Abstract
Leaves constitute a substantial fraction of the total resistance to water flow through plants. A key question is how hydraulic resistance within the leaf is distributed among petiole, major veins, minor veins, and the pathways downstream of the veins. We partitioned the leaf hydraulic resistance (Rleaf) for sugar maple (Acer saccharum) and red oak (Quercus rubra) by measuring the resistance to water flow through leaves before and after cutting specific vein orders. Simulations using an electronic circuit analog with resistors arranged in a hierarchical reticulate network justified the partitioning of total Rleaf into component additive resistances. On average 64% and 74% of the Rleaf was situated within the leaf xylem for sugar maple and red oak, respectively. Substantial resistance—32% and 49%— was in the minor venation, 18% and 21% in the major venation, and 14% and 4% in the petiole. The large number of parallel paths (i.e. a large transfer surface) for water leaving the minor veins through the bundle sheath and out of the leaf resulted in the pathways outside the venation comprising only 36% and 26% of Rleaf. Changing leaf temperature during measurement of Rleaf for intact leaves resulted in a temperature response beyond that expected from changes in viscosity. The extra response was not found for leaves with veins cut, indicating that water crosses cell membranes after it leaves the xylem. The large proportion of resistance in the venation can explain why stomata respond to leaf xylem damage and cavitation. The hydraulic importance of the leaf vein system suggests that the diversity of vein system architectures observed in angiosperms may reflect variation in whole-leaf hydraulic capacity.
Water flow through the leaf is one of the most important but least understood components of the whole-plant hydraulic system. The leaf hydraulic resistance (Rleaf) constitutes a significant hydraulic bottleneck, correlates with leaf structure, and apparently constrains gas exchange (Tyree and Zimmermann, 2002; Sack et al., 2003b; Sack and Tyree, 2004). Rleaf is an aggregate measure; once past the petiole, water flows through a reticulate network of veins, across the bundle sheath cells, and through or around mesophyll cells before evaporation and diffusion from the stomata (Esau, 1965). Recent work has focused primarily on measurement of Rleaf and its functional correlates. Few studies have attempted to determine quantitatively how the various components of the water flow pathway through the leaf contribute to its total resistance.
Partitioning of Rleaf is a crucial step for understanding leaf hydraulic design. Cavitation in the leaf vein xylem can substantially increase Rleaf, as can physical damage to major veins; both drive reductions of leaf water potential and gas exchange (Salleo et al., 2000; Nardini et al., 2001, 2003; Cochard et al., 2002; Huve et al., 2002; Nardini and Salleo, 2003; Sack et al., 2003a). These observations suggest that a large proportion of Rleaf is situated in the veins. However, a number of studies have reported that most of the leaf resistance is located outside the xylem, i.e. in the pathways of water to the sites of evaporation (Boyer, 1974; Tyree and Cheung, 1977; Yang and Tyree, 1994; Tyree et al., 2001; Martre et al., 2001; Salleo et al., 2003). This apparent contradiction may result from the fact that these studies used cutting or freezing treatments to quantify the removal of the extravascular resistance, without taking explicit account of the hierarchical architecture of angiosperm venation. In this study, we examine the contribution of vascular and nonvascular components of the leaf water pathway through to Rleaf by hydraulically isolating the various parts of the pathway based on the architecture of the vein system.
This study focuses on the within-leaf hydraulic architecture of sugar maple (Acer saccharum) and red oak (Quercus rubra). We partitioned Rleaf into four component resistances—that of the petiole (Rpetiole), the major vein system (Rmajor veins), the minor veins (Rminor veins), and the pathways outside of the vein xylem (Routside veins)—by quantifying the reduction in Rleaf when specific vein orders were severed. We combined these measurements with an analog circuit model to determine whether these component resistances, set in reticulate hierarchical venation with water leaking to the mesophyll and eventually to the sites of evaporation, can be considered as resistors additive in series. Temperature responses were used to diagnose whether water moving through the leaf crosses cell membranes into the symplast, as opposed to taking place entirely in the apoplast (Haines, 1994; Martre et al., 2002).
RESULTS
Leaf Hydraulic Resistance
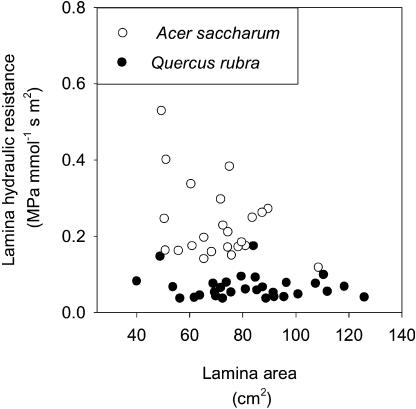
The hydraulic resistance of the leaf lamina (Rlamina = Rleaf − Rpetiole) in sugar maple and red oak were, respectively, 0.258 MPa mmol−1 s m2 ± 0.0265 se and 0.0593 MPa mmol−1 s m2 ± 0.0037 se (Table I; compare with mean values of 0.145 and 0.0666, respectively, in Sack et al., 2002, using three different methods for the same trees the previous summer). Absolute lamina hydraulic resistance (not normalized by leaf area) decreased with increasing leaf area for the mature leaves sampled (for sugar maple, r = −0.59, n = 24, and P = 0.003; for red oak, r = −0.52, n = 31, andP = 0.002). Consequently, Rlamina (normalized by leaf area) did not vary systematically with leaf size (r = 0.32 and P = 0.12, and r = 0.095 and P = 0.61, respectively; Fig. 1).
