Abstract
The brain is comprised of neurons and its support system including astrocytes, glial cells and microglia, thereby forming neurovascular units. Neurons require support from glial cells to establish and maintain functional circuits, but microglia are often overlooked. Microglia function as the immune cell of the central nervous system, acting to monitor the microenvironment for changes in signaling, pathogens and injury. More recently, other functional roles for microglia within the healthy brain have been identified, including regulating synapse formation, elimination and function. This review aims to highlight and discuss these alternate microglial roles in the healthy and in contrast, diseased brain with a focus on two acute neurological diseases, traumatic brain injury and epilepsy. In these conditions, microglial roles in synaptic stripping and stabilization as part of neuronal:glial interactions may position them as mediators of the transition between injury-induced circuit dismantling and subsequent reorganization. Increased understanding of microglia roles could identify therapeutic targets to mitigate the consequences of neurological disease.
Microglia: Amoeboid to ramified and back again
Microglial morphology has long been interpreted to follow function, with surface antigens changing dependent on the stimulus for activation and the role required to play in the brain. This simplistic view is now being challenged, with data collected over the previous decades indicating alternate roles for microglia, particularly in the uninjured brain. During development of the brain, microglial precursors undergo three developmental milestones toward becoming fully integrated microglia. Microglia proliferate and migrate to populate different central nervous system (CNS) regions, and then differentiate from an amoeboid-like form into their ramified morphology. Within the non-pathological brain, ramified microglia constantly survey the microenvironment by movement of their fine processes, sampling the surface of cells and interstitial fluid in their immediate vicinity [1, 2] and rapidly activating in response to changes in the microenvironment, for example the presence of a pathogen.
Acute neurological diseases that disrupt local microenvironments lead to disturbances in ionic homeostasis, enzyme activation and associated signaling molecules. These disease disturbances signal the activation of the microglia. Through regulatory proteins, such as cytokines and chemokines, microglia morph from the ramified state to become “activated”. The activated morphology of the microglia may have different forms including: hyper-ramified, active, amoeboid or rod (see Figure 1). As with macrophages outside of the CNS, depending on the stimulus (i.e.) interferon gamma or interleukin-4, microglia will classically (M1) or alternatively (M2) activate. In general terms, M1 microglia have an inflammatory role, whereas M2 are predominantly anti-inflammatory; for this review we will not discuss in detail M1 versus M2.
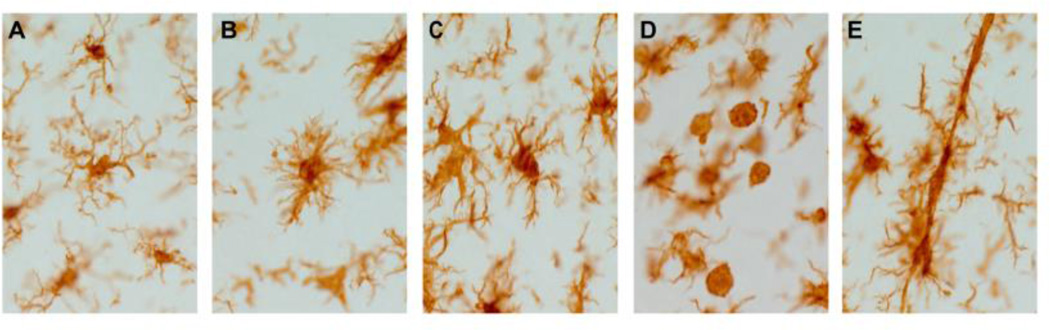
Figure 1.
(A) Ramified microglia in healthy rat cortex, following brain-injury microglia morphology rapidly changes (A–E). (B) Hyper-ramified, bushy microglia. (C) Activated microglia with fewer processes than either ramified form. (D) Fully activated microglia/infiltrating macrophages have no processes. (E) Rod microglia, this morphology has thin soma and polarized processes.
