SUMMARY
Epstein-Barr virus (EBV) and Kaposi’s sarcoma-associated herpesvirus (KSHV) are two γ-herpesviruses identified in humans and are strongly associated with the development of malignancies. Murine γ-herpesvirus (MHV-68) is a naturally occurring rodent pathogen, representing a unique experimental model for dissecting γ-herpesvirus infection and the immune response. These γ-herpesviruses actively antagonize the innate and adaptive antiviral responses, thereby efficiently establishing latent or persistent infections, and even promoting development of malignancies. In this review, we summarize immune evasion strategies of γ-herpesviruses. These include suppression of MHC-I- and MHC-II-restricted antigen presentation, impairment of dendritic cell functions, downregulation of costimulatory molecules, activation of viral-specific regulatory T cells, and induction of inhibitory cytokines. There is a focus on how both γ-herpesvirus-derived and host-derived immunomodulators interfere with adaptive antiviral immunity. Understanding immune-evasive mechanisms is essential for developing future immunotherapies against EBV- and KSHV-driven tumors.
Keywords: Immune escape, γ-herpesviruses, EBV, KSHV, MHV-68, viral immunomodulators
INTRODUCTION
Persistent viral infection depends on the balance between host immune responses and viral immune evasion. To establish a persistent infection efficiently, viruses have evolved a number of strategies to avoid immune elimination. These include: hiding in certain cell types to escape encounters with the immune system; inhibiting their replication to limit antigen production; subverting cytokine-mediated intercellular communication; and increasing the generation of viral mutants. Latency is a successful mechanism of evasion for all herpesviruses, allowing them to survive in their hosts for a lifetime. Two γ-herpesviruses have been identified in humans: Epstein-Barr virus (EBV) [1] and Kaposi’s sarcoma-associated herpesvirus (KSHV) [2]. EBV and KSHV are strongly associated with a range of malignancies, mostly in immune suppressed populations [3].
EBV, also called human herpesvirus 4 (HHV-4), belongs to the genus Lymphocryptovirus. Its genome, ~172 kbp [4], encodes 86 proteins [5]. EBV undergoes lytic replication mainly in epithelial cells of the oropharynx, subsequently establishes latent infection in B cells, and can reactivate periodically from latency. EBV is an orally transmitted ubiquitous virus and infects more than 90% of the adult population worldwide. In healthy individuals, EBV can cause infectious mononucleosis, and is a significant risk factor for Burkitt’s lymphoma and nasopharyngeal carcinoma. In AIDS and other immunocompromised patients, EBV is directly associated with the development of malignancies, such as non-Hodgkin’s lymphoma, CNS lymphomas and post-transplant lymphoproliferative disease [6].
KSHV, also called human herpesvirus 8 (HHV-8), belongs to the genus Rhadinovirus. Like EBV, its linear dsDNA consists of 160 to 170 kbp and is stably maintained in latently infected B cells [7]. Its genome contains a central unique region of 140.5 kbp with at least 81 ORFs [8]. KSHV, a unique human tumor virus, has pirated numerous genes from host cells, which may help the virus to escape from the immune system and provoke host cell proliferation [9]. This virus can be transmitted both sexually and through body fluids, such as saliva [10], in addition to bloodborne transmission, which is important in the setting of intravenous drug abuse. The prevalence of KSHV infection is not as high as EBV and is greatly dependent on regional and social factors, such as intravenous drug use. Prevalence ranges from 2 to 40% in the general population, 20 to 50% in HIV-infected individuals without Kaposi’s sarcoma, and over 90% in patients with Kaposi’s sarcoma and other KSHV associated diseases [11]. KSHV is etiologically linked to the development of Kaposi’s sarcoma, primary effusion lymphoma and multicentric Castleman’s disease [10].
Murine γ-herpesvirus (MHV-68) is a naturally occurring rodent pathogen, originally isolated from two species of free living small rodents: Apodemus flavicollis (yellow-necked-mouse) and Clethrionomys glareolus (bank vole) [12]. Later studies indicated this virus is endemic in wood mice (Apodemus sylvaticus) in the UK, suggesting this may be the natural host species [13]. MHV-68 belongs to the genus Rhadinovirus and is genetically closely related to EBV and KSHV [14, 15]. Its genome contains a region of ~118 kbp with at least 80 genes, of which more than 60 ORFs are homologous to those of KSHV [15]. MHV-68 initially replicates in the lung after intranasal infection [16] and then establishes lifelong latency, primarily in B cells [17], but also in dendritic cells (DCs), macrophages [18] and lung epithelial cells [19]. MHV-68 provides an important model to explore the γ-herpesvirus infections and host responses [20-26].
These γ-herpesviruses evade the immune response by producing certain viral proteins and non-coding RNAs. Gammaherpesvirus-derived immunomodulators can interfere with antigen presentation, attenuate IFN signaling, alter host chemokine networks, inhibit complement-mediated effector activity, and block apoptotic and autophagic pathways. These help viral escape from innate and adaptive antiviral responses [27-32]. In addition, suppression of antiviral responses by regulatory T cells (Tregs) is a host factor associated with viral persistence and the development of malignancies [33]. In this review, we summarize how γ-herpesviruses escape from adaptive immunity by releasing viral immunomodulators and by manipulating the immunological environment of the host.
INTERFERENCE WITH MHC CLASS I-RESTRICTED ANTIGEN PRESENTATION
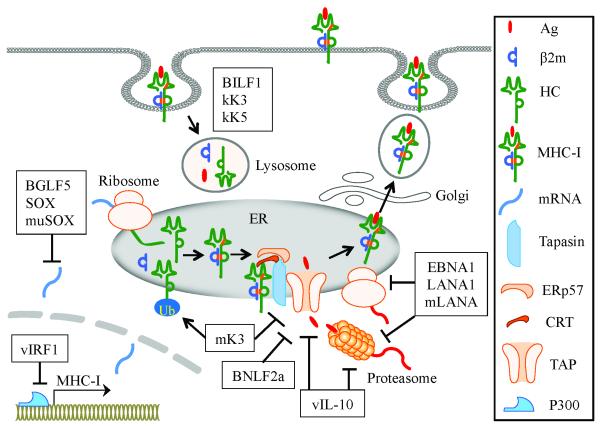
CD8+ T cells play a critical role in the control of viral infections by recognizing viral peptides presented by MHC class I (MHC-I) molecules. Downregulation of MHC-I expression on the cell surface is an important strategy for γ-herpesviruses to avoid being recognized by CD8+ T cells. Antigenic peptides are generated in the cytosol by the proteasome, shuttled into the ER and loaded onto newly synthesized MHC-I molecules. This process is controlled by the peptide-loading complex (PLC), which contains TAP (TAP1 and TAP2, transporters associated with antigen processing), tapasin (a transmembrane glycoprotein), ERp57 (an oxidoreductase) and CRT (calreticulin, a glycoprotein chaperone) [34]. The peptide-MHC-I complex is transported to the cell surface where it will be recognized by CD8+ T cells. Gammaherpesviruses effectively interfere with the adaptive antiviral response by producing immunomodulators that act at various stages during MHC-I-mediated antigen presentation (Figure 1).
Figure 1.
Gammaherpesviruses inhibit MHC-I-restricted antigen presentation. vIRF1 interacts with the transcriptional coactivator p300 and thereby interferes with the MHC-I transcription. BGLF5, SOX and muSOX promote the degradation of host mRNAs and shutoff of MHC-I gene expression. EBNA1, LANA1 and mLANA escape being processed by the proteasome and inhibit their own translation. BNLF2a shuts down TAP-mediated import of antigenic peptides. mK3 directs immature MHC-I molecules to the cytosol and also degrades TAP and tapasin. BILF1, kK3 and kK5 trigger endocytosis of cell surface MHC-I molecules and promote their lysosomal degradation. vIL-10 downregulates TAP1 and bli/LMP2 (a subunit of proteasome) at the mRNA level.
Inhibition of MHC-I molecule transcription by KSHV vIRF1
Activation of the IFN-mediated antiviral pathway is one of the major defensive strategies for the host immune system. KSHV-encoded IFN regulatory factors 1 to 4 (vIRF1 to 4) are homologous to cellular IRFs. Generally, the vIRFs function as negative regulators for the antiviral response and apoptosis mediated by cellular IRFs [35]. vIRF1 and vIRF3 contribute to viral immune evasion by interfering with MHC-I and MHC-II antigen presentation pathways, respectively. vIRF1 (KSHV k9) functions as a repressor of IFN-mediated signal transduction, inhibiting the expression of IFN-inducible genes by blocking IRF1- and IRF3-mediated transcription. vIRF1 downregulates basal and IFN-induced MHC-I transcription in lymphatic endothelial cells through its interaction with the transcriptional coactivator p300 [35]. Moreover, vIRF1 can antagonize the upregulation of MHC-I transcription induced by KSHV-encoded vFLIP (viral FLICE inhibitory protein) [36].
Degradation of cellular mRNA by KSHV SOX, EBV BGLF5 and MHV-68 muSOX
Viral infection often results in a global shutoff of host cellular gene expression, which confers a selective advantage to the virus by facilitating redirection of cellular resources toward virus replication and by dampening immune stimulatory signals. SOX, a shutoff factor for host gene expression, is encoded by KSHV ORF37 and named after its functions shutoff and exonuclease [37]. SOX induces host mRNA degradation by hyperadenylation of the transcripts, thereby retaining host mRNAs in the nucleus. SOX also induces relocalization of cytoplasmic poly(A)-binding proteins into the nucleus, and these proteins are important for stability and translation of cytoplasmic mRNAs. Therefore, SOX may prevent nascent mRNA export and deplete preexisting cytoplasmic mRNAs [38, 39]. BGLF5, an EBV-derived DNase, displays a high degree of homology to KSHV SOX at the amino acid level. BGLF5 can promote mRNA degradation and induce host MHC-I gene shutoff [40]. Both BGLF5 and SOX have also been reported to have intrinsic RNase activity [41, 42]. Impairment of endogenous antigen recognition by virus-specific CD8+ T cells is observed following expression of BGLF5 or SOX [43]. MuSOX, a SOX homolog from MHV-68, mimics all known activities of SOX and BGLF5, underscoring functional conservation within the γ-herpesviruses [44].