Table I.
Mean hydraulic resistances of intact leaf lamina ± se (n), treated leaves and petioles
| Hydraulic Resistance | Sugar Maple | Red Oak |
|---|---|---|
| MPa mmol−1 s m2 | ||
| Intact leaf lamina | 0.258 ± 0.0265 (25) | 0.0593 ± 0.0037 (25) |
| Lamina with minor veins cut | 0.129 ± 0.0177 (8) | 0.0380 ± 0.0040 (10) |
| Lamina with tertiary veins cut | 0.0662 ± 0.0065 (4) | 0.0182 ± 0.0080 (5) |
| Petiole | 0.0416 ± 0.0026 (27) | 0.00295 ± 0.00019 (32) |
In a two-way ANOVA (with factors species and treatments), significant differences were found among treatments (F = 13.37; P < 0.001), among species (F = 23.96; P < 0.001), and for the species-treatment interaction (F = 5.07; P = 0.009).
Figure 1.
Lamina hydraulic resistance for leaves of a range of lamina area at maturity for sugar maple (A. saccharum) and red oak (Q. rubra).
Effects of Treatments: Partitioning Leaf Hydraulic Resistance
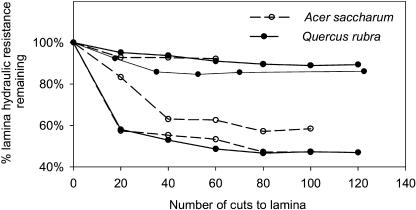
For both species, the cutting treatments significantly reduced hydraulic resistance (Table I; Fig. 2). When minor veins were incrementally severed at random locations throughout the leaf, the resistance dropped until 40 to 80 cuts were made; further cuts produced no significant drop in resistance (Fig. 2). Across the tested leaves, the final percentage of reduction in hydraulic resistance was not significantly related to the final number of cuts per area (for sugar maple, R2 = 0.39 and P = 0.10; for red oak, R2 = 0.001 and P = 0.94), further indicating that in each treated leaf the measurable resistance outside the venation was removed.
Figure 2.
Effects of sequentially cutting minor veins on leaf hydraulic resistance of individual test leaves of sugar maple and red oak. Each cut severed at least 5 to 7 minor veins (see “Materials and Methods”).
The degree to which shorting minor veins reduced leaf lamina hydraulic resistance ranged substantially, from 8% to 63% (mean 41% ± 7 se) in sugar maple and 1% to 53% (mean 27% ± 6 se) in red oak. This variation is similar to, or much lower than, the variation found in previous studies applying cutting treatments without discriminating vein orders. The variation may arise from natural diversity among leaves of a species in the membrane conductivity of leaf cells and the arrangement and dimensions of leaf xylem conduits (Jeje, 1985; Martre et al., 2002). Although extreme care was taken not to cut any visible tertiary vein, the percentage of resistance outside of the venation might in some cases have been overestimated due to the cutting of a tertiary vein submerged in the lamina or an uncommonly large and conductive minor vein; any such effects would amplify the variability of the measurement. However, the variation in the drop of hydraulic resistance after vein cutting was normally distributed (Anderson-Darling test for non-normality; P = 0.27 − 0.72), making the average a robust indicator of the central tendency. Removing the resistance of the minor veins and everything downstream of them by cutting the tertiary veins decreased the resistance of the leaf lamina by 68% to 92% (mean 79% ± 5% se) in sugar maple and by 66% to 84% (mean 78% ± 3% se) in red oak.
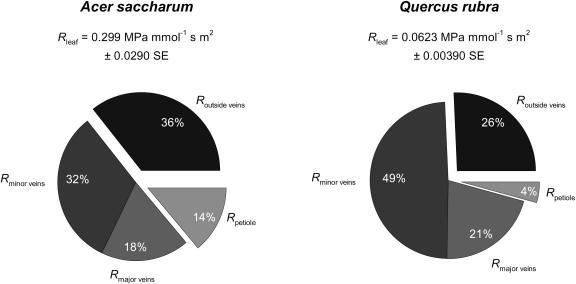
There were significant differences between species in the ways in which Rleaf is distributed among its component parts (Table I). However, for both species, on average, the bulk of Rleaf was situated in the xylem (of the petiole and the major and minor veins), 64% for sugar maple and 74% for red oak (Fig. 3).
Figure 3.
Mean values ± se for Rleaf for sugar maple and red oak, and average partitioning of hydraulic resistance in petiole (Rpetiole), major vein network (Rmajor veins), and minor veins (Rminor veins), and in pathways outside the vein xylem (Routside veins).