Presently, microglia activation is thought to be a continuum between ramified activated, amoeboid and back again with the first stage in microglial activation typically described as the “alert”, hyper-ramified or bushy microglia morphology [3]. Hyper-ramified microglia retract their processes from the surrounding tissue and thicken, acquiring a "bushy-like" appearance and display immunoreactivity for CD11b, suggesting that these cells have a functional inflammatory role. As the microglia become activated, their cell bodies become more spherical, and the number of processes greatly reduced. The activated morphology has immunoreactivity to CD11c, MHCII, CD68/ED1. Fully activated microglia are amoeboid, have no processes and are indistinguishable from blood-borne macrophages; they act as scavenging cells by engulfing debris. Amoeboid microglia have immunoreactivity to markers similar to macrophages, including those previously listed as well as CD45. Recently, we reported on another morphologically distinct microglia population following diffuse traumatic brain injury (TBI), rod microglia [4]. The role for rod microglia in neurological disease has yet to be elucidated, however due to their rod-shape and position adjacent to neuronal elements, an involvement in synaptic plasticity is suspected. Rod microglia formation occurs early after experimental diffuse brain injury in the primary somatosensory barrel field (S1BF); this same region harbors significant neuropathology identified by silver accumulation [5]. Microglial activation is ongoing for weeks post-injury, with dynamic shifts in the population distribution between the subpopulations of activated microglia. Moreover, the S1BF of the cortex comprises part of the whisker barrel circuit which deciphers sensory input from the whiskers. Indeed, the extent of neuronal activation (cFos staining) in response to whisker stimulation shows hypo-activation followed by hyper-activation in the S1BF over time after experimental diffuse TBI, indicating periods of circuit dismantling followed by reorganization [6]. During the phase of circuit reorganization, animals develop behavior changes manifested as sensory sensitivity to whisker stimulation [7]. Microglial activation likely contributes to all phases in the development of behavioral morbidity through circuit reorganization. Although we mention discuss five microglial morphologies, it is important to remain sufficiently flexible in recognizing there may be more additional morphologies, depending on the which would define neurological conditions and injury in relevant CNS compartments, as have been shown recently for ‘honeycomb’ and ‘jellyfish’ microglia subjacent to the dura [8].
In any situation where normal homeostasis becomes disturbed or impaired, microglia rapidly activate and proliferate towards reestablishing homeostasis. At these times, activated microglia constitute a heterogeneous population of cells that dynamically change in phenotype depending on the type of stimulus and the other elements of the microenvironment. Microglial populations increase from the infiltration of monocytes, proliferation of the resident population of the microglia, and the migration of microglia from uninjured regions [9]. Infiltrating monocytes tend to be minimal in number following experimental diffuse TBI, denervation or neurodegeneration [10], but present in focal and mixed models of TBI. Once activated, microglia may participate not only in mechanisms of injury by releasing proinflammatory cytokines, but also in neuroprotection, repair and circuit refinement. In disease, activated microglia release cytokines, present antigens to T-cells as well as phagocytose tissue debris, damaged cells and microbes [11–16], enabling the restoration of homeostasis.
Specific cytokines are associated with microglial proliferation and migration. Following injury to the CNS, microglia become activated within hours of brain injury and prior to increased cytokine production in the periphery [17]. This suggests that microglia are responding to locally produced cytokines and chemokines. Indeed, several cytokines, including IL-3, Il-6, GM-CSF and CSF-1, can stimulate proliferation of microglia (reviewed in [18]). In contrast, chemokines such as CCL2 attract microglia to the site of injury or infection. This orchestrated process of microglial migration combined with proliferation allows microglia to reach sufficient numbers at the site of injury to restore homeostasis by removing tissue debris or damaged cells [19–21]. Once homeostasis has been restored microglia have the ability to return to the ramified state [22, 23]. This can, to some extent, be followed by immuno-labeling with CD11b, MHCI and MHCII, lectin and CD45, which decrease or disappear in the transition from amoeboid back to ramified morphologies [24].
Neuronal-microglial signaling modulates microglial activation in health and disease
Microglial activation and consequently deactivation occurs during normal functioning of the CNS. In adapting to environmental cues, circuits and their connections are continuously modified. During modification of the synapse, degradation and apoptotic material are generated and can be found within microglia [25]. This suggests intricate molecular communication between neurons and glia. More specifically, “on/off” signals have been proposed which either signal microglia to remain in a ramified state or transition into an active morphology. Additionally, there may be “find me” signals to attract microglia to doomed neurons and synapses, while the subsequent phagocytosis is mediated by “eat me” signals presented on the cell surface. These signals are only partially known and may depend on region, insult and timing unique to each neurological condition. Undeniably, microglia express major classes and subtypes of excitatory and inhibitory neurotransmitter receptors and ion channels, promoting the interpretation that microglial function are regulated through specific signaling.
“On” signaling occurs when a novel signal is recognized by microglia to trigger activation. Many “on” signals trigger the innate immune response. Typically, “on” signals activate microglia through toll-like receptors (TLRs) or pro-inflammatory cytokines. In general, “on” signals trigger nuclear translocation of nuclear factor κB (NFκB) and subsequent transcriptional activation of various pro-inflammatory genes including those encoding cytokines, chemokines, cyclooxygenase-2, inducible nitric oxide (NO) synthase, adhesion molecules and major histocompatibility complex receptors (MHC) [26]. Adenosine triphosphate (ATP) is released from injured or activated neurons or astrocytes and acts as a paracrine signal that activates microglia via purigenic ionotropic P2X and metabotropic P2Y receptors (reviewed in [27]). ATP signaling has been shown to be necessary and sufficient for microglia in surveillance, synaptic plasticity, and response to injury.
