Abstract
Protein toxins, such as gelonin, are highly desirable anti-cancer drug candidates due to their unparalleled potency and repetitive reaction mechanism in inhibiting protein translation. However, for its potential application in cancer therapy, there remains the cell membrane barrier that allows permeation of only small molecules, which must be overcome. To address this challenge, we conjugated gelonin with a protein transduction domain (PTD), the TAT peptide, via genetic recombination. The chimeric TAT-gelonin fusion protein (TAT-Gel) retained equipotent N-glycosidase activity yet displayed greater cell uptake than unmodified recombinant gelonin (rGel), thereby yielding a significantly augmented cytotoxic activity. Remarkably, TATGel displayed up to 177-fold lower IC50 (avg. 54.3 nM) than rGel (avg. IC50: 3640 nM) in tested cell lines. This enhanced cytotoxicity, however, also raised potential toxicity concerns due to the non-selectivity of PTD in its mediated cell transduction. To solve this problem, we investigated the plausibility of regulating the cell transduction of TAT-Gel via a reversible masking using heparin and protamine. Here, we demonstrated, both in vitro and in vivo, that the cell transduction of TAT-Gel can be completely curbed with heparin and yet this heparin block can be efficiently reversed by the addition of protamine. This reversible tight regulation of the cell transduction of TAT-Gel by heparin and protamine sheds light of possible application of TATGel in achieving a highly effective yet safe drug therapy for the treatment of tumors.
Keywords: Gelonin, TAT, Heparin, Protamine, Tumor
INTRODUCTION
Protein enzymes have been drawing significant interest as a new class of anti-cancer drugs.1-5 Due to superior efficiency and a repetitive reaction mechanism, protein drugs can supersede the potency barrier which most of the small molecule drugs suffer, providing the opportunity to eradicate tumor at an exceedingly low bioavailable drug concentrations at the tumor target.1 Among those proteins, gelonin, a 30-kDa size plant derived toxin, exemplifies a class of ribosome inactivating proteins (RIP) with extreme potency in inhibiting protein synthesis. Gelonin cleaves the adenine group at a specific position (A-4324) in the conserved sarcin/ricin loop of the eukaryotic 28S ribosomal RNA, resulting in an irreversible inactivation of the ribosomes.6, 7 The efficiency in ribosome inactivation by gelonin is so high that, according to numerous literature reports, only a few molecules of gelonin that gained access to its cytosolic substrate ribosomes would be sufficient to kill a tumor cell.1, 8 Yet, clinical application of gelonin is still limited because of its poor uptake by tumor cells.8, 9
To date, various approaches have been attempted to overcome the cell membrane barrier for gelonin. Most of these methods were by linking gelonin to a protein or peptide ligands (e.g., monoclonal antibodies (mAb), growth factors, cytokines, etc), so that it could enter cells via the receptor-mediated endocytosis pathway.1, 10-12 The success of such methods, however, relied heavily on the extent of internalization of the ligand-receptor complex, which could vary significantly dependent upon the type of ligands and receptors involved.12 Moreover, once endocytosed, the drugs would be entrapped in the endosomes and, unless finding a way to escape, they would not have access to the cell cytoplasm - a pre-requisite for gelonin to exert cytotoxic effects.12, 13 More critically, tumor penetration by antibody is often quite poor, due to their large size (150 kDa) and high binding affinity to the antigens, with the latter being termed as the binding-site barrier.14
In the past twenty years, the discovery of a group of short basic peptides, so-called “protein transduction domains (PTDs)” have revolutionized the way to deliver cell-impermeable macromolecules into cells.15, 16 Numerous literatures reported that these PTDs, such as the TAT peptide derived from HIV viral protein, can ferry attached cargos (almost any type of macromolecules or nano-carriers) into all organ/tissue types, without causing membrane perturbation or damage.15, 17 The PTD-mediated cell internalization was so potent that it could not be matched by the conventional receptor-mediate endocytosis method.16, 18-20 Nevertheless, the lack of selectivity in cell-entry has presented a formidable challenge to successfully apply this PTD-based strategy, because of concerns related to drug associated toxicity on normal tissues.15, 17
To address this selectivity issue, various prodrug-type strategies have been attempted.15, 17 Since direct adsorption of PTD to the anionic constituents of the cell surface (e.g., glycosaminoglycans or phospholipids) was considered to be an essential step for initiation of cell transduction, many of the approaches relied on blocking this event.15, 17 A simple yet effective way to achieve this goal is by reversibly masking the cationic PTD with anionic materials (e.g., hyaluronic acid, glutamate oligomers, heparin, etc.).21-23 Indeed, Yang and co-workers suggested a prodrug strategy based on the reversible masking/demasking of PTD using clinically approved anionic heparin and cationic protamine drugs, and demonstrated the in vitro feasibility of this strategy in regulating the cell internalization of PTD-linked asparaginase, an approved enzyme drug for treatment of leukemia.23
In this research, we synthesized a recombinant chimeric TAT-gelonin fusion toxin (TATGel) using the genetic engineering method. The functionality of TAT-Gel was assessed in vitro using the rabbit reticulocyte lysate and cellular assays. Furthermore, we also conducted preliminary proof-of-concept animal studies to validate the plausibility of regulating the cell uptake event of TAT-Gel through the use of heparin and protamine. Overall, both in vitro and in vivo studies provided strong evidence to support our hypothesis, i.e. cell transduction of TATGel could be effectively inhibited by heparin masking while protamine was able to reverse this heparin-induced block. Results presented in this paper suggest the potential of achieving an effective yet safe delivery of TAT-Gel for cancer therapy.
MATERIALS AND METHODS
Materials
Fluorescein isothiocyanate (FITC), rhodamine B isothiocyanate (TRITC), heparin sulfate and protamine sulfate were purchased from Sigma-Aldrich (St. Louis, MO). Isopropyl-β-thiogalactopyranoside (IPTG), kanamycin and carbenicillin were purchased from Fisher Scientific (Pittsburg, PA). E. coli cells (TOP10 and BL21star (DE3)), fetal bovine serum albumin (FBS), PBS (pH 7.4), Dulbecco's Modified Eagle Medium (DMEM), Hoechst 33342, pEXP-5-NT/TOPO TA expression kit and AcTEV™ protease were purchased from Invitrogen (Carlsbad, CA). DNA primers were purchased from Integrated DNA Technologies Inc. (Coralville, IA). DNA restriction enzymes (BamHI and XhoI) and T4 DNA ligase were purchased from New England Biolabs (Ipswich, MA). Rabbit reticulocyte lysate assay system was purchased from Promega Corporation (Madison, WI). Cell proliferation kit II (XTT) was purchased from Roche Applied Science (Indianapolis, IN). BCA assay kit was purchased from Bio-Rad Laboratories (Hercules, CA). LS174T human adenocarcinoma cells, CT26 murine colon cancer cells, 9L rat glioma cells, U87 MG human glioblastoma-astrocytoma cells, HeLa human cervical cancer cells, PC-3 human prostate cancer cells, Madin-Darby Canine Kidney (MDCK) cells and 293 human embryonic kidney (HEK) cells were purchased from American Type Culture Collection (ATCC, Manassas, VA). The pET28a-Gel plasmid vector for the expression of recombinant gelonin (rGel) was a generous gift from Dr. Wolfgang E. Trommer (University of Kaiserslautern, Germany).24
Plasmid Construction
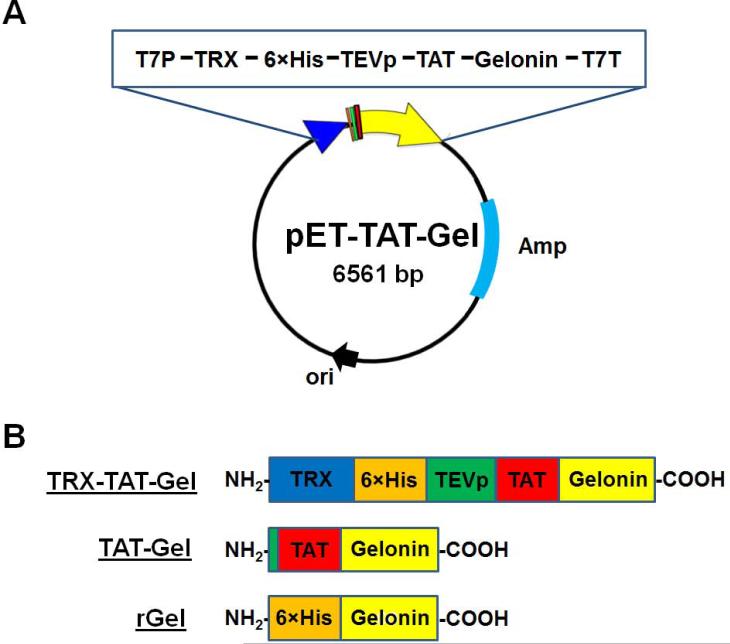
The plasmid (pET-TAT-Gel) for expression of chimeric TAT-gelonin fusion protein (TAT-Gel) was prepared in two steps. First, TAT-gelonin gene (810 bp) was constructed by PCR with TG-forward and TG-backward primers (please refer to the supporting information; Table S1), using pET-Gel vector (pET28a-Gel) as the template. After reaction, the PCR product was purified on 1% agarose gel and ligated into a pEXP-5-NT/TOPO vector using the vendor's protocol (Invitrogen, Carlsbad, CA). Next, by 3-step sequential PCR reactions using the prepared pNT/TOPO-TAT-Gel vector as the initial template, we engineered genes encoding TEV protease cleavable peptide (TEVp) and 6×His, as well as a BamHI cleavage site at the 5’-end of TAT-gelonin gene, and created an XhoI cleavage site at the 3’-end. The primers (pTG-forward 1-3 & pTG-backward) used for these PCR reactions are summarized in the supporting information (Table S1). The final PCR product (BamHI-6×His-TEVp-TAT-gelonin-XhoI) was digested (BamHI & XhoI) and, after purification on 1% agarose gel, ligated into the linearized pET21a-TRX vector (ProMab Biotechnologies, Inc., Richmond, CA) containing a thioredoxin (TRX) gene to produce the pET-TAT-Gel. The prepared pET-TAT-Gel vector was submitted for DNA sequencing analysis. The schematic design of pET-TAT-Gel vector and schematic images of recombinant gelonin toxins are shown in Fig. 1A and 1B, respectively.
