Summary
Anatomical variations in lumbosacral plexus or nerves to genitourinary structures in dogs are under described, despite their importance during surgery and potential contributions to neuromuscular syndromes. Gross dissection of 16 female mongrel hound dogs showed frequent variations in lumbosacral plexus classification, sympathetic ganglia, ventral rami input to nerves innervating genitourinary structures and pudendal nerve (PdN) branching. Lumbosacral plexus classification types were mixed, rather than pure, in 13 (82%) of dogs. The genitofemoral nerve (GFN) originated from ventral ramus of L4 in 67% of nerves, differing from the expected L3. Considerable variability was seen in ventral rami origins of pelvic (PN) and Pd nerves, with new findings of L7 contributions to PN, joining S1 and S2 input (23% of sides in 11 dogs) or S1–S3 input (5%), and to PdN, joining S1–S2, unilaterally, in one dog. L7 input was confirmed using retrograde dye tracing methods. The PN also received CG1 contributions, bilaterally, in one dog. The PdN branched unusually in two dogs. Lumbosacral sympathetic ganglia had variant intra-, inter- and multisegmental connectivity in 6 (38%). Thus, the anatomy of mongrel dogs had higher variability than previously described for purebred dogs. Knowledge of this variant innervation during surgery could aid in the preservation of nerves and reduce risk of urinary and sexual dysfunctions.
Introduction
In our recent dog studies examining surgical strategies for reinnervation of the bladder after injury of spinal roots, we observed several variations in the neuroanatomy of the lumbosacral plexus (plexus lumbosacralis), and nerves supplying the detrusor muscle of the bladder (vesica urinaria) and external urethral sphincter (ostium urethrae externum) (Ruggieri et al., 2006, 2008a,b, 2011). These variations differed from prior reports examining the lumbosacral plexus in dogs (Fletcher, 1970; Bailey et al., 1988) and from studies examining the innervation of lower urinary tract structures (Evans, 1936; Oliver et al., 1970; Purinton and Oliver, 1979; Tanagho et al., 1982; Yoshimura and De Groat, 1997). Such variations may contribute to neuromuscular syndromes in dogs and humans. Furthermore, anatomical variations of communicating rami in the sympathetic chain are thought to explain some surgical failures when performing sympathectomies in humans (Umeda et al., 1987; Cho et al., 2005; Zhang et al., 2009), although variations in the sympathetic chain has been incompletely described in dogs.
It is known that dogs with spinal cord injury (SCI) in the lower lumbar and sacral segments present with poor prognoses, based on clinical signs of loss of perception, locomotor ability and voluntary micturition. Forty percentage of dogs cases with inter-vertebral disc disease present with mild intermittent urinary incontinence after decompressive surgery as a treatment (Webb et al., 2010a, b). We hypothesize that there are undescribed anatomical variations and anomalies in the lumbosacral plexus or ventral rami of spinal nerves contributing to the origin of the nerves supplying genitourinary structures that could be injured during surgery on the lumbosacral vertebra column. Such injuries could contribute to post-surgical functional losses. Further clarification of these variations is needed to avoid urinary and faecal incontinence in dogs undergoing surgery for SCI or inter-vertebral disc disease.
Thus, we sought here to identify anatomical variations in neurological structures that contribute to the innervation of the genitourinary system in female mongrel dogs [where the term mongrel is used to refer to dogs of descended from multiple breeds of the same species (i.e. mixed) or undetermined breed (Merriam-Webster Inc., 2005; Houghton Mifflin Company, 2011)]. This is in contrast to purebred (a single breed within the same species) and cross-breed animals (the cross of two breeds within the same species) (Merriam-Webster Inc., 2005; Houghton Mifflin Company, 2011). Specifically, we examined for variations in the following: (1) the classification of the lumbosacral plexus; (2) the ventral rami of spinal nerves from which they originate; (3) their branching patterns to end organs; and (4) the lumbosacral sympathetic chain from which they arise. Neuronal tract tracing techniques were used in six of the 16 dogs to confirm whether lumbar and coccygeal spinal cord segments also contribute to the origin of presumed sacral nerves. Data from this study will clarify the innervation of genitourinary structures for the improvement of bladder reinnervation studies using dogs. These results will also improve awareness of the frequency of topographic variations and anomalies for veterinarians performing other surgeries in this region in mongrel canine dogs.
Materials and Methods
Animals
A total of 16 female mongrel hound dogs were used that were part of other studies. Three of these dogs were unoperated controls, and three were sham controls. Only structures unaltered by any prior surgeries or procedures were included in this anatomical study. The study subjects were fully conditioned with a mean age of 59 weeks (36.6–99 weeks) and a mean weight of 18.6 kg, range 16.7–20 kg (Marshall BioResources, North Rose, NY, USA). All studies were approved by the Temple University Institutional Animal Care and Use Committee, and carried out in accordance with the Guidelines of the National Institute of Health (NIH) in the USA regarding the care and use of animals for experimental procedures, and the laboratory animal care guidelines of both the United States Department of Agriculture and the Association for Assessment and Accreditation of Laboratory Animal Care.