On the other hand, microglial “off” signals are produced constitutively in the healthy brain microenvironment to maintain microglia in a ramified state. These “off” signals include CX3CL1 and members of the immunoglobulin (IgSF), such as CD200. Accumulating evidence shows that neurons express several IgSF molecules that potentially suppress microglial immune function (reviewed in [28]). The best characterized molecules of the IgSF in the CNS are CD200 and CD47, both are constitutively expressed at the neuronal membrane surface [29, 30]. CD200, formerly called Ox2, is expressed on neurons and binds to the CD200 receptor on microglia, a receptor that contains an ITIM (inhibitory tyrosine immunoregulatory motif). This motif initiates a signaling cascade in macrophages which in turn leads to the down-regulation of macrophage activation [29, 31]. The disappearance of “off” signals leads to microglial responses and likely activation.
Healthy neurons constitutively express high levels of chemokine CX3CL1/Fractalkine, with the corresponding receptor, CX3CR1, found in microglia. CX3CL1-CX3CR1 signaling has been documented in neuron-microglia interactions [32]. The lack of CX3CL1 signaling is thought to indicate neuronal distress and a “find me” signal. Exposure of neuron-microglia co-cultures to CX3CL1 reduced inflammatory neuronal death in vitro [33, 34]. These findings have been repeated in vivo where CX3CR1 deficient mice had higher levels of microglia activity in three models of brain disease including Parkinson disease, amyotrophic lateral sclerosis (ALS) and toxic insult via lipopolysaccharide (LPS). This increase in microglia activity was accompanied by increased neuronal cell death [35]. Neurotoxic microglia activities are suppressed by CX3CL1-CX3CR1 signaling. Whereas, phosphatidylserine (PtdSer) on the outer membrane of apoptotic cells is amongst a few established “eat me” signals (reviewed in [36]).
Studies suggest that microglia proliferation is not essential in remodeling or deafferentation of pre-synaptic contacts from an injured neuron in the adult CNS; plasticity following synaptic pathology can occur without involving microglia initiated synaptic stripping. Reducing synaptic activity in the visual cortex, either by removal of sensory input or the use of tetrodotoxin, reduced the frequency of microglia contacts with synapses by about one third [37]. Inhibition of injury-induced microglia proliferation by the application of intraventricular cytosine arabinoside (Ara C) after transection of the hypoglossal nerve had no effect on the retraction of synapses from the motor neuron cell body and importantly, had no discernable impact on the subsequent success of regeneration of peripheral nerve [38]. Moreover, in mice lacking macrophage-colony stimulating factor 1 (CSF-1), synaptic withdrawal or removal still occurs despite the absence of this growth factor. However, microglia in these knockout mice do not proliferate after peripheral nerve transection [39].
Microglia and Synapses
The idea of what constitutes a synapse has changed since they were first described (reviewed in [40]). The concept of a synapse being a neuron communicating with another cell has been set aside, with the knowledge that glial cells, particularly microglia and astrocytes, also contribute to synaptic plasticity and function in the healthy and diseased brain. A role for the extracellular matrix as an integral member of the synapse has also been proposed [41].
In addition to their inflammatory role, microglia function in the development and maintenance of synapses. Initially during development, the first migration of microglia coincides with the first wave of synaptogenesis (in rodents around embryonic day 14–15), with synapses developing in the absence of astrocytes which have not yet formed. In this phase, microglia assist and promote early synaptogenesis through the secretion of trophic factors such as brain derived neurotrophic factor (BDNF). During later development, microglial processes actively engulf synaptic structures and exert a major role in controlling the number of synapses through synaptic pruning [42].
In the injured brain, microglial inflammatory and synaptic maintenance roles are not mutually exclusive. The complement cascade has been proposed as a potential mediator of microglia-synapse interactions and developmental synaptic pruning [25, 43, 44]. Complement proteins are locally synthesized by neurons and glial cells, however microglia and astrocytes are the major producers of complement in healthy and diseased brain [45, 46]. Throughout the CNS, microglia express high levels of C1q, as well as CR3 (CD11b) and CR5. These complement receptors are critical for inducing phagocytosis of complement-coated structures and regulating cytokine signaling as well as chemotaxis [47]. Other pathways which have been implicated as modulators of developmental synaptic pruning include major histocompatibility complex (MHC) and neuronal pentraxins (reviewed in [48]), which execute their effects on synapses similar to the complement pathways. It is clear that microglia respond to and propagate inflammatory signals, however as research continues into other functions for microglia new roles are being described.
