Abstract
We examined the synaptic structure, quantity and distribution of AMPA- and NMDA-type glutamate receptors (AMPARs and NMDARs, respectively) in rat cochlear nuclei by a highly sensitive freeze-fracture replica labeling technique. Four excitatory synapses formed by two distinct inputs, auditory nerve (AN) and parallel fibers (PF), on different cell types were analyzed. These excitatory synapse types included AN synapses on bushy cells (AN-BC synapses) and fusiform cells (AN-FC synapses) and PF synapses on FC (PF-FC synapses) and cartwheel cell spines (PF-CwC synapses). Immunogold labeling revealed differences in synaptic structure as well as AMPAR and NMDAR number and/or density in both AN and PF synapses, indicating a target-dependent organization. The immunogold receptor labeling also identified differences in the synaptic organization of FCs based on AN or PF connections, indicating an input-dependent organization in FCs. Among the four excitatory synapse types, the AN-BC synapses were the smallest and had the most densely packed IMPs, whereas the PF-CwC synapses were the largest and had sparsely-packed IMPs. All four synapse types showed positive correlations between the IMP-cluster area and the AMPAR number, indicating a common intra-synapse-type relationship for glutamatergic synapses. Immunogold particles for AMPARs were distributed over the entire area of individual AN synapses, PF synapses often showed synaptic areas devoid of labeling. The gold-labeling for NMDARs occurred in a mosaic fashion, with less positive correlations between the IMP-cluster area and the NMDAR number. Our observations reveal target- and input-dependent features in the structure, number, and organization of AMPARs and NMDARs in AN and PF synapses.
Keywords: SDS-freeze fracture immunolabeling, electron microscopy, GluN1, ventral cochlear nucleus, dorsal cochlear nucleus, synapses, RRID:AB_94946, RRID: nif-0000-30467
Introduction
Synapse size varies between synaptic contacts and often correlates with fundamental properties of synaptic transmission (Rollenhagen and Lübke, 2006; Xu-Friedman and Regehr, 2004). Synapse size has been shown to be proportional to the number of ionotropic glutamate receptors (iGluRs), whose content and spatial arrangements in the synapse primarily determine synaptic quantal size in many glutamatergic synapses in the CNS (Franks et al., 2003; Ganeshina et al., 2004; Matsuzaki et al., 2001; Takumi et al., 1999; Tarusawa et al., 2009). However, these anatomical, molecular, and functional parameters, as well as their correlation to the electrophysiological properties of synaptic transmission in glutamatergic synapses in the binaural and monaural auditory pathways, remain unknown. This information is important for understanding the mechanisms of both normal and impaired auditory processing.
The auditory nerve (AN) transmits all auditory information from the cochlea to the brain. In the cochlear nucleus (CN), AN fibers bifurcate to innervate multiple cell populations, including bushy cells (BCs) in the ventral CN (VCN) and fusiform cells (FCs) in the dorsal CN (DCN) (Rhode et al., 1983a,b; Lorente de No, 1981; Fig. 1). These two cell types differ significantly in their ability to encode temporal properties of sound stimuli. BCs project to binaural circuits in the superior olivary complex and encode spectral and temporal characteristics that allow sounds to be localized in the horizontal plane (Irvine, 1987). FCs project to monaural circuits in the inferior colliculus and encode spectral cues for localizing sounds in the vertical plane (Kanold and Young, 2001). While the main glutamatergic inputs on BC somata are from AN fibers, FCs also receive glutamatergic inputs from granule cells via the parallel fibers (PFs) (Wouterlood and Mugnaini, 1984). PF synapses deliver somatosensory information through FC apical dendrites and cartwheel cell (CwC) spines within the molecular layer of the DCN (Davis and Young, 1997). Some auditory neurons maintain precise temporal representations through their synaptic networks (Isaacson and Walmsley, 1996; Gardner et al., 1999). In contrast, multimodal inputs to the spiny dendrites of FCs and CwCs through PF synapses are subject to long-term potentiation and long-term depression (Fujino and Oertel, 2003). We hypothesize that there is an anatomical substrate and distinct iGluR organization at these CN synapses that correlates with their specific electrophysiological properties.
Figure 1. Cochlear nucleus circuit and glutamatergic synapses in the FRIL replica.
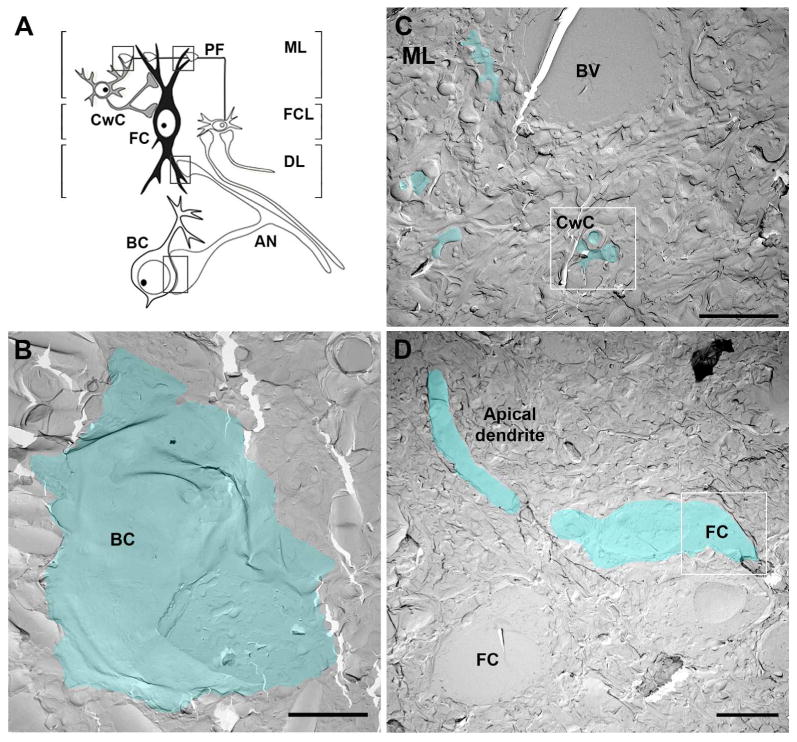
A. Schematic of the AVCN and DCN circuit and synapses analyzed with FRIL (boxed areas). B-D. Electromicrographs show low magnifications of AVCN and DCN replicas. E-face membranes of one bushy cell: BC in AVCN (B), some dendrites and spines of CwCs in the molecular layer (C) and one fusiform cell: FC with the apical dendrite and proximal basal dendrite (D) are pseudo-colored in blue to aid visualization. Boxed white areas in C and D are shown at higher magnification in figures 3C and 2B, respectively. Scales bar: B and C: 5 μm, D: 10 μm. BV: blood vessel; CwC: cartwheel cells; DL: deep layer; FCL: fusiform cell layer; ML: molecular layer; PF: parallel fibers.
AMPARs and NMDARs mediate excitatory transmission at AN and PF synapses in the cochlear nucleus (Manis and Molitor, 1996; Raman et al., 1994). Previously, the expression of AMPARs and NMDARs at AN and PF synapses has been analyzed with pre-embedding immunolabeling or postembedding immunogold labeling methods (Petralia et al., 1996; Rubio and Wenthold, 1997, Rubio, 2006; Wang et al., 1998). However, neither of these techniques provides faithful demonstration of iGluR numbers and synaptic structures, due to the sectioning of synapses as well as low iGluR detection sensitivity. In this study, we used freeze-fracture replica immunogold labeling (FRIL) to reveal morphological and molecular characteristics of AN and PF synapses that could predict properties of synaptic transmission at individual synapses. FRIL allows for the highly sensitive and quantitative detection of molecules of interest in the synapse (nearly 100% detection sensitivity to functional AMPARs) and provides a two dimensional (2D) landscape of the plasma membrane and receptor arrangement (Masugi-Tokita et al., 2007; Tarusawa et al., 2009). We observed substantial differences in the synaptic intra-membrane particle (IMP) cluster size and AMPAR and NMDAR content between the AN and PF synapses on their postsynaptic targets. Our results suggest that synapse size, AMPAR and NMDAR numbers, and the 2D-distribution of these receptors are regulated in an input-target specific manner in the CN.
Material and Methods
Animals used for morphological analysis
For this study, male Sprague Dawley rats (n=3) at postnatal day 30 were used. Rats were raised on a 12 h light/dark cycle with water and food ad libitum. All animal experiments were conducted in accordance with the guidelines of the National Institute for Physiological Science's Animal Care and Use Committee.
FRIL
Rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and perfused transcardially with 25 mM phosphate buffer saline (PBS) for 1 min, followed by perfusion with 2% paraformaldehyde (PFA) and 15% saturated picric acid solution in 0.1 M phosphate buffer (PB) for 12 min. Coronal slices (150 μm thick) were cut using a vibrating microslicer (DTK-1000; Dosaka EM) in 25 mM PBS. The rostral anteroventral and dorsal cochlear nucleus (AVCN and DCN, respectively) were trimmed from the slice. The trimmed slices were immersed in 30% glycerol/25 mM PBS at 4°C overnight and rapidly frozen by a high pressure freezing machine (HPM010; BAL-TEC, Balzers). Frozen samples were then fractured into two parts at −120°C and replicated by deposition of carbon (5 nm thick), platinum (uni-direction from 60°, 2 nm), and carbon (20 nm) in a freeze-fracture replica machine (BAF 060; BAL-TEC). After thawing, tissue debris attached to the replicas was dissolved with gentle rocking at 80°C for 18 h in a solution containing 15 mM Tris-HCl (pH8.3), 20% sucrose, and 2.5% SDS. The replicas were then washed in 50 mM Tris-buffered saline (TBS) (pH7.4) containing 0.05% bovine serum albumin (BSA) and blocked with 5% BSA in the washing buffer for 1 h at room temperature (∼20°C). The replicas were incubated with a rabbit primary antibody against GluA1–4 (panAMPAR; Nusser et al., 1998) or mouse primary antibody against GluN1 NMDAR subunit (MAB363, Millipore) (see below for the antibodies characterization) overnight at 15°C, followed by incubation with anti-rabbit [British Biocell International (BBI)] or anti-mouse (GE Healthcare) secondary antibodies conjugated with 5 nm gold particles for 1 h at 37°C. To label AN and PF endings, the replicas were double gold labeled with an anti-guinea pig antibody against vGluT1, which is a marker for both types of glutamatergic endings (Rubio et al., 2008; Gómez-Nieto and Rubio, 2009) (see below for the antibody characterization). The VgluT1 antibody was labeled with an anti-guinea pig secondary antibody conjugated with 10 nm gold particles (BBI). Although vGluTs are vesicular proteins, they are often detected on presynaptic plasma membrane and, thus, have been used to identify the origin of presynaptic profiles (Hagiwara et al., 2005; Masugi-Tokita et al., 2007). The reliability of AMPAR localization by FRIL under our fixing condition has been discussed previously (Tarusawa et al., 2009).
Antibody characterization
Please see Table 1 for a list of all primary antibodies used in the present study. A rabbit polyclonal antibody against GluA1–4 (panAMPAR), a mouse monoclonal antibody against GluN1 NMDAR subunit (MAB363, Millipore) and a guinea pig antisera against BNPI (vGluT1) were used.
