Abstract
Over the last decade there has been considerable progress in the discovery and development of biomarkers of kidney disease, and several have now been evaluated in different clinical settings. While there is a growing literature on the performance of various biomarkers in clinical studies, there is limited information on how these biomarkers would be utilized by clinicians to manage patients with acute kidney injury (AKI). Recognizing this gap in knowledge, we convened the 10th Acute Dialysis Quality Initiative (ADQI) meeting to review the literature on biomarkers in AKI and their application in clinical practice. We asked an international group of experts to assess four broad areas for biomarker utilization for AKI: risk assessment, diagnosis and staging; differential diagnosis; prognosis and management and novel physiological techniques including imaging. This article provides a summary of the key findings and recommendations of the group, to equip clinicians to effectively use biomarkers in AKI.
Keywords: Acute kidney injury, acute renal failure, biomarkers, diagnosis, prognosis, surveillance, monitoring, staging, differential diagnosis, management
Introduction
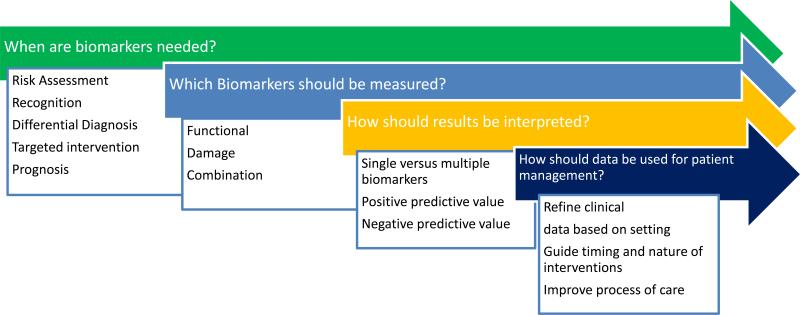
A rise in serum creatinine or a reduction in urine output are the current hallmarks for recognizing acute kidney injury (AKI). Recent standardization of diagnostic and staging criteria for AKI has defined the epidemiology of this syndrome in outpatient clinics, emergency rooms, hospital wards and intensive care units (ICUs) worldwide1-4. Clinicians are now better informed on the consequences of even small changes in renal function, however in most circumstances this has not translated into an improvement in management of AKI 5. Several authoritative publications have lamented that the lack of biomarkers for kidney injury has limited progress in improving outcomes of this devastating disorder6-8. This has led to an intense interest in the discovery and validation of novel AKI biomarkers. However, despite their availability, kidney-specific biomarkers have seen very limited clinical application, despite availability for clinical use in several regions worldwide9, 10. Most studies have focused on demonstrating that kidney biomarkers appear at earlier time points than serum creatinine, however they have not been integrated with creatinine and urine output changes to enhance management of AKI. We believe the lack of utilization reflects the absence of specific clinical recommendations for applying these emerging biomarkers to optimize patient management. Given the multitude of emerging biomarkers with different test characteristics (in serum and urine), diverse platforms for evaluation, and the large number of studies emphasizing the potential benefit of one biomarker over another, it is not surprising that clinicians refrain from using these assays in clinical practice. Additionally, concern about the costs and reimbursement for biomarker assays can dampen enthusiasm for clinical implementation. For clinical biomarker utility, clinicians must ascertain when biomarkers are needed, which ones to use, and how to interpret the data and utilize the information to improve patient management. Clinicians managing patients with AKI require information on when biomarkers are needed, which ones should be used, how to interpret the results and how to utilize the information to manage patients through the course of AKI (Fig 1) These key issues are pertinent for the efficient adoption of biomarkers in clinical practice but have not previously been well defined in AKI diagnostics.
Fig 1. Clinical need for biomarkers to improve management of acute kidney injury.
Several components typically need to be considered for each decision that guides biomarker utilization. These key issues are pertinent for the efficient adoption of biomarkers in clinical practice.
Recognizing this gap in knowledge, we convened the 10th Acute Dialysis Quality Initiative (ADQI) meeting to review the literature on biomarkers in AKI and their application in clinical practice. We recognized that the term “biomarker” is inclusive of any “characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” 11. Based on the methodology from prior ADQI conferences (detailed in Appendix), we convened an interdisciplinary, international group of experts and asked them to perform a critical analysis of the evidence available and to develop evidence-based consensus recommendations for the use of AKI biomarkers in clinical practice and identify areas for future research. This report summarizes the key discussion topics and conclusions of the conference.
