Abstract
Using the 1993–2011 data from the Wisconsin Longitudinal Study (N = 5,218), we examine prostate cancer screening, mortality after the diagnosis, and health behaviors as potential mechanisms explaining the paradoxical association between men’s higher education and higher prostate cancer risk. Our study combines within-cohort longitudinal hazard models predicting a prostate cancer diagnosis with Monte Carlo simulations estimating the joint effects of socioeconomic differences in prostate cancer screening and mortality after the diagnosis. Our findings strongly suggest that higher utilization of prostate cancer screening and lower mortality after the diagnosis are important explanations for higher prostate rates among more educated men. In addition to applying an innovative method to the issues of prostate cancer incidence and survival, our results have potentially important implications for the current debate about the utility of prostate cancer screening as well as for accurate predictions of future mortality and morbidity trends in the expanding older population.
The study was approved by the Internal Review Board of the Pennsylvania State University on 01/04/2012 (IRB #37586 “Childhood Socioeconomic Status and Late-Life Mortality: Sex and Race Differences”).
Prostate cancer is the most common non-skin cancer among men in the United States, with a lifetime diagnosis risk of nearly 16% (Moyer et al., 2010). Yet because most cases of prostate cancer are non-aggressive and have a favorable prognosis, the lifetime risk of prostate cancer mortality is estimated at 2.8% (Moyer et al., 2010). Whereas median age at a prostate cancer diagnosis is 67 years, the median age at death is 80 years (National Cancer Institute, 2012). Prostate cancer is a chronic disease of central interest to gerontologists because its incidence dramatically increases with age. The risk of prostate cancer is very low before age 50, and almost 90% of diagnoses occur after age 50 (National Cancer Institute, 2012). Thus, the issues of prostate cancer screening, diagnosis, treatment, and mortality are highly salient in the older population, both from a clinical perspective and with respect to understanding current and future trends in morbidity and mortality of older men.
Research indicates considerable population heterogeneity in prostate cancer incidence and survival after the diagnosis. A particularly striking pattern is that the rates of prostate cancer are significantly elevated among men of higher socioeconomic status (SES) compared to men with lower SES (Clegg et al., 2009; Scales et al., 2007). This pattern presents a provocative challenge because it is contrary to the lower morbidity of socially advantaged men with respect to almost all other chronic diseases (Elo, 2009; Phelan, Link, & Tehranifar, 2010). The most commonly offered explanation for the positive association between men’s SES and prostate cancer incidence is a higher frequency of regular prostate-specific antigen (PSA) screening among higher-SES men compared to men with lower socioeconomic resources (Sanderson et al., 2006; Steenland, Rodriguez, Mondul, Calle, & Thun, 2004). Although compelling, this mechanism warrants a more nuanced exploration because direct empirical evidence is relatively limited due to the paucity of within-individual studies following the same cohort(s) of men over a sufficiently long time period to capture the onset of prostate cancer and the length of survival after the diagnosis. Most previous studies of men’s SES and prostate cancer incidence were based on small clinical samples of participants recruited after the diagnosis and lack information about pre-diagnosis characteristics and prospective measures of prostate cancer screening (Rapiti et al., 2009; Sanderson et al., 2006; Steenland et al., 2004). When information on PSA testing prior to the diagnosis is not available, it may be problematic to distinguish a screening PSA test from a test conducted after the diagnosis (Steenland et al., 2004). Finally, because cancer registries do not contain nuanced measures of individual socioeconomic characteristics, including education, most studies that examined the association between SES and prostate cancer overwhelmingly used area-level SES as a proxy for individual-level SES (Howrey, Kuo, Lin, & Goodwin, 2012; Liu, Cozen, Bernstein, Ross, & Deapen, 2001).
Moreover, there are other potential mechanisms, specifically, mortality and health behaviors, that are strongly patterned by SES and can underlie SES differences in population patterns of prostate cancer. Yet these mechanisms have not been considered jointly with prostate cancer screening in previous research; thus it is not clear how the interplay of multiple influences creates the paradoxical positive association between higher SES and higher prostate cancer rates.
Using the 1993–2011 data from the Wisconsin Longitudinal Study (WLS), we examine prostate cancer screening, mortality after the diagnosis, and health behaviors as potential mechanisms linking men’s socioeconomic advantage to higher prostate cancer risk. In our study, SES is defined as education, which is the most consistent and powerful indicator of socioeconomic standing (Elo, 2009). This measure is not subject to reverse causality because education is typically completed in young adulthood. Moreover, education is particularly relevant to our study because high educational attainment enhances knowledge of preventive and curative options as well as the ability to effectively navigate the health care system (Phelan et al., 2010). This consequence of education is central to our argument about the PSA screening. In addition, higher education is strongly associated with reduced mortality (Masters, Hummer, & Powers, 2012), which is emphasized in our argument about survival after the prostate cancer diagnosis. We use Weibull parametric hazard models to estimate the effect of men’s education on the prostate cancer diagnosis between 1993 and 2005 as well as to examine whether health behaviors in 1993–1994 explain the effect of education. Further, we modify and extend a Monte Carlo (MC) simulation approach (Shlyakhter, Mirny, Vlasov, & Wilson, 1996) to explore whether and how educational differences in prostate cancer screening and survival after the diagnosis may contribute to the observed positive association between education and prostate cancer. Another strength of our study is that we explore the joint role of screening and mortality as intertwined mechanisms underlying the focal association. To our knowledge, MC simulations have not been applied to the analysis of SES and prostate cancer, yet this method can potentially improve our understanding of the meaning and sources of socioeconomic differences in prostate cancer when data for direct estimation are limited or unavailable.
Education and Prostate Cancer Screening
In our study, prostate cancer screening refers mostly to the PSA test because of its critical importance for raising the number of prostate cancer diagnoses in the population. The role of PSA screening has recently come to the forefront of medical discourse and public attention because of the U.S. Preventive Services Task Force (USPSTF) recommendation against PSA screening for men of all ages (Moyer et al., 2012). This recommendation is based on the lack of sensitivity and specificity of this test, the risks of overdiagnosis and overtreatment associated with prostate cancer screening, and the lack of consistent evidence of improved survival (Moyer et al., 2012). The USPSTF recommendation became a subject of a heated debate, with many urologists opposing the Task Force’s conclusion and emphasizing the benefits of PSA screening (Catalona et al., 2012; Schöder, 2012).
