Abstract
Objectives
This study examined whether metabolic syndrome (MetS) would moderate the association of cognition with frailty in middle and old age.
Methods
A cross-sectional design was used. Six hundred and ninety participants (age ≥ 50 years) from an on-going national survey were included in the study. Confirmatory factor analysis was applied to determine latent variables of executive function (EF), episodic memory (EM), and MetS based on relevant measurements. Frailty was defined using a modified form of Fried’s criteria.
Results
Applying structural equation modeling, having MetS significantly increased the likelihood of being frail. Better performance on EM tasks, but not EF, was significantly associated with lower likelihood of MetS. Worse performance on EF, but not EM, significantly increased the likelihood of being frail. There was a significant interacting effect between MetS and EF, but not EM, on frailty. Further contrast analysis indicated that having MetS strengthened the negative association between EF and frailty.
Conclusion
MetS moderates the relationship between EF and frailty. A prospecitve study is needed to validate such relationships before developing interventions targeting the prevention or treatment of EF and frailty in individuals with MetS.
Keywords: Frailty, Executive Function, Episodic Memory, Metabolic Syndrome
Introduction
Frailty presents as a declined ability to respond to stressful events and an increased vulnerability to adverse health outcomes (Fried, et al. 2001). Frail individuals perform poorly on cognitive assessments; more importantly, a number of longitudinal studies suggest baseline cognitive function predicts the incidence of frailty prospectively (Aranda, et al. 2011; Doba, et al. 2012; Raji, et al. 2011). Although these studies provide support for a strong relationship between cognition and frailty, whether other health conditions moderate this relationship is not known. Given the high prevelance of co-morbidity in middle and old age, it is likely the relationship between frailty and cogntion does not occur in isolation but rather in a context of other diseases and chronic conditions that may exert influence on the relationship between frailty and cognition (Robertson, et al. 2013). Understanding how the relationship between cognition and frailty is affected by other health conditions may shed light on shared underlying mechanisms and identify those individuals for whom the relationship between frailty and cognition is particularly strong, leading to improved targeting of preventative and/or management strategies.
An area of great interest is whether cognitive decline and the development of frailty share biological risk factors. Robertson et al., proposed several potential factors including Alzheimer’s disease pathology, hormones, nutrition, chronic inflammation, mental health, and cardiovascular risk, but there is a lack of experimental evidence to support these suggestions (Robertson et al. 2013). Metabolic syndrome is a condition involving the co-occurrence of several biological risk factors and is recognized as an important health condition indicative of risk for future development of adverse cardiovascular events (Grundy, et al. 2004). MetS has also been associated with declines in cognition and incident frailty, separately (Adults 2001; Afilalo, et al. 2009; Panza, et al. 2011). The prevalence of MetS ranges between 23% to 46% in middle and old age and MetS develops earlier than most chronic conditions, thus making it an important target for early prevention (Weiss et al. 2013). In a recent review, Barzilay and Stein proposed that MetS leads to “accelerated aging” processes of which frailty and cognitive decline are two of the major noncardiovascular complications (Barzilay and Stein 2011). However, whether the presense of MetS affects the relationship between cognition and frailty has not been examined. If MetS does affect the relationship, it may be an important marker for identifying those individuals most at risk for experiencing cognitive decline and becoming frail.
The purpose of the current study was to examine the relationships among cognition, MetS, and frailty in a group of adults aged 50 years or older; specifically, we tested whether MetS would moderate the relationship between cognition and frailty. To maximize the sensitivity of measurements for cognition and MetS, instead of simply using composite scores, we applied a confirmatory factor analysis (CFA) approach to develop latent variables of MetS and cognition using relevant indicators (ATP III, 2001; Lachman, et al. 2011). We also employed structural equation modeling (SEM) to simultaneously examine the main and interacting effects of these latent variables on frailty.