Opportunities and challenges for utilizing Biomarkers for AKI management
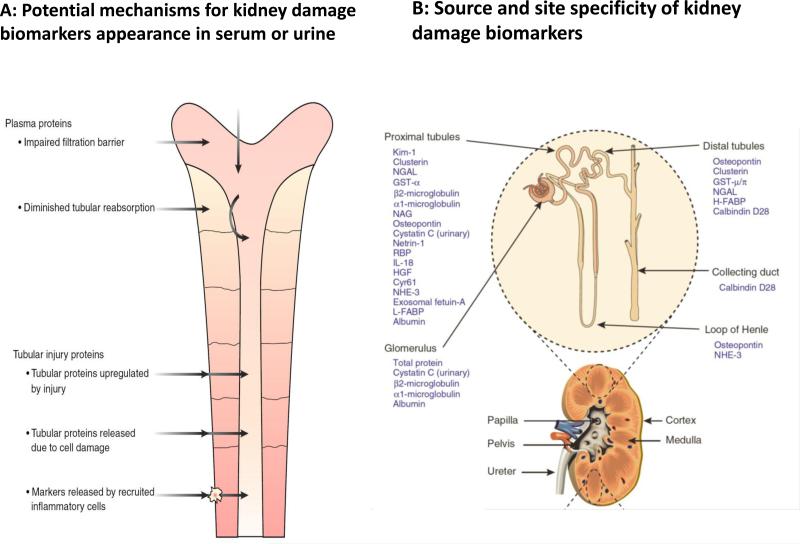
Over the last few years, the biomarker field for AKI has rapidly expanded with the identification of different molecules emanating from the injured kidney or reflecting altered kidney function10, 12, 13. These molecules have ranged from constitutive proteins released by the damaged kidney to molecules up regulated in response to injury or non-renal tissue products that are filtered, reabsorbed, or secreted by the kidney14 (Fig 2). These biomarkers also include proteins or encapsulated molecules in exosomes and more recently microRNA's 12. These biomarkers of kidney damage can be utilized to delineate the nature, magnitude and site of injury based on their specificity. Several studies have described the predictive utility of the biomarkers in earlier diagnosis of AKI, differentiation of the nature and severity of injury or providing prognostic information on the course and outcomes of AKI 4, 9, 15-17. Recent data from several studies have shown that specific markers of kidney damage including KIM-1, NGAL, L-FABP, interleukin-18: IL-18 may be elevated prior to an increase in serum creatinine18-20. Similarly, changes in urine flow may occur earlier than serum creatinine 21 and urinary sediment analysis for cellular and cast scores can serve as valid measures of AKI severity. Some studies have already focused on utilizing these biomarkers to guide interventions and define the optimal time points for initiating dialysis 22. Finally, in studies incorporating biomarker patterns, levels of change have been correlated with the severity (stage) of AKI and assessed for their predictive value for acute dialysis initiation, mortality, or renal functional recovery15. Based on this large body of accumulating data, it is evident that biomarkers have potential utility in several domains of the assessment and management of AKI (Fig 3)23. However, in most biomarker studies, proof of the specificity of biomarker changes to diagnose actual changes in renal pathology (the “gold standard”) has been lacking. Instead, the current, functional AKI biomarkers (serum creatinine and urine output changes) have served as the “bronze standard” for the majority of clinical validation studies of novel AKI biomarkers, in some cases supplemented by adjudicated case definitions using additional clinical information, but only exceptionally including kidney biopsy information 24. Additionally, biomarker studies have generally not included newer modes of imaging e.g. Doppler ultrasound, resulting in poor correlations of functional changes with kidney damage 25, 26. Finally, very limited information exists on the utility of incorporating these newer biomarkers with creatinine and urine output data to enhance the clinical care of patients with AKI. For instance, The TRIBE (Translational Research Involving Biomarkers and Endpoints) study evaluated 1219 adults undergoing cardiac surgery and reported that urine IL-18 and urine and plasma NGAL levels peaked within 6 hours after surgery19. A clinical prediction model for AKI had an area under the receiver-operating characteristic curve (AUC) of 0.69. Urine IL-18 and plasma NGAL improved the AUC to 0.76 and 0.75, respectively.
Fig 2. Potential mechanisms and specificity of urinary biomarkers of kidney damage.
Panel A: Biomarkers may appear in the urine through several mechanisms including filtration across the glomerular basement membrane (e.g. proteinuria, micro albuminuria); increased (or decreased) passive release (e.g. alpha and pi GST); active induction (or repression) followed by release and/or secretion (e.g. NGAL, KIM-1) and decreased (or increased) resorption/catabolism (e.g. cystatin C, Beta 2 macroglobulin). (modified from ref 39) Panel B: Damage biomarkers are also site specific and the magnitude and duration of biomarker change can potentially identify the extent of damage (modified from Ref 14).
Fig 3. Potential utilization of Biomarkers for AKI.
I (reprinted with permission from ref 17) Several biomarkers are now available for assessing changes in kidney function and detecting kidney damage. They can be utilized for initial diagnosis and staging, differential diagnosis and prognosis.
Biomarker utilization has also been limited by the identification of the best biomarkers for each purpose (risk assessment, diagnosis, determination of cause for differential diagnosis and prognosis), and the recognition that the thresholds may be different in each setting. Moreover, the relevance of differential changes in various markers representing different sites and mechanisms of injury in relation to changes in renal function over time is not well delineated. The utility of biomarkers in distinguishing de novo AKI from AKI superimposed on underlying CKD is an additional area of uncertainty. Biomarkers levels are generally higher in patients with CKD and consequently thresholds for identifying biomarker elevations are likely to be different 27. The ADQI group addressed these issues by assigning groups focused on four broad areas for biomarker utilization in AKI management 1) risk assessment, diagnosis and staging; 2) differential diagnosis; 3) assessment of prognosis and management; and 4) novel techniques including imaging. As part of the pre-conference activities, each group reviewed the literature and developed pertinent questions that would need to be addressed to define a strategy for clinical use. During the conference, it was apparent that a unifying approach was required to enable clinicians to understand the value of biomarkers and assist them in effective utilization in each domain.