Table 1. Antibodies used in this study.
| Antigen | Description of Immunogen | Source, Host species, Cat.#, Clon or Lot, RRID | Concentration or dilution |
|---|---|---|---|
| GluA1-4 (panAMPAR) | Recombinant proteins corresponding to aa 724-781 of rat GluA1 flop | Raised in Dr. Elek Molnár's laboratory (Nusser et al., 1998). Rabbit, Affinity purified polyclonal antibody | 3 μg/ml |
| GluN1 | Recombinant protein corresponding to aa 660-811 of mouse GluN1 | Millipore, Mouse, MAB363 Clone 54.1, RRID: AB_94946 | 4 μg/ml |
| BNPI/vGluT1 | Recombinant protein corresponding to aa 493-560 of mouse BNPI | Raised in Dr. Ryuichi Shigemoto's laboratory, Guinea pig antibody | 1:100 |
The rabbit anti-AMPAR antibody (anti-GluA1-4 or anti-panAMPAR) was raised against a glutathione S-transferase (GST) fusion protein containing the extracellular 58 amino acid residues (724–781, Table 1) preceding the last membrane-spanning segment of GluR1flop (GST-GluA1flop(724-781)). The preparation, purification and full characterization of this antibody is described in previous publications (Nusser et al., 1998; Pickard et al., 2000). The antisera were pre-adsorbed with the un-fused GST protein and subsequently affinity purified with the GST-GluA1flop(724-781) fusion protein (Pickard et al., 2000). The affinity-purified rabbit polyclonal antibody detected the GST-GluA1flop(724-781) fusion protein on immunoblots, whereas no cross-reactivity was observed to GST (Pickard et al., 2000). In immunoblots of rat brain membranes, this antibody specifically recognized a single band with an approximate size of 110 kDa, corresponding to the molecular weight of AMPAR subunit proteins taking into account subunit glycosylation (Pickard et al., 2000). COS-7 cells expressing individual subunits showed that the antibody raised against the conserved extracellular amino acid residues 724-781 of GluA1flop recognized all AMPAR subunits (GluA1-4 flip and flop), with no cross-reactivity with the closely related kainate receptor subunits (Pickard et al., 2000). All immunoreactivity was blocked by pre-adsorbing the antibody with 100 μg/ml of GST-GluA1flop(724-781), and no specific staining was detected by replacing the antibody with the pre-immune serum (Pickard et al., 2000). The selectivity of the anti-GluA1-4 antibody was further investigated using a guinea pig polyclonal anti-GluA1-4 antibody raised against GST-GluA1flop(724-781) (Pickard et al., 2001). Both rabbit and guinea pig anti-GluA1-4 antibodies produced the same staining patterns in rat brain samples (Pickard et al., 2001) and cultured hippocampal neurons (Pickard et al., 2001; Noel et al., 1999). Furthermore, both antibodies immunoprecipitated the same 110 kDa proteins from solubilized rat brain membrane fractions, which were identified on immunoblots as AMPAR subunits by a panel of GluA1-4, GluA1, GluA2 and GluA3 protein selective antibodies (Moult et al., 2006; Gladding et al., 2009). The obtained FRIL patterns obtained with the rabbit anti-GluA1-4 antibody were entirely consistent with our previous reports (Tanaka et al., 2005; Masugi-Tokita et al., 2007; Antal et al., 2008; Tarusawa et al., 2009; Wang et al., 2014). In FRIL, no labeling was found in parallel fiber Purkinje cell synapses of GluA2/3 null mice (Masugi-Tokita and Shigemoto, 2007). One to one relationship between the density of functional AMPAR channels in climbing fiber-Purkinje cell synapses estimated by an electrophysiological approach and the average labeling density obtained by this panAMPAR in the FRIL was confirmed (Tanaka et al., 2006). Moreover, selective immunosignal in the postsynaptic membrane specialization of various synaptic connections have been repeatedly observed (rat spinal cord in Antal et al., 2008, rat lateral geniculate nucleus in Tarusawa et al., 2009, mouse amygdala in Dong et al., 2010). These observations strongly suggest specific labeling of this antibody to all four subunit of AMPARs.
The specificity of the GluN1 antibody was confirmed by absence of labeling in replicas obtained from forebrain-specific GluN1 KO mice (Iwasato et al., 2000; Szabadits et al., 2011). The guinea pig antiserum against brain-specific Na+- dependent inorganic phosphate glutamate transporter 1 (BNPI/vGluT1) was raised against a GST fusion protein containing a C-terminal sequence (amino acid residues 493–560) of a brain-specific Na+-dependent inorganic phosphate transporter (BNPI) or vesicular glutamate transporter 1 (vGluT1) as described in Kulik et al (2002). The anti-BNPI/vGluT1 antibody showed a single ∼60-kDa immunoreactive product in rat whole brain in immunoblot analyses and immunohistochemical staining pattern in the rat brain obtained by the antiserum was identical to that previously described by Bellocchio et al. (1998) (Kulik et al., 2002).
Quantification of immunoparticles
Images of excitatory postsynaptic specializations, indicated by the presence of intramembrane particle clusters (IMP-clusters) on the exoplasmic face (E-face) (Sandri et al., 1972; Harris and Landis, 1986) and accompanied by presynaptic protoplasmic face (P-face) labeling by vGluT1 immunoparticles, were captured at a magnification of 93,000× with a digital camera [MegaView III; Soft Imaging System (SIS) or Orius 830W, Gatan]. IMP-clusters were defined as densely packed IMPs at a distance of <15 nm from each other. The IMP-clusters were demarcated freehand, and the areas of individual IMP-clusters were measured using ImageJ (NIH; RRID: nif-0000-30467). Immunoparticles within demarcated IMP-clusters and those located outside but within 30 nm from the edge of the IMP-clusters were regarded as synaptic labeling, considering possible deviation of the immunoparticles from antigens (Matsubara et al., 1996). The total number and density of immunoparticles for GluA1-4 or GluN1 in each IMP-cluster was compared with data obtained only from complete IMP-clusters. The density of the immunoparticles for GluA1-4 in each IMP-cluster was calculated by dividing the number of the immunoparticles by the area of the IMP-cluster.
Identification of auditory nerve (AN) and parallel fiber (PF) synapses on the replica
Identification of AN-BC synapses on replicas of the AVCN
Only the most rostral sections of the anteroventral cochlear nucleus (AVCN) were used because this area is enriched with BCs. The auditory nerve is the main glutamatergic ending on cell bodies and dendrites of BCs (Gómez-Nieto and Rubio, 2009, 2011; Ryugo and May, 1993). Membranes of BC dendrites were rare in the AVCN replicas. In this study we analyzed the IMP-clusters of the AN synapses on the E-face membranes of BC somata (Fig. 1B). Identification of the IMP-clusters on the E-face membrane of the BC somata was performed as described previously (Gulley et al., 1997).
Identification of AN-FC and PF-FC synapses on replicas of the DCN
The DCN is a layered nucleus that it is divided into a molecular or superficial layer (ML or layer I), a fusiform cell layer (FCL or layer II), and a deep layer (DL or layers III-IV) (Fig. 1). The procedure to identify FCs and their basal and apical dendrites was similar to that used previously (Rubio and Wenthold, 1997; Rubio and Juiz, 2004). The cell bodies of the FCs are located in the FCL of the DCN and extend their apical and basal dendritic arborizations towards the ML and DL, respectively.
AN-FC synapses
The AN fibers are the primary glutamatergic input within the FCL and DL that contact the FCs (Kane, 1974; Smith and Rhode, 1985; Ryugo and May, 1993; Rubio and Wenthold,1997; Rubio and Juiz, 2004). AN inputs make multiple synaptic contacts on the basal pole of the cell body and on basal dendrites of FCs (Smith and Rhode, 1985; Zhang and Oertel, 1994; Rubio and Wenthold, 1997). The analysis of IMP-clusters was performed on the basal pole of the cell body of identified FCs and on the proximal basal dendrites that were observed extending from the cell body (Fig. 1D).
PF-FC synapses on apical dendrites
Excitatory synaptic inputs from PFs are the predominant synaptic population on apical dendrites (dendritic spines and shafts) of FCs and are the only excitatory input to these dendrites (Smith and Rhode, 1985; Oertel and Wu, 1989; Manis, 1989; Osen et al., 1995; Rubio and Wenthold, 1997, 1999; Rubio and Juiz, 2004). Apical FC dendrites were identified by their location in the FCL-ML of the DCN. In this study, we analyzed only the dendritic membranes (∼2-3 μm in diameter) that were perpendicular to the longitudinal axis of the nucleus. These membranes usually emerged from elongated cell bodies of identified FCs located in the FCL (Fig. 1D). Apical dendrites of FCs also contain spines, although smaller in size (unpublished observations). However, in our analysis, we did not observe membranes of spines extending from or near FC apical dendrites. Thus, it is possible that the IMP-clusters of the PF-FC synapses on FC spines are rarely represented in FRIL.
PF-CwC synapses
PFs on cartwheel cells (CwC) in the DCN replica were identified by their location in the nucleus (Wouterlood and Mugnaini, 1984, Berrebi and Mugnaini, 1991; Rubio and Juiz, 2004). PFs are the sole glutamatergic inputs to CwCs, which are the main inhibitory interneuron in the molecular layer of the DCN (Wouterlood and Mugnaini, 1984). PFs primarily make synaptic contact on the dendritic spines of CwCs (Wouterlood and Mugnaini, 1984; Rubio and Juiz, 2004). CwC somata were located between the FCL and ML and extended their robust dendritic arborization towards the surface of the DCN. Dendritic spines of CwC are abundant in the molecular layer and are easily recognized because of their large size. On the replica, the E-face membranes of the analyzed spines were located in the most apical 100 μm of the DCN (this measurement was estimated based on grid bar distance) (Fig. 1C).
Measurement of postsynaptic membrane specialization width from ultrathin sections
Rats were anesthetized with a ketamine/zylaxine mix and perfused transcardially with 2% paraformaldehyde and 2.5% glutaraldehyde in 0.15 M cacodylate buffer (pH 7.4) with 2 mM calcium chloride at room temperature (∼20°C). Brains were then removed and post-fixed two hours in the same fixative solution at 4°C. Coronal brainstem slices (80 μm thickness) were vibratome-sectioned in an ice-cold solution (0.15 M cacodylate buffer and 2 mM calcium chloride). Sections were washed with the same ice-cold solution and then incubated in a solution containing 3% potassium ferrocyanide in 0.3 M cacodylate buffer with 4 mM calcium chloride and 4% aqueous osmium tetroxide. Tissues were washed with ddH2O and incubated in a filtered thiocarbohydrazide solution. Sections were then washed with ddH2O and placed in 2% osmium tetroxide in ddH20. Sections were washed again with ddH20 and then placed overnight in 1% uranyl acetate. Sections were stained en bloc using the Walton's lead aspartate solution and washed with ddH2O. Sections were dehydrated in a series of ethanol (50%, 70%, 85%, 95%, and 100%), infiltrated with epoxy resin (EMbed-812; Electron Microscopy Science; Redding, CA), embedded between acetate sheets, and polymerized at 60°C for 48 hours. Serial ultrathin sections were prepared at a thickness of 70 nm (Ultracut S; Leica). AN-BC, AN-FC, PF-FC and PF-CwC synapses were identified by their morphological features as previously described (Rubio and Wenthold, 1997; Rubio and Juiz, 2004; Gómez-Nieto and Rubio, 2009). Serial images of identified synapses were captured from the beginning to the end of each synapse at a magnification of 30,000× with the digital camera. The edge of postsynaptic density (PSD) was defined either by the thickening of the postsynaptic membrane or by the visible synaptic cleft, in addition to the rigid alignment of the presynaptic and postsynaptic membranes. The width of the PSD in each section was measured using ImageJ (http://rsbweb.nih.gov) software. The maximum PSD width in each synapse was used for analysis.