Surprisingly missing from both sides of the debate are socioeconomic disparities in prostate cancer screening and the fact that some population subgroups are systematically more likely to utilize preventive screening, which may create uneven population patterns of prostate cancer incidence. Of particular interest to this study is that the PSA test has a strong socioeconomic gradient (Sanderson et al., 2006). More educated men are more likely than their peers with less education to use regular prostate cancer screening (Liu et al., 2001; Sanderson et al., 2006; Steenland et al., 2004). The PSA test can detect prostate cancer at early asymptomatic stages and, thus, elevate the incidence of prostate cancer among well-educated men exposed to more frequent screening (Hsing & Devesa, 2001). Both in the United States and UK, SES was negatively related to prostate cancer incidence before 1987 when PSA screening became widely available, whereas a positive association emerged after 1987 (Hall, Holman, Wisniewski, & Semmes, 2005; Liu et al., 2001).
Given the Task Force recommendation and the ensuing controversy, it is particularly important to refine evidence of the role of education in the trends of prostate cancer overdiagnosis. This evidence can potentially inform modifications of the recommendation to take into account population heterogeneity in screening utilization and implications of this heterogeneity for the fact that overdiagnosis of prostate cancer is socially patterned. Yet, despite the potential importance of the screening explanation for higher rates of prostate cancer diagnosis among more educated men, it may be difficult to test this explanation empirically. Given the limited information about men’s education, PSA testing prior to the diagnosis, and longitudinal mortality follow-ups in most data sets, we modify and extend a MC simulation method (Shlyakhter et al., 1996) to evaluate indirectly the screening explanation for the positive association between education and prostate cancer based on men’s knowledge of their prostate cancer diagnosis. The diagnosis of prostate cancer in our study is self-reported. We make an assumption that reporting of prostate cancer by our participants is based on their knowledge of the diagnosis. Because prostate cancer is often indolent and asymptomatic, it is very likely that men may have prostate cancer but do not report it simply because they are unaware of it. At the early asymptomatic stage, the major source of knowledge of the diagnosis is preventive screening. More educated men have higher rates of prostate cancer screening (Rapiti et al., 2009; Steenland et al., 2004) and, thus, are more likely to be diagnosed with prostate cancer than low-SES men even if the true risk of the disease is the same regardless of education. Our MC simulations evaluate which proportion of less educated men should underreport a prostate cancer diagnosis to wipe out the observed positive effect of education. Under our assumption that prostate cancer screening is the major source of educational differences in the knowledge of a prostate cancer diagnosis, this analysis essentially explores whether the positive association between education and prostate cancer is spurious due to higher screening utilization by more educated men.
Education and Mortality after the Prostate Cancer Diagnosis
Whereas higher rates of prostate cancer screening can potentially inflate the rates of prostate cancer diagnosis among well-educated men, socioeconomic differences in survival after the diagnosis can lead to the overrepresentation of higher-SES men among prostate cancer survivors. Central to our argument is that highly educated men have lower mortality than men with low education both in the absence and presence of prostate cancer. Among men diagnosed with prostate cancer, higher-SES men live longer regardless of their cause of death, whereas low-SES men have higher mortality after the diagnosis both from prostate cancer and from all other causes (Du, Lin, Johnson, & Altekruse, 2011; Rapiti et al., 2009; Steenland et al., 2004). Because well-educated men live longer after the diagnosis, they remain longer in the population and inflate the rates of prostate cancer in socially advantaged groups. In contrast, men with lower education are more likely to die from all causes and, thus, are removed from the population earlier, often for reasons unrelated to prostate cancer.
Although the differential mortality explanation alone can potentially account for the positive association between education and prostate cancer, it can be a particularly powerful mechanism when acting synergistically with differential prostate screening by education. Using MC simulations, we examine the extent to which the positive association between education and prostate cancer is explained by educational differences in mortality after the diagnosis. We further evaluate how the effect of men’s education on prostate cancer rates changes when educational differences in mortality are considered jointly with educational differences in prostate cancer screening.
Education, Health Behaviors, and Prostate Cancer
The differential screening and mortality explanations suggest that the positive association between education and prostate cancer risk is spurious. Alternatively, it is possible that the effect of education is real and mediated by health behaviors. If certain health habits that increase the risk of prostate cancer are more prevalent among men with higher than lower education, then the elevated risk of a prostate cancer diagnosis among more educated men will be explained by these lifestyle factors. Specifically, research suggests that compared to those with little schooling, well-educated persons are more likely to drink moderately than to abstain from alcohol (Mirowsky & Ross, 1998). Although reported associations between alcohol use and prostate cancer are mixed (Chao et al., 2010; Velicer, Kristal, & White, 2006), some studies suggest that alcohol use may increase the risk of prostate cancer. Compared to men reporting almost never drinking alcohol, moderate alcohol consumption is associated with an increased risk of prostate cancer (Hayes et al., 1996; Sesso, Paffenbarger, & Lee, 2001). Because more educated men are less likely to abstain from alcohol and more likely to be moderate drinkers than their less educated peers, alcohol use may be a potential link between higher levels of education and elevated prostate cancer risk. With respect to body weight, some studies suggest that obesity may reduce the risk of localized and indolent prostate cancer while increasing the risk of aggressive disease or cancer fatality (Buschemeyer & Freedland, 2007; Giovannucci et al., 2003). Lower levels of obesity among more educated men (Schieman, Pudrovska, & Eccles, 2006) may explain the fact that education is associated positively with prostate cancer incidence (especially nonaggressive indolent tumors) but negatively with prostate cancer mortality. Thus, we examine the extent to which higher levels of education increase the risk of a prostate cancer diagnosis through alcohol use and body weight.
In addition, research indicates that physical activity can decrease the risk of prostate cancer (Wolin & Stoll, 2012), whereas smoking tends to increase this risk (Rolison, Hanoch, & Miron-Shatz, 2012). Yet, we do not expect exercise and smoking to explain the positive association between education and prostate cancer because well-educated men have a positive profile of these behaviors, which would have reduced their risk of prostate cancer. Therefore, smoking and physical activity are used as control variables in our models but not as focal mediators.
In sum, the purpose of the present study is to evaluate three potential explanations for the positive association between men’s education and prostate cancer. According to the screening and mortality explanations, men’s education does not affect the risk of prostate cancer. Any observed association between education and prostate cancer is an artifact of educational differences in prostate cancer screening and survival after the diagnosis. Alternatively, the health behaviors explanation suggests that the effect of education is real and mediated by obesity and alcohol use that are differentially distributed by education. In addition to improving our knowledge of the relationship between education and prostate cancer, this study introduces the application of MC simulations to the social epidemiology of prostate cancer.