Retardation of antigen processing by EBV EBNA1, KSHV LANA1 and MHV-68 mLANA
The proteasome is a protein complex that functions to degrade unneeded or damaged proteins by proteolysis. The ubiquitin-proteasome system degrades intracellular proteins into peptide fragments that can be presented by MHC-I molecules [45]. Some viral proteins employ effective strategies to escape from CD8+ T cell recognition by self-inhibition of antigen processing. EBNA1 (Epstein-Barr nuclear antigen 1), a virus episome maintenance protein, regulates the replication of episomes in synchrony with cellular DNA, allowing proper segregation during cell division [46]. EBNA1-specific CD8+ T cell responses are present in the majority of EBV-infected healthy individuals, but the number of EBNA1-specific CD8+ T cells is significantly decreased in nasopharyngeal carcinoma patients [47]. Loss of EBNA1-specific memory CD8+ and CD4+ T cells are also observed in patients progressing to AIDS-related non-Hodgkin lymphoma [48]. These data suggest that EBNA1-specific T-cell immunity is important for control of EBV-associated malignancies. EBNA1 restricts its own proteasomal degradation. A glycine-alanine repeat (GAr) domain of EBNA1 plays an important role in interfering with antigen processing by proteasome and MHC-I-restricted presentation [49, 50]. In addition, the GAr domain also regulates EBNA1 synthesis by inhibiting its own translation [51].
KSHV LANA (latency-associated nuclear antigen) tethers viral DNA to chromosomes during mitosis to ensure correct partition of the viral genome into progeny cells [52]. It also plays a critical role in maintaining latency by repressing Rta (replication and transcription activator)-mediated transactivation, resulting in decreased lytic cycle replication [53]. Although LANA1 does not share amino acid homology with EBNA1, the QED central repeat domain of LANA1 has similar function in immune evasion by inhibiting its own proteasomal degradation and retarding its own translation [54]. mLANA encoded by MHV-68 ORF73 is homologous to KSHV LANA [15], and is involved in the establishment and maintenance of latency [55]. Similar to EBNA1 and LANA1, mLANA is also associated with inhibition of its own protein degradation and synthesis [56].
Impairment of peptide-MHC-I assembly by MHV-68 mK3 and EBV BNLF2a
MHC-I molecule consists of two polypeptide chains, heavy α chain (HC) and β2-microglobulin (β2m). MHV-68-encoded K3 (mK3) protein is a homolog of KSHV-encoded K3 and K5 (kK3 and kK5), having E3 ubiquitin ligase activity. kK3 and kK5 induce rapid endocytosis and lysosomal degradation of cell surface glycoproteins (see next section). In contrast, mK3 is predominantly expressed in the ER membrane where it ubiquitinates the cytoplasmic tails of newly synthesized H-2Db glycoproteins for proteasomal degradation. This leads to the rapid degradation of nascent MHC-I molecules [57]. mK3 can also degrade the TAP and tapasin [58]. The mK3-induced reduction of MHC-I expression is sufficient to block antigen presentation [59]. However, mK3 fails to interfere with the assembly of MHC-I molecule in TAP/tapasin deficient cells, suggesting that the ubiquitination is dependent on TAP/tapasin [60].
BNLF2a, an EBV lytic cycle protein, inhibits TAP-mediated import of antigenic peptides into the ER by suppressing the binding of both ATP and peptide to TAP [61]. BNLF2a is a membrane-associated protein, composed of two specialized domains. Its C-terminal tail anchor mediates membrane integration and ER retention, and its cytosolic N-terminus inhibits TAP function [62].
Interference with cell surface expression of peptide-MHC-I complexes by EBV BILF1 and KSHV K3/K5
Once antigenic peptide is loaded onto MHC-I molecule, the peptide-MHC-I complex dissociates from the PLC and then leaves the ER to the cell surface. EBV-encoded BILF1, a lytic cycle protein, is a G-protein coupled receptor and localizes predominantly in the plasma membrane [63]. BILF1 can downregulate the expression of MHC-I molecules on the cell surface by enhancing their endocytosis and promoting lysosomal degradation [64]. BILF1 selectively downregulates cell surface expression of HLA-A, -B and -E, while HLA-C expression is less affected [65]. kK3 and kK5 (also termed MIR1 and MIR2, modulator of immune recognition) are two KSHV-encoded membrane-bound E3 ubiquitin ligases [66]. kK3 and kK5 lead to ubiquitination of the cytosolic tail of MHC-I molecules, thus enhancing their endocytosis from the cell surface. Subsequently, the internalized MHC-I molecules are delivered to endolysosomal vesicles where they undergo degradation [67, 68]. kK3 and kK5 display different specificities in downregulation of HLA alleles. kK3 downregulates the expression of HLA-A, -B, -C and -E, whereas kK5 downregulates HLA-A and -B significantly, HLA-C weakly, but not HLA-E [69].
INTERFERENCE WITH MHC CLASS II-RESTRICTED ANTIGEN PRESENTATION
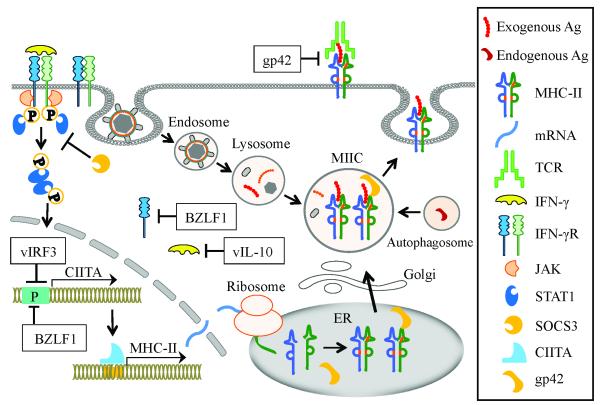
CD4+ T helper cells are important for the induction and maintenance of effective CD8+ T cell response and humoral immunity by recognizing antigens presented by MHC-II molecules [70]. Generally, peptide antigens presented by MHC-II are derived from exogenous proteins that are endocytosed, degraded in lysosomes, then delivered to the MHC-II loading compartment (MIICs) [71]. In addition, endogenous antigens can be presented through autophagy by the fusion of autophagosomes with MIICs [72]. These antigenic peptides in MIICs are loaded onto MHC-II molecules and the peptide-MHC-II complexes are then transported to the cell surface where they will be recognized by CD4+ T helper cells. Gammaherpesviruses encode a variety of immunomodulators to interfere with MHC-II-restricted antigen presentation (Figure 2).
Figure 2.
Gammaherpesviruses inhibit MHC-II-restricted antigen presentation. SOCS3 expression is upregulated by KSHV infection, which inhibits IFN-γ-induced phosphorylation of STAT1, thereby downregulating the transactivation of CIITA gene. BZLF1 and vIRF3 bind to the promoters of CIITA genes and thus interfere with its transactivation. BZLF1 reduces IFN-γR α expression at both the mRNA and protein level, and in that way shuts down the entire IFN-γ-induced CIITA signaling cascade. gp42 blocks TCR and MHC-II engagement. vIL-10 reduces IFN-γ synthesis and inhibits both basal and IFN-γ-induced expressions of MHC-II.
Attenuation of MHC-II expression by KSHV vIRF3 and EBV BZLF1
As a master regulator, the class II transactivator (CIITA) promotes expression of MHC-II molecules at the transcriptional level [73]. The CIITA gene is under the control of several promoters. Promoter I (PI) and PIII direct the specific constitutive expression of CIITA with a preferential use in DCs and B cells, respectively. In contrast, PIV mediates the IFN-γ-inducible expression of CIITA [74]. STAT1 and IRF1 are required for the function of PIV [75].
SOCS (suppressor of cytokine signaling) can inhibit the JAK/STAT signaling pathway, and SOCS1 and SOCS3 play important roles as regulators of adaptive immunity [76]. KSHV infection triggers transcriptional upregulation of SOCS3 in human endothelial cells, which results in a reduction of HLA-DR at the transcriptional level. This is because SOCS3 inhibits IFN-γ-induced phosphorylation of STAT1 and transcription of CIITA [77]. vIRF3 (also called LANA2) is expressed in latently infected primary effusion lymphoma cells functioning as an oncogene and is required for the cell survival [78]. vIRF3 downregulates the surface expression of MHC-II on KSHV-infected lymphoma cells by inhibiting the activity of CIITA PIV and PIII [79]. In addition, vIRF3 interacts with cellular IRF5, thereby blocking IRF5-mediated IFN promoter activation and interfering with IRF5-mediated regulation of cell proliferation. This contributes to immune evasion and sustained proliferation of primary effusion lymphoma cells [80, 81].
BZLF1 (BamH fragment Z left frame 1, also called Zta, ZEBRA or EB1), the EBV immediate-early lytic protein, is a transcription factor that activates lytic cycle cascade leading to switch from latency to lytic cycle [82]. BZLF1 reduces IFN-γ receptor α (IFN-γRα) expression at both the mRNA and protein levels, and thus reduces IFN-γ-induced MHC-II expression [83]. BZLF1 also inhibits transcription of the MHC-II gene by binding to and repressing CIITA PIII [84]. In addition, BZLF1 downregulates surface CD74 (a chaperone for MHC-II antigen presentation) post-transcriptionally [85].
