Abstract
The ozone-sensitive Arabidopsis mutant vitamin c-1 (vtc1) is deficient in l-ascorbic acid (AsA) due to a mutation in GDP-Man pyrophosphorylase (Conklin et al., 1999), an enzyme involved in the AsA biosynthetic pathway (Smirnoff et al., 2001). In this study, the physiology of this AsA deficiency was initially investigated in response to biotic (virulent pathogens) stress and subsequently with regards to the onset of senescence. Infection with either virulent Pseudomonas syringae or Peronospora parasitica resulted in largely reduced bacterial and hyphal growth in the vtc1 mutant in comparison to the wild type. When vitamin c-2 (vtc2), another AsA-deficient mutant, was challenged with P. parasitica, growth of the fungus was also reduced, indicating that the two AsA-deficient mutants are more resistant to these pathogens. Induction of pathogenesis-related proteins PR-1 and PR-5 is significantly higher in vtc1 than in the wild type when challenged with virulent P. syringae. In addition, the vtc1 mutant exhibits elevated levels of some senescence-associated gene (SAG) transcripts as well as heightened salicylic acid levels. Presumably, therefore, low AsA is causing vtc1 to enter at least some stage(s) of senescence prematurely with an accompanying increase in salicylic acid levels that results in a faster induction of defense responses.
Aerobic organisms experience oxidative stress caused by the production of reactive oxygen species (ROS), such as singlet oxygen, superoxide anion radicals, hydrogen peroxide, and hydroxyl radicals. In plants, ROS are formed as a byproduct of metabolic pathways, such as photosynthesis and respiration (Asada and Takahashi, 1987), and by processes that are enhanced during abiotic and/or biotic stress (Foyer and Harbinson, 1994; Dat et al., 2000; Grant and Loake, 2000). Under optimal growth conditions, the production of ROS in cells is low but increases dramatically when cells are subjected to unfavorable environmental conditions (Polle, 2001), such as high light stress, UV radiation, chilling, drought, heavy metals, air pollutants, e.g. ozone and sulfur dioxide, and pathogen attack. On the one hand, ROS are toxic metabolites that can damage cellular components and therefore need to be detoxified by ROS-scavenging mechanisms (Asada, 1999; Niyogi, 2000). On the other hand, ROS serve as secondary messengers involved in signaling transduction pathways to control pathogen defense responses and programmed cell death (Hammond-Kosack and Jones, 1996; Desikan et al., 2001; Neill et al., 2002). Therefore, under optimal conditions the level of ROS needs to be tightly controlled.
ROS generated by either biotic or abiotic stresses can be toxic to the cell. Therefore, mechanisms are required that limit their production and/or that scavenge them to prevent their excessive accumulation (for recent reviews on antioxidant mechanisms, see Niyogi, 2000; Mittler, 2002). ROS-scavenging pathways in plants include the water-water cycle (Mehler-peroxidase pathway) in chloroplasts (Asada, 1999), the ascorbate-glutathione cycle present in the cytosol, chloroplasts, mitochondria, apoplast and peroxisomes, and catalase in peroxisomes (for review, see Mittler, 2002). The antioxidant l-ascorbic acid (AsA) plays a crucial role in these complex antioxidant processes. Moreover, AsA is a cosubstrate of many enzymes, e.g. ascorbate peroxidase, which detoxifies hydrogen peroxide, and 2-oxoacid-dependent dioxygenases, which are involved in the biosynthesis of plant hormones, such as abscisic acid (ABA) and GA (Arrigoni and De Tullio, 2000, 2002; Smirnoff, 2000). Hence, mutant plants with decreased AsA levels, such as vitamin c-1 (vtc1), have been shown to be hypersensitive to oxidative stress, e.g. ozone, freezing, UV-B radiation, and sulfur dioxide (Conklin et al., 1996). The vtc1 mutant, isolated by its sensitivity to ozone, has lower activity of the AsA biosynthetic enzyme GDP-Man pyrophosphorylase, resulting in a 70% lower AsA content compared to the wild type (Conklin et al., 1999). Two recent studies on this mutant have demonstrated that both leaf photosynthesis and the capacity of the antioxidant system are largely unchanged (Veljovic-Jovanovic et al., 2001), while some plant defense-related transcripts are up-regulated (Pastori et al., 2003). The effect of low AsA on xanthophyll cycle activity was studied recently in the AsA-deficient mutant vitamin c-2 (vtc2; Müller-Moulé et al., 2002). The VTC2 gene has been cloned, but it encodes a protein of unknown function (Levin et al., 2000).
A variety of microorganisms may cause symptoms of disease in plants (biotic stress). Following avirulent pathogen attack, a rapid increase in ROS, known as the oxidative burst, is observed. High concentrations of ROS are thought to directly kill the invading pathogen (Peng and Kuc, 1992), activate cell wall cross-linking to strengthen the cell wall (Brisson et al., 1994), and activate defense genes (Levine et al., 1994). In addition, biosynthesis of salicylic acid (SA), which induces the expression of pathogenesis-related (PR) proteins (Malamy et al., 1990; Conrath et al., 1997), and other antimicrobial compounds is initiated (Terras et al., 1995). Following these initial responses, a hypersensitive response occurs, characterized by localized cell death. During a hypersensitive response, local defense mechanisms are induced, which lead to the formation of necrotic lesions at the infection site and thus prevent further spread of the pathogen (Dangl et al., 1996; Hammond-Kosack and Jones, 1996). Virulent pathogens have been reported to induce nonspecific resistance responses via the induction of SA synthesis and PR proteins (Glazebrook et al., 1997; Rogers and Ausubel, 1997). However, the defense responses elicited by virulent pathogens are either activated more slowly, and/or they are activated to lower levels than the defense response induced by avirulent pathogens (Crute et al., 1994, and references therein). There is strong evidence that plants can transduce pathogenic signals through alternative, SA-independent pathways, such as the jasmonic acid (JA) and ethylene pathway (Dong, 1998; Pieterse and van Loon, 1999). In addition, nitric oxide has been implicated as another signaling molecule to activate plant defenses (Klessig et al., 2000).
The goal of this study was to provide more insights into the physiology of an AsA deficiency in response to pathogen exposure. We describe below the effects of low leaf AsA content on virulent Pseudomonas syringae pv maculicola ES4326 and the downy mildew pathogen Peronospora parasitica pv Noco using the previously described AsA-deficient mutants vtc1-1 and vtc2-1 (hereafter referred to as vtc1 and vtc2; Conklin et al., 1999, 2000; Levin et al., 2000). Finally, we investigate the possibility that vtc1 may be entering senescence prematurely, which could provide some explanation for its observed altered pathogen sensitivity.