Rationale and Design of Biomarker-assisted approaches for AKI management
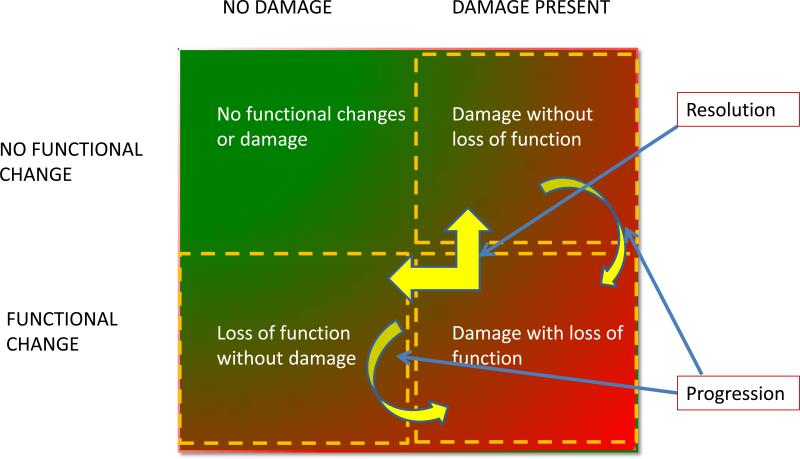
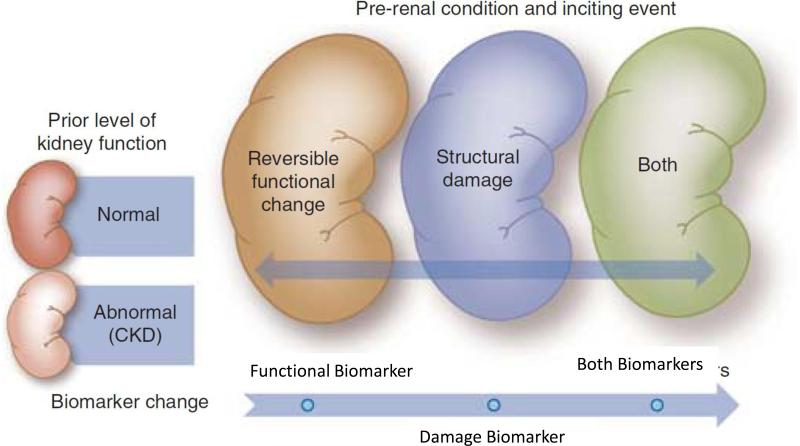
Our current understanding of the pathophysiology of human AKI is largely based on knowledge from experimental models extrapolated to the clinical arena. Large epidemiologic studies have enhanced our understanding of events related to the development and course of AKI. However, until recently, when biomarkers have emerged, it has been difficult to clearly define the pathophysiologic mechanisms corresponding to clinical AKI. We propose that available biomarkers can be classified as those representing changes in renal function e.g. serum creatinine or cystatin C, urine flow rates and those reflecting kidney damage e.g. KIM-1, NGAL, L-FABP, IL-18, etc. This delineation permits the simultaneous utilization of biomarkers from each category to delineate the spectrum of AKI. As shown in Fig 4, at any given point in time, patients would fall into one of the 4 quadrants, based on the changes in the representative functional and damage marker tests. Furthermore, sequential assessment of functional and damage markers would allow tracking of the course of AKI and provide information on the events and potential mechanisms involved, based on the specificity of the biomarkers used to identify the site, nature and magnitude of injury discussed further below. A combination of kidney functional and damage markers simultaneously provides an easy method to stratify patients with AKI. At initial presentation patients would be classified in one of the 4 groups and then could be assessed over time to see their transitions across the categories. This framework provides a novel approach to assessing patients with AKI for diagnosis and staging, differential diagnosis and prognosis. We believe this unique framework of the combined use of functional and damage biomarkers provide a potent tool to enable clinicians and researchers to most effectively utilize biomarkers in AKI. We asked each of the four work groups to utilize this novel structure to develop their recommendations. In each instance, the groups addressed which biomarkers would be most helpful and would work best in this framework. Some of the key concepts are summarized below.
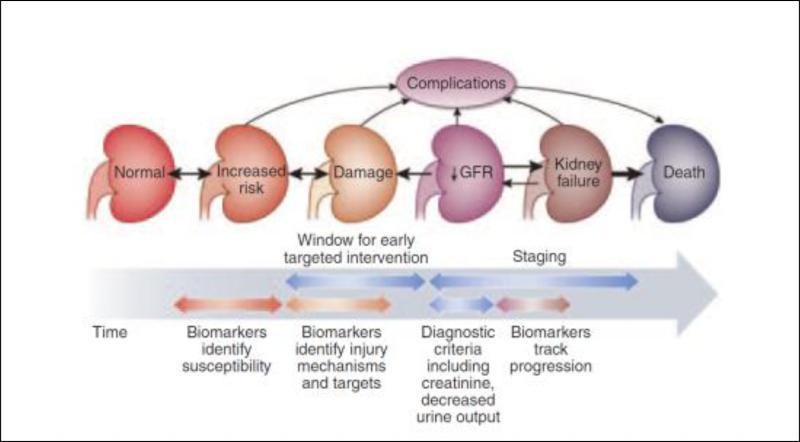
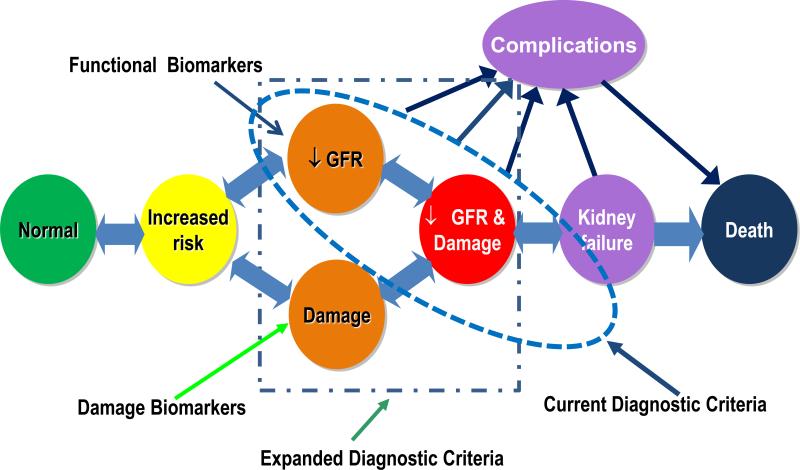
AKI diagnosis and staging: The availability of damage markers allows the identification of patients who manifest kidney damage without evidence of functional change (Fig 3, upper right quadrant). In contrast, in patients with no detectable markers of decreased kidney function or kidney damage (the upper left quadrant of Fig 4); there is no evidence of AKI. Accordingly, we have modified the prior “conceptual model of AKI” (Fig 5) to demonstrate how the combined use of functional and damage biomarkers can be used to expand the diagnostic criteria for AKI The precise diagnostic criteria for AKI defined by the detection of damage markers alone, in the absence of detectable kidney dysfunction, will need more research to define thresholds that are context- and disease-specific. The individual sensitivity and specificity of different AKI damage markers would permit their utilization in combination for establishing a diagnosis of AKI when an individual biomarker may be influenced by the underlying disease state. For instance, NGAL levels may be influenced by underlying infections however if levels of NGAL were elevated along with elevations of KIM-1 and LFABP the diagnostic likelihood of AKI would be enhanced. Combination of damage markers to be used concurrently or as confirmatory evidence would need additional research. For example, the international consensus definition of myocardial infarction, requires an increase of serum troponin concentration above the 99th percentile for the population in a compatible clinical context 28. Whether similar thresholds are applicable for kidney damage markers in the appropriate clinical context but without demonstrably decreased kidney function defined will need to be evaluated. In the appropriate clinical setting, this new damage biomarker criterion will enhance the ability of RIFLE/AKIN to define AKI. However, there are currently insufficient damage biomarker data to support staging of AKI, however, AKI stages basing on renal function changes are suggested to remain. Identification of a damage biomarker positive, creatinine negative state may reflect a phase of renal injury and could serve as a specific parameter for drug development to ascertain nephrotoxic potential. Several on-going studies with collaborations from the Predictive Safety Testing consortium with the FDA and EMEA will likely inform this process29-31. We anticipate that validation of a damage biomarker positive state will be required linking these changes to pertinent clinical outcomes independent of changes in serum creatinine13. This expansion of the diagnostic criteria for AKI will also mandate standardization of assay methods and comparisons across platforms (e.g. lab-based, point of care, etc.). The staging criteria for severity of AKI would also need to be further delineated for the “damage alone” category as further data emerge (Fig 6). The potential inclusion of damage markers to establish the independent diagnosis and staging of AKI will require additional research to establish appropriate thresholds which will likely be influenced by the site specificity, phase of disease, the underlying renal mass and the magnitude and duration of injury. In the absence of these specific data at the current time diagnosis and staging of AKI would remain based on creatinine and urine output criteria, however as evidence accumulates for damage markers individually and in combination criteria for establishing diagnosis and staging of AKI based solely on damage markers could be tested and validated.
