Thus, Prox1 facilitates the transition of NPCs from self-renewal to neuronal differentiation [21].
MicroRNAs (miRNAs) are short 20–22 nucleotide RNA molecules that are expressed in a tissue-specific and developmentally regulated manner. They function as negative regulators of gene expression in a variety of eukaryotic organisms, and are key post-transcriptional regulators in stem/progenitor cell self-renewal and fate determination [22]. In our original miRNA array screens, we have observed multiple hippocampal miRNAs that are regulated by μ-opioid receptor (OPRM1) agonists [11, 23]. These miRNAs can either modulate signals downstream of OPRM1 (miR-190) [11] or modulate the receptor level post-transcriptionally (miR339, miR23b and let-7), thereby affecting the overall receptor signaling process [23–25]. Also, in our previous studies, we have observed that morphine decreases the hippocampal Notch1 level. Such a decrease in Notch1 level could be the consequence of a morphine-mediated attenuation of NeuroD1 activity in response to the agonist-dependent inhibition of CaMKIIα activity [12], and/or the decrease in Notch1 level was the consequence of Prox1 activity. According to the microRNA.org target prediction database, Prox1 is a candidate target of miR-181 and that direct evidence has shown that Prox1 expression is negatively regulated by miR-181 in endothelial cells [26]. The miR-181 family includes four isoforms, miR-181a, miR-181b, miR-181c and miR-181d, with their mature sequences named miR-181a-5p, miR-181b-5p, miR-181c-5p and miR-181d-5p [27]. In our original miRNA array screens, miR-181a is one of the hippocampal microRNAs that is being differentially regulated by OPRM1 agonists. Thus, in our current study, we examined whether morphine can regulate hNPC’s fate determination via its regulation of miR-181a and subsequent modulation of Prox1 and Notch1 activity.
Materials and Methods
Animal, primary cultures and in vitro differentiation analysis
Eight-week-old CD1 (ICR) male mice were obtained from Charles River Laboratories, Inc. (Wilmington, MA) two weeks before experiments. Primary cultures and differentiation of mouse hippocampal neurospheres were carried out as previously described [28, 29], with slight modifications. Briefly, glass coverslips were coated with 1 mg/ml Matrigel for 2 h at room temperature. Neurospheres were triturated to form a single-cell suspension and cultured in the complete differentiation medium for approximately 4 days until fully differentiated. EGF and FGF2 were obtained from Sigma-Aldrich (St Louis, MO). NeuroCult™ NSC Medium for proliferation and differentiation, and the Enzymatic Dissociation Kit were purchased from STEMCELL Technologies (Vancouver, Canada). Matrigel was from BD Biosciences (San Jose, CA).
Immunoblotting
Immunoblotting was performed as described previously [30]. Briefly, chemifluorescence was detected by using the ECF Reagent (GE Healthcare, UK) and the fluorescence intensity was measured with Storm 860 Molecular Imager (GE Healthcare). The intensity of individual bands was determined with ImageQuant software (GE Healthcare). Antibodies are listed in supporting information Table S1.
Quantitative Real-Time PCR and Transfection
The total RNAs were extracted and reverse transcribed with the miScript system (Qiagen, Germany). Real-time PCR was performed according to the instructions in the miScript system, which included a SYBR Green PCR kit (Qiagen). GAPDH was used as an internal control. Primer sets used in real-time PCR assays are listed in supporting information Table S2.
The over-expression of the microRNAs and transgenes was performed by using Lipofectamine 2000 (Life technologies, CA) according to the manufacturer’s instructions. Briefly, the DNA-lipid complex solution containing 7.5 μl transfection reagent and 2.5 μg DNA or 1.5 μg siRNA was added to each well of a 6-well plate. The cells were incubated in the transfection medium for 24 h before the initiation of differentiation by using the complete differentiation medium. Transfection efficiency was determined to be ≥ 60% by quantifying the number of GFP+ cells in transfection studies using a GFP expression vector (Fig. S1).
Immunocytochemistry and in vitro quantification
Immunocytochemistry was performed as described previously [31]. Labeled cells were mounted on slides with DAPI Fluormount G (SouthernBiotech, Birmingham, AL) and visualized using an upright microscope (model DM5500 B; Leica, Germany) with a CCD camera. Cells were counted using ImageJ (NIH, MA). The total cell numbers were counted according to DAPI-positive particles and those of each cell type were counted according to indicated markers. For single-color images, cells were counted by using the “Analyze Particles” module of ImageJ. Double positive cells were confirmed by overlapped color and counted manually by the “Cell Counter” plugin. The final data represent at least four individual experiments. For each single experiment, at least three individual fields of a single sample were selected randomly to reach a total number of at least 1,000 cells. Antibodies are listed in Table S1.
Luciferase Assay
A 1.35-kb element of the Prox1 3′UTR was amplified by PCR using forward 5′-CTCGAGTAGAGATTGCAACGCTCTTTTG-3′ and reverse 5′-CGCCGGCGGGGCCTGGATCACACTCTTA-3′ primers, and cloned into downstream of a firefly luciferase gene. A mutated miR-181a binding site (Prox1 3′UTR mutation, PRless) was constructed using the QuickChange site-directed mutagenesis kit (Stratagene, CA), using forward 5′-CTCGAGTAGAGATTGCAACGCTCTTTTATCAGTATGGATAGAAGAATTCC-3′ and reverse 5′-CGCCGGCGGGGCCTGGATCACACTCTTA-3′ primers. 0.4 μg Notch1-luc and PRless-luc constructs were used in each transfection, while 1.6 μg of Prox1 expression vector was used in transfecting the cells. The mmu-miR-181a mimic, mmu-miR-181a inhibitor and control RNAs were obtained from Dharmacon RNA Technologies (Lafayette, CO). HEK293T cells were transfected using Lipofectamine 2000. Luciferase activity was determined 24 h after transfection using the Dual-Luciferase Assay System (Promega, WI) and normalized against the Renilla reniformis luciferase expressed by pRL-CMV (Promega).
Chromatin Immunoprecipitation (ChIP)
ChIP was performed as previously described [32]. Briefly, samples were sonicated using a Sonicator Cell Disruptor model W-220F (Heat Systems-Ultrasonic, Inc., Plainview, NY). Immunoprecipitated DNA was purified with QIAquick PCR purification kit (Qiagen) and used in further PCR analysis.
BrdU Labeling, Animal Training and Morphine Administration
BrdU was dissolved in 0.1 M phosphate-buffered saline, pH 7.4 (PBS), and heated to 50–60°C. Eight-week-old male CD-1 mice were given a single intraperitoneal injection of 300 mg/kg BrdU daily for 3 consecutive days. Conditioned place preference (CPP) training was carried out as previously described [12]. In the conditioning section, half of the mice received a subcutaneous injection of 5 mg/kg morphine daily while the other half received an equal volume of saline. The mice were sacrificed 14 days after the first BrdU injection and brains were fixed for immunohistochemistry.
Histology, Immunohistochemistry and Cell Quantification
Tissue preparation and immunohistochemistry were performed as described [12]. Briefly, brain sections (30 μm) were prepared using a cryostat at −20°C. For BrdU staining, the samples were pre-treated with 2M HCl for 30 min at 45°C, followed by a 2-minute neutralization with 0.1M sodium borate (pH 8.5). Every eighth section across the hippocampus was stained with antibodies listed in Table S1. Sections were mounted on slides with DAPI Fluormount G (SouthernBiotech). BrdU+Tuj1+ and BrdU+GFAP+ cells were quantified throughout the DG. The average number of positive cells per section was then normalized by multiplying this average by the number of 30-μm sections corresponding to the entire DG [33].