RESULTS
Susceptibility of vtc1 to Virulent P. syringae and P. parasitica Is Diminished
At 2 weeks of age, the AsA-deficient mutants vtc1 and vtc2 both contain approximately one-third the wild-type level of total AsA (Conklin et al., 2000). To assess interactions of the AsA-deficient vtc1 mutant with virulent pathogens, we challenged the wild type and mutant with the virulent bacterium P. syringae pv maculicola ES4326 (Dong et al., 1991) and the oomycete P. parasitica pv Noco, the cause of downy mildew (Koch and Slusarenko, 1990).
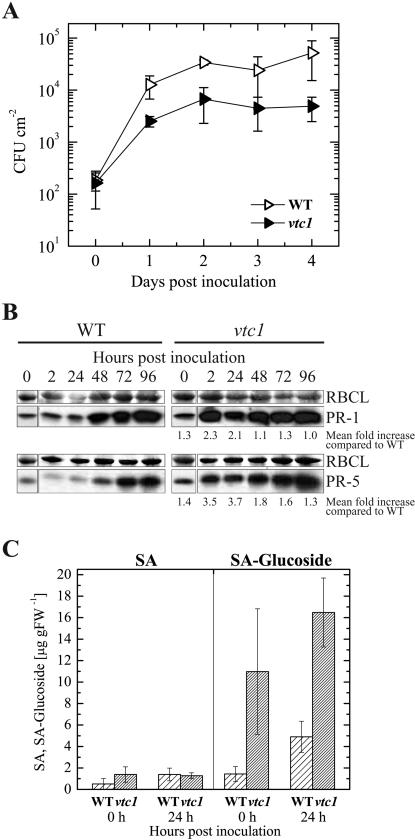
The initial rate of bacterial growth was similar in the wild type and vtc1. However, 1 to 3 d postinoculation, the bacterial titer was approximately 5-fold and on day 4, 10-fold lower in vtc1 compared to the wild type (Fig. 1A), indicating that vtc1 supports less growth of this pathogen than the wild type. The increased resistance of vtc1 to virulent P. syringae correlates with higher levels of both PR proteins and SA. PR-1 and PR-5 are more abundant in vtc1 than in the wild type, especially very early in the infection process (Fig. 1B). During the first 24 h postinoculation, the PR-1 and PR-5 levels were more than 2-fold and 3-fold higher than in the wild type, respectively (Fig. 1B). Similarly, the level of free SA is slightly higher (about 2-fold) in vtc1 compared to the wild type. However, SA-glucoside is more than 7-fold elevated in noninfected leaves of vtc1 (Fig. 1C), resulting in an overall higher content of total SA in the mutant compared to the wild type. The mutant maintains a high level of SA-glucoside 2 h (data not shown) and 24 h after inoculation (Fig. 1C), during the same period when the level of PR proteins is increased in the mutant compared to the wild type. The content of SA-glucoside is also elevated in the wild type at 24 h postinoculation, but significantly higher SA-glucoside levels are observed in vtc1. These results suggest a stronger SA-dependent induction of PR-1 and PR-5 in vtc1 compared to the wild type upon challenge with P. syringae pv maculicola ES4326.
Figure 1.
Responses of the wild type and vtc1 upon infection with virulent P. syringae pv maculicola ES4326. Leaves of 5-week-old plants were inoculated with a bacterial suspension containing 105 CFU (colony forming units) mL−1. A, Growth curve of ES4326 in leaves of the wild type and vtc1. Mean values ± sd of three to four different plants are depicted. Similar results were obtained in four additional independent experiments. B, Western-blot analysis of wild-type and vtc1 leaves after infiltration with ES4326 was performed to demonstrate the induction of PR-1 and PR-5. Ten micrograms of total protein were loaded. To show equal loading, the blot was stained with Ponceau red to visualize the large subunit of Rubisco (RBCL). Western-blot analyses were performed two to three times with similar results. Numbers below western blots of vtc1 indicate mean fold increase of PR-1 and PR-5 protein expression, respectively, in the mutant compared to the wild type. C, Endogenous levels of free SA and SA-glucoside (the conjugated form) in 5-week-old leaves of the wild type and vtc1 not infected with ES4326 and 24 h postinoculation. Data represent means ± sd of three independent samples each. Similar results were observed in two additional experiments.
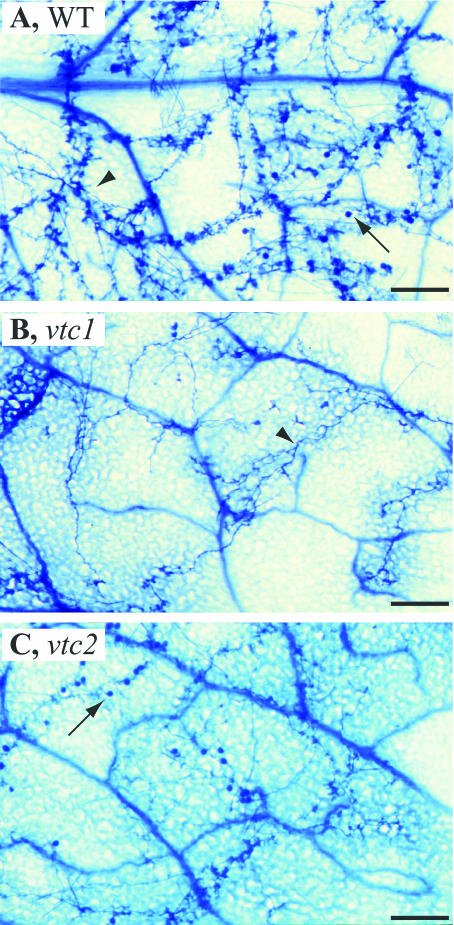
The resistance of vtc1 to virulent P. syringae raised the question of whether this phenotype is specific to virulent Pseudomonas or is a response to virulent pathogens in general. We have discovered that vtc1 also exhibits strikingly increased resistance to a virulent strain of P. parasitica. The AsA-deficient mutant vtc2 shares this phenotype. We inoculated leaves of wild-type and mutant plants with a conidiophore suspension of P. parasitica pv Noco and examined infected leaves 7 d postinoculation. Noco is virulent on Columbia (Col-0) wild-type plants, as indicated by the high conidiophore production and massive hyphal growth (Table I; Fig. 2A). By contrast, both vtc1 and vtc2 leaves supported little growth and reproduction of this fungus (Table I; Fig. 2, B and C). A higher titer of the initial inoculum used in the first replicate probably explains the slightly higher infection rate in replicate 1 versus replicate 2. In a parallel experiment, conidiophore formation and hyphal development in vtc2 were also found to be significantly lower than in the wild type. Production of conidiophores and hyphal growth were similar in vtc1 and vtc2 (Table I), indicating that both these AsA-deficient mutants are much more resistant to this otherwise virulent fungus.