It is likely that further refinement will link changes in biomarkers to outcomes and permit the delineation of severity stages on a continuous scale. However, this will need to be established in future studies.
Determination of Cause for Differential diagnosis: As discussed by Group 2, the combined use of functional and damage AKI biomarkers enables more accurate and useful differential diagnosis of the aetiology and mechanisms of AKI when a case is detected clinically. A key advance is the identification of a functional change without damage, corresponding to the lower left quadrant in Fig 4; this improves our ability to further delineate what is currently considered in the rather non-specific syndrome of “pre-renal azotemia”, which has traditionally been equated with volume-responsive, reversible alterations in kidney function 32. The category of “functional change alone” would also group the traditional “post-renal” category under this single domain, thereby focusing attention on the potentially time-sensitive reversibility of the underlying lesions prior to damage occurring. In other cases, AKI will be characterised by a combination of decreased function and the presence of damage, which would currently be characterised as a case of acute tubular necrosis (“ATN”), but would also be typical of more severe, late stage urinary tract obstruction, or other renal parenchymal injuries (lower right quadrant of Fig 4). In these circumstances, the variety of damage and functional markers could be applied effectively to map out the underlying pathophysiology and sequence of events without restricting the broad differential diagnosis to purely anatomic pre-, intra-, and post-renal categories. The proposed approach allows an assessment not only of mechanism i.e. dysfunction, damage, or both, but also of time course, evolution and prognosis to fully characterise any case of AKI. Finally, the clinical application of combination of functional and damage markers must account for the potential impact of presence or absence of pre-existing CKD influencing thresholds for detection and risk of progression in the evaluation of new AKI cases (Fig 7). The utilization of biomarkers to evaluate changes in kidney function in the presence of pre-existing CKD offers unique challenges. A combination of function and damage markers can be used to classify patients in the groups defined in Fig 4. However the thresholds for biomarkers will likely be different based on the underlying pre-existing level of renal function. This distinction will be important mechanistically to define outcomes and understand the pathophysiology.
Monitoring, prognosis, and guidance of management: As discussed by Group 3, the time sequence of functional vs. damage marker changes provides unique information on subsets of patients who progress from one phase to another; for example, when functional change leads to damage e.g. severe hypoperfusion leading to functional AKI with a GFR decrease and then ischemic AKI resulting in kidney damage. In Fig 4, such a case would evolve counter-clockwise from the upper left normal, in the absence of pre-existing CKD to lower left delineating dysfunction, and then to lower right with combined damage and dysfunction quadrants. The biomarker profile encountered in the evaluation of such a case would depend on when the evaluation began, and the frequency and components of serial monitoring. Urinary tract obstruction could be evaluated in the same way: purely functional at first, but characterised by a combination of damage and dysfunction if not promptly and effectively reversed. Conversely, in other cases where damage precedes a change in function (e.g. AKI caused by tubular injury inflicted by nephrotoxins whether exogenous- such as aminoglycosides, or endogenous- such as inflammatory mediators in sepsis, or heme pigments, a case of AKI would typically evolve clockwise from upper left to upper right quadrant in what are currently subclinical cases of nephrotoxic AKI, progressing to the lower right quadrant where damage leads to clinically detectable dysfunction. This framework is applicable for all the current and emerging biomarkers and can be used across the spectrum of AKI irrespective of the disease states and conditions that contribute to AKI. The thresholds for various biomarkers that categorize patients in one group or the other will need to be defined in future studies. In addition to determining the clinical relevance of each category, transitions among the quadrants may provide further information concerning the course and prognosis for renal e, g, recovery of kidney function, or AKI progression and dialysis requirement and non-renal e.g. mortality, other organ dysfunction, complications outcomes.
Delineation of the functional space: As discussed by group 4, the availability of instruments and imaging techniques provides new tools for characterizing the underlying pathophysiology during the course of AKI. Physiologic markers of normal renal function can thus be used to characterize derangements in renal function during AKI. The conceptual frame work of physiological biomarkers is superimposed upon the previously established concept of clinical phases of acute kidney injury 1. Thus, physiological biomarkers are not only needed in the early phase of AKI but throughout the continuum of AKI. The ability to measure these physiological variables may lead to identification of patients at risk for AKI, or early diagnosis of AKI, and may lead to the use of variables that inform therapeutic decisions. These physiological processes represent an integrative environment for the interaction of inflammatory mediators, imbalance in the homeostasis of oxygen, nitric oxide and oxygen radicals causing microcirculatory dysfunction and impaired tissue oxygenation leading to AKI. The use of imaging techniques(e.g. contrast ultrasound, MRI) as well as other physiologic markers (e.g. real-time GFR), which may become markers of renal dysfunction or damage when they become abnormal, expands the clinician's ability to understand the relationship between damage and functional AKI biomarker profiles and the evolution of renal and non-renal clinical outcomes (Fig 5). Determining which of the emerging candidate imaging or physiologic monitoring techniques is/are best suited for evaluation in specific circumstances and disease states will require new research to help refine our understanding and develop an approach for renal functional monitoring that can be applied in practice.