Methods
Participants
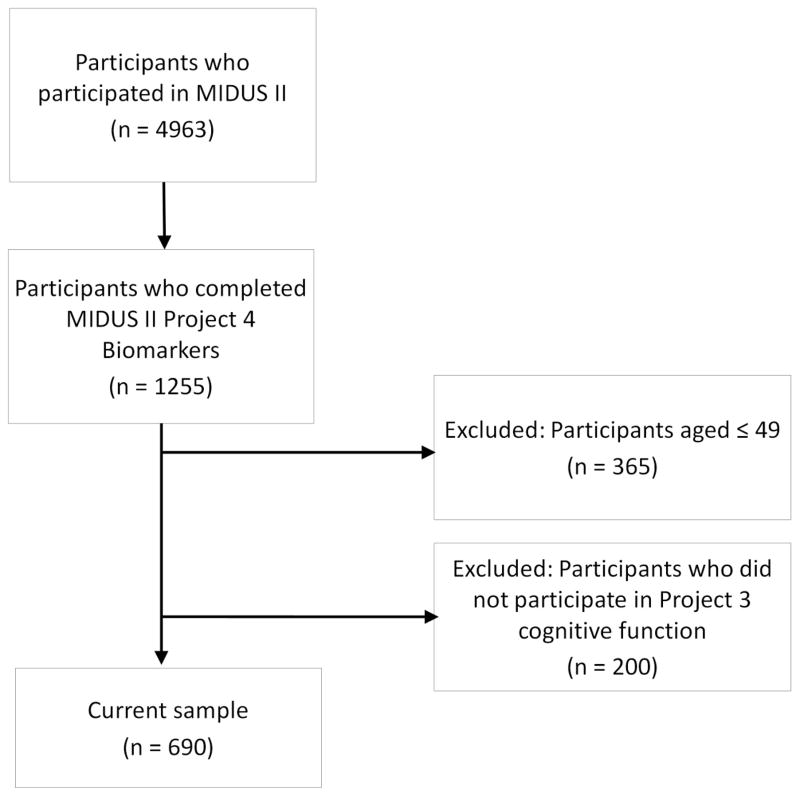
This cross-sectional study used data from the second wave of the Survey of Midlife Development in the United States (MIDUS II), a nationally representative database. There are five categories of assessments in MIDUS II: socio-demographic and psycho-behavioral survey (project 1), daily diaries (project 2), cognitive function (project 3), biomarkers (project 4), and neuroscience (project 5). A total of 4,963 individuals participated in MIDUS II. Data from projects 1, 3, and 4 was used for this analysis. There were a total of 1255 participants who attended project 4. We excluded those aged 49 or younger (n = 365) and who did not attend project 3 (n = 200). The final sample for the current study was 690 (see Appendix Flow Chart).
Appendix Flow Chart.
Procedure
MIDUS II Project 1 included self-administered questionnaires on socio-demographic information. Project 3, which included a series of cognitive tests, was administered over the telephone. Project 4 included a two-day visit to one of the participating General Clinical Research Centers (GCRCs). Of relevance to the current study, on Day 1, participants completed a detailed medical history interview, medication review, and physical assessment with GCRC clinicians as well as self-administered questionnaire on psycho-behavioral characteristics. On Day 2, a fasting blood sample was collected between 08:00 A.M. and 10:00 A.M., and appropriately stored. Institutional Review Board approval was obtained for each study project at each study site and informed written consent was obtained from all participants (Dienberg Love, et al. 2011).
Measures
Frailty was measured based on the validated Fried Frailty measure, which assess individuals on five frailty characteristics: physical inactivity, weakness, exhaustion, unintentional weight loss, and slowness (Fried et al. 2001). We first developed four continuous (i.e., grip strength, exhaustion, weight change, and walking speed) and one dichotomous (i.e., physical inactivity) variable for the individual indicators of frailty. Except physical inactivity, all other indicators were dichotomized into criteria variables based on cut-off scores of Fried et al. (Fried et al. 2001) (see Appendix Table 1). For the five frailty criteria, those with zero criteria were considered non frail, with 1– 2 criteria were pre-frail, and 3 or more criteria were frail. However, only 19 participants were classified as frail (2.8%); therefore, the subgroups with pre-frail and frail were combined. Pre-frail and frail participants were similar in their demographic and health characteristics, except that sleep quality was significantly worse among frail participants. Data on at least one of the frailty indicators was missing for 26 participants; these individuals were not included in the analyses. There were no significant differences in any demographic information between participants with and without frailty data.
Appendix Table 1.