Microglia modulate synapses
Classical synaptic receptors, including the metabotropic and ionotropic glutamate receptors as well as GABA, are found on microglia. As a result, synaptic transmission may influence microglial responses to inflammation. It has been hypothesized that microglia could regulate synaptic transmission through the activation of astrocytes (reviewed in [48]). Indeed, ramified microglia are essential to normal synaptic function by sampling the surface of cells and the interstitial fluid [48]. It is therefore plausible to suggest that ramified microglia have organized synaptic specializations not dissimilar to those found surrounding synapses to sense, monitor and interact with neurons at synapses. In this way, microglia contribute to a quad-partite synapse; an extension of the tri-partite synapse described a decade ago (review article by [48–50]). Moreover, microglia may actively remove astrocytes and extracellular matrix from synapses to allow for deafferentation or plasticity of neurons, however, this concept has yet to be thoroughly investigated.
Microglial processes specifically interact with subsets of synaptic structures, leading to the reciprocal interactions between microglia and synapses under non-pathological conditions. Elegant two-photon imaging studies have shown that there are various modes of dynamic microglial behavior including interactions with synaptic elements which are regulated through neurotransmission, neuronal activity and sensory experience. Microglial processes can interact with both pre-synaptic axon terminals and post-synaptic dendritic spines in the somatosensory cortex of adult mice [9]. Microglial processes have been shown to contact synapses in an experience-dependent manner. Employing the thin-skull window strategy in juvenile mice, and by fluorescently labelling both neurons and microglia, Wake and colleagues were able to clearly demonstrate microglial processes making direct transient contacts with synapses for 5 minute periods once every hour [37]. The amount of time spent in contact with synapses increased to approximately an hour following cerebral ischemia. The authors proposed that the prolonged contact by microglia initiated a cascade of events which ultimately lead to the disappearance of synapses. These events in the living brain suggest continued interaction between microglial and neuronal elements.
During development, amoeboid microglia have long been known to phagocytose the apoptotic neurons associated with programmed cell death, but the role of microglia extends beyond simply cleaning up debris (reviewed in [9]). In the adult, ramified or unchallenged microglia have an emerging role in synaptic plasticity by clearing synapses. Ramified microglia form phagocytic pouches in their distal extensions to engulf synaptic components [51], rather than engulfment by the amoeboid cell soma. Activated and amoeboid microglia seem to have dual roles in restoring homeostasis while modulating inflammatory responses. Furthermore, experiments investigating microglial response to visual experience showed microglial processes localizing to small, transiently growing dendritic spines in the visual cortex of juvenile mice in response to light stimulus. When the light stimulus was removed microglia became less motile and contacted a different, larger subset of spines [52].
Microglia: Are they synaptic strippers?
Before circuit reorganization can occur, synapses at the foundation of circuits must be removed – namely stripped from their synaptic partners. Some 45 years ago, a possible but still controversial role for activated microglia in synaptic stripping emerged. Electron microscope images depicted microglial processes structurally intervening between pre-synaptic axon terminals and post-synaptic dendrites or neuronal cell bodies under pathological conditions [53]. It was inferred that microglia could provide trophic support to overcome minor disturbances in the microenvironment, thereby reducing the risk of excitotoxic damage to neurons. Microglia may also reduce the risk of excitotoxicity by a mechanism of synaptic stripping first shown in a model of motor neuron axotomy [53]. Depending on whether an experimental model of facial nerve is reversible or irreversible, microglia will respond differently [54]. Most axotomized motor neurons survive and regrow their axons, suggesting that the burst of reactive microgliosis that accompanies axotomy (reversible) is beneficial to support neuronal regeneration. Microglial response to ricin (irreversible) is more phagocytic in order to remove dead and dying neurons. It has been hypothesized that synaptic stripping also occurs in models of cerebral inflammation associated with synaptic loss in the neocortex [55]. A decrease in synaptic activity precedes and may potentially initiate, synaptic stripping [56]. These findings can be extended to surmise that phagocytic microglia would predominate in focal brain injury, whereas reactive microgliosis would be associated with diffuse brain injury.
Lines of evidence in the facial nerve transection model suggest a simple guilty-by-association for microglial involvement at degenerating synapses [57]. Chronic or Wallerian degeneration in the CNS can occur without microglial processes enveloping synaptic boutons [58, 59]. Additionally, three dimensional reconstructions from both conventional and dual-beam electron microscopy rule out the presence of non-neuronal processes between the pre-synaptic and post-synaptic elements [60]. However, others have suggested that the simple act of collecting tissue maybe enough for microglia to retract from synapses, cofounding results [11]. In this case, live imaging through a cranial window or multi-photon approaches may be optimal for studying microglial interactions with synapses. Regardless, mounting evidence implicates microglia in altering synapses and therefore neuronal circuits. Therefore, it is tempting to speculate that microglia ultimately impact behavior.