Data Analysis
All statistical and curve-fitting analyses were performed using GraphPad Prism 6.0 software. Data represent mean ± SEM of at least three separate experiments. Statistical significance was determined by one-way ANOVA followed by post hoc comparison using Dunnett’s tests.
Results
Morphine modulates lineage-specific differentiation of neural progenitor cells
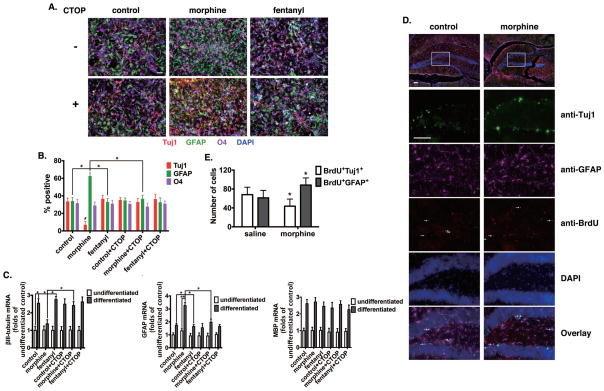
To examine the effects of μ-opioid receptor (OPRM1) agonists on neural progenitor cells, we compared the effects of 1 μM morphine and 10 nM fentanyl on hNPC differentiation, with or without 10 μM OPRM1-specific antagonist Cys2-Tyr3-Orn5-Pen7-amide (CTOP). Using glial fibrillary acidic protein (GFAP), βIII-tubulin (Tuj1) and O4 as markers for astrocytes, neurons and oligodendrocytes respectively, there were equal percentage of cells stained positive for these markers after hNPC differentiation (Fig 1A and B). Morphine treatment resulted in a large percentage of GFAP+ cells (62.7±2.9%) but only very few Tuj1+ neurons (7.0±3.1%), with no change in O4+ cells (29.0±3.1%)(Fig 1, A and B). Fentanyl, on the other hand, did not modulate the cell lineage significantly compared to control group. CTOP blocked the effect of morphine, resulting in non-preferential differentiation of hNPCs.
Figure 1. Morphine modulates lineage-specific differentiation of neural progenitor cells.
(A) Adult hippocampus-derived neural progenitor cells were cultured in complete differentiation medium for 4 d, with or without the treatment of 1 μM morphine or 10 nM fentanyl, and 10 μM CTOP. Cells were stained with markers for neurons (Tuj1), astrocytes (GFAP), oligodendrocytes (O4) and with DAPI. Scale bar, 25 μm. Images are representative of at least three independent experiments with similar results.
(B) Quantification of cells stained with each marker, calculated as the percentage of the total number of cells stained with DAPI. Red: Tuj1; Green: GFAP; Purple: O4. *, p<0.05 between indicated groups. #, p<0.05 compared to Tuj1 in control group. Data are the mean ± SEM of at least three independent experiments.
(C) The expression of βIII-tubulin, GFAP and MBP were determined by real-time PCR after 4 d of differentiation. The results were normalized against those of GAPDH, and further normalized against the result obtained from untreated cultures in the control group. All data represent the mean ± SEM of four independent experiments. *, p<0.05; **, p<0.01 between indicated groups.
(D) CD-1 mice (8-week-old males) received daily i.p. injections of 300 mg/kg BrdU for 3 consecutive days, followed by CPP training with subcutaneous injection of 5 mg/kg morphine or an equal volume of saline. Brains were fixed at day 14 and then sectioned for BrdU staining and co-immunostaining with Tuj1 and GFAP antibodies, and DAPI. Cells co-stained with antibodies of both BrdU and Tuj1 or GFAP are indicated by white arrows. Scale bars, 50 μm.
(E) Total numbers of BrdU-labeled cells co-stained with Tuj1 or GFAP antibodies in mouse hippocampus sections were counted and compared. Data represent the mean ± SEM of six independent experiments. *, p<0.05 compared to the number of cells with the same positive marker in the saline group.
Although morphine was able to alter the percentage of different cell linages after NPC differentiation, it remains uncertain whether morphine induced lineage-preferential differentiation or promoted proliferation of astrocytes by modulating the cell-cycling mechanism. Thus, we counted the number of viable cells after the treatment of morphine or fentanyl, before and after NPC differentiation. As shown in Fig. S2, although both morphine and fentanyl significantly promoted proliferation of undifferentiated NPCs, the total cell number remained constant after the initiation of differentiation. These results indicate that morphine changes the percentage of differentiated cell linages by preferentially inducing astrocyte differentiation via OPRM1, but not by modulating cell proliferation.
To further confirm the immunofluorescence results, we examined the levels of lineage-specific markers βIII-tubulin, GFAP and myelin basic protein (MBP, oligodendrocyte marker), both before and after differentiation, by quantitative real-time PCR (qRT-PCR). As shown in Fig. 1C, although all three markers increased after differentiation, morphine treatment resulted in a significant increase of GFAP and a decrease in βIII-tubulin levels, but did not affect the MBP level. On the other hand, fentanyl did not affect the any of these markers. The effect of morphine was blocked by 10 μM CTOP, indicating a mechanistic role of OPRM1. qRT-PCR analyses of these markers with the hippocampi isolated from mice implanted with either placebo or morphine base (75 mg) pellets also showed similar results (Fig. S3, D and E).
Bromodeoxyuridine (5-bromo-2′-deoxyuridine, BrdU) labeling was carried out to investigate whether morphine can influence lineage-specific differentiation of hNPCs in vivo. Since morphine-induced conditioned place preference (CPP) was confirmed to influence adult neurogenesis [12], we carried out CPP training with 5 mg/kg morphine or an equal volume of saline injected subcutaneously. The fate of BrdU+ cells in the DG were examined by immunohistochemistry on day 14 after BrdU injection (Fig. 1, D and E). In the saline group, BrdU+ hNPCs gave rise to similar populations of Tuj1+ (68.0±14.4) and GFAP+ (61.3±14.3) cells. Morphine administration resulted in a significant decrease in the number of BrdU+Tuj1+ cells (45.3±17.1) and increase in that of BrdU+GFAP+ cells (86.7±17.5), suggesting that morphine preferentially induces astrocyte differentiation in vivo. Together, these results suggested that morphine could promote astrocyte-preferential differentiation of hNPCs.
Morphine promotes astrocyte-preferential differentiation via Notch1
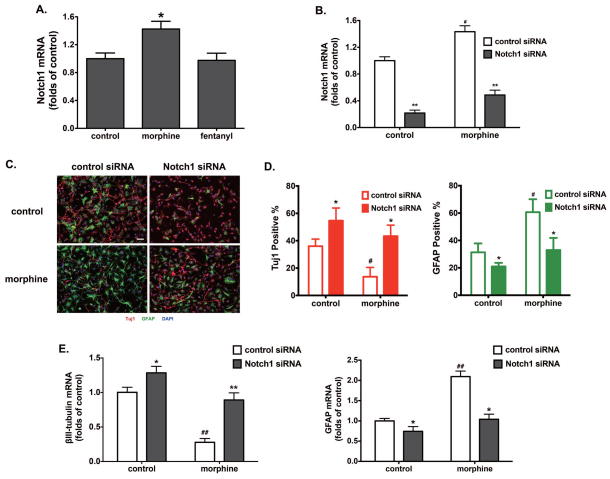
We have previously reported that morphine and fentanyl differentially regulate NeuroD1 [11]. Since Notch1 is one of the putative downstream targets of NeuroD1 [16] and has been demonstrated to promote astrogliogenesis [18, 19], morphine likely promotes hNPC differentiating into astrocytes via regulation of Notch1 expression. As shown in Fig. 2A, Notch1 expression was significantly increased upon morphine- but not by fentanyl-treatment. qRT-PCR analyses of the hippocampal tissue isolated from mice implanted with either placebo or morphine pellets also showed similar morphine-induced increases in Notch1 levels (Fig. S3C).