Retrograde in vivo dye tracing from the bladder neck, urethra and clitoris: Six of the sixteen dogs were injected with retrograde-labelling dyes at 3 weeks prior euthanasia. The six dogs were divided into two groups of three each. All of these six dogs were controls (did not undergo denervation or reinnervation surgeries). Prior to dye injections, dogs were sedated with 6 mg/kg of propofol, i.v., to allow endotracheal tube insertion, and then anaesthesia was maintained using isoflurane at 2–3% maximum alveolar concentration (MAC), as previously described (Ruggieri et al., 2008a). The bladder was then cystoscoped. Dogs in group 1 received injections of Fluorogold (Fluorochrome, LLC, Denver, CO, USA) into the detrusor muscle at four different sites lateral to each ureteral orifice (4–5% w/v in 0.9% saline solution, 50 µl/injection, eight injections/dog, 400 µl total per animal) and injections of Nuclear Yellow (Life Technologies Corporation, Grand Island, NY, USA) at four different sites of the external urethral sphincter (2% w/v in 74% dimethyl sulfoxide (DMSO), 50 µl/injection, 200 µl total per animal). Dogs in group 2 received injections of Fluorogold into detrusor muscle lateral to the ureteral orifices at four different sites (dosed as above), injections of True Blue (Life Technologies Corporation) at four sites of the external urethral sphincter (2% w/v in 74% DMSO, 50 µl/injection, 200 µl total per animal) and injections of Nuclear Yellow into the left and right sides of the body of the clitoris (2% w/v in 74% DMSO, 50 µl/injection, 100 µl total per animal). The dogs were then allowed to recover from anaesthesia and were returned to their home cages. Three weeks later, after euthanasia, the dorsal root ganglia (DRG), lumbosacral sympathetic ganglia and lumbosacral spinal cords of these six female dogs (12 sides) were collected and examined for neuronal cell bodies labelled with one or more of these retrograde dyes, using previously described methods (Ruggieri et al., 2006, 2008a,b).
Anaesthesia and euthanasia
All 16 canines were sedated with 6 mg/kg of propofol i.v. to allow endotracheal tube insertion for inhalational anaesthesia of isoflurane at 2–3% mean alveolar concentration with oxygen as the carrier gas. We then identified the genitofemoral (GFN), pelvic nerve (PN) and pudendal nerve (PdN) in the abdominal, pelvic and perineal regions prior to administration of a terminal dose of sodium pentobarbital (i.v., 360 mg/kg body weight). After euthanasia, tissues were collected for the remaining dissections.
Gross dissection
At the end of the study, the dogs were anesthetized, as described above, and then euthanized using a terminal dose of sodium pentobarbital (i.v., 360 mg/kg body weight), as previously described (Ruggieri et al., 2008a). In each canine dog, the PN and the PdN were dissected and their topographical anatomy examined. The GFN was also examined, as it is sensory to the skin of the proximal medial thigh, prepuce and scrotum/major labia, and motor to the cremasteric muscle. First, the PdN was located using a perineal approach. Each dog was placed in a supine position, and the pelvic limbs were tied cranially to expose the perineum region. Perineum fat tissue was carefully removed. The PdN was located, bilaterally, within planes of the levator ani and coccygeus muscle, medial to the superficial gluteus muscle and dorsal to the internal pudendal artery and vein. The PdN was labelled on both the sides with a loop of string. Next, the PN and GFN were identified using an abdominal approach. A midline incision from the xiphoid cartilage to the pubic symphysis was performed by cutting through the skin, subcutaneous fat and linea alba to access to the abdominal cavity. The greater omentum and abdominal organs were moved cranially within the abdominal cavity to allow a clear view. Posteriorly, the bladder (apex, body and neck) and median ligament of the bladder were identified in the pelvic cavity inlet. Both right and left side ureters, hypogastric nerve and lateral ligament of the bladder were identified as reference points. The vesical branches of the PN innervating the detrusor muscle were labelled and identified bilaterally on the lateral-ventral surface of the bladder, towards the ureter. After identification and careful labelling of these nerves, animals were euthanized, as described above.
After euthanasia, both pelvic limbs were removed, while attempting to keep the coccygeus muscle and part of the superficial gluteal muscle intact so that the path of the PdN from the pelvis to the perineum could be examined for variations. Posteriorly, ventral rami of spinal nerves contributing to the origin of the PN and PdN were identified. The anatomical classification of the lumbosacral plexus was assessed, as was the anatomy of the lumbosacral sympathetic ganglia, noting the presence of rami communicans between ganglia or ganglionic fusions, or absence of ganglia. In the pelvic and perineal regions, anatomical variations in the pudendal nerve trunk and branching patterns of nerve from this trunk to the dog bladders and external urethral sphincters were assessed.
As 10 of these specimens were part of other surgical studies, not all tissues were available for all anatomical analyses. However, as stated above, only structures unaltered by the surgeries were included in this anatomical study. The number used for each type of assay is as follows: (1) the classification of the lumbosacral plexus, n = 11, as indicated in Table 2; (2) the ventral rami of spinal nerves from which they originate, n = 6–11, depending on the nerve, as indicated in Table 3; (3) their branching patterns to genitourinary end organs, n = 16; and (4) the lumbosacral sympathetic chain from which they arise, n = 16.
Table 2.
