Abstract
Heterosis has been widely used in agriculture to increase yield and to broaden adaptability of hybrid varieties and is applied to an increasing number of crop species. We performed a systematic survey of the extent and degree of heterosis for dry biomass in 63 Arabidopsis accessions crossed to three reference lines (Col-0, C24, and Nd). We detected a high heritability (69%) for biomass production in Arabidopsis. Among the 169 crosses analyzed, 29 exhibited significant mid-parent-heterosis for shoot biomass. Furthermore, we analyzed two divergent accessions, C24 and Col-0, the F1 hybrids of which were shown to exhibit hybrid vigor, in more detail. In the combination Col-0/C24, heterosis for biomass was enhanced at higher light intensities; we found 51% to 66% mid-parent-heterosis at low and intermediate light intensities (60 and 120 μmol m−2 s−1), and 161% at high light intensity (240 μmol m−2 s−1). While at the low and intermediate light intensities relative growth rates of the hybrids were higher only in the early developmental phase (0–15 d after sowing [DAS]), at high light intensity the hybrids showed increased relative growth rates over the entire vegetative phase (until 25 DAS). An important finding was the early onset of heterosis for biomass; in the cross Col-0/C24, differences between parental and hybrid lines in leaf size and dry shoot mass could be detected as early as 10 DAS. The widespread occurrence of heterosis in the model plant Arabidopsis opens the possibility to investigate the genetic basis of this phenomenon using the tools of genetical genomics.
The term heterosis describes increased size and yield in crossbred as compared to the corresponding inbred lines (Shull, 1948). It has also been applied to the expression of adaptive traits such as increased fertility and resistance to biotic and abiotic stress (Dobzhansky, 1950). Maximum heterosis is observed in the F1. In subsequent generations, obtained through successive selfing, the superiority of the progeny over their parents is progressively lost. Heterosis is often expressed as mid-parent heterosis (MPH), comparing the average trait value of the F1 hybrid to the average trait value of the parents. In an agricultural context, the hybrid must exceed the best parent to be useful. For this purpose best-parent heterosis (BPH) is determined.
Three principal genetic models have been suggested as explanation for the extreme hybrid phenotype: dominance, (pseudo) overdominance, and epistasis (Crow, 1952; Geiger, 1988; Tsaftaris, 1995). The dominance hypothesis attributes increased vigor to the action of favorable dominant alleles (usually at multiple loci) from both parents combined in the hybrid (Xiao et al., 1995). The overdominance hypothesis postulates the existence of loci at which the heterozygous state is superior to either homozygote. Pseudo-overdominance, in contrast, refers to the situation of tightly linked genes with favorable dominant alleles linked in repulsion. There is also evidence for the role of epistasis in heterosis, i.e. the interaction of favorable alleles at different loci contributed by the two parents, which themselves may show additive, dominant, or overdominant action (Yu et al., 1997; Monforte and Tanksley, 2000; Li et al., 2001; Luo et al., 2001).
In addition to formal genetic hypotheses, numerous physiological and molecular mechanisms underlying the heterosis phenomenon have been proposed (Comings and MacMurray, 2000; de Vienne et al., 2001). Griffing and Zsiros (1971) considered heterosis as the result of interaction between genetic and environmental stimuli. They dissected the complex phenomenon of heterosis into environment-dependent component parts, such as temperature-dependent heterosis (Langridge, 1962). Riday et al. (2003) suggested that in many cases heterosis can be accounted for by the interaction of genes controlling morphologically divergent traits between the parents. This has been shown in Arabidopsis for phosphate acquisition (Narang and Altmann, 2001), where the F1 hybrids inherited beneficial root traits from both parents.
Parental genetic distance is often regarded as a useful indicator for hybrid performance (Melchinger, 1999). A number of methods exist to estimate genetic distance based on pedigree data, morphological data, agronomic performance data, biochemical data, and DNA data (Mohammadi and Prasanna, 2003). Several studies have reported a positive correlation between genetic distance of the parental lines and the superior hybrid performance (Liu et al., 2002; Barbosa et al., 2003). However, in maize (Zea mays), heterosis is known to culminate at an optimum of parental genetic distance before declining again (Moll et al., 1965).
In Arabidopsis, heterosis for rosette diameter (El Asmi 1974, 1975; Barth et al., 2003), stem length and biomass (Rédei, 1962; Griffing and Langridge, 1963; Corey et al., 1976; Barth et al., 2003), photosynthetic efficiency (Sharma et al., 1979), seedling viability (Mitchell-Olds, 1995), seed number (Alonso-Blanco et al., 1999), and phosphate efficiency (Narang and Altmann, 2001) has been reported for only a limited number of crosses. If heterosis is a widespread occurring phenomenon in Arabidopsis, the vast genome and technological resources available for this model species could be used to rapidly advance our understanding of underlying physiological and molecular processes and a precedence could be established that may support the analysis of heterosis in crops.
We performed a systematic survey of the extent and degree of heterosis for dry biomass in 63 Arabidopsis accessions crossed to three reference lines (Col-0, C24, and Nd). Furthermore, we analyzed two divergent accessions, C24 and Col, in more detail. F1 hybrids of these crosses were shown to exhibit strong hybrid vigor depending on light conditions and developmental stages.
RESULTS
Occurrence and Degree of Heterosis for Shoot Biomass in Arabidopsis
A large survey of the occurrence and the degree of heterosis was conducted with 63 different Arabidopsis accessions crossed to the three reference lines C24, Col-0, and Nd. Major effects of the pollination procedure (hand versus self-pollination) on seed size and subsequently on shoot weight of the plants grown from these seeds were observed. As determined for the two accessions Col-0 and C24, seeds obtained by hand pollination had almost double the weight of seeds from self-pollination. At 15 and 28 d after sowing (DAS), C24 and Col-0 plants grown from selfed seeds reached less than one-half the weight of those from manually pollinated seeds (Table I). Therefore, for each of the 169 crosses analyzed, F1 seeds from both reciprocal crosses and seeds from parents, produced by manual fertilisation, were used for the analyses. If the number of siliques on self-pollinated mother plants was restricted to the same number as for the hand pollinated mother plants, the seed weights were again similar. We did not detect a significant difference in dry shoot mass at 15 DAS between plants of the parental lines grown from manually pollinated or restricted siliques (Table I).
Table I.
Weight and size of seeds from different pollination methods and dry shoot mass at 15 and 28 DAS from plants grown from the same seed lots at 120 μmol m−2 s−1
| Cross | Pollination | SW ± sd | Sig. | PW15 ± sd | Sig. | PW28 ± sd | Sig. |
|---|---|---|---|---|---|---|---|
| C24×C24 | Self | 17.3 ± 2.4 | a | 0.19 ± 0.03 | a | 7.8 ± 1.6 | a |
| Col-0×Col-0 | Self | 17.6 ± 0.6 | a | 0.19 ± 0.04 | a | 8.9 ± 1.4 | b |
| C24×C24 | Manual | 32.7 ± 1.3 | b | 0.73 ± 0.22 | b | 15.0 ± 2.3 | c |
| Col-0×Col-0 | Manual | 31.5 ± 1.6 | b | 0.79 ± 0.21 | c | 24.4 ± 3.8 | d |
| C24×C24 | Self restr. | 30.4 ± 1.5 | b | 0.72 ± 0.17 | b | ||
| Col-0×Col-0 | Self restr. | 29.4 ± 1.1 | b | 0.82 ± 0.25 | c | ||
| C24×Col-0 F1 | Manual | 37.8 ± 1.2 | c | 1.04 ± 0.32 | d | 32.5 ± 6.1 | e |
| Col-0×C24 F1 | Manual | 32.3 ± 1.3 | b | 0.97 ± 0.25 | d | 31.8 ± 6.5 | e |
Data shown are means of 100 seeds/20 plants from five different lots ± sd. SW, mean thousand seed weight in mg; PW15, mean dry shoot mass at 15 DAS in mg/plant; PW28, mean dry shoot mass at 28 DAS in mg/plant; sd, standard deviation. Self, self-pollination; manual, manual pollination of emasculated flowers; self restr. = self-pollination of a restricted number of flowers (five to six) per plant. Sig., Different letters indicate significant differences between the lines (P < 0.001).