Figure 1.
Scheme of recombinant toxins. (A) Schematic design of pET-TAT-Gel vector. The pET-TAT-Gel vector was constructed by inserting the full sequence of TAT-gelonin gene into a pET-21a vector containing thioredoxin (TRX) gene; (B) Schematic images of TRX-TAT-Gel, TAT-Gel and rGel proteins (TRX-TAT-Gel: recombinant thioredoxin-6×His tagged-TAT-gelonin fusion protein, TAT-Gel: recombinant TAT-gelonin fusion protein, rGel: recombinant gelonin)
Expression and Purification of Recombinant Gelonin Toxins
For expression of recombinant gelonin (rGel) and TAT-Gel, BL21 (DE3) E. coli transformed of pET-Gel and pET-TAT-Gel were grown at 37°C in LB broth containing 50 μg/mL carbenicillin and 80 μg/mL kanamycin, respectively. At absorbance (OD600) of 0.7 – 1.0, isopropyl-D-thiogalactoside (IPTG) was added to the cultures as an inducer to a final concentration of 0.5 mM. After 6 h incubation at 37°C with shaking at 250 rpm, cells were harvested by centrifugation and suspended in phosphate buffer saline (20 mM PBS, 300 mM NaCl, pH 7). The cells were then lysed by sonication (4 × 30 s, with 50% output in ice bath) and centrifuged. The supernatants were loaded onto HisPure® Ni-NTA resin (Bio-Rad Laboratories, Hercules, CA) and, after wash with PBS, rGel and thioredoxin-6×His tagged TAT-gelonin (a.k.a. TRX-TAT-Gel) were eluted with 400 mM imidazole (20 mM PBS, 300 mM NaCl, 400 mM imidazole, pH 7). For further purification of rGel, the eluent was loaded onto a cation exchange column (HiTrap Sepharose CM-FF, GE Healthcare Bio-Sciences, Pittsburgh, PA) connected to an HPLC (Alltech 526 HPLC pump, Deerfield, IL) and the final rGel product was purified by salt gradient elution (0 to 2 M NaCl at a rate of 0.02 M/min, flow rate: 1 mL/min). TRX-TATGel was incubated with TEV protease (AcTEV™ protease, Invitrogen) for the removal of thioredoxin-6×His tag, and TAT-Gel was purified on heparin column using a salt gradient (0 to 1.4 M NaCl at a rate of 0.02 M/min, flow rate: 1 mL/min). The purification of rGel and TAT-Gel was monitored by SDS-PAGE on 10% Tris-HCl gel. The purities of final recombinant gelonin toxin products were determined based on densitometry analysis using ImageJ software (National Institutes of Health, Bethesda, MD) and the production yields were calculated by standard BCA assay.
Protein Synthesis Inhibition Assay
Inhibition of protein translation by TAT-Gel was evaluated, in comparison with rGel, following the procedure of Shin et al using a modified rabbit reticulocyte lysate assay.9
Fluorescence Dye Labeling
Fluorescence dye labeling of recombinant gelonin toxins (i.e. rGel and TAT-Gel) was carried out by mixing toxins (2 mg/mL in 0.1 M bicarbonate buffer, pH 9.0) with 5-fold molar excess of a fluorescence dye (e.g., TRITC or FITC) and incubation for 3 h at room temperature. After incubation, the dye-labeled rGel and TAT-Gel were purified by using dye removal resins following the vendor's protocol (Bio-Rad, Hercules, CA).
Transduction of TAT-Gel into LS174T Cells
LS174T cells were seeded onto an 8-well chambered coverglass (Thermo Scientific, Rockford, IL) at 105 cells/well and incubated in complete DMEM medium with 10% FBS. When the cells were attached to the bottom of the chambers, TRITC-labeled rGel and TAT-Gel were separately added to the wells and incubated at 37°C for 3 h in humidified CO2 incubator. After incubation, the cells were rinsed three times with heparin/PBS (10 mg/mL heparin, 20 mM phosphate buffer, 0.15 M NaCl, pH 7.4) and their nuclei were counterstained with Hoechst 33342. Following the Hoechst staining, the cells were washed three times with PBS and the images of the live cells were taken by a Nikon A1R-A1 confocal laser microscope with a 60×objective (Nikon Instruments Inc., Melville, NY). Cell images were acquired and analyzed using NIS-Elements Microscope Imaging software (Nikon Instruments Inc., Melville, NY).
Cytotoxicity Assay
The cytotoxic activity of TAT-Gel was assessed with various cancer cell lines (LS174T, CT26, 9L, U87 MG, HeLa and PC3) and noncancerous cell lines (MDCK and 293 HEK) by XTT assay. Briefly, cells were dispensed into 96-well plates at a density of 5×103 cells per well and incubated in complete DMEM medium with 10% FBS. When the cells were bound to the bottom of the plates, rGel and TAT-Gel were added to the wells at varying final concentrations (10−11 – 10−5 M) and incubated for 72 h. After incubation, the relative cell viability was determined by XTT assay following the vendor's protocol (Roche Applied Science, Indianapolis IN). The concentrations of rGel and TAT-Gel to inhibit 50% of cell proliferation (IC50) were calculated using Prism software (Prism version 5.0, GraphPad, CA).