The Fried Frailty Characteristics in the Present Study
| Characteristics | Measurements and Frailty Indicators | Frailty Criteria |
|---|---|---|
| Physical inactivity* | Using one item, “Do you engage in at least 20 minutes of physical activity at least 3 times per week?” Participants reporting “No” were considered to have physical inactivity. | Participants reporting “No” were considered to have physical inactivity. |
| Weakness/grip strength | Grip strength was assessed using a handheld dynamometer. The average of three trials in the dominant hand was used. | The lowest 20% by body-mass index and gender were used to determine the weakness. |
| Exhaustion | Using two items from the CES-D Scale: (a) “I felt that everything I did was an effort” and (b) “I could not get going.” | Participants responding “a moderate amount of the time (3–4 days)” or “most of the time (> 4 days)” were considered to have exhaustion. |
| Unintentional weight loss | Calculating percent weight loss in the participants between their completion of the SAQ at project 1 and their overnight stay at project 4. | In an attempt to capture intentionality, individuals who lost 5% or more of their body weight and did not report losing weight due to diet and exercise were considered to have unintentionally lost weight. |
| Slowness/walking speed | Calculating feet per second using the time, in seconds, it took participants to walk 50 feet at their usual pace. | The slowest 20% by gender and height were used to determine the slowness. |
Note.
Physical inactivity was the only variable that frailty indicator and criteria were the same variable.
Cognition
Two sets of neuropsychological tests were conducted over the phone: the Brief Test of Adult Cognition by Telephone (BTACT) (Tun and Lachman 2006) and the Stop and Go Switch Task (SGST) (Tun and Lachman 2008). Two domains, episodic memory (EM) and executive function (EF) had been derived using exploratory and confirmatory factor analysis of the seven cognitive tests previously (Lachman et al. 2011). EM and EF decline earliest in the aging process (Park and Reuter-Lorenz 2009). Two tests of episodic verbal memory (Word List Immediate and Delayed Recall) from BTACT were used to determine EM; the measures of working memory span (Digits Backward), verbal fluency (Category Fluency), inductive reasoning (Number Series), and processing speed (Backward Counting) from BTACT and attention switch and inhibitory control (called “inhibition” thereafter) from SGST were used to determine EF (Lachman et al. 2011).
MetS was originally defined using the National Cholesterol Education Program Adult Treatment Panel III guidelines (2001), including (a) abdominal obesity (waist circumference > 102cm in men and > 88cm in women), (b) triglyceride level ≥ 150 mg/dL, (c) low high-density lipoprotein (HDL) cholesterol (< 40 mg/dL in men and <50 mg/dL in women), (d) systolic blood pressure (BP) ≥ 130mm Hg and/or diastolic BP ≥ 85mm Hg or use of antihypertensive medication, and (e) high fasting glucose (≥ 110 mg/dL or use of anti-diabetic medication). Fasting glucose was unavailable; hence, we used blood hemoglobin A1c (HbA1c) ≥ 7% or use of anti-diabetic medication as the criterion for hyperglycemia. BP was averaged over three measurements during the physical exam. Lipid and HbA1c levels were measured using standard fasting blood draw procedures. All prescription medications were documented based on the original bottles the participants brought to the GCRC.
Additionally, literature consistently supports a positive association between the level of C-reactive protein (CRP) and adverse cardiovascular events, and suggests that adding CRP to the profile of MetS would enhance the predictive value of MetS (Abraham, et al. 2007; Ridker, et al. 2004). In 2004, the National Heart Lung Blood Institute/American Heart Association report emphasized the importance of considering CRP as a “metabolic risk factor” (Grundy et al. 2004). Therefore, in the present study, we utilized a modified MetS profile that includes CRP. CRP was analyzed using a particle enhanced immunonepholometric assay (BNII nephelometer, Dade Behring Inc., Deerfield, IL) with acceptable intra-assay and inter-assay coefficients (< 10%). There is no consistent clinically meaningful cut-off score for CRP; thus, we analyzed CRP as a continuous variable. In the present study, we added CRP to the five established MetS components to determine MetS using CFA. The CFA approach, instead of simply applying a cutoff score for MetS, was used in previous study without considering CRP (Stevenson, et al. 2012).
Covariates
Demographic and health characteristics were chosen based on the frailty related literature (Robertson et al. 2013). Demographic characteristics included age, sex, and education. Active alcohol intake was considered if the participant was drunk one or more days/week. The use of corticosteroids medications was recorded based on the original bottles brought in by participants for their overnight stay at the GCRC. History of stroke and cancer were collected based on participant self-report. Sleep quality was assessed with the Pittsburg Sleep Quality Inventory (37). A global sleep quality score was calculated by summing the responses to all items across 7 domains with higher scores indicating better sleep.