Xylem Conduit Dimensions
Sugar maple had narrower vein xylem conduits than red oak, consistent with the differences in Rleaf and its components. For sugar maple, the three largest midrib vessels had a mean maximum width of23.3 μm ± 1.8 se; for red oak, 46.2 μm ± 2.8 se (difference significant at P < 0.001; t test). For sugar maple the three largest minor vein tracheary elements had a mean maximum width of 4.6 μm ± 0.5 se; for red oak, 6.2 μm ± 0.3 se (P < 0.05; t test).
Temperature Responses
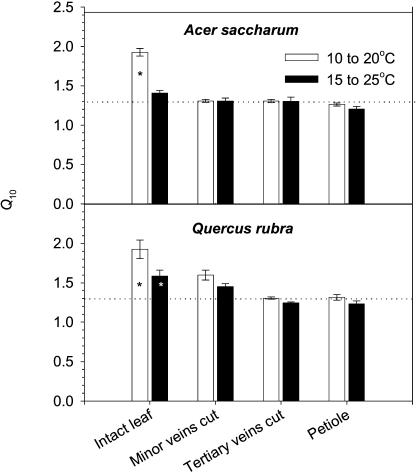
The effect of temperature on water flow through intact (i.e. untreated) leaves of both species was significantly greater than that expected from the effects of temperature on the viscosity of water (Fig. 4). In intact leaves of sugar maple, the Q10 values for 10°C/20°C and 15°C/25°C, here calculated in terms of the effect of temperature on flow rate through the leaf for a constant delivery pressure, were 1.92 ± 0.05 and 1.41 ± 0.03; in leaves of red oak, the values were 1.93 ± 0.12 and 1.59 ± 0.07. For leaves with cut veins as well as for petiole segments, Q10 values were not statistically different from 1.3, indicating that the effect of temperature could be entirely accounted for by the expected changes in viscosity.
Figure 4.
Temperature responses (Q10) of water flow through intact and treated leaves of sugar maple and red oak. The delivery pressure was held constant while the temperature was varied. Dotted lines indicate the Q10 values attributable solely to the effects of temperature on the viscosity of pure water; *, significantly different at P < 0.05.
DISCUSSION
Partitioning of Leaf Hydraulic Resistance: The Leaf Vascular System
The methods used to partition Rleaf in this study suggest that the venation constitutes a significant fraction of Rleaf (on average, 64% in sugar maple and 74% in red oak). These findings are at odds with a number of studies on dicotyledonous leaves, in which the majority of Rleaf was reported to occur within the mesophyll. For example, Rvenation of A. saccharum, A. rubrum, Fagus grandifolia, Helianthus annuus, Quercus petraea, and Viburnum tinus was estimated to be, on average, 17%, 26%, 9%, 18%, 9%, and 50% of Rleaf (Boyer, 1974; Tyree and Cheung, 1977; Yang and Tyree, 1994; Tyree et al., 2001; Salleo et al., 2003; discussion of studies of grasses is below). An important difference between these studies and the work presented here is that in this study the vein severing treatments used were applied to specific vein orders. By contrast, the partitioning of resistance by making substantial cuts to the lamina (Tyree et al., 2000) or by removing the entire leaf margin (Boyer, 1974; Tyree and Cheung, 1977; Yang and Tyree, 1994) is likely to have severed major veins, leading to an underestimation of Rvenation. An alternative approach to estimating the contribution of vascular versus nonvascular components of Rleaf involved freezing the leaf (Tyree et al., 2001; Salleo et al., 2003), which led to an estimation of Rvenation as 10%, 45%, 26%, and 43% of Rleaf for, respectively, Juglans regia, Prunus laurocerasus, Q. petraea, and V. tinus. Although it is likely that this treatment achieved the goal of removing mesophyll resistance by shearing cell membranes, it also may have increased the radial permeability of the entire venation system. If in the treated leaves water was able to leak easily from major veins, much of the flow would have bypassed the minor veins, resulting in underestimation of Rvenation.
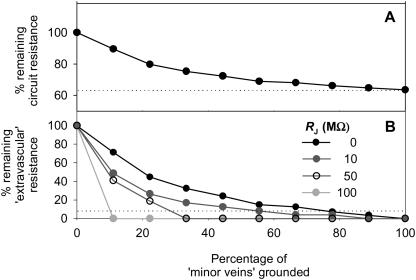
A surprisingly small number of cuts were sufficient to remove all significant resistance outside the minor vein system. Indeed, if one approximates the minor vein system as a square grid, the minor vein densities of sugar maple and red oak (5 mm mm−2 and 7 mm mm−2, respectively; L. Sack, unpublished data) predict ≈1250 and 2450 minor vein segments cm−2 (in a square grid, the segment number per area = vein density2/2). Our treatments would have opened less than 1% of segments. One hypothesis that can explain why cutting so few minor vein segments, relative to the total density, removes the hydraulic resistance outside the xylem is that the topology of the minor vein network places the segments essentially in parallel. Because resistors in parallel are inversely additive, shorting even a small number has a large effect on the total network resistance. One aspect of the hydraulic architecture of leaf venation that contributes to the minor veins acting as in parallel, irrespective of their position, is a relatively large resistance at the major-minor vein junctions. Tests of this idea with the electronic circuit model (Fig. 5) confirmed, in principle, that larger resistances at the major-minor vein junctions reduce the number of shorted minor veins necessary to remove virtually all the resistance downstream of the veins (see “Materials and Methods”; Fig. 7B). Measurements of pressure dissipation as a function of vein order and position in Laurus nobilis leaves demonstrated relatively large pressure drops occurring between tertiary and minor veins (Zwieniecki et al., 2002). The partitioning of resistance within the minor venation network merits further investigation.