Figure 4. Proposed framework for evaluating AKI based on Biomarkers.
A combination of kidney functional and damage markers simultaneously provides a simple method to stratify patients with AKI. At initial presentation, patients would be evaluated in terms of these two domains, and then could be assessed over time to monitor their transitions across the domains.
Figure 5. Modified conceptual model of acute kidney injury.
The availability of specific biomarkers permits recognition of kidney damage separately from changes in kidney function. Kidney damage and changes in function may precede each other or occur concurrently. The time sequence of events depends on the nature and duration of the insult and the underlying state of health of the kidney. Consequently, we propose a modified conceptual framework to include evidence of isolated kidney damage as a potential criterion for diagnosis of AKI. The timing of diagnosis will depend on the frequency with which specific biomarkers of kidney damage and function are assessed.
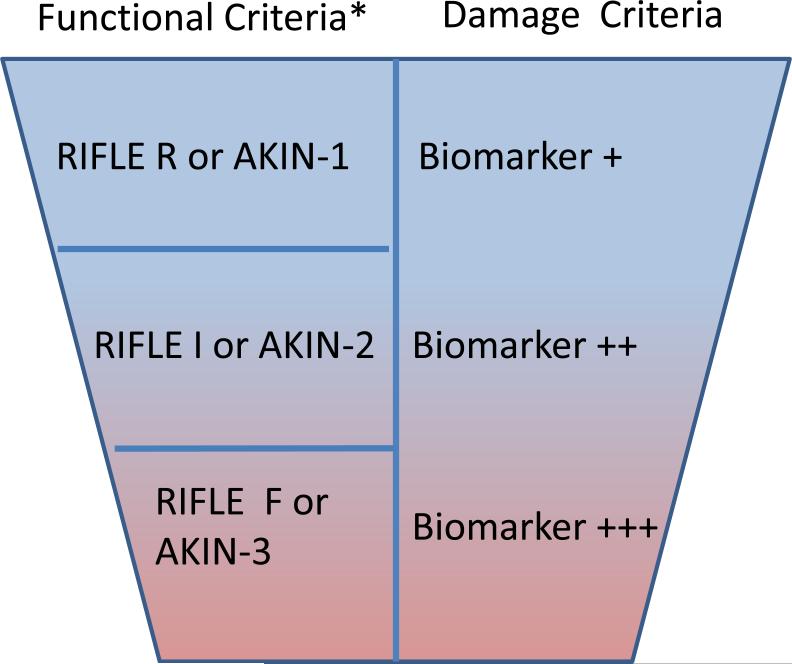
Figure 6. Proposed new criteria for AKI Diagnosis and Staging using Biomarkers.
New criteria for AKI diagnosis are displayed. In order to diagnose AKI selecting the worst criterion (function [RIFLE/AKIN] or damage) is recommended. In the appropriate clinical setting, this new damage biomarker criterion will enhance the ability of RIFLE/AKIN to define AKI. There are currently insufficient injury biomarker data to support staging of AKI, however, AKI stages basing on renal function changes are suggested to remain. The semi-quantitative trend for increasing biomarker severity associated with increasing kidney damage is suggested by the literature and is displayed by darkening background color as well as the symbols: +/++/+++.
*Adapted from RIFLE/AKIN criteria. AKIN= acute kidney injury Network; sCrea=serum creatinine; UO=urine output; RRT=renal replacement therapy.
Figure 7. Utilization of functional and damage markers concurrently to manage patients with AKI and CKD (modified from reference17).
The utilization of biomarkers to evaluate changes in kidney function in the presence of pre-existing CKD offers unique challenges. A combination of function and damage markers can be used to evaluate patients as shown in Fig 4. However, the thresholds for biomarkers will likely be different based on the underlying pre-existing level of renal function. This distinction will be important mechanistically to define outcomes and understand the pathophysiology.
Interpretation of data
The rapid expansion of the biomarker field has prompted development of analytic strategies to convert the data to actionable knowledge. Recognising the value of a biomarker in a particular situation requires knowledge of the sensitivity, specificity, positive and negative predictive values of the test, and the thresholds at which this is effective. For example, Bagshaw and colleagues have demonstrated that NGAL levels are higher in septic AKI than in non-septic AKI33. Similarly there may be non-specific elevations in biomarkers in patients with co-morbidities such as CKD and heart failure resulting in different thresholds for defining AKI in these conditions27, 34. Combining biomarkers adds additional challenges, including the possibility that their net signals maybe in different directions. For instance, in a given case and time point, damage biomarker A may be elevated while damage biomarker B values are within normal range, or vice versa. Similarly, urine output maybe low without concurrent elevation in serum creatinine, or vice versa. Given the dynamic nature of AKI, sequential changes in biomarkers also need to be considered with respect to an initial reference point. Another practical issue is how best to quantify and express urinary biomarkers in relation to the underlying GFR. Normalizing the value of the urinary biomarker to urinary creatinine excretion has been suggested however can result in over or underestimation of the level of the damage marker depending on changes in creatinine excretion. Timed urine collections to quantify actual biomarker excretion are potentially more accurate however assume that biomarker production and excretion are constant in the time period35. In these situations, biomarkers need to demonstrate additional value incremental beyond what is provided by clinical data. New data from the SAPPHIRE study suggest that biomarker combinations used in concert with clinical models may improve the diagnostic performance of novel AKI biomarkers36. Additionally, the influence of multiple methods and platforms for measurement on assay ranges, levels and thresholds is an area of critical importance and requires standardization. Finally, predictive models relating biomarker values to clinical outcomes on a continuous scale may further enhance the practicality of biomarker measurements to inform clinical decisions.