Data Analysis
Due to its skewed distribution, CRP was log-transformed. Descriptive analysis was performed. To examine the association between MetS, cognition (EF or EM), and frailty, we conducted SEM with the measurement model consisting of a CFA on the items used for the MetS and the cognition measures and the structural model consisting of an interaction between two latent variables (see Figure 1) using Mplus 7.0 (Muthen and Muthen 1998). The model was estimated using maximum likelihood estimation (Klein and Moosbrugger 2000), including estimating a random effect and maximum likelihood estimator with robust standard errors. The dependent variable (i.e., frailty) and MetS components (except CRP) were defined as dichotomous variables; while CRP and cognition measures were continuous variables. Age, sex, corticosteroids, sleep quality, history of cancer, history of stroke, and alcohol intake were controlled setting them as correlates of the dependent variable. For multilevel analysis including an interacting term between latent variables, comparative model fit indices have not been developed in Mplus. We reported absolute model fit indices (Akaike Information Criteria, AIC, and Bayesian Information Criteria, BIC), and likelihood ratio chi-square test defined as the difference between the loglikelihood of the present model (with interacting term) and nested model (without interacting term) multiplied by (−2), as suggested by Dr. Muthen (http://www.statmodel.com/discussion/messages/11/862.html?1193666052). If there were any significant interactions between cognition (EF or EM) and MetS, we next examined the association between cognition and frailty by stratifying MetS at 0 vs. 1. Statistical significance was evaluated using an overall α level of 0.05.
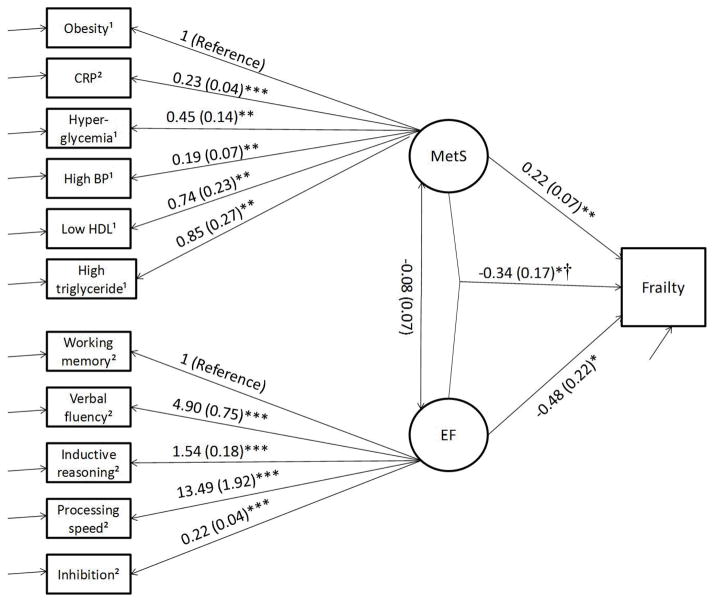
Figure 1.
Figure 1a Interaction between MetS and EF on Frailty. AIC = 25237.08, BIC = 25433.934. Note. 5 cases were missing. Age, sex, corticosteroids, sleep quality, history of cancer, history of stroke, and alcohol taken were controlled. * p < .05; ** p < .01; *** p < .001. † Interaction term between MetS and EF. 1 dichotomous variable; 2 continuous variable.
Equations for contrast analysis:
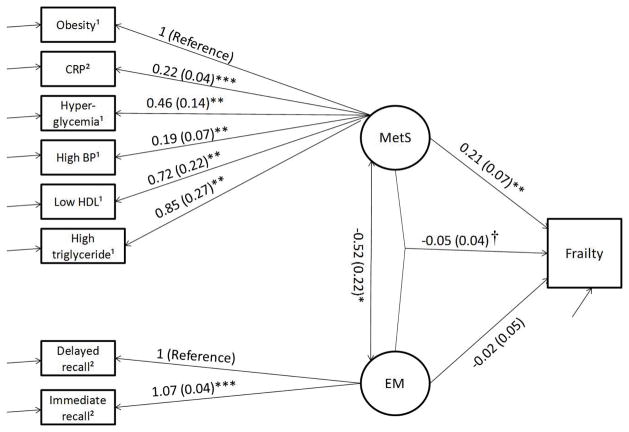
Figure 1b Interaction between MetS and EM on Frailty. AIC = 14511.16, BIC = 14667.74. Note. 6 cases were missing. Age, sex, corticosteroids, sleep quality, history of cancer, history of stroke, and alcohol taken were controlled. ** p < .01; *** p < .001. † Interaction term between MetS and EM. 1 dichotomous variable; 2 continuous variable.