Figure 2. Morphine promotes astrocyte-preferential differentiation via Notch1.
(A) The expression of Notch1 was determined by real-time PCR after 4 d of differentiation, in the presence of 1 μM morphine or 10 nM fentanyl. The results were normalized against those of GAPDH, and further normalized against the result obtained from the control group. *, p<0.05 compared to control.
(B) The expression of Notch1 was determined by real-time PCR after transfection with control and Notch1 siRNA. The results were normalized against those of GAPDH. **, p<0.01 compared to control siRNA transfected group with the same treatment; #, p<0.05 compared to the control siRNA transfected group without morphine treatment.
(C) Adult hippocampus-derived neural progenitor cells were transfected with control siRNA or Notch1 siRNA, and cultured in complete differentiation medium with 1 μM morphine for 4 d. Cells were stained with markers for neurons (Tuj1), astrocytes (GFAP) and with DAPI. Scale bar, 25 μm. Images are representative of at least three independent experiments with similar results.
(D) Quantification of cells stained with each marker, calculated as the percentage of the total number of cells stained with DAPI. Red: Tuj1; Green: GFAP. *, p<0.05 compared to the control siRNA group with the same treatment; #, p<0.05 compared to the control group transfected with control siRNA.
(E) The expression of βIII-tubulin and GFAP were determined by real-time PCR after 4 d of differentiation with indicated treatments. The results were normalized against those of GAPDH. *, p<0.05, **, p<0.01, compared to control-siRNA-transfected group with the same treatment. ##, p<0.01 compared to the control group transfected with control siRNA. All data represent mean ± SEM of four independent experiments.
To determine whether Notch1 is involved in morphine-induced cell lineage modulation, we inhibited Notch1 expression by small interference RNA (siRNA) and quantified the percentage of GFAP+ and Tuj1+ cells after differentiation. Transfection of Notch1 siRNA significantly decreased Notch1 levels in hNPCs, which remained higher in morphine-treated cells than control cells after siRNA expression (Fig. 2B). Notably, the percentage of GFAP+ cells treated with or without morphine decreased in the presence of Notch1 siRNA, by 27.7±7.2% and 10.3±4.1%, respectively (Fig 2, C and D). In contrast, the quantity of Tuj1+ neurons increased significantly in response to Notch1 down-regulation (Fig. 2, C and D). qRT-PCR analyses also showed that Notch1 siRNA could significantly inhibit GFAP expression and promoted βIII-tubulin expression in hNPCs with or without morphine treatment (Fig. 2E). These results implicate Notch1 in morphine-induced astrocyte differentiation of hNPCs.
Morphine induces Notch1-mediated astrocyte-preferential differentiation by down-regulating Prox1
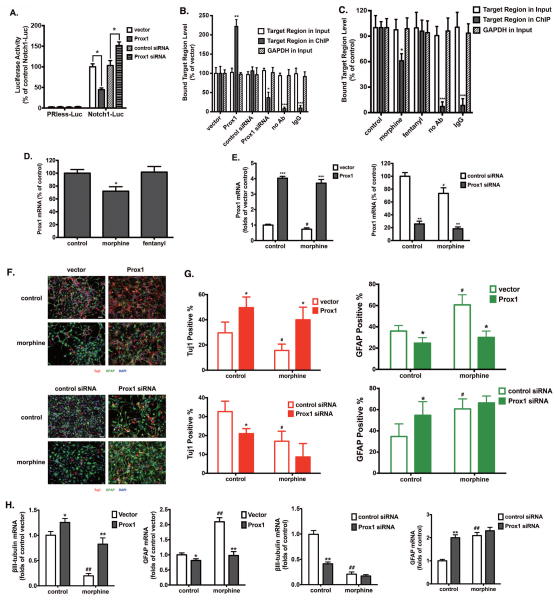
Because Prox1 is implicated in Notch1 gene suppression during neurogenesis and specifies neuronal direction in NPC fate determination [20, 21], we examined whether Prox1 could suppress Notch1 activity by directly interacting with its promoter. The Notch1 promoter activity was determined by transient transfection of a mammalian Notch1 promoter-luciferase construct [21] into HEK293T cells. After expressing pcDNA3.0-Flag-mProx1 via transient transfection, the luciferase activity was significantly decreased. On the other hand, over-expression of Prox1 siRNA resulted in increased luciferase activity, reflecting an increase in Notch1 promoter activity (Fig. 3A).
Figure 3. Morphine induces Notch1-mediated astrocyte-preferential differentiation by down-regulating Prox1.
(A) Transcriptional assays in HEK293T cells co-transfected with pcDNA3.0-Flag-mProx1 and the empty pcDNA3.0 vector, or control siRNA and Prox1 siRNA, along with luciferase reporter constructs containing human Notch1 and empty vector (PRless). *, p<0.05 between indicated groups.
(B–C) The binding of Prox1 to Notch1 promoter was determined by qRT-PCR after ChIP assay. No Ab (without Prox1 antibody) and IgG control (with an antibody of rabbit host that reacts with an irrelevant and non-nuclear antigen) were used to confirm that the binding is specific to Prox1. Real-time PCR performed for the inputs was used to confirm equal amounts of samples were used. The results were normalized against those in vector (B) or control (C) samples in each group. *, p<0.05; **, p<0.01; ***, p<0.001 compared to vector or control groups. All data represent mean ± SEM of four independent experiments.
(D) The expression of Prox1 was determined by real-time PCR after 4 d of differentiation, in the presence of 1 μM morphine or 10 nM fentanyl. The results were normalized against those of GAPDH, and further normalized against the result obtained from the control group. *, p<0.05 compared to control.
(E) The expression of Prox1 was determined by real-time PCR after transfection with vector or Prox1-expressing plasmid (left panel), or control or Notch1 siRNA (right panel). The results were normalized against those of GAPDH. **, p<0.01 compared to control siRNA transfected group with the same treatment; ***, p<0.001 compared to vector group with the same treatment; #, p<0.05 compared to the vector (left panel) or control siRNA (right panel) transfected group without morphine treatment.
(F) Adult hippocampus-derived neural progenitor cells were transfected with vector or Prox1-expressing plasmid (top panel), or control siRNA or Prox1 siRNA (bottom panel), and cultured in complete differentiation medium with 1 μM morphine for 4 d. Cells were stained with markers for neurons (Tuj1), astrocytes (GFAP) and with DAPI. Scale bar, 25 μm. Images are representative of at least three independent experiments with similar results.
(G) Quantification of cells stained with each marker, calculated as the percentage of the total number of cells stained with DAPI. Red: Tuj1; Green: GFAP. *, p<0.05, **, p<0.01, compared to vector or control siRNA transfected groups with the same treatment. #, p<0.05 compared to the control group transfected with vector or control siRNA.
(H) The expression of βIII-tubulin and GFAP were determined by real-time PCR after 4 d of differentiation with indicated treatments. The results were normalized against those of GAPDH. *, p<0.05, **, p<0.01, compared to vector or control siRNA transfected groups with the same treatment. ##, p<0.05 compared to the control group transfected with control siRNA. All data represent mean ± SEM of four independent experiments.