Table I.
P. parasitica conidiophore production in 4-week-old wild-type, vtc1, and vtc2 plants
| Genotype | Replicate | Number of Plants with Observed Disease Levels
|
|||||
|---|---|---|---|---|---|---|---|
| 0 | + | ++ | +++ | ++++ | Total Plants | ||
| WT | 1 | 0 | 0 | 6 | 6 | 13 | 25 |
| 2 | 0 | 0 | 5 | 12 | 7 | 24 | |
| vtc1 | 1 | 5 | 3 | 16 | 0 | 0 | 24 |
| 2 | 12 | 7 | 5 | 0 | 0 | 24 | |
| vtc2 | 1 | 6 | 5 | 9 | 3 | 1 | 24 |
| WT | Total | 0 | 0 | 11 | 18 | 20 | 49 |
| vtc1 | Total | 17 | 10 | 21 | 0 | 0 | 48 |
Conidiophore production was assessed on leaves of the wild type as well as the AsA-deficient mutants vtc1 and vtc2 7 d after inoculation with P. parasitica pv Noco at 5 × 104 conidiophores mL−1 in water (replicate 1) or with 7 to 8 × 109 conidiophores mL−1 (replicate 2). For each replicate, 12 plants were scored within one pot, three from each corner of the pot 7 d after inoculation. Disease levels were scored as follows: 0, no conidiophores on leaf; +, at least one leaf with one to five conidiophores; ++, at least one leaf with 5 to 20 or more conidiophores; +++, majority of leaves with 5 to 20 or more conidiophores; ++++, all leaves (approximately five true leaves at this age) with 20 or more conidiophores.
Figure 2.
Conidiophore production in wild-type, vtc1, and vtc2 plants inoculated with P. parasitica pv Noco. A, Pronounced conidiophore production (arrow) and massive hyphae spread (arrowhead) in the wild type. B, In vtc1 and C, in vtc2, conidiophore production and hyphal development were much lower than in the wild type after infection with Noco. See Table I for quantitative analyses of conidiophore production in the wild type, vtc1, and vtc2. Several leaves of individual plants (4 weeks old) were sprayed with conidiophores of Noco and analyzed 7 d after inoculation as described in “Materials and Methods.” Scale bar = 100 μm.
A Subset of Senescence-Associated Gene Transcripts Is Elevated in vtc1
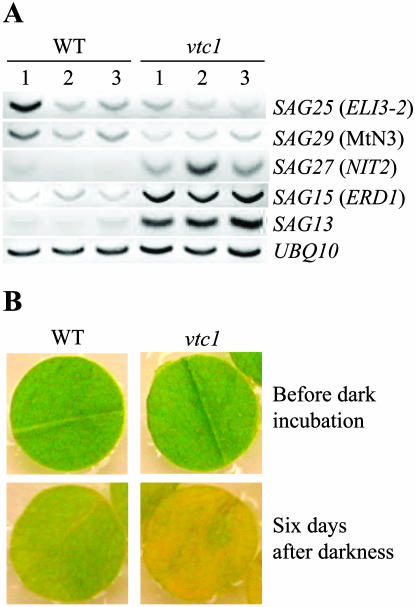
It has been shown previously that a decline in AsA correlates positively with senescence in postharvest spinach (Spinacea oleracea) leaves (Hodges and Forney, 2000) and during dark-induced as well as natural nodule senescence in lupin plants (Hernandez-Jimenez et al., 2002), suggesting that an AsA deficiency promotes senescence. Furthermore, during senescence SA levels increase, resulting in the induction of defense-related genes (Quirino et al., 1999; Morris et al., 2000). To test whether leaves of vtc1 used for P. syringae infection are in an early stage of senescence, expression levels of several senescence-associated genes (SAGs) were tested using semiquantitative reverse transcription (RT)-PCR (Fig. 3A). Although transcript levels were not significantly different for SAG25 and SAG29, much higher expression levels of SAG27, SAG15, and SAG13 were detected in vtc1 compared to the wild type (Fig. 3A). SAG27 is a defense-related gene that can also be induced by SA (Quirino et al., 1999). SAG15 and SAG13 have been reported to be induced by ABA and ethylene, which promote and modulate the timing of senescence, respectively (Nakashima et al., 1997; Weaver et al., 1998). When leaf discs of those wild-type and vtc1 plants were floated on water and incubated in the dark for 6 d (dark-induced senescence), discs of vtc1 senesced much faster than those of the wild type, as indicated by the accelerated chlorophyll loss in vtc1 (Fig. 3B). Under normal growth conditions, symptoms of senescence also appeared earlier in vtc1 than in the wild type when plants were 6 to 7 weeks old (data not shown). These data suggest that vtc1 enters at least some stages of senescence prematurely, although no obvious signs of senescence were visible in the 5-week-old mutant plants used for pathogen inoculation experiments (Fig. 3B, Before dark incubation). We have preliminary results indicating that transcription levels of SAG13 in vtc1 are decreased to wild-type levels (both in the dark and in the light) when the endogenous AsA content is artificially elevated. Similar results have been reported recently for PR-1 (Pastori et al., 2003), suggesting that elevated expression of senescence-associated and defense-related genes in vtc1 is directly related to the AsA deficiency.
Figure 3.
AsA deficiency causes premature senescence in vtc1. A, Semiquantitative RT-PCR was performed on 1 μg of total RNA isolated from 5-week-old plants of the wild type and vtc1 used for bacterial infection with P. syringae ES4326. Transcription levels of cDNA fragments amplified from SAG are depicted for three independent replicates of the wild type and vtc1. B, Phenotype of wild-type and vtc1 leaf discs after dark-induced senescence. Leaf discs of 5-week-old plants were floated on water and kept in the dark for 6 d.