Shoot dry weights were determined from 35-d-old plants (five individuals per genotype) for the 169 crosses. Heritability (h2) of biomass production, estimated by parent-offspring regression, was 0.69 ± 0.05 with P < 0.001. Mid-parent-heterosis (MPH) determined in these 169 crosses varied between −33.8% and 150.9% (Fig. 1), and best-parent-heterosis (BPH) ranged from −42.6% to 140.5%. Of these, 44 crosses with high heterosis for shoot biomass production (the upper quartile with MPH ranging from 39% to 150.9%), and eight additional crosses with lower heterosis were selected for further analysis. In five replicated experiments shoot dry weight of 28-d-old plants, all of which were still in their vegetative phase, was determined. Twenty-nine (56%) of these 52 crosses showed significant (P < 0.05) MPH, and 23 (44%) crosses also showed significant (P < 0.05) BPH (Table II).
Figure 1.
Mid-parent-heterosis (MPH) for dry shoot mass. MPH shows continuous variation in 169 F1 hybrids derived from 63 Arabidopsis accessions crossed to three reference accessions Col-0, C24, and Nd. The upper quartile of 44 crosses with high heterosis and additional 8 crosses with lower heterosis were selected for further analysis.
Table II.
Mid-parent-heterosis and best-parent-heterosis in 29 F1 hybrids
| Cross | MPH ± sd | Sig. | BPH ± sd | Sig. |
|---|---|---|---|---|
| Ak-1 × C24 | 53.0 ± 30.9 | ** | 18.2 ± 15.6 | ** |
| Cl-0 × C24 | 47.6 ± 13.8 | ** | 30.3 ± 20.6 | * |
| Col-0 × C24 | 61.0 ± 22.9 | ** | 39.7 ± 22.6 | ** |
| Cvi × C24 | 30.2 ± 17.9 | * | −0.7 ± 23.4 | ns |
| Da(1)-12 × C24 | 95.2 ± 48.3 | ** | 90.5 ± 37.2 | ** |
| Dijon M × C24 | 71.6 ± 40.1 | * | 70.8 ± 40.7 | * |
| Dr-0 × C24 | 53.2 ± 22.6 | ** | 37.7 ± 24.4 | ** |
| Dra-0 × C24 | 50.8 ± 6.3 | ** | 33.7 ± 12.4 | * |
| El-0 × Nd | 35.4 ± 7.7 | * | 29.9 ± 8.5 | * |
| Enkh D × C24 | 63.7 ± 34.5 | ** | 53.3 ± 42.0 | * |
| Ep-0 × C24 | 65.8 ± 21.5 | ** | 41.1 ± 27.5 | * |
| Er-0 × C24 | 26.1 ± 17.8 | ** | 4.7 ± 22.8 | ns |
| Gr × C24 | 44.3 ± 16.1 | * | 30.9 ± 10.5 | * |
| Gr × Col | 36.2 ± 13.2 | * | 12.3 ± 13.7 | * |
| HOG × C24 | 60.3 ± 18.8 | * | 42.1 ± 21.4 | ns |
| Ler × C24 | 96.8 ± 28.1 | ** | 85.4 ± 26.8 | ** |
| Ler × Col | 68.4 ± 39.6 | ** | 58.7 ± 45.6 | * |
| Lu × C24 | 33.0 ± 25.0 | * | 19.2 ± 15.3 | ns |
| Nd × C24 | 45.4 ± 30.3 | * | 32.6 ± 30.1 | * |
| Old × C24 | 49.6 ± 22.1 | * | 32.2 ± 22.0 | * |
| Oy × C24 | 95.2 ± 37.1 | * | 89.1 ± 33.9 | * |
| RLD-1 × C24 | 79.2 ± 13.5 | ** | 63.8 ± 18.3 | * |
| RLD-1 × Col | 64.5 ± 19.6 | ** | 64.0 ± 14.7 | ** |
| RLD-1 × Nd | 36.8 ± 8.2 | ** | 33.4 ± 10.1 | ** |
| Rsch × C24 | 40.6 ± 12.5 | * | 32.4 ± 16.5 | * |
| Rubezhnoe-1 × C24 | 54.7 ± 15.4 | * | 36.2 ± 22.3 | ns |
| Sorbo × C24 | 48.2 ± 20.7 | * | 34.8 ± 18.3 | * |
| Te × C24 | 30.7 ± 12.0 | ** | 9.7 ± 15.6 | ns |
| Ws × C24 | 51.2 ± 8.5 | ** | 36.5 ± 12.8 | * |
MPH was calculated from mean dry shoot weight of four plants in five replicated experiments. MPH, mid-parent-heterosis in %; sd, standard deviation; sig., significance level. ** significant at P < 0.01; * significant at P < 0.05; ns, not significant.
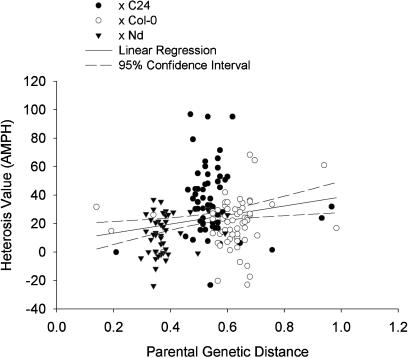
We estimated the parental genetic distances between the 63 accessions and the three parental reference lines for the 169 crosses. A distance matrix was deduced from pairwise comparisons of genotypic data based on 115 single nucleotide polymorphism (SNP)-based markers. We performed a linear regression of heterosis for shoot biomass against genetic distance between the parental lines, using absolute MPH (AMPH) as heterosis measure. While the regression was significant (P < 0.05), it accounted for only 1.9% of the variance. The scatter plot (Fig. 2) illustrates lack of correlation between parental genetic distance and mid-parent-heterosis for dry shoot mass.
Figure 2.
Lack of correlation between parental genetic distance and MPH for dry shoot mass. Parental genetic distance (GD) was calculated from SNP-typing data (115 markers), absolute mid-parent-heterosis (AMPH) was calculated as (mean F1 − mean P) from means of five plants per parental and reciprocal hybrid line, in 169 Arabidopsis F1 hybrids. Data points are labeled according to the reference line used in the cross (C24, Col-0, or Nd).
The cross Col-0/C24 exhibited highly significant MPH (61.0% ± 22.9%) and BPH (39.7% ± 22.6%). For this cross, a recombinant inbred line (RIL) population has been established in the authors' lab. Therefore, it was chosen for a detailed analysis of: (1) the F1 and F2 shoot dry mass values (mean and variance), (2) the developmental stage at which shoot biomass heterosis occurs, and (3) the influence of different light conditions (intensity) on the degree of heterosis.
Shoot Dry Mass Heterosis in the Combination Col-0/C24
Comparison of P, F1, and F2
To estimate biomass production in the F1 and the F2 of the combination Col-0/C24, shoot dry weights were determined 15 and 28 DAS for plants cultivated at 120 μmol m−2 s−1 light. Plants grown from manually pollinated seeds were used for comparisons between reciprocal F1 (C24 × Col-0 F1, Col-0 × C24 F1) and parents (C24 × C24, Col-0 × Col-0), as the F1 were produced by manual pollination of the respective mother. Comparisons of the F2 (C24 × Col-0 F2, Col-0 × C24 F2) and the parents (C24 and Col-0) were done with plants from self-pollinated seeds, as the F2 were obtained through self-pollination of F1 plants. While the F1 showed 33.3% to 63.2% higher means of shoot dry weights but similar coefficient of variation (CV) in comparison to the parents, the F2 had only 17.5% to 23.7% higher mean shoot dry weight but larger CV (Fig. 3).