Results
Table 1 shows the descriptive data. The average age of the total sample was 63.23 years and 45.1% was male. A total of 287 (43.2%) participants were considered pre-frail/frail. The most prevalent frailty characteristic was physical inactivity (21.4%), while the least prevalent was slowness (5.1%).
Table 1.
Demographic and Health Data
| Age (Mean, SD) | 63.23 (9.15) |
| Education a | |
| • High school graduate or less | 177 (25.7%) |
| • Some college | 196 (28.4%) |
| • College graduate or more | 315 (45.6%) |
| Male (n, %) | 311 (45.1%) |
| Alcohol intake (n, %) | 464 (67.2%) |
| Corticosteroids (n, %) | 99 (14.3%) |
| Stroke (n, %) b | 3 (0.4%) |
| Cancer (n, %) c | 102 (14.8%) |
| Sleep quality (Mean, SD) | 5.75 (3.35) |
| MetS component | |
| • Obesity (n, %) | 365 (52.9%) |
| • CRP (Mean, SD) ‡ | 0.24 (1.02) |
| • High triglycerides (n, %) | 194 (28.1%) |
| • High BP (n, %) | 426 (61.7%) |
| • Low HDL (n, %) | 191 (27.7%) |
| • Hyperglycemia (n, %) | 85 (12.3%) |
| EF | |
| • Working memory (Mean, SD) | 4.95 (1.39) |
| • Verbal fluency (Mean, SD) | 19.23 (5.60) |
| • Inductive reasoning (Mean, SD) | 2.40 (1.49) |
| • Processing speed (Mean, SD) | 37.19 (10.15) |
| • Inhibition† (Mean, SD) | −3.82 (0.63) |
| EM | |
| • Immediate recall (Mean, SD) | 6.76 (2.16) |
| • Delayed recall (Mean, SD) | 4.36 (2.46) |
| Frailty (n, %) | 287 (43.2%) |
| • Physical inactivity (n, %) | 148 (21.4%) |
| • Exhaustion (n, %) | 84 (12.2%) |
| • Unintentional weight loss (n, %) | 62 (9.0%) |
| • Weakness (n, %) | 57 (8.3%) |
| • Slowness (n, %) | 35 (5.1%) |
Note.
log-transformed.
reversed coded, higher indicating better.
3 cases were missing;
5 cases were missing;
3 cases were missing.
Figure 1a shows the association between MetS, EF, and frailty. For the CFA (measurement) portion of the model, when setting obesity as the reference, all the relevant factor indicators (CRP, high triglycerides, high BP, low HDL, and hyperglycemia) were significant for MetS. When setting working memory as reference, all the relevant factor indicators (verbal fluency, inductive reasoning, processing speed, and inhibition) were significant for EF. For the structural model, there was no significant association between MetS and EF, the two latent variables (B = −0.80, SE = 0.07, p = .25). There were significant main effects of MetS (B = 0.22, SE = 0.07, OR = 1.24, p = .003) and EF (B = −0.48, SE = 0.22, OR = 0.62, p = .033) as well as interacting effect between MetS and EF (B = −0.34, SE = 0.17, OR = 0.71, p = .049) on frailty, controlling for covariates. Chi-square testing (χ2 = 5640.72, df = 3, p < .001) indicated the present model was a significant improvement to the nested model (without interacting term). To further interpret the interaction effect between EF and MetS, stratification analysis was conducted. MetS was set to equal 0 (MetS not present) or 1 (Mets present) (see Figure 1a). A stronger association of lower EF and increased likelihood of frailty existed in individuals with MetS than without MetS.
Figure 1b shows the association between MetS, EM, and frailty. For the measurement model, factors were all significant for MetS, and when setting immediate recall as reference, delayed recall was significant for EM. For the SEM part, there was significant association between MetS and EM (B = −0.59, SE = 0.25, p = .017), and significant association between MetS and frailty (B = 0.21, SE = 0.07, OR = 1.23, p = .002). There was no significant main effect of EM (B = −0.02, SE = 0.05, OR = 0.98, p = .70) or interacting effect between MetS and EM (B = −0.04, SE = 0.03, OR = 0.96, p = .18) on frailty. Chi-square testing (χ2 = −2.01, df = 1, p = 0.16) indicated the present model was not a significant improvement to the nest model.