DISCUSSION
Ascorbate Deficiency Is Associated with Resistance to Virulent Pathogens
Despite the heightened constitutive level of total SA in vtc1, PR proteins are not induced constitutively in the mutant. However, in a recent study of vtc1 (Pastori et al., 2003), transcript levels of PR-1, which were determined by RT-PCR, were found to be slightly elevated in the AsA-deficient mutant when compared to the wild type. Pastori et al. (2003) did not observe a concurrent constitutive up-regulation in genes involved in SA biosynthesis, such as Phe ammonium lyase. Levels of SA biosynthetic enzymes were not analyzed here. Instead, measurement of SA content itself revealed a slight increase in free SA and a significant increase in SA-glucoside in the mutant relative to the wild type in the absence of pathogen. It is possible that the constitutively elevated SA level in the mutant causes activation of SA glycosyl transferase, an enzyme that can be activated by SA and converts SA to the SA-glucoside (Lee et al., 1995). This would explain the elevated level of SA-glucoside in vtc1 versus the wild type.
Surprisingly, the AsA-deficient mutants vtc1 and vtc2 are more resistant to the virulent pathogens P. syringae pv maculicola ES4326 and P. parasitica pv Noco. This resistance may be in part due to the increased induction of an SA-dependent defense network including pathogenesis-related proteins PR-1 and PR-5, as seen in the vtc1 mutant. Inoculation with virulent P. syringae ES4326 caused a faster accumulation of SA in vtc1 than in the wild type (compare with Fig. 1, B and C), which could at least partially explain the more pronounced pathogen-induced elevation of PR proteins in vtc1 than in the wild type.
Therefore, resistance of vtc1 to P. syringae ES4326 may at least partially be due to a stronger, SA-dependent up-regulation of defense responses. SA levels somewhat lower compared to vtc1 have been reported for the turnip crinkle virus-resistant Arabidopsis mutant Di-17, showing that low doses of SA are sufficient to induce certain defense genes (Dempsey et al., 1997). On the other hand, mutants, such as the constitutive expression of PR-1 (cep; Silva et al., 1999), the lesion simulating disease mutants lsd1, lsd6 and lsd7 (Weymann et al., 1995; Dietrich et al., 1997), as well as the accelerated cell death mutant acd2 (Mach et al., 2001), possess much higher levels of SA and also show increased disease resistance to virulent pathogens (Silva et al., 1999). All these mutants spontaneously form hypersensitive response-like lesions on leaves under normal growth conditions. This does not occur in vtc1, presumably because the SA levels in vtc1 are lower relative to those in the other mutants.
Nevertheless, vtc1 is more resistant to virulent pathogens but sensitive to ozone. How can this be explained? In Col-0 wild-type plants, ozone exposure causes a low oxidative burst, resulting in a low but sufficient accumulation of SA, leading to the induction of defense genes conferring ozone resistance but not triggering programmed cell death. This response is presumably attenuated by JA in the wild type. The JA-insensitive mutant jar1 is more sensitive to ozone compared to the wild type because of the higher accumulation of hydrogen peroxide and SA after ozone exposure (Rao et al., 2000). In vtc1, ozone fumigation could boost already elevated SA levels well above the wild type, triggering programmed cell death and ozone sensitivity, which is apparent as chlorotic lesions and tissue collapse. However, when challenged with virulent pathogens, the increased levels of SA and other plant hormones, along with a decrease in AsA that confers ozone sensitivity, may result in defense responses in vtc1 that paradoxically confer pathogen resistance. This pathogen resistance may be at least partially a result of this mutant's premature entry into senescence.
Ascorbate Deficiency in vtc1 Is Associated with Promotion of Premature Senescence
We propose that the AsA-deficient vtc1 mutant is entering at least partially into senescence earlier than its wild-type parent. Many independent lines of evidence lead us to this proposal. First, a faster senescence phenotype in potato (Solanum tuberosum) plants with reduced GDP-Man pyrophosphorylase activity (the enzyme that is mutated in vtc1) and lowered AsA level has been reported previously (Keller et al., 1999). Conversely, there are reports that an elevation of AsA results in a delay in senescence (Garg and Kapoor, 1972). Second, as mentioned above, SA levels have been shown to increase during senescence in Arabidopsis (Morris et al., 2000) and are also elevated in vtc1. It follows from this phenotype that SAG27, which is induced by SA (Quirino et al., 1999), is up-regulated in vtc1 (Fig. 3A). In addition, at least two other SAGs (SAG13 and SAG15) are also up-regulated. In conjunction with other hormones (ABA, JA, ethylene, and brassinosteroid), SA has been proposed to be a promoter of senescence, as exogenous treatment with these hormones results in differential induction of SAGs (Morris et al., 2000; He et al., 2001). Indeed, at least two senescence promoters may be acting prematurely in vtc1, as in addition to the elevation in SA that we report here, Pastori et al. (2003) reported elevated levels of ABA in this mutant. In addition, we have preliminary results that in the presence of excess AsA, PR-1, and SAG13 transcripts elevated in the AsA-deficient state of the untreated mutant are diminished to wild-type levels (data not shown). Third, at least two transcription factors (a putative zinc finger protein, gene identification no. At3g28210; and R2R3-MYB transcription factor, gene identification no. At1g18570) that are induced during senescence are up-regulated in vtc1 (Chen et al., 2002; Pastori et al., 2003). Fourth, several publications have demonstrated that senescence can be induced by darkness (Oh et al., 1997; Simpson et al., 2003). We have shown that vtc1 senesces faster upon dark-induced senescence (Fig. 3B). As it has been proposed that multiple senescence promoters are needed to induce senescence (Miller et al., 1999; He et al., 2001), perhaps the combination of elevated SA, elevated ABA, and darkness is enough to induce visible senescence in vtc1. Finally, numerous studies report that susceptibility to pathogens decreases with increasing leaf age, a phenomenon referred to as age-related resistance (ARR; Koch and Mew, 1991; Roumen et al., 1992; Kus et al., 2002). SA accumulation is necessary for the ARR response against virulent P. syringae, as plant lines deficient in SA accumulation, such as NahG, sid1, and sid2, do not exhibit ARR (Kus et al., 2002). These authors furthermore demonstrated that PR-1 and SAG13 transcripts increase during ARR, supporting the findings reported here and suggesting that vtc1 exhibits ARR.