Data analysis
All measurement values are reported as mean ± SEM unless otherwise noted. Statistical analyses were conducted using Prism 6 (GraphPad Software, Inc.), and the level for statistical significance was set at 0.05. The normality of the data was assessed by applying Shapiro-Wilk's W-test. Statistical evaluation of immunogold densities was performed using the Mann-Whitney U-test or Kruskal Wallis test where appropriate. Statistical evaluation of the maximum PSD and IMP-cluster lengths was performed using the Mann-Whitney U-test. For multiple group comparison of data sets, Steel-Dwass tests were employed. Correlations were examined using Pearson's correlation test or Spearman's rank order test.
Results
Identification of AN synapses on bushy and fusiform cells and PF synapses on fusiform and cartwheel cells by their location and morphological characteristics and by labeling for vGluT1 in freeze-fracture replicas prepared from rat cochlear nucleus
In this study, we only included rostral regions of AVCN and DCN samples in which the three main layers could be identified (Fig. 1). Rostral AVCN regions are enriched with BCs, and the cell bodies of BCs were often observed fractured through the cytoplasm (cross-fracture), although the plasma membranes (E-face, P-face) of BC somata were also observed. In general, dendritic profiles were rarely seen in the AVCN replicas. To avoid the inclusion of membranes of stellate cells that receive AN input on their thin and large dendrites in the AVCN (Cao and Oertel, 2010), we only collected large E-faces of putative BC somata that receive AN inputs for our analysis (Figs. 1B; 2A). In the DCN replicas, the three main layers were clearly distinguishable (Fig. 1). The cell bodies of FCs were easily identified within the FCL and were often observed in cross-fracture. But contrary to the AVCN, basal and apical dendritic membranes were clearly seen either in E-face or P-face, extending from the FC somata (Figs. 1D; 2B; 3A). Another major input within the FCL is from the mossy fibers that make synaptic contacts exclusively on very thin dendrites of the granule cells (MF-GC synapses) within the FCL but not on FCs or CwCs (Weinberg and Rustioni 1989; Wright, Ryugo, 1996; Rubio and Juiz, 1998). Thus, AN-FC synapses can be investigated selectively by observation of synapses on large somatic or dendritic plasma membranes in the FCL without contamination of MF-GC synapses. All of the IMP-clusters within the FCL included in the study belonged to identified proximal basal dendrites of FCs (see Materials and Methods).
Figure 2. Auditory nerve synapses in the FRIL replica.
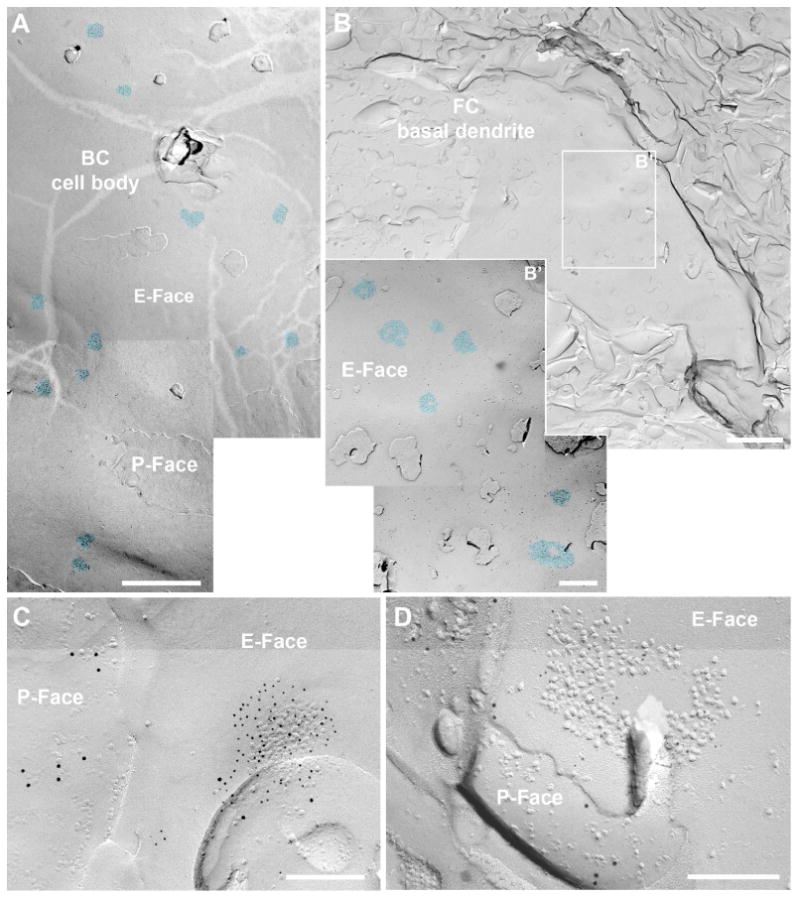
A-B. FRIL electromicrographs at low magnification showing the IMP-cluster distribution of auditory nerve synapses (AN) on bushy cell soma (A) and on basal dendrites of fusiform cells (B). B' shows higher magnification of the boxed area in B. The IMP-clusters are pseudocolored in blue to aid visualization. C-D. Images of PSDs of AN synapses on the cell body of a BC (C) and on a basal dendrite of a FC (D) immunolabeled with N-terminus antibodies for pan-AMPAR (GluA1-4) antibody (5 nm gold; C) or GluN1 (5 nm gold; D), indicating the presence of IMP-clusters on the E-face, accompanied by a presynaptic protoplasmic face (P-face) that was labeled by vGluT1 immunoparticles (vesicular glutamate transporter 1; 10 nm gold) as a marker for AN endings. Scale bars: A: 1 μm, B: 2 μm, B': 500 nm, C-D: 200 nm.
Figure 3. Parallel fiber synapses in the FRIL replica.
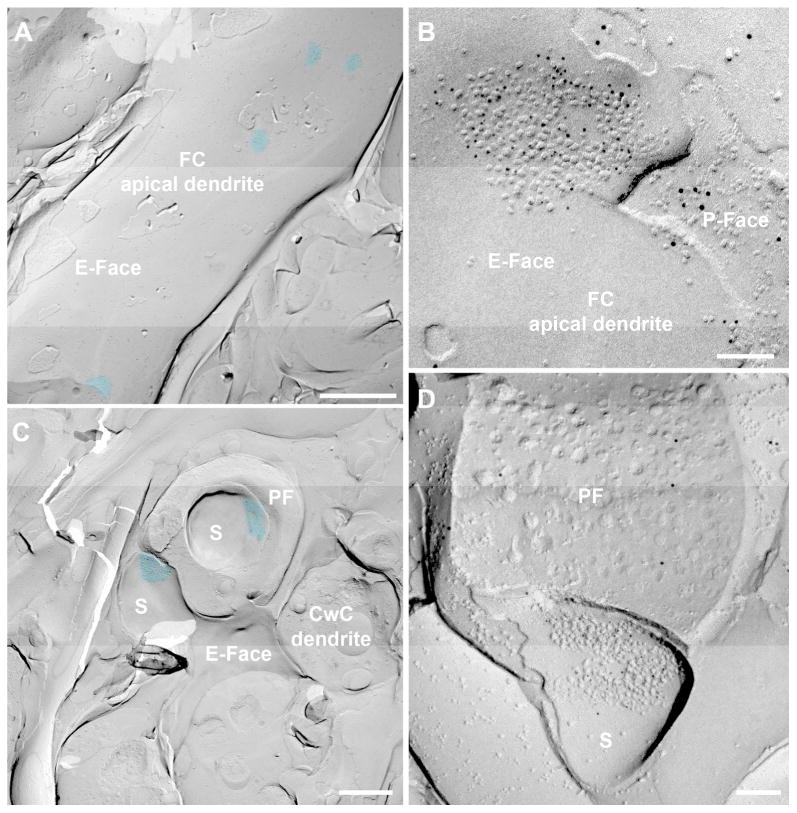
A and C. Low mag to show the IMP-cluster distribution of the parallel fiber (PF) synapses on an apical dendrite of an FC (A) and on CWC spines (C). The IMP-clusters are pseudocolored in blue to aid visualization. B and D. Images of PSDs of PF synapses on an apical dendrite of an FC and on a CwC spine immunolabeled with a N-terminus pan-AMPAR (GluA1-4) antibody (5 nm gold), indicating the presence of IMP-clusters on the E-face, accompanied by a presynaptic protoplasmic face (P-face) that was labeled by vGluT1 immunoparticles (10 nm gold) as a marker for PF endings. The PF ending in (D) is partially fractured. S: spine. Scale bars A: 1 μm; B and D: 100 nm; C: 500 nm.
In the ML, cell bodies and dendrites of CwCs were observed (Fig. 1C), and numerous membrane expansions that resembled dendritic spines were seen in either E-face or P-face (Figs. 3C,D). These cells receive inputs exclusively from PFs (PF-CwC synapses, Wouterlood and Mugniani, 1984; Rubio and Juiz, 2004).
The postsynaptic membrane specialization of glutamatergic synapses in a FRIL is indicated by a cluster of IMPs on the E-face of the plasma membrane (Sandri et al., 1972; Gulley et al., 1977; Harris and Landis, 1986), and it is often accompanied by the P-face of its presynaptic plasma membrane (Tarusawa et al., 2009). The replicas were immunolabeled with specific antibodies for a common extracellular region of all AMPAR subunits (GluA1-4) or GluN1. In the rostral AVCN replicas, such IMP-clusters were observed in the putative cell bodies of the BCs, and they were always immunopositive for the anti-GluA1-4 antibody, confirming that these IMP-clusters represent the postsynaptic specialization of glutamatergic synapses (Figs. 2A,C, 5A; Table 3). On the other hand, GluN1 labeling was only observed in 41% of the IMP-clusters in the AVCN (n = 58 AN-BC synapses, Table 3). In the DCN, the IMP-clusters were observed in putative dendritic shafts of FCs and CwC spines and were always positive for GluA1-4 or GluN1 (Figs. 2B-D, 3, 5; Table 3).
Figure 5. Differential distribution of AMPARs and NMDARs in IMP-clusters of AN and PF synapses.
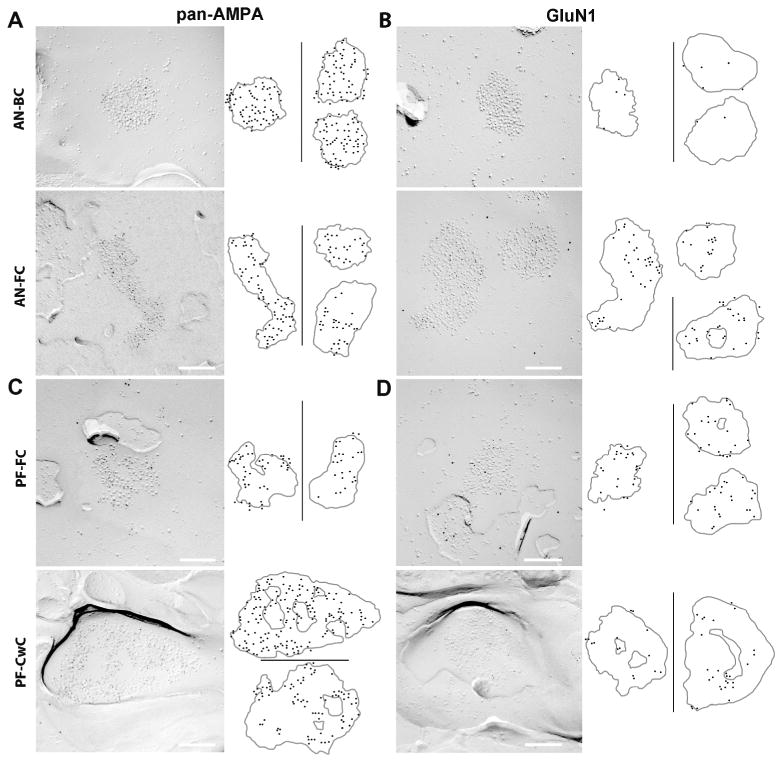
A-B. FRIL images of IMP-clusters for auditory nerve-bushy cell (AN-BC) and auditory nerve-fusiform cell (AN-FC) synapses that were gold labeled (5 nm gold particles) for pan-AMPAR (GluA1-4) (A) or GluN1 (B). The cartoons on the right show the distribution of the gold particles within several IMP-clusters. The original size (5 nm) of the gold particles has been enlarged to aid visualization. Scale bars: 200 nm.