Binding of TAT-Gel to Heparin Sepharose Beads and Protamine-Triggered Release
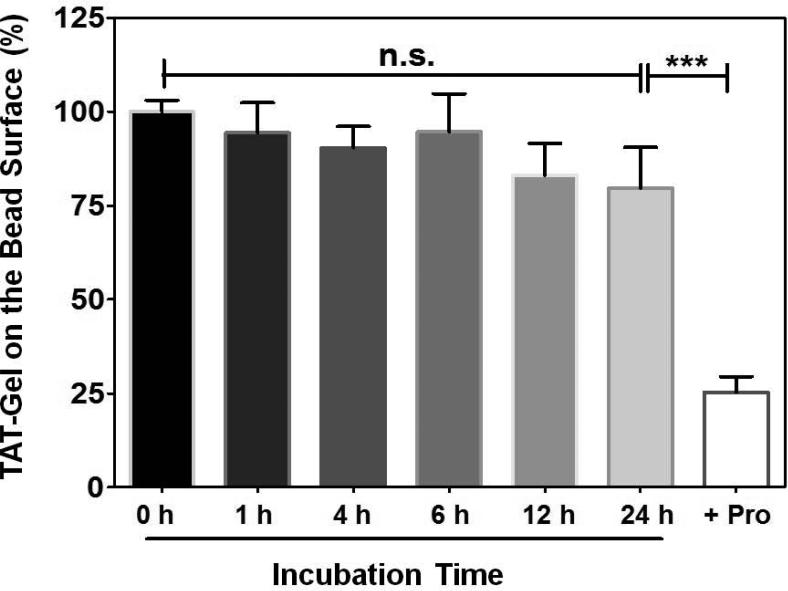
Heparin bead slurries (Heparin HyperD® M, Pall Corporation, Port Washington, NY; 50% (v/v) in PBS) were dispensed to eppendorf tubes (100 μL per tube), followed by addition of 250 μL aliquots of FITC-labeled TAT-Gel (200 μg/mL in PBS). After incubation of the bead mixtures for 1 h at room temperature, the beads were centrifuged at 10,000 rpm for 5 min and supernatants were discarded. The beads loaded with FITC-labeled TAT-Gel were then re-dispersed in 250 μL of rat plasma and incubated up to 24 h at 37°C. At intended time points (0, 1 h, 4 h, 6 h, 12 h and 24 h), the beads (N = 3 per time point) were washed with PBS for three times, and the FITC-labeled TAT-Gel remaining on the beads’ surface were eluted with 2 M NaCl solution. To test the protamine-triggered release of TAT-Gel, 100 μL of protamine solution (10 mg/mL) was added to a group of beads loaded with FITC-labeled TAT-Gel and incubated for 30 min. After incubation, the beads were washed with PBS for three times, and the remaining FITC-labeled TAT-Gel on the beads’ surface was eluted with 2 M NaCl solution. For the control, FITC-labeled TAT-Gel was loaded to beads and, after PBS wash, eluted with 2 M NaCl solution, without incubation in plasma. The fluorescence intensities of the eluents were measured by a plate reader (excitation/emission wave length: 485 nm/530 nm, BioTEK® Synergy™ BioTEK, co., Winooski, VT) and the bound fractions of FITC-labeled TAT-Gel remaining on the heparin beads’ surface (%) were calculated by dividing the mean fluorescence intensities by the mean fluorescence intensity of the control group. Statistical significant differences in the bound fraction of TAT-Gel among the groups were compared by 1-way ANOVA with Tukey's multiple comparison test as the post hoc test using Prism software (Prism version 5.0, GraphPad, CA).
Heparin/Protamine-Regulated Cell Transduction of TAT-Gel
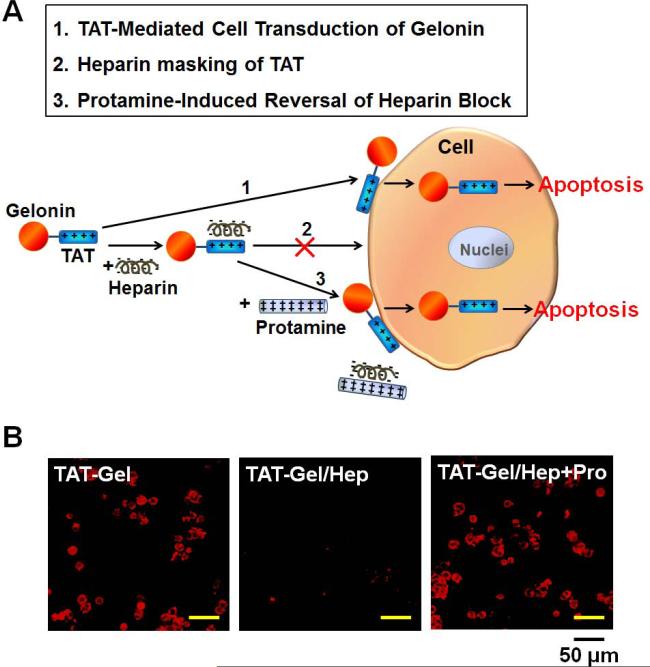
LS174T cells were seeded onto an 8-well chambered coverglass (Thermo Scientific, Rockford, IL) at a density of 105 cells/well and incubated in complete DMEM medium with 10% FBS. When the cells were bound to the bottom of the chambers, TRITC-labeled TAT-Gel was added to the wells either: 1) alone, 2) with heparin (TAT-Gel/Hep) or 3) with heparin and protamine (“TAT-Gel/Hep+Pro”), and incubated at 37°C for 3 h. The TAT-Gel/Hep complex was prepared by mixing TRITC-labeled TAT-Gel with 3-fold molar excess of heparin and incubation at 4°C for 30 min. The “TAT-Gel/Hep+Pro” treatment was by addition of protamine (3-fold molar excess to heparin) to the wells immediately after the cells were treated with TATGel/Hep. After incubation, cells were stringently washed for three times with heparin/PBS, followed by another wash with PBS, and the images of the live cells were acquired by a Nikon A1R-A1 confocal laser microscope with a 20×objective (Nikon Instruments Inc., Melville, NY) and analyzed using NIS-Elements Microscope Imaging software (Nikon Instruments Inc., Melville, NY).
Heparin/Protamine-Mediated Regulation on the Cytotoxicity of TAT-Gel
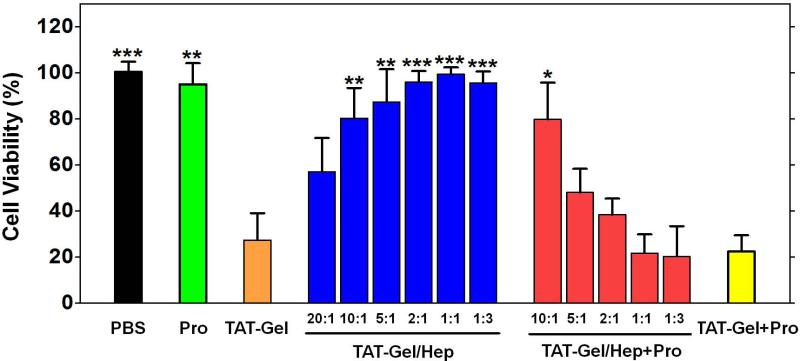
LS174T cells were dispensed into 96-well plates at 5×103 cells per well and incubated in complete DMEM medium with 10% FBS. When the cells were bound to the bottom of the plates, the cells were treated with either: 1) TAT-Gel, 2) TAT-Gel/Hep, 3) “TAT-Gel/Hep+Pro”, 4) “TAT-Gel+Pro” or 5) protamine (a.k.a. Pro). The final concentration of TAT-Gel used in all the groups was 100 nM. The TAT-Gel/Hep samples were prepared by mixing TAT-Gel with varied molar ratios of heparin (TAT-Gel : heparin = 20:1, 10:1, 5:1, 2:1, 1:1 or 1:3) and incubation at 4°C for 30 min. The “TAT-Gel/Hep+Pro” treatment was by addition of protamine to the cells pre-treated with TAT-Gel/Hep (prepared by TAT-Gel : heparin molar ratio of 1:3) with different heparin to protamine molar ratios of 10:1, 5:1, 2:1, 1:1 or 1:3, and the “TATGel+Pro” treatment was by addition of 10 molar excess (1 μM) of protamine to the TAT-Gel-treated cells. In case of protamine treatment, cells were incubated with 1 μM protamine. After treatment with the test compounds, the cells were incubated at 37°C for 72 h and then the relative cell viability was measured by XTT assay. Statistical significant differences in the relative cell viability of TAT-Gel-treated group and those of other groups were compared by Student's t-test using Prism software (Prism version 5.0, GraphPad, CA).