Lumbosacral plexus classification and variation in 22 sides of 11 mongrel (mixed breed) hound female dogs
| Genitofemoral nerve origin | Pelvic nerve origin | Pudendal nerve origin | Plexus classification type | |||||
|---|---|---|---|---|---|---|---|---|
| Right side | Left side | Right side | Left side | Right side | Left side | Right side | Left side | |
| Dog 1 | _ | _ | S3, CG1 (Post) | S3, CG1 (Post) | S3 (Post) | S3 (Post) | Post | Post |
| Dog 2 | _ | _ | L7, S1, S2 (Pre) | S2, S3 (Post) | S3 (Post) | S1, S2 (Pre) | Pre–Post | Pre–Post |
| Dog 3 | _ | _ | S2, S3 (Post) | S2, S3 (Post) | S2, S3 (Post) | S1, S2 (Pre) | Post | Pre–Post |
| Dog 4 | _ | _ | L7, S1–3 (Median) | S1–3 (Median) | _ | _ | Median | Median |
| Dog 5 | L3, L4 (Median) | L3, L4 (Median) | S1–S3 (Median) | S1–3 (Median) | S2, S3 (Post) | S2, S3 (Post) | Median–Post | Median–Post |
| Dog 6 | _ | _ | S1, S2 (Pre) | S1, S2 (Pre) | S2 (Pre) | S2, S3 (Post) | Pre | Pre–Post |
| Dog 7 | L3, L4 (Median) | L3, L4 (Median) | S2, S3 (Post) | S2, S3 (Post) | S2 (Pre) | S3 (Post) | Pre–Median–Post | Median–Post |
| Dog 8 | L4 (Post) | L4 (Post) | S1–3 (Median) | S1–3 (Median) | S1–3 (Median) | S1–3 (Median) | Median–Post | Median–Post |
| Dog 9 | L4 (Post) | L4 (Post) | S1, S2 (Pre) | L7, S1, S2 (Pre) | S2, S3 (Post) | S2, S3 (Post) | Pre–Post | Pre–Post |
| Dog 10 | L4 (Post) | L4 (Post) | L7, S1, S2 (Pre) | L7, S1, S2 (Pre) | S2, S3 (Post) | S2, S3 (Post) | Pre–Post | Pre–Post |
| Dog 11 | L4 (Post) | L4 (Post) | S2, S3 (Post) | L7, S1, S2 (Pre) | S2, S3 (Post) | L7, S1–3 (Median) | Post | Pre–Median–Post |
| Summary Classification | 33% Median 66% Post |
36.3% Pre 27.2% Median 36.3% Post |
20% Pre 15% Median 65% Post |
4.5% Pre 9% Median 18.1% Post 36.6% Pre–Post 22.7% Median–Post 9% Pre–Median–Post |
||||
Pre, pre-fixed; Median, median-fixed; Post, post-fixed type plexus; L, lumbar; S, sacral; CG, coccygeal.
Each spinal nerve ventral rami contributing to the nerves listed were classified (in parenthesis) according to Fletcher’s system (Fletcher, 1970). % frequency is also listed of individual nerves and for the plexus overall.
Retrograde-labelling methods were used to verify L7 contributions.
Table 3.
Ventral rami origins of pelvic, pudendal and genitofemoral nerves in mongrel (mixed breed) hound female dogs (n = 6–11 dogs, as indicated)
| Nerve | Range of spinal nerve ventral rami origin(s) | |||||
|---|---|---|---|---|---|---|
| Genitofemoral | L3–L4 33.3% (4) | L4 66.7% (8) | No sacral contribution observed | Total sides 12 sides of 6 dogs | ||
| Pelvic | L7–S2 (L7–S3a) 27.3% (6) | S1–S2 13.6% (3) | S1–S3 22.7% (5) | S2–S3 27.3% (6) | S3-CG1 9.1% (2) | Total sides 22 sides 11 dogs |
| Pudendal | S1–S2 (L7-S2a) 10% (2) | S1–S3 15% (3) | S2 10% (2) | S2–S3 45% (9) | S3 20% (4) | Total sides 20 sides of 10 dogs |
L, lumbar; S, sacral; CG, coccygeal.
For each nerve, the variations in origin are listed in bold, as are % frequency of observations and number of observations per side of dog (in parentheses) in total number of spinal column sides examined.
Observed in one side of dog only.
Data analysis
Descriptive statistics were performed, and percentage incidence of anatomical variations was generated.
Results
The classification of the lumbosacral plexus shown in Table 1 is as described previously by Fletcher (1970). Our lumbosacral plexus shows mixed-type classifications rather than the pure median, pre- or post-fixed types in 11 dogs (Table 2). We observed that 33% (4/12 sides) of the genitofemoral nerves (GFN) studied presented with median-fixed plexus type contributions and 67% (8/12 sides) with post-fixed plexus type contributions, but no pre-fixed plexus type contributions. Regarding the pelvic nerve (PN), 36% (8/22 sides) presented with pre-fixed plexus type contributions, 27% (6/22 sides) with median-fixed plexus type contributions and 36% (8/22 sides) with post-fixed plexus type contributions. Regarding the pudendal nerve (PdN), 20% (4/20 sides) presented with pre-fixed plexus type contributions and 15% (3/20 sides) with median-fixed plexus type contributions, but most commonly with post-fixed plexus type contributions (65%, 13/20 sides). When we combined these data and examined the overall classification type of the lumbosacral plexus, bilaterally, we observed that 82% (9/11 dogs) had mixed classification types (Table 2). Only two dogs matched the classification types described by Fletcher in both the right and left sides: 1 post-fixed and 1 median-fixed. A mixed pre–post type was the most commonly observed (37%), median–post type was next frequent in presentation (23%), and pre-fixed was most rare (5%, 1 side of one dog). See Table 2 for remaining observations.