The elevated SA levels in vtc1 are most likely contributing to pathogen resistance via an up-regulation of an SA-inducible defense network. However, SA-independent signaling pathways via ABA, JA, and/or ethylene (and perhaps other senescence-associated factors) may also contribute to promote pathogen resistance in vtc1. In fact, recent studies report a specific requirement of AsA as a cofactor in the synthesis of ABA, GA, and ethylene. ABA and GA biosynthesis require the activity of AsA-dependent 2-oxoacid-dependent dioxygenases, enzymes that regulate the synthesis of Hyp-containing proteins and hormones in plants (and animals; Arrigoni and De Tullio, 2000, 2002). For example, transcript levels of 9-cis-epoxycarotenoid dioxygenase (NCED), an AsA- and Fe3+-dependent dioxygenase catalyzing the formation of xanthoxin, the precursor of ABA (Schwartz et al., 1997), are up-regulated in vtc1 compared to the wild type (Pastori et al., 2003). This suggests that low levels of AsA in vtc1 may decrease the flux through the dioxygenase reaction. The authors hypothesized that elevated NCED transcript levels in vtc1 compensate for the decreased cofactor (AsA) availability, resulting in increased ABA biosynthesis (Pastori et al., 2003). ABA induces PR-1 in other plant species, such as rice (Oryza sativa; Agrawal et al., 2001).
Furthermore, ethylene biosynthesis could be altered in vtc1 and affect pathogen resistance of this mutant. AsA is a co-factor for 1-aminocyclopropane-1-caroxylate oxidase that forms ethylene (Dong et al., 1992). In their microarray study of vtc1, Pastori et al. (2003) found an up-regulation of an ethylene-responsive transcription factor when the endogenous AsA content in vtc1 was artificially elevated (Pastori et al., 2003). Therefore, AsA availability may control ethylene biosynthesis/signaling. Ethylene is involved in the induction of PR genes (Knoester et al., 1995; Grimmig et al., 2003).
As the AsA deficiency in vtc1 is the result of reduction in GDP-Man pyrophosphorylase activity, the vtc1 mutant may also have alterations in GDP-Man-dependent protein glycosylation (Conklin et al., 1999). However, all the evidence outlined above suggest that the premature senescence phenotype of this mutant and its related pathogen resistance stem from the mutant's AsA deficiency. This is further supported by the concurrent pathogen resistance of the AsA-deficient vtc2 mutant. It is formally possible that the vtc2 is also deficient in GDP-Man, as the AsA deficiency in this mutant is the result of a mutation in a gene of unknown function. Further confirmation of the above phenotypes by experimentation with additional AsA-deficient lines with defects downstream of GDP-Man synthesis (such as the antisense l-Gal dehydrogenase lines generated by N. Smirnoff; Gatzek et al., 2002) will be necessary. Additionally, possible reversion of the premature senescence and pathogen resistance of vtc1 and vtc2 by artificial elevation of AsA requires further investigations.
Conclusion
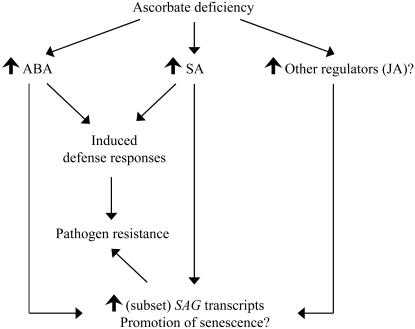
We have tried to tie together the phenotypes of AsA deficiency, elevated SA, elevated ABA, increased pathogen resistance, and increased SAG transcription of vtc1 in a model shown in Figure 4. Future work is required to determine the specific role of AsA in cell signaling and how AsA can potentially affect gene expression. There are indications that the redox state of AsA in the apoplast is important in modulating redox-sensitive proteins, thus controlling the biosynthesis of signaling molecules (SA, ABA, and GA) that influence plant development and defense responses (Pignocchi and Foyer, 2003). Furthermore, the expression of developmental and defense genes can be altered in vtc1 depending on the AsA pool (Pastori et al., 2003). This study indicates for two AsA-deficient mutants, vtc1 and vtc2, that low AsA decreases susceptibility to two virulent pathogens. Further study of the response of vtc1 and vtc2 to pathogen infection in the background of mutants known to be affected in pathogen- and senescence-induced signaling pathways should add to our understanding of the role of AsA in this complex response network.
Figure 4.
A model describing the relationship between AsA deficiency, pathogen resistance, and premature senescence in vtc1. AsA deficiency causes the induction of signaling molecules, such as ABA, SA, and presumably other signaling factors such as JA, as indicated by the upright solid arrows. We provide evidence that elevated levels of SA result in the induction of defense responses and, therefore, in pathogen resistance of vtc1. ABA is presumably also involved in the induction of defense responses. We propose that ABA, SA, and/or other regulators cause an up-regulation of SAGs, promoting senescence and possibly contributing to pathogen resistance of vtc1.
Finally, one must consider the power of mutant analysis with respect to these ozone-sensitive mutants. Analysis of these mutants has aided in the study of several basic aspects of plant biology, including AsA biosynthesis, antioxidant signaling networks, photooxidative stress, pathogen resistance, and senescence.
MATERIALS AND METHODS
Plant Material and Growth Conditions
The vtc1 mutant has been described previously (Conklin et al., 1996; Conklin et al., 1999). Five- or 4-week-old F4 plants from a backcross between Arabidopsis L. Heynh. (Col-0) wild type and vtc1 were used for experiments. Plants were grown in 8-cm-round pots in Cornell mix soil (Landry et al., 1995) in a light room at a photosynthetically active radiation (PAR) of 90 to 120 μmol photons m−2 s−1 provided by 400 W metal halide bulbs. Photoperiod was 16 h, temperature was 21°C to 23°C, and relative humidity was 50% or 60%, respectively.
Infection of Plants with Virulent Pseudomonas syringae pv maculicola ES4326
Plants for bacterial infection were germinated in a light room for 2 weeks (120 μmol photons m−2 s−1 PAR, 16-h photoperiod) and grown for another 3 weeks in a controlled-environment growth chamber (Conviron, Winnipeg, Canada). Conditions in the growth chamber were 120 μmol photonsm−2 s−1 PAR, 8-h photoperiod, 21°C, and 70% relative humidity. Inoculation of wild-type and vtc1 plants with P. syringae pv maculicola ES4326 was performed according to Dong et al. (1991). At the times given, bacterial growth in leaves was determined in 0.55 cm2 leaf discs that were extracted by macerating the discs in 300 mL of 10 mm MgCl2. Serial dilutions were plated onKing's B plates containing 100 μg mL−1 streptomycin. In addition, leaf tissue was collected for western-blot analysis. The infection experiment was carried out independently five times.