To confirm the direct interaction between Prox1 and the Notch1 promoter, we measured their association by ChIP after transfecting hNPCs with either pcDNA3.0-Flag-mProx1 or Prox1 siRNA. Primers were used to amplify the Notch1 promoter region (−621 to −340 relative to the translation initiation site) cross-linked and immunoprecipitated with Prox1. As indicated by Fig. 3B, the binding of Prox1 to the Notch1 promoter was enhanced upon Prox1 over-expression and reduced upon Prox1 siRNA transfection, suggesting that Prox1 directly interacts with and suppresses the transcription of the Notch1 gene. Moreover, morphine was able to reduce the binding of Prox1 to the Notch1 promoter while fentanyl had no significant effect (Fig. 3C), suggesting that morphine might induce an increase in Notch1 expression by decreasing the availability of Prox1.
If Prox1 directly interacts with the Notch1 promoter and suppresses Notch1 expression, morphine might modulate Notch1 expression and hNPC differentiation by its regulation of Prox1. As shown in Fig. 3D, Prox1 expression in hNPCs was significantly decreased by 28.1±5.7% upon morphine but not fentanyl treatment. In vivo morphine treatment also showed a significant decrease in Prox1 level (Fig. S3B). Therefore, we hypothesize that Prox1 is involved in morphine-induced lineage-specific differentiation. In order to test our hypothesis, Prox1 expression in hNPCs was modulated by over-expression of either pcDNA3.0-Flag-mProx1 or Prox1 siRNA (Fig. 3, E–G). After transient pcDNA3.0-Flag-mProx1 transfection, the percentage GFAP+ astrocytes with or without morphine treatment decreased from 60.7±4.5% and 36.0±2.5% (control vector) to 30.3±3.9% and 24.7±2.4%, respectively. Meanwhile, the percentage of Tuj1+ neurons in the presence of morphine increased correspondingly (Fig. 3, F and G, top panels). On the other hand, transfection of Prox1 siRNA increased the percentage GFAP+ astrocytes from 34.7±5.6% (vector control) to 54.7±6.1%, with concomitant decrease of Tuj1+ neurons (32.6±2.6% to 21.0±1.2%). Interestingly, morphine treatment did not increase further the percentage of hNPC differentiated into GFAP+ astrocytes (Fig. 3, F and G, bottom panels). In order to substantiate these observations, qRT-PCR analyses were carried out to quantify βIII-tubulin and GFAP. As shown in Fig. 3H, over-expression of Prox1 significantly increased βIII-tubulin mRNA level and decreased GFAP mRNA level in hNPCs treated with or without morphine. On the other hand, expression of Prox1 siRNA decreased βIII-tubulin mRNA level and increased GFAP mRNA level in hNPCs without morphine treatment, but did not show significant effects in morphine-treated hNPCs. Taken together, these data suggest that morphine regulates Notch1 expression and facilitates astrocyte differentiation of hNPCs by decreasing the Prox1 level.
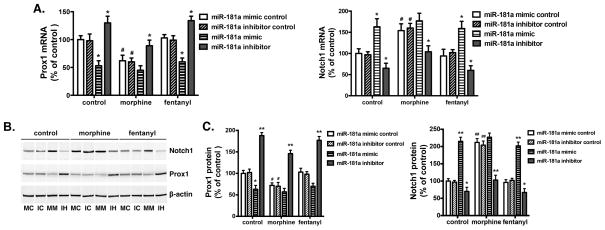
MiR-181a regulates Prox1 and Notch1 expression
Since morphine and fentanyl differentially regulate various microRNAs [11], the changes in Prox1 and Notch1 could reflect agonist-dependent microRNA regulation. By using the microRNA target prediction on microRNA.org, we found that miR-181a was one of the candidate inhibitors of Prox1, with the mirSVR score of −1.0985. In our original microRNA array screens, we also observed that morphine could increase miR-181a level. In order to confirm the effect of miR-181a on Prox1 and Notch1, we transfected the hNPCs with either the miR-181a mimic or inhibitor. As indicated in Fig. 4, over-expression of miR-181a mimic decreased both mRNA and protein levels of Prox1 and increased those of Notch1. Morphine treatment of miR-181a mimic- transfected hNPCs had no further effects on Prox1 or Notch1. Similarly, miR-181a mimic had no further effect on Prox1 or Notch1 after morphine treatment. On the other hand, miR-181a inhibitor increased both mRNA and protein levels of Prox1 and decreased those of Notch1 in hNPCs of all groups, thus neutralizing the effect of morphine. In fentanyl-treated cells, miR-181a mimic decreased the protein level of Prox1 by 33.1±7.2% and increased that of Notch1 by 105.8±9.7% (Fig. 4C). In cells without any treatment, Prox1 protein level was decreased by 36.9±8.4% while Notch1 was increased by 114.6±10.6%. On the contrary, miR-181a inhibitor increased the protein level of Prox1 by 79.2±9.1% and decreased that of Notch1 by 54.8±9.6% in fentanyl-treated cells. In cells without any treatment, Prox1 was increased by 86.3±7.1% and Notch1 was decreased by 27.1±8.2% (Fig. 4, B and C). With the over-expression of the miR-181a inhibitor, the level of Notch1 protein in hNPCs treated with morphine was similar to those of control cells (Fig. 4C), suggesting that such Notch1 protein increase was a direct reflection of miR-181a’s activity.
Figure 4. Prox1 and Notch1 expression change correspondingly with miR-181a expression.
Mouse NPC primary cultures were transfected with miR-181a mimic control (double strand), miR-181a inhibitor control (single strand), miR-181a mimic, or miR-181a inhibitor by using Lipofectamine 2000. Two days after transfection, the mRNA (A) and protein (B–C) levels of Prox1 and Notch1 were determined by real-time PCR and western blot, respectively. The results were normalized against internal controls (GAPDH for mRNA and β-actin for protein). MC: miR-181a mimic control; IC: miR-181a inhibitor control; MM: miR-181a mimic; IH: miR-181a inhibitor. *, p<0.05, **, p<0.01 compared to cultures transfected with control RNA and with the same drug treatment. #, p<0.05, ##, p<0.01 compared to cultures transfected with control RNA without drug treatment. All data represent mean ± SEM of four independent experiments.