Discussion
To the best of our knowledge, this is the first study examining the relationship between MetS, cognition, and frailty using both measurement and structural models via SEM. Such modeling techniques allow a sophisticated examination of the complex associations between multiple components of MetS and cognition in relation to frailty. That is, by including measurement models for cognition and MetS, the estimation of measurement error that is derived from the CFA is then incorporated into the estimates of the parameters within the structural model making the estimates of the relationships within the structural model more robust. Our findings support previous investigators’ suggestions that CRP be considered an indicator of MetS (Grundy et al. 2004), and replicated the CFA for the two domains of cognition (Lachman et al. 2011). We found that having MetS significantly increased the likelihood of being frail and that performing better on EM tasks, but not EF, was associated with a significantly lower likelihood of MetS. Worse performance on EF tasks, but not EM, was associated with a significantly higher likelihood of being frail. There was a significant interacting effect between MetS and EF, but not EM, on frailty. Further stratification analysis indicated that having MetS strengthened the relationship between EF and being frail in the individuals with MetS, and individuals with executive dysfunction were those more likely to be frail.
We found several significant bi-correlations between frailty, MetS, and cognition that are consistent with the literature. First, although previous work found those with MetS are, in general, more likely to exhibit poor cognition (Panza et al. 2011), memory may have a more prominent association with MetS than other cognitive domains, such as EF (Hassenstab, et al. 2010; Komulainen, et al. 2007), which was replicated in the present study. Second, consistent to previous studies, frailty was associated with executive dysfunction (Robertson et al. 2013). Through the function of the frontal cortex and white matter, EF regulates motor planning, which is critical for carrying out movements (i.e., grip and gait) that can maintain both upper and lower extremity function (Ble, et al. 2005; MacDonald, et al. 2011). When declines in EF are experienced, declines in physical function may manifest as decreased grip strength and walking speed. EM was not significantly related to frailty. This is likely due to our approach to frailty as a physical phenomenon. Encoding and storaging memory is not in motor planning. Finally, we found a significant association between MetS and frailty regardless of the type of cognition. Such a relationship is likey attributed to the degrading effects of metbolic risk factors (e.g., altered markers of carbohydrate metabolism, elevated inflammation markers, hypertension and coagulation, and insulin resistance) on bone and muslce tissue that are related to frailty (Barzilay, et al. 2007; Barzilay and Stein 2011).
MetS was supported as a moderator of the relationship between EF, but not EM, and frailty. Chronic inflammation, insulin resistance, and blood pressure regulation from MetS may be involved in supporting the association. First, individuals with MetS are predisposed to developing chronically elevated levels of inflammation that can negatively affect EF. CRP is one of the most consistently identified inflammatory factors regulating the relationship between cognition and frailty (Barzilay and Stein 2011). A recent study found that CRP mediated the relationship between muscle strength and cognitive decline in women (Canon and Crimmins 2011). Second, Abbatecola and colleagues (2007) proposed insulin resistance might also explain the relationship between cognition and frailty. That is, insulin modulates glucose use through receptors located in the hippocampus and frontal cortex, which is in particular related to EF; meanwhile, insulin resistance causes an imbalance toward catabolism, presenting as problems related to muscle mass and strength (Abbatecola, et al. 2007). Although we did not directly measure insulin resistance, it is usually highly correlated to hemoglobin A1c levels. Finally, there is no study examining disrupted circulation as a mechanism. However, arterial blood pressure regulates the changes in tissue perfusion, which potentially explain the degree of physiological reserve/frailty (Fattori, et al. 2013). Meanwhile, several studies suggest that arterial blood pressure is linked to cerebral blood flow and can directly influence cognitive function (Panza et al. 2011). In individuals with MetS, these three pathways may be disrupted and increase the likelihood of experiencing declines in EF and developing frailty. In contrast, in those without MetS, these pathological processes may not reach the threshold to disturbing the brain’s capacity in regulating motor process that likely play a role in the development of frailty. Very few studies have tested these potential biological pathways as links between cognitive decline and frailty, especially in groups vulnerable to disruption in these biological pathways (i.e., individuals with MetS).
Presently, there is an emphasis in aging research to develop interventions that can address comorbidities simultaneously. A recent aerobic exercise intervention simultaneously improved frailty indicators and cognitive function (Langlois, et al. 2013). A well-established lifestyle intervention, Diabetes Prevention Program, which includes both dietary and physical activity interventions, improves MetS related conditions such as obesity and glucose level (Goldberg and Mather 2012). Given the success of these interventions and the close relationships observed in this current study among EF, MetS and frailty, it is reasonable to propose that any of these interventions may be effective in preventing or managing MetS or preventing cognitive decline and frailty in individuals with MetS.