In Vivo Evaluation of Heparin/Protamine-Mediated Regulation using LS174T Xenograft Tumor Mouse Model
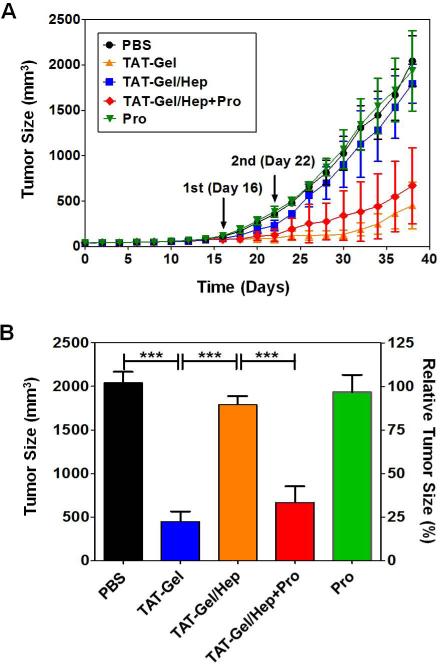
Six-week-old male athymic nude mice (body weight: 23 - 26 g) were purchased from Charles Rivers Laboratories (Raleigh, NC). Three days after arrival (day 0), LS174T cells were implanted at the left hind region of the mice legs (5×106 cells/mice). When the tumor size reached 100 mm3 (day 16), the mice were randomly divided into 5 groups (N = 5 per group) and separately treated with either: 1) PBS, 2) TAT-Gel (injected dose: 2 μg), 3) TAT-Gel/Hep (TATGel 2 μg; heparin 3 μg), 4) “TAT-Gel/Hep+Pro” (TAT-Gel 2 μg; heparin 3 μg; protamine 3 μg) or 5) protamine (a.k.a. Pro; 3 μg). The samples were administered via intra-tumor injection at day 16 and 22. The TAT-Gel/Hep complex was freshly prepared, prior to every treatment, by mixing TAT-Gel with 3-fold molar excess of heparin and incubation at 4°C for 30 min. The “TAT-Gel/Hep+Pro” treatment was by administration of protamine (3-fold molar excess to heparin) into the tumor, 5 min after tumor injection of TAT-Gel/Hep. Tumor size was monitored daily with a vernier caliper and the tumor volume (mm3) was calculated using the following formula: V = (w2 × l) / 2. In this equation, w is the width and l is the length of the tumor. When the average tumor volume of PBS-treated mice reached 2000 mm3, Statistical significant differences in the tumor sizes among the groups were compared by 1-way ANOVA with Tukey's multiple comparison test as the post hoc test using Prism software (Prism version 5.0, GraphPad, CA). All animal experiments were conducted according to the protocols approved by the University of Michigan Committee on the Use and Care of Animals (UCUCA: protocol No. 08945-3).
Statistical Analysis
All data were expressed as mean ± standard deviation. Unless otherwise noted, statistically significant differences between groups were determined by Student's t-test (Prism version 5.0, GraphPad, San Diego, CA) and results that yielded p value lower than 0.05 were considered statistically significant.
RESULTS
Expression and Purification of Recombinant Gelonin Toxins
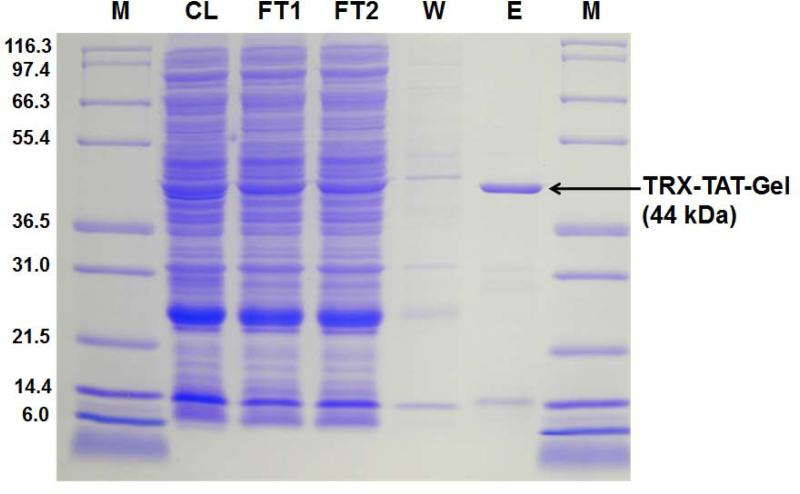
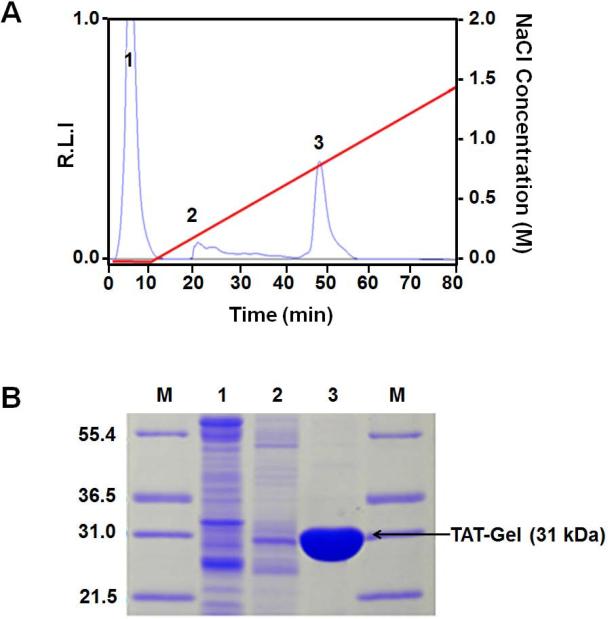
Recombinant gelonin (rGel) and chimeric TAT-gelonin fusion protein (TAT-Gel) were successfully expressed from E. coli as soluble proteins. The rGel was completely purified by sequential Ni-NTA metal affinity and cation exchange chromatography; as it appeared as a single band at 31 kDa on SDS-PAGE (data not shown). Thioredoxin-6×His tagged TAT-gelonin (a.k.a. TRX-TAT-Gel) was also successfully purified on Ni-NTA metal affinity resins; as evidenced by the appearance of an intense band at 44 kDa (Lane E) on the SDS-PAGE (Fig. 2). By incubation of TRX-TAT-Gel with TEV protease (AcTEV™, Carlsbard, CA), thiredoxin-6×His tag was cleaved and the TAT-Gel was acquired after heparin column purification using salt gradient elution. As shown in Fig. 3A, while endogenous bacterial proteins (Fraction 1 & 2) were eluted at 0 and 0.1 M NaCl (retention time: 20 min); as identified by the presence of multiple bands from the SDS-PAGE result (Lane 1 and 2; Fig. 3B), TAT-Gel was eluted as a single peak (Fraction 3) at 0.7 M NaCl with a longer retention time of 45 min. As seen in Lane 3 of Fig. 3B, this Fraction 3 yielded a single band at 31 kDa, which was consistent with the expected size of TAT-Gel. The yields of rGel and TAT-Gel were 1 and 3 mg/L culture, respectively, based on BCA assay. The purity of both compounds was determined to be higher than 95%, according to results from densitometry analysis.
Figure 2.
SDS-PAGE results for Ni-NTA column purification of TRX-TAT-Gel. Lane M: markers of the protein molecular weight standard (Mark 12™ standard, Invitrogen), Lane CL: supernatant fraction of the cell lysate. Lane FT 1 and 2: flow through fractions 1 and 2. Lane W: wash fraction. Lane E: elution fraction representing TRX-TAT-Gel. (TRX-TAT-Gel: recombinant thioredoxin-6×His tagged-TAT-gelonin fusion protein)
Figure 3.
Heparin column purification of TAT-Gel after cleavage of thioredoxin-6×His tag from the TRX-TAT-Gel by incubation with TEV protease. (A) Chromatogram. Using a NaCl salt gradient from 0 to 1.4 M (red line), three major fractions labeled as Peak 1 – 3 eluted at 0, 0.2 and 0.7 M NaCl (with retention time of 2, 20 and 45 min, respectively). (B) SDS-PAGE results for the fractions eluted from the heparin column. Lane M: markers of the protein molecular weight standard (Mark 12™ standard, Invitrogen). Lane 1, 2, and 3 represented results from the three peak fractions (1, 2, and 3, respectively). Results showed that TAT-Gel was eluted from the 3rd peak at 0.7 M NaCl. (TRX-TAT-Gel: recombinant thioredoxin-6×His tagged-TAT-gelonin fusion protein, TAT-Gel: recombinant TAT-gelonin fusion protein)
Inhibition of Protein Synthesis by TAT-Gel
Since gelonin's N-glycosidase activity to inhibit protein synthesis was essential for exerting anti-cancer activity, we examined whether it was well-reserved following the conjugation of gelonin with TAT, using the rabbit reticulocyte lysate assay. Both rGel and TAT-Gel yielded similar inhibitory profiles for the translation of luciferase (data not shown), and their IC50 values (rGel: 20 ± 12 pM vs. TAT-Gel: 15 ± 4 pM, p > 0.05 by Student's t-test) were statistically identical; suggesting that incorporation of TAT did not affect the inherent activity of gelonin in the TAT-Gel conjugate.