Figure 5.
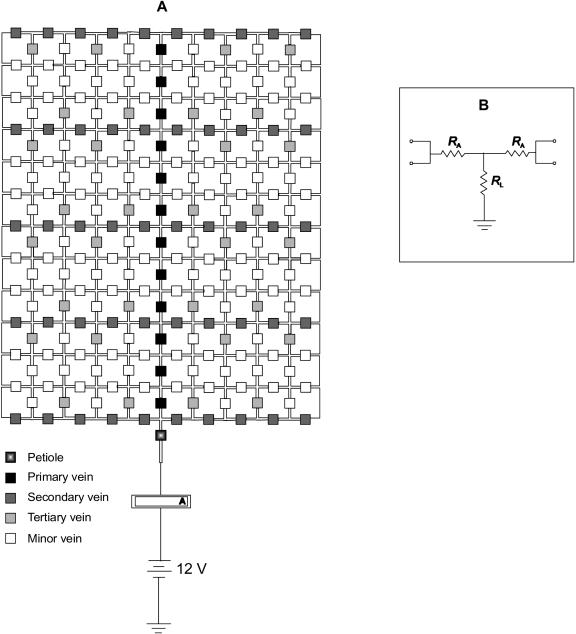
An electronic circuit model of the leaf hydraulic architecture, including reticulate hierarchical vein orders. A, Whole-leaf circuit. B, Each component includes two axial resistors of resistance, RA, and a radial leak resistance, RL, representing leaks out of the xylem to the mesophyll and eventually to sites of evaporation. For different vein orders, RA and RL can be set to different values. An ammeter proximal to the petiole allows determination of current; the resistance of the circuit can be calculated as the supply voltage/current.
Figure 7.
A, The effects of grounding minor veins randomly in analog electrical circuit (Fig. 5) With successive minor veins shorted out, the line converges to the percentage of xylem resistance, shown by the dotted line (position of this line is a function of the parameterization; as for Fig. 6, with RL of the minor veins set to 2 MΩ). B, For the same simulation, the decline of percentage of extravascular resistance (black circles) compared with that for circuits with added resistance RJ at the junctions of major and minor veins, i.e. at junctions of black and white or of gray and white squares in Figure 5 (RL in minor veins was successively reduced with increasing RJ, to maintain the percentage of Rleaf in the xylem within bounds at 64% to 71%; for the dark gray circles, RL = 10 MΩ; for the white circles, 50 MΩ; for the light gray circles, 115 MΩ). Below the dotted line, <5% of the extravascular resistance remains.
The anatomy of major veins and minor veins is consistent with the partitioning of hydraulic resistance reported here. An increase in resistance from major to minor veins is expected from the fact that higher vein orders have a lower percentage of cross-sectional area represented by xylem and fewer, narrower conduits (Plymale and Wylie, 1944; Canny, 1993). The minor veins, which typically make up 93% to 96% of the total vein density (Armacost, 1944; Plymale and Wylie, 1944; Pray, 1954; Dengler and Mackay, 1975; Russin and Evert, 1984), contain only tracheids (Plymale and Wylie, 1944; Canny, 1993). We note that sugar maple had a substantially higher hydraulic resistance than red oak in the whole leaf, as well as in each component—in the major and minor vein systems and in the pathways outside the vein xylem. Consistent with this finding, sugar maple had narrower xylem conduits than red oak in the midrib and minor veins. This pattern parallels that for European species Acer platanoides and Quercus robur, of comparable ecology and leaf form; A. platanoides has narrower midrib vessels and higher shoot hydraulic resistance than Q. robur (Aasamaa et al., 2001). Despite these parallels, it is still not clear that leaf xylem conduit dimensions would directly scale up to hydraulic resistance in a predictable fashion. Further work will be needed in combining anatomy, microstructure, and modeling, accounting for the multiple hydraulic pathways in series and parallel, within the venation and in the pathways outside the xylem.