Protein Extraction and Western-Blot Analysis
Leaf tissue (between 30 and 100 mg) was extracted in 1× Tris-buffered saline containing 1 mm phenylmethylsulfonyl flouride, 1 mm benzamidine, and 5 mm ɛ-amino caproic acid and centrifuged for 5 min at 10,000g. In the resulting supernatant, protein concentrations were adjusted to 10 μg of total protein. Protein separation and blotting were performed on 15% polyacrylamide gels and nitrocellulose membranes (Gelman Sciences, Ann Arbor, MI), respectively. Membranes were stained with Ponceau red to check equal loading. After blocking in 5% milk, membranes were incubated with polyclonal PR-1 and PR-5 antibodies, respectively, followed by anti-rabbit antibody conjugated horseradish peroxidase. Blots were developed by using the ECL detection kit from Roche (Indianapolis), and films were scanned and quantified with ImageQuant 5.0 software (Amersham Biosciences, Piscataway, NJ).
Infection of Plants with Peronospora parasitica pv Noco and Trypan Blue Staining
The first replicate was performed with wild-type plants and vtc1 (M5 seed), whereas for the second replicate, the wild type and vtc1 (from the second backcross) and vtc2 (M4 seed) were used. On both occasions, plants were grown in 10-cm-square pots in a growth chamber set to 16°C at night, 18°C during the day, 12-h photoperiod. Plants were 3 weeks old when inoculated by spraying. Plants were either inoculated with P. parasitica pv Noco at 5 × 104 conidiophores mL−1 water (replicate 1) or with 7 to 8 × 109 conidiophores mL−1 (replicate 2). Seven days after inoculation, plants were evaluated for conidiophores production using a dissection microscope.
Plants were stained by trypan blue 5 d after inoculation (replicate 1) and 7 d after inoculation (replicate 2) according to Keogh et al. (1980).
Determination of Salicylic Acid
Extraction and quantitation of SA was performed with 0.25 to 0.55 g of leaf tissue of 5-week-old leaves of the wild type and vtc1 as described by Bowling et al. (1994).
Isolation of Total RNA and Expression Analysis of Senescence-Associated Genes by RT-PCR
Total RNA from approximately 1 g fresh weight of 5-week-old plants of the wild type and vtc1 used for pathogen infection with P. syringae ES4326 was isolated using Trizol (Invitrogen, Carlsbad, CA). Primers used to amplify a cDNA fragment of SAG are 5′-CAGCTTGCCCACCCATTGTTA-3′ and 5′-GTCGTACGCACCGCTTCTTTCTTA-3′ for SAG13, 5′-ACGATCCACCGCTTCTCCACAACT-3′ and 5′-GCCGGCGCTACCATCATCAAC-3′ forSAG15, 5′-AGGCGGTTTAGGTCATGTAGGAGTG-3′ and 5′-GGCGGTGTTGACATAATCGGCAGAG-3′ for SAG25, 5′-TCCTGGCCCTGAAGTAGAAA-3′ and 5′-GTCCCGCAAGAACCTGTCC-3′ for SAG27, and 5′-CCCTATGTGGTGGCGCTCTTCAG-3′ and 5′-CCGACGGCGTTTTGCAGTATTTG-3′ for SAG29. For more information on SAG13 and SAG15, see Miller et al. (1999); for all other SAGs, refer to Quirino et al. (1999). cDNA fragments were generated from 1 μg of total RNA by using the Access RT-PCR kit from Promega (Madison, WI), running 20 amplification cycles (linear range of amplification). The linear range of amplification was determined by running increasing cycle numbers and analyzing the amount of cDNA fragments (loaded as 1:3 dilutions in distilled water) on 2% agarose gels containing ethidium bromide. Band intensities were quantified with ImageQuant 5.0 (Amersham Biosciences). A cDNA fragment generated from UBQ10 (5′-GATCTTTGCCGGAAAACAATTGGAGGATGGT-3′ and 5′-CGACTTGTCATTAGAAAGAAAGAGATAACAGG-3′) served as an internal control.
Acknowledgments
We thank Dr. David Stern for helpful comments on the manuscript, Dr. Greg Martin (Boyce Thompson Institute for Plant Research at Cornell University, Ithaca, NY) for providing the Pseudomonas strain, Dr. Dan Kliebenstein (University of California, Davis, CA) for providing PR-1 and PR-5 antibodies, and Dr. Terrence Delaney (Cornell University, Ithaca, NY) for providing equipment and help for the fungal infection experiment.
This work was supported by the German Academic Exchange Service (postdoctoral fellowship D/00/22216 to C.B.), the National Science Foundation (grant no. MCB–0110404 to D.F.K.), and the U.S. Department of Agriculture National Research Initiative Competitive Grants Program (grant no. 96–35100–3212 to P.L.C.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.032185.