Prox1 is one of the miR-181a’s targets
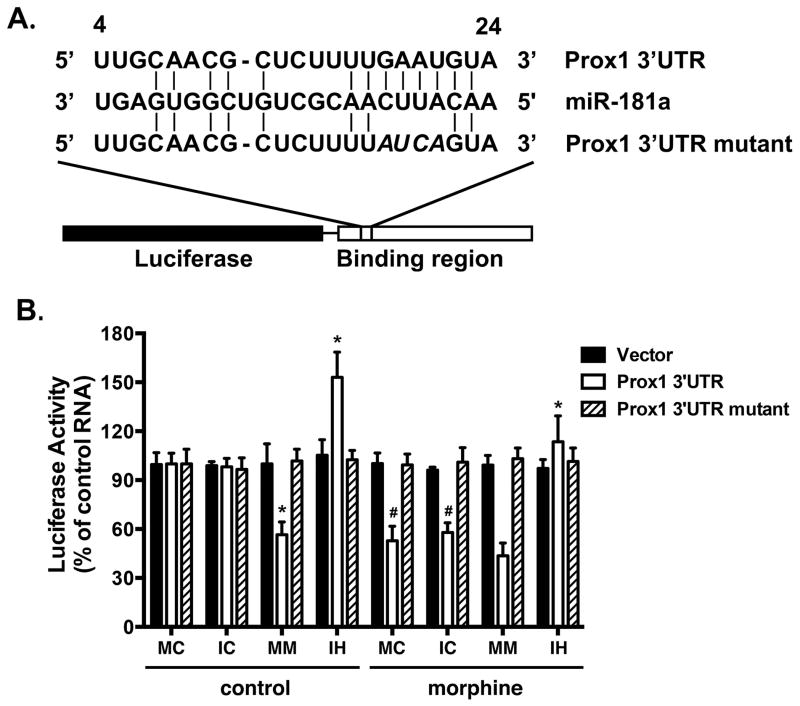
MiR-181a is predicted to bind to nucleotides 4 to 24 of the 3′UTR of mouse Prox1 mRNA. To assess whether miR-181a was capable of binding to and repressing Prox1 transcript level, a 1.35-kb fragment of the Prox1 3′UTR containing the single predicted consensus miR-181a binding site was cloned into the 3′UTR of a firefly luciferase reporter construct. Another luciferase reporter was constructed with the mutant miR-181a targeting site and used as a control (Fig. 5A). The reporters were then co-transfected with miR-181a mimic or inhibitor into HEK293T cells. As expected, the miR-181a mimic suppressed luciferase expression from the Prox1 3′UTR construct, whereas the miR-181a inhibitor enhanced the luciferase expression, presumably by blocking the inhibitory effects of endogenous miR-181a. In contrast, miR-181a mimic or inhibitor did not affect luciferase expression from the mutant Prox1 3′ UTR construct. With morphine treatment, the luciferase expression in hNPCs transfected with the miR-181a inhibitor was similar to those of control cells (Fig. 5B). These data provide compelling evidence that miR-181a directly binds to and negatively regulates Prox1 transcript level.
Figure 5. Prox1 is one of miR-181a’s targets.
(A) Schematics of the Prox1 3′UTR and Prox1 3′UTR mutant reporters. The first nucleotide after the stop codon of mouse Prox1 mRNA is designated as number 1.
(B) HEK293T cells were transfected with one of the RNAs, one of the reporters, and the luciferase reporter system by using Lipofectamine 2000. RNAs included control RNA, miR-181a mimic and miR-181a inhibitor. Reporters included vector, Prox1 3′UTR, and Prox1 3′UTR mutant. The luciferase expression was determined as described under “Materials and Methods”. The results were normalized against internal control (R. reniformis luciferase) and further normalized against the results obtained from cultures transfected with control RNA in each group. MC: miR-181a mimic control; IC: miR-181a inhibitor control; MM: miR-181a mimic; IH: miR-181a inhibitor. *, p<0.05 compared to cells transfected with Prox1 3′UTR and control RNA, and treated with the same agonist. #, p<0.05 compared to cells transfected with Prox1 3′UTR and the same control RNA, and without agonist treatment. Data represent mean ± SEM of four independent experiments.
Morphine increases the level of miR-181a-5p but not other isoforms or pri- or pre-miR-181a via OPRM1
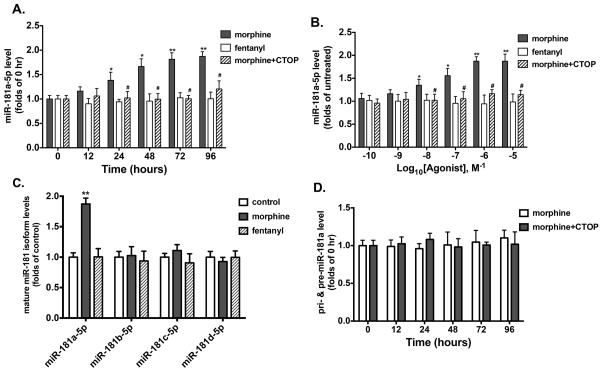
Since miR-181a targets Prox1 and negatively regulates it, whether this microRNA participates in morphine-induced astrocyte-specific differentiation was examined. hNPCs were treated with 1 μM morphine or 10 nM fentanyl with or without 10 μM CTOP. The level of miR-181a-5p, a major form of mature miR-181a, was monitored by qRT-PCR at different time points after initiation of agonist treatment (Fig. 6A). Fentanyl exhibited no significant effect on miR-181a-5p level at any time point. In contrast, a significant increase in miR-181a-5p level was observed 24 h after initiation of morphine treatment and persisted for at least 96 h. This morphine-induced increase in miR-181a-5p level was not observed in the presence of 10 μM CTOP. In vivo studies carried out with hippocampi isolated from morphine-treated mice also showed similar effects on miR-181a-5p levels with qRT-PCR analyses (Fig. S3A).
Figure 6. Morphine increases the expression of miR-181a via OPRM1.
(A) Time-dependent abilities of morphine and fentanyl to modulate the expression of miR-181a-5p in mouse NPCs cultured in complete differentiation medium. Cultures were treated with 1 μM morphine or 10 nM fentanyl for indicated times with or without the treatment of 10 μM CTOP. The expression of miR-181a-5p was determined by real-time PCR and normalized against the mRNA level of GAPDH. The normalized results were further normalized against the results in untreated cultures (0 h). *, p<0.05, **, p<0.01 compared to untreated cultures in the absence of CTOP. #, p<0.05 compared to the morphine-treated group with the same time point or dose, but without CTOP treatment.
(B) Dose-dependent curves of morphine and fentanyl to modulate the expression of miR-181a-5p in mouse NPCs cultured in complete differentiation medium. Cultures were treated with indicated doses of agonists for 96 h with or without the treatment of 10 μM CTOP. The expression of miR-181a-5p was determined by qRT-PCR. *, p<0.05, **, p<0.01 compared to untreated cultures in the absence of CTOP. #, p<0.05 compared to the morphine-treated group with the same time point or dose, but without CTOP treatment.
(C) The abilities of morphine and fentanyl to modulate the expression of the four isoforms of miR-181 in mouse NPCs cultured in complete differentiation medium. Cultures were treated with 1 μM morphine or 10 nM fentanyl for 96 h. The expression of miR-181a-5p was determined by qRT-PCR. **, p<0.01 compared to control.
(D) Time-dependent abilities of morphine to modulate the expression of pri- and pre-miR-181a in mouse NPCs cultured in complete differentiation medium. Cultures were treated with 1 μM morphine for indicated times with or without the treatment of 10 μM CTOP. The expression of pri- and pre-miR-181a was determined by qRT-PCR. All data represent mean ± SEM of four independent experiments.
To further analyze the agonists’ effects on miR-181a-5p level, a dose-dependent effect on miR-181a-5p level was examined. As shown in Fig. 6B, morphine significantly increased miR-181a-5p level at concentrations > 10 nM, with an EC50 value of 57±9 nM, whereas fentanyl exhibited no observable effect at any concentration tested. The effect of morphine was significantly blocked by CTOP.
As the miR-181 family consists of four isoforms (a, b, c and d) that share homologous sequences, it is likely that other isoforms other than miR-181a may participate in morphine-induced astrocyte-specific differentiation. We thus examined whether morphine was able to modulate the levels of all four isoforms of mature miR-181. As shown in Fig. 6C, although 1 μM morphine promoted miR-181a-5p maturation after 96 h, no significant effect was observed for other isoforms. Fentanyl, as expected, exhibited no significant effect on any isoform. Therefore, miR-181a is the only relevant isoform up-regulated by morphine.