Blockade of TCR and MHC-II engagement by EBV gp42
EBV gp42 (also called BZLF2), a lytic-phase protein, functions as an entry mediator in a complex with glycoproteins gH and gL during B cell infection [86]. gp42 triggers fusion of the EBV envelope with the host cell membrane by binding to MHC-II molecules on the surface of B cells [87]. gp42 has two forms, full length type II membrane protein and a shorter soluble protein, and both of them can bind to immature MHC-II protein or peptide-MHC-II complex. gp42 does not affect peptide-loading on MHC-II molecules, but can block the interaction of TCR with peptide-MHC-II complex, thereby inhibiting antigenic recognition by CD4+ T helper cells [88, 89].
Limited presentation of EBNA1 antigen to CD4+ T helper cells
EBNA1 can be processed and loaded onto MHC-II molecules as an endogenous antigen [90], and this progress requires autophagy [91]. EBNA1 is predominantly located in the nucleus, which restricts its accessibility to the autophagy pathway. In consequence, limited EBNA1 epitopes are presented on the surface of infected cells for CD4+ T cell recognition [92].
IMPAIRMENT OF DC FUNCTIONS AND DOWNREGULATION OF COSTIMULATORY MOLECULES
DCs are professional antigen-presenting cells (APCs) and are essential for the initiation of immune responses as messengers between innate and adaptive immunity [93]. DCs are very efficient at antigen processing for both MHC-I and -II pathways by capturing antigens in infected tissues and then migrating to lymphoid organs to prime T cells. Interference with the function of DCs is a common feature of γ-herpesviruses. EBV inhibits the development of DCs by promoting apoptosis of their monocyte precursors, leading to reduced numbers of mature DCs [94]. Monocyte-derived DCs in patients with Kaposi’s sarcoma have been shown to be functionally impaired [95]. Cytokines including IL-10, released by primary effusion lymphoma cells, can suppress DC differentiation [96]. KSHV inhibits monocyte differentiation into DCs [97] and reduces DC migration by downregulating CCR6 and CCR7 expression on the cell surface and by inducing cytoskeleton modifications [98]. KSHV also downregulates DC-SIGN (dendritic cell-specific ICAM-3 grabbing nonintegrin), a receptor for virus attachment, thereby decreases endocytic activity of DCs and inhibits antigen-specific activation of CD8+ T cells [99].
Costimulation provided by APCs as signal 2 is essential to induce full activation of T cells and prevent them from becoming refractory to antigenic stimulation. The most important costimulatory pathways include CD28/CD80-CD86, CD40L/CD40, CD27/CD70, 4-1BB/4-1BBL and OX-40/OX-40L [100]. The CD28/CD80-CD86 pathway is crucial in the antiviral CD8+ T cell response and immune surveillance against MHV-68 [101]. Interaction of ICAM-1 (intercellular adhesion molecule 1) with LFA-1 (lymphocyte function-associated antigen 1) also provides an important costimulatory signaling for T cell activation [102]. Viruses have evolved the ability to downregulate these costimulatory molecules. kK5 can downregulate CD86 and ICAM-1 expression on B cells by inducing their endocytosis and degradation, thereby impairing the ability to activate T cells [103]. Moreover, the kK5-triggered downregulation of CD86 and ICAM-1 expression on the BJAB (a B lymphoma cell line) results in a reduced natural killer (NK) cell-mediated cytotoxicity [104]. kK5 also protects KSHV-infected cells against NK cytotoxicity by decreasing expression of the ligands for NK cell activating receptors, MICA (MHC class I polypeptide-related sequence A), MICB and AICL (activation-induced C-type lectin) [105]. This strategy is very important for evasion from NK cytotoxicity because downregulation of MHC-I molecules renders KSHV-infected cells sensitive to NK-mediated recognition and killing.
Unlike many viral infections, MHV-68 infection does not upregulate the expression of CD80, CD86, MHC-I, and MHC-II molecules on immature DCs, indicating that MHV-68 cannot induce DC maturation. Furthermore, LPS and poly I:C stimulations fail to promote the expression of these molecules on MHV-68-infected immature DCs, suggesting that MHV-68 can block DC maturation [106].
REGULATORY T CELLS INDUCED BY γ-HERPESVIRUS INFECTIONS
Tregs play an important role in the maintenance of immunologic homeostasis by suppressing immune responses in infection and autoimmunity [107]. Tregs are composed of phenotypically and functionally distinct populations, and their differentiation and function are controlled by specific signals in the immune environment. Tregs suppress immune responses by several mechanisms, including direct cell-cell contact, modulation of APC functions, and production of anti-inflammatory cytokines such as IL-10, TGF-β and IL-35 [108]. Consequently, Tregs can play a dual role in infectious diseases by preventing immunopathology and impeding pathogen clearance.
EBV-specific Tregs
Enhanced number and function of Tregs are observed in many disease conditions associated with the γ-herpesviruses. CD4+CD25highFoxp3+ Tregs increase in the peripheral blood of EBV positive patients with nasopharyngeal carcinoma and suppress the proliferation of autologous CD4+CD25- T cells [109]. Reactivation of EBV-specific CD8+ T cells in transplant patients results in an expansion of CD8+ Tregs that show upregulated Foxp3 expression, produce both IL-10 and IFN-γ, and suppress proliferation of noncognate CD4+ T cells via cell-cell contact [110]. CCR4+Foxp3+ Tregs are abundantly present in the infiltrating cells in age-related EBV-positive B cell lymphoproliferative disorder [111]. EBNA1 upregulates production of the chemokine CCL20 by EBV positive Hodgkin’s lymphoma cells and thereby increases the migration of CD4+Foxp3+ Tregs [112]. EBNA1 can induce both Th1 and CD4+CD25+Foxp3+GITR+ Tregs in vitro, and the Tregs display suppressive activity by cell-cell contact or partially by soluble inhibitory factors [113]. Taken together, these EBV-specific Tregs may be associated with immune escape of tumor cells and affect the clinical progression of malignancies. In contrast, it has also been reported that an increase in CD4+CD25+FOXP3+ Tregs correlates with EBV infection, but has no impact on survival in patients with classical Hodgkin lymphoma [114].
MHV-68-specific Tregs
CD8+ Tregs can be induced in a range of different systems and exhibit different phenotypes and functions. Naturally occurring CD8+CD122+ Tregs mediate suppression through IL-10 [115]. HIV-specific IL-10-positive CD8+ Tregs mediate suppression through direct cell-cell contact [116]. In MHV-68-infected mice, IL-10-producing CD8+ T cells account for 1% of total CD8+ T cells, of which around 10% are specific to ORF61524-531, a dominant epitope of MHV-68. In the absence of CD4+ T cell help, a similar situation to AIDS patients, the population of IL-10-producing CD8+ T cells increases up to 4.5% of total CD8+ T cells during the chronic phase of MHV-68 infection, and these cells function as Tregs [117]. IL-10-producing CD8+ Tregs are partially responsible for erosion in immune surveillance against MHV-68, which leads to spontaneous viral reactivation in the lungs of CD4+ T cell-depleted mice [118]. The CD8+ Tregs suppress proliferation of naïve CD8+ T cells in vitro in a cell contact-independent manner, and the suppression cannot be antagonized by blockade of IL-10R, suggesting the existence of suppressive factors besides IL-10 [117]. Interestingly, it is also reported that MHV-68 can reduce the frequency and activity of naturally occurring CD4+CD25+ Tregs following infection [119].
INDUCTION AND PRODUCTION OF IL-10 IN γ-HERPESVIRUS INFECTIONS
IL-10 plays a pivotal role in controlling inflammation by suppressing the functions of APCs and T cells and the production of inflammatory cytokines [120]. Accordingly, IL-10 plays a dual role in infectious disease. In acute virus infections, such as influenza virus, effector CD8+ and CD4+ T cells attenuate lung inflammation by producing IL-10, thus preventing immunopathology [121]. In persistent virus infections, such as HIV, IL-10 inhibits virus-specific T cells, thereby impeding pathogen control [122]. It has been demonstrated that γ-herpesvirus infections can induce IL-10 production. The percentage of IL-10-expressing cells in EBV-positive cases of Hodgkin’s disease is significantly higher than in EBV-negative cases [123]. B cell lines derived from EBV positive Burkitt’s lymphoma, especially those from patients with AIDS, constitutively secrete large quantities of IL-10 [124]. High levels of IL-10 expression are also induced by EBV infection of B lymphocytes [125]. IL-10 and viral IL-6 (vIL-6) have been shown to be autocrine growth factors for autonomous proliferation of KSHV-infected primary effusion lymphoma cell lines [126]. MHV-68 infection also results in increased levels of IL-10 [127]. MHV-68-infected mice show elevated IL-10 expression in DCs, which interferes with the ability of DCs to activate T cells, and this action can be partially reversed by blocking IL-10 [128]. It is notable that IL-10 can be encoded not only by the host cells (cIL-10), but also by some viruses (vIL-10). IL-10-like ORFs have been identified in multiple members of the two families Herpesviridae and Poxviridae. Several γ-herpesviruses, including EBV, rhesus lymphocryptovirus, equine herpesvirus 2 and ovine herpesvirus 2, have been shown to encode IL-10 homologs (vIL-10) [129]. The acquisition of IL-10-like sequences likely equips the viruses with a powerful tool to modulate host immune function.
cIL-10 induced by γ-herpesvirus-derived immunomodulators
LMP1 (latent membrane protein 1) is a key factor for EBV-mediated transformation of primary B cells and plays multiple roles in EBV induced immune escape [130]. LMP1 induces high levels of IL-10 secretion by CD4+ T cells from EBV sero-positive individuals, and thus inhibits T cell proliferation and IFN-γ secretion induced both by mitogen and recall antigen [131]. LMP2A can upregulate IL-10 production in mitogen-stimulated primary B cells and B cell lymphomas, and the increased IL-10 promotes the survival of these cells [132]. BZLF1 activates both transcription and translation of human IL-10 (hIL-10) in B cells by binding directly to specific DNA sequences in the hIL-10 promoter [133]. EBERs are EBV-encoded non-coding RNAs with ~170 nucleotides, are expressed at high levels in infected cells, and play a role in the pathogenesis of this infection [134]. EBERs also induce IL-10 expression in Burkitt’s lymphoma cells, and the IL-10 acts as an autocrine growth factor for Burkitt’s lymphoma [135].