Influence of microglia on circuit function
As previously mentioned, microglia provide key functions during development, ranging from immune-related duties, including removal of non-functional tissue, to classical glia functions, such as release of trophic factors and synaptic development and maintenance with later synaptic pruning. Novel roles are currently emerging which link microglia with higher order brain function and show that microglia may ultimately influence behavior. For example, microglia are the only brain cells to express Hoxb8, for which mice lacking Hoxb8 exhibit obsessive grooming behavior [61]. Recently, sophisticated studies with Hoxb8 mice have demonstrated the abnormal grooming behavior is not due to neuronal pathology, but rather from losing Hoxb8 microglia [62]. Although the morphology of microglia remained similar between wild-type and mutant animals, the phenotype and number of microglia was dramatically altered. Furthermore, the activation of microglia from their ramified state has been implicated in many neurological disorders, including stroke [63], trauma [4, 64, 65], neurodegenerative diseases (reviewed in [18]) and more recently epilepsy [66, 67]. Indeed, early-life activation of microglia by LPS, infection, or stress can result in cognitive disabilities that persist through to adulthood [68]. Depression, autism spectrum disorders, bipolar disorder, and schizophrenia are known to be associated with microglial activation [68–71]. By acknowledging microglial effects on circuit function, new therapeutic targets emerge for the prevention, treatment or cure of neurological conditions.
Microglia reformat circuits following traumatic brain injury
TBI initiates complex molecular cascades which ultimately lead to circuit dismantling and subsequent reorganization. Reorganized circuits can evolve into behavioral morbidities with the formation of maladaptive circuits as evidenced by post-traumatic seizures and sensory sensitivity.
Following experimental diffuse brain injury using fluid percussion, the mechanical forces result in diffuse axonal injury and perisomatic axotomy [72, 73], particularly within the ventral posterior medial (VPM) nucleus resulting in disconnection of thalamocortical projection neurons. Silver staining, indicative of neuropathology, extends into the S1BF a region of the VPM relay [5]. Furthermore, there is functional hyperactivation in the VPM and S1BF to whisker stimulation [6] and the development of behavioral sensory sensitivity to whisker stimulation [7, 74]. These data indicate maladaptive circuit reorganization post-TBI.
Additional studies have demonstrated the activation of microglia along the whisker barrel circuit in regions where silver pathology is most abundant [4, 64]. The robust presentation of rod microglia, which form trains across the cortical layers in regions with dense neuropathology, indicate neuronal:glial interactions. Rod microglia are reported to be in highest numbers in the S1BF at time points prior to the evolution of aberrant behaviors, suggesting these microglia may play a role in synaptic plasticity and circuit reorganization.
Microglia may influence seizure development
Epilepsy is a prevalent neurological disease, and is a common end point of many forms of acquired brain pathology including tumors, infection, stroke and traumatic brain injury. Epilepsy has been associated with a number of autoimmune diseases that affect the CNS, supporting the hypothesis that immune activation in the brain is not only a consequence but may be involved in the etiology of this condition [75]. Specific neuronal cell types such as GABAergic interneurons are being increasingly recognized as playing a mechanistic role in epilepsy [67]. As we have previously discussed, with neuronal dysfunction comes microglial activation, as has been demonstrated in epilepsy.
Kainic acid models of epilepsy show microglia activation by day 8 post-injection, which remained elevated until 30 days, with seizures beginning on day 15 [66]. In a different form of epilepsy, the Rasmussen’s encephalitis (RE), neuroinflammation has been linked with progressive stages of neuropathology including neuronal loss and cortical atrophy particularly in late stage of the disease (stage 3) [76]. Areas of tissue pathology revealed that axonal swelling/retraction bulbs coincided with leukocyte accumulation and astrocytosis. Most interesting was the finding that inflammation was identified in the early stage of the disease when the neuronal anatomy was still normal. This however deteriorated at later stages, corroborating neuroinflammation in the pathogenesis of epilepsy. Yet, in this and other studies, sparse information was provided on the role of microglial cells, changes in their activation state and morphology as well as their co-localization with degenerating neuronal structures. Regardless of the etiology of RE, microglia activation is an essential hallmark of RE, a disease associated with seizures and chronic inflammation. Additionally, rod microglia were documented along neuronal apical dendrites of patients with RE [77], suggesting neuronal:microglial interactions. Furthermore, the magnitude of microglial reactivity in focal cortical dysplasia correlated with the duration of epilepsy and seizure frequency [78].
Although a role for astrocytes in modulating neuronal activity and synapses has been documented [79–81], data from brain injury and epilepsy studies indicate that microglial activation precedes the onset of behavior changes. Microglia may modulate synapses, with consequences for reorganization of circuits and impacting function.
Conclusions
Traditionally, neurons have been implicated solely as the targets of microglial cytotoxicity, innocent victims of over-activated immune cells. Now it is becoming clear that neurons actively regulate microglial function, and thereby contribute to the inflammatory cascades of the CNS. However, the opposite is equally likely, with microglia activating neurons. The dynamic neuronal-microglial interactions can regulate healthy brain function, respond to local disturbances or suffer the consequences of disease. In this way, the interplay between these CNS compartments can lead to behavioral changes, indicating microglia are essential for proper brain function. This defines a role for microglia beyond the much investigated pathological sensor. In this way, microglia can serve as a source for biomarkers and a target for therapeutic intervention.