C-D. FRIL images of IMP-clusters for parallel fiber-fusiform cell (PF-FC) and parallel fiber-cartwheel cell (PF-CwC) synapses that were gold labeled (5 nm gold particles) for pan-AMPAR (GluA1-4) and GluN1. The cartoons on the right show the distribution of the gold particles within the IMP-clusters. Scale bars: 200nm.
Table 3.
The total number of gold particles for GluA1-4 (panAMPAR) and GluN1 at AN and PF synapses, together with the total number of IMP-clusters.
| AVCN | DCN | |||
|---|---|---|---|---|
|
| ||||
| AN-BC | AN-FC | PF-FC | PF-CwC | |
| GluA1-4 | ||||
| Total gold | 3551 | 2564 | 1374 | 3969 |
| IMP cluster N (positive/total) | 90/90 | 62/62 | 46/46 | 52/52 |
| GluN1 | ||||
| Total gold | 52 | 1642 | 815 | 690 |
| IMP clusters N (positive/total) | 24/58 | 60/60 | 50/50 | 61/61 |
To confirm the identity of AN and PF synapses on the replica, we labeled for vGluT1. It has been reported that AN endings in the AVCN and within the FCL-DL of the DCN, along with the PF endings within the ML of the DCN, express vGluT1 (Gomez-Nieto and Rubio, 2009; Rubio et al., 2008). In contrast, somatosensory endings within the AVCN and DCN express vGluT2 but not vGluT1 (Zhou et al., 2007; Zheng et al., 2011). In agreement with these molecular features of presynaptic plasma membranes, the E-faces of replicated membranes with IMP-clusters were often accompanied by P-faces of membranes labeled with vGluT1 both in the AVCN (Fig. 2C) and DCN (Figs. 2D, 3B,D, 5C-D).
AN-BC and AN-FC synapses were often found as multiple IMP-clusters on large postsynaptic E-face membranes (Fig. 2). The minimum distance between the peripheral edges of two of these IMP-clusters was ∼0.2 μm. In contrast, PF-FC and PF-CwC synapses were observed as single IMP-clusters on small postsynaptic E-face membranes (Fig. 3). These findings are in agreement with the notion that a single AN fiber makes multiple synaptic contacts on a single postsynaptic cell (O'Neil et al., 2011; Rubio and Wenthold, 1997).
The size and shape of glutamatergic postsynaptic membrane specializations vary between synaptic connections in the CN
We studied the synaptic morphology of glutamatergic postsynaptic membrane specializations using FRIL replicas of the rostral AVCN and DCN. To compare the size of the synapses formed by the AN on BCs and FCs and those of PFs on FCs and CwCs, we measured the area of IMP-clusters that were replicated in their entirety. We found that putative PSDs of AN and PF synapses had qualitatively different morphologies in the arrangement of IMPs (Figs. 2, 3, 5). The size of the four connections was statistically significantly different from each other (Fig. 4, Table 2, p < 0.05, Steel-Dwass test). We observed round shaped IMP-clusters with densely packed IMPs in AN-BC synapses in the rostral AVCN. Within the DCN, more elliptical shaped IMP-clusters with a much more sparse IMP distribution were found frequently in AN-FC, PF-FC, and PF-CwC synapses. It is notable that PF-CwC synapses frequently showed IMP-clusters in complex shapes and contained an area devoid of IMPs within the IMP-clusters (Figs. 3D, 5C-D).
Figure 4. Mean synaptic size in AN and PF synapses.
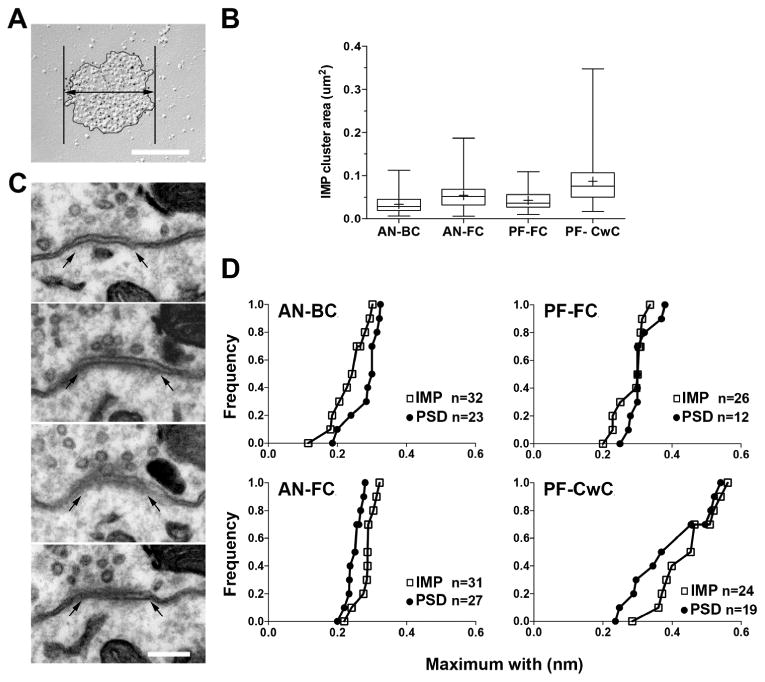
A. FRIL image of the IMP-clusters of AN-BC synapses. The thick line with a double arrow indicates the maximum width of an IMP-cluster. B. Whisker-box histogram showing the distribution of the IMP-cluster area for AN and PF synapses. Horizontal bars show the median, boxes indicate the 25-75 percentiles, and whiskers indicate the minimum and maximum. Crosses indicate the mean. C. Serial ultrathin sections of an AN-BC synapse. Arrows indicate the edge of the PSD. D. Cumulative frequency plots of the maximum widths of the PSDs and IMP-clusters. The maximum widths of the PSD and IMP-clusters were not significantly different (AN-BC: p = 0.70, AN-FC: p = 0.33, PF-FC p = 0.06, PF-CwC p = 0.30, Mann–Whitney U test). Scale bar in A and C: 200 nm.
Table 2.
IMP-cluster areas analyzed for AN-BC in the AVCN and AN-FC, PF-FC and PF-CwC in DCN.
| AVCN | DCN | |||
|---|---|---|---|---|
|
| ||||
| AN-BC | AN-FC | PF-FC dendrites | PF-CwC spines | |
| Mean | 0.033*** | 0.054*** | 0.043*** | 0.087*** |
| SEM | 0.001 | 0.002 | 0.002 | 0.004 |
| Median | 0.027 | 0.052 | 0.037 | 0.076 |
| Kurtosis | 0.79 | 3.05 | 0.26 | 3.52 |
| Skewness | 1.11 | 1.32 | 0.85 | 1.57 |
| Minimum | 0.006 | 0.006 | 0.01 | 0.017 |
| Maximum | 0.112 | 0.187 | 0.109 | 0.347 |
| CV | 0.59 | 0.53 | 0.59 | 0.61 |
| Count | 207 | 183 | 130 | 172 |
Statistically different between AN-BC vs AN-FC synapses and PF-FC vs PF-CwC synapses. Kruskall-Wallis test, P<0.001.
To verify whether the area of the IMP-clusters in the replicas was comparable to that of PSD visualized in conventional ultrathin sections, we also analyzed the maximum PSD widths of AN-BC, AN-FC, PF-FC, and PF-CwC synapses in serial ultrathin sections (Fig. 4C). Our data show that the maximum PSD width of the AN-BC synapses on the cell body (median = 0.30 um, n = 23 synapses), AN-FC synapses on proximal and distal basal dendrites (median = 0.28 um, n= 27), PF-FC synapses on apical dendrites (median = 0.30 um, n = 12), and PF-CwC on spines (median = 0.36 um, n = 19) were not significantly different from the maximum width of the IMP-clusters in each of the four types of synapses (Fig. 4D); (maximum width of the IMP-clusters for the AN-BC synapse, median = 0.34 um, n = 32 synapses, and p = 0.70; for AN-FC synapses, median = 0.31 um, n = 31 synapses, and p = 0.33; for PF-FC synapses, median = 0.34 um, n = 26 synapses, and p = 0.06 ; and for PF-CwC synapses, median = 0.56 um, n = 24, and p = 0.30; Mann– Whitney U test). These results indicate that the IMP-clusters on the E-face correspond to PSDs and that, on average and by two different analyses, the synapse size is unique among the four excitatory synapse types.
Distribution, number, and density of AMPARs and NMDARs on AN-BC and AN-FC synapses
To determine the distribution and the number of AMPARs and NMDARs at AN-BC and AN-FC synapses, we took advantage of the superb molecular visualization power of FRIL for membrane proteins, which allows for the reliable detection and precise localization of molecules of interest at nano-scale spatial resolution (labeling efficiency close to unity for functional AMPARs in electron microscopy; Masugi-Tokita et al., 2007; Tarusawa et al., 2009). Our results show that distribution and quantity of AMPARs and NMDARs within the IMP-cluster areas of AN synapses vary between synapse types.
AMPARs
The GluA1-4 AMPAR immunoparticles were homogenously distributed almost entirely over the IMP-clusters of all AN-BC synapses and a majority of AN-FC synapses. However, some AN-FC synapses clearly showed a subregion within a synaptic plasma membrane devoid of AMPAR labeling (Fig. 5A). Nevertheless, all the IMP-clusters of AN-BC and AN-FC synapses were gold labeled for GluA1-4 (Table 3). As expected from the large variability in PSD areas, the number of labeled AMPARs differed between the IMP-cluster areas of AN-BC and AN-FC synapses (CV = 0.53 for both synapses, Fig. 6A). The densities of labeled AMPARs were less variable between IMP-clusters of both synapse types (CV = 0.23 for AN-BC, CV = 0.29 for AN-FC).
Figure 6. Differential density of AMPARs and NMDARs in IMP-clusters.