MicroRNAs (miRNAs) are a class of short (~20-23 nucleotides), single-stranded and non-coding RNAs that regulate gene expression post-transcriptionally. Gammaherpesvirus-encoded miRNAs and their contribution to pathogenesis and tumorigenesis have recently been reviewed [136]. KSHV encodes over 25 miRNAs, and these miRNAs participate in a highly complex network of interactions with the cellular and viral transcriptomes [137, 138]. Of these, miR-K12-3 and miR-K12-7 can induce the transcription and secretion of IL-10 and IL-6 by macrophages [139].
MHV-68-encoded M2, a latency-associated protein, induces an antigen-specific CD8+ T cell response, which contributes to the control of latency establishment in the infected animal [22]. However, M2 has also been reported to play a role in MHV-68 latency establishment and reactivation in splenic B cells [140]. M2 increases the pool of germinal center B cells by inhibiting apoptosis of the infected cells [141], and suppresses IFN-mediated antiviral activity by downregulating STAT1/2 signaling [142]. In addition, M2 induces B cells to secrete high levels of IL-10, which leads to the proliferation and differentiation of M2-expressing B cells [143].
vIL-10 encoded by EBV
In addition to inducing IL-10 expression by host cells, EBV encodes vIL-10, a product of the lytic phase BCRF-1 gene [144]. vIL-10 shares 84% amino acid identity with hIL-10, while the identity between hIL-10 and murine IL-10 is 73% [145]. vIL-10, together with BNLF2a, contributes to immune evasion during the early phase of infection [146]. vIL-10 downregulates TAP1 and bli/LMP2 (a subunit of the proteasome) at the mRNA level, thereby resulting in a reduction of MHC-I expression [147] (Figure 1). vIL-10 inhibits IFN-γ synthesis by activated mouse Th1 clones and human peripheral blood mononuclear cells [148]. It reduces antigen-specific T cell proliferation by downregulating basal and IFN-γ- or IL-4-induced MHC-II expression on monocytes [149] (Figure 2). Like hIL-10, vIL-10 is also a potent growth factor for activated B cells [150]. However, vIL-10 does not stimulate the proliferation of thymocytes [151] and mast cells [152] and does not upregulate MHC-II on B cells [153]. Substitution of isoleucine at position 87 with alanine (corresponding to the vIL-10 residue) abrogates the immunostimulatory activity of cIL-10 but preserves its immunosuppressive activity [145]. These data suggest that vIL-10 may derive from a captured and mutated cIL-10 gene, and the alteration is beneficial to the virus by disrupting host immune responses.
CONCLUSIONS
Gammaherpesviruses establish persistent infections and trigger the development of malignancies by antagonizing the adaptive antiviral responses in their hosts. Immune evasion mechanisms are associated with the suppression of MHC-I- and MHC-II-restricted antigen presentation, impairment of DC functions, downregulation of costimulatory molecules, activation of viral-specific Tregs, and induction of inhibitory cytokines, such as IL-10. By summarizing the literature above, we aim to gain a clearer picture regarding how γ-herpesviruses escape from adaptive immunity by the concerted actions of γ-herpesvirus-derived immunomodulators and host-derived regulatory factors.
Gammaherpesvirus infections utilize an exceptionally broad array of strategies to evade the immune response, involving multiple viral and host proteins. These are potential targets for drug development, as inhibition of these pathways has the potential to attenuate the infection. However in order to have a significant impact on disease, it may be necessary to target multiple pathways simultaneously. Progress in this area has been hampered by the lack of tractable animal models to study EBV and KSHV infection and immunity in vivo. The MHV-68 model offers a system in which the effects of potential therapies on infection, pathogenesis and the immune response can be monitored very readily. Other factors for consideration are that the outcomes of γ-herpesvirus infections depend not only on the properties of the virus and its immunomodulators, but also on the presence of other pathogens such as HIV and the individual immune response of the host. Accordingly, more in-depth study of immune modulation during γ-herpesvirus infection is necessary, and will likely ultimately lead to new therapeutic approaches for this important class of infection.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants R01 grants R01AI069943 and R01CA103642.
Abbreviations used
- CIITA
class II transactivator
- DC
dendritic cell
- EBNA
Epstein-Barr nuclear antigen
- IRF
IFN regulatory factor
- KSHV
Kaposi’s sarcoma-associated herpesvirus
- LANA
latency-associated nuclear antigen
- MHV-68
murine γ-herpesvirus
- Treg
regulatory T cell
REFERENCES
- 1.Epstein MA, Achong BG, Barr YM. Virus Particles in Cultured Lymphoblasts from Burkitt’s Lymphoma. Lancet. 1964;1:702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- 2.Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 3.Martin D, Gutkind JS. Human tumor-associated viruses and new insights into the molecular mechanisms of cancer. Oncogene. 2008;27(Suppl 2):S31–42. doi: 10.1038/onc.2009.351. DOI: 10.1038/onc.2009.351. [DOI] [PubMed] [Google Scholar]
- 4.Baer R, Bankier AT, Biggin MD, et al. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984;310:207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- 5.Tarbouriech N, Buisson M, Geoui T, Daenke S, Cusack S, Burmeister WP. Structural genomics of the Epstein-Barr virus. Acta Crystallogr D Biol Crystallogr. 2006;62:1276–1285. doi: 10.1107/S0907444906030034. DOI: 10.1107/S0907444906030034. [DOI] [PubMed] [Google Scholar]
- 6.Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350:1328–1337. doi: 10.1056/NEJMra032015. DOI: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- 7.Renne R, Lagunoff M, Zhong W, Ganem D. The size and conformation of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) DNA in infected cells and virions. J Virol. 1996;70:8151–8154. doi: 10.1128/jvi.70.11.8151-8154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russo JJ, Bohenzky RA, Chien MC, et al. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coscoy L. Immune evasion by Kaposi’s sarcoma-associated herpesvirus. Nat Rev Immunol. 2007;7:391–401. doi: 10.1038/nri2076. DOI: 10.1038/nri2076. [DOI] [PubMed] [Google Scholar]
- 10.Ablashi DV, Chatlynne LG, Whitman JE, Jr., Cesarman E. Spectrum of Kaposi’s sarcoma-associated herpesvirus, or human herpesvirus 8, diseases. Clin Microbiol Rev. 2002;15:439–464. doi: 10.1128/CMR.15.3.439-464.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatlynne LG, Ablashi DV. Seroepidemiology of Kaposi’s sarcoma-associated herpesvirus (KSHV) Semin Cancer Biol. 1999;9:175–185. doi: 10.1006/scbi.1998.0089. DOI: 10.1006/scbi.1998.0089. [DOI] [PubMed] [Google Scholar]
- 12.Blaskovic D, Stancekova M, Svobodova J, Mistrikova J. Isolation of five strains of herpesviruses from two species of free living small rodents. Acta Virol. 1980;24:468. [PubMed] [Google Scholar]
- 13.Blasdell K, McCracken C, Morris A, et al. The wood mouse is a natural host for Murid herpesvirus 4. J Gen Virol. 2003;84:111–113. doi: 10.1099/vir.0.18731-0. [DOI] [PubMed] [Google Scholar]
- 14.Efstathiou S, Ho YM, Hall S, Styles CJ, Scott SD, Gompels UA. Murine herpesvirus 68 is genetically related to the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri. J Gen Virol. 1990;71:1365–1372. doi: 10.1099/0022-1317-71-6-1365. [DOI] [PubMed] [Google Scholar]
- 15.Virgin HWt, Latreille P, Wamsley P, et al. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J Virol. 1997;71:5894–5904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sunil-Chandra NP, Efstathiou S, Arno J, Nash AA. Virological and pathological features of mice infected with murine gammaherpesvirus 68. J Gen Virol. 1992;73:2347–2356. doi: 10.1099/0022-1317-73-9-2347. [DOI] [PubMed] [Google Scholar]
- 17.Sunil-Chandra NP, Efstathiou S, Nash AA. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J Gen Virol. 1992;73:3275–3279. doi: 10.1099/0022-1317-73-12-3275. [DOI] [PubMed] [Google Scholar]
- 18.Flano E, Husain SM, Sample JT, Woodland DL, Blackman MA. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J Immunol. 2000;165:1074–1081. doi: 10.4049/jimmunol.165.2.1074. [DOI] [PubMed] [Google Scholar]