References
- 1.Davalos D, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 2.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 3.Ladeby R, et al. Microglial cell population dynamics in the injured adult central nervous system. Brain Res Brain Res Rev. 2005;48(2):196–206. doi: 10.1016/j.brainresrev.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Ziebell JM, et al. Rod microglia: elongation, alignment, and coupling to form trains across the somatosensory cortex after experimental diffuse brain injury. J Neuroinflammation. 2012;9:247. doi: 10.1186/1742-2094-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lifshitz J, Lisembee AM. Neurodegeneration in the somatosensory cortex after experimental diffuse brain injury. Brain Struct Funct. 2012;217(1):49–61. doi: 10.1007/s00429-011-0323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall KD, Lifshitz J. Diffuse traumatic brain injury initially attenuates and later expands activation of the rat somatosensory whisker circuit concomitant with neuroplastic responses. Brain Res. 2010;1323:161–173. doi: 10.1016/j.brainres.2010.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNamara KC, Lisembee AM, Lifshitz J. The whisker nuisance task identifies a late-onset, persistent sensory sensitivity in diffuse brain-injured rats. J Neurotrauma. 2010;27(4):695–706. doi: 10.1089/neu.2009.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roth TL, et al. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505(7482):223–228. doi: 10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wake H, et al. Microglia: actively surveying and shaping neuronal circuit structure and function. Trends Neurosci. 2013;36(4):209–217. doi: 10.1016/j.tins.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Ajami B, et al. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10(12):1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 11.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 12.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10(11):1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 13.Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 14.Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9(6):429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- 15.Graeber MB. Changing face of microglia. Science. 2010;330(6005):783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- 16.Prinz M, et al. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci. 2011;14(10):1227–1235. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- 17.Morganti-Kossmann MC, et al. Role of cerebral inflammation after traumatic brain injury: a revisited concept. Shock. 2001;16(3):165–177. doi: 10.1097/00024382-200116030-00001. [DOI] [PubMed] [Google Scholar]
- 18.Tambuyzer BR, Ponsaerts P, Nouwen EJ. Microglia: gatekeepers of central nervous system immunology. J Leukoc Biol. 2009;85(3):352–370. doi: 10.1189/jlb.0608385. [DOI] [PubMed] [Google Scholar]
- 19.Brockhaus J, Moller T, Kettenmann H. Phagocytozing ameboid microglial cells studied in a mouse corpus callosum slice preparation. Glia. 1996;16(1):81–90. doi: 10.1002/(SICI)1098-1136(199601)16:1<81::AID-GLIA9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 20.Carthew HL, Ziebell JM, Vink R. Substance P-induced changes in cell genesis following diffuse traumatic brain injury. Neuroscience. 2012;214:78–83. doi: 10.1016/j.neuroscience.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 21.Lazar G, Pal E. Removal of cobalt-labeled neurons and nerve fibers by microglia from the frog's brain and spinal cord. Glia. 1996;16(2):101–107. doi: 10.1002/(SICI)1098-1136(199602)16:2<101::AID-GLIA2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Hailer NP, et al. Fluorescent dye prelabelled microglial cells migrate into organotypic hippocampal slice cultures and ramify. Eur J Neurosci. 1997;9(4):863–866. doi: 10.1111/j.1460-9568.1997.tb01436.x. [DOI] [PubMed] [Google Scholar]
- 23.Hailer NP, Jarhult JD, Nitsch R. Resting microglial cells in vitro: analysis of morphology and adhesion molecule expression in organotypic hippocampal slice cultures. Glia. 1996;18(4):319–331. doi: 10.1002/(sici)1098-1136(199612)18:4<319::aid-glia6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 24.Wang CC, et al. Immunohistochemical study of amoeboid microglial cells in fetal rat brain. J Anat. 1996;189(Pt 3):567–574. [PMC free article] [PubMed] [Google Scholar]
- 25.Schafer DP, Stevens B. Synapse elimination during development and disease: immune molecules take centre stage. Biochem Soc Trans. 2010;38(2):476–481. doi: 10.1042/BST0380476. [DOI] [PubMed] [Google Scholar]
- 26.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 27.Benarroch EE. Microglia: Multiple roles in surveillance, circuit shaping, and response to injury. Neurology. 2013;81(12):1079–1088. doi: 10.1212/WNL.0b013e3182a4a577. [DOI] [PubMed] [Google Scholar]