DATA AND METHODS
The Wisconsin Longitudinal Study (WLS) is a long-term study of a random sample of 10,317 men and women who graduated from Wisconsin high schools in 1957. Participants were interviewed at ages 18 (in 1957), 36 (in 1975), 54 (in 1993), and 65 (in 2004). Survey data were also collected from a selected sibling in 1977, 1994, and 2005. The overwhelming majority of the WLS participants are non-Hispanic White because very few members of racial or ethnic minority groups graduated from Wisconsin high schools in the 1950s. The WLS sample retention is exceptionally high. The baseline 1957 sample of main participants comprised 4,991 men, 87 % of whom (4,330 men) participated in the 1975 wave. About 86% of the 1975 male participants were re-interviewed in 1993, and 74% of the 1975 men participated also in the 2004 interview. In addition, 553 brothers of the main participants were interviewed in all waves between 1977 and 2005. Our analytic sample contains men who participated in the 1993–1994 and 2004–2005 waves: 259 men (151 main respondents and 108 siblings) who have been diagnosed with prostate cancer by 2005 and 4,959 men who have never been diagnosed with prostate cancer. This sample also subsumes 455 men (36 with and 419 without a prostate cancer diagnosis) who died by 2011. The cause of death and date of death was established via the National Death Index (NDI) link.
Measures
The dependent variable
All participants were asked, “Has a doctor ever told you that you have cancer or a malignant tumor, not including minor skin cancers?” If participants answered affirmatively, further questions about the cancer site and year of diagnosis were included. Prostate cancer is coded 1 for men who reported being diagnosed with prostate cancer and 0 for men who did not report a cancer diagnosis. Age at prostate cancer diagnosis is measured in years. The median age at diagnosis is 67 years, the youngest age is 50 years, and the oldest age is 80 years. We conducted additional verification of self-reports of prostate cancer diagnosis using information from death certificates obtained via the NDI link. Among deceased men, 29 died of prostate cancer as the primary cause. In addition, death certificates of 56 men indicated prostate cancer either as an underlying cause (Item 32, Part I) or other significant condition contributing to death (Item 32, Part II). A comparison of self-reported cancer to death certificates suggests a high level of congruence, with 81 men (94%) correctly reporting their diagnosis.
Independent variables
Our focal predictor variable is men’s education reported in 1993–1994, which is represented with a dummy variable coded 1 if a man obtained at least some college education and coded 0 for men who had a high school diploma or did not graduate from high school. Men’s occupation is represented with several mutually exclusive dummy variables: professional/managerial; sales, administrative, or service occupation; crafts, operative, technician; farmer, laborer. Men’s financial resources are reflected with a natural log of wealth in 1993–1994. Marital status is coded 1 if a man was married and 0 if he was unmarried. Parental status is represented with the total number of children.
Health behaviors were reported in 1993–1994. Alcohol use is categorized into non-drinkers, moderate drinkers (1–2 drinks per day), and heavy drinkers (>2 drinks per day). Body weight is coded based on body mass index (BMI) as normal weight (BMI < 25), overweight (BMI 25–29.9), and obese (BMI ≥ 30). Smoking is represented with three dummy variables: current smoker, former smoker, and nonsmoker. Physical activity is coded as a monthly frequency of moderate and vigorous exercise (1 = less than once per month, 2 = about one to three times per month, 3 = once or twice per week, 4 = three or more times per week).
We also include a measure of a digital rectal prostate exam coded 1 if a man had a prostate exam in the 12 months prior to the 2004–2005 interview. Among men with prostate cancer, 52 men reported the exam before the diagnosis and 207 after the diagnosis. Among men not diagnosed with prostate cancer, 2,722 reported having a prostate exam. Finally, all models adjust for having a first-degree relative with prostate cancer and birth year. Main participants were born in 1939, and siblings between 1924 and 1955, with 1940 as the median birth year.
Analytic Plan
We start with a comparison of means of all variables between men who have been diagnosed with prostate cancer and men without prostate cancer. Further, to predict the diagnosis of prostate cancer, we use a continuous-time parametric survival model assessing education as a focal predictor of a prostate cancer diagnosis until men’s age at the latest wave of data collection in 2005. The hazard function for man i at time j represents the instantaneous probability that a man is diagnosed with prostate cancer at a given age conditional on not having been diagnosed before that age and is modeled as:
| (1) |
where h(tij) is the hazard of prostate cancer diagnosis evaluated at exact age t, h0 is a baseline hazard, β and ω are vectors of parameters, and Zi is a vector of control variables. The baseline hazard h0 is represented with the Weibull distribution chosen over other functional forms based on the model fit:
| (2) |
where p is a shape parameter and β0 is a level parameter. In all survival models, robust standard errors are used to account for nonindependence of observations between main participants and their brothers. We estimate our models using stepwise regression by adding blocks of variables sequentially and comparing changes in the model fit with the addition of each block.
We use MC simulations (Shlyakhter et al., 1996) to explore whether the effect of education can be driven by differences in prostate cancer reporting and mortality between men with higher and lower levels of education. First, we assume that both men with college education and men without college education might have had prostate cancer but did not report it because they were unaware of it. We further assume that the two groups of men differed in their underreporting of a prostate cancer diagnosis by fraction fH for men with college education and fraction fL for men without college education. Fractions fH and fL were assumed to follow a normal distribution with the mean equal zero and standard deviations σH and σL. The distributions were truncated at zero, so that only positive values of fH and fL were allowed to exclude over-reporting of prostate cancer. The following formula was used to calculate fH and fL:
| (3) |
A cross-tabulation of education and prostate cancer status divided the sample into four groups: college-educated men with prostate cancer (a), men without college education with prostate cancer (b), college-educated men without prostate cancer (c), and men without college education without prostate cancer (d). The odds ratio observed from this contingency table was 1.37, which means that college-educated men had 37% higher odds of prostate cancer than men without college education. Under assumption that education does not have an effect on prostate cancer, the odds of prostate cancer among college-educated men (a/c) equal the odds of prostate cancer among men without college education (b/d). If underreport of prostate cancer was present, the observed numbers in each cell would be aO, bO, cO, and dO, where aO = a − fH × a, cO = c + fH × a, bO = b − fL × b, and dO = d + fL × b. For every combination of σH and σL we simulated aO, bO, cO, and dO 100,000 times, calculated the odds ratio (aOcO/bOdO) for each simulation, and estimated the fraction exceeding the observed odds ratio of 1.37. The range of variation of σH and σL was 0–0.5.