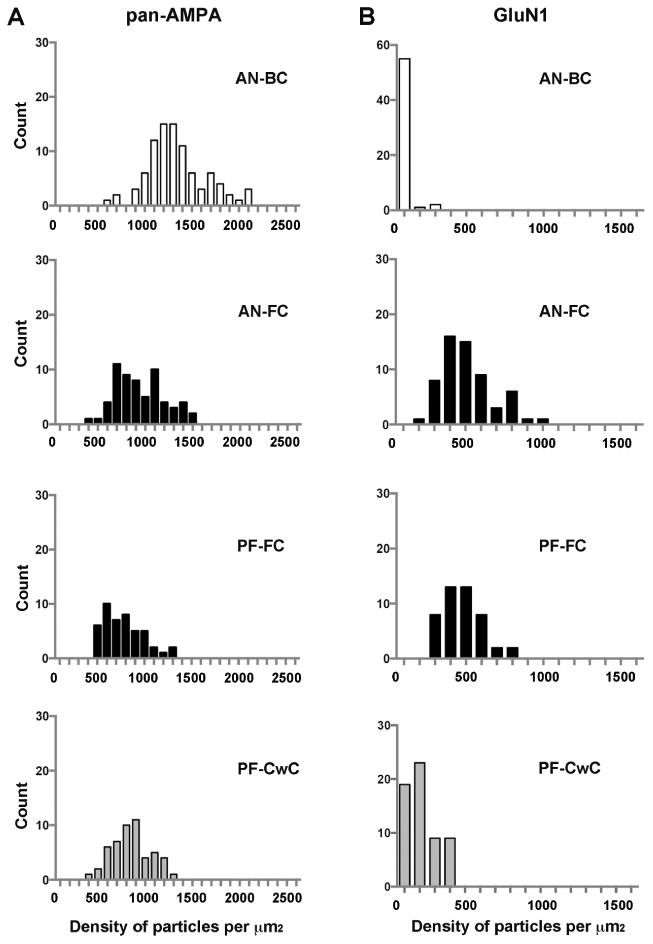
Histograms showing the distribution of densities of gold particles for AMPARs (A) and NMDARs (B) in SDS-FRIL replicas of individual IMP-clusters in the rostral AVCN (AN-BC synapses) and FCL of the DCN (AN-FC synapses), as well as, within the ML of the DCN (PF-FC and PF-CwC synapses). Replicas from which data were obtained were immunolabeled with antibodies that recognize all 4 subunits of AMPARs (A) or GluN1 subunits of NMDARs (B).
The mean number of immunogold particles for AMPAR in the AN-BC synapses was 38 ± 2.1 (median = 33.5, range 6 -107 particles/IMP-cluster; n= 90), and the mean number of particles for AN-FC synapses was 41 ± 2.8 (median = 41, range 8 - 87 particles/IMP-cluster; n = 62). Thus, these two types of synapses created by AN fiber inputs express a similar average number of AMPARs (p = 0.4; Mann-Whitney test; Fig. 7A). As the area of the IMP-clusters was significantly smaller in AN-BC synapses than in AN-FC synapses (Fig. 4, Table 2), the average density of AMPARs was significantly higher in AN-BC synapses (Fig. 7A, AN-BC: mean = 1281 ± 31.4, median = 1245, range 596 – 2,000 particles/μm2; AN-FC: mean = 895 ± 32.9, median = 846.5, range 356 - 1471 particles/μm2, p < 0.001, Steel-Dwass test).
Figure 7. Number and density of AMPARs and NMDARs in IMP-clusters of AN and PF synapses.
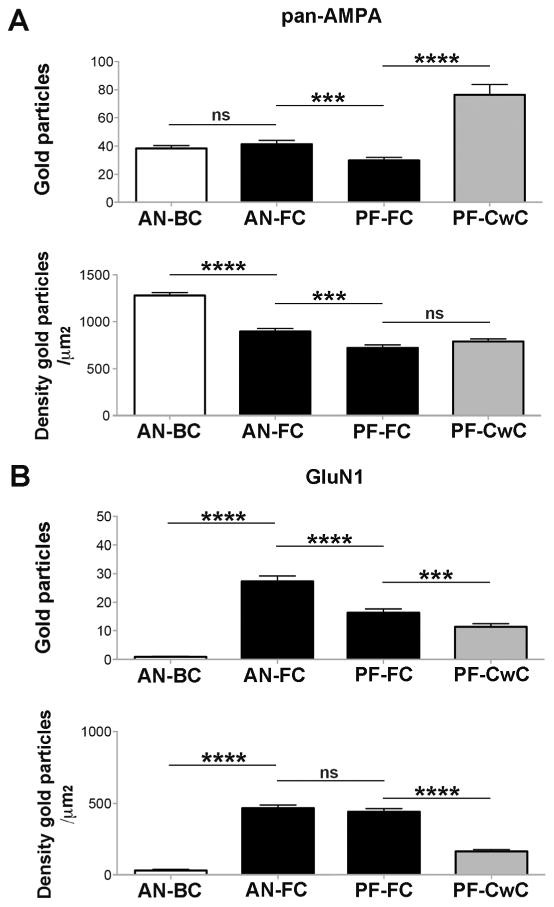
A-B. Histograms show the density and number of gold particles per IMP-cluster for AMPAR (A) and GluN1 (B) at AN-BC and AN-FC synapses and PF-FC and PF-CwC synapses. The number of AMPAR gold particles was similar for AN-BC and AN-FC synapses, although the density was higher for AN-BC synapses. For PF-FC and PF-CwC synapses, the density of AMPAR gold particles was similar, although the number of gold particles was higher for PF-CwC synapses. The number and density of gold particles for AMPARs was higher in AN synapses on the basal dendrites of FCs than in PF synapses on the apical dendrites of FCs. B) GluN1 labeling was stronger at the IMP-clusters of AN-FC synapses, while it was almost undetectable at the IMP-clusters of AN-BC synapses. GluN1 labeling was stronger at IMP-clusters of PF-FC synapses compared to those of PF-CwC synapses. GluN1 expression was higher in AN synapses on the basal dendrites of FCs than in PF synapses on the apical dendrites of FCs (Mann-Whitney U test (p < 0.001).
Both AN-BC and AN-FC synapses showed a strong positive correlation between the number of labeled AMPARs and the IMP-cluster area size, indicating that the AMPAR density was constant across synapses of the same connection type (Fig. 8A; AN-BC: r = 0.84; AN-FC: r = 0.70) and that the number of AMPARs in individual synapses is primarily dependent on the synaptic size in AN synapses.
Figure 8. Correlation of the number of gold particles and IMP-cluster area.
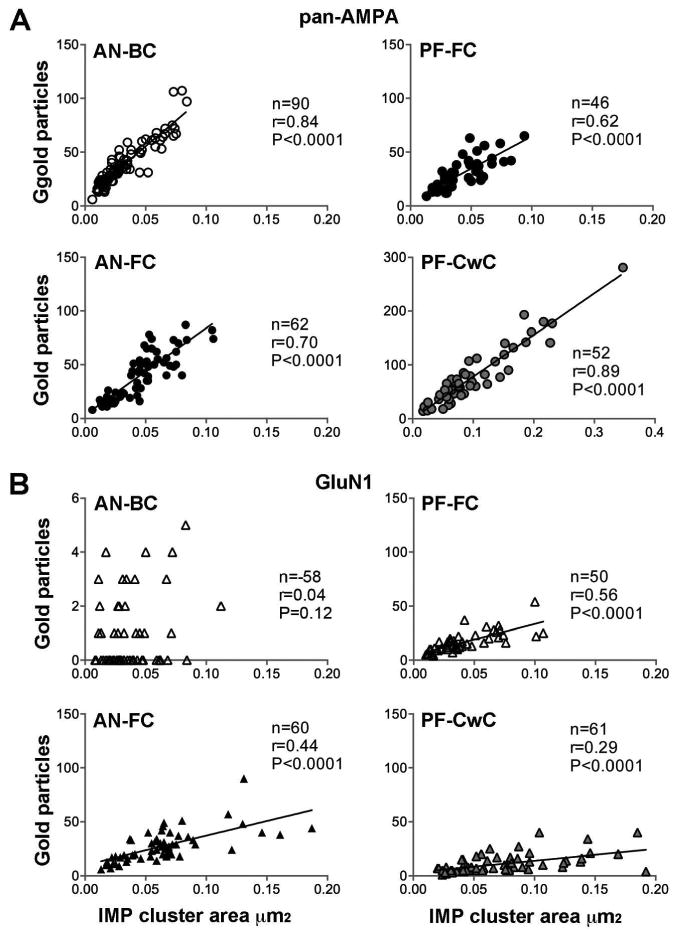
Scatterplots of the number of gold particles for AMPARs (A) and GluN1 (B) vs. the IMP-cluster areas of AN-BC, AN-FC, PF-FC and PF-CwC synapses (Spearman's rank-order test).
NMDARs
To label NMDARs in the AVCN and DCN replicas, we used a monoclonal antibody against the obligatory GluN1 subunit, which is constituent of all NMDARs (Cull-Candy et al., 2001; Gibb 2004). We found a highly inhomogeneous distribution of NMDAR gold labeling in the IMP-cluster areas of AN-BC and AN-FC synapses. In the former synapses, only 41% of the IMP-cluster areas showed labeling for GluN1 (Table 2), and the number of gold particles was negligible (p < 5, mean = 0.86 ± 0.17, median = 0) in individual IMP-cluster areas (Figs. 5B, 6B, 7B, 8B). Labeling density for GluN1 in the AN-BC synapses (mean = 31.6 ± 7.30, median = 0) was not significantly different from GluN1 labeling in the extrasynaptic plasma membrane and background labeling measured on the P-face of plasma membrane, suggesting no expression of NMDARs at AN-BC synapses (Table 4, p = 0.14 vs extrasynaptic, p = 0.14 vs background, Mann-Whitney test). In contrast, all IMP-cluster areas of AN-FC synapses were labeled for GluN1 (Table 3), and the immunoparticles were distributed sparsely and partially over the IMP-cluster area, so the IMP-clusters presented large open spaces devoid of gold labeling (Figs. 5B, 6B, 7B, 8B; Table 2; mean = 27 ± 1.9, median = 25, range 6 – 90 particles/IMP-cluster). The density of labeling in individual IMP-clusters (mean = 468 ± 21.9, median = 434.5, range 198 – 919 particles/μm2) was significantly higher than the extrasynaptic plasma membrane or background labeling (Table 4, p < 0.0001 vs extrasynaptic, p < 0.0001 vs background, Mann-Whitney test).
Table 4.
Extrasynaptic and background labeling of AMPARs and NMDARs.
| AVCN | DCN | |
|---|---|---|
| Extrasynaptic | ||
| GluA1-4 | ||
| Average density/um2 | 3.40 ± 0.30 | 2.4 ± 0.70 |
| Average number gold N= 3 animals | 10.20 ± 0.51 | 5.5 ± 1.90 |
| GluN1 | ||
| Average density/um2 | 0.61 ± 0.22 | 2.17 ± 0.27 |
| Average number gold N= 3 animals | 0.81 ± 0.32 | 5.84 ± 1.27 |
| Background | ||
| GluA1-4 | ||
| Average density/um2 | 2.88 ± 1.44 | 0.72 ± 0.54 |
| Average number gold N= 3 animals | 0.61 ± 0.26 | 0.50 ± 0.32 |
| GluN1 | ||
| Average density/um2 | 1.37 ± 0.90 | 1.77 ± 0.65 |
| Average number gold N= 3 animals | 0.25 ± 0.16 | 0.41 ± 0.25 |
Results are expressed ± SEM; Comparison of the extrasynaptic vs background gold labeling density for AMPARs or NMDARs were not found statistically significant; P > 0.05.
Distribution, number, and density of AMPARs and NMDARs on PF-FC and PF-CwC synapses
AMPARs
All of the IMP-clusters of PF-FC and PF-CwCs synapses were gold labeled for GluN1 (Table 2). At IMP-cluster areas of PF-FC and PF-CwCs synapses, the AMPAR immunoparticles were arranged inhomogeneously and often appeared in a mosaic-like distribution, in which each IMP-cluster contained an area devoid of AMPAR labeling (Fig. 5C). As expected from the large variability in PSD areas, the number of labeled AMPARs was variable among PF-FC and PF-CwC synapses (CV = 0.49 and CV = 0.7, respectively; Fig. 6A). The densities of labeled AMPARs were less variable among IMP-clusters in both synapse types (CV = 0.29 for PF-FC, CV = 0.25 for PF-CwC).