Future Directions
Based on our evaluation, the combined use of biomarkers of kidney dysfunction and damage may facilitate an earlier diagnosis of AKI, along with more accurate differential diagnosis and prognostic assessment, particularly when such markers are monitored serially over time and are combined with clinical parameters. However, these concepts will need to be validated with future studies in the following areas:
Confirm that the proposed expansion of the diagnostic criteria for AKI to include the isolated presence of damage biomarkers, with preserved function (Fig 5, and upper right quadrant of Fig 4) is clinically relevant. This requires demonstration that isolated functional and damage situations do exist in practice and have different outcomes. There is emerging evidence that transient elevations of serum creatinine and isolated changes in damage biomarkers are associated with outcomes that are intermediate between non-AKI and AKI based on standard criteria4, 37. Further research will be needed to define the specific thresholds for damage biomarkers that will correspond to the current stages of AKI based on creatinine and urine output changes. These thresholds will likely vary with the site specificity of the biomarkers, the magnitude of response based on the severity of injury and their temporal profile during the evolution of AKI. These factors will need to be considered in the design and interpretation of studies.
Determine the mechanistic pathways that are involved in the development of AKI and its natural course. Functional and damage markers are useful probes to understand the pathways contributing to the pathophysiology of AKI. For example, the combination of site-specific damage markers with functional testing could be needed to understand the contribution of changes in auto-regulatory mechanisms in the response to an insult to the observed loss of renal function. Additionally, these parameters would need to be tested in different disease states e.g. nephrotoxic vs. ischemia-reperfusion injury.
Define the prognostic value of the combined use of functional and damage markers in sequential measurements to confirm the prognostic significance of these categories of functional change alone, damage alone and combined functional change and damage. These paradigms will also need to be tested to understand the pathophysiology and natural history of AKI. For instance, understanding if functional change precedes damage vs. damage preceding functional change and what are the thresholds for these events would help define the best time points and strategies for targeted intervention.
Ascertain how well the combination of damage and functional markers can improve recognition of AKI in the setting of CKD17, 38. Studies will need to address the thresholds for each set of markers and demonstrate the value of combining specific markers for differential diagnosis in the presence of other co-morbidities that often accompany CKD. The role of damage markers in establishing a diagnosis of AKI in a setting of CKD will need to be developed further to identify the best performing markers and establish relevant thresholds which are specific for new injury and are correlated with changes in outcomes
Large population-based studies would be required across multiple centres enrolling patients in the wide spectrum of AKI and different disease states, to determine if operationalizing the approach to AKI with a simple 2×2 table to mechanistically define AKI cases and their evolution (Fig 4) usefully influences patient management and ultimately improves outcomes.
Discover and confirm the sensitivity and specificity of damage and functional markers for specific situations e.g. for the biomarkers that are most efficient for differentiating damage in cardio renal syndrome may be different in patients with renal dysfunction in setting of cirrhosis. Similarly, imaging and other physiological markers would need to be validated in the context of various conditions leading to AKI and for distinguishing AKI from CKD.
Establish standard techniques for collection, handling and presentation of biomarker data that permits appropriate interpretation across settings. While individual protocols have described techniques for handling and preserving urine and blood samples for biomarker assessment in AKI there is a great need to develop a uniform standardized protocol that can be easily accessed and followed for future studies. Additionally, further research is needed to optimize the reporting of urinary biomarker data in spot and timed collection samples as simple concentrations, or mass per unit time, or as a ratio to urinary creatinine or as fractional urinary excretion 35. ” Additionally it would be necessary to validate techniques for normalizing urinary biomarkers to urinary creatinine in the setting of changing renal function.
(ref 35).
Summary
The availability of new biomarkers of kidney damage and functional change offers an unprecedented opportunity for improved evaluation and management of patients with AKI. To a large extent the current evidence base of AKI biomarkers has been limited and has focused on establishing the performance of various damage markers in comparison to creatinine thereby limiting the clinical utility to establishing an earlier diagnosis of AKI. The evidence-based deliberations from this ADQI conference provide a novel conceptual framework for combining functional and damage markers to equip researchers and clinicians with the tools to better understand the syndrome of AKI. We have proposed utilizing combinations of functional and damage markers to evaluate patients with AKI in terms of the two domains (function and damage) (Fig 4). This initial delineation permits an improved understanding of the mechanisms and pathophysiology of AKI, and facilitates the determination of prognosis and selection of time points for interventions. We anticipate that as different damage and functional biomarkers are discovered, they will further refine the syndrome of AKI and lead to new strategies for diagnosis and intervention in AKI. This conceptual framework will need to be validated through future studies and additional evidence will be required to establish the best combinations of damage and functional biomarkers for their utilization is clinical practice. We believe the proposed approach is an important step towards improving the adoption of biomarkers for AKI in clinical practice, and will ultimately enable clinicians to improve outcomes from AKI.
Acknowledgements
We wish to acknowledge University College Dublin School of Medicine & Medical Science, and specifically Mos. Sinead Cleary and Ms Ruth-Anne Kilty, for their organizational support. In addition, the conference was supported in part by unrestricted educational grants from the following companies (listed alphabetically): Abbott Laboratories, Alere, Argutus, Astute Medical, CMIC (Japan), FAST Diagnostics, Gambro, Roche.
APPENDIX
Appendix A
Appendix A.