Because mortality affects the number of men living with prostate cancer, selective survival may create a difference in the number of college-educated men and men without college education living with prostate cancer. To estimate the range of this effect, we also simulated a scenario where a fraction of men diagnosed with prostate cancer had died before their cancer was registered in the study. If both mortality and underreport of a prostate cancer diagnosis were present, the observed numbers in each cell would be aO = a − fH × a − fHma× (a − fH × a), cO = c + fH × a − (fHmc ×c+ fHma× fH × a), bO = b − fL × b − fLmb× (b − fL × b), and dO = d + fL × b − (fLmd ×d+ fLmb×fL × b). This formula includes mortality from all causes. Because all-cause mortality for men diagnosed with prostate cancer incorporates mortality from prostate cancer, we used two different mortality fractions for men with prostate cancer diagnosis (a and b) and without the diagnosis (c and d). The fractions of all-cause mortality were set to fixed numbers estimated from our data: fHma = 0.121, fLmb = 0.164, fHmc = 0.065, and fLmd = 0.104. In contrast, the fractions of men underreporting prostate cancer (fH and fL) were allowed to vary randomly with the variability described by σH and σL.
RESULTS
A comparison of men with and without prostate cancer shown in Table 1 reveals that men with prostate cancer were significantly more likely to have at least some college education than their peers without prostate cancer (58% versus 50%, p < .05), whereas the two groups were similar with respect to their occupation, wealth, marital status, and the number of children. In terms of health behaviors, there was no difference by prostate cancer diagnosis in smoking and BMI. In contrast, men diagnosed with prostate cancer were less likely to abstain from alcohol, more likely to consume 1–2 drinks per day, and had lower levels of vigorous exercise than men without a diagnosis. Finally, men who reported a prostate cancer diagnosis in 2004–2005 were significantly more likely to have a prostate exam in the same year than men without a prostate cancer diagnosis (72% versus 55%, p < .001). These patterns remain very similar when variables are summarized separately for main participants and siblings (available upon request).
Table 1.
Summary Statistics for the Study Variables: The Wisconsin Longitudinal Study, Men (N = 5,218)
| Variables | Diagnosed with prostate cancer (n = 259) | Not diagnosed with prostate cancer (n = 4,959) |
|---|---|---|
| Age | 64.375 (1.692) | 64.412 (1.737) |
| At least some college education | .575* | .497 |
| Occupation: | ||
| Professional, managerial | .333 | .321 |
| Sales, clerical, service | .184 | .205 |
| Crafts, operative, technician, | .190 | .162 |
| Wealth (ln) | 11.732 (3.021) | 11.487 (3.220) |
| Married | .911 | .868 |
| Number of children | 2.988 (1.699) | 2.844 (1.606) |
| Health behaviors: | ||
| Never smoked | .441 | .412 |
| Current smoker | .145 | .128 |
| Former smoker | .419 | .459 |
| Abstains from drinking | .204* | .274 |
| Moderate drinking (1–2 drinks per day) | .653* | .593 |
| Heavy drinking | .143 | .132 |
| Light physical activity | 3.220 (.850) | 3.217 (.775) |
| Vigorous physical activity | 1.938* (1.089) | 2.100 (1.181) |
| Normal weight (BMI <25) | .166 | .175 |
| Overweight (BMI 25–29.9) | .633 | .621 |
| Obese (BMI ≥ 30) | .201 | .204 |
| Prostate exam in 2004–2005 | .718*** | .549 |
Note. Each cell contains means/proportions and standard errors for means (in parentheses).
Asterisks denote significant differences between men with and without prostate cancer:
p < .05;
p < .01;
p < .001.
Weibull Survival Models Predicting the Prostate Cancer Diagnosis
Table 2 shows the effect of education on the risk of prostate cancer until 2005. As indicated in Model 1, men who had at least some college education were 53% more likely to be diagnosed with prostate cancer than men with a high school diploma or less (HR = 1.532, p < .001). After adjustment for occupation, wealth, marital status, and the number of children in Model 2, the effect of education changes only trivially. Unlike education, men’s wealth, occupation, and marital and parental statuses are not significantly associated with prostate cancer hazard. Finally, men’s health behaviors are added in Model 3, which indicates that smoking, alcohol use, and BMI are neither related to prostate cancer nor explain the significant effect of education. Although exercise is associated with a reduced prostate cancer risk, physical activity does not explain the effect of education because more educated men have higher levels of physical activity; thus, their higher risk of diagnosis is despite their more frequent exercise.
Table 2.
Hazard Ratios and 95% Confidence Intervals from Weibull Parametric Hazard Models Predicting the Diagnosis of Prostate Cancer: The Wisconsin Longitudinal Study, Men (N = 5218, Prostate Cancer Cases = 259)
| Variables | (1) | (2) | (3) | (4) |
|---|---|---|---|---|
| At least some college education vs ≤ high school | 1.532*** (1.189, 1.975) | 1.503*** (1.123, 2.009) | 1.544*** (1.192, 2.000) | 1.528*** (1.183, 1.972) |
| Occupation: | ||||
| Professional, managerial | 1.000 | |||
| Sales, clerical, service | 1.171 (.829. 1.654) | |||
| Crafts, operative, technician, | 1.043 (.747, 1.456) | |||
| Wealth (ln) | 1.034 (.984, 1.086) | |||
| Married | 1.123 (.822, 1.533) | |||
| Number of children | .958 (.889, 1.033) | |||
| Health behaviors: | ||||
| Never smoked | 1.000 | |||
| Current smoker | 1.200 (.811, 1.775) | 1.295 (.892, 1.878) | ||
| Former smoker | .912 (.687, 1.210) | |||
| Moderate drinking | 1.000 | 1.041 (.795, 1.362) | ||
| Abstain | .887 (.641, 1.227) | |||
| Heavy drinking | 1.068 (.738, 1.543) | |||
| Frequency of light physical activity | .836* (.707, .987) | |||
| Frequency of vigorous physical activity | .872* (.733, .998) | |||
| Frequency of physical activity | .847* (.712, .991) | |||
| Normal | 1.000 | |||
| Overweight | 1.214 (.849, 1.734) | |||
| Obese | 1.234 (.800, 1.902) | |||
| Overweight or Obese | 1.241 (.874, 1.760) | |||
| Family history of prostate cancer | 1.642*** (1.271, 2.119) | 1.647*** (1.275, 2.127) | 1.641*** (1.269, 2.122) | 1.638*** (1.267, 2.117) |
| β0 (Level) | −51.016 | −51.766 | −51.135 | −51.385 |
| p (Shape) | 11.373 | 11.458 | 11.444 | 11.366 |
| Log likelihood (df) | −571 (4) | −568 (9) | −566 (12) | −568 (8) |
| AIC | 1150 | 1155 | 1156. | 1153 |
| BIC | 1176 | 1214 | 1234 | 1206 |
Note: All models adjust for birth year and family history of prostate cancer. Standard errors are robust to the nonindependence of observations between siblings. df = degrees of freedom. BIC = Bayesian information criterion. AIC = Akaike information criterion.
p < .05;
p < .01;
p < .001.