Table 1.
Lumbosacral plexus classification according to Fletcher (1970) (based on 30 purebred dogs)
| Nerve | ||||||
|---|---|---|---|---|---|---|
| Plexus type | Genitofemoral | Femoral | Obturator | Superior gluteal |
Sciatic | Pelvic/Pudendal |
| Pre-fixed | L3 | L4, L5 | L4–L6 | L6, L7 | L6, L7, S1 | S1, S2 |
| Median-fixed | L3, L4 | L3–L5 | L4–L6 | L6, L7, S1 | L6, L7, S1, S2 | S1–S3 |
| Post-fixed | L4 | L4–L6 | L4–L6 | L7, S1 | L6, L7, S1, S2 | S2, S3 |
L, lumbar; S, sacral.
For each classification type, the spinal nerve ventral rami contributing to each nerve is listed.
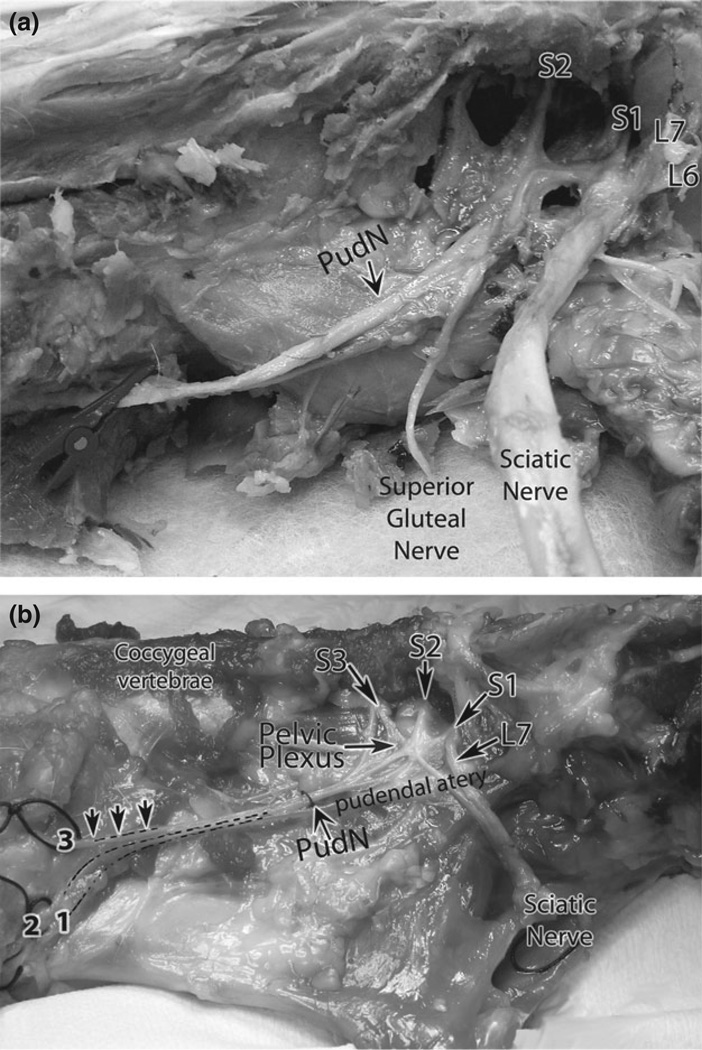
A summary of the individual ventral branches of spinal nerves contributing to the GFN, PN and PdN origin is shown in Tables 2 and 3 (n = 6 = 11/nerve, as indicated). We observed contributions from ventral rami of spinal nerves to the GFN from L4 spinal nerves in 67% (8/12 sides) and from L3 and L4 ventral rami in 33% (4/12 sides). More variability was observed in the ventral rami contributions to the PN and PdN (Tables 2 and 3). For the PN, 27% (6/22 sides) had contributions from the ventral rami of spinal nerves of L7, S1 and S2 segments (one of these dogs also received a contribution from S3 to the PN), 27% from S2 and S3 segments, 23% from S1 to S3 segments, 14% from S1 and S2 and from S3 and CG1 in one dog, bilaterally (9% of sides). For the PdN, 45% (9/20 sides) presented with contributions from ventral rami of spinal nerves of S2 and S3 segments (Fig. 1a), 20% (4/12 sides) from S3 only, 15% from S1 to S3 segments (Fig. 1b), 10% from S1 and S2 segments, with one dog also having contributions from L7 to the PdN, unilaterally, and 10% from S2 only (2/20 sides).
Fig. 1.