The findings from this study must be interpreting in light of certain study limitations. First, The sample was relatively young and reported relatively high levels of physical functioning which resulted in reduced variability in our frailty measuring requiring the combination of the pre-frail and frail groups. However, since MetS occurs relatively early compared to many other chronic conditions, it is important to examine its influence on cognition and frailty in other age groups than merely older adults (Weiss et al. 2013). Second, although SEM is appropriate for testing established conceptual framework, the cross-sectional nature of this study still limits our ability to deduce the directionality of the tested relationships. Third, major cardiovascular events (e.g., heart attack) are closely linked to MetS. Future studies may consider take into account the effect of this factor in confounding the relationships between MetS, frailty, and cognition. Finally, we were not able to replicated the MetS or Fried frailty measure exactly. For MetS measure, given the unavailable of fasting glucose, we used HbA1c, which may not be sensitive to capture the recent onset of elevation in glucose. Regarding frailty measure, previous studies have also used modified versions of this measure and found significant associations between frailty and various markers of poor health (i.e., number of chronic diseases) (e.g., (Espinoza, et al. 2012)). Previously observed relationships between frailty and various markers of health (i.e., lower levels of EF and MetS) were observed in our study, providing concurent validity for our frailty measure.
Conclusion
This study provides a new perspect on the relationship between cognition and frailty within the context of specific health condition, MetS. If these same associations are observed in a prospective study, targeting individual with MetS for interventions designed for the prevention of EF and/or frailty may be a worthwhile endeavour in an attempt to prevent multiple chronic conditions through a single intervention.
Key points.
Executive function and metabolic syndrome are associated with the likelihood of being frail;
Metabolic syndrome moderates the association between executive function and being frail.
Acknowledgments
Funding: MIDUS II was supported by from the National Institute on Aging (P01-AG020166).
The manuscript development was supported by the University of Rochester CTSA award number KL2 TR000095 from the National Center for Advancing Translational Sciences of the National Institutes of Health to F. Lin.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Abbatecola AM, Ferrucci L, Marfella R, Paolisso G. Insulin resistance and cognitive decline may be common soil for frailty syndrome. Arch Intern Med. 2007;167:2145–2146. doi: 10.1001/archinte.167.19.2145-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham J, Campbell CY, Cheema A, Gluckman TJ, Blumenthal RS, Danyi P. C-reactive protein in cardiovascular risk assessment: a review of the evidence. J Cardiometab Syndr. 2007;2:119–123. doi: 10.1111/j.1559-4564.2007.05950.x. [DOI] [PubMed] [Google Scholar]
- Expert Panel on Detection Evaluation Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol. 2009;103:1616–1621. doi: 10.1016/j.amjcard.2009.01.375. [DOI] [PubMed] [Google Scholar]
- Aranda MP, Ray LA, Snih SA, Ottenbacher KJ, Markides KS. The protective effect of neighborhood composition on increasing frailty among older Mexican Americans: a barrio advantage? J Aging Health. 2011;23:1189–1217. doi: 10.1177/0898264311421961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilay JI, Blaum C, Moore T, Xue QL, Hirsch CH, Walston JD, Fried LP. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med. 2007;167:635–641. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- Barzilay JI, Stein PK. Association of the metabolic syndrome with age-related, nonatherosclerotic, chronic medical conditions. Metab Syndr Relat Disord. 2011;9:327–335. doi: 10.1089/met.2011.0027. [DOI] [PubMed] [Google Scholar]
- Ble A, Volpato S, Zuliani G, Guralnik JM, Bandinelli S, Lauretani F, Bartali B, Maraldi C, Fellin R, Ferrucci L. Executive function correlates with walking speed in older persons: the InCHIANTI study. J Am Geriatr Soc. 2005;53:410–415. doi: 10.1111/j.1532-5415.2005.53157.x. [DOI] [PubMed] [Google Scholar]