Education and the Prostate Cancer Exam
Although not perfect, there is a direct measure of prostate cancer screening available in our data. All men reported whether or not they had a digital rectal prostate exam (DRE) during the 12 months prior to the interview. We analyze this direct measure to compare its effect to the results of our indirect estimation via MC simulations. Among men not diagnosed with prostate cancer, men with higher education were 32% more likely to have a prostate exam than less educated men (OR = 1.32, 95% CI: 1.18, 1.47). Among men diagnosed with prostate cancer who reported the prostate exam before the diagnosis, adjustment for DRE reduces the effect of education from HR = 1.53 to HR = 1.24 (or by 47%), and it becomes significant at the .05 level. The mediation analysis (VanderWeele, 2011) indicates that this reduction is significant at the .001 level.
Monte Carlo Simulations
To extend and refine the results from our regression models, we apply MC simulations to examine whether the observed effect of education may reflect differences in prostate cancer knowledge and mortality. Findings reveal that for all simulated combinations of σH and σL, the minimum fraction of men without college education that should underreport their diagnosis to produce the observed odds ratio of 1.37 is 0.26. In other words, if 26% of men with lower education had cancer but did not report it, the observed effect of education on prostate cancer could be driven entirely by differential reporting of a prostate cancer diagnosis (Type I error). A review of recent studies (Ross, Bernheim, Bradley, Teng, & Gallo, 2007; Ross, Berkowitz, & Ekwueme, 2008; Scales et al., 2007; Steenland et al., 2004) suggests that higher-SES men are, on average, about 28.3% more likely than men of lower SES to undergo regular prostate cancer screening, which is the main source of differential knowledge of a prostate cancer diagnosis. SES differences in prostate cancer screening reported in the literature are greater than the difference of 26% required to wipe out the effect of education. Thus, the differential knowledge of a prostate cancer diagnosis resulting from education difference in prostate cancer screening is a plausible explanation for a higher risk of a prostate cancer diagnosis among men with college education.
It is also possible that the fraction of men underreporting prostate cancer may differ across samples. Figure 1 illustrates how the probability of Type I error changes depending on the values of σH and σL (see Eq. 3). When σL < 0.05 (Figure 1A), the probability of Type I error is essentially zero regardless of the value of σH. When σL > 0.1, the risk of a Type I error increases and reaches 5% at σL = 0.12 if there is no variation in fH (σH = 0). As σH increases, the risk of Type I error declines. At the highest simulated value of σH = 0.5, the probability of Type I error reaches 5% at σL = 0.21. For comparison, our review of recent studies based on samples drawn from different populations suggests a value of σ = 0.20 (Cheng et al., 2009; Rapiti et al., 2009; Schwartz et al., 2009; Steenland et al., 2004; Tewari et al., 2009). If we assume both σH and σL to be 0.20, there is a 9% chance of Type I error. Thus, it appears that the observed effect of education is sensitive to sampling error and may reflect random fluctuation in prostate cancer knowledge across samples.
Figure 1.
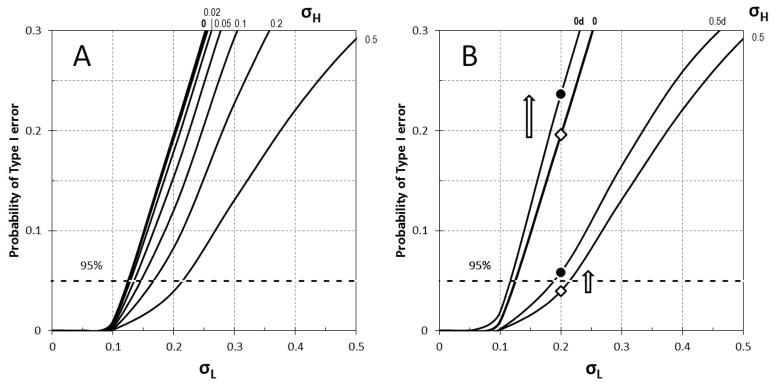
Monte Carlo Simulations of the Risk of Type I Error Based on Differential Knowledge of a Prostate Cancer Diagnosis and Differential Mortality by Education
Note: σH denotes the variation in underreporting of prostate cancer among men with at least some college education; σL denotes the variation in underreporting of prostate cancer among men with high school education or less. Panel A shows the probability of Type I error without the inclusion of mortality. Panel B shows an increase in this probability when differential mortality by education is taken into account (black dots) compared to simulations without the effect of mortality (white diamonds).
Source: The Wisconsin Longitudinal Study, main participants and siblings, 1993–2011.
Differential mortality among men with different levels of education can affect the number of men living with prostate cancer and, thus, lead to the observed difference in prostate cancer prevalence even if cancer incidence rates were the same. We used simulations to quantify the combined effects of cancer underreporting and mortality on the probability of Type I error. (Figure 1B), prostate cancer mortality was assumed to differ by education: fHma = 0.121 and fLmb = 0.164 based on the observed data. The addition of differential mortality significantly increased the probability of Type I error. When σH = 0 and σL = 0.2 probability of type I error increases from 0.196 to 0.237 (by 21%) and when σH = 0.5, at the same value σL = 0.2 the error probability increases from 0.0396 to 0.0584, by 47% (Figure 1B). If we set both σH and σL equal to 0.20, the addition of differential mortality by education (Figure 1B) increases the probability of Type I error by 36%. This results support our hypothesis that the observed higher rates of prostate cancer among more educated men reflect not only differential screening but also selective mortality and, especially, a combination of screening and mortality.
DISCUSSION
Using data from the Wisconsin Longitudinal Study (WLS), we evaluate potential explanations for the positive association between men’s education and the risk of prostate cancer, including educational differences in prostate cancer screening, mortality after a prostate cancer diagnosis, and health behaviors. We applied a parametric survival model and Monte Carlo simulations to explore the meaning and sources of differences in prostate cancer rates by educational level. Men who had at least some college education were 53% more likely to be diagnosed with prostate cancer than men with a high school diploma or less adjusting for occupation, wealth, marital status, and number of children, health behaviors, and family history of prostate cancer. Our findings strongly suggest that this positive effect of education is an artifact of educational differences in prostate cancer screening and mortality after the diagnosis.
Prostate Cancer Screening
Socioeconomic differences in prostate cancer screening are very likely to produce the observed positive effect of education. The knowledge of a prostate cancer diagnosis was significantly higher among well-educated men compared to men with lower levels of education. According to our assumption, prostate cancer screening is the major source of knowledge of a prostate cancer diagnosis because most prostate cancers are characterized by an indolent asymptomatic nature. Low-SES men are less likely to utilize regular screening (Liu et al., 2001; Sanderson et al., 2006) and, thus, are less likely to be aware of a prostate cancer diagnosis. Our simulations suggest that if 26% of men with lower education had cancer but did not report it, the observed effect of education on prostate cancer could be driven entirely by differential reporting of a prostate cancer diagnosis (Type I error). This proportion is not significantly different from the difference of 28.3% required to wipe out the effect of education, as suggested by recent research on PSA screening (Ross et al., 2007; Ross et al., 2008; Scales et al., 2007; Steenland et al., 2004). Moreover, the observed effect of education is sensitive to sampling error and may reflect random fluctuation in prostate cancer reporting across samples. Therefore, the differential knowledge of a prostate cancer diagnosis resulting from education difference in prostate cancer screening is a plausible explanation for a higher risk of prostate cancer among men with college education.