Photographs of the ventral rami branches of the lumbosacral plexus contributing to the pudendal and sciatic nerves. (a) Contributions to the pudendal nerve that are pre-fixed in type (i.e. from sacral (S) 2 and S3 ventral rami). This photograph also shows contribution to the sciatic nerve from lumbar (L) 6, L7 and S1 ventral rami (also pre-fixed in type), and contributions to the superior gluteal nerve that appear to be from only S1 and S2. (b). Contributions to the pudendal nerve that are median-fixed in type (i.e. from S1, S2 and S3 ventral rami). This photograph also shows contribution to the sciatic nerve from L7 and S1 (L6 contributions were also observed, but are not shown in the photograph). The dashed lines indicate branches of the pudendal nerve (PudN) arising from a main trunk (a classic Type I pudendal nerve). 1 = dorsal nerve of the clitoris; 2 = perineal nerve; 3 = caudal rectal nerves.
Contributions from L7 to the PN and PdN were confirmed using retrograde dye tracing labelling methods in six dogs. We observed retrograde-labelled motor neurone cell bodies in the DRG, lumbosacral sympathetic ganglia or ventral horn of the spinal cord in the L7 segment, as appropriate for dye placement in five dogs after injections of Fluorogold into the detrusor muscle and in one dog after injection of Nuclear Yellow into the body of the clitoris. Examples of fluorogold retrogradely labelled cells are shown in Fig. 2 (nuclear yellow labelling not shown).
Fig. 2.
Fluorogold retrogradely labelled neuronal cell bodies in the ventral horn of lumbar (L7) segments in spinal cord sections following injection of fluorogold into the bladder near the ureteral orifice 3 weeks before euthanasia (n = 6 dogs examined for this assay). (a, c) A cross-section of a L7 segment in the spinal cord showing fluorogold labelled neuronal cell bodies (arrows) in the ventral horn. (b, d) Higher-power field of neuronal cell bodies showing cytoplasmic localization of the fluorogold dye in neuronal cell bodies (arrows). The objective used to capture the image is indicated in each panel. VH, ventral horn; WM, white matter.
We next examined the branching patterns of nerves to the lower urinary tract end organs and its variations in all 16 dogs. All but one dog had the classic Type I PdN, with one trunk that then branched into three nerves: caudal rectal nerves, perineal nerve and dorsal nerve of the clitoris (shown in Fig. 1b). In the remaining one specimen, we observed that the caudal rectal nerves, perineal nerve and dorsal nerve of the clitoris arose directly from the ventral rami of S2–S3 spinal nerves and innervated these organs without first merging into a principal PdN trunk, a branching style that matched the PdN Type V described by Olszewski (1982). With regard to the path of these nerves to their end organs, a vesical branch of the PN to the wall of the bladder was observed in all specimens. Branches of the PdN to the urethra (perineal nerve) and clitoris (dorsal nerve of the clitoris) were observed in all specimens. Interestingly, one specimen had an additional anomalous branch that passed from the PdN to the superficial gluteus muscle.
The anatomy of the lumbosacral sympathetic ganglia and their connectivity was examined in all 16 dogs (Fig. 3a–d). In 63% of the dogs examined (10/16 dogs), lumbosacral sympathetic ganglia were present for each segment, bilaterally (Fig. 3d). Intra-, inter- or multisegmental connectivity was observed in the remaining 37% of dogs. These observations were clearly the connections between ganglia rather than fused or impar ganglia, which were not observed (Fig. 3). Intrasegmental connectivity was observed in four dogs (Fig. 3a), with one dog presenting with connectivity between left and right L7 segmental ganglia, two dogs with connectivity between left and right S1 segmental ganglia and one dog with connectivity between left and right S2 segmental ganglia. Inter-segmental connectivity was present in only one specimen (Fig. 2b), with the right S2 ganglia connected to the left S3 ganglia. Multisegmental connectivity was observed in one other specimen, with connections between right and left L7 ganglia and between the right S1 ganglion and the left L7 ganglion (Fig. 3c; Fig. 4a, b).
Fig. 3.
Diagrams of the observed intra-, inter- and multi-segmental connectivity between lumbosacral sympathetic ganglia (n = 16 dogs examined for this assay). Right (R) versus left (L) sides are indicated in the far left upper panel. Different types of (a) intra-, (b) inter- and (c) multi-segmental connectivity (red arrows) were observed between lumbosacral sympathetic ganglia (red letters) in the lower lumbar and sacral vertebrae column segments (black letters). (d) Normal “regular type” lumbosacral sympathetic ganglia anatomy, in which no between-ganglia connectivity is observed. We did not observe the presence of fused ganglia, impar ganglia or missing ganglia. The number of observations in 16 female mongrel hound dogs is shown in parenthesis.
Fig. 4.
Photograph of multi-segmental connectivity between lumbosacral sympathetic ganglia in one dog. (a) L7 sympathetic ganglia are shown on the right and left sides of the vertebral column and are connected at this point, indicating intrasegmental connectivity. However, as indicated by the forceps in panel (a) and in the enlarged photo shown in panel (b), there are several inter-segmental processes connecting to more caudal sympathetic ganglia.