- 28.Biber K, et al. Neuronal 'On' and 'Off' signals control microglia. Trends Neurosci. 2007;30(11):596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Hoek RM, et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290(5497):1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 30.Wright GJ, et al. The unusual distribution of the neuronal/lymphoid cell surface CD200 (OX2) glycoprotein is conserved in humans. Immunology. 2001;102(2):173–179. doi: 10.1046/j.1365-2567.2001.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barclay AN, et al. CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol. 2002;23(6):285–290. doi: 10.1016/s1471-4906(02)02223-8. [DOI] [PubMed] [Google Scholar]
- 32.Biber K, et al. Chemokines and their receptors in central nervous system disease. Curr Drug Targets. 2006;7(1):29–46. doi: 10.2174/138945006775270196. [DOI] [PubMed] [Google Scholar]
- 33.Mizuno T, et al. Production and neuroprotective functions of fractalkine in the central nervous system. Brain Res. 2003;979(1–2):65–70. doi: 10.1016/s0006-8993(03)02867-1. [DOI] [PubMed] [Google Scholar]
- 34.Zujovic V, et al. Fractalkine modulates TNF-alpha secretion and neurotoxicity induced by microglial activation. Glia. 2000;29(4):305–315. [PubMed] [Google Scholar]
- 35.Cardona AE, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9(7):917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 36.Schlegelmilch T, Henke K, Peri F. Microglia in the developing brain: from immunity to behaviour. Curr Opin Neurobiol. 2011;21(1):5–10. doi: 10.1016/j.conb.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Wake H, et al. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29(13):3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Svensson M, Aldskogius H. Synaptic density of axotomized hypoglossal motorneurons following pharmacological blockade of the microglial cell proliferation. Exp Neurol. 1993;120(1):123–131. doi: 10.1006/exnr.1993.1046. [DOI] [PubMed] [Google Scholar]
- 39.Kalla R, et al. Microglia and the early phase of immune surveillance in the axotomized facial motor nucleus: impaired microglial activation and lymphocyte recruitment but no effect on neuronal survival or axonal regeneration in macrophage-colony stimulating factor-deficient mice. J Comp Neurol. 2001;436(2):182–201. [PubMed] [Google Scholar]
- 40.Morris GP, et al. Microglia: a new frontier for synaptic plasticity, learning and memory, and neurodegenerative disease research. Neurobiol Learn Mem. 2013;105:40–53. doi: 10.1016/j.nlm.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Dityatev A, Rusakov DA. Molecular signals of plasticity at the tetrapartite synapse. Curr Opin Neurobiol. 2011;21(2):353–359. doi: 10.1016/j.conb.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 2013;77(1):10–18. doi: 10.1016/j.neuron.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 43.Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- 44.Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131(6):1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 45.Veerhuis R, Nielsen HM, Tenner AJ. Complement in the brain. Mol Immunol. 2011;48(14):1592–1603. doi: 10.1016/j.molimm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woodruff TM, et al. The role of the complement system and the activation fragment C5a in the central nervous system. Neuromolecular Med. 2010;12(2):179–192. doi: 10.1007/s12017-009-8085-y. [DOI] [PubMed] [Google Scholar]
- 47.Veerhuis R, et al. Cytokines associated with amyloid plaques in Alzheimer's disease brain stimulate human glial and neuronal cell cultures to secrete early complement proteins, but not C1-inhibitor. Exp Neurol. 1999;160(1):289–299. doi: 10.1006/exnr.1999.7199. [DOI] [PubMed] [Google Scholar]
- 48.Schafer DP, Lehrman EK, Stevens B. The "quad-partite" synapse: microglia-synapse interactions in the developing and mature CNS. Glia. 2013;61(1):24–36. doi: 10.1002/glia.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Araque A, et al. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22(5):208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 50.Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2(3):185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- 51.Sierra A, et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7(4):483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8(11):e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blinzinger K, Kreutzberg G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z Zellforsch Mikrosk Anat. 1968;85(2):145–157. doi: 10.1007/BF00325030. [DOI] [PubMed] [Google Scholar]
- 54.Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Prog Neurobiol. 1999;57(6):563–581. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 55.Trapp BD, et al. Evidence for synaptic stripping by cortical microglia. Glia. 2007;55(4):360–368. doi: 10.1002/glia.20462. [DOI] [PubMed] [Google Scholar]