We next examined the level of primary (pri-) and precursor (pre-) miR-181a at different time points after the treatment with 1 μM morphine, with or without 10 μM CTOP. As shown in Fig. 6D, morphine had no significant effect at any time point on pri- and pre-miR-181a level, either with or without CTOP treatment. These data indicate that morphine but not fentanyl was capable of promoting miR-181a maturation via OPRM1 in hNPCs, by regulating the processing rather than transcription of miR-181a.
miR-181a plays an essential role in morphine-induced astrocyte-preferential hNPC differentiation
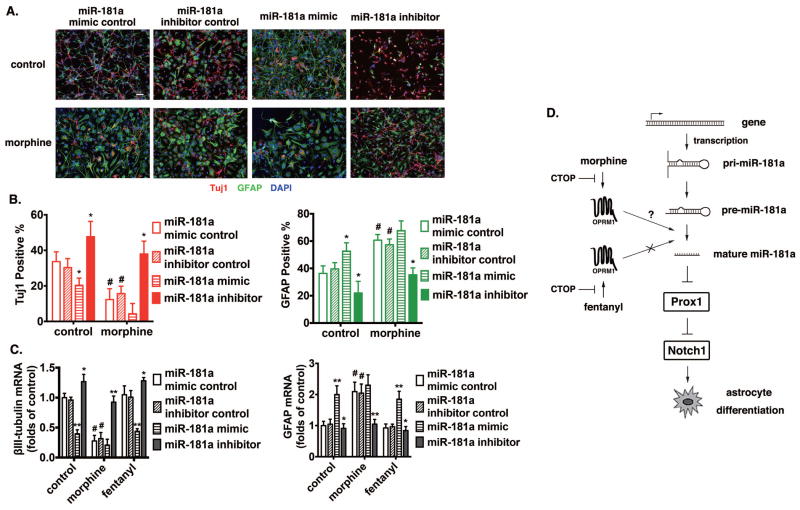
Because of miR-181a’s direct interaction with Prox1’s 3′UTR region, we hypothesize that morphine-dependent regulation of miR-181a level in hNPCs is crucial for morphine-induced astrocyte differentiation. In order to test our hypothesis, miR-181a mimic and inhibitor were transiently transfected into hNPCs and the cells were then treated with or without morphine for 4 days in full differentiation medium. Subsequently, the percentages of hNPCs differentiated into neurons, astrocytes and oligodendrocytes were determined with specific cell markers. As summarized in Fig. 7A and 7B, miR-181a mimic significantly increased the proportion of GFAP+ astrocytes to 52.7±2.9% from 36.2±2.6% observed in control. A parallel decrease was observed in Tuj1+ neurons from 33.8±2.5% to 20.1±1.9% in the miR-181a mimic-transfected cells. These observed miR-181a mimic responses resembled the effect of morphine on lineage-specific differentiation. In contrast, miR-181a inhibitor significantly decreased GFAP+ astrocytes and increased Tuj1+ neurons, regardless of morphine treatment. Thus, interrupting miR-181a activity can block the ability of morphine to induce astrocyte-preferential differentiation.
Figure 7. MiR-181a plays an essential role in morphine-induced astrocyte-preferential NPC differentiation.
(A) Mouse NPC primary cultures were transfected with control RNA, miR-181a mimic, or miR-181a inhibitor by using Lipofectamine 2000, with or without the treatment of 1 μM morphine for 4 d. Cells were stained with markers for neurons (Tuj1), astrocytes (GFAP) and with DAPI. Scale bar, 25 μm. Images are representative of at least three independent experiments with similar results.
(B) Quantification of cells stained with each marker, calculated as the percentage of the total number of cells stained with DAPI. Red: Tuj1; Green: GFAP. *, p<0.05 compared to cultures transfected with control RNA with the same treatment. #, p<0.05 compared to the control group transfected with the control RNA. Data are the mean ± SEM of at least three independent experiments.
(C) The expression of βIII-tubulin and GFAP were determined by real-time PCR after 4 d of differentiation. The results were normalized against those of GAPDH, and further normalized against the results obtained from cultures transfected with control RNA in each group. *, p<0.05; **, p<0.01 compared to cultures transfected with control RNA and treated with the same agonist. #, p<0.05 compared to the control group transfected with control RNA. Data represent the mean ± SEM of four independent experiments.
(D) Schematic representation of the miR-181a/Prox1/Notch regulation pathway modulated by OPRM1 activation induced by morphine but not fentanyl.
The overall effect of miR-181a mimic or inhibitor on morphine-induced astrocyte differentiation can be observed at the transcript level by qRT-PCR. As shown in Fig. 7C, miR-181a mimic significantly decreased the level of βIII-tubulin and increased that of GFAP in control and fentanyl-treated cells, to a level similar to that observed after morphine treatment. On the contrary, miR-181a inhibitor increased βIII-tubulin levels and decreased GFAP levels in all three groups. These results suggest an essential role of miR-181a in morphine-induced astrocyte-preferential NPC differentiation.
Discussion
Adult neurogenesis, especially lineage-preferential differentiation of neural stem and progenitor cells, is modulated by multiple external factors. However, its relation with GPCRs, such as the OPRM1, has not yet been extensively reported. In our current study, we demonstrate that morphine, rather than fentanyl, is capable of inducing astrocyte-preferential differentiation of hNPCs. The underlying mechanism was further elucidated to include miR-181a, a member of the important family of miRNAs, which regulate a variety of biological processes. Morphine, but not fentanyl, increases the miR-181a expression, which in turn suppresses the expression of one of its targets, Prox1, a known inhibitor of Notch1. The decrease in Prox1 expression thus gives rise to an increase in Notch1 expression. This ultimately results in the phenotype change of hNPCs that turns out to be astrocyte-preferential differentiation.
As indicated in our current study, morphine inhibits adult neurogenesis by modulating the lineage of hNPCs differentiation and decreasing the number of neurons. This conclusion is consistent with our previous studies showing that morphine attenuates the progenitor cell differentiation into mature neurons and subsequently the drug memory retention [12]. This morphine effect was a result of its incapability to activate NeuroD1 via the regulation of miR-190 [11]. Thus, the morphine-mediated attenuation of the NPC differentiation into mature neurons can proceed via dual mechanisms. One is through the decrease in the miR-190/NeuroD1 pathway activation, which is attributed to its inability to induce the phosphorylation and activation of Yin Yang 1, the nuclear transcription activator [32]. The other is via the activation of the miR-181a/Prox1/Notch1 pathway, which alters cell lineage to result in decreased numbers of neurons and increased astrocytes, as indicated in the current study. Since Notch1 is also a target of NeuroD1 [16], it is likely that crosstalk may occur between the two pathways. Therefore, it is probable that morphine-induced lineage-preferential hNPC differentiation is one of the targets by which morphine attenuates the differentiation into mature neurons, and hence adult neurogenesis and subsequent behavioral alterations related to hippocampal functions.
So far we have established that downstream targets of miR-181a, Prox1 and subsequently Notch1, play an important role in morphine-induced astrocyte-preferential differentiation. However, we have not yet identified the mechanism by which morphine modulates the expression of miR-181a. Since our current observations indicate that morphine treatment does not alter the primary and precursor miR-181a level in hNPCs (Fig. 6C), it is probable that morphine regulates miR-181a at the levels of processing, editing or functioning [34]. For example, the transactivation-responsive RNA-binding protein (TRBP), which is a partner of Dicer, is crucial for Dicer accumulation and therefore contributes to proper miRNA processing. It has been demonstrated that TRBP is stabilized through serine phosphorylation, catalyzed by extracellular regulated kinase (ERK)[35]. It was also observed that p38 kinase could mediate the Ser387 phosphorylation of Argonaute 2 (AGO2), thus resulting in increased recruitment of AGO2 to processing bodies [36]. The observed changes in miR-181a level during morphine treatment could be mediated via similar protein kinase-dependent control mechanisms.