- 19.Stewart JP, Usherwood EJ, Ross A, Dyson H, Nash T. Lung epithelial cells are a major site of murine gammaherpesvirus persistence. J Exp Med. 1998;187:1941–1951. doi: 10.1084/jem.187.12.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doherty PC, Christensen JP, Belz GT, Stevenson PG, Sangster MY. Dissecting the host response to a γ-herpesvirus. Philos Trans R Soc Lond B Biol Sci. 2001;356:581–593. doi: 10.1098/rstb.2000.0786. DOI: 10.1098/rstb.2000.0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nash AA, Dutia BM, Stewart JP, Davison AJ. Natural history of murine γ-herpesvirus infection. Philos Trans R Soc Lond B Biol Sci. 2001;356:569–579. doi: 10.1098/rstb.2000.0779. DOI: 10.1098/rstb.2000.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Usherwood EJ, Roy DJ, Ward K, et al. Control of gammaherpesvirus latency by latent antigen-specific CD8+ T cells. J Exp Med. 2000;192:943–952. doi: 10.1084/jem.192.7.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Usherwood EJ, Meadows SK, Crist SG, Bellfy SC, Sentman CL. Control of murine gammaherpesvirus infection is independent of NK cells. Eur J Immunol. 2005;35:2956–2961. doi: 10.1002/eji.200526245. DOI: 10.1002/eji.200526245. [DOI] [PubMed] [Google Scholar]
- 24.Usherwood EJ, Ward KA, Blackman MA, Stewart JP, Woodland DL. Latent antigen vaccination in a model gammaherpesvirus infection. J Virol. 2001;75:8283–8288. doi: 10.1128/JVI.75.17.8283-8288.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai CY, Hu Z, Zhang W, Usherwood EJ. Strain-dependent requirement for IFN-γ for respiratory control and immunotherapy in murine gammaherpesvirus infection. Viral Immunol. 2011;24:273–280. doi: 10.1089/vim.2011.0004. DOI: 10.1089/vim.2011.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai CY, Allie SR, Zhang W, Usherwood EJ. MicroRNA miR-155 affects antiviral effector and effector Memory CD8 T cell differentiation. J Virol. 2013;87:2348–2351. doi: 10.1128/JVI.01742-12. DOI: 10.1128/JVI.01742-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barton E, Mandal P, Speck SH. Pathogenesis and host control of gammaherpesviruses: lessons from the mouse. Annu Rev Immunol. 2011;29:351–397. doi: 10.1146/annurev-immunol-072710-081639. DOI: 10.1146/annurev-immunol-072710-081639. [DOI] [PubMed] [Google Scholar]
- 28.Horst D, Verweij MC, Davison AJ, Ressing ME, Wiertz EJ. Viral evasion of T cell immunity: ancient mechanisms offering new applications. Curr Opin Immunol. 2011;23:96–103. doi: 10.1016/j.coi.2010.11.005. DOI: 10.1016/j.coi.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Speck SH, Ganem D. Viral latency and its regulation: lessons from the γ-herpesviruses. Cell Host Microbe. 2010;8:100–115. doi: 10.1016/j.chom.2010.06.014. DOI: 10.1016/j.chom.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Areste C, Blackbourn DJ. Modulation of the immune system by Kaposi’s sarcoma-associated herpesvirus. Trends Microbiol. 2009;17:119–129. doi: 10.1016/j.tim.2008.12.001. DOI: 10.1016/j.tim.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Hansen TH, Bouvier M. MHC class I antigen presentation: learning from viral evasion strategies. Nat Rev Immunol. 2009;9:503–513. doi: 10.1038/nri2575. DOI: 10.1038/nri2575. [DOI] [PubMed] [Google Scholar]
- 32.Liang C, Lee JS, Jung JU. Immune evasion in Kaposi’s sarcoma-associated herpes virus associated oncogenesis. Semin Cancer Biol. 2008;18:423–436. doi: 10.1016/j.semcancer.2008.09.003. DOI: 10.1016/j.semcancer.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Qian CN, Zeng YX. Regulatory T cells and EBV associated malignancies. Int Immunopharmacol. 2009;9:590–592. doi: 10.1016/j.intimp.2009.01.015. DOI: 10.1016/j.intimp.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Elliott T, Williams A. The optimization of peptide cargo bound to MHC class I molecules by the peptide-loading complex. Immunol Rev. 2005;207:89–99. doi: 10.1111/j.0105-2896.2005.00311.x. DOI: 10.1111/j.0105-2896.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- 35.Baresova P, Pitha PM, Lubyova B. Distinct roles of Kaposi’s sarcoma-associated herpesvirus-encoded vIRFs in inflammatory response and cancer. J Virol. 2013;87:9398–9410. doi: 10.1128/JVI.03315-12. DOI: 10.1128/JVI.03315-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lagos D, Trotter MW, Vart RJ, et al. Kaposi sarcoma herpesvirus-encoded vFLIP and vIRF1 regulate antigen presentation in lymphatic endothelial cells. Blood. 2007;109:1550–1558. doi: 10.1182/blood-2006-05-024034. DOI: 10.1182/blood-2006-05-024034. [DOI] [PubMed] [Google Scholar]
- 37.Glaunsinger B, Ganem D. Lytic KSHV infection inhibits host gene expression by accelerating global mRNA turnover. Mol Cell. 2004;13:713–723. doi: 10.1016/s1097-2765(04)00091-7. [DOI] [PubMed] [Google Scholar]
- 38.Lee YJ, Glaunsinger BA. Aberrant herpesvirus-induced polyadenylation correlates with cellular messenger RNA destruction. PLoS Biol. 2009;7:e1000107. doi: 10.1371/journal.pbio.1000107. DOI: 10.1371/journal.pbio.1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar GR, Glaunsinger BA. Nuclear import of cytoplasmic poly(A) binding protein restricts gene expression via hyperadenylation and nuclear retention of mRNA. Mol Cell Biol. 2010;30:4996–5008. doi: 10.1128/MCB.00600-10. DOI: 10.1128/MCB.00600-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rowe M, Glaunsinger B, van Leeuwen D, et al. Host shutoff during productive Epstein-Barr virus infection is mediated by BGLF5 and may contribute to immune evasion. Proc Natl Acad Sci U S A. 2007;104:3366–3371. doi: 10.1073/pnas.0611128104. DOI: 10.1073/pnas.0611128104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buisson M, Geoui T, Flot D, et al. A bridge crosses the active-site canyon of the Epstein-Barr virus nuclease with DNase and RNase activities. J Mol Biol. 2009;391:717–728. doi: 10.1016/j.jmb.2009.06.034. DOI: 10.1016/j.jmb.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 42.Bagneris C, Briggs LC, Savva R, Ebrahimi B, Barrett TE. Crystal structure of a KSHV-SOX-DNA complex: insights into the molecular mechanisms underlying DNase activity and host shutoff. Nucleic Acids Res. 2011;39:5744–5756. doi: 10.1093/nar/gkr111. DOI: 10.1093/nar/gkr111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuo J, Thomas W, van Leeuwen D, et al. The DNase of gammaherpesviruses impairs recognition by virus-specific CD8+ T cells through an additional host shutoff function. J Virol. 2008;82:2385–2393. doi: 10.1128/JVI.01946-07. DOI: 10.1128/JVI.01946-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Covarrubias S, Richner JM, Clyde K, Lee YJ, Glaunsinger BA. Host shutoff is a conserved phenotype of gammaherpesvirus infection and is orchestrated exclusively from the cytoplasm. J Virol. 2009;83:9554–9566. doi: 10.1128/JVI.01051-09. DOI: 10.1128/JVI.01051-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rock KL, Goldberg AL. Degradation of cell proteins and the generation of MHC class I-presented peptides. Annu Rev Immunol. 1999;17:739–779. doi: 10.1146/annurev.immunol.17.1.739. DOI: 10.1146/annurev.immunol.17.1.739. [DOI] [PubMed] [Google Scholar]
- 46.Yates JL, Warren N, Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature. 1985;313:812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]
- 47.Fogg MH, Wirth LJ, Posner M, Wang F. Decreased EBNA-1-specific CD8+ T cells in patients with Epstein-Barr virus-associated nasopharyngeal carcinoma. Proc Natl Acad Sci USA. 2009;106:3318–3323. doi: 10.1073/pnas.0813320106. DOI: 10.1073/pnas.0813320106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piriou E, van Dort K, Nanlohy NM, van Oers MH, Miedema F, van Baarle D. Loss of EBNA1-specific memory CD4+ and CD8+ T cells in HIV-infected patients progressing to AIDS-related non-Hodgkin lymphoma. Blood. 2005;106:3166–3174. doi: 10.1182/blood-2005-01-0432. DOI: 10.1182/blood-2005-01-0432. [DOI] [PubMed] [Google Scholar]
- 49.Levitskaya J, Coram M, Levitsky V, et al. Inhibition of antigen processing by the internal repeat region of the Epstein-Barr virus nuclear antigen-1. Nature. 1995;375:685–688. doi: 10.1038/375685a0. DOI: 10.1038/375685a0. [DOI] [PubMed] [Google Scholar]
- 50.Levitskaya J, Sharipo A, Leonchiks A, Ciechanover A, Masucci MG. Inhibition of ubiquitin/proteasome-dependent protein degradation by the Gly-Ala repeat domain of the Epstein-Barr virus nuclear antigen 1. Proc Natl Acad Sci USA. 1997;94:12616–12621. doi: 10.1073/pnas.94.23.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yin Y, Manoury B, Fahraeus R. Self-inhibition of synthesis and antigen presentation by Epstein-Barr virus-encoded EBNA1. Science. 2003;301:1371–1374. doi: 10.1126/science.1088902. DOI: 10.1126/science.1088902. [DOI] [PubMed] [Google Scholar]
- 52.Ballestas ME, Chatis PA, Kaye KM. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science. 1999;284:641–644. doi: 10.1126/science.284.5414.641. [DOI] [PubMed] [Google Scholar]