Transduction of TAT-Gel into LS174T cells
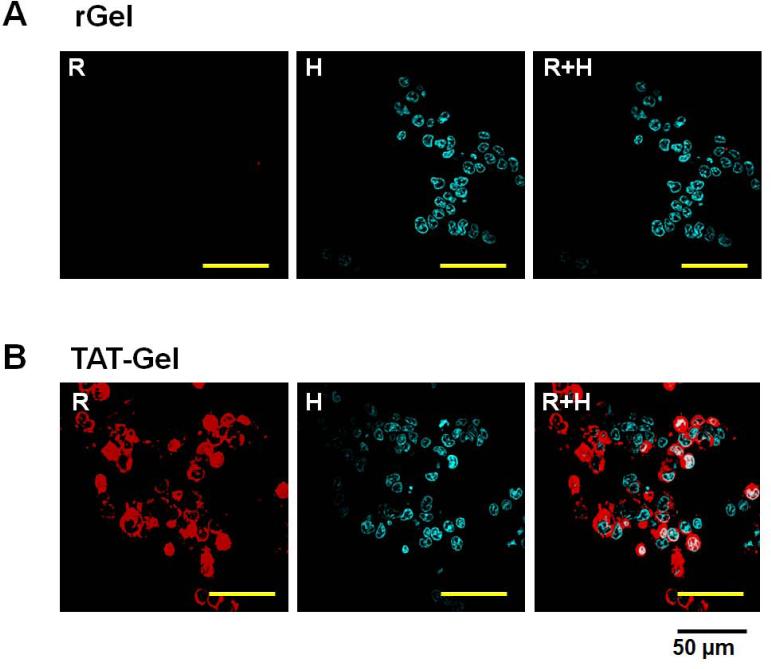
To examine whether TAT can induce transduction of the fused gelonin into cells, an uptake study was conducted with LS174T cells. The confocal microscopic images of the cells, taken after incubation with TRITC-labeled recombinant gelonin toxins and subsequent nuclei counter-stain with Hoechst 33342, are displayed in Fig. 4. As seen, while virtually no fluorescence signal was visible from the rGel-treated cells (Fig. 4A), strong fluorescence intensity was observed from cells incubated with TAT-Gel (Fig. 4B). These results indicated that TAT indeed promoted the cellular uptake of the cell-impermeable gelonin; presumably through its cell transduction mechanism.
Figure 4.
Cell uptake study results of rGel and TAT-Gel on LS174T cells. The cells were incubated with either TRITC-labeled (A) rGel or (B) TAT-Gel for 3 h at 37°C. After incubation, the cells were washed for three times with 10 mg/mL heparin/PBS solution for stringent wash, counterstained the nuclei with Hoechst 33342, and, after three more wash with PBS, the cell images were taken by a confocal microscope utilizing TRITC (red), Hoechst (blue) channels and merged. (rGel: recombinant gelonin, TAT-Gel: recombinant TAT-gelonin fusion protein)
Cytotoxic Activity of TAT-Gel
Given the cell penetrating ability of TAT and the well-reserved N-glycosidase activity of gelonin, we hypothesized that TAT-Gel would yield significantly greater cytotoxic effects than rGel. To test this hypothesis, cell viability studies were carried out on various cancer cell lines (e.g., LS174T, CT26, 9L, U87 MG, HeLa and PC-3) and noncancerous cell lines (e.g., MDCK and 293 HEK). As displayed in Table 1, among all the tested cell lines, while cell death was observed from rGel-treated cells only at above micro-molar concentrations (avg. IC50: 4110 ± 734 nM), significantly augmented cytotoxicity (avg. IC50: 54.3 ± 13.8 nM) was observed from cells incubated with TAT-Gel. This enhanced cytotoxic activity of TAT-Gel provided clear evidence to the effectiveness of the TAT fusion strategy in facilitating cell internalization of the barely permeable gelonin.
Table 1.
Cytotoxic activity (IC50) of TAT-Gel
| Cell Line | IC50a, nM |
|||
|---|---|---|---|---|
| rGel | TAT-Gel | Fold Difference | ||
| Cancer Cells | LS174T | 5130 ± 802 | 29.0 ± 15.0*** | 177 |
| CT26 | 4190 ± 1120 | 72.2 ± 26.6** | 58 | |
| U87 MG | 3100 ± 409 | 46 ± 13.7*** | 67 | |
| 9L | 4260 ± 856 | 57.8 ± 26.3** | 74 | |
| HeLa | 4980 ± 920 | 66.4 ± 31.4*** | 75 | |
| PC-3 | 3330 ± 761 | 63.4 ± 25.1** | 53 | |
| Non Cancer Cells | MDCK | 3580 ± 846 | 53 ± 16.3** | 68 |
| 293 HEK | 4300 ± 1020 | 46.7 ± 14.0** | 92 | |
IC50 values were calculated by nonlinear regression using Prism software (GraphPad). All experiments were performed with three different batches (N=3).
P < 0.001
P < 0.0001 by Student's t-test. (rGel: recombinant gelonin, TAT-Gel: recombinant TAT-gelonin fusion protein)
Binding of TAT-Gel to Heparin Sepharose Beads and Protamine-Triggered Release
To verify the avid binding of TAT-Gel to anionic heparin via electrostatic interaction, and its protamine-induced release, FITC-labeled TAT-Gel was loaded onto heparin beads, and the release was monitored during incubation with rat plasma at 37°C with or without the addition of protamine. As shown in Fig. 5, the majority (80%) of added TAT-Gel were found stably binding onto surface of the heparin beads up to 24 h. There was no statistically significant difference between the bound fractions of TAT-Gel between 0 h and 24 h (P > 0.05). After the addition of protamine, however, significant amount of TAT-Gel (75%) was instantly released within a period of 30 min.
Figure 5.
Binding of TAT-Gel to heparin sepharose beads and protamine-triggered release. FITC-labeled TAT-Gel was loaded onto heparin beads (Heparin HyperD® M, Pall Corporation, Port Washington, NY; 50% (v/v) in PBS) and incubated up to 24 h at 37°C in rat plasma. At intended time points (0, 1 h, 4 h, 6 h, 12 h and 24 h), beads (N = 3 per time point) were washed, and the bound FITC-labeled TAT-Gel remaining on the beads’ surface were eluted with 2 M NaCl solution. Protamine-triggered release of TAT-Gel was tested by addition of excessive protamine (1 mg) to a group of heparin beads loaded with FITC-labeled TAT-Gel and incubation for 30 min at 37°C. The fluorescence intensities of all the eluents were measured and the bound fraction of FITC-labeled TAT-Gel on the heparin bead (%) for each test tube was calculated by dividing the fluorescence intensity of the eluent by the mean fluorescence intensity of the control group's eluents. Statistical significant differences in the bound fractions of TAT-Gel among the groups were compared by 1-way ANOVA with Tukey's multiple comparison test as the post hoc test using Prism software (GraphPad). *** P < 0.0001. (TAT-Gel: recombinant TAT-gelonin fusion protein)
Heparin/Protamine-Regulated Cell Transduction of TAT-Gel
Since direct adsorption of TAT to the cell membranes is an essential requisite for initiating cell transduction, we hypothesized that cell internalization of TAT-Gel can be effectively regulated by physically blocking/deblocking of the TAT on TAT-Gel with heparin and protamine, respectively. To test the hypothesis, we examined the cell uptake behavior of TAT-Gel in the presence of heparin but with or without protamine. The scheme of this heparin/protamine-mediated regulation on TAT-Gel cell uptake and the uptake results are shown in Fig. 6A and 6B, respectively. As seen, while strong fluorescence was visible in the cells incubated with TRITC-labeled TAT-Gel, virtually no fluorescence signal was observed from cells treated with the mixture of TAT-Gel and heparin (TAT-Gel/Hep); suggesting that TAT-mediated cell transduction of gelonin was completely abolished due to heparin masking of the TAT peptide. In a sharp contrast, cells pre-treated with TAT-Gel/Hep displayed a markedly enhanced fluorescence intensity following the addition of protamine (“TAT-Gel/Hep+Pro”); indicating that protamine was able to lift the heparin block and subsequently resume the cell transduction of TAT-Gel.
Figure 6.