Our results show that, on average, the IMP-clusters area of PF-CwC synapses had 2.5 fold greater number of gold particles for AMPARs than PF-FC synapses (Fig. 7A), but because the average IMP-cluster area of PF-CwC synapses was larger (Fig. 4; Table 2), the density of immunolabeling in both synapse types was similar (Fig. 7A). The mean number and density of immunogold particles for AMPAR in IMP-cluster areas of PF-FC synapses were 29.8 ± 2.1 (median = 28.5, range 9 - 65 particles/IMP-cluster; n = 46) and 724.7 ± 31.2 (median = 699.0, range 400 – 1285.7 particles/μm2), respectively. The mean number and density of immunogold particles in the IMP-cluster areas of PF-CwC synapses were 76.3 ± 7.4 (median = 60.5, range 14 - 281 particles/IMP-cluster; n= 52) and 791.8 ± 28.0 (median = 792.3, range 360 – 1200 particles/μm2), respectively. These results reveal that, despite common inputs, PF-CwC synapses express significantly higher numbers of AMPARs than PF-FC synapses in the postsynaptic membrane specializations (p < 0.0001, Mann-Whitney test) due to the larger synaptic area in the PF-CwC synapses.
For both synapse types, the number of immunogold particles in individual IMP-clusters was found to be strongly, positively correlated with the size of the IMP-clusters (PF-FC: r = 0.62, p < 0.0001; PF-CwC: r = 0.89, p < 0.0001, Pearson's correlation test; Fig. 8A).
NMDARs
NMDAR labeling was scattered and most of surface area of the synaptic IMP-clusters appeared free of labeling (Fig. 5D); however, all of the IMP-clusters in the analyzed PF-FC and PF-CwC synapses were gold labeled for GluN1 (Table 3). The density of labeling varied from cluster to cluster (Fig. 6B).
Our results show that the IMP-cluster areas of PF-FC synapses had a higher number and density of GluN1 gold particles than PF-CwC synapses (p < 0.0001, Mann-Whitney test, Fig. 7B). The mean number and density of GluN1 immunogold particles in synaptic sites of PF-FC synapses were 16.3 ± 1.3 (median = 14, range 4 - 54 particles/IMP-cluster; n= 50) and 441.7 ± 22.3 (median = 424.6, range 210 – 880.9 particles/μm2), respectively. The mean number and density of immunogold particles in the IMP-cluster areas of PF-CwC synapses were 11.3 ± 1.0 (median = 8, range 1 - 40 particles/IMP-cluster; n= 61) and 163.3 ± 12.5 (median = 120.3, range 20.8 – 396.8 particles/μm2), respectively. In both synapse types, the density of labeling in individual IMP-clusters was significantly higher than the extrasynaptic plasma membrane or background labeling (Table 4, p < 0.0001 vs extrasynaptic, p < 0.0001 vs background, Mann-Whitney test).
For both synapses, the number of immunogold particles for GluN1 in individual IMP-cluster areas was proportional to the size of the IMP-clusters, but the correlation was strong for PF-FC synapses where it was weak for PF-CwC synapses (PF-FC: r = 0.56, p < 0.0001; PF-CwC: r = 0.29, p < 0.0001; Fig. 8B).
Labeling for extrasynaptic AMPARs and NMDARs in the PF-FC and PF-CwC synapses was very low (Table 4). The background estimated on the P-face of FCs and CwCs was also low (Table 4) and the labeling densities for extrasynaptic and background labeling were not significantly different (p > 0.05).
Number and density of synaptic AMPARs and NMDARs on fusiform cells
Next we determined whether the number and/or density for AMPARs and NMDARs were input-specific on FCs (Fig. 7). Our data showed that the number of AMPARs and NMDARs was higher for AN synapses on basal dendrites than for PF synapses on apical dendrites (AMPAR: AN-FC mean = 41 ± 2.8, PF-FC mean = 29.8 ± 2.1 p < 0.0003; NMDAR: AN-FC mean = 27 ± 1.9, PF-FC mean = 16.3 ± 1.3, p < 0.0001). The density of AMPAR gold particles was also higher at AN synapses on basal dendrites than at PF synapses on apical dendrites (AN-FC mean = 895 ± 32.9, PF-FC mean = 724.7 ± 31.2, p < 0.006), whereas the NMDAR density was similar (AN-FC mean = 468 ± 21.9, PF-FC mean = 441.7 ± 22.3, p > 0.05) at both synapse types.
Discussion
We analyzed the organization of two types of glutamatergic projections in the cochlear nucleus. One is a primary input, the AN fibers synapsing on BCs and FCs, and the other is an input from an interneuron, the PFs synapsing on FCs and CwCs. Our results show that IMP-cluster areas, AMPAR and NMDAR numbers, and the receptors' 2D-distribution are target-dependent in both AN and PF synapses. Our data also show that AMPAR and NMDAR numbers are input-dependent in FCs. These morphological and molecular properties may play important roles in shaping neuronal transmission at individual connections during normal auditory processing.
The size of glutamatergic synaptic IMP clusters is distinct between the four synaptic connections
The physiological response of synapses has been shown to correlate with synapse size and the number of postsynaptic iGluRs (Matsuzaki et al, 2001; Tanaka et al., 2005). The analysis of the synaptic area of AN and PF synapses revealed large variability even between synapses from the same input source. These differences in size could explain the large variability of neuronal responses found in the AVCN and DCN (Isaacson and Wimsley, 1996; Gardner et al., 1999). Additionally, we found that the size of the IMP-clusters was distinct between the four synaptic connections, indicating that synapse size is controlled in a connection-dependent manner.
To respond to acoustic stimulation, auditory neurons must maintain rapid transmission rates and maximize temporal fidelity through their synaptic networks. The AN fibers bifurcate to innervate BCs and FCs; however, the synaptic conductance of AN-BC synapses are quicker than AN-FC synapses (Gardner et al., 1999, 2001; Fujino and Oertel, 2003). Our FRIL analysis of the size and shape of these two AN synapse types showed clear ultrastructural differences. In the AVCN, as previously shown (Gulley et al., 1997), we found that the IMP-clusters of AN-BC synapses were small and densely packed with intramembrane particles. These characteristics were not present at the IMP-clusters of AN-FC synapses, which suggests that small, dense IMP-clusters are unique to the ultrafast AN-BC synapses. Likewise, the plastic PF synapses (Fujino and Oertel, 2003) should be more variable in size and likely have larger synaptic area than those of AN synapses. Our FRIL analysis supports this hypothesis in part, as we found that the synaptic area of PFs on CwCs were the largest of the four types. However, we also found that the synaptic areas of the AN-FC-basal dendrite synapses, which lack plastic properties (Fujino and Oertel, 2003), were significantly larger than the PF-FC synapses on the apical dendrites. Thus, other factors in addition to pure morphology should be taken into account before correlating the anatomy of a synaptic input to its specific function. The surface area of the synapse can be related to levels of synaptic activity (Xu-Friedman and Regehr, 2004). A decrease in PSD size has been shown at the calyx synapse on medial nucleus of the trapezoid body (MNTB) neurons after the onset of hearing (Taschenberger et al., 2002), and larger surface area of endbulb synapses exists in congenital deaf cats and mice (Lee et al., 2003; Ryugo et al., 1997, 2005). Therefore, a dense and small synapse size could contribute to more reliable synaptic responses and efficient activation of AMPARs by quantal release of glutamate (Tarusawa et al., 2009; Budisantoso et al., 2012).
The number of AMPARs determines the synaptic strength
The size of the PSD is proportional to the total number of iGluRs (Ganeshina et al., 2004). The number of labeled AMPARs is direct measure of synaptic efficacy (Tanaka et al., 2005; Tarusawa et al., 2009). In this study, we used the same antibody and immunogold labeling protocol of a previous study, in which we estimated the detection sensitivity of FRIL for AMPARs as high as one immunogold particle per functional AMPAR channel (Tanaka et al., 2005). Using this approach, we estimated the number of AMPARs at the AN synapses on BCs and FCs, and those of the PFs on FCs and CwCs. In these four types of synaptic connections, all IMP-clusters on the E-face were strongly labeled for AMPARs, confirming that they were glutamatergic synapses. Similar to the synapse size data (above), the number of gold labeled AMPARs in individual synapses showed a high degree of variability between synapses from the same input source. This variability in the number of AMPARs between release sites, together with the variations in vesicular glutamate content (Wang and Manis, 2008; Yang and Xu-Friedman, 2008) could contribute to the measured variability in the amplitude of quantal excitatory postsynaptic currents (EPSCs; Isaacson and Walmsley, 1996; Gardner et al., 1999; Yang and Xu-Friedman, 2009). We found a positive correlation between synapse size and the number of AMPARs in the four types of synapses analyzed. Such correlations have been found in other synapses, such as granule cell-CA3 pyramidal cell synapses, CA3-CA1 synapses (Nusser et al., 1998), layer I-II synapses in the sensory cortex (Kharazia and Weinberg, 1999), and retinogeniculate-relay and corticogeniculate-relay synapses (Tarusawa et al., 2009; Fukazawa and Shigemoto, 2012). Together with our results in the CN, this set of data indicates that the relationship between synapse size and AMPAR expression is a common feature of excitatory synaptic connections in the CNS.
Based on previous studies (Tarusawa et al., 2009), AN synapses that contain a similar number of AMPARs should have the same synaptic strength (amplitude). However, it has been shown that AN-BC synapses have larger amplitudes than AN-FC synapses (Gardner et al., 1999). The average rise and decay times of AMPAR responses correlate with synaptic area (Tarusawa et al., 2009) and with a specific synaptic receptor subunit composition (Geiger et al., 1995). The kinetics are also faster for responses produced at synapses with smaller synaptic areas, as they have fewer AMPARs exposed to low concentrations of glutamate. Here, we show that, on average, AN-BC synapses were almost half the size of AN-FC synapses, which may help explain the differences in kinetics that have been found between the two synapse types (Gardner et al., 1999; 2001).
AMPAR distribution is target-dependent for AN and PF synapses
Based on recent observations in other synapses in the CNS, synaptic connections can be classified into two types based on AMPAR arrangement: homogeneous and mosaic (Fukazawa and Shigemoto, 2012). The former feature tends to appear in synaptic connections with high AMPAR densities (Tarusawa et al., 2009; Fukazawa and Shigemoto, 2012). In our study, we show that AN inputs onto BCs make synapses with very high AMPAR density and wide and homogeneous AMPAR distribution in the IMP-clusters. Conversely, AN-FC, PF-FC, and PF-CwC synapses all showed lower AMPAR density and a mosaic AMPAR distribution. It appears as though synaptic connections with highly plastic properties show large synaptic areas, low AMPAR density, and a mosaic arrangement of AMPARs in the synapse (Fukazawa and Shigemoto, 2011). Our results are consistent with this notion not only in the case of PF synapses, which have plastic properties, but also for AN-FC synapses, which lack such plastic properties (Fujino and Oertel, 2003). Thus, the mosaic type arrangement of AMPARs may have or reflect biological consequences other than synaptic plasticity.
The expression of NMDARs is target-dependent for AN and PF synapses
Our study demonstrates that NMDAR expression at AN and PF synapses is postsynaptic target-dependent. Within a single DCN replica, the mean number of labeled NMDARs at individual connections was significantly different between AN-FC, PF-FC, and PF-CwC synapses.