Information regarding workgroups and work product
| Co-Chairs | Group 1: | Group 2: | Group 3: | Group 4: | Special Observers |
|---|---|---|---|---|---|
|
Patrick Murray (Dublin, Ireland); Ravindra L Mehta (San Diego, USA) |
Use of biomarkers for risk assessment, early diagnosis and staging of AKI | Use of biomarkers for differential diagnosis of AKI in clinical practice | Use of biomarkers to assess prognosis and guide management of AKI | Imaging and other biomarkers for AKI | Rotating through each group |
| Facilitators | Andrew Shaw (Durham, NC, USA | Zoltan Endre (Sydney, Australia; | Lakhmir Chawla (Washington, DC, USA | Can Ince (Rotterdam, Netherlands | |
| Claudio Ronco (Vicenza, Italy | John Kellum (Pittsburgh, PA | Dinna Cruz (Vicenza, Italy; | Mark Okusa (Charlottesvill e, VA, USA | ||
| Members | Michael Haase (Magdeburg, Germany) Peter McCullough (Novi, MI, USA Josee Bouchard (Montréal, Qc, CA Sus Waikar (Boston, MA, USA Eddie Siew (Nashville, TN, USA |
Stuart Goldstein (Cincinnati, OH, USA Jay Koyner (Chicago, IL, USA Etienne Macedo (São Paulo, SP, Brazil Kent Doi (Tokyo, Japan Salvatore Di Somma (Rome, Italy |
Alan Maisel (San Diego, CA, USA Andy Lewington (Leeds, UK Ravi Thadhani (Boston, MA, USA Sean Bagshaw (Edmonton, Alberta, CA Raj Chakravarthi (Hyderabad, India |
Jacques Duranteau (Paris, France Peter Doran (Dublin, Ireland Li Yang (Beijing, China; Bertrand Jaber (Cambridge, MA, USA |
Paul Clopton (San Diego, CA, US Joe Bonventre (Boston, MA, USA Gary Cutter PhD (Birmingham, AL, USA Bruce Molitoris (Indiana, IN, USA Prasad Devarajan (Cincinnati, OH, USA Maria Fitzgibbon (Dublin, Ireland; Eisei Noiri (Tokyo, Japan Chirag Parikh (New Haven, CT, USA |
Information regarding workgroups and work product
Appendix B
ADQI Methodology
Our consensus process relied on evidence where available and, in the absence of evidence, consensus expert needed opinion where possible as described previously. We conducted the consensus process in three stages: (1) pre-conference, (2) conference, and (3) post-conference. Prior to the conference, we identified four topics for discussion pertaining to the utilization of kidney biomarkers. For each topic, we outlined a preliminary set of key questions. We then invited an international panel, predominantly from the fields for nephrology and intensive care based on their expertise in acute kidney injury. Panelists were assigned to 7 person work groups, with two members serving as the group facilitators. Each workgroup addressing one key topic. For each group topic studies were identified via MEDLINE search, bibliographies of review articles and participants' files. Searches were limited to English language articles. Evidence was classified according to levels per EBM methodology. Qualitative commentary was provided when deemed necessary by the group. However, there was no critical appraisal of individual studies during this phase. Outcomes were grouped into the major categories: physiologic (eg. blood pressure, BUN, etc.), clinical (short-term morbidity/mortality, long-term morbidity/mortality, renal recovery, functional class/quality of life) and economic. Different types of outcomes were considered separately for each intervention. Animal research was not considered as evidence except that as contributing to commentary.
Summary statements were developed through a series of breakout sessions where individual work group members were required to identify key issues for which recommendations were needed and to classify current state of consensus and identify supporting evidence for each issue. Workgroup members were then required to present their findings to the entire group, revising each statement as needed until a final version was agreed upon. The responsibility for presenting the findings of the work group to the rest of the participants was shared by each member on a rotating basis. Group facilitators revised work group findings as needed after each plenary session. Directives for future research were achieved by asking the participants to: a) identify deficiencies in the literature, b) determine if more evidence is necessary, and c) if so, and articulate general research questions. When possible, pertinent study design issues are also considered. Special observers had a scheduled rotation through each of the four workgroups to provide input. In each breakout session, the workgroups refined the key questions, identified the supporting evidence, and generated recommendations and/or directions for future research as appropriate. Summary statements were developed through these series of alternating breakout and plenary sessions and were further refined until final versions were agreed upon. A writing committee assembled the individual reports from the work groups. Each report was edited to conform to a uniform style and for length. The final reports were mailed to each participant for comment and revision. Once final reports were completed, the writing committee summarized the individual reports into a final conference document that is submitted for publication.
References
- 1.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singbartl K, Kellum JA. AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney Int. 2011 doi: 10.1038/ki.2011.339. [DOI] [PubMed] [Google Scholar]
- 3.Wang HE, Muntner P, Chertow GM, et al. Acute Kidney Injury and Mortality in Hospitalized Patients. Am J Nephrol. 2012;35:349–355. doi: 10.1159/000337487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nickolas TL, Schmidt-Ott KM, Canetta P, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol. 2012;59:246–255. doi: 10.1016/j.jacc.2011.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart J FG, Smith N, Kelly K, Mason M. Adding insult to injury: a review of the care of patients who died in hospital with a primary diagnosis of acute kidney injury (acute renal failure). A report by the National Confidential Enquiry into Patient Outcome and Death. 2009 http://www ncepodorguk/2009akihtm 2009.