References
- Agrawal GK, Rakwal R, Jwa NS, Agrawal VP (2001) Signaling molecules and blast pathogen attack activates rice OsPR1a and OsPR1b genes: a model illustrating components participating during defense/stress response. Plant Physiol Biochem 39: 1095–1103 [Google Scholar]
- Arrigoni O, De Tullio MC (2000) The role of ascorbic acid in cell metabolism: between gene-directed functions and unpredictable chemical reactions. J Plant Physiol 157: 481–488 [Google Scholar]
- Arrigoni O, De Tullio MC (2002) Ascorbic acid: much more than just an antioxidant. Biochim Biophys Acta 1569: 1–9 [DOI] [PubMed] [Google Scholar]
- Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50: 601–639 [DOI] [PubMed] [Google Scholar]
- Asada K, Takahashi M (1987) Production and scavenging of active oxygen in photosynthesis. In CJ Arntzen, ed, Photoinhibition: Topics in Photosynthesis. Elsevier, Amsterdam, pp 227–287
- Bowling SA, Guo A, Cao H, Gordon AS, Klessig DF, Dong X (1994) A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. Plant Cell 6: 1845–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson LF, Tenhaken R, Lamb C (1994) Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance. Plant Cell 6: 1703–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Provart NJ, Glazebrook J, Katagiri F, Chang HS, Eulgem T, Mauch F, Luan S, Zou G, Whitham SA, Budworth PR, Tao Y, et al (2002) Expression profile matrix of Arabidopsis transcription factor genes suggests their putative functions in response to environmental stresses. Plant Cell 14: 559–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Norris SR, Wheeler GL, Williams EH, Smirnoff N, Last RL (1999) Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis. Proc Natl Acad Sci USA 96: 4198–4203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Saracco SA, Norris SR, Last RL (2000) Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics 154: 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Williams EH, Last RL (1996) Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proc Natl Acad Sci USA 93: 9970–9974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrath U, Silva H, Klessig DF (1997) Protein dephosphorylation mediates salicylic acid-induced expression of PR-1 genes in tobacco. Plant J 11: 747–757 [Google Scholar]
- Crute I, Beynon J, Dangl JL, Holub EB, Mauch-Mani B, Slusarenko A, Staskawicz B, Ausubel FM (1994) Microbial pathogenesis of Arabidopsis. In EM Meyerowitz, CR Somerville, eds, Arabidopsis. Cold Spring Harbor Laboratory Press, Plainview, NY, pp 705–747
- Dangl JL, Dietrich RA, Richberg MH (1996) Death don't have no mercy: cell death programs in plant-microbe interactions. Plant Cell 8: 1793–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dat J, Vandenabeele S, Vranova E, Van Montagu M, Inze D, Van Breusegem F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57: 779–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey DA, Pathirana MS, Wobbe KK, Klessig DF (1997) Identification of an Arabidopsis locus required for resistance to turnip crinkle virus. Plant J 11: 301–311 [DOI] [PubMed] [Google Scholar]
- Desikan R, A-H-Mackerness S, Hancock JT, Neill SJ (2001) Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol 127: 159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich RA, Richberg MH, Schmidt R, Dean C, Dangl JL (1997) A novel zinc finger protein is encoded by the Arabidopsis LSD1 gene and functions as a negative regulator of plant cell death. Cell 88: 685–694 [DOI] [PubMed] [Google Scholar]
- Dong JG, Fernandez-Maculet JC, Yang SF (1992) Purification and characterisation of 1-aminocyclopropane-1-carboxylate oxidase from apple fruit. Proc Natl Acad Sci USA 89: 9789–9793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X (1998) SA, JA, ethylene, and disease resistance in plants. Curr Opin Plant Biol 1: 316–323 [DOI] [PubMed] [Google Scholar]
- Dong X, Mindrinos M, Davis KR, Ausubel FM (1991) Induction of Arabidopsis defense genes by virulent and avirulent Pseudomonas syringae strains and by a cloned avirulence gene. Plant Cell 3: 61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Harbinson J (1994) Oxygen metabolism and the regulation of photosynthetic electron transport. In PM Mullineaux, ed, Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants. CRC Press, Boca Raton, FL, pp 1–42
- Garg OP, Kapoor V (1972) Retardation of leaf senescence by ascorbate. J Exp Bot 23: 699–704 [Google Scholar]
- Gatzek S, Wheeler GL, Smirnoff N (2002) Antisense suppression ofL-galactose dehydrogenase in Arabidopsis thaliana provides evidence for its role in ascorbate synthesis and reveals light modulated L-galactose synthesis. Plant J 30: 541–553 [DOI] [PubMed] [Google Scholar]
- Glazebrook J, Zook M, Mert F, Kagan I, Rogers EE, Crute IR, Holub EB, Hammerschmidt R, Ausubel FM (1997) Phytoalexin-deficient mutants of Arabidopsis reveal that PAD4 encodes a regulatory factor and that four PAD genes contribute to downy mildew resistance. Genetics 146: 381–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JJ, Loake GJ (2000) Role of reactive oxygen intermediates and cognate redox signaling in disease resistance. Plant Physiol 124: 21–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimmig B, Gonzalez-Perez MN, Leubner-Metzger G, Vogeli-Lange R, Meins F, Hain R, Penuelas J, Heidenreich B, Langebartels C, Ernst D, et al (2003) Ozone-induced gene expression occurs via ethylene-dependent and -independent signalling. Plant Mol Biol 51: 599–607 [DOI] [PubMed] [Google Scholar]
- Hammond-Kosack KE, Jones JDG (1996) Resistance gene-dependent plant defense responses. Plant Cell 8: 1773–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Tang W, Swain JD, Green AL, Jack TP, Gan S (2001) Networking senescence-regulating pathways by using Arabidopsis enhancer trap lines. Plant Physiol 126: 707–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Jimenez MJ, Lucas MM, de Felipe MR (2002) Antioxidant defense and damage in senescing lupin nodules. Plant Physiol Biochem 40: 645–657 [Google Scholar]
- Hodges DM, Forney CF (2000) The effects of ethylene, depressed oxygen and elevated carbon dioxide on antioxidant profiles of senescing spinach leaves. J Exp Bot 51: 645–655 [DOI] [PubMed] [Google Scholar]
- Keller R, Renz FS, Kossmann J (1999) Antisense inhibition of the GDP-mannose pyrophosphorylase reduces the ascorbate content in transgenic plants leading to developmental changes during senescence. Plant J 19: 131–141 [DOI] [PubMed] [Google Scholar]
- Keogh RC, Deverall BJ, Mcleod S (1980) Comparison of histological and physiological-responses to Phakopsora pachyrhizi in resistant and susceptible soybean. Trans Br Mycol Soc 74: 329–333 [Google Scholar]
- Klessig DF, Durner J, Noad R, Navarre DA, Wendehenne D, Kumar D, Zhou JM, Shah J, Zhang S, Kachroo P, Trifa Y, Pontier D, et al (2000) Nitric oxide and salicylic acid signaling in plant defense. Proc Natl Acad Sci USA 97: 8849–8855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoester M, Bol JF, Vanloon LC, Linthorst HJM (1995) Virus-induced gene-expression for enzymes of ethylene biosynthesis in hypersensitively reacting tobacco. Mol Plant Microbe Interact 8: 177–180 [DOI] [PubMed] [Google Scholar]