Figure 3.
Mean dry shoot mass and coefficient of variation (CV) in P, F1 and F2 of the combination Col-0/C24. A, Mean dry shoot mass at 15 DAS (left axis) and 28 DAS (right axis). Means of at least 16 plants ± sd are shown. B, Coefficients of variation at 15 and 28 DAS. The analysis shows the defining characteristics of heterosis; superior performance of the F1, and reduction of the effect in the F2. For comparisons between F1 and parents, plants grown from manually pollinated seeds were used, comparisons of the F2 and the parents were done with plants from self-pollinated seeds.
Occurrence of Heterosis in Different Phases of Vegetative Growth and under Different Light Intensities in the Combination Col-0/C24
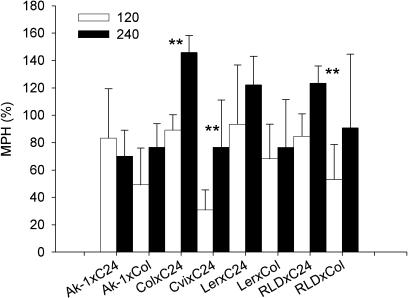
Differences in shoot dry weight between parental lines and F1 of the combination Col-0/C24 could be detected as early as 10 DAS in material grown at photon flux densities of 60, 120, or 240 μmol m−2 s−1 (Fig. 4). The superior performance of the Col-0/C24 F1 hybrids in comparison to their parents ranged from 42% to 60% for plants 10 DAS at both low (60 μmol m−2 s−1) and intermediate (120 μmol m−2 s−1) light intensities. A similar MPH was observed for plants cultivated for 25 d under these conditions (Fig. 5). In sharp contrast, plants grown at 240 μmol m−2 s−1 had significantly (P < 0.001) higher MPH than those grown at lower light intensities. This enhanced performance of the Col-0/C24 F1 hybrids is highlighted by an MPH of 161% for shoot dry mass (Fig. 5). In an additional experiment, eight F1 hybrids and their parents were grown at 120 and 240 μmol m−2 s−1, and dry shoot mass determined after 25 d (Fig. 6). In addition to Col-0 × C24, only two further crosses, Cvi × C24, RLD-1 × C24, showed a significant difference (P < 0.01) in MPH between light intensities.
Figure 4.
Mean dry shoot mass at 10 DAS of plants grown at three photon flux densities. Photon flux densities are expressed in PAR. Data represent means of at least 14 plants ± sd.
Figure 5.
MPH for dry shoot mass in Col-0/C24 at different phases of vegetative growth and under different light intensities. Photon flux densities are expressed in PAR. MPH was calculated from three replicates with 12 plants each, data shown are means ± sd.
Figure 6.
MPH for shoot biomass in 8 crosses grown at 120 and 240 μmol m−2 s−1. Photon flux densities are expressed in PAR. Heterosis was calculated as mean of 9 plants from 3 replicates. **, Significant difference (P < 0.01).
Table III displays the relative and absolute growth rates (RGR and AGR) of parental and hybrid lines of the cross Col-0/C24 until 25 DAS. The growth rates at 120 and 240 μmol m−2 s−1 were broken down into two phases, an early vegetative phase (0–15 DAS), i.e. until the earliest time point at which significant weight differences were found, and a late vegetative phase (15–25 DAS) until just before flowering of the parents. At 120 μmol m−2 s−1 RGRs differed significantly between parents and F1 hybrids in the early phase only, indicating that major differences in plant size are established early in development and only maintained in later developmental stages. At 240 μmol m−2 s−1, RGRs are significantly different between parents and F1 hybrids throughout the entire vegetative phase.
Table III.
Relative and absolute growth rates of parental and hybrid lines in two developmental phases and at two different light intensities
| Phase | 0–15 | 15–25 | 0–15 | 15–25 | |
|---|---|---|---|---|---|
| Genotype | PFD | 120 | 120 | 240 | 240 |
| C24 | RGR | 0.20 ± 0.02 a | 0.27 ± 0.01 a | 0.20 ± 0.01 a | 0.28 ± 0.01 a |
| Col-0 | RGR | 0.22 ± 0.01 a | 0.30 ± 0.01 a | 0.21 ± 0.02 a | 0.31 ± 0.01 a |
| C24 × Col-0 | RGR | 0.26 ± 0.01 b | 0.28 ± 0.01 a | 0.23 ± 0.02 b | 0.32 ± 0.01 b |
| Col-0 × C24 | RGR | 0.27 ± 0.01 b | 0.29 ± 0.01 a | 0.24 ± 0.01 b | 0.33 ± 0.01 b |
| C24 | AGR | 0.03 ± 0.02 a | 0.45 ± 0.08 a | 0.07 ± 0.02 a | 0.82 ± 0.10 a |
| Col-0 | AGR | 0.04 ± 0.02 a | 0.47 ± 0.14 a | 0.06 ± 0.01 a | 0.90 ± 0.11 a |
| C24 × Col-0 | AGR | 0.07 ± 0.03 b | 0.68 ± 0.12 b | 0.11 ± 0.02 b | 2.30 ± 0.13 b |
| Col-0 × C24 | AGR | 0.07 ± 0.02 b | 0.63 ± 0.13 b | 0.10 ± 0.01 b | 2.12 ± 0.34 b |
RGR, Relative growth rate in d−1. AGR, Absolute growth rate in mg d−1. PFD, Photon flux density in μmol m−2 s−1. 0–15: early vegetative phase (0–15 DAS); 15–25: late vegetative phase (15–25 DAS). Different letters indicate significant differences between the lines (P < 0.05).
Analysis of Heterosis in Different Plant Organs in the Combination Col-0/C24
Growth of the aerial parts of a plant also depends on the development of the root system. We analyzed root growth in F1 and parents of the cross Col-0/C24 in an in vitro system on vertical petri dishes (Stitt and Feil, 1999). The roots grow on the agar surface, allowing easy access to the root system. This is in contrast to Müssig et al. (2003), who optimized their experimental system for prolonged root growth in the agar of vertical plates. At 7 DAS the Col-0/C24 F1 hybrids displayed intermediate root length, and at 10 and 15 DAS the Col-0/C24 F1 hybrids had reached a root length similar to the (better) parent Col-0 (Table IV). Shoot and root dry mass were determined at 15 DAS from vertical plates. Results for shoot growth were comparable to those obtained in soil (Fig. 3, Table II): significant differences between parents (P < 0.001), and between parents and F1 hybrids (P < 0.001), and a significant (P < 0.001) MPH for shoot mass (54.6% ± 15.4%). We observed significant heterosis for root mass at 15 DAS, with MPH = 56.9% ± 25.9% (P < 0.001). No significant MPH for root length at 15 DAS could be detected (P = 0.069). Linear regression of shoot mass against root mass was significant (P < 0.001) with R2 = 0.724. Linear regression of shoot mass against root length was not significant (P = 0.192). Length and density of root hairs were determined on horizontal plates where the roots grew into the agar-solidified medium. At 15 DAS, root hairs of the Col-0/C24 F1 hybrids were significantly (P < 0.05) longer than those of either parent (Table V), with MPH = 41.3% ± 1.9%. Root hair density of the F1 hybrids was similar to that found in parent C24, which showed higher root hair density than Col-0.
Table IV.