This finding can contribute to the ongoing debate about PSA screening recommendations because we emphasize an aspect that is missing from the entire discussion – the unequal rates of cancer screening by SES (Buelow, Zimmer, Mellor, & Sax, 1998). The wide implementation of PSA screening after 1987 inflated prostate cancer diagnoses among all men but this effect was particularly pronounced among higher-SES men. Because overdiagnosis of prostate cancer is greater among the well-educated, prostate cancer screening can lead to the underestimation of prostate cancer among low-SES men who are more vulnerable to the disease and have higher prostate cancer mortality after the diagnosis (Rapiti et al., 2009; Steenland et al., 2004).
Higher-SES men’s adoption of PSA screening when it was universally recommended is consistent with extensive research documenting that the more educated are better able to navigate the health care system and take advantage of medical innovations (Schnittker, 2004; Phelan et al., 2010). Given the recent change in recommendations about prostate cancer screening (Moyer et al., 2012), well-educated men may be once again more likely to adopt the new knowledge and be at the forefront of reduced screening because persons with high levels of education make more informed decisions regarding prevention due to their more accurate understanding of health risks (Phelan et al., 2010; Schnittker, 2004). This potential reversal of SES differences in prostate cancer screening can have implications for future population trends of prostate cancer incidence, which can repeat pre-1987 patterns when higher-SES men had a lower risk of a prostate cancer diagnosis compared to low-SES men.
Education and Mortality after the Prostate Cancer Diagnosis
It is even more likely that the positive effect of education reflects the joint influence of higher prostate cancer screening and lower mortality among more educated men. When we added the assumption that men with less education are more likely to die after a prostate cancer diagnosis from all causes than men with higher levels of education, the probability that the observed effect of education is not statistically significant (Type I error) increased by 36% compared to simulations estimating the effect of only prostate cancer screening. Because most prostate cancers are typically associated with a favorable survival prognosis, men tend to die with prostate cancer rather than from prostate cancer. All-cause and cause-specific mortality is markedly lower among well-educated men compared to men with lower education (Elo, 2009; Montez, Hayward, Brown, & Hummer, 2009) because education contributes to a wide array of health-enhancing resources, including cognitive ability, problem-solving, self-efficacy, healthy lifestyle, financial stability, and access to high-quality health care (Phelan et al., 2010). Therefore, in addition to higher rates of PSA screening, longer survival of more educated men after the diagnosis inflates prostate cancer rates among socioeconomically advantaged groups. This finding suggests that studies evaluating the effectiveness of PSA screening in improving prostate cancer outcomes should consider not only prostate cancer mortality but also mortality from all causes after the diagnosis and the marked socioeconomic differences in men’s survival. A failure to address socioeconomic differentials in screening and mortality may create a distorted picture of trends in prostate cancer in the population of older men.
Our findings also have important policy implications. Given an unprecedented increase in the number of people 65 and older, understanding trends in older adults’ morbidity and mortality is of critical importance for addressing the anticipated increase in the burden on the healthcare system in general and Medicare in particular. Researchers, policymakers, and other stakeholders express concerns about the expansion of morbidity reflecting an increasingly large number of people living with chronic diseases and disability (Robine & Michel, 2005). An unintended consequence of large-scale PSA screening has been an artificial expansion of morbidity, especially among high-SES men who are typically diagnosed with indolent asymptomatic cancers and live many years after the diagnosis. Addressing socioeconomic inequality in prostate cancer screening and survival is important for making accurate predictions of future health trends in the older population that will facilitate the development of effective aging-related policies.
Health Behaviors
Contrary to our hypothesis, educational differences in alcohol use and obesity do not explain the positive association between education and prostate cancer diagnosis. Of all health behaviors included in our models (body weight, alcohol use, smoking, and physical activity), only light and vigorous exercise were significantly associated with the lower risk of prostate cancer. Yet, the effect of education persists after adjustment for physical activity, which is consistent with our expectations because higher levels of exercise among more educated men would have resulted in a lower risk of a prostate cancer diagnosis. An interesting question is why obesity and alcohol use do not explain the effect of education. One possibility is that health behaviors at earlier life course stages are more important influences on prostate cancer etiology than lifestyle in late midlife that was assessed in our study. For example, in a cohort study of 47,781 men, obesity in adulthood was not related to prostate cancer incidence, whereas body mass at ages 5, 10, and 21 was strongly inversely related to the risk of advanced and metastatic carcinoma (Giovannucci et al., 1997). The scope of lifestyle measures in childhood and young adulthood is limited in the WLS. Ideally, future studies of prostate cancer should include information about health behaviors assessed prospectively starting in early life.
Limitations and Future Directions
To our knowledge, this is the first study to examine the joint implications of prostate cancer screening and mortality for the positive association between men’s education and prostate cancer. Our Monte Carlo simulations suggest that higher utilization of prostate cancer screening and lower mortality after the diagnosis may be important explanations for higher prostate cancer rates among more educated men. Future research should explore these explanations further, if possible, with data that are nationally representative and contain an assessment of prostate cancer screening prior to the diagnosis.
The incidence of prostate cancer varies by race and ethnicity in the United States (Crawford, 2009). African American men are particularly at risk even after adjusting for dietary and lifestyle risk factors (Platz, Rimm, Willett, Kantoff, & Giovannucci, 2000). These disparities might be related to differences in genetic background and physiologic status, such as sex steroid hormones (Platz & Giovannucci, 2004). If biological factors play a different role in prostate cancer etiology among African American men, the importance of social factors may also vary by race. Yet, our data do not allow comparisons across racial and ethnic groups, which is an important direction for future research. Moreover, because most men in our sample graduated from high school, our findings do not fully reflect the experiences of men with lower functional literacy for whom it may be more problematic to interpret preventive care guidelines.