- 53.Lan K, Kuppers DA, Verma SC, Robertson ES. Kaposi’s sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen inhibits lytic replication by targeting Rta: a potential mechanism for virus-mediated control of latency. J Virol. 2004;78:6585–6594. doi: 10.1128/JVI.78.12.6585-6594.2004. DOI: 10.1128/JVI.78.12.6585-6594.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kwun HJ, da Silva SR, Shah IM, Blake N, Moore PS, Chang Y. Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mimics Epstein-Barr virus EBNA1 immune evasion through central repeat domain effects on protein processing. J Virol. 2007;81:8225–8235. doi: 10.1128/JVI.00411-07. DOI: 10.1128/JVI.00411-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fowler P, Marques S, Simas JP, Efstathiou S. ORF73 of murine herpesvirus-68 is critical for the establishment and maintenance of latency. J Gen Virol. 2003;84:3405–3416. doi: 10.1099/vir.0.19594-0. [DOI] [PubMed] [Google Scholar]
- 56.Bennett NJ, May JS, Stevenson PG. Gamma-herpesvirus latency requires T cell evasion during episome maintenance. PLoS Biol. 2005;3:e120. doi: 10.1371/journal.pbio.0030120. DOI: 10.1371/journal.pbio.0030120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boname JM, Stevenson PG. MHC class I ubiquitination by a viral PHD/LAP finger protein. Immunity. 2001;15:627–636. doi: 10.1016/s1074-7613(01)00213-8. [DOI] [PubMed] [Google Scholar]
- 58.Boname JM, de Lima BD, Lehner PJ, Stevenson PG. Viral degradation of the MHC class I peptide loading complex. Immunity. 2004;20:305–317. doi: 10.1016/s1074-7613(04)00047-0. [DOI] [PubMed] [Google Scholar]
- 59.Stevenson PG, Efstathiou S, Doherty PC, Lehner PJ. Inhibition of MHC class I-restricted antigen presentation by γ2-herpesviruses. Proc Natl Acad Sci USA. 2000;97:8455–8460. doi: 10.1073/pnas.150240097. DOI: 10.1073/pnas.150240097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lybarger L, Wang X, Harris MR, Virgin HWt, Hansen TH. Virus subversion of the MHC class I peptide-loading complex. Immunity. 2003;18:121–130. doi: 10.1016/s1074-7613(02)00509-5. [DOI] [PubMed] [Google Scholar]
- 61.Hislop AD, Ressing ME, van Leeuwen D, et al. A CD8+ T cell immune evasion protein specific to Epstein-Barr virus and its close relatives in Old World primates. J Exp Med. 2007;204:1863–1873. doi: 10.1084/jem.20070256. DOI: 10.1084/jem.20070256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Horst D, Favaloro V, Vilardi F, et al. EBV protein BNLF2a exploits host tail-anchored protein integration machinery to inhibit TAP. J Immunol. 2011;186:3594–3605. doi: 10.4049/jimmunol.1002656. DOI: 10.4049/jimmunol.1002656. [DOI] [PubMed] [Google Scholar]
- 63.Paulsen SJ, Rosenkilde MM, Eugen-Olsen J, Kledal TN. Epstein-Barr virus-encoded BILF1 is a constitutively active G protein-coupled receptor. J Virol. 2005;79:536–546. doi: 10.1128/JVI.79.1.536-546.2005. DOI: 10.1128/JVI.79.1.536-546.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zuo J, Currin A, Griffin BD, et al. The Epstein-Barr virus G-protein-coupled receptor contributes to immune evasion by targeting MHC class I molecules for degradation. PLoS Pathog. 2009;5:e1000255. doi: 10.1371/journal.ppat.1000255. DOI: 10.1371/journal.ppat.1000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Griffin BD, Gram AM, Mulder A, et al. EBV BILF1 evolved to downregulate cell surface display of a wide range of HLA class I molecules through their cytoplasmic tail. J Immunol. 2013;190:1672–1684. doi: 10.4049/jimmunol.1102462. DOI: 10.4049/jimmunol.1102462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lehner PJ, Hoer S, Dodd R, Duncan LM. Downregulation of cell surface receptors by the K3 family of viral and cellular ubiquitin E3 ligases. Immunol Rev. 2005;207:112–125. doi: 10.1111/j.0105-2896.2005.00314.x. DOI: 10.1111/j.0105-2896.2005.00314.x. [DOI] [PubMed] [Google Scholar]
- 67.Coscoy L, Ganem D. Kaposi’s sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc Natl Acad Sci U S A. 2000;97:8051–8056. doi: 10.1073/pnas.140129797. DOI: 10.1073/pnas.140129797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coscoy L, Sanchez DJ, Ganem D. A novel class of herpesvirus-encoded membrane-bound E3 ubiquitin ligases regulates endocytosis of proteins involved in immune recognition. J Cell Biol. 2001;155:1265–1273. doi: 10.1083/jcb.200111010. DOI: 10.1083/jcb.200111010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ishido S, Wang C, Lee BS, Cohen GB, Jung JU. Downregulation of major histocompatibility complex class I molecules by Kaposi’s sarcoma-associated herpesvirus K3 and K5 proteins. J Virol. 2000;74:5300–5309. doi: 10.1128/jvi.74.11.5300-5309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4+ T cells in immunity to viruses. Nat Rev Immunol. 2012;12:136–148. doi: 10.1038/nri3152. DOI: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pieters J. MHC class II compartments: specialized organelles of the endocytic pathway in antigen presenting cells. Biol Chem. 1997;378:751–758. [PubMed] [Google Scholar]
- 72.Schmid D, Pypaert M, Munz C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity. 2007;26:79–92. doi: 10.1016/j.immuni.2006.10.018. DOI: 10.1016/j.immuni.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ting JP, Trowsdale J. Genetic control of MHC class II expression. Cell. 2002;109(Suppl):S21–33. doi: 10.1016/s0092-8674(02)00696-7. [DOI] [PubMed] [Google Scholar]
- 74.Muhlethaler-Mottet A, Otten LA, Steimle V, Mach B. Expression of MHC class II molecules in different cellular and functional compartments is controlled by differential usage of multiple promoters of the transactivator CIITA. EMBO J. 1997;16:2851–2860. doi: 10.1093/emboj/16.10.2851. DOI: 10.1093/emboj/16.10.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morris AC, Beresford GW, Mooney MR, Boss JM. Kinetics of a gamma interferon response: expression and assembly of CIITA promoter IV and inhibition by methylation. Mol Cell Biol. 2002;22:4781–4791. doi: 10.1128/MCB.22.13.4781-4791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tamiya T, Kashiwagi I, Takahashi R, Yasukawa H, Yoshimura A. Suppressors of cytokine signaling (SOCS) proteins and JAK/STAT pathways: regulation of T-cell inflammation by SOCS1 and SOCS3. Arterioscler Thromb Vasc Biol. 2011;31:980–985. doi: 10.1161/ATVBAHA.110.207464. DOI: 10.1161/ATVBAHA.110.207464. [DOI] [PubMed] [Google Scholar]
- 77.Butler LM, Jeffery HC, Wheat RL, et al. Kaposi’s sarcoma-associated herpesvirus inhibits expression and function of endothelial cell major histocompatibility complex class II via suppressor of cytokine signaling 3. J Virol. 2012;86:7158–7166. doi: 10.1128/JVI.06908-11. DOI: 10.1128/JVI.06908-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wies E, Mori Y, Hahn A, et al. The viral interferon-regulatory factor-3 is required for the survival of KSHV-infected primary effusion lymphoma cells. Blood. 2008;111:320–327. doi: 10.1182/blood-2007-05-092288. DOI: 10.1182/blood-2007-05-092288. [DOI] [PubMed] [Google Scholar]
- 79.Schmidt K, Wies E, Neipel F. Kaposi’s sarcoma-associated herpesvirus viral interferon regulatory factor 3 inhibits gamma interferon and major histocompatibility complex class II expression. J Virol. 2011;85:4530–4537. doi: 10.1128/JVI.02123-10. DOI: 10.1128/JVI.02123-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bi X, Yang L, Mancl ME, Barnes BJ. Modulation of interferon regulatory factor 5 activities by the Kaposi sarcoma-associated herpesvirus-encoded viral interferon regulatory factor 3 contributes to immune evasion and lytic induction. J Interferon Cytokine Res. 2011;31:373–382. doi: 10.1089/jir.2010.0084. DOI: 10.1089/jir.2010.0084. [DOI] [PubMed] [Google Scholar]
- 81.Wies E, Hahn AS, Schmidt K, et al. The Kaposi’s sarcoma-associated herpesvirus-encoded vIRF-3 inhibits cellular IRF-5. J Biol Chem. 2009;284:8525–8538. doi: 10.1074/jbc.M809252200. DOI: 10.1074/jbc.M809252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miller G, El-Guindy A, Countryman J, Ye J, Gradoville L. Lytic cycle switches of oncogenic human gammaherpesviruses. Adv Cancer Res. 2007;97:81–109. doi: 10.1016/S0065-230X(06)97004-3. DOI: 10.1016/S0065-230X(06)97004-3. [DOI] [PubMed] [Google Scholar]
- 83.Morrison TE, Mauser A, Wong A, Ting JP, Kenney SC. Inhibition of IFN-γ signaling by an Epstein-Barr virus immediate-early protein. Immunity. 2001;15:787–799. doi: 10.1016/s1074-7613(01)00226-6. [DOI] [PubMed] [Google Scholar]
- 84.Li D, Qian L, Chen C, et al. Down-regulation of MHC class II expression through inhibition of CIITA transcription by lytic transactivator Zta during Epstein-Barr virus reactivation. J Immunol. 2009;182:1799–1809. doi: 10.4049/jimmunol.0802686. DOI: 10.4049/jimmunol.0802686. [DOI] [PubMed] [Google Scholar]