Root dry weight and length of primary root of Arabidopsis grown on vertical agar plates at 120 μmol m−2 s−1
| Root Length | Root Length | Root Length | Root Dry Mass | Shoot Dry Mass | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 7 DAS | 10 DAS | 15 DAS | 15 DAS | 15 DAS | ||||||
| C24 | 9.0 ± 2.6 | a | 11.6 ± 2.2 | a | 12.5 ± 3.2 | a | 0.19 ± 0.04 | a | 0.50 ± 0.06 | a |
| Col-0 | 15.9 ± 3.4 | c | 18.3 ± 4.1 | b | 18.9 ± 3.9 | b | 0.29 ± 0.07 | b | 0.61 ± 0.06 | b |
| C24 × Col-0 F1 | 13.7 ± 2.4 | b | 16.8 ± 3.3 | b | 17.7 ± 4.2 | b | 0.35 ± 0.06 | b | 0.85 ± 0.09 | c |
| Col-0 × C24 F1 | 13.8 ± 2.3 | b | 16.4 ± 3.3 | b | 17.3 ± 3.6 | b | 0.34 ± 0.06 | b | 0.84 ± 0.07 | c |
Data represent means of 60 plants ± sd, of length of primary root (in mm) at 7, 10, and 15 DAS, and of root and shoot dry weight (in mg) at 15 DAS, from two independent experiments. Different letters indicate significant differences between the genotypes (P < 0.001).
Table V.
Root hair length and density of Arabidopsis grown on horizontal agar plates at 120 μmol m−2 s−1
| Line | Root Hair Length | Root Hair Density | ||
|---|---|---|---|---|
| C24 | 0.86 ± 0.30 | b | 65 ± 11 | ab |
| Col-0 | 0.62 ± 0.17 | a | 59 ± 7 | a |
| C24 × Col-0 F1 | 1.08 ± 0.24 | c | 68 ± 6 | b |
| Col-0 × C24 F1 | 1.03 ± 0.24 | c | 67 ± 7 | b |
Data represent means of 30 roots ± sd, of length (in mm) and density (in mm−1) of root hairs at 15 DAS. Significant differences between lines were determined by ANOVA and Tukey's HSD (P < 0.01), and are indicated by different letters.
We investigated a possible relationship between leaf area or rosette diameter versus shoot dry mass, which is a prerequisite for nondestructive analysis of biomass heterosis. Area of the largest leaf and rosette diameter was measured at 10 DAS, and shoot biomass determined at 15 DAS. Significant differences between genotypes in all traits measured could be detected (Table VI). Area of the largest leaf appeared to be the better indicator for shoot mass than rosette diameter; linear regression of shoot dry weight against leaf area revealed a significant positive relationship with R2 = 0.61 and P < 0.001. In contrast, linear regression of shoot dry weight against rosette diameter only gave R2 = 0.27, P < 0.001. There was a significant Pearson correlation between heterosis for shoot biomass and heterosis for leaf area (R2 = 0.85; P < 0.01).
Table VI.
Leaf area, rosette diameter, and dry shoot biomass
Area of the largest leaf (in mm2) and rosette diameter (in cm) at 10 DAS, and dry shoot mass (in mg) at 15 DAS of Arabidopsis seedlings grown at 120 μmol m−2 s−1. Data shown are means of 135 seedlings ± sd. Significant differences (Sig.) between lines (P < 0.01) were determined by ANOVA and Tukey's HSD, and are indicated by different letters.
| Line | Leaf Area | Sig. | Rosette Diameter | Sig. | Shoot Biomass | Sig. |
|---|---|---|---|---|---|---|
| C24 | 0.10 ± 0.03 | a | 0.83 ± 0.14 | b | 2.18 ± 0.632 | a |
| Col-0 | 0.09 ± 0.02 | a | 0.63 ± 0.11 | a | 2.10 ± 0.425 | a |
| C24 × Col-0 | 0.15 ± 0.03 | b | 0.84 ± 0.11 | b | 3.12 ± 0.488 | b |
| Col-0 × C24 | 0.14 ± 0.04 | b | 0.74 ± 0.08 | ab | 2.84 ± 0.550 | b |
DISCUSSION AND CONCLUSION
The study presented here constitutes the largest and most systematic survey of heterosis of biomass production hitherto reported in Arabidopsis. The data collected confirm the widespread occurrence of heterosis in Arabidopsis, and identify numerous useful crosses for detailed analyses of the phenomenon.
Systematic surveys for heterosis of agronomic characters have been performed in several crop species, e.g. grain amaranths (Amaranthus cruentus, A. hypockondriacus; Lehmann et al., 1991), maize (Parentoni et al., 2001; Betran et al., 2003), tomato (Lycopersicon esculentum; Makesh et al., 2002), and rice (Oryza sativa; Jiang et al., 2002; Verma et al., 2002). The number of lines analyzed in these studies are comparable to those used in our survey in Arabidopsis. Previous studies in Arabidopsis analyzed diallels of 5 to 7 ecotypes (Griffing and Langridge, 1963; El Asmi, 1974; Corey et al., 1976). In our analysis of 169 Arabidopsis crosses we detected a high heritability (69%) for biomass production, confirming the suitability of this trait for genetic studies. In crop plants heritabilities for biomass production ranging from 50% to 85% have been reported (Alza and Fernandez-Martinez, 1997 in wheat [Triticum aestivum]; Hoi et al., 1999 in oat [Avena sativa]; Annicchiarico et al., 1999 in clover [Trifolium pretense]; Przulj and Momcilovic, 2001 in barley [Hordeum vulgare]). We found surprisingly large heterosis for shoot biomass in F1 hybrids of several Arabidopsis accessions, up to 97% for Ler × C24 under standard conditions, and 161% for Col-0 × C24 under high light conditions. As an inbreeding species, Arabidopsis is expected to display only low levels of heterosis (Becker and Link, 1999). Arabidopsis accessions could be considered inbred populations with very rare outcrossing events (Hoffmann et al., 2003) that were selected in/adapted to differing ecological conditions. Crosses between Arabidopsis accessions therefore mimic crosses between inbred lines of outbreeders. Another or an additional explanation could be the controlled growth conditions that were optimized to allow maximum growth. Barth et al. (2003) analyzed heterosis for six traits, including biomass, in five Arabidopsis hybrids. They found a comparable level of heterosis for biomass in the crosses Col-0 × C24 (60% versus 61% in our study) and C24 × Ws (55% versus 51%). Differing results occurred in the cross C24 × Aa-0 (140% versus 9%). This difference may be due to the use of different parental lines in the crosses, as Arabidopsis accessions are not always genetically homogeneous (Breyne et al., 1999).
In hybrid breeding programs, the most important and difficult task is the selection of parental lines and prediction of hybrid performance. In well documented breeding lines, relatedness, and consequently genetic distance, can be deduced from pedigree data (Helms et al., 1997). The development of molecular marker systems such as AFLPs, SSRs, and SNPs considerably facilitated the estimation of genetic distance, based on marker diversity, between any genotypes (Milbourne et al., 1997; Virk et al., 1999; Barth et al., 2002). The genetic distance estimates between the 63 Arabidopsis accessions analyzed in this study were derived from a similarity matrix calculated from 115 SNPs (Törjék et al., 2003, and unpublished data). These SNPs were developed to identify differences between accessions C24 and Col-0. Their use to estimate genetic distances between other accessions introduces an ascertainment bias. We could detect only an extremely weak relationship between parental genetic distance and amount of heterosis in the 63 Arabidopsis accessions studied. Similarly, Barth et al. (2003) could not detect a relationship between parental genetic distance and heterosis for biomass in five Arabidopsis hybrids. A positive correlation between genetic distance and heterosis has been reported for oilseed rape (Brassica napus; Riaz et al., 2001) and maize (Barbosa et al., 2003). In contrast, studies in other plant species often failed to detect a relationship between these two parameters (Cerna et al., 1997 in soybean [Glycine max]; Joyce et al., 1999 in clover; Liu et al., 1999 in wheat; Riday et al., 2003 in Medicago). Zhao et al. (1999) showed that in rice the relationship between molecular marker heterozygosity and heterosis is variable, depending on the germplasm used and the character analyzed. They concluded that a detailed characterization of the germplasm and an in-depth comprehension of the genetic basis of heterosis would be needed to develop strategies for utilizing molecular markers in hybrid performance prediction.