Most men in our sample were born in the late 1930s. The PSA screening recommendation was widely implemented in 1987 when these men were about 50 years old. Thus, they have been exposed to the messages of the universal prostate cancer screening for over 20 years and are now confronted with the opposite recommendations in their 70s. An important direction for future research will be to explore socioeconomic differences in prostate cancer diagnosis and survival after diagnosis in younger cohorts of men who are now in their adult and middle-aged years and have just started receiving the information about the risks of PSA screening. Further, it is important to address other potential influences on prostate cancer that we could not evaluate with our data, for example, human papilloma virus (Adami, Kuper, Andersson, Bergström, & Dillner, 2003), cholesterol (Batty, Kivimäki, Clarke, Davey Smith, & Shipley, 2011), or unmarried status (Batty et al., 2011). Moreover, because findings about lifestyle and prostate cancer are mixed, the role of health behaviors in the etiology of prostate cancer still remains uncertain. Even if some studies report a relationship between alcohol use or body weight and prostate cancer, others do not (Baillargeon et al., 2006; Chao et al., 2010). More research is needed about lifestyle factors at different life stages and prostate cancer etiology.
Despite these limitations, this is the first study to our knowledge to combine within-cohort longitudinal hazard models predicting prostate cancer diagnosis with MC simulations estimating the joint effects of SES differences in prostate cancer screening and mortality after the diagnosis. In addition to applying an innovative method to the issues of prostate cancer incidence and survival, our findings have potentially important implications for the current debate about the utility of PSA screening as well as for accurate understanding of future health trends for effective policies addressing health care needs of the expanding older population.
Contributor Information
Tetyana Pudrovska, University of Texas-Austin.
Andriy Anishkin, University of Maryland-College Park.
References
- Adami HO, Kuper H, Andersson SO, Bergström R, Dillner J. Prostate cancer risk and serologic evidence of human papilloma virus infection: A population-based case-control study. Cancer Epidemiology, Biomarkers and Prevention. 2003;12:872–5. [PubMed] [Google Scholar]
- Baillargeon J, Platz EA, Rose DP, Pollock BH, Ankerst DP, Haffner S, Higgins B, Lokshin A, Troyer D, Hernandez J, Lynch S, Leach RJ, Thompson IM. Obesity, adipokines, and prostate cancer in a prospective population-based study. Cancer Epidemiology, Biomarkers & Prevention. 2006;15:1331–35. doi: 10.1158/1055-9965.EPI-06-0082. [DOI] [PubMed] [Google Scholar]
- Batty GD, Kivimäki M, Clarke R, Davey Smith G, Shipley MJ. Modifiable risk factors for prostate cancer mortality in London: Forty years of follow-up in the Whitehall study. Cancer Causes and Control. 2011;22:311–8. doi: 10.1007/s10552-010-9691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buelow JR, Zimmer AH, Mellor MJ, Sax R. Mammography screening for older minority women. Journal of Applied Gerontology. 1998;17:133–49. [Google Scholar]
- VanderWeele T. Causal mediation analysis with survival data. Epidemiology. 2011;22:582–585. doi: 10.1097/EDE.0b013e31821db37e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschemeyer WC, Freedland SJ. Obesity and prostate cancer: Epidemiology and clinical implications. European Urology. 2007;52:331–43. doi: 10.1016/j.eururo.2007.04.069. [DOI] [PubMed] [Google Scholar]
- Catalona WJ, D’Amico AV, Fitzgibbons WF, Kosoko-Lasaki O, Leslie SW, Lynch HT, Moul JW, Rendell MS, Walsh PC. What the U.S. Preventive Services Task Force missed in its prostate cancer screening recommendation. Annals of Internal Medicine. 2012;157:137–139. doi: 10.7326/0003-4819-157-2-201207170-00463. [DOI] [PubMed] [Google Scholar]
- Chao C, Haque R, Van Den Eeden S, Caan BJ, Poon KY, Quinn VP. Red wine consumption and risk of prostate cancer: The California Men’s Health Study. International Journal of Cancer. 2010;126:171–79. doi: 10.1002/ijc.24637. [DOI] [PubMed] [Google Scholar]
- Cheng I, Witte JS, McClure LA, Shema S, Cockburn M, John E, Clarke CA. Socioeconomic status and prostate cancer incidence and mortality rates among the diverse population of California. Cancer Causes and Control. 2009;20:1431–40. doi: 10.1007/s10552-009-9369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg LX, Reichman ME, Miller BA, Hankey BF, Singh GK, Lin YD, Goodman MT, Lynch CF, Schwartz SM, Chen VW, Bernstein L, Gomez SL, Graff JJ, Lin CC, Johnson NJ, Edwards BK. Impact of socioeconomic status on cancer incidence and stage at diagnosis: Selected findings from the Surveillance, Epidemiology, and End Results: National Longitudinal Mortality Study. Cancer Causes and Control. 2009;20:417–35. doi: 10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford ED. Understanding the epidemiology, natural history, and key pathways involved in prostate cancer. Urology. 2009;73(Supplement 5A):S4–10. doi: 10.1016/j.urology.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Du XL, Lin CC, Johnson NJ, Altekruse S. Effects of individual-level socioeconomic factors on racial disparities in cancer treatment and survival: Findings from the National Longitudinal Mortality Study, 1979–2003. Cancer. 2011;117:3242–51. doi: 10.1002/cncr.25854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elo IT. Social Class Differentials in Health and Mortality: Patterns and Explanations in Comparative Perspective. Annual Review of Sociology. 2009;35:553–72. [Google Scholar]
- Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Height, body weight, and risk of prostate cancer. Cancer Epidemiology, Biomarkers & Prevention. 1997;6:557–63. [PubMed] [Google Scholar]
- Giovannucci E, Rimm EB, Liu Y, Leitzmann M, Wu K, Stampfer MJ, Willett WC. Body mass index and risk of prostate cancer in U.S. health professionals. Journal of the National Cancer Institute. 2003;95:1240–4. doi: 10.1093/jnci/djg009. [DOI] [PubMed] [Google Scholar]
- Hayes RB, Brown LM, Schoenberg JB, Greenberg RS, Silverman DT, Schwartz AG, Swanson GM, Benichou J, Liff JM, Hoover RN, Pottern LM. Alcohol use and prostate cancer risk in US blacks and whites. American Journal of Epidemiology. 1996;143:692–7. doi: 10.1093/oxfordjournals.aje.a008802. [DOI] [PubMed] [Google Scholar]