- 85.Zuo J, Thomas WA, Haigh TA, et al. Epstein-Barr virus evades CD4+ T cell responses in lytic cycle through BZLF1-mediated downregulation of CD74 and the cooperation of vBcl-2. PLoS Pathog. 2011;7:e1002455. doi: 10.1371/journal.ppat.1002455. DOI: 10.1371/journal.ppat.1002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Q, Turk SM, Hutt-Fletcher LM. The Epstein-Barr virus (EBV) BZLF2 gene product associates with the gH and gL homologs of EBV and carries an epitope critical to infection of B cells but not of epithelial cells. J Virol. 1995;69:3987–3994. doi: 10.1128/jvi.69.7.3987-3994.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li Q, Spriggs MK, Kovats S, et al. Epstein-Barr virus uses HLA class II as a cofactor for infection of B lymphocytes. J Virol. 1997;71:4657–4662. doi: 10.1128/jvi.71.6.4657-4662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ressing ME, van Leeuwen D, Verreck FA, et al. Interference with T cell receptor-HLA-DR interactions by Epstein-Barr virus gp42 results in reduced T helper cell recognition. Proc Natl Acad Sci U S A. 2003;100:11583–11588. doi: 10.1073/pnas.2034960100. DOI: 10.1073/pnas.2034960100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ressing ME, van Leeuwen D, Verreck FA, et al. Epstein-Barr virus gp42 is posttranslationally modified to produce soluble gp42 that mediates HLA class II immune evasion. J Virol. 2005;79:841–852. doi: 10.1128/JVI.79.2.841-852.2005. DOI: 10.1128/JVI.79.2.841-852.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Munz C, Bickham KL, Subklewe M, et al. Human CD4+ T lymphocytes consistently respond to the latent Epstein-Barr virus nuclear antigen EBNA1. J Exp Med. 2000;191:1649–1660. doi: 10.1084/jem.191.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paludan C, Schmid D, Landthaler M, et al. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. DOI: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 92.Leung CS, Haigh TA, Mackay LK, Rickinson AB, Taylor GS. Nuclear location of an endogenously expressed antigen, EBNA1, restricts access to macroautophagy and the range of CD4 epitope display. Proc Natl Acad Sci U S A. 2010;107:2165–2170. doi: 10.1073/pnas.0909448107. DOI: 10.1073/pnas.0909448107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. DOI: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 94.Li L, Liu D, Hutt-Fletcher L, Morgan A, Masucci MG, Levitsky V. Epstein-Barr virus inhibits the development of dendritic cells by promoting apoptosis of their monocyte precursors in the presence of granulocyte macrophage-colony-stimulating factor and interleukin-4. Blood. 2002;99:3725–3734. doi: 10.1182/blood.v99.10.3725. [DOI] [PubMed] [Google Scholar]
- 95.Stebbing J, Gazzard B, Portsmouth S, et al. Disease-associated dendritic cells respond to disease-specific antigens through the common heat shock protein receptor. Blood. 2003;102:1806–1814. doi: 10.1182/blood-2003-03-0891. DOI: 10.1182/blood-2003-03-0891. [DOI] [PubMed] [Google Scholar]
- 96.Cirone M, Lucania G, Aleandri S, et al. Suppression of dendritic cell differentiation through cytokines released by Primary Effusion Lymphoma cells. Immunol Lett. 2008;120:37–41. doi: 10.1016/j.imlet.2008.06.011. DOI: 10.1016/j.imlet.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 97.Cirone M, Lucania G, Bergamo P, Trivedi P, Frati L, Faggioni A. Human herpesvirus 8 (HHV-8) inhibits monocyte differentiation into dendritic cells and impairs their immunostimulatory activity. Immunol Lett. 2007;113:40–46. doi: 10.1016/j.imlet.2007.07.013. DOI: 10.1016/j.imlet.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 98.Cirone M, Conte V, Farina A, et al. HHV-8 reduces dendritic cell migration through down-regulation of cell-surface CCR6 and CCR7 and cytoskeleton reorganization. Virol J. 2012;9:92. doi: 10.1186/1743-422X-9-92. DOI: 10.1186/1743-422X-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rappocciolo G, Jenkins FJ, Hensler HR, et al. DC-SIGN is a receptor for human herpesvirus 8 on dendritic cells and macrophages. J Immunol. 2006;176:1741–1749. doi: 10.4049/jimmunol.176.3.1741. [DOI] [PubMed] [Google Scholar]
- 100.Bertram EM, Dawicki W, Watts TH. Role of T cell costimulation in anti-viral immunity. Semin Immunol. 2004;16:185–196. doi: 10.1016/j.smim.2004.02.006. DOI: 10.1016/j.smim.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 101.Fuse S, Obar JJ, Bellfy S, Leung EK, Zhang W, Usherwood EJ. CD80 and CD86 control antiviral CD8+ T-cell function and immune surveillance of murine gammaherpesvirus 68. J Virol. 2006;80:9159–9170. doi: 10.1128/JVI.00422-06. DOI: 10.1128/JVI.00422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dustin ML, Springer TA. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341:619–624. doi: 10.1038/341619a0. DOI: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- 103.Coscoy L, Ganem D. A viral protein that selectively downregulates ICAM-1 and B7-2 and modulates T cell costimulation. J Clin Invest. 2001;107:1599–1606. doi: 10.1172/JCI12432. DOI: 10.1172/JCI12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ishido S, Choi JK, Lee BS, et al. Inhibition of natural killer cell-mediated cytotoxicity by Kaposi’s sarcoma-associated herpesvirus K5 protein. Immunity. 2000;13:365–374. doi: 10.1016/s1074-7613(00)00036-4. [DOI] [PubMed] [Google Scholar]
- 105.Thomas M, Boname JM, Field S, et al. Down-regulation of NKG2D and NKp80 ligands by Kaposi’s sarcoma-associated herpesvirus K5 protects against NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2008;105:1656–1661. doi: 10.1073/pnas.0707883105. DOI: 10.1073/pnas.0707883105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hochreiter R, Ptaschinski C, Kunkel SL, Rochford R. Murine gammaherpesvirus-68 productively infects immature dendritic cells and blocks maturation. J Gen Virol. 2007;88:1896–1905. doi: 10.1099/vir.0.82931-0. DOI: 10.1099/vir.0.82931-0. [DOI] [PubMed] [Google Scholar]
- 107.Wan YY. Regulatory T cells: immune suppression and beyond. Cell Mol Immunol. 2010;7:204–210. doi: 10.1038/cmi.2010.20. DOI: 10.1038/cmi.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. DOI: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 109.Lau KM, Cheng SH, Lo KW, et al. Increase in circulating Foxp3+CD4+CD25high regulatory T cells in nasopharyngeal carcinoma patients. Br J Cancer. 2007;96:617–622. doi: 10.1038/sj.bjc.6603580. DOI: 10.1038/sj.bjc.6603580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Popescu I, Macedo C, Abu-Elmagd K, et al. EBV-specific CD8+ T cell reactivation in transplant patients results in expansion of CD8+ type-1 regulatory T cells. Am J Transplant. 2007;7:1215–1223. doi: 10.1111/j.1600-6143.2007.01740.x. DOI: 10.1111/j.1600-6143.2007.01740.x. [DOI] [PubMed] [Google Scholar]
- 111.Takegawa S, Jin Z, Nakayama T, et al. Expression of CCL17 and CCL22 by latent membrane protein 1-positive tumor cells in age-related Epstein-Barr virus-associated B-cell lymphoproliferative disorder. Cancer Sci. 2008;99:296–302. doi: 10.1111/j.1349-7006.2007.00687.x. DOI: 10.1111/j.1349-7006.2007.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baumforth KR, Birgersdotter A, Reynolds GM, et al. Expression of the Epstein-Barr virus-encoded Epstein-Barr virus nuclear antigen 1 in Hodgkin’s lymphoma cells mediates Up-regulation of CCL20 and the migration of regulatory T cells. Am J Pathol. 2008;173:195–204. doi: 10.2353/ajpath.2008.070845. DOI: 10.2353/ajpath.2008.070845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Voo KS, Peng G, Guo Z, et al. Functional characterization of EBV-encoded nuclear antigen 1-specific CD4+ helper and regulatory T cells elicited by in vitro peptide stimulation. Cancer Res. 2005;65:1577–1586. doi: 10.1158/0008-5472.CAN-04-2552. DOI: 10.1158/0008-5472.CAN-04-2552. [DOI] [PubMed] [Google Scholar]
- 114.Assis MC, Campos AH, Oliveira JS, et al. Increased expression of CD4+CD25+FOXP3+ regulatory T cells correlates with Epstein-Barr virus and has no impact on survival in patients with classical Hodgkin lymphoma in Brazil. Med Oncol. 2012;29:3614–3619. doi: 10.1007/s12032-012-0299-4. DOI: 10.1007/s12032-012-0299-4. [DOI] [PubMed] [Google Scholar]
- 115.Endharti AT, Rifa IM, Shi Z, et al. Cutting edge: CD8+CD122+ regulatory T cells produce IL-10 to suppress IFN-γ production and proliferation of CD8+ T cells. J Immunol. 2005;175:7093–7097. doi: 10.4049/jimmunol.175.11.7093. [DOI] [PubMed] [Google Scholar]
- 116.Elrefaei M, Ventura FL, Baker CA, Clark R, Bangsberg DR, Cao H. HIV-specific IL-10-positive CD8+ T cells suppress cytolysis and IL-2 production by CD8+ T cells. J Immunol. 2007;178:3265–3271. doi: 10.4049/jimmunol.178.5.3265. [DOI] [PubMed] [Google Scholar]
- 117.Hu Z, Zhang W, Usherwood EJ. Regulatory CD8+ T cells associated with erosion of immune surveillance in persistent virus infection suppress in vitro and have a reversible proliferative defect. J Immunol. 2013;191:312–322. doi: 10.4049/jimmunol.1201773. DOI: 10.4049/jimmunol.1201773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Molloy MJ, Zhang W, Usherwood EJ. Suppressive CD8+ T cells arise in the absence of CD4 help and compromise control of persistent virus. J Immunol. 2011;186:6218–6226. doi: 10.4049/jimmunol.1003812. DOI: 10.4049/jimmunol.1003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gasper-Smith N, Marriott I, Bost KL. Murine γ-herpesvirus 68 limits naturally occurring CD4+CD25+ T regulatory cell activity following infection. J Immunol. 2006;177:4670–4678. doi: 10.4049/jimmunol.177.7.4670. [DOI] [PubMed] [Google Scholar]