NMDARs are expressed in the adult DCN (Petralia et al, 1996) and at adult AN-FC synapses (Rubio and Wenthold, 1997). However, the expression of NMDARs in the AVCN is developmentally regulated (Caicedo and Eybalin, 1999) and, until the present study, the expression level of NMDARs at adult AN-BC synapses was unclear. Electrophysiological studies have shown that the EPSCs of AN-FC synapses are mediated through AMPARs and NMDARs (Fujino and Oertel, 2003). At AN-BC synapses in 3-week rodents, studies have shown a large AMPAR component and a clear but small NMDAR component; this NMDAR component decreases by postnatal day 25 (Cao and Oertel, 2010; Isaacson and Walmsley, 1995; Pliss et al., 2009). This small NMDAR component in young adult mice at AN-BC synapses has been suggested to promote firing probability and improve temporal precision (Pliss et al., 2009). Our results for postnatal day 30 rats show that only AN-FC synapses present positive NMDAR gold-labeling, whereas AN-BC synapses lack immunolabeling for NMDAR. These results suggest that the NMDAR component of the EPSC is likely to decrease with age, and that NMDAR in AN-BC synapses might be important for wiring inputs with approximately coincident synaptic activity during development.
The EPSCs of PF synapses have a strong AMPAR component that is blocked by CNQX (Manis and Molitor, 1996; Fujino and Oertel, 2003). Electrophysiological data also indicate that PFs have some NMDARs because there is a clear effect of APV on current and on plasticity (Manis and Molitor, 1996; Fujino and Oertel, 2003). These authors showed PF-FC and PF-CwC EPSCs at positive potentials, but when compared to AMPAR/NMDAR ratios at other CNS synapses (Myme et al., 2003; Tarusawa et al., 2009), PF synapses have a more prominent slow NMDAR component. Thus, this set of data suggests that NMDARs are present in low levels at both PF synapse types. At PF synapses, we found NMDAR expression levels were lower than those at AN-FC synapses, which, remarkably, do not demonstrate long-term plasticity. Thus, our results suggest that small numbers of NMDARs will produce a substantial current that is sufficient to generate long-term changes in the PF synaptic properties during auditory processing.
The expression of AMPAR and NMDARs is input-specific in fusiform cells
FCs receive two distinct glutamatergic inputs on their basal and apical dendrites; AN fibers synapse on basal dendrites while the PF fibers synapse on apical dendrites (Rubio and Wenthold, 1997). Using FRIL, we show that the number and density of AMPARs and the number of NMDARs in the IMP-clusters of AN synapses and PFs synapses differ. Thus, in neurons that receive different glutamatergic inputs, the differential expression of AMPARs and NMDARs may be necessary to maintain the basic electrophysiological properties.
Acknowledgments
The authors thank Sanae Hara for her technical help with some of the sample preparations and Jennifer Chikar for proof editing the manuscript. This research was supported by grants from NIH (1R01DC013048-01 to M.E.R), Biotechnology and Biological Sciences Research Council, UK (grant BB/J015938/1 to E.M.).
Footnotes
Conflict of interest statement: The authors declare no competing financial interests.
Role of authors: All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: MER. Acquisition of data: MER. Analysis and interpretation of data: MER, NK, YK, RS. Drafting of the manuscript: MER. Critical revision of the manuscript for important intellectual content: MER, NK, YK, RS. Statistical analysis: MER, YK. Obtained funding: MER, RS. Technical, and material support: CK. Study supervision: MER. Development and characterization of anti-GluA1-4 antibody: EM
References
- Antal M, Fukazawa Y, Eördögh M, Muszil D, Molnár E, Itakura M, Takahashi M, Shigemoto R. Numbers, densities, and colocalization of AMPA- and NMDA-type glutamate receptors at individual synapses in the superficial spinal dorsal horn of rats. J Neurosci. 2008;28:9692–9701. doi: 10.1523/JNEUROSCI.1551-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocchio EE, Reimer RJ, Fremeau RTJ, Edwards RH. Science. PVW; 2000. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter; pp. 957–960. [DOI] [PubMed] [Google Scholar]
- Berrebi AS, Mugnaini E. Distribution and targets of the cartwheel cell axon in the dorsal cochlear nucleus of the guinea pig. Anat Embryol (Berl) 1991;183:427–425. doi: 10.1007/BF00186433. [DOI] [PubMed] [Google Scholar]
- Budisantoso T, Matsui K, Kamasawa N, Fukazawa Y, Shigemoto R. Mechanisms underlying signal filtering at a multisynapse contact. J Neurosci. 2012;32:2357–2376. doi: 10.1523/JNEUROSCI.5243-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A, Eybalin M. Glutamate receptor phenotypes in the auditory brainstem and mid-brain of the developing rat. Eur J Neurosci. 1999;11:51–74. doi: 10.1046/j.1460-9568.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- Cao XJ, Oertel D. Auditory nerve fibers excite targets through synapses that vary in convergence, strength, and short-term plasticity. J Neurophysiol. 2010;104:2308–2230. doi: 10.1152/jn.00451.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Davis KA, Young ED. Granule cell activation of complex-spiking neurons in dorsal cochlear nucleus. J Neurosci. 1997;17:6798–67806. doi: 10.1523/JNEUROSCI.17-17-06798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong YL, Fukazawa Y, Wang W, Kamasawa N, Shigemoto R. Differential postsynaptic compartments in the laterocapsular division of the central nucleus of amygdala for afferents from the parabrachial nucleus and the basolateral nucleus in the rat. J Comp Neurol. 2010;518:4771–4791. doi: 10.1002/cne.22487. [DOI] [PubMed] [Google Scholar]
- Franks KM, Stevens CF, Sejnowski TJ. AMPAR arrangement on transmission properties. J Neurosci. 2003;23:3186–3195. doi: 10.1523/JNEUROSCI.23-08-03186.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino K, Oertel D. Bidirectional synaptic plasticity in the cerebellum-like mammalian dorsal cochlear nucleus. Proc Natl Acad Sci USA. 2003;100:265–270. doi: 10.1073/pnas.0135345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa Y, Shigemoto R. Intra-synapse-type and inter-synapse-type relationships between synaptic size and AMPAR expression. Curr Opin Neurobiol. 2012;22:446–452. doi: 10.1016/j.conb.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y. Differences in the expression of AMPA and NMDA receptors between axospinous perforated and nonperforated synapses are related to the configuration and size of postsynaptic densities. J Comp Neurol. 2004;468:86–95. doi: 10.1002/cne.10950. [DOI] [PubMed] [Google Scholar]
- Gardner SM, Trussell L, Oertel D. Correlation of AMPA receptor subunit composition with synaptic input in the mammalian cochlear nuclei. J Neurosci. 2001;21:7428–7437. doi: 10.1523/JNEUROSCI.21-18-07428.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner SM, Trussell L, Oertel D. Time course and permeation of synaptic AMPA receptors in cochlear nuclear neurons correlate with input. J Neurosci. 1999;19:8721–8729. doi: 10.1523/JNEUROSCI.19-20-08721.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb AJ. NMDA receptor subunit gating—uncovered. Trends Neurosci. 2004;27:7–10. doi: 10.1016/j.tins.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburgh PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;1:193–2014. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Gladding CM, Collett VJ, Jia Z, Bashir ZI, Collingridge GL, Molnar E. Tyrosine dephosphorylation regulates AMPAR internalisation in mGluR-LTD. Mol Cell Neurosci. 2009;40:267–279. doi: 10.1016/j.mcn.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Gómez-Nieto R, Rubio ME. A bushy cell network in the rat ventral cochlear nucleus. Journal of Comparative Neurology. 2009;516:241–263. doi: 10.1002/cne.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Nieto R, Rubio ME. Ultrastructure, synaptic organization, and molecular components of bushy cell networks in the anteroventral cochlear nucleus of the rhesus monkey. Neuroscience. 2011;179:188–207. doi: 10.1016/j.neuroscience.2011.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley RL, Wenthold RJ, Neises GR. Remodeling of neuronal membranes as an early response to deafferentation. A freeze-fracture study. J Cell Biol. 1977;75:837–850. doi: 10.1083/jcb.75.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Landis DM. Membrane structure at synaptic junctions in area CA1 of the rat hippocampus. Neuroscience. 1986;19:857–872. doi: 10.1016/0306-4522(86)90304-0. [DOI] [PubMed] [Google Scholar]
- Hagiwara A, Fukazawa Y, Deguchi-Tawarada M, Ohtsuka T, Shigemoto R. Differential distribution of release-related poteins in the hippocampal CA3 area as revealed by freeze-fracture replica labeling. J Comp Neurol. 2005;489:195–216. doi: 10.1002/cne.20633. [DOI] [PubMed] [Google Scholar]
- Irvine DR. Interaural intensity differences in the cat: changes in sound pressure level at the two ears associated with azimuthal displacements in the frontal horizontal plane. Hear Res. 1987;26:267–86. doi: 10.1016/0378-5955(87)90063-3. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Walmsley B. Receptors underlying excitatory synaptic transmission in slices of the rat anteroventral cochlear nucleus. J Neurophysiol. 1995;73:964–973. doi: 10.1152/jn.1995.73.3.964. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Walmsley B. Amplitude and time course of spontaneous and evoked excitatory postsynaptic currents in bushy cells of the anteroventral cochlear nucleus. J Neurophysiol. 1996;76:1566–1571. doi: 10.1152/jn.1996.76.3.1566. [DOI] [PubMed] [Google Scholar]
- Iwasato T, Datwani A, Wolf AM, Nishiyama H, Taguchi Y, Tonegawa S, Knöpfel T, Erzurumlu RS, Itohara S. Cortex-restricted disruption of NMDAR1 impairs neuronal patterns in the barrel cortex. Nature. 2000;406:726–731. doi: 10.1038/35021059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane EC. Synaptic organization in the dorsal cochlear nucleus of the cat: a light and electron microscopic study. J Comp Neurol. 1974;155:301–329. doi: 10.1002/cne.901550303. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Young ED. Proprioceptive information from the pinna provides somatosensory input to cat dorsal cochlear nucleus. J Neurosci. 2001;21:7848–7858. doi: 10.1523/JNEUROSCI.21-19-07848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharazia VN, Weinberg RJ. Immunogold localization of AMPA and NMDA receptors in somatic sensory cortex of albino rat. J Comp Neurol. 1999;412:292–302. doi: 10.1002/(sici)1096-9861(19990920)412:2<292::aid-cne8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Kulik A, Nakadate K, Nyiri G, Notomi T, Malitschek B, Bettler B, Shigemoto R. Distinct localization of GABA(B) receptors relative to synaptic sytes in the rat cerebellum and ventrobasal thalamus. EuR J Neurosci. 2002;15:291–307. doi: 10.1046/j.0953-816x.2001.01855.x. [DOI] [PubMed] [Google Scholar]
- Lee DJ, Cahill HB, Ryugo DK. Effects of congenital deafness in the cochlear nuclei of Shaker-2 mice: an ultrastructural analysis of synapse morphology in the endbulbs of Held. J Neurocytol. 2003;32:229–243. doi: 10.1023/B:NEUR.0000010082.99874.14. [DOI] [PubMed] [Google Scholar]
- Lorente de No R. The primary acoustic nuclei. Raven Press; NY: 1981. [Google Scholar]
- Manis PB. Responses to parallel fiber stimulation in the guinea pig dorsal cochlear nucleus in vitro. J Neurophysiol. 1989;61:149–161. doi: 10.1152/jn.1989.61.1.149. [DOI] [PubMed] [Google Scholar]