In our survey, no indication for the existence of separate heterotic groups in Arabidopsis was obtained. While hybrids of Col-0 and C24 show highly significant heterosis, these two varieties apparently do not define separate heterotic groups, because several accessions (including Cvi, Gr, Ler, and RLD) showed significant heterosis in crosses to both of them. Heterotic groups have been well characterized from pedigree and molecular marker analyses in maize (Smith et al., 1990; Barbosa et al., 2003), and have been proposed for sunflower (Helianthus annuus; Hongtrakul et al., 1997; Cheres and Knapp, 1998). Heterotic groups are initially identified through a series of combining ability studies, including diallel schemes that permit estimation of general and specific combining ability (Lehmann et al., 1991; Revilla et al., 2002). To correctly identify heterotic groups, a diallel between distantly and closely related Arabidopsis lines should be evaluated. Our analysis was a test-cross scheme that allows determination of general combining ability and selection of appropriate lines for a diallel to assess specific combining ability and heterotic groups.
The detailed analysis of the Col-0/C24 cross showed the defining characteristics of heterosis, i.e. superior performance of F1 and reduction in F2. Special care had to be taken to compare plants originating from similarly sized seeds produced by either manual pollination or selfing; C24 and Col-0 parental plants grown from selfed seeds reached less than one-half the weight of those from manually pollinated seeds. Ashby (1937) showed in tomato that hybrid seeds and embryos were larger than those of the parental lines, due to a larger cell number. Alonso-Blanco et al. (1999) reported that the Arabidopsis accession Cvi yielded 40% fewer seeds than Ler, but that Cvi seeds were almost twice as heavy. This is in agreement with our findings that reducing the number of developing siliques in hand pollinated parental lines C24 and Col-0 leads to seeds whose weight is similar to that of the hybrid seeds obtained by manual pollination.
We wanted to determine if rosette diameter and/or leaf area could be used as indicators of dry biomass production in Arabidopsis parental and heterotic hybrid lines. At 10 DAS, the time point of our leaf area and rosette diameter measurements, the relative growth rates of the F1 lines are significantly higher than those of the parents. The plants of all lines were in developmental stage 1.04 (Boyes et al., 2001), in agreement with Pérez-Pérez et al. (2002), who showed that most of the 188 Arabidopsis accessions in their analysis of leaf architecture, including Col-0 and C24, displayed the same vegetative developmental rates when cultured under the same conditions. A positive correlation between total leaf area and total dry mass has been reported for maize (Pavlikova and Rood, 1987), cotton (Gossypium hirsutum; Bhatt, 1987), and tomato (Rao et al., 1992). In contrast, Titok et al. (1994) found a discrepancy between biomass accumulation and leaf area development in hybrid tomato plants grown in vitro. Leister et al. (1999) showed in Arabidopsis that plant size measured by plant area estimation correlates with fresh weight. Rosette diameter does not only depend on leaf blade area, but to a large extent on petiole length. Leaf shape and the relative size of blade and petiole have been shown to vary between accessions (Pérez-Pérez et al., 2002) and depending on growth conditions. Tsukaya et al. (2002) found a differential genetic control of leaf petiole length and leaf blade expansion. At low light Arabidopsis plants show a shade avoidance phenotype characterized by increased petiole length and reduced leaf blade surface (Vandenbussche et al., 2003). However, due to their restricted size, petioles usually contribute less to dry biomass than leaf blades. In our experiments area of the largest leaf at this early stage showed better correlation with shoot biomass than rosette diameter, both in the F1 hybrids and the parental lines. Our findings indicate that image sequence analysis of total leaf area could be a suitable noninvasive method to estimate growth rates during early vegetative development of Arabidopsis.
We restricted the analyses of the Col-0/C24 crosses to the vegetative phase, until 28 DAS at 120 μmol m−2 s−1 and until 25 DAS at 240 μmol m−2 s−1 to avoid interference by different flowering times between parental and hybrid lines. A survey of incremental RGR (every 3 d) revealed a sharp decline after 35 and 32 DAS, respectively, for the parental lines (data not shown). Pérez-Pérez et al. (2002) noted in a survey of natural variation of leaf architecture in Arabidopsis that lamina growth was fastest in the early stages of leaf expansion in all studied leaves. The cold-night long-day pregermination regime used in our study lead to enhanced homogeneity of seed germination in different genotypes. Parental and hybrid lines from the cross Col-0/C24 all germinated at the same day. Events leading to the onset of heterosis, i.e. to the establishment of size differences between parents and hybrids, took place very early during development. Differences in shoot biomass, leaf size, and root growth could be detected as early as 10 DAS.
The occurrence of heterosis for biomass in early stages, and its maintenance until later stages has been reported for several plant species, including sorghum (Sorghum bicolor; Miller and Atkins, 1979), tomato (Rao et al., 1992), lisianthus (Eustoma grandiflorum; Ecker and Barzilay, 1993), and sweet pepper (Capsicum annuum; Mulge and Anand, 1997). El Asmi (1975) reported heterosis for rosette diameter in Arabidopsis at 19 DAS. Analyses aiming at the identification of genes involved in the onset of biomass heterosis in Arabidopsis should therefore concentrate on the early developmental stages. During the early vegetative growth phase, parents and hybrids displayed small but significant differences in RGR at all light intensities. However, only at 240 μmol m−2 s−1 were these differences in RGR maintained during the late vegetative growth phase. In concordance with these findings, the MPH for biomass changed only marginally between 15 and 25 DAS at 120 μmol m−2 s−1, whereas at elevated light intensity (240 μmol m−2 s−1), the superior performance of the Col-0/C24 F1 hybrids in comparison to their parents was enhanced dramatically. Taken together, these results indicate that differences in plant size are established early in development, and are then maintained throughout the vegetative growth period. Under beneficial conditions, e.g. higher light intensities, the F1 hybrids are able to sustain a higher relative growth rate to the end of the vegetative growth period, resulting in substantially higher heterosis values. A correlation between light intensity and expression of heterosis has also been reported for Antirrhinum majus (Haney et al., 1953). Small increases in relative growth rates between parental and hybrid lines have been shown to lead to large differences in size (Milborrow, 1998). A larger leaf area during seedling growth allows the F1 hybrids to absorb more light than their parents, potentially resulting in increased photosynthetic activity per plant. This has been demonstrated for cotton (Wells et al., 1988) and tomato (Rao et al., 1992).
In the Col-0/C24 combination, the F1 hybrids combined beneficial root traits from both parents: long roots of Col-0, longer root hairs and higher root hair density of C24. These results are in agreement with those obtained by Narang and Altmann (2001) for the same lines under phosphate deficient conditions. In contrast, in our experiments root hair length of the F1 hybrids surpassed that of both parents. This could be due to our phosphate sufficient growth conditions. Enlargement of the root system is a morphological adaptation that allows plants to efficiently acquire nutrients from the soil (Lynch, 1995). The better developed root system of the F1 hybrids could potentially lead to increased nutritional uptake to support elevated growth rates, thus contributing to heterosis for biomass production.
Our results also hint to the possible involvement of two different mechanisms leading to increased biomass production in the hybrids. Size differences are established very early during seedling development, independent of light intensity. Later during the vegetative phase a light-dependent mechanism seems to become active. This could be due to increased photosynthetic efficiency of the F1 hybrids, as indicated by the differential reaction to higher light intensity. The light-dependent mechanism appears to be genotype specific; only three of eight crosses analyzed displayed increased heterosis for biomass production at the high light intensity. A differential contribution of QTL depending on developmental stages has been described by several authors. In rice, Price and Tomos (1997) observed that root-length QTLs varied greatly with developmental stage. They identified one major QTL for seminal root growth at the early developmental stages, and one major QTL for adventitious root growth that became active at a later stage. Pérez-Pérez et al. (2002) detected 16 and 13 QTL affecting architecture of juvenile and adult leaves in Arabidopsis, respectively. Only 8 QTL were common to both developmental stages. Quesada et al. (2002) described a lack of correlation between the salinity responses during germination and vegetative growth. The map positions of the salt tolerance QTL detected for germination did not coincide with those obtained for vegetative growth. Their results suggested that different genetic controls regulate salt tolerance in different developmental stages in Arabidopsis.