- 120.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 121.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15:277–284. doi: 10.1038/nm.1929. DOI: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brockman MA, Kwon DS, Tighe DP, et al. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood. 2009;114:346–356. doi: 10.1182/blood-2008-12-191296. DOI: 10.1182/blood-2008-12-191296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dukers DF, Jaspars LH, Vos W, et al. Quantitative immunohistochemical analysis of cytokine profiles in Epstein-Barr virus-positive and -negative cases of Hodgkin’s disease. J Pathol. 2000;190:143–149. doi: 10.1002/(SICI)1096-9896(200002)190:2<143::AID-PATH519>3.0.CO;2-5. DOI: 10.1002/(SICI)1096-9896(200002)190:2<143::AID-PATH519>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 124.Benjamin D, Knobloch TJ, Dayton MA. Human B-cell interleukin-10: B-cell lines derived from patients with acquired immunodeficiency syndrome and Burkitt’s lymphoma constitutively secrete large quantities of interleukin-10. Blood. 1992;80:1289–1298. [PubMed] [Google Scholar]
- 125.Burdin N, Peronne C, Banchereau J, Rousset F. Epstein-Barr virus transformation induces B lymphocytes to produce human interleukin 10. J Exp Med. 1993;177:295–304. doi: 10.1084/jem.177.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jones KD, Aoki Y, Chang Y, Moore PS, Yarchoan R, Tosato G. Involvement of interleukin-10 (IL-10) and viral IL-6 in the spontaneous growth of Kaposi’s sarcoma herpesvirus-associated infected primary effusion lymphoma cells. Blood. 1999;94:2871–2879. [PubMed] [Google Scholar]
- 127.Peacock JW, Bost KL. Murine gammaherpesvirus-68-induced interleukin-10 increases viral burden, but limits virus-induced splenomegaly and leukocytosis. Immunology. 2001;104:109–117. doi: 10.1046/j.0019-2805.2001.01286.x. DOI: imm1286 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Flano E, Kayhan B, Woodland DL, Blackman MA. Infection of dendritic cells by a γ2-herpesvirus induces functional modulation. J Immunol. 2005;175:3225–3234. doi: 10.4049/jimmunol.175.5.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Slobedman B, Barry PA, Spencer JV, Avdic S, Abendroth A. Virus-encoded homologs of cellular interleukin-10 and their control of host immune function. J Virol. 2009;83:9618–9629. doi: 10.1128/JVI.01098-09. DOI: 10.1128/JVI.01098-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Middeldorp JM, Pegtel DM. Multiple roles of LMP1 in Epstein-Barr virus induced immune escape. Semin Cancer Biol. 2008;18:388–396. doi: 10.1016/j.semcancer.2008.10.004. DOI: 10.1016/j.semcancer.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 131.Marshall NA, Vickers MA, Barker RN. Regulatory T cells secreting IL-10 dominate the immune response to EBV latent membrane protein 1. J Immunol. 2003;170:6183–6189. doi: 10.4049/jimmunol.170.12.6183. [DOI] [PubMed] [Google Scholar]
- 132.Incrocci R, McCormack M, Swanson-Mungerson M. Epstein-Barr virus LMP2A increases IL-10 production in mitogen-stimulated primary B-cells and B-cell lymphomas. J Gen Virol. 2013;94:1127–1133. doi: 10.1099/vir.0.049221-0. DOI: 10.1099/vir.0.049221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mahot S, Sergeant A, Drouet E, Gruffat H. A novel function for the Epstein-Barr virus transcription factor EB1/Zta: induction of transcription of the hIL-10 gene. J Gen Virol. 2003;84:965–974. doi: 10.1099/vir.0.18845-0. [DOI] [PubMed] [Google Scholar]
- 134.Iwakiri D, Takada K. Role of EBERs in the pathogenesis of EBV infection. Adv Cancer Res. 2010;107:119–136. doi: 10.1016/S0065-230X(10)07004-1. DOI: 10.1016/S0065-230X(10)07004-1. [DOI] [PubMed] [Google Scholar]
- 135.Kitagawa N, Goto M, Kurozumi K, et al. Epstein-Barr virus-encoded poly(A)- RNA supports Burkitt’s lymphoma growth through interleukin-10 induction. EMBO J. 2000;19:6742–6750. doi: 10.1093/emboj/19.24.6742. DOI: 10.1093/emboj/19.24.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhu Y, Haecker I, Yang Y, Gao SJ, Renne R. γ-Herpesvirus-encoded miRNAs and their roles in viral biology and pathogenesis. Curr Opin Virol. 2013;3:266–275. doi: 10.1016/j.coviro.2013.05.013. DOI: 10.1016/j.coviro.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gottwein E. Kaposi’s sarcoma-associated herpesvirus microRNAs. Front Microbiol. 2012;3:165. doi: 10.3389/fmicb.2012.00165. DOI: 10.3389/fmicb.2012.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bai Z, Huang Y, Li W, et al. Genomewide mapping and screening of Kaposi’s sarcoma-associated herpesvirus (KSHV) 3’ untranslated regions identify bicistronic and polycistronic viral transcripts as frequent targets of KSHV microRNAs. J Virol. 2014;88:377–392. doi: 10.1128/JVI.02689-13. DOI: 10.1128/JVI.02689-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Qin Z, Kearney P, Plaisance K, Parsons CH. Pivotal advance: Kaposi’s sarcoma-associated herpesvirus (KSHV)-encoded microRNA specifically induce IL-6 and IL-10 secretion by macrophages and monocytes. J Leukoc Biol. 2010;87:25–34. doi: 10.1189/jlb.0409251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Herskowitz JH, Jacoby MA, Speck SH. The murine gammaherpesvirus 68 M2 gene is required for efficient reactivation from latently infected B cells. J Virol. 2005;79:2261–2273. doi: 10.1128/JVI.79.4.2261-2273.2005. DOI: 10.1128/JVI.79.4.2261-2273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.de Oliveira VL, Almeida SC, Soares HR, Parkhouse RM. Selective B-cell expression of the MHV-68 latency-associated M2 protein regulates T-dependent antibody response and inhibits apoptosis upon viral infection. J Gen Virol. 2013;94:1613–1623. doi: 10.1099/vir.0.050013-0. DOI: 10.1099/vir.0.050013-0. [DOI] [PubMed] [Google Scholar]
- 142.Liang X, Shin YC, Means RE, Jung JU. Inhibition of interferon-mediated antiviral activity by murine gammaherpesvirus 68 latency-associated M2 protein. J Virol. 2004;78:12416–12427. doi: 10.1128/JVI.78.22.12416-12427.2004. DOI: 10.1128/JVI.78.22.12416-12427.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Siegel AM, Herskowitz JH, Speck SH. The MHV68 M2 protein drives IL-10 dependent B cell proliferation and differentiation. PLoS Pathog. 2008;4:e1000039. doi: 10.1371/journal.ppat.1000039. DOI: 10.1371/journal.ppat.1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Moore KW, Vieira P, Fiorentino DF, Trounstine ML, Khan TA, Mosmann TR. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990;248:1230–1234. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- 145.Ding Y, Qin L, Kotenko SV, Pestka S, Bromberg JS. A single amino acid determines the immunostimulatory activity of interleukin 10. J Exp Med. 2000;191:213–224. doi: 10.1084/jem.191.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jochum S, Moosmann A, Lang S, Hammerschmidt W, Zeidler R. The EBV immunoevasins vIL-10 and BNLF2a protect newly infected B cells from immune recognition and elimination. PLoS Pathog. 2012;8:e1002704. doi: 10.1371/journal.ppat.1002704. DOI: 10.1371/journal.ppat.1002704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zeidler R, Eissner G, Meissner P, et al. Downregulation of TAP1 in B lymphocytes by cellular and Epstein-Barr virus-encoded interleukin-10. Blood. 1997;90:2390–2397. [PubMed] [Google Scholar]
- 148.Hsu DH, de Waal Malefyt R, Fiorentino DF, et al. Expression of interleukin-10 activity by Epstein-Barr virus protein BCRF1. Science. 1990;250:830–832. doi: 10.1126/science.2173142. [DOI] [PubMed] [Google Scholar]
- 149.de Waal Malefyt R, Haanen J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rousset F, Garcia E, Defrance T, et al. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci USA. 1992;89:1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.MacNeil IA, Suda T, Moore KW, Mosmann TR, Zlotnik A. IL-10, a novel growth cofactor for mature and immature T cells. J Immunol. 1990;145:4167–4173. [PubMed] [Google Scholar]
- 152.Vieira P, de Waal-Malefyt R, Dang MN, et al. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI. Proc Natl Acad Sci USA. 1991;88:1172–1176. doi: 10.1073/pnas.88.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Go NF, Castle BE, Barrett R, et al. Interleukin 10, a novel B cell stimulatory factor: unresponsiveness of X chromosome-linked immunodeficiency B cells. J Exp Med. 1990;172:1625–1631. doi: 10.1084/jem.172.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]