- Manis PB, Molitor SC. N-methyl-D-aspartate receptors at parallel fiber synapses in the dorsal cochlear nucleus. J Neurophysiol. 1996;76:1639–1656. doi: 10.1152/jn.1996.76.3.1639. [DOI] [PubMed] [Google Scholar]
- Masugi-Tokita M, Tarusawa E, Watanabe M, Molnár E, Fujimoto K, Shigemoto R. Number and density of AMPA receptors in individual synapses in the rat cerebellum as revealed by SDS-digested freeze-fracture replica labeling. J Neurosci. 2007;27:2135–2144. doi: 10.1523/JNEUROSCI.2861-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara A, Laake JH, Davanger S, Usami S, Ottersen OP. Organization of AMPA receptor subunits at a glutamate synapse: a quantitative immunogold analysis of hair cell synapses in the rat organ of Corti. J Neurosci. 1996;16:4457–4467. doi: 10.1523/JNEUROSCI.16-14-04457.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. AMPAR Number vs Amplitude. Nat Neurosci. 2001;11:1086–11092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moult PR, Gladding CM, Sanderson T, Fitzjohn SM, Bashir ZI, Molnar E, Collingridge GL. Tyrosine phosphatases regulate AMPA receptor trafficking during metabotropic glutamate receptor-mediated long-term depression. J Neurosci. 2006;26:2544–2554. doi: 10.1523/JNEUROSCI.4322-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myme CI, Sugino K, Turrigiano GG, Nelson SB. The NMDA-to-AMPA ratio at synapses onto layer 2/3 pyramidal neurons is conserved across prefrontal and visual cortices. J Neurophysiol. 2003;90:771–779. doi: 10.1152/jn.00070.2003. [DOI] [PubMed] [Google Scholar]
- Noel J, Ralph GS, Pickard L, Williams J, Molnar E, Uney JB, Collingridge GL, Henley JM. Surface expression of AMPA receptors in hippocampal neurons is regulated by an NSF-dependent mechanism. Neuron. 1999;23:365–376. doi: 10.1016/s0896-6273(00)80786-2. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Lujan R, Laube G, Roberts JD, Molnar E, Somogyi P. Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron. 1998;21:545–59. doi: 10.1016/s0896-6273(00)80565-6. [DOI] [PubMed] [Google Scholar]
- Oertel D, Wu SH. Morphology and physiology of cells in slice preparations of the dorsal cochlear nucleus of mice. J Comp Neurol. 1989;283:228–247. doi: 10.1002/cne.902830206. [DOI] [PubMed] [Google Scholar]
- Osen KK, Storm-Mathisen J, Ottersen OP, Dihle B. Glutamate is concentrated in and released from parallel fiber terminals in the dorsal cochlear nucleus: a quantitative immunocytochemical analysis in guinea pig. J Comp Neurol. 1995;357:482–500. doi: 10.1002/cne.903570311. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Zhao HM, Wenthold RJ. Ionotropic and metabotropic glutamate receptors show unique postsynaptic, presynaptic, and glial localizations in the dorsal cochlear nucleus. J Comp Neurol. 1996;372:356–383. doi: 10.1002/(SICI)1096-9861(19960826)372:3<356::AID-CNE3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Pickard L, Noël J, Henley JM, Collingridge GL, Molnar E. Developmental changes in synaptic AMPA and NMDA receptor distribution and AMPA receptor subunit composition in living hippocampal neurons. J Neurosci. 2000;20:7922–7931. doi: 10.1523/JNEUROSCI.20-21-07922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard L, Noel J, Duckworth JK, Fitzjohn SM, Henley JM, Collingridge GL, Molnar E. Transient synaptic activation of NMDA receptors leads to the insertion of native AMPA receptors into hippocampal neuronal plasma membrane. Neuropharmacology. 2001;41:700–713. doi: 10.1016/s0028-3908(01)00127-7. [DOI] [PubMed] [Google Scholar]
- Pliss L, Yang H, Xu-Friedman MA. Context-Dependent Effects of NMDA Receptors on Precise Timing Information at the Endbulb of Held in the Cochlear Nucleus. J Neurophysiol. 2009;102:2627–2637. doi: 10.1152/jn.00111.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Zhang S, Trussell LO. Pathway-specific variants of AMPA receptors and their contribution to neuronal signaling. J Neurosci. 1994;14:4998–5010. doi: 10.1523/JNEUROSCI.14-08-04998.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode WS, Smith PH, Oertel D. Physiological response properties of cells labeled intracellularly with horseradish peroxidase in cat dorsal cochlear nucleus. J Comp Neurol. 1983a;213:426–447. doi: 10.1002/cne.902130407. [DOI] [PubMed] [Google Scholar]
- Rhode WS, Oertel D, Smith PH. Physiological response properties of cells labeled intracellularly with horseradish peroxidase in cat ventral cochlear nucleus. J Comp Neurol. 1983b;213:448–463. doi: 10.1002/cne.902130408. [DOI] [PubMed] [Google Scholar]
- Rollenhagen A, Lübke JH. The morphology of excitatory central synapses: from structure to function. Cell Tissue Res. 2006;326:221–237. doi: 10.1007/s00441-006-0288-z. [DOI] [PubMed] [Google Scholar]
- O'Neil JN, Connelly CJ, Limb CJ, Ryugo DK. Synaptic morphology and the influence of auditory experience. Hear Res. 2011;279(1-2):118–130. doi: 10.1016/j.heares.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio ME. Redistribution of synaptic AMPA receptors at glutamatergic synapses in the dorsal cochlear nucleus as an early response to cochlear ablation in the rat. Hearing Research. 2006:216–217. 154–167. doi: 10.1016/j.heares.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Wenthold RJ. Glutamate receptors are selectively targeted to postsynaptic sites in neurons. Neuron. 1997;18:939–950. doi: 10.1016/s0896-6273(00)80333-5. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Juiz JM. Chemical anatomy of excitatory endings in the dorsal cochlear nucleus: synaptic distribution of aspartate amino-transferase, glutamate and zinc. Journal of Comparative Neurology. 1998;399:341–358. doi: 10.1002/(sici)1096-9861(19980928)399:3<341::aid-cne4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Juiz JM. Differential distribution of synaptic endings containing glutamate, glycine, and GABA in the rat dorsal cochlear nucleus. Journal of Comparative Neurology. 2004;477:253–272. doi: 10.1002/cne.20248. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Gudsnuk KA, Smith Y, Ryugo DK. Revealing the molecular layer of the primate dorsal cochlear nucleus. Neuroscience. 2008;154:99–113. doi: 10.1016/j.neuroscience.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryugo DK, May SK. The projections of intracellularly labeled auditory nerve fibers to the dorsal cochlear nucleus of cats. J Comp Neurol. 1993;329:20–35. doi: 10.1002/cne.903290103. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Kretzmer EA, Niparko JK. Restoration of auditory nerve synapses in cats by cochlear implants. Science. 2005;310:1490–1492. doi: 10.1126/science.1119419. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Pongstaporn T, Huchton DM, Niparko JK. Ultrastructural analysis of primary endings in deaf white cats: morphologic alterations in endbulbs of Held. J Comp Neurol. 1997;385:230–44. doi: 10.1002/(sici)1096-9861(19970825)385:2<230::aid-cne4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Szabadits E, Cserép C, Szonyi A, Fukazawa Y, Shigemoto R, Watanabe M, Itohara S, Freund TF, Nyiri G. NMDA receptors in hippocampal GABAergic synapses and their role in nitric oxide signaling. J Neurosci. 2011:5893–5904. doi: 10.1523/JNEUROSCI.5938-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri C, Akert K, Livingston RB, Moor H. Particle aggregations at specialized sites in freeze-etched postsynaptic membranes. Brain Res. 1972;41:1–16. doi: 10.1016/0006-8993(72)90612-9. [DOI] [PubMed] [Google Scholar]
- Smith PF, Rhode WS. Electron microscopic features of physiologically characterized, HRP-labeled fusiform cells in the cat dorsal cochlear nucleus. J Comp Neurol. 1985;237:127–143. doi: 10.1002/cne.902370110. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Matsuzaki M, Tarusawa E, Momiyama A, Molnar E, Kasai H, Shigemoto R. Number and density of AMPA receptors in single synapses in immature cerebellum. J Neurosci. 2005;25:799–807. doi: 10.1523/JNEUROSCI.4256-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarusawa E, Matsui K, Budisantoso T, Molnár E, Watanabe M, Matsui M, Fukazawa Y, Shigemoto R. Input-specific intrasynaptic arrangements of ionotropic glutamate receptors and their impact on postsynaptic responses. J Neurosci. 2009;29:12896–12908. doi: 10.1523/JNEUROSCI.6160-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi Y, Matsubara A, Rinvik E, Ottersen OP. The arrangement of glutamate receptors in excitatory synapses. Ann N Y Acad Sci. 1999;868:474–82. doi: 10.1111/j.1749-6632.1999.tb11316.x. [DOI] [PubMed] [Google Scholar]
- Taschenberger H, Leão RM, Rowland KC, Spirou GA, von Gersdorff H. Neuron. 2002;36:1127–1143. doi: 10.1016/s0896-6273(02)01137-6. [DOI] [PubMed] [Google Scholar]
- Wang YX, Wenthold RJ, Ottersen OP, Petralia RS. Enbulb synapses in the anteroventral cochlear nucleus express specific subset of AMPA-type glutamate receptor subunits. J Neurosci. 1998;18:1148–1160. doi: 10.1523/JNEUROSCI.18-03-01148.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Manis PB. Short-Term Synaptic Depression and Recovery at the Mature Mammalian Endbulb of Held Synapse in Mice. J Neurophysiol. 2008;100:1255–1264. doi: 10.1152/jn.90715.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Nakadate K, Masugi-Tokita M, Shutoh F, Aziz W, Tarusawa E, Lorincz A, Molnár E, Kesaf S, Li YQ, Fukuzawa Y, Nagao S, Shigemoto R. Distinct cerebellar engrams in short-term and long-term motor learning. Proc Natl Acad Sci USA. 2014;111:E188–E193. doi: 10.1073/pnas.1315541111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg RJ, Rustioni A. Brainstem projections to the rat cuneate nucleus. J Comp Neurol. 1989;282:142–56. doi: 10.1002/cne.902820111. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Mugnaini E. Cartwheel neurons of the dorsal cochlear nucleus: a Golgi-electron microscopic study in rat. J Comp Neurol. 1984;227:136–157. doi: 10.1002/cne.902270114. [DOI] [PubMed] [Google Scholar]
- Wright DD, Ryugo DK. Mossy fiber projections from the cuneate nucleus to the cochlear nucleus in the rat. J Comp Neurol. 1996;365:159–72. doi: 10.1002/(SICI)1096-9861(19960129)365:1<159::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Xu-Friedman MA, Regehr WG. Ultrastructural contributions to desensitization at cerebellar mossy fiber to granule cell synapses. J Neurosci. 2003;23:2182–2192. doi: 10.1523/JNEUROSCI.23-06-02182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Xu-Friedman M. Relative roles of different mechanisms of depression at the mouse endbulb of Held. J Neurophysiol. 2008;99:2510–2521. doi: 10.1152/jn.01293.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Xu-Friedman MA. Impact of synaptic depression on spike timing at the endbulb of Held. J Neurophysiol. 2009;102:1699–1710. doi: 10.1152/jn.00072.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Oertel D. Neuronal circuits associated with the output of the dorsal cochlear nucleus through fusiform cells. J Neurophysiol. 1994;71:914–30. doi: 10.1152/jn.1994.71.3.914. [DOI] [PubMed] [Google Scholar]
- Zeng C, Shroff H, Shore SE. Cuneate and spinal trigeminal nucleus projections to the cochlear nucleus are differentially associated with vesicular glutamate transporter-2. Neuroscience. 2011;176:142–151. doi: 10.1016/j.neuroscience.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Nannapaneni N, Shore S. Vesicular glutamate transporters 1 and 2 are differentially associated with auditory nerve and spinal trigeminal inputs to the cochlear nucleus. J Comp Neurol. 2007;500:777–787. doi: 10.1002/cne.21208. [DOI] [PubMed] [Google Scholar]