Discussion
Most (81%) of the lumbosacral plexi examined in these female mongrel dogs examined in this study had contribution patterns from ventral rami of spinal nerves to GFN, PN and PdN that varied from Fletcher (1970) schema developed in 30 purebred dogs. We observed more contributions from L7 ventral rami to the PN and PdN using anatomical and retrograde dye tracing methods, than described previously, indicating greater L7 innervation to the bladder detrusor and external urethral sphincter muscles than previously understood. Most specimens had the classic Type I PdN, in which there is one main PdN trunk that branches into three nerves: a caudal rectal, perineal and dorsal nerve of the clitoris. However, one specimen lacked a principal PdN trunk; instead, these three nerves rose directly from the ventral rami of S2–S3, a variation described in humans (Olszewski, 1982). Another specimen had an additional anomalous branch passing from the PdN to the superficial gluteus muscle that has not been previously described in human or dog anatomy. Lastly, several points of unexpected connectivity were observed between lumbosacral sympathetic ganglia in over one-third of the dogs, although no fused or impar ganglia were observed. We consider these results relevant for understanding of some idiopathic neurogenic syndromes and for improved post-surgery function of genitourinary organs in veterinarian medicine and human clinical medicine.
Textbook anatomical descriptions of variations in the lumbosacral plexus follow a classification schema developed by Fletcher in 1970 from a study on 30 dogs of different purebreds and sizes (Fletcher, 1970): pre-fixed, median-fixed or post-fixed plexus, with a ratio of presentation of 1:3:1, respectively. Despite the continued use of this schema in textbooks (Evans, 2007; Evans and DeLahunta, 2010), Fletcher reported high variability from this schema in the 30 dogs that he examined. Baily repeated a portion of this study in 12 male purebred dogs in 1988 and found that the ventral rami contributing to cutaneous nerves of the pelvic limb presented with a ratio of 1:8:1 for pre-, median- and post-fixed types (Bailey et al., 1988). More recently, Mihelic performed a cadaveric study on 212 purebred, cross-breed and mongrel dogs of both genders and different sizes (Mihelić et al., 2007) and also found considerable variation from the Fletcher classification schema in ventral rami of spinal nerves contributing to femoral, obturator and sciatic nerves (Mihelić et al., 2007). Some of the patterns corresponded to Fletcher’s scheme, although the ratio differed (5:6:1 for pre-, median- and post-fixed types), as did the pattern of ventral nerve contributing rami to femoral, obturator and sciatic nerves in many specimens (Bailey et al., 1988). No studies have repeated Fletcher’s (1970) study with regard to investigating variability in ventral rami contributing to genitofemoral, pelvic and pudendal nerves.
We had originally postulated that the mixture of two and even three classifications of lumbosacral plexus within each dog was due to our use of mongrel hounds, rather than purebred dogs. A closer look at Fletcher’s study showed that he had reported that only two of 30 dogs presented with lumbosacral plexi that met his median-fixed definition (Fletcher, 1970). In a dog study examining cutaneous nerves innervating the pelvic limb in 12 purebred dogs, Baily concluded that the lumbosacral plexus had a continuous spectrum of variability (Bailey et al., 1988). An even greater number of possible variations were observed in a recent study examining segmental contributions to femoral, obturator and sciatic nerves (Mihelić et al., 2007). These results combined indicate that the lumbosacral plexus has a continuous spectrum of variability and that these differences are not a result of breed, cross-breed or mixed breed/mongrel. Additional findings from our study is the lack of bilateral symmetry (right versus left) in most specimens examined and a lack of ipsilateral symmetry in contributions of ventral rami of spinal nerves to GFN, PN and PdN (Table 2). These findings suggest that dog anatomy texts require updating.
With regard to the pelvic and pudendal nerves, we observed more frequent contributions from L7 ventral rami than previously described (Evans, 2007; Evans and DeLahunta, 2010). We have previously reported the presence of retrograde-labelled neurones from the bladder in coccygeal one (CG1) spinal cord segments of one dog (Ruggieri et al., 2008a). Our results here, combined with this past study, confirm that CG1 ventral rami can occasionally contribute branches to the pudendal nerve. The frequency of L7 and coccygeal contributions to the detrusor and external urethral sphincter muscles should be examined further in future studies examining reinnervation strategies to these structures in order to enhance their success.
We did not find any reports concerning gender-based variability to the pelvic and pudendal nerves (PN or PdN). The effect of these variations on lower urinary tract function is unknown. Also, we cannot establish whether gender contributed to the anatomical variations observed in this study examining only female dogs. Perhaps, mongrel dogs have more variability in contributions to nerves of the lower urinary tract than previously reported. Significant anatomical variations have been observed in the upper part of the left ventricle of the heart in mongrel dogs, compared with German Shepherd dogs (Ozkaya et al., 2013). Mongrel dogs also have larger inferior right ventricles than German Shepherds, which have larger middle right ventricles than mongrel dogs (Ozkaya et al., 2013). Thus, at least with regard to heart and genitourinary structures, cross-bred/mongrel dogs may have more anatomical variations than purebred dogs.
Our observation of a predominant input from L4 ventral rami to the GFN nerve in female mongrel dogs differs from that published previously by Fletcher (1970), who described the origin of this nerve as mainly from L4 ventral rami in male dogs, but from L3 in female dogs. Varied contributions to the GFN have been reported previously: contributions from L3 ventral rami only (Goss, 1960), from L3 and L4 in 10 of 13 dogs (83%) and from L2 and L3 in 3 dogs (25%) (Bailey et al., 1988). This nerve has been used as a nerve graft in human patients with prostate cancer to optimize recovery of penile erectile potency and possibly continence (Nelson et al., 2006) and in our dog model of bladder reinnervation to restore detrusor muscle function after denervation (Ruggieri et al., 2008a,b). Understanding the frequency of variations in contributions to the GFN would aid its use as a nerve graft.