- Canon ME, Crimmins EM. Sex differences in the association between muscle quality, inflammatory markers, and cognitive decline. J Nutr Health Aging. 2011;15:695–698. doi: 10.1007/s12603-011-0340-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienberg Love G, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS national study: protocol, measures, sample, and comparative context. J Aging Health. 2011;22:1059–1080. doi: 10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doba N, Tokuda Y, Goldstein NE, Kushiro T, Hinohara S. A pilot trial to predict frailty syndrome: the Japanese Health Research Volunteer Study. Exp Gerontol. 2012;47:638–643. doi: 10.1016/j.exger.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Espinoza SE, Jung I, Hazuda H. Frailty transitions in the San Antonio Longitudinal Study of Aging. J Am Geriatr Soc. 2012;60:652–660. doi: 10.1111/j.1532-5415.2011.03882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattori A, Santimaria MR, Alves RM, Guariento ME, Neri AL. Influence of blood pressure profile on frailty phenotype in community-dwelling elders in Brazil - FIBRA study. Arch Gerontol Geriatr. 2013;56:343–349. doi: 10.1016/j.archger.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Goldberg RB, Mather K. Targeting the consequences of the metabolic syndrome in the Diabetes Prevention Program. Arterioscler Thromb Vasc Biol. 2012;32:2077–2090. doi: 10.1161/ATVBAHA.111.241893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- Hassenstab JJ, Sweat V, Bruehl H, Convit A. Metabolic syndrome is associated with learning and recall impairment in middle age. Dement Geriatr Cogn Disord. 2010;29:356–362. doi: 10.1159/000296071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Moosbrugger H. Maximum likelihood estimation of latent interaction effects with the LMS method. Psychometrika. 2000;65:457–474. [Google Scholar]
- Komulainen P, Lakka TA, Kivipelto M, Hassinen M, Helkala EL, Haapala I, Nissinen A, Rauramaa R. Metabolic syndrome and cognitive function: a population-based follow-up study in elderly women. Dement Geriatr Cogn Disord. 2007;23:29–34. doi: 10.1159/000096636. [DOI] [PubMed] [Google Scholar]
- Lachman ME, Agrigoroaei S, Murphy C, Tun PA. Frequent cognitive activity compensates for education differences in episodic memory. Am J Geriatr Psychiatry. 2011;18:4–10. doi: 10.1097/JGP.0b013e3181ab8b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois F, Vu TT, Chasse K, Dupuis G, Kergoat MJ, Bherer L. Benefits of physical exercise training on cognition and quality of life in frail older adults. J Gerontol B Psychol Sci Soc Sci. 2013;68:400–404. doi: 10.1093/geronb/gbs069. [DOI] [PubMed] [Google Scholar]
- MacDonald SW, DeCarlo CA, Dixon RA. Linking biological and cognitive aging: toward improving characterizations of developmental time. J Gerontol B Psychol Sci Soc Sci. 2011;66(Suppl 1):i59–70. doi: 10.1093/geronb/gbr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. MPlus (Version 7.0)[Computer Software] Los Angeles, CA: Muthen and Muthen; 1998. [Google Scholar]
- Panza F, Frisardi V, Capurso C, Imbimbo BP, Vendemiale G, Santamato A, D’Onofrio G, Seripa D, Sancarlo D, Pilotto A, et al. Metabolic syndrome and cognitive impairment: current epidemiology and possible underlying mechanisms. J Alzheimers Dis. 2011;21:691–724. doi: 10.3233/JAD-2010-091669. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raji MA, Al Snih S, Ostir GV, Markides KS, Ottenbacher KJ. Cognitive status and future risk of frailty in older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2011;65:1228–1234. doi: 10.1093/gerona/glq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Wilson PW, Grundy SM. Should C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? Circulation. 2004;109:2818–2825. doi: 10.1161/01.CIR.0000132467.45278.59. [DOI] [PubMed] [Google Scholar]
- Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment-A review of the evidence and causal mechanisms. Ageing Res Rev. 2013 doi: 10.1016/j.arr.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Stevenson JE, Wright BR, Boydstun AS. The metabolic syndrome and coronary artery disease: a structural equation modeling approach suggestive of a common underlying pathophysiology. Metabolism. 2012;61:1582–1588. doi: 10.1016/j.metabol.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Tun PA, Lachman ME. Telephone assessment of cognitive function in adulthood: the Brief Test of Adult Cognition by Telephone. Age Ageing. 2006;35:629–632. doi: 10.1093/ageing/afl095. [DOI] [PubMed] [Google Scholar]
- Tun PA, Lachman ME. Age differences in reaction time and attention in a national telephone sample of adults: education, sex, and task complexity matter. Dev Psychol. 2008;44:1421–1429. doi: 10.1037/a0012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R, Bremer AA, Lustig RH. What is metabolic syndrome, and why are children getting it? Ann N Y Acad Sci. 2013;1281:123–140. doi: 10.1111/nyas.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]