The widespread occurrence of heterosis in the model plant Arabidopsis opens the possibility to investigate the genetic basis of this phenomenon using the tools of genetical genomics (Jansen and Nap, 2001). To this end we will analyze 400 Col-0/C24 RIL and their test-cross hybrids and subject selected lines to transcriptome and metabolome analyses together with parents and F1 hybrids.
MATERIALS AND METHODS
Plant Material
Seeds of 63 analyzed accessions were obtained from various sources: Col-0 from G. Rédei (University of Missouri at Columbia, MO); C24 from J.P. Hernalsteens (Vrije Universiteit Brussels); Ler from M. Koornneef (Wageningen University, The Netherlands); Cvi, Bch-1, Eil-0, Gr, Hi, Lip-0, Lm, Lu, Oy, Per, Rsch, Te, and Yo from S. Misera (Institut für Pflanzengenetik und Kulturpflanzenforschung, Gatersleben, Germany); all others from the Nottingham Stock Centre (NASC). Accessions were homogenised by single-seed propagation and bulk-amplified (Törjék et al., 2003). Reciprocal F1 hybrids were produced by hand-pollinating emasculated flowers of the respective mother plant, five to six flowers per plant. Production of F2 and propagation was by self-pollination.
Plant Cultivation
For growth and light experiments, plants were grown in 1:1 mixture of GS 90 soil and vermiculite (Gebrüder Patzer, Sinntal-Jossa, Germany). Seeds were germinated in growth chambers under a cold-night long-day regime (16 h fluorescent light [60, 120, or 240 μmol m−2 s−1] at 20°C and 75% relative humidity [RH]/8 h dark at 6°C and 75% RH) for 3 to 5 d before the seedlings were transferred to a long-day regime (16 h fluorescent light [60, 120, or 240 μmol m−2 s−1] at 20°C and 60% RH/8 h dark at 18°C and 75% RH). To avoid position effects, trays were rotated around the growth chamber every two days. For heterosis experiments, plants were grown in 96-well-trays under the same conditions as above in a randomized block design with six blocks and four replicates. Three plants were grown per replicate. To determine growth parameters at different light intensities, plants were grown at 60, 120, and 240 μmol m−2 s−1 in four independent experiments with four replicates of three plants each. Plants for leaf area and rosette diameter measurements were grown in a randomized block design with three blocks and five replicates. Nine plants were grown per replicate.
Data Collection
Shoot Dry Weight
Shoot dry weight was determined at several time points until flowering. Plants were placed in a vacuum oven at 80°C for 48 h. Relative growth rates were estimated by linear regression of the natural logarithm of shoot dry weight versus time (Wareing and Phillips, 1981), and seed weight was used for time point 0 DAS.
Root Growth
Seeds were surface sterilized in 70% ethanol and 20% NaOCl + 0.02% Triton X-100 prior to pregermination on damp filter paper for 2 d at 4°C. Seeds were then transferred to vertical plates containing half-strength Murashige and Skoog medium with 1% Suc and 0.8% agar. For each line, six plants were grown in five replicated plates in two independent experiments. The seedlings were cultivated in a growth chamber under the same conditions as soil grown plants. Primary root length was marked on the petri dish daily until 15 DAS. Root and shoot dry weight was determined 15 DAS. Root hair length and density was determined according to Narang and Altmann (2001). For each line, five plants were grown in three replicated horizontal plates in two independent experiments. A Leica Stereomicroscope MZ12.5 coupled to a Spot Camera, and Meta Imaging Series 4.6 Software (Universal Imaging, Downington, PA) was used for data acquisition and analysis.
Calculation of Heterosis
MPH and BPH were calculated as: MPH = (mean F1 − mean P)/mean P in %; BPH = (mean F1 − mean best P)/mean best P in % (Falconer and Mackay 1996). Expressing heterosis values relative to parental performance allows comparison of different crosses. Absolute MPH and BPH values, calculated as (mean F1 − mean P) and (mean F1 − mean best P), respectively, were used for statistical analyses (Lamkey and Edwards, 1999).
Estimation of Heritability
Heritability of biomass production was estimated by linear regression of the mean dry mass of the F1 hybrids against the mean dry mass of the parents (Falconer and Mackay, 1996).
Genetic Distance
Genetic distance (GD) was calculated as follows: GD = 1 − identity values. The identity values between the accessions were obtained with the BioEdit Sequence Alignment Editor (Hall, 1999) by pairwise comparison of genotype data determined for 115 SNP-based markers (Törjék et al., 2003) and K.J. Schmid, O. Törjék, R.C. Meyer, H. Schmuths, M.H. Hoffmann, T. Altmann, unpublished data.
Data Analyses
Statistical analyses were performed with Genstat for Windows V6.1 (Payne et al., 2002). Linear measures were square-root transformed, weight was log transformed. For comparisons between crosses, ANOVA and appropriate multiple comparison and two-sided t tests were used. Significant heterosis values were identified by t tests. Differences in RGR between generations were analyzed comparing the slopes of the linear regressions using a covariance analysis (Meerts and Garnier, 1996; Antunez et al., 2001).
Acknowledgments
We thank Melanie Lück, Monique Zeh, Cindy Marona, Anke Kalkbrenner, and Katrin Seehaus for excellent technical assistance and plant care.
This work was supported by the Bundesministerium für Bildung und Forschung GABI project (grant no. FK 0312275A/9), by the EU-Natural project (grant no. QLRT-2000-01097 to T.A.), by the Deutsche Forschungsgemeinschaft (grant no. AL387/6-1 to T.A. and R.C.M.), and by the Max-Planck-Society.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.033001.