The hierarchical leaf venation is best developed in dicotyledons, raising the question of how the hydraulic resistance is partitioned in leaves with fewer vein orders. Two studies have examined Rleaf in monocots by measuring longitudinal resistance in leaf segments (effectively driven by the dimensions and numbers of the largest longitudinal conduits) to parameterize a model of the hydraulic architecture as a single longitudinal vein, with lateral radial leaks (Wei et al., 1999; Martre et al., 2001). These studies concluded that most of the resistance to flow was outside the venation. Although it is possible that in grasses Routside veins is a larger component of Rleaf than in dicotyledonous leaves with more vein orders, modeling the vein system of grasses as a collection of parallel pipes may, to some degree, neglect the actual distribution network. In grass leaves the major longitudinal veins constitute as little as ≈30% of the total vein density (Canny, 1990). Large veins may dominate axial transport, but smaller longitudinal and transverse veins with conduit diameters and numbers only 20% to 50% of those in the large longitudinal veins may in fact distribute most water to the mesophyll (Colbert and Evert, 1982; Altus and Canny, 1985; Altus et al., 1985; Canny, 1990).
If Routside venation is lower than Rminor veins, as shown for sugar maple and red oak, why should water leaving the major veins flow through the network of minor veins at all? We note that at the scale of the single tracheid, there may be very high resistance to radial leakage. However, Routside veins is an aggregate value for all the pathways outside of the vein system, determined by the combination of axial resistance and the number and resistance of the parallel leaks in the circuit (i.e. RA and RL in the minor veins in Fig. 5; for the same principle applied to the root system, see Landsberg and Fowkes, 1978). Though the resistance of each radial leak may be large, the large number of leaks in parallel across the entire minor vein system leads to a relatively low overall integrated resistance.
Partitioning of Leaf Hydraulic Resistance: Water Flow through the Mesophyll
The resistance to water flow through the mesophyll appears to arise, at least in part, from cell membranes. Both species exhibited a temperature response greater than expected solely from viscosity. It is unlikely that stomatal closure induced by chilling could account for this effect. As shown in previous studies, stomatal closure to ≈3% or less of maximum aperture would be needed to significantly influence the measurement of Rleaf (Sack et al., 2002); by contrast, for many species for which data are available, chilling from 25°C to 10°C under high irradiance in air reduced stomatal aperture at most to ≈50% maximum (e.g. Meidner and Mansfield, 1968; Willmer and Fricker, 1996; Yang et al., 1998; Cochard et al., 2000; Wilkinson et al., 2001). Additionally, stomata would be expected to take several minutes to close, while the increase in Rleaf with chilling occurred smoothly over time and ceased when temperature was held constant at any chilling temperature. The greater-than viscosity temperature response of Rleaf is consistent with the flow path including a transcellular component (Haines, 1994). This temperature response is the first to be reported for steady-state water flow through individual leaves; previously, milder effects (but still greater than those expected for viscosity) have been reported for leafy shoots (Fredeen and Sage, 1999; Cochard et al., 2000; Matzner and Comstock, 2001). Responses greater than expected from viscosity have also been found for the dehydration of shoots and leaves using the pressure bomb (Tyree et al., 1973, 1975; Boyer, 1974; Tyree and Cheung, 1977). From these studies an average activation energy was calculated as 26 to 27 kJ mol−1, equivalent to Q10 values of 1.4 to 1.5 in our temperature ranges, and the activation energy dropped to about 17 kJ mol−1 (equivalent to a Q10 value of 1.3) when water was forced through only branch and leaf xylem, consistent with the loss of a nonviscosity response in leaves with cut veins in this study. The Q10 values for intact leaves are low relative to values reported for plant plasma membranes—2 to 3 in this temperature range (Martre et al., 2002). However, given that the temperature response is found only in the Routside venation component (≈30% of Rleaf), the response is consistent with what would be expected.
The most likely location for a transcellular component of water flow across leaves is the bundle sheath, which surrounds the minor veins along virtually their entire length (Armacost, 1944; Plymale and Wylie, 1944). Dye experiments have suggested that most, if not all, water leaving the minor vein tracheary elements enters the bundle sheath parenchyma (Canny, 1990); this entry into the symplast might be forced by a Casparian strip; histochemical studies have sometimes indicated the presence of suberized walls (Van Fleet, 1950; Canny, 1990; Lersten, 1997). Some early studies concluded that membranes are too resistive to occur in the transpirational pathways (e.g. Boyer, 1974). However, the actual resistance of crossing a membrane is reduced by a high transfer area and the presence of aquaporins. For example, if we use a value for membrane hydraulic conductivity of 278 mmol m−2 s−1 MPa−1, obtained from measurements of Arabidopsis leaf mesophyll protoplasts (Martre et al., 2002), and an estimate of total minor vein surface area per leaf surface area of 0.30 m2 m−2 leaf, calculated assuming minor veins to be round in cross section with a diameter of 12 μm (Armacost, 1944) and a minor vein density of 8 × 103 m m−2 leaf (Plymale and Wylie, 1944), the calculated resistance is 0.012 MPa mmol−1 s m2. This value is 10% and 75% of Routside veins for sugar maple and red oak, respectively. Crossing membranes is therefore quantitatively consistent. Routside veins would clearly be sensitive to the membrane hydraulic conductivity.