Heparin/protamine modulation of TAT-Gel cell transduction. (A) Schematic illustration of regulating TAT-Gel cell transduction via reversible masking and demasking of TAT on the TAT-Gel by anionic heparin and cationic protamine, respectively. (B) Confocal microscopic images of LS174T cells treated with TATGel, TAT-Gel/Hep or “TAT-Gel/Hep+Pro”. LS174T cells were plated onto 8-well chambered coverglass (Thermo Scientific, Rockford, IL) at a density of 105 cells/well and, when cells were attached to the bottom of the chambers, TRITC-labeled TAT-Gel (5 μM) was added to the wells either 1) alone, 2) with heparin (TAT-Gel/Hep) or 3) with heparin and protamine (“TAT-Gel/Hep+Pro”). After 3 h incubation, cells were washed and the live cell images were acquired by a Nikon A1R-A1 confocal laser microscope with a 20×objective. The TAT-Gel/Hep complex was prepared by mixing TAT-Gel with 3-fold molar excess of heparin and incubation at 4°C for 30 min. For “TAT-Gel/Hep+Pro” treatment, cells were treated with TAT-Gel/Hep (with 3-fold molar excess of heparin to TAT-Gel), followed by immediate addition of protamine (3-fold molar excess against heparin). (TAT-Gel: recombinant TAT-gelonin fusion protein)
Heparin/Protamine-Mediated Regulation on the Cytotoxicity of TAT-Gel
The effects of heparin/protamine regulation on the cytotoxicity of TAT-Gel were evaluated on LS174T cells via the XTT assay. As shown in Fig. 7, while TAT-Gel yielded significant cell death (27.4% cell viability at 100 nM), cells incubated with TAT-Gel/Hep complex exhibited dramatically attenuated cytotoxicity when the molar ratio of TAT-Gel to heparin was reduced below 10:1. Indeed, when the TAT-Gel : heparin molar ratio reached to 5:1,virtually no cell death (> 90% of viability) was observed; suggesting that cell transduction of TAT-Gel was completely inhibited by heparin masking, and therefore all of TAT-Gel conjugates remained outside the cells. Results in Fig. 7 also showed that protamine by itself was not toxic to cells (95% cell viability at 1 μM), nor the addition of protamine to TAT-Gel-treated cells further enhanced the cytotoxicity by TAT-Gel (22.4% of relative cell viability). However, when protamine was added to cells treated with TAT-Gel/Hep (i.e. “TAT-Gel/Hep+Pro” treatment), at a heparin : protamine molar ratio below 5:1, the cytotoxic effects of TAT-Gel was almost fully recovered, reflecting the protamine-induced reversal of heparin block, and thus the freed TATGel could internalize cells to cause cell death.
Figure 7.
Heparin/protamine-mediated regulations on cytotoxicity by TAT-Gel. LS174T cells were treated with: 1) PBS, 2) protamine (a.k.a. Pro; 1 μM), 3) TAT-Gel, 4) TATGel/Hep, 5) “TAT-Gel/Hep+Pro” or 6) “TAT-Gel+Pro”, and, after incubation at 37°C for 48 h, the relative cell viability was determined by XTT assay. The concentration of TAT-Gel was 100 nM for all the tested groups. TAT-Gel/Hep complex was prepared by mixing TAT-Gel and heparin in varied molar ratios (20:1, 10:1, 5:1, 2:1, 1:1, 1:3) and incubation at 4°C for 30 min. For “TAT-Gel/Hep+Pro” treatment, protamine was added to TAT-Gel/Hep pre-treated cells (TAT-Gel : heparin = 1:3) with varied heparin : protamine molar ratios (10:1, 5:1, 2:1, 1:1 or 1:3). In case of “TAT-Gel+Pro” treatment, 10-fold molar excess of protamine (final 1 μM concentration) was added to the TAT-Gel pre-treated cells. For all experiments, N=3. Statistical significant differences in the relative cell viability of TAT-Gel-treated group and those of other groups were compared by Student's t-test. * P < 0.01, ** P < 0.001, *** P < 0.0001. (TAT-Gel: recombinant TAT-gelonin fusion protein)
In Vivo Evaluation of Heparin/Protamine-Mediated Regulation using LS174T Xenograft Tumor Mouse Model
The in vivo feasibility of heparin/protamine-mediated regulation on cell transduction of TAT-Gel was examined using an LS174T s.c. xenograft tumor mouse model. Tumor growth profiles of mice that were administered twice with different test compounds through intra-tumor injection are shown in Fig. 8A and the average tumor sizes at day 38 (38 days after tumor implantation) are displayed in Fig. 8B. Compared with the PBS-treated control group (tumor size: 2042 ± 279 mm3 at day 38), significant inhibition on tumor growth (78%) was observed in mice treated with TAT-Gel (tumor size: 450 ± 258 mm3 at day 38). However, there was no statistically significant difference between mice treated with TAT-Gel/Hep (tumor size: 1793 ± 215 mm3, 12% inhibition) and those of control; indicating the effectiveness of heparin blocking on cell transduction of TAT-Gel in vivo. In sharp contrast, while no reduction in tumor size was observed for mice treated with protamine alone (tumor size: 1934 ± 443 mm3, 5% inhibition), substantial tumor suppression (tumor size: 668 ± 419 mm3, 67% inhibition) was observed from mice treated with “TAT-Gel/Hep+Pro”. These results clearly demonstrated the feasibility of protamine-induced reversal of heparin block under the in vivo tumor environment.
Figure 8.
In vivo evaluation of heparin/protamine regulation on TAT-Gel cell transduction using LS174T s.c. xenograft tumor mouse model. (A) Tumor growth profiles. When the average tumor size reached 100 mm3 (at day 16; 16 days after tumor implantation at day 0), mice were divided into 5 groups (N=5), and treated twice with PBS (circle), TAT-Gel (triangle), TAT-Gel/Hep (square), “TATGel/Hep+Pro” (diamond) or protamine (a.k.a. Pro; reverse triangle) by intra-tumor injection at day 16 and 22. TAT-Gel/Hep complex was prepared by mixing TATGel with 3-fold molar excess of heparin and incubation at 4°C for 30 min. For “TAT-Gel/Hep+Pro” treatment, administration of TAT-Gel/Hep was followed by injection of protamine (3-fold molar excess against heparin) to the tumor with 5 min interval. Tumor sizes were measured daily since tumor implantation (at day 0). (B) Average tumor sizes at day 38 when the average tumor volume of PBS-treated mice reached 2000 mm3. Statistical significant differences in the tumor sizes among the groups were compared by 1-way ANOVA with Tukey's multiple comparison test as the post hoc test. *** P < 0.0001. (TAT-Gel: recombinant TAT-gelonin fusion protein).
DISCUSSION
Protein transduction domains (PTDs) have drawn significant interest as a promising tool to deliver biologically active macromolecules into intact cells. Numerous reports have been found in literatures that PTDs are capable of ferrying small molecule drugs, macromolecules (protein, gene) and even nanoparticles into almost all types of cells with such an efficient transduction mechanism that is unmatched by any other cell internalization pathways (e.g., endocytosis).15, 25, 26 Nevertheless, the lack of cell selectivity of PTD in their mediated cell transduction presents a major obstacle to their clinical applications, because of concerns of causing drug-induced cytotoxicity on normal tissues. Thus, selectively directing the cell penetrating activity of the PTDs to only diseased cells has been an imperative challenge that must be addressed. In this research, we explored the feasibility of improving the drugability of gelonin, a highly potent but cell-impermeable ribosome inactivating protein (RIP) toxin, by linking it to a renowned PTD, TAT peptide. To resolve the selectivity issue, we tested the plausibility of regulating the cell internalization of TAT-Gel conjugate via reversible masking and demasking of TAT using heparin and protamine, respectively.
For the first time, functional recombinant TAT-gelonin chimeric fusion protein (TATGel) was successfully produced as a soluble protein from the E. coli by adopting the co-expression strategy based on the use of thioredoxin (TRX), a 12-kD oxidoreductase enzyme, as a fusion partner. During the early stage of producing TAT-Gel from the E. coli system, the major obstacles that we encountered were the poor expression and insolubility of the recombinant protein, which was presumably attributed to the unique amino acid composition of TAT which consists of clusters of highly basic arginine residues that are translated by one of the rarest codons in E. coli. 27, 28 Certain literatures have noted that cationic PTDs such as low molecular weight protamine (LMWP) and TAT are inclined to form insoluble aggregates that deleteriously affect the expression yields of the fusion proteins. Lee et al. reported that, by the fusion with TAT (or LMWP), the expression yield of mutated cocaine esterase was markedly reduced.27 Indeed, in our early attempt to express TAT-Gel without the help of TRX, SDS-PAGE results showed that there was virtually no apparent soluble expression (data not shown). For the improvement of both the solubility and expression yield of TAT-Gel, TRX, as a fusion partner, possesses several important merits, such as: 1) producing an extraordinarily high expression (40% of the total protein) from E. coli and 2) greatly enhancing the solubility of the fused proteins during expression.29 Indeed, the utility of this TRX strategy was demonstrated by our previous success in expressing soluble recombinant gelonin-LMWP chimera.9 Consistent with these early findings, by employing the TRX as the fusion partner, a reasonably high yield (3 mg TAT-Gel/L culture) of TAT-Gel was successfully expressed as a soluble protein from the E. coli system; as confirmed by the results from the SDS-PAGE (Fig. 2).