- Hall SE, Holman CDJ, Wisniewski ZS, Semmens J. Prostate cancer: Socioeconomic, geographical and private-health insurance effects on care and survival. British Journal of Urology International. 2005;95:51–8. doi: 10.1111/j.1464-410X.2005.05248.x. [DOI] [PubMed] [Google Scholar]
- Howrey BT, Kuo YF, Lin YL, Goodwin JS. The impact of PSA screening on prostate cancer mortality and overdiagnosis of prostate cancer in the United States. The Journal of Gerontology: Medical Sciences. 2012 doi: 10.1093/gerona/gls135. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsing AW, Devesa SS. Trends and patterns of prostate cancer: What do they suggest? Epidemiological Review. 2001;23:3–13. doi: 10.1093/oxfordjournals.epirev.a000792. [DOI] [PubMed] [Google Scholar]
- Liu L, Cozen W, Bernstein L, Ross RK, Deapen D. Changing relationship between socioeconomic status and prostate cancer incidence. Journal of the National Cancer Institute. 2001;93:705–9. doi: 10.1093/jnci/93.9.705. [DOI] [PubMed] [Google Scholar]
- Masters RK, Hummer RA, Powers DA. Educational Differences in U.S. Adult Mortality: A Cohort Perspective. American Sociological Review. 2012;77:548–72. doi: 10.1177/0003122412451019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirowsky J, Ross CE. Education, personal control, lifestyle and health: A human capital hypothesis. Research on Aging. 1998;20:415–35. [Google Scholar]
- Montez JK, Hayward MD, Brown DC, Hummer RA. Why is the educational gradient of mortality steeper for men? Journal of Gerontology: Social Sciences. 2009;64B:625–34. doi: 10.1093/geronb/gbp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer VA on behalf of the U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine. 2012;157:120–34. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- Phelan JC, Link BG, Tehranifar P. Social conditions as fundamental causes of health inequalities: Theory, evidence, and policy implications. Journal of Health and Social Behavior. 2010;51:28–40. doi: 10.1177/0022146510383498. [DOI] [PubMed] [Google Scholar]
- Platz EA, Rimm EB, Willett WC, Kantoff PW, Giovannucci E. Racial variation in prostate cancer incidence and in hormonal system markers among male health professionals. Journal of the National Cancer Institute. 2000;92:2009–17. doi: 10.1093/jnci/92.24.2009. [DOI] [PubMed] [Google Scholar]
- Platz EA, Giovannucci E. The epidemiology of sex steroid hormones and their signaling and metabolic pathways in the etiology of prostate cancer. The Journal of Steroid Biochemistry and Molecular Biology. 2004;92:237–53. doi: 10.1016/j.jsbmb.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Rapiti E, Fioretta G, Schaffar R, Neyroud-Caspar I, Verkooijen HM, Schmidlin F, Miralbell R, Zanetti R, Bouchardy C. Impact of socioeconomic status on prostate cancer diagnosis, treatment, and prognosis. Cancer. 2009;115:5556–65. doi: 10.1002/cncr.24607. [DOI] [PubMed] [Google Scholar]
- Robine JM, Michel JP. Looking forward to a general theory on population aging. Journal of Gerontology: Medical Sciences. 2004;59A:590–97. doi: 10.1093/gerona/59.6.m590. [DOI] [PubMed] [Google Scholar]
- Rolison JJ, Hanoch Y, Miron-Shatz T. Smokers: At risk for prostate cancer but unlikely to screen. Addictive Behavior. 2012;37:736–8. doi: 10.1016/j.addbeh.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Ross JS, Bernheim SM, Bradley EH, Teng HM, Gallo WT. Use of preventive care by the working poor in the United States. Preventive Medicine. 2007;44:254–9. doi: 10.1016/j.ypmed.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LE, Berkowitz Z, Ekwueme DU. Use of the prostate-specific antigen test among U.S. men: Findings from the 2005 National Health Interview Survey. Cancer Epidemiology, Biomarkers, and Prevention. 2008;17:636–44. doi: 10.1158/1055-9965.EPI-07-2709. [DOI] [PubMed] [Google Scholar]
- Sanderson M, Coker AL, Perez A, Du XL, Peltz G, Fadden MK. A multilevel analysis of socioeconomic status and prostate cancer risk. Annals of Epidemiology. 2006;16:901–907. doi: 10.1016/j.annepidem.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scales CD, Moul JW, Curtis LH, Elkin EP, Hughes ME, Carroll PR. Prostate cancer in the Baby Boomer generation: Results from CaPSURE. Urology. 2007;70:1162–67. doi: 10.1016/j.urology.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Schieman S, Pudrovska T, Eccles R. Perceptions of body weight among older adults: Analyses of the intersection of gender, race, and socioeconomic status. Journal of Gerontology: Social Sciences. 2007;62:S415–23. doi: 10.1093/geronb/62.6.s415. [DOI] [PubMed] [Google Scholar]
- Schnittker J. Education and the changing shape of the income gradient in health. Journal of Health and Social Behavior. 2004;45:286–305. doi: 10.1177/002214650404500304. [DOI] [PubMed] [Google Scholar]
- Schöder FH. Landmarks in prostate cancer screening. British Journal of Urology International. 2012;110(Supplement 1):3–7. doi: 10.1111/j.1464-410X.2012.011428.x. [DOI] [PubMed] [Google Scholar]
- Schwartz K, Powell IJ, Underwood W, George J, Yee C, Banerjee M. Interplay of race, socioeconomic status, and treatment on survival of patients with prostate cancer. Urology. 2009;74:1296–302. doi: 10.1016/j.urology.2009.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute. SEER Cancer Statistics 2012 [Google Scholar]
- Sesso HD, Paffenbarger RS, Lee IM. Alcohol consumption and risk of prostate cancer: The Harvard Alumni Health Study. International Journal of Epidemiology. 2001;30:749–55. doi: 10.1093/ije/30.4.749. [DOI] [PubMed] [Google Scholar]
- Shlyakhter A, Mirny L, Vlasov A, Wilson R. Monte Carlo modeling of epidemiological studies. Human and Ecological Risk Assessment. 1996;2:920–38. [Google Scholar]
- Steenland K, Rodriguez C, Mondul A, Calle EE, Thun M. Prostate cancer incidence and survival in relation to education (United States) Cancer Causes and Control. 2004;15:939–45. doi: 10.1007/s10552-004-2231-5. [DOI] [PubMed] [Google Scholar]
- Tewari AK, Gold HT, Demers RY, Johnson CC, Yadav R, Wagner EH, Yood MU, Field TS, Divine G, Menon M. Effect of socioeconomic factors on long-term mortality in men with clinically localized prostate cancer. Urology. 2009;73:624–30. doi: 10.1016/j.urology.2008.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velicer CM, Kristal A, White E. Alcohol use and the risk of prostate cancer: Results from the VITAL cohort study. Nutrition and Cancer. 2006;56:50–6. doi: 10.1207/s15327914nc5601_7. [DOI] [PubMed] [Google Scholar]
- Wolin KY, Stoll C. Physical activity and urologic cancers. Urologic Oncology. 2012;30:729–34. doi: 10.1016/j.urolonc.2012.07.009. [DOI] [PubMed] [Google Scholar]