References
- Alonso-Blanco C, Blankestijn-de Vries H, Hanhart CJ, Koornneef M (1999) Natural allelic variation at seed size loci in relation to other life history traits of Arabidopsis thaliana. Proc Natl Acad Sci USA 96: 4710–4717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alza JO, Fernandez-Martinez JM (1997) Genetic analysis of yield and related traits in sunflower (Helianthus annuus L) in dryland and irrigated environments. Euphytica 95: 243–251 [Google Scholar]
- Annicchiarico P, Piano E, Rhodes I (1999) Heritability of, and genetic correlations among, forage and seed yield traits in Ladino white clover. Plant Breed 118: 341–346 [Google Scholar]
- Antunez I, Retamosa EC, Villar R (2001) Relative growth rate in phylogenetically related deciduous and evergreen woody species. Oecologia 128: 172–180 [DOI] [PubMed] [Google Scholar]
- Ashby E (1937) Studies in the inheritance of physiological characters. III. hybrid vigour in the tomato. Part 1. manifestation of hybrid vigour from germination to the onset of flowering. Ann Bot (Lond) 1: 11–41 [Google Scholar]
- Barbosa AMM, Geraldi IO, Benchimol LL, Garcia AAF, Souza CL, Souza AP (2003) Relationship of intra- and interpopulation tropical maize single cross hybrid performance and genetic distances computed from AFLP and SSR markers. Euphytica 130: 87–99 [Google Scholar]
- Barth S, Busimi AK, Utz HF, Melchinger AE (2003) Heterosis for biomass yield and related traits in five hybrids of Arabidopsis thaliana L. Heynh. Heredity 91: 36–42 [DOI] [PubMed] [Google Scholar]
- Barth S, Melchinger AE, Lubberstedt T (2002) Genetic diversity in Arabidopsis thaliana L. Heynh. investigated by cleaved amplified polymorphic sequence (CAPS) and inter-simple sequence repeat (ISSR) markers. Mol Ecol 11: 495–505 [DOI] [PubMed] [Google Scholar]
- Becker HC, Link W (1999) Nutzen und Schaden der Heterosis in der Pflanzenzüchtung. In 50. Arbeitstagung der Vereinigung Österreichischer Pflanzenzüchter, Gumpenstein, pp 141–146
- Betran FJ, Ribaut JM, Beck D, de Leon DG (2003) Genetic diversity, specific combining ability, and heterosis in tropical maize under stress and nonstress environments. Crop Sci 43: 797–806 [Google Scholar]
- Bhatt JG (1987) Leaf growth, reproduction, growth and yield in cotton (Gossypium hirsutum L.). Z Acker Pflanzenbau 159: 264–268 [Google Scholar]
- Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, Gorlach J (2001) Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell 13: 1499–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breyne P, Rombaut D, Van Gysel A, Van Montagu M, Gerats T (1999) AFLP analysis of genetic diversity within and between Arabidopsis thaliana ecotypes. Mol Gen Genet 261: 627–634 [DOI] [PubMed] [Google Scholar]
- Cerna FJ, Cianzio SR, Rafalski A, Tingey S, Dyer D (1997) Relationship between seed yield heterosis and molecular heterozygosity in soybean. Theor Appl Genet 95: 460–467 [Google Scholar]
- Cheres MT, Knapp SJ (1998) Ancestral origins and genetic diversity of cultivated sunflower: coancestry analysis of public germplasm. Crop Sci 38: 1476–1482 [Google Scholar]
- Comings DE, MacMurray JP (2000) Molecular heterosis: a review. Mol Genet Metab 71: 19–31 [DOI] [PubMed] [Google Scholar]
- Corey LA, Matzinger DF, Cockerham CC (1976) Maternal and reciprocal effects on seedling characters in Arabidopsis thaliana. Genetics 82: 677–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow J (1952) Dominance and overdominance. In JW Gowen, ed, Heterosis. Iowa State College Press, Ames, IA, pp 282–297
- de Vienne D, Bost B, Fievet J, Zivy M, Dillmann C (2001) Genetic variability of proteome expression and metabolic control. Plant Physiol Biochem 39: 271–283 [Google Scholar]
- Dobzhansky T (1950) Genetics of natural populations. XIX. origin of heterosis through natural selection in populations of Drosophila pseudobscura. Genetics 35: 288–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker R, Barzilay A (1993) Quantitative genetic analysis of growth rate in lisianthus. Plant Breed 111: 253–256 [Google Scholar]
- El Asmi H (1974) Quantitative studies on heterosis in Arabidopsis thaliana (L.) Heynh. Arabidopsis Information Service 11: 15–16 [Google Scholar]
- El Asmi H (1975) Further analysis of heterosis and its expression for the rosette diameter length in Arabidopsis thaliana (L.) Heynh. Arabidopsis Information Service 12: 24–25 [Google Scholar]
- Falconer DS, Mackay TFC (1996) Introduction to quantitative genetics, Ed 4. Prentice Hall, Harlow, UK
- Geiger HH (1988) Epistasis and heterosis. In BS Weir, EJ Eisen, MM Goodman, G Namkoong, eds, 2nd International Conference on Quantitative Genetics. Sinauer, Sunderland, MA
- Griffing B, Langridge J (1963) Phenotypic stability of growth in the self-fertilized species Arabidopsis thaliana. In WD Hanson, HF Robinson, eds, Statistical Genetics and Plant Breeding, Vol 982. N A S – N C R Publ, Washington, DC, pp 368–394
- Griffing B, Zsiros E (1971) Heterosis associated with genotype environment interactions. Genetics 68: 443–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41: 95–98 [Google Scholar]
- Haney WJ, Gartner JB, Wilson GB (1953) The effect of light on the expression of heterosis. J Hered 44: 10–12 [Google Scholar]
- Helms T, Orf J, Vallad G, McClean P (1997) Genetic variance, coefficient of parentage, and genetic distance of six soybean populations. Theor Appl Genet 94: 20–26 [DOI] [PubMed] [Google Scholar]
- Hoffmann MH, Bremer M, Schneider K, Burger F, Stolle E, Moritz G (2003) Flower visitors in a natural population of Arabidopsis thaliana. Plant Biol 5: 491–494 [Google Scholar]
- Hoi SW, Holland JB, Hammond EG (1999) Heritability of lipase activity in oat caryopses. Crop Sci 39: 1055–1059 [Google Scholar]
- Hongtrakul V, Huestis GM, Knapp SJ (1997) Amplified fragment length polymorphisms as a tool for DNA fingerprinting sunflower germplasm: genetic diversity among oilseed inbred lines. Theor Appl Genet 95: 400–407 [Google Scholar]
- Jansen RC, Nap JP (2001) Genetical genomics: the added value from segregation. Trends Genet 17: 388–391 [DOI] [PubMed] [Google Scholar]
- Jiang TB, Li RH, Sun CQ, Wang XK (2002) Utilization of diverse rice ecotypes in heterosis breeding. Breed Sci 52: 107–113 [Google Scholar]
- Joyce TA, Abberton MT, Michaelson-Yeates TPT, Forster JW (1999) Relationships between genetic distance measured by RAPD-PCR and heterosis in inbred lines of white clover (Trifolium repens L.). Euphytica 107: 159–165 [Google Scholar]
- Lamkey KR, Edwards JW (1999) The quantitative genetics of heterosis. In JG Coors, S Pandey, eds, Genetics and Exploitation of Heterosis in Crops. American Society of Agronomy: Crop Science Society of America: Soil Science Society of America, Madison, WI, pp 31–48
- Langridge J (1962) A genetic and molecular basis for heterosis in Arabidopsis and Drososphila. Am Nat 96: 5–27 [Google Scholar]
- Lehmann JW, Clark RL, Frey KJ (1991) Biomass heterosis and combining ability in interspecific and intraspecific matings of grain amaranths. Crop Sci 31: 1111–1116 [Google Scholar]
- Leister D, Varotto C, Pesaresi P, Niwergall A, Salamini F (1999) Large-scale evaluation of plant growth in Arabidopsis thaliana by non-invasive image analysis. Plant Physiol Biochem 37: 671–678 [Google Scholar]
- Li ZK, Luo LJ, Mei HW, Wang DL, Shu QY, Tabien R, Zhong DB, Ying CS, Stansel JW, Khush GS, Paterson AH (2001) Overdominant epistatic loci are the primary genetic basis of inbreeding depression and heterosis in rice. I. Biomass and grain yield. Genetics 158: 1737–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XC, Ishiki K, Wang WX (2002) Identification of AFLP markers favorable to heterosis in hybrid rice. Breed Sci 52: 201–206 [Google Scholar]
- Liu Z-Q, Pei Y, Pu Z-J (1999) Relationship between hybrid performance and genetic diversity based on RAPD markers in wheat, Triticum aestivum L. Plant Breed 118: 119–123 [Google Scholar]