Our findings indicated that the intrinsic N-glycosidase activity of gelonin was minimally affected by the incorporation of TAT; as reflected by the indistinctive inhibition profiles on protein synthesis between rGel and TAT-Gel based on the rabbit reticulocyte lysate assay. Apparently, the small size of TAT and the intrinsic stability of gelonin's 3-D conformational structure appeared to account for this phenomenon.9, 24, 30 Moreover, the strategic selection of the N-terminus of gelonin for TAT incorporation might have also played an important role on this event. It was reported that gelonin consisted predominantly of a flexible β-sheet structure at the N-terminus, while it was composed of a relatively rigid α-helix near the C-terminus.30 Thus, the N-terminus of gelonin was thought to be the preferable site for TAT gene insertion so that the intrinsic 3-D conformation of the catalytic center could be retained. Whereas no difference was observed between recombinant gelonin (rGel) and TAT-Gel in their activities to inhibit protein synthesis in the absence of cells, the TAT-Gel displayed significantly enhanced cell uptake (Fig. 4) and cell-killing ability than rGel, when both were being confronted with the cell membrane barrier. Remarkably, the IC50 values were found to be 53 to 177-fold lower for TAT-Gel when compared with rGel (Table 1).
Following successful synthesis and characterizations of TAT-Gel, the next issue remained was how to direct its cytotoxic effects towards only the targeted tumor cells. As revealed by the cell viability results (Table 1), TAT-Gel yielded indiscriminative cytotoxicity against all the cell lines tested, due to the nonselective cell transduction mechanism of TAT that was initiated by its interaction with negatively charged cell surface glycosaminoglycans which were present on the membranes of the vast majority of cells.23, 31 Despite continuing efforts to develop an ideal delivery strategy for directing the PTD-linked drugs selectively to the diseased cells,17, 32 so far there has been very little success in achieving this goal. Here, we presented a new strategy by regulating the cell transduction of TAT-Gel via heparin masking and then the reversal of the heparin block by protamine. Heparin, a clinically used polymeric drug with an exceptionally high density of negative charges on the surface, has been reported to bind strongly with the highly cationic PTDs thereby neutralizing the positive charges that are essential for PTD-mediated cell penetration.23, 33-35 Hence, mediated by this tight heparin binding to TAT, the TAT-Gel will be deprived of the cell-penetrating ability and subsequently its cytotoxic activity towards normal cells during targeting of the tumor tissue. Indeed, the avid binding of heparin and TAT-Gel was confirmed by results from heparin bead binding assay (Fig. 5). As seen, via heparin binding, cell internalization of TAT-Gel was almost completely prohibited; as evidenced by in vitro cell uptake (Fig. 6B) and cytotoxicity results (Fig. 7). While heparin was able to completely block the cell transduction of TAT-Gel, this blockade could be readily lifted by the administration of protamine, a clinical heparin antidote possessing a much stronger binding affinity to heparin than TAT. The feasibility of protamine-triggered relief of heparin masking was confirmed by results from the heparin bead binding assay (Fig. 5). As shown, addition of an excess amount of protamine induced a significant release (75%) of TAT-Gel within 30 min. Moreover, via this protamine-triggered detachment of heparin, the freed TAT-Gel could re-gain its cell penetration ability thereby yielding its therapeutic effects toward cancer cells (Fig. 6B & 7).
Encouraged by the in vitro findings, the functionality of heparin and protamine regulating cell transduction of TAT-Gel was further evaluated in vivo using the LS174T s.c. xenograft tumor mouse model. As demonstrated in Fig. 8, the intra-tumorally administered TAT-Gel/Hep complex appeared to remain stable and undissociated under the in vivo environment; as evidenced by the insignificant reduction in tumor size of the treated animals due to the inability of the TAT-Gel/Hep complex to internalize cells. In a sharp contrast, animals receiving protamine after the administration of the TAT-Gel/Hep (“TAT-Gel/Hep+Pro”) displayed a significant inhibition (67%) of tumor growth, confirming that the later-administered protamine was able to reverse heparin blocking thus resuming the cell-penetration activity of TAT-Gel. Further extensive animal studies that involve a specific antibody for tumor targeting as well as the tail vein injection of both the TAT-Gel/Hep complex and protamine using LS174T s.c. xenograft tumor mouse model are currently in progress in our laboratory.
CONCLUSIONS
In this research, we report the first synthesis of soluble and functional recombinant TAT-gelonin chimeric fusion protein (TAT-Gel) from E. coli. Owing to its cell internalization function, the TAT-Gel conjugate appeared to be a far superior anti-tumor agent than the native unmodified gelonin (rGel); as evidenced by its several orders of magnitude lower IC50 than rGel.
Yet, the indiscriminative cytotoxicity against all the tested cell lines raised concerns for the use of TAT-Gel in cancer therapy, due to the strong likelihood of triggering gelonin-induced potential toxic effects toward normal tissues. To overcome this non-selectivity and safety issue, we examined the feasibility of a new delivery strategy by regulating the cell transduction function of TAT on TAT-Gel via heparin and protmaine-mediated masking and demasking events, respectively. Both our in vitro and in vivo results confirmed that heparin could completely inhibit cell uptake of the TAT-Gel conjugate, while protamine could efficiently reverse this heparin blockade on TAT, thereby resuming the potent cytotoxic effects of TAT-Gel towards target cancer cells. Overall, this study provided a proof-of-concept demonstration of the feasibility and utility of the proposed TAT-aided intracellular delivery of gelonin and, virtually all protein drugs.
Supplementary Material
ACKNOWLEDGEMENTS
This work was partially supported by the NSFC 2013 A3 Foresight Program (81361140344) and National Key Basic Research Program of China (2013CB932502). In addition, this work was also supported in part by National Institutes of Health R01 Grants CA114612. We thank Dr. Wolfgang E. Trommer (University of Kaiserslautern, Germany) for the gelonin expression vector (pET28a-Gel).