We were unable to find any previous reports on anatomical variations in the path of the GFN and PN to their end organs in dogs. However, variations in the PdN were observed in this study. We observed an anomalous branch from the PdN to the superficial gluteus muscle, a finding that has not been previously described in humans or dogs. We also observed a lack of a main PdN trunk in one dog. The variant matches the PdN Type V variant described by Olszewski in a study of 50 human fetuses (Olszewski, 1982) in which there is only one four-trunked PdN: one trunk is the caudal rectal nerve, a second is the dorsal nerve of the clitoris/penis, a third is the superficial perineal nerve and the fourth is the deep perineal nerve. Furthermore, the presence of fluorogold retrogradely labelled neuronal cell bodies in the ventral horn of spinal cord sections following injection in the detrusor muscle (Fig. 1) is a consistent finding from our group (Ruggieri et al., 2006, 2008a,b, 2011). These findings combined suggest that there are differences in the neuroanatomy of the PdN from those previously reported (Olszewski, 1982; Evans, 2007; Evans and DeLahunta, 2010) that surgeons need to be aware of during surgery to the vertebral or perineal regions.
Lastly, a study by Woźniak in 1966 showed variable fusions between left and right sides of the sympathetic ganglia at lower lumbar and sacral segments in dogs, cats and humans (Woźniak, 1966), as did Dyce in 1958 in horses (Dyce, 1958). Mizeres postulated that these were not “fusions”, but were single “impar” ganglia (Mizeres, 1955). We did not observe any fused or impar ganglia. Instead, we observed intra- and inter-segmental communicating fibres between ganglia in over one-third of specimens. Some ganglia were in close proximity; our investigation of unfixed tissues allowed us to discern the presence of communicating fibres connecting the ganglia. In one specimen, we also observed multisegmental connectivity between left- and right-side sympathetic ganglia (Fig. 1c). Perhaps, these anatomical variations of sympathetic ganglia underlie losses in urinary continence following vertebral column decompression surgery in dogs (Umeda et al., 1987; Cho et al., 2005; Zhang et al., 2009).
Limitations of this study include a small sample size of 16 dogs and the study of only female dogs. We are not reporting variations in sensory innervation to the genitourinary organs in this study, as our main goal was to examine the gross anatomical variations in neural structures and gross dissections does not discern sensory versus motor innervation. The results of retrograde-labelling studies are beyond the scope of this study. One strength of our study is the examination of mongrel hound dogs of similar size, as this is the first study of its type.
In conclusion, we observed high variability in the anatomy of the lumbosacral plexus, with a predominant mixed classification type for individual nerves to the genitourinary organs within a single plexus and when comparing left versus right plexus within a specimen, rather than pure pre-, median- or post-fixed type plexus. This finding highlights the need to readdress the use of this classification schema. The ventral rami of spinal nerves contributing to PN and PdN origin showed greater contributions from lower lumbar segment (L7) than previously reported and a contribution from a coccygeal segment (CG1) in one specimen. We suspect this variability is due to the mongrel (mixed breed) dogs studied here, rather than the purebred dogs used in prior studies. We also observed an anomalous branch from the PdN to the superficial gluteus muscle, a novel finding. We also observed inter-, intra- and even multisegmental connectivity in lumbosacral sympathetic ganglia. It is likely that each of these anatomical variations affects functional recovery, or lack thereof (i.e. urinary and faecal incontinence), in neurogenic syndromes and following spinal cord or nerve root injury.
Acknowledgments
Source of funding
The project described was supported by Award Number NS070267 to MRR and MFB, and NS070267-S1 to SG from the National Institute of Neurological Disorders and Stroke. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Footnotes
Conflict of interests
The authors have no financial conflict of interests or disclaimers to declare.