Many questions remain about how the transpiration stream moves in the mesophyll. Once in the bundle sheath, water may move apoplastically (i.e. in nanopaths in the cell walls), symplastically (i.e. through plasmodesmata cell to cell), or even transcellularly (i.e. crossing cell membranes; see Tyree et al., 1999; Tyree, 2003). Water might flow from one mesophyll cell to another or flow largely through the bundle sheath extensions in species that possess them (Armacost, 1944; Wylie, 1946; McClendon, 1992) to the epidermis. Finally, evaporation might occur only near the stomata (Wylie, 1943, 1946; Tyree and Yianoulis, 1980; Shackel and Brinckmann, 1985) or, alternatively, take place throughout the spongy mesophyll (Davies, 1986; Nonami and Schulze, 1989; Nonami et al., 1991). There is a close agreement between measurements of Rleaf using methods that flood the air spaces of the leaf mesophyll once water leaves the xylem and bundle sheath (e.g. in the high-pressure method [HPM] or high-pressure flowmeter; Tyree et al., 1999) and methods that do not (e.g. based on evaporation rates from leaves and driving force estimated from the pressure bomb water potential; Cochard et al., 2000; Tsuda and Tyree, 2000; Nardini et al., 2001; Sack et al., 2002). This agreement suggests that the resistance to water flow beyond the bundle sheath is small (Sack et al., 2002). On the other hand, it is possible that both approaches underestimate the resistance to water flow across the mesophyll. In the HPM, flooding the air spaces short-circuits the normal liquid phase pathway, while evaporative flux measurements that use the pressure bomb may underestimate the driving force using a volume-weighted average water potential for the equilibrated leaf, rather than the unknown actual water potential at the sites of evaporation during transpiration (Yang and Tyree, 1994). However, the likelihood that both methods should be in error to the same degree is small.
Leaf Hydraulic Design
The hydraulic architecture of dicotyledonous leaves reflects the advantages of minimizing construction costs relative to hydraulic capacity. Although the highest capacity system would have an independent vein to each mesophyll cell (Givnish, 1979), branched systems reduce cost (Cuenca, 1989). In systems with fully minimized cost in relation to conductive capacity, resistance increases with each order of branching; such a pattern is the basis for Murray's law (Sherman, 1981; Canny, 1993; McCulloh et al., 2003). Narrow terminal conduits with high axial resistance are also expected because of their relatively high surface area to volume for water transfer to the mesophyll (LaBarbera, 1990). Additional advantages accrue with reticulation, such as the stabilization of water potential throughout the lamina because water can flow where it is needed; there are flow paths around sites of damage or cavitation. The veins may also play a supportive and protective mechanical role (Niklas, 1999).
The hydraulic design of the leaf is consistent with the observed functional importance of the leaf xylem. Vein xylem cavitation—diurnally or during leaf desiccation—as well as major vein damage can increase Rleaf to the extent that it substantially reduces leaf water potential and drives stomatal closure (Salleo et al., 2000, 2003; Nardini et al., 2001, 2003; Cochard et al., 2002; Huve et al., 2002; Brodribb and Holbrook, 2003; Sack et al., 2003a). For instance, in Cercis siliquastrum, when leaves desiccated to leaf water potential of −2.5 MPa, Rleaf nearly doubled due to embolism of the minor veins, and stomata closed (Nardini et al., 2003). Such a marked impact of increases in Rvenation is expected if Rvenation is a substantial component of Rleaf, but not if the bulk of leaf resistance were external to the venation (Meinzer, 2002).
Across plant species, Rleaf is negatively coordinated with peak rates of gas exchange (Aasamaa et al., 2001; Sack et al., 2003b). The importance of the vein system in determining Rleaf, as found in this study, suggests potentially important functional consequences at the whole-leaf and plant level for the diversity of leaf venation architecture in angiosperms (Roth-Nebelsick et al., 2001), i.e. in the density and topology of major and minor veins, in the geometry, number and size of vascular bundles in the veins, as well as in the numbers and sizes of the xylem conduits inside. These systems may represent configurations that diverge in hydraulic capacity and gas exchange, as well as in biomechanical support, damage tolerance, and construction cost. Future work is needed to test for functional consequences in diverse vein system anatomies.
MATERIALS AND METHODS
Plant Material
From June 2002 to August 2002 at Harvard Forest in Petersham, Massachusetts (42′54°N, 72′18°W), trees of sugar maple (Acer saccharum; Sapindaceae family; Gleason and Cronquist, 1991; Judd et al., 2002) and red oak (Quercus rubra; Fagaceae family), growing along roads at the edge of the forest, were selected for study. Diameters at breast height ranged between 56 to 91 cm for sugar maple and 20 to 51 cm for red oak. Exposed branches with mature leaves were sampled 5 to 8 m above the ground for five trees per species. Material collected in the field was recut under water and allowed to hydrate overnight by placing the cut ends of the branches in water and covering leaves with plastic.