- Koch E, Slusarenko A (1990) Arabidopsis is susceptible to infection by a downy mildew fungus. Plant Cell 2: 437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch MF, Mew TW (1991) Effect of plant age and leaf maturity on the quantitative resistance of rice cultivars to Xanthomonas campestris pv Oryzae. Plant Dis 75: 901–904 [Google Scholar]
- Kus JV, Zaton K, Sarkar R, Cameron RK (2002) Age-related resistance in Arabidopsis is a developmentally regulated defense response to Pseudomonas syringae. Plant Cell 14: 479–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry LG, Chapple CC, Last RL (1995) Arabidopsis mutants lacking phenolic sunscreens exhibit enhanced ultraviolet-B injury and oxidative damage. Plant Physiol 109: 1159–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HI, Leon J, Raskin I (1995) Biosynthesis and metabolism of salicylic acid. Proc Natl Acad Sci USA 92: 4076–4079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin IMR, Norris SR, Ramamoorthy J, Last RL (2000) Map-based cloning of the VTC2 (Vitamin C 2) locus. 11th International Conference on Arabidopsis Research, http://www.arabidopsis.org/servlets/TairObject?type=publication&id=1547090
- Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79: 583–593 [DOI] [PubMed] [Google Scholar]
- Mach JM, Castillo AR, Hoogstraten R, Greenberg JT (2001) The Arabidopsis-accelerated cell death gene ACD2 encodes red chlorophyll catabolite reductase and suppresses the spread of disease symptoms. Proc Natl Acad Sci USA 98: 771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy J, Carr JP, Klessig DF, Raskin I (1990) Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science 250: 1002–1004 [DOI] [PubMed] [Google Scholar]
- Miller JD, Arteca RN, Pell EJ (1999) Senescence-associated gene expression during ozone-induced leaf senescence in Arabidopsis. Plant Physiol 120: 1015–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7: 405. [DOI] [PubMed] [Google Scholar]
- Morris K, Mackerness SAH, Page T, John CF, Murphy AM, Carr JP, Buchanan-Wollaston V (2000) Salicylic acid has a role in regulating gene expression during leaf senescence. Plant J 23: 677–685 [DOI] [PubMed] [Google Scholar]
- Müller-Moulé P, Conklin PL, Niyogi KK (2002) Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo. Plant Physiol 128: 970–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Kiyosue T, Yamaguchi-Shinozaki K, Shinozaki K (1997) A nuclear gene, erd1, encoding a chloroplast-targeted Clp protease regulatory subunit homolog is not only induced by water stress but also developmentally up-regulated during senescence in Arabidopsis thaliana. Plant J 12: 851–861 [DOI] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT (2002) Hydrogen peroxide and nitric oxide as signaling molecules in plants. J Exp Bot 53: 1237–1247 [PubMed] [Google Scholar]
- Niyogi KK (2000) Safety valves for photosynthesis. Curr Opin Plant Biol 3: 455–460 [DOI] [PubMed] [Google Scholar]
- Oh SA, Park JH, Lee GI, Paek KH, Park SK, Nam HG (1997) Identification of three genetic loci controlling leaf senescence in Arabidopsis thaliana. Plant J 12: 527–535 [DOI] [PubMed] [Google Scholar]
- Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH (2003) Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell 15: 939–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M, Kuc J (1992) Peroxidase-generated hydrogen peroxide as a source of antifungal activity in vitro and on tobacco leaf disks. Phytopathology 82: 696–699 [Google Scholar]
- Pieterse CM, van Loon LC (1999) Salicylic acid-independent plant defence pathways. Trends Plant Sci 4: 52–58 [DOI] [PubMed] [Google Scholar]
- Pignocchi C, Foyer CH (2003) Apoplastic ascorbate metabolism and its role in the regulation of cell signalling. Curr Opin Plant Biol 6: 379–389 [DOI] [PubMed] [Google Scholar]
- Polle A (2001) Dissecting the superoxide dismutase-ascorbate-glutathione-pathway in chloroplasts by metabolic modeling. Computer simulations as a step towards flux analysis. Plant Physiol 126: 445–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirino BF, Normanly J, Amasino RM (1999) Diverse range of gene activity during Arabidopsis thaliana leaf senescence includes pathogen-independent induction of defense-related genes. Plant Mol Biol 40: 267–278 [DOI] [PubMed] [Google Scholar]
- Rao MV, Lee H, Creelman RA, Mullet JE, Davis KR (2000) Jasmonic acid signaling modulates ozone-induced hypersensitive cell death. Plant Cell 12: 1633–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EE, Ausubel FM (1997) Arabidopsis enhanced disease susceptibility mutants exhibit enhanced susceptibility to several bacterial pathogens and alterations in PR-1 gene expression. Plant Cell 9: 305–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumen EC, Bonman JM, Parlevliet JE (1992) Leaf age-related partial resistance to Pyricularia oryzae in tropical lowland rice cultivars as measured by the number of sporulating lesions. Phytopathology 82: 1414–1417 [Google Scholar]
- Schwartz SH, Tan BC, Gage DA, Zeevaart JA, McCarty DR (1997) Specific oxidative cleavage of carotenoids by VP14 of maize. Science 276: 1872–1874 [DOI] [PubMed] [Google Scholar]
- Silva H, Yoshioka K, Dooner HK, Klessig DF (1999) Characterization of a new Arabidopsis mutant exhibiting enhanced disease resistance. Mol Plant Microbe Interact 12: 1053–1063 [DOI] [PubMed] [Google Scholar]
- Simpson SD, Nakashima K, Narusaka Y, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Two different novel cis-acting elements of erd1, a clpA homologous Arabidopsis gene function in induction by dehydration stress and dark-induced senescence. Plant J 33: 259–270 [DOI] [PubMed] [Google Scholar]
- Smirnoff N (2000) Ascorbic acid: metabolism and functions of a multi-faceted molecule. Curr Opin Plant Biol 3: 229–235 [PubMed] [Google Scholar]
- Smirnoff N, Conklin PL, Loewus FA (2001) Biosynthesis of ascorbic acid in plants: a renaissance. Annu Rev Plant Physiol Plant Mol Biol 52: 437–467 [DOI] [PubMed] [Google Scholar]
- Terras FR, Eggermont K, Kovaleva V, Raikhel NV, Osborn RW, Kester A, Rees SB, Torrekens S, Van Leuven F, Vanderleyden J, et al (1995) Small cysteine-rich antifungal proteins from radish: their role in host defense. Plant Cell 7: 573–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veljovic-Jovanovic SD, Pignocchi C, Noctor G, Foyer CH (2001) Low ascorbic acid in the vtc-1 mutant of Arabidopsis is associated with decreased growth and intracellular redistribution of the antioxidant system. Plant Physiol 127: 426–435 [PMC free article] [PubMed] [Google Scholar]
- Weaver LM, Gan S, Quirino B, Amasino RM (1998) A comparison of the expression patterns of several senescence-associated genes in response to stress and hormone treatment. Plant Mol Biol 37: 455–469 [DOI] [PubMed] [Google Scholar]
- Weymann K, Hunt M, Uknes S, Neuenschwander U, Lawton K, Steiner HY, Ryals J (1995) Suppression and restoration of lesion formation in Arabidopsis lsd mutants. Plant Cell 7: 2013–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]