- 56.Yamada J, Nakanishi H, Jinno S. Differential involvement of perineuronal astrocytes and microglia in synaptic stripping after hypoglossal axotomy. Neuroscience. 2011;182:1–10. doi: 10.1016/j.neuroscience.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 57.Perry VH, O'Connor V. The role of microglia in synaptic stripping and synaptic degeneration: a revised perspective. ASN Neuro. 2010;2(5):e00047. doi: 10.1042/AN20100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adalbert R, Gilley J, Coleman MP. Abeta, tau and ApoE4 in Alzheimer's disease: the axonal connection. Trends Mol Med. 2007;13(4):135–142. doi: 10.1016/j.molmed.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 59.Scott DA, et al. A pathologic cascade leading to synaptic dysfunction in alpha-synuclein-induced neurodegeneration. J Neurosci. 2010;30(24):8083–8095. doi: 10.1523/JNEUROSCI.1091-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siskova Z, et al. Degenerating synaptic boutons in prion disease: microglia activation without synaptic stripping. Am J Pathol. 2009;175(4):1610–1621. doi: 10.2353/ajpath.2009.090372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greer JM, Capecchi MR. Hoxb8 is required for normal grooming behavior in mice. Neuron. 2002;33(1):23–34. doi: 10.1016/s0896-6273(01)00564-5. [DOI] [PubMed] [Google Scholar]
- 62.Chen SK, et al. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141(5):775–785. doi: 10.1016/j.cell.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Annunziato L, Boscia F, Pignataro G. Ionic transporter activity in astrocytes, microglia, and oligodendrocytes during brain ischemia. J Cereb Blood Flow Metab. 2013;33(7):969–982. doi: 10.1038/jcbfm.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cao T, et al. Morphological and genetic activation of microglia after diffuse traumatic brain injury in the rat. Neuroscience. 2012;225:65–75. doi: 10.1016/j.neuroscience.2012.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ziebell JM, et al. Attenuated neurological deficit, cell death and lesion volume in Fas-mutant mice is associated with altered neuroinflammation following traumatic brain injury. Brain Res. 2011;1414:94–105. doi: 10.1016/j.brainres.2011.07.056. [DOI] [PubMed] [Google Scholar]
- 66.Drexel M, Preidt AP, Sperk G. Sequel of spontaneous seizures after kainic acid-induced status epilepticus and associated neuropathological changes in the subiculum and entorhinal cortex. Neuropharmacology. 2012;63(5):806–817. doi: 10.1016/j.neuropharm.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goldberg EM, Coulter DA. Mechanisms of epileptogenesis: a convergence on neural circuit dysfunction. Nat Rev Neurosci. 2013;14(5):337–349. doi: 10.1038/nrn3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beumer W, et al. The immune theory of psychiatric diseases: a key role for activated microglia and circulating monocytes. J Leukoc Biol. 2012;92(5):959–975. doi: 10.1189/jlb.0212100. [DOI] [PubMed] [Google Scholar]
- 69.Blank T, Prinz M. Microglia as modulators of cognition and neuropsychiatric disorders. Glia. 2013;61(1):62–70. doi: 10.1002/glia.22372. [DOI] [PubMed] [Google Scholar]
- 70.Derecki NC, et al. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature. 2012;484(7392):105–109. doi: 10.1038/nature10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frick LR, Williams K, Pittenger C. Microglial dysregulation in psychiatric disease. Clin Dev Immunol. 2013;2013:608654. doi: 10.1155/2013/608654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kelley BJ, et al. Traumatic axonal injury in the perisomatic domain triggers ultrarapid secondary axotomy and Wallerian degeneration. Exp Neurol. 2006;198(2):350–360. doi: 10.1016/j.expneurol.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 73.Lifshitz J, Kelley BJ, Povlishock JT. Perisomatic thalamic axotomy after diffuse traumatic brain injury is associated with atrophy rather than cell death. J Neuropathol Exp Neurol. 2007;66(3):218–229. doi: 10.1097/01.jnen.0000248558.75950.4d. [DOI] [PubMed] [Google Scholar]
- 74.Learoyd AE, Lifshitz J. Comparison of rat sensory behavioral tasks to detect somatosensory morbidity after diffuse brain-injury. Behav Brain Res. 2012;226(1):197–204. doi: 10.1016/j.bbr.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vezzani A, Balosso S, Ravizza T. Inflammation and epilepsy. Handb Clin Neurol. 2012;107:163–175. doi: 10.1016/B978-0-444-52898-8.00010-0. [DOI] [PubMed] [Google Scholar]
- 76.Pardo CA, et al. The pathology of Rasmussen syndrome: stages of cortical involvement and neuropathological studies in 45 hemispherectomies. Epilepsia. 2004;45(5):516–526. doi: 10.1111/j.0013-9580.2004.33103.x. [DOI] [PubMed] [Google Scholar]
- 77.Wirenfeldt M, et al. Increased activation of Iba1+ microglia in pediatric epilepsy patients with Rasmussen's encephalitis compared with cortical dysplasia and tuberous sclerosis complex. Neurobiol Dis. 2009;34(3):432–440. doi: 10.1016/j.nbd.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boer K, et al. Evidence of activated microglia in focal cortical dysplasia. J Neuroimmunol. 2006;173(1–2):188–195. doi: 10.1016/j.jneuroim.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 79.Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81(2):229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sofroniew MV. Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist. 2014;20(2):160–172. doi: 10.1177/1073858413504466. [DOI] [PubMed] [Google Scholar]
- 81.Wanner IB, et al. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci. 2013;33(31):12870–12886. doi: 10.1523/JNEUROSCI.2121-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]