Although both morphine and fentanyl are agonists of OPRM1, they behave differently in their regulation of miR-181a levels and subsequently fate determination of differentiated hNPCs. It is very likely that morphine and fentanyl generate diverse pathways downstream of the receptor but upstream of miR-181a. One possible mechanism is the observed pathway selectivity in morphine- and fentanyl-mediated ERK activation. It was demonstrated by earlier studies that morphine activates ERKs via the G-protein dependent pathway without inducing nuclear translocation of ERKs. However, fentanyl activates ERKs in a β-arrestin-dependent manner, and the phosphorylated ERKs translocate into the nucleus [30]. Moreover, the agonist-selective ERK activation results in agonist-selective regulation on miR-190 [11] through regulation on the transcription factor Yin Yang 1 [32]. Probably, the agonist-selective regulation of post-transcriptional processing of miR-181a is also a result of pathway-selective ERK activation by morphine and fentanyl. Further studies are needed to thoroughly elucidate the detailed mechanism.
Since miR-181a has a large variety of targets other than Prox1, its functions might not be limited to modulating the expression of Prox1 and Notch1. In our current study, it is clear that although Prox1 siRNA is more capable of down-regulating Prox1 level compared to morphine treatment, morphine seems to be more effective in inducing astrocyte differentiation (Fig. 3, B–D). Therefore, it is likely that some other agonist-selective functions that are related to miR-181a will be discovered in future investigations. For example, miR-181a has been reported to regulate the GluA2 subunit of AMPA receptors, which contributes to neurogenesis [37]. Similarly, the Notch signaling pathway impinges on a wide array of cellular processes including cell proliferation, differentiation and apoptosis [38]. It has been reported that Notch signaling can mediate the self-renewal of adult neural stem cells via modification of the cell cycle [39]. It was also found that Notch activation induces apoptosis in neural progenitor cells through a p53-dependent pathway [40]. Therefore, the morphine-induced regulation of miR-181a and Notch1 may modulate a variety of neural stem/progenitor cells functions. Our current studies and others suggest that multiple pathways in addition to morphine’s agonist-induced OPRM1 modulation of miRNAs result in the eventual decrease in the adult neurogenesis by morphine.
Conclusion
We demonstrate that morphine regulates adult neurogenesis by inducing astrocyte-preferential differentiation of hNPCs. This occurs via OPRM1 up-regulation of miR-181a at a post-transcriptional level. miR-181a targets Prox1 mRNA by directly interacting with its 3′UTR, thus inhibiting Prox1 expression. Since Prox1 directly interacts with the Notch1 promoter and suppresses Notch1 expression, decreased Prox1 expression induced by morphine results in increased Notch1 expression, and finally astrocyte-preferential differentiation of hNPCs.
Supplementary Material
Figure S1 hNPCs transfected with a GFP-expressing DNA construct showing the transfection efficiency of Lipofectamine 2000 in the current study.
Mouse hNPCs were transfected with a GFP-expressing DNA construct by using Lipofectamine 2000, and cultured in complete differentiation medium with 1 μM morphine for 4 d. Cells were stained with DAPI. Scale bar, 50 μm. The image is representative of at least three independent experiments with similar results.
Figure S2 Morphine and fentanyl promote NPC proliferation before but not after differentiation.
Mouse hNPCs were treated with 1 μM morphine or 10 nM fentanyl for indicated time periods. Medium for differentiation was added on day 4 to initiate differentiation. The numbers of viable cells were counted by the TC10 automated cell counter. Data represent four independent experiments. *, p<0.05; **, p<0.01 compared to control group of the same time point.
Figure S3 Morphine modulates in vivo mRNA levels of miR-181a, Prox1, Notch1, βIII-tubulin and GFAP.
Mice were treated by subcutaneous implantation of one morphine pellet (75 mg free base per mouse) or placebo pellets for 4 days. The hippocampi of treated mice were collected and the expression of miR-181a, Prox1, Notch1, βIII-tubulin and GFAP in hippocampus samples were determined by real-time PCR. The results were normalized against those of GAPDH, and further normalized against the result obtained from the placebo group. Data represent the mean ± SEM of three independent experiments. *, p<0.05 compared to placebo.
Acknowledgments
This work was supported by National Institutes of Health, National Institute of Drug Abuse (Grant DA031442-02).
We thank Dr. Panagiotis K. Politis (Center for Basic Research, Biomedical Research Foundation of the Academy of Athens, Athens, Greece) for the DNA constructs of Notch1-Luc, PRless-Luc and pcDNA3.0-Flag-mProx1. We thank Patrick W McGarrah and Brittany J Servant (Department of Pharmacology, University of Minnesota) for proofreading and editing the manuscript. Research is supported by NIDA Grant DA031442-02.
Footnotes
Author Contribution:
Chi Xu: Conception and design, Provision of study material, Collection and assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript
Yue Zhang: Provision of study material, Collection and assembly of data
Hui Zheng: Conception and design
Horace H. Loh: Conception and design
Ping-Yee Law: Conception and design, Financial support, Provision of study material, Data analysis and interpretation, Manuscript writing, Final approval of manuscript
References
- 1.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 2.Kempermann G, Jessberger S, Steiner B, et al. Milestones of neuronal development in the adult hippocampus. Trends in neurosciences. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiological reviews. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 4.Canales JJ. Adult neurogenesis and the memories of drug addiction. European archives of psychiatry and clinical neuroscience. 2007;257:261–270. doi: 10.1007/s00406-007-0730-6. [DOI] [PubMed] [Google Scholar]
- 5.Eisch AJ, Barrot M, Schad CA, et al. Opiates inhibit neurogenesis in the adult rat hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teuchert-Noodt G, Dawirs RR, Hildebrandt K. Adult treatment with methamphetamine transiently decreases dentate granule cell proliferation in the gerbil hippocampus. Journal of neural transmission. 2000;107:133–143. doi: 10.1007/s007020050012. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi M, Suzuki T, Seki T, et al. Repetitive cocaine administration decreases neurogenesis in adult rat hippocampus. Annals of the New York Academy of Sciences. 2004;1025:351–362. doi: 10.1196/annals.1316.043. [DOI] [PubMed] [Google Scholar]
- 8.Jang MH, Shin MC, Jung SB, et al. Alcohol and nicotine reduce cell proliferation and enhance apoptosis in dentate gyrus. Neuroreport. 2002;13:1509–1513. doi: 10.1097/00001756-200208270-00004. [DOI] [PubMed] [Google Scholar]
- 9.Rueda D, Navarro B, Martinez-Serrano A, et al. The endocannabinoid anandamide inhibits neuronal progenitor cell differentiation through attenuation of the Rap1/B-Raf/ERK pathway. The Journal of biological chemistry. 2002;277:46645–46650. doi: 10.1074/jbc.M206590200. [DOI] [PubMed] [Google Scholar]
- 10.Kamboj SK, Tookman A, Jones L, et al. The effects of immediate-release morphine on cognitive functioning in patients receiving chronic opioid therapy in palliative care. Pain. 2005;117:388–395. doi: 10.1016/j.pain.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 11.Zheng H, Zeng Y, Zhang X, et al. mu-Opioid receptor agonists differentially regulate the expression of miR-190 and NeuroD. Molecular pharmacology. 2010;77:102–109. doi: 10.1124/mol.109.060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng H, Zhang Y, Li W, et al. NeuroD modulates opioid agonist-selective regulation of adult neurogenesis and contextual memory extinction. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2013;38:770–777. doi: 10.1038/npp.2012.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho JH, Tsai MJ. The role of BETA2/NeuroD1 in the development of the nervous system. Molecular neurobiology. 2004;30:35–47. doi: 10.1385/MN:30:1:035. [DOI] [PubMed] [Google Scholar]