Leaf Hydraulic Resistance
Measurements of leaf hydraulic properties were made using the HPM (which uses the high-pressure flowmeter of Tyree et al., 1993; see Sack et al., 2002). We report hydraulic resistance (Rleaf), rather than hydraulic conductance (=1/resistance), because resistances add in series, enabling measured values for leaves to be partitioned into their component resistances. The HPM measures Rleaf as the force required to push water through a leaf for a given flow rate. Pressurized (0.5–0.6 MPa) degassed water was forced through a system of tubing, which includes a high-resistance segment, into the petiole (which had been previously cut under water and attached by compression fitting), through the leaf, and eventually out of the stomata. The high-resistance tubing was a 145-cm segment of red (0.125 mm internal diameter) polyetheretherketone (PEEK) tubing (Upchurch Scientific, Oak Harbor, WA). The hydraulic resistance of the high-resistance tubing (RT) was determined from the slope of delivery pressure versus flow rate measured with an analytic balance (±0.1 mg; Mettler AG104; Mettler-Toledo GmbH, Greifensee, Switzerland). Pressure transducers (Omega PX-180; Omega Engineering, Stamford, CT) before (P1) and after (P2) the high-resistance tubing allowed calculation of the flow rate as equal to (P1 – P2)/RT. Rleaf (MPa mmol−1 s m2) is calculated by dividing the pressure drop across the leaf (P2) by the flow rate and normalizing by the area of the leaf lamina determined using a leaf area meter (Li-Cor, Lincoln, NE). To prevent growth of microorganisms, the entire tubing system was bleached and rinsed at regular (approximately 4–5 d of measurement) intervals.
Rleaf was recorded once measurements (each 2–5 min) were stable to a coefficient of variation <5% for 20 min, which took typically 30 to 45 min for an intact leaf and 10 to 15 min after veins were cut. Tests on three leaves per species showed that Rleaf, once stable, remained so for >2 h, the period during which cutting treatments and/or temperature responses were made. Deionized filtered degassed water was used as the flow solution, refiltered to 0.2-μm pore diameter on introduction to the system; Rleaf determined using water was statistically similar to that found using a 10 mm KCl flow solution for test leaves of each species. All measured leaves were submerged in a temperature-controlled water bath (25°C, except during temperature responses). Leaves were illuminated (>1,200 μmol photons m−2 s−1 of photosynthetically active irradiance inside the emptied water bath at the leaf position; LI-250 light meter; Li-Cor) to stimulate stomatal opening. Failure to illuminate leaves resulted in Rleaf values that far exceeded values in high irradiance, as well as values determined using two independent techniques (Sack et al., 2002, for red oak; L. Sack, unpublished data, for sugar maple). Following determination of Rleaf, the lamina was severed at its connection to the petiole, and the hydraulic resistance of the portion of the petiole connected to the flowmeter measured. Rlamina (MPa mmol−1 s m2) was calculated by subtracting this resistance from Rleaf.
Partitioning of Leaf Hydraulic Resistance
Leaves were measured before and after applying one of two vein cutting treatments. To remove the resistance downstream of the minor veins (i.e. veins of higher order than tertiaries, embedded in the lamina; Dengler and Kang, 2001), minor veins were cut at random locations throughout the lamina by making 1.5- to 2-mm incisions into the lamina with a scalpel. Given the minor vein densities in sugar maple and red oak of, respectively, 5 mm mm−2 and 7 mm mm−2 (L. Sack, unpublished data), each cut would have severed at least 5 to 7 minor veins (assuming a minor vein grid). When cutting the minor veins, great care was taken to avoid all major veins (i.e. the primary, secondary, and tertiary veins). For a first set of leaves (three per species), cuts were applied 20 to 25 at a time until there was no further change in the measured resistance; for additional leaves (5–7 per species), a number of cuts far in excess (120–150 cuts) of that needed to achieve the maximum response was used. When fully treated, the leaves typically had, on average, 2 cuts cm−2 leaf. Although it is unlikely that the cuts themselves introduced additional resistance by crushing or pinching of the tracheids in the severed minor veins, any such resistance would have been rendered negligible in the fully treated leaves due to the incremental cutting method (i.e. cutting until resistance no longer declined). The reduction in hydraulic resistance was used to calculate the fraction of the total resistance from the xylem in the minor veins to the point where water exits the leaf (Routside veins). The remaining hydraulic resistance (expressed as percentage of Rlamina) was considered to be the resistance of the venation (Rvenation). The normality of the distribution of values for Rvenation as a percentage of Rlamina was assessed using Anderson-Darling tests (using Minitab Release 13.32; Minitab, State College, PA). On a different set of leaves, all the obvious tertiary veins throughout the lamina (50–80 veins) were cut. The hydraulic resistance remaining was considered that of the major veins (Rmajor veins), and Rminor veins was calculated as Rvenation − Rmajor veins. Because the petiole must be severed and recut so that it can be sealed to the flow meter, Rpetiole was estimated using the resistance of the petiole segment divided by its length and then multiplied by the intact petiole length, as estimated using regressions of petiole length versus leaf area (Sack et al., 2003b). Estimates of Rpetiole were normalized by leaf area to have the same units as Rlamina.