- Luo LJ, Li ZK, Mei HW, Shu QY, Tabien R, Zhong DB, Ying CS, Stansel JW, Khush GS, Paterson AH (2001) Overdominant epistatic loci are the primary genetic basis of inbreeding depression and heterosis in rice. II. grain yield components. Genetics 158: 1755–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J (1995) Root architecture and plant productivity. Plant Physiol 109: 7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makesh S, Puddan M, Ashok S, Rizwana BM (2002) Heterosis studies for quality and yield in tomato (Lycopersicon esculentum Mill.). Adv Plant Sci 15: 597–601 [Google Scholar]
- Meerts P, Garnier E (1996) Variation in relative growth rate and its components in the annual Polygonum aviculare in relation to habitat disturbance and seed size. Oecologia 108: 438–445 [DOI] [PubMed] [Google Scholar]
- Melchinger AE (1999) Genetic diversity and heterosis. In JG Coors, S Pandey, eds, The Genetics and Exploitation of Heterosis in Crops. American Society of Agronomy: Crop Science Society of America: Soil Science Society of America, Madison, WI, pp 99–118
- Milborrow BV (1998) A biochemical mechanism for hybrid vigour. J Exp Bot 49: 1063–1071 [Google Scholar]
- Milbourne D, Meyer R, Bradshaw JE, Baird E, Bonar N, Provan J, Powell W, Waugh R (1997) Comparison of PCR-based marker systems for the analysis of genetic relationships in cultivated potato. Mol Breed 3: 127–136 [Google Scholar]
- Miller IL, Atkins RE (1979) Comparisons of embryo weight and seedling growth in grain Sorghum parents and hybrids. Iowa State J Res 53: 273–290 [Google Scholar]
- Mitchell-Olds T (1995) Interval mapping of viability loci causing heterosis in Arabidopsis. Genetics 140: 1105–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi SA, Prasanna BM (2003) Analysis of genetic diversity in crop plants: salient statistical tools and considerations. Crop Sci 43: 1235–1248 [Google Scholar]
- Moll RH, Lonnquist JH, Fortuna JV, Johnson CE (1965) The relationship of heterosis and genetic divergence in maize. Genetics 52: 139–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monforte AJ, Tanksley SD (2000) Fine mapping of a quantitative trait locus (QTL) from Lycopersicon hirsutum chromosome 1 affecting fruit characteristics and agronomic traits: breaking linkage among QTLs affecting different traits and dissection of heterosis for yield. Theor Appl Genet 100: 471–479 [Google Scholar]
- Mulge R, Anand N (1997) Prediction of heterosis and combining ability for yield and yield characters at seedling stage in sweet pepper (Capsicum annuum L.). Indian J Genet Pl Br 57: 180–185 [Google Scholar]
- Müssig C, Shin GH, Altmann T (2003) Brassinosteroids promote root growth in Arabidopsis. Plant Physiol 133: 1261–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narang RA, Altmann T (2001) Phosphate acquisition heterosis in Arabidopsis thaliana: a morphological and physiological analysis. Plant Soil 234: 91–97 [Google Scholar]
- Parentoni SN, Magalhaes JV, Pacheco CAP, Santos MX, Abadie T, Gama EEG, Guimaraes PEO, Meirelles WF, Lopes MA, Vasconcelos MJV, Paiva E (2001) Heterotic groups based on yield-specific combining ability data and phylogenetic relationship determined by RAPD markers for 28 tropical maize open pollinated varieties. Euphytica 121: 197–208 [Google Scholar]
- Pavlikova E, Rood SB (1987) Cellular basis of heterosis for leaf area in maize. Can J Plant Sci 67: 99–104 [Google Scholar]
- Payne R, Baird D, Cherry M, Gilmour A, Harding S, Kane A, Lane P, Murray D, Soutar D, Thompson R, et al (2002) Genstat Release 6.1 Reference Manual. VSN International, Oxford
- Pérez-Pérez JM, Serrano-Cartagena J, Micol JL (2002) Genetic analysis of natural variations in the architecture of Arabidopsis thaliana vegetative leaves. Genetics 162: 893–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AH, Tomos AD (1997) Genetic dissection of root growth in rice (Oryza sativa L.). II: mapping quantitative trait loci using molecular markers. Theor Appl Genet 95: 143–152 [Google Scholar]
- Przulj N, Momcilovic V (2001) Genetic variation for dry matter and nitrogen accumulation and translocation in two-rowed spring barley. I. dry matter translocation. Eur J Agron 15: 241–254 [Google Scholar]
- Quesada V, Garcia-Martinez S, Piqueras P, Ponce MR, Micol JL (2002) Genetic architecture of NaCl tolerance in Arabidopsis. Plant Physiol 130: 951–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao NKS, Bhatt RM, Anand N (1992) Leaf area, growth and photosynthesis in relation to heterosis in tomato. Photosynthetica 26: 449–454 [Google Scholar]
- Rédei GP (1962) Single locus heterosis. Z Vererbungsl 93: 164–170 [Google Scholar]
- Revilla P, Malvar RA, Cartea ME, Soengas P, Ordas A (2002) Heterotic relationships among European maize inbreds. Euphytica 126: 259–264 [Google Scholar]
- Riaz A, Li G, Quresh Z, Swati MS, Quiros CF (2001) Genetic diversity of oilseed Brassica napus inbred lines based on sequence-related amplified polymorphism and its relation to hybrid performance. Plant Breed 120: 411–415 [Google Scholar]
- Riday H, Brummer EC, Campbell TA, Luth D, Cazcarro PM (2003) Comparisons of genetic and morphological distance with heterosis between Medicago sativa subsp sativa and subsp falcata. Euphytica 131: 37–45 [Google Scholar]
- Sharma RK, Griffing B, Scholl RL (1979) Variations among races of Arabidopsis thaliana for survival in limited carbon dioxide. Theor Appl Genet 54: 11–16 [DOI] [PubMed] [Google Scholar]
- Shull GH (1948) What is heterosis? Genetics 33: 439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith OS, Smith JSC, Bowen SL, Tenborg RA, Wall SJ (1990) Similarities among a group of elite maize inbreds as measured by pedigree, F1 grain yield, grain yield, heterosis, and RFLPs. Theor Appl Genet 80: 833–840 [DOI] [PubMed] [Google Scholar]
- Stitt M, Feil R (1999) Lateral root frequency decreases when nitrate accumulates in tobacco transformants with low nitrate reductase activity: consequences for the regulation of biomass partitioning between shoots and root. Plant Soil 215: 143–153 [Google Scholar]
- Titok VV, Lemesh VA, Rusinova OV, Podlisskikh VL (1994) Leaf area, chlorophyll content and biomass of tomato plants and their heterotic hybrids under in vitro culture. Photosynthetica 30: 255–260 [Google Scholar]
- Törjék O, Berger D, Meyer R, Müssig C, Schmid K, Rosleff-Sörensen T, Weisshaar B, Mitchell-Olds T, Altmann T (2003) Establishment of a high-efficiency SNP-based framework marker set for Arabidopsis. Plant J 36: 122–140 [DOI] [PubMed] [Google Scholar]
- Tsaftaris SA (1995) Molecular aspects of heterosis in plants. Physiol Plant 94: 362–370 [Google Scholar]
- Tsukaya H, Kozuka T, Kim GT (2002) Genetic control of petiole length in Arabidopsis thaliana. Plant Cell Physiol 43: 1221–1228 [DOI] [PubMed] [Google Scholar]
- Vandenbussche F, Vriezen WH, Smalle J, Laarhoven LJJ, Harren FJM, Van Der Straeten D (2003) Ethylene and auxin control the Arabidopsis response to decreased light intensity. Plant Physiol 133: 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma OP, Santoshi US, Srivastava HK (2002) Heterosis and inbreeding depression for yield and certain physiological traits in hybrids involving diverse ecotypes of rice (Oryza sativa L.). J Genet Breed 56: 267–278 [Google Scholar]
- Virk PS, Pooni HS, Syed NH, Kearsey MJ (1999) Fast and reliable genotype validation using microsatellite markers in Arabidopsis thaliana. Theor Appl Genet 98: 462–464 [Google Scholar]
- Wareing PF, Phillips IDJ (1981) Growth and differentiation in plants, Ed 3. Pergamon Press, Oxford
- Wells R, Meredith WRJ, Williford JR (1988) Heterosis in upland cotton II. relationship of leaf area to plant photosynthesis. Crop Sci 28: 522–525 [Google Scholar]
- Xiao J, Li J, Yuan L, Tanksley SD (1995) Dominance is the major genetic basis of heterosis in rice as revealed by QTL analysis using molecular markers. Genetics 140: 745–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SB, Li JX, Tan YF, Gao YJ, Li XH, Zhang QF, Maroof MAS (1997) Importance of epistasis as the genetic basis of heterosis in an elite rice hybrid. Proc Natl Acad Sci USA 94: 9226–9231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao MF, Li XH, Yang JB, Xu CG, Hu RY, Liu DJ, Zhang Q (1999) Relationship between molecular marker heterozygosity and hybrid performance in intra- and inter-subspecific crosses of rice. Plant Breed 118: 139–144 [Google Scholar]