- 14.Hou YY, Lu B, Li M, et al. Involvement of actin rearrangements within the amygdala and the dorsal hippocampus in aversive memories of drug withdrawal in acute morphine-dependent rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:12244–12254. doi: 10.1523/JNEUROSCI.1970-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ables JL, Breunig JJ, Eisch AJ, et al. Not(ch) just development: Notch signalling in the adult brain. Nature reviews Neuroscience. 2011;12:269–283. doi: 10.1038/nrn3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seo S, Lim JW, Yellajoshyula D, et al. Neurogenin and NeuroD direct transcriptional targets and their regulatory enhancers. The EMBO journal. 2007;26:5093–5108. doi: 10.1038/sj.emboj.7601923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beatus P, Lundkvist J, Oberg C, et al. The origin of the ankyrin repeat region in Notch intracellular domains is critical for regulation of HES promoter activity. Mechanisms of development. 2001;104:3–20. doi: 10.1016/s0925-4773(01)00373-2. [DOI] [PubMed] [Google Scholar]
- 18.Tanigaki K, Nogaki F, Takahashi J, et al. Notch1 and Notch3 instructively restrict bFGF-responsive multipotent neural progenitor cells to an astroglial fate. Neuron. 2001;29:45–55. doi: 10.1016/s0896-6273(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 19.Ge W, Martinowich K, Wu X, et al. Notch signaling promotes astrogliogenesis via direct CSL-mediated glial gene activation. Journal of neuroscience research. 2002;69:848–860. doi: 10.1002/jnr.10364. [DOI] [PubMed] [Google Scholar]
- 20.Karalay O, Jessberger S. Translating niche-derived signals into neurogenesis: the function of Prox1 in the adult hippocampus. Cell cycle. 2011;10:2239–2240. doi: 10.4161/cc.10.14.15850. [DOI] [PubMed] [Google Scholar]
- 21.Kaltezioti V, Kouroupi G, Oikonomaki M, et al. Prox1 regulates the notch1-mediated inhibition of neurogenesis. PLoS biology. 2010;8:e1000565. doi: 10.1371/journal.pbio.1000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 23.Hwang CK, Wagley Y, Law PY, et al. MicroRNAs in opioid pharmacology. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2012;7:808–819. doi: 10.1007/s11481-011-9323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Q, Law PY, Wei LN, et al. Post-transcriptional regulation of mouse mu opioid receptor (MOR1) via its 3′ untranslated region: a role for microRNA23b. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2008;22:4085–4095. doi: 10.1096/fj.08-108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Q, Zhang L, Law PY, et al. Long-term morphine treatment decreases the association of mu-opioid receptor (MOR1) mRNA with polysomes through miRNA23b. Molecular pharmacology. 2009;75:744–750. doi: 10.1124/mol.108.053462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kazenwadel J, Michael MZ, Harvey NL. Prox1 expression is negatively regulated by miR-181 in endothelial cells. Blood. 2010;116:2395–2401. doi: 10.1182/blood-2009-12-256297. [DOI] [PubMed] [Google Scholar]
- 27.Lagos-Quintana M, Rauhut R, Meyer J, et al. New microRNAs from mouse and human. RNA. 2003;9:175–179. doi: 10.1261/rna.2146903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pacey LK, Stead S, Gleave JA, et al. Neural Stem Cell Culture: Neurosphere generation, microscopical analysis and cryopreservation. PROTOCOL EXCHANGE. 2006 doi: 10.1038/nprot.2006.215. [DOI] [Google Scholar]
- 29.Chojnacki A, Weiss S. Production of neurons, astrocytes and oligodendrocytes from mammalian CNS stem cells. Nature protocols. 2008;3:935–940. doi: 10.1038/nprot.2008.55. [DOI] [PubMed] [Google Scholar]
- 30.Zheng H, Loh HH, Law PY. Beta-arrestin-dependent mu-opioid receptor-activated extracellular signal-regulated kinases (ERKs) Translocate to Nucleus in Contrast to G protein-dependent ERK activation. Molecular pharmacology. 2008;73:178–190. doi: 10.1124/mol.107.039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng H, Chu J, Qiu Y, et al. Agonist-selective signaling is determined by the receptor location within the membrane domains. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9421–9426. doi: 10.1073/pnas.0802253105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng H, Chu J, Zeng Y, et al. Yin Yang 1 phosphorylation contributes to the differential effects of mu-opioid receptor agonists on microRNA-190 expression. The Journal of biological chemistry. 2010;285:21994–22002. doi: 10.1074/jbc.M110.112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kee N, Teixeira CM, Wang AH, et al. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nature neuroscience. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 34.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nature reviews Genetics. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 35.Paroo Z, Ye X, Chen S, et al. Phosphorylation of the human microRNA-generating complex mediates MAPK/Erk signaling. Cell. 2009;139:112–122. doi: 10.1016/j.cell.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng Y, Sankala H, Zhang X, et al. Phosphorylation of Argonaute 2 at serine-387 facilitates its localization to processing bodies. The Biochemical journal. 2008;413:429–436. doi: 10.1042/BJ20080599. [DOI] [PubMed] [Google Scholar]
- 37.Saba R, Storchel PH, Aksoy-Aksel A, et al. Dopamine-regulated microRNA MiR-181a controls GluA2 surface expression in hippocampal neurons. Molecular and cellular biology. 2012;32:619–632. doi: 10.1128/MCB.05896-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou ZD, Kumari U, Xiao ZC, et al. Notch as a molecular switch in neural stem cells. IUBMB life. 2010;62:618–623. doi: 10.1002/iub.362. [DOI] [PubMed] [Google Scholar]
- 39.Hitoshi S, Alexson T, Tropepe V, et al. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes & development. 2002;16:846–858. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang X, Klein R, Tian X, et al. Notch activation induces apoptosis in neural progenitor cells through a p53-dependent pathway. Developmental biology. 2004;269:81–94. doi: 10.1016/j.ydbio.2004.01.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 hNPCs transfected with a GFP-expressing DNA construct showing the transfection efficiency of Lipofectamine 2000 in the current study.
Mouse hNPCs were transfected with a GFP-expressing DNA construct by using Lipofectamine 2000, and cultured in complete differentiation medium with 1 μM morphine for 4 d. Cells were stained with DAPI. Scale bar, 50 μm. The image is representative of at least three independent experiments with similar results.
Figure S2 Morphine and fentanyl promote NPC proliferation before but not after differentiation.
Mouse hNPCs were treated with 1 μM morphine or 10 nM fentanyl for indicated time periods. Medium for differentiation was added on day 4 to initiate differentiation. The numbers of viable cells were counted by the TC10 automated cell counter. Data represent four independent experiments. *, p<0.05; **, p<0.01 compared to control group of the same time point.
Figure S3 Morphine modulates in vivo mRNA levels of miR-181a, Prox1, Notch1, βIII-tubulin and GFAP.
Mice were treated by subcutaneous implantation of one morphine pellet (75 mg free base per mouse) or placebo pellets for 4 days. The hippocampi of treated mice were collected and the expression of miR-181a, Prox1, Notch1, βIII-tubulin and GFAP in hippocampus samples were determined by real-time PCR. The results were normalized against those of GAPDH, and further normalized against the result obtained from the placebo group. Data represent the mean ± SEM of three independent experiments. *, p<0.05 compared to placebo.