We tested whether the partitioning of Rleaf into additive components as described above is consistent with the leaf hydraulic architecture as conceived in Figure 5 using circuit model simulations (Electronics Workbench version 5.0c; Interactive Image Technologies, Toronto). In the simulations presented here, RL of major veins was set at infinity, such that the only leaks to the mesophyll occurred through minor veins, to best approximate the flow paths in real leaves, where the major veins account for <5% of vein density and would thus account for a very low proportion of the total transfer area to mesophyll (see “Discussion”).
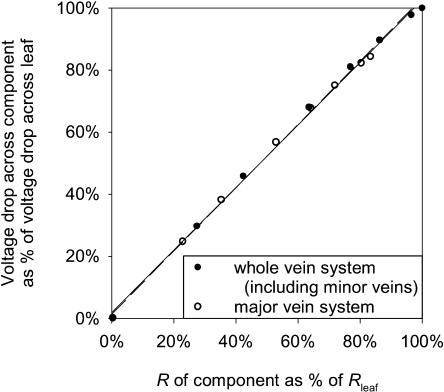
Our goal was to test whether the component resistances (such as the major veins considered together and the minor veins considered together) behave as simple resistors in series in an Ohmic circuit, despite their branching, and the reticulation of the circuit. For simple resistors in series, the percentage of the total circuit voltage drop that occurs across any resistor equals the percentage of the total circuit resistance contributed by the resistor. Simulations were run with the circuit model, changing only the RL of the minor veins. For each simulation, Rvenation and Rmajor veins were calculated as a percentage of Rleaf. The voltage drop from petiole to the tertiary veins and the voltage drop from petiole to minor veins were determined as the difference between input voltage (12 V) and averaged probed voltages for respectively 16 tertiary veins and 16 minor veins throughout one side of the circuit. As expected for simple resistors in series, the percentage of voltage drop between petiole and tertiary veins was the same value as Rmajor veins as a percentage of Rleaf, and the percentage of the total voltage drop between petiole and minor veins was the same value as Rvenation as a percentage of Rleaf (Fig. 6; relationships not different from 1:1).
Figure 6.
The additivity of partitioned resistance components for leaves modeled with the circuit in Figure 1 (RA and RL were parameterized for petiole 5 kΩ and ∞, for primaries 2 kΩ and ∞, for secondaries 10 kΩ and ∞, for tertiaries 200 kΩ and ∞, and for minor veins 1 MΩ and values ranging 0.001–10,000 MΩ; other hierarchical parameterizations produced similar patterns). Black circles and solid regression line, Vminor veins/total V drop (12 V) versus Rvenation/Rleaf (y = 1.01x + 0.012;R2 = 0.997; P < 0.001); white circles and dashed regression line,Vtertiary veins/total V drop (12 V) versus Rmajor veins/Rleaf (y = 1.02x +0.013; R2 = 0.998; P < 0.001). Regressions not significantly different from 1:1 line.
To confirm that incrementally severing leaf minor veins would cause the measured hydraulic resistance to converge with the resistance of the vein network, in the model circuit the RL of minor veins was shorted out (i.e. set to 0 Ω) in successive groups of 16 minor veins, selected at random across the circuit (Fig. 7A). The measured resistance, expressed as a percentage of the initial resistance, followed a trajectory similar in shape to that observed in severing groups of minor veins in real leaves (i.e. an initially steep slope evening off; compare with Fig. 2).
Finally, we tested whether the number of shorted veins required to remove the extravascular resistance was sensitive to resistance RJ at the junctions of major and minor veins (i.e. at junctions of black and white or of gray and white squares in Fig. 5). With increasing RJ, a smaller number of shorted minor veins was required to remove >95% of the extravascular resistance (Fig. 7B).
Temperature Responses
The effect of temperature on water flow through intact and treated leaves of each species (n = 4–6 for each treatment per species) was determined by varying the temperature of leaves supplied with water at a constant delivery pressure. Once flow through the leaf had stabilized, the temperature of the water bath was lowered from 25°C to 10°C at a rate of 1°C per 2 to 5 min. Changes in measured flow rates through leaves were insensitive to the cooling rate. Rleaf declined smoothly during the chilling and stabilized when temperature was held constant at any chilling temperature. Q10 values for were calculated for two temperature intervals, 15°C to 25°C and 10°C to 20°C, as the flow rate at higher temperature divided by that at lower temperature. t tests were used to determine differences of Q10 values from 1 (using Minitab Release 13.32; Minitab), including sequential Bonferroni adjustment for an overall P = 0.05 for each species (Rice, 1989).
Anatomy
Measurements were made to compare the correspondence of xylem anatomy and hydraulic resistance for five leaves per species that were subjected to hydraulics measurements. Cross-sections from the center of the lamina perpendicular to the midrib leaf were prepared using a cryotome, stained with toluidine blue, and digitally imaged under a microscope. The maximum widths of the three largest conduits were measured (using ImageJ freeware; http://rsb.info.nih.gov/ij/) within the midrib (after Aasamaa et al., 2001) and centrally in the clearest adjacent minor vein (compare with Esau, 1965; p. 426). Visualization with polarized light was often helpful to resolve individual xylem conduits.
Acknowledgments
We thank Michael Burns, Maggie Dietrich, David Sanchez