REFERENCES
- 1.Antignani A, Fitzgerald D. Immunotoxins: the role of the toxin. Toxins. 2013;5:1486–1502. doi: 10.3390/toxins5081486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pieters R, Hunger SP, Boos J, Rizzari C, Silverman L, Baruchel A, Goekbuget N, Schrappe M, Pui CH. L-asparaginase treatment in acute lymphoblastic leukemia: a focus on Erwinia asparaginase. Cancer. 2011;117:238–249. doi: 10.1002/cncr.25489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng PN, Lam TL, Lam WM, Tsui SM, Cheng AW, Lo WH, Leung YC. Pegylated recombinant human arginase (rhArg-peg5,000mw) inhibits the in vitro and in vivo proliferation of human hepatocellular carcinoma through arginine depletion. Cancer Res. 2007;67:309–317. doi: 10.1158/0008-5472.CAN-06-1945. [DOI] [PubMed] [Google Scholar]
- 4.Pastan I, FitzGerald D. Recombinant toxins for cancer treatment. Science. 1991;254:1173–1177. doi: 10.1126/science.1683495. [DOI] [PubMed] [Google Scholar]
- 5.Pasut G, Sergi M, Veronese FM. Anti-cancer PEG-enzymes: 30 years old, but still a current approach. Adv Drug Deliv Rev. 2008;60:69–78. doi: 10.1016/j.addr.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Stirpe F, Olsnes S, Pihl A. Gelonin, a new inhibitor of protein synthesis, nontoxic to intact cells. Isolation, characterization, and preparation of cytotoxic complexes with concanavalin A. J Biol Chem. 1980;255:6947–6953. [PubMed] [Google Scholar]
- 7.Puri M, Kaur I, Perugini MA, Gupta RC. Ribosome-inactivating proteins: current status and biomedical applications. Drug Discov Today. 2012;17:774–783. doi: 10.1016/j.drudis.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Atkinson SF, Bettinger T, Seymour LW, Behr JP, Ward CM. Conjugation of folate via gelonin carbohydrate residues retains ribosomal-inactivating properties of the toxin and permits targeting to folate receptor positive cells. J Biol Chem. 2001;276:27930–27935. doi: 10.1074/jbc.M102825200. [DOI] [PubMed] [Google Scholar]
- 9.Shin MC, Zhang J, David AE, Trommer WE, Kwon YM, Min KA, Kim JH, Yang VC. Chemically and biologically synthesized CPP-modified gelonin for enhanced anti-tumor activity. J Control Release. 2013;172:169–178. doi: 10.1016/j.jconrel.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borthakur G, Rosenblum MG, Talpaz M, Daver N, Ravandi F, Faderl S, Freireich EJ, Kadia T, Garcia-Manero G, Kantarjian H, Cortes JE. Phase 1 study of an anti-CD33 immunotoxin, humanized monoclonal antibody M195 conjugated to recombinant gelonin (HUM-195/rGEL), in patients with advanced myeloid malignancies. Haematologica. 2013;98:217–221. doi: 10.3324/haematol.2012.071092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao Y, Marks JW, Liu Z, Cheung LH, Hittelman WN, Rosenblum MG. Design optimization and characterization of Her2/neu-targeted immunotoxins: comparative in vitro and in vivo efficacy studies. Oncogene. 2014;33:429–439. doi: 10.1038/onc.2012.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldmacher VS, Scott CF, Lambert JM, McIntyre GD, Blattler WA, Collnhson AR, Stewart JK, Chong LD, Cook S, Slayter HS, et al. Cytotoxicity of gelonin and its conjugates with antibodies is determined by the extent of their endocytosis. J Cell Physiol. 1989;141:222–234. doi: 10.1002/jcp.1041410129. [DOI] [PubMed] [Google Scholar]
- 13.Pirie CM, Liu DV, Wittrup KD. Targeted cytolysins synergistically potentiate cytoplasmic delivery of gelonin immunotoxin. Mol Cancer Ther. 2013;12:1774–1782. doi: 10.1158/1535-7163.MCT-12-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudnick SI, Lou J, Shaller CC, Tang Y, Klein-Szanto AJ, Weiner LM, Marks JD, Adams GP. Influence of affinity and antigen internalization on the uptake and penetration of Anti-HER2 antibodies in solid tumors. Cancer Res. 2011;71:2250–2259. doi: 10.1158/0008-5472.CAN-10-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin MC, Zhang J, Min KA, Lee K, Byun Y, David AE, He H, Yang VC. Cell-penetrating peptides: achievements and challenges in application for cancer treatment. J Biomed Mater Res A. 2014;102:575–587. doi: 10.1002/jbm.a.34859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bechara C, Sagan S. Cell-penetrating peptides: 20 years later, where do we stand? FEBS Lett. 2013;587:1693–1702. doi: 10.1016/j.febslet.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y, Jiang Y, Wang H, Wang J, Shin MC, Byun Y, He H, Liang Y, Yang VC. Curb challenges of the “Trojan Horse” approach: Smart strategies in achieving effective yet safe cell-penetrating peptide-based drug delivery. Adv Drug Deliv Rev. 2013;65(10):1299–1315. doi: 10.1016/j.addr.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He H, Ye J, Wang Y, Liu Q, Chung HS, Kwon YM, Shin MC, Lee K, Yang VC. Cell-penetrating peptides meditated encapsulation of protein therapeutics into intact red blood cells and its application. J Control Release. 2013;176:123–132. doi: 10.1016/j.jconrel.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JY, Choi YS, Suh JS, Kwon YM, Yang VC, Lee SJ, Chung CP, Park YJ. Cell-penetrating chitosan/doxorubicin/TAT conjugates for efficient cancer therapy. Int J Cancer. 2011;128:2470–2480. doi: 10.1002/ijc.25578. [DOI] [PubMed] [Google Scholar]
- 20.Li YT, Kwon YM, Spangrude GJ, Liang JF, Chung HS, Park YJ, Yang VC. Preliminary in vivo evaluation of the protein transduction domain-modified ATTEMPTS approach in enhancing asparaginase therapy. J Biomed Mater Res A. 2009;91:209–220. doi: 10.1002/jbm.a.32204. [DOI] [PubMed] [Google Scholar]
- 21.Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc Natl Acad Sci U S A. 2004;101:17867–17872. doi: 10.1073/pnas.0408191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang T, Zhang Z, Zhang Y, Lv H, Zhou J, Li C, Hou L, Zhang Q. Dual-functional liposomes based on pH-responsive cell-penetrating peptide and hyaluronic acid for tumor-targeted anticancer drug delivery. Biomaterials. 2012;33:9246–9258. doi: 10.1016/j.biomaterials.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 23.Kwon YM, Li YT, Liang JF, Park YJ, Chang LC, Yang VC. PTD-modified ATTEMPTS system for enhanced asparaginase therapy: a proof-of-concept investigation. J Control Release. 2008;130:252–258. doi: 10.1016/j.jconrel.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hossann M, Li Z, Shi Y, Kreilinger U, Buttner J, Vogel PD, Yuan J, Wise JG, Trommer WE. Novel immunotoxin: a fusion protein consisting of gelonin and an acetylcholine receptor fragment as a potential immunotherapeutic agent for the treatment of Myasthenia gravis. Protein Expr Purif. 2006;46:73–84. doi: 10.1016/j.pep.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 25.Lindgren M, Hallbrink M, Prochiantz A, Langel U. Cell-penetrating peptides. Trends Pharmacol Sci. 2000;21:99–103. doi: 10.1016/s0165-6147(00)01447-4. [DOI] [PubMed] [Google Scholar]
- 26.Jiao CY, Delaroche D, Burlina F, Alves ID, Chassaing G, Sagan S. Translocation and endocytosis for cell-penetrating peptide internalization. J Biol Chem. 2009;284:33957–33965. doi: 10.1074/jbc.M109.056309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee TY, Park YS, Garcia GA, Sunahara RK, Woods JH, Yang VC. Cell permeable cocaine esterases constructed by chemical conjugation and genetic recombination. Mol Pharm. 2012;9:1361–1373. doi: 10.1021/mp200623w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kane JF. Effects of rare codon clusters on high-level expression of heterologous proteins in Escherichia coli. Curr Opin Biotechnol. 1995;6:494–500. doi: 10.1016/0958-1669(95)80082-4. [DOI] [PubMed] [Google Scholar]
- 29.LaVallie ER, DiBlasio-Smith EA, Collins-Racie LA, Lu Z, McCoy JM. Thioredoxin and related proteins as multifunctional fusion tags for soluble expression in E. coli. Methods Mol Biol. 2003;205:119–140. doi: 10.1385/1-59259-301-1:119. [DOI] [PubMed] [Google Scholar]
- 30.Li Z, Qu Y, Li H, Yuan J. Truncations of gelonin lead to a reduction in its cytotoxicity. Toxicology. 2007;231:129–136. doi: 10.1016/j.tox.2006.11.074. [DOI] [PubMed] [Google Scholar]
- 31.Console S, Marty C, Garcia-Echeverria C, Schwendener R, Ballmer-Hofer K. Antennapedia and HIV transactivator of transcription (TAT) “protein transduction domains” promote endocytosis of high molecular weight cargo upon binding to cell surface glycosaminoglycans. J Biol Chem. 2003;278:35109–35114. doi: 10.1074/jbc.M301726200. [DOI] [PubMed] [Google Scholar]
- 32.Yuan X, Lin X, Manorek G, Howell SB. Challenges associated with the targeted delivery of gelonin to claudin-expressing cancer cells with the use of activatable cell penetrating peptides to enhance potency. BMC Cancer. 2011;11:61. doi: 10.1186/1471-2407-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calnan BJ, Tidor B, Biancalana S, Hudson D, Frankel AD. Arginine-mediated RNA recognition: the arginine fork. Science. 1991;252:1167–1171. doi: 10.1126/science.252.5009.1167. [DOI] [PubMed] [Google Scholar]
- 34.Eiklid K, Olsnes S, Pihl A. Entry of lethal doses of abrin, ricin and modeccin into the cytosol of HeLa cells. Exp Cell Res. 1980;126:321–326. doi: 10.1016/0014-4827(80)90270-0. [DOI] [PubMed] [Google Scholar]
- 35.Brooks H, Lebleu B, Vives E. Tat peptide-mediated cellular delivery: back to basics. Adv Drug Deliv Rev. 2005;57:559–577. doi: 10.1016/j.addr.2004.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.