References
- Bailey C, Kitchell R, Haghighi S, Johnson R. Spinal nerve root origins of the cutaneous nerves of the canine pelvic limb. Am. J. Vet. Res. 1988;49:115. [PubMed] [Google Scholar]
- Cho HM, Lee DY, Sung SW. Anatomical variations of rami communicantes in the upper thoracic sympathetic trunk. Eur. J. Cardiothorac. Surg. 2005;27:320–324. doi: 10.1016/j.ejcts.2004.10.057. [DOI] [PubMed] [Google Scholar]
- Dyce KM. The splanchnic nerves and major abdominal ganglia of the horse. J. Anat. 1958;92:62–73. [PMC free article] [PubMed] [Google Scholar]
- Evans JP. Observations on the nerves of supply to the bladder and urethra of the cat, with a study of their action potentials. J. Physiol. 1936;86:396–414. doi: 10.1113/jphysiol.1936.sp003375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans HE. Miller’s Anatomy of the Dog. Philadelphia, PA: Elsevier Science Health Science Division; 2007. [Google Scholar]
- Evans HE, DeLahunta A. Guide to the Dissection of the Dog. 7th edn. St. Louis, MO: Saunders/Elsevier; 2010. [Google Scholar]
- Fletcher T. Lumbosacral plexus and pelvic limb myotomes of the dog. Am. J. Vet. Res. 1970;31:35–41. [PubMed] [Google Scholar]
- Goss CM. Topographical anatomy of the dog. By O. Charnock Bradley. revised by Tom Grahame. The Macmillan Co., New York. 1959. Anat. Rec. 1960;138:79. [Google Scholar]
- Houghton Mifflin Company. The American Heritage dictionary of the English language. 5th edn. Boston: Houghton Mifflin Harcourt; 2011. [Google Scholar]
- Merriam-Webster Inc. The Merriam-Webster Dictionary. Springfield, Mass: Merriam-Webster; 2005. [Google Scholar]
- Mihelić D, Slavica A, Deždek D, Trbojević-Vukičević T, Džaja P, Majić-Balić I. N. Femoralis, N. Obturatorius and N. Ischiadicus: Deviation in Creation in the Dogs. Anat. Histol. Embryol. 2007;36:401–407. doi: 10.1111/j.1439-0264.2007.00784.x. [DOI] [PubMed] [Google Scholar]
- Mizeres NJ. The anatomy of the autonomic nervous system in the dog. Am. J. Anat. 1955;96:285–318. doi: 10.1002/aja.1000960205. [DOI] [PubMed] [Google Scholar]
- Nelson BA, Chang SS, Cookson MS, Smith JA. Morbidity and efficacy of genitofemoral nerve grafts with radical retropubic prostatectomy. Urology. 2006;67:789–792. doi: 10.1016/j.urology.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Oliver J, Bradley W, Fletcher T. Spinal cord distribution of the somatic innervation of the external urethral sphincter of the cat. J. Neurol. Sci. 1970;10:11–23. doi: 10.1016/0022-510x(70)90088-2. [DOI] [PubMed] [Google Scholar]
- Olszewski J. Variations of the pudendal nerve in man. Folia Morphol. 1982;41:245. [PubMed] [Google Scholar]
- Ozkaya G, Ozyigit G, Ercan I, Arican I. Comparing the Hearts of German Shepherd and Mongrel Dogs Using Statistical Shape Analysis. Pak. Vet. J. 2013;33:187–190. [Google Scholar]
- Purinton P, Oliver J. Spinal cord origin of innervation to the bladder and urethra of the dog. Exp. Neurol. 1979;65:422–434. doi: 10.1016/0014-4886(79)90109-2. [DOI] [PubMed] [Google Scholar]
- Ruggieri MR, Braverman AS, D’Andrea L, Simpkiss B, Kozin SH, Pontari MA, Betz R, Barbe MF. Functional reinnervation of the canine bladder after spinal root transection and immediate end-on-end repair. J. Neurotrauma. 2006;23:1125–1136. doi: 10.1089/neu.2006.23.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggieri MR, Braverman AS, D’Andrea L, Betz R, Barbe MF. Functional reinnervation of the canine bladder after spinal root transection and genitofemoral nerve transfer at one and three months after denervation. J. Neurotrauma. 2008a;25:401–409. doi: 10.1089/neu.2007.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggieri MR, Braverman AS, D’Andrea L, McCarthy J, Barbe MF. Functional reinnervation of the canine bladder after spinal root transection and immediate somatic nerve transfer. J. Neurotrauma. 2008b;25:214–224. doi: 10.1089/neu.2007.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggieri MR, Sr, Braverman AS, Bernal RM, Lamarre NS, Brown JM, Barbe MF. Reinnervation of urethral and anal sphincters with femoral motor nerve to pudendal nerve transfer. Neurourol. Urodyn. 2011;30:1695–1704. doi: 10.1002/nau.21171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanagho EA, Schmidt RA, Gomes de Araujo C. Urinary striated sphincter: what is its nerve supply? Urology. 1982;20:415–417. doi: 10.1016/0090-4295(82)90468-x. [DOI] [PubMed] [Google Scholar]
- Umeda S, Arai T, Hatano Y, Mori K, Hoshino K. Cadaver anatomic analysis of the best site for chemical lumbar sympathectomy. Anesth. Analg. 1987;66:643–646. [PubMed] [Google Scholar]
- Webb AA, Ngan S, Fowler D. Spinal cord injury II: prognostic indicators, standards of care, and clinical trials. Can. Vet. J. 2010a;51:598. [PMC free article] [PubMed] [Google Scholar]
- Webb AA, Ngan S, Fowler JD. Spinal cord injury I: a synopsis of the basic science. Can. Vet. J. 2010b;51:485. [PMC free article] [PubMed] [Google Scholar]
- Woźniak W. Sacral segments of the sympathetic trunks in the dog, cat, and man. Folia Morphol. 1966;25:433. [PubMed] [Google Scholar]
- Yoshimura N, De Groat WC. Neural control of the lower urinary tract. Int. J. Urol. 1997;4:111–125. doi: 10.1111/j.1442-2042.1997.tb00156.x. [DOI] [PubMed] [Google Scholar]
- Zhang B, Li Z, Yang X, Li G, Wang Y, Cheng J, Tang X, Wang F. Anatomical variations of the upper thoracic sympathetic chain. Clin. Anat. 2009;22:595–600. doi: 10.1002/ca.20803. [DOI] [PubMed] [Google Scholar]