- 6.Devarajan P. Biomarkers for the early detection of acute kidney injury. Curr Opin Pediatr. 2011;23:194–200. doi: 10.1097/MOP.0b013e328343f4dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonventre JV, Vaidya VS, Schmouder R, et al. Next-generation biomarkers for detecting kidney toxicity. Nat Biotechnol. 2010;28:436–440. doi: 10.1038/nbt0510-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parikh CR, Garg AX. Acute kidney injury: Better biomarkers and beyond. Kidney Int. 2008;73:801–803. doi: 10.1038/ki.2008.17. [DOI] [PubMed] [Google Scholar]
- 9.Singer E, Elger A, Elitok S, et al. Urinary neutrophil gelatinase-associated lipocalin distinguishes pre-renal from intrinsic renal failure and predicts outcomes. Kidney Int. 2011;80:405–414. doi: 10.1038/ki.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaidya VS, Waikar SS, Ferguson MA, et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci. 2008;1:200–208. doi: 10.1111/j.1752-8062.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz R. Biomarkers and surrogate markers: an FDA perspective. NeuroRx. 2004;1:189–195. doi: 10.1602/neurorx.1.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chawla LS, Kellum JA. Acute kidney injury in 2011: Biomarkers are transforming our understanding of AKI. Nat Rev Nephrol. 2012;8:68–70. doi: 10.1038/nrneph.2011.216. [DOI] [PubMed] [Google Scholar]
- 13.Vaidya VS, Bonventre JV. Mechanistic biomarkers for cytotoxic acute kidney injury. Expert Opin Drug Metab Toxicol. 2006;2:697–713. doi: 10.1517/17425255.2.5.697. [DOI] [PubMed] [Google Scholar]
- 14.Bonventre JV, Vaidya VS, Schmouder R, et al. Next-generation biomarkers for detecting kidney toxicity. Nat Biotechnol. 2010;28:436–440. doi: 10.1038/nbt0510-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srisawat N, Wen X, Lee M, et al. Urinary biomarkers and renal recovery in critically ill patients with renal support. Clin J Am Soc Nephrol. 2011;6:1815–1823. doi: 10.2215/CJN.11261210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Endre ZH, Walker RJ, Pickering JW, et al. Early intervention with erythropoietin does not affect the outcome of acute kidney injury (the EARLYARF trial). Kidney Int. 2010;77:1020–1030. doi: 10.1038/ki.2010.25. [DOI] [PubMed] [Google Scholar]
- 17.Mehta RL. Biomarker explorations in acute kidney injury: the journey continues. Kidney Int. 2011;80:332–334. doi: 10.1038/ki.2011.181. [DOI] [PubMed] [Google Scholar]
- 18.Siew ED, Ikizler TA, Gebretsadik T, et al. Elevated urinary IL-18 levels at the time of ICU admission predict adverse clinical outcomes. Clin J Am Soc Nephrol. 2010;5:1497–1505. doi: 10.2215/CJN.09061209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parikh CR, Coca SG, Thiessen-Philbrook H, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22:1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coca SG, Yalavarthy R, Concato J, et al. Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int. 2008;73:1008–1016. doi: 10.1038/sj.ki.5002729. [DOI] [PubMed] [Google Scholar]
- 21.Macedo E, Malhotra R, Claure-Del Granado R, et al. Defining urine output criterion for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2011;26:509–515. doi: 10.1093/ndt/gfq332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cruz DN, de Geus HR, Bagshaw SM. Biomarker strategies to predict need for renal replacement therapy in acute kidney injury. Semin Dial. 2011;24:124–131. doi: 10.1111/j.1525-139X.2011.00830.x. [DOI] [PubMed] [Google Scholar]
- 23.Mehta RL. Timed and targeted therapy for acute kidney injury: a glimpse of the future. Kidney Int. 2010;77:947–949. doi: 10.1038/ki.2010.79. [DOI] [PubMed] [Google Scholar]
- 24.Stillman IE, Lima EQ, Burdmann EA. Renal biopsies in acute kidney injury: who are we missing? Clin J Am Soc Nephrol. 2008;3:647–648. doi: 10.2215/CJN.01110308. [DOI] [PubMed] [Google Scholar]
- 25.Davison DL, Patel K, Chawla LS. Hemodynamic Monitoring in the Critically Ill: Spanning the Range of Kidney Function. Am J Kidney Dis. 2012 doi: 10.1053/j.ajkd.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Sharfuddin AA, Sandoval RM, Molitoris BA. Imaging techniques in acute kidney injury. Nephron Clin Pract. 2008;109:c198–204. doi: 10.1159/000142929. [DOI] [PubMed] [Google Scholar]
- 27.Bolignano D, Coppolino G, Campo S, et al. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is associated with severity of renal disease in proteinuric patients. Nephrol Dial Transplant. 2008;23:414–416. doi: 10.1093/ndt/gfm541. [DOI] [PubMed] [Google Scholar]
- 28.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Eur Heart J. 2007;28:2525–2538. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 29.Sistare FD, DeGeorge JJ. Promise of new translational safety biomarkers in drug development and challenges to regulatory qualification. Biomark Med. 2011;5:497–514. doi: 10.2217/bmm.11.52. [DOI] [PubMed] [Google Scholar]
- 30.Ozer JS, Dieterle F, Troth S, et al. A panel of urinary biomarkers to monitor reversibility of renal injury and a serum marker with improved potential to assess renal function. Nat Biotechnol. 2010;28:486–494. doi: 10.1038/nbt.1627. [DOI] [PubMed] [Google Scholar]
- 31.Dieterle F, Sistare F, Goodsaid F, et al. Renal biomarker qualification submission: a dialog between the FDA-EMEA and Predictive Safety Testing Consortium. Nat Biotechnol. 2010;28:455–462. doi: 10.1038/nbt.1625. [DOI] [PubMed] [Google Scholar]
- 32.Schrier RW. Diagnostic value of urinary sodium, chloride, urea, and flow. J Am Soc Nephrol. 2011;22:1610–1613. doi: 10.1681/ASN.2010121289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bagshaw SM, Bennett M, Haase M, et al. Plasma and urine neutrophil gelatinase-associated lipocalin in septic versus non-septic acute kidney injury in critical illness. Intensive Care Med. 2010;36:452–461. doi: 10.1007/s00134-009-1724-9. [DOI] [PubMed] [Google Scholar]
- 34.Yndestad A, Landro L, Ueland T, et al. Increased systemic and myocardial expression of neutrophil gelatinase-associated lipocalin in clinical and experimental heart failure. Eur Heart J. 2009;30:1229–1236. doi: 10.1093/eurheartj/ehp088. [DOI] [PubMed] [Google Scholar]
- 35.Waikar SS, Sabbisetti VS, Bonventre JV. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int. 2010;78:486–494. doi: 10.1038/ki.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uchino S, Bellomo R, Bagshaw SM, et al. Transient azotaemia is associated with a high risk of death in hospitalized patients. Nephrol Dial Transplant. 2010;25:1833–1839. doi: 10.1093/ndt/gfp624. [DOI] [PubMed] [Google Scholar]
- 38.Belcher JM, Parikh CR. Is it time to evolve past the prerenal azotemia versus acute tubular necrosis classification? Clin J Am Soc Nephrol. 2011;6:2332–2334. doi: 10.2215/CJN.08570811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Briggs JP. The hunt for the perfect biomarker for acute kidney injury: back to gamma-trace? Kidney Int. 2008;74:987–989. doi: 10.1038/ki.2008.426. [DOI] [PubMed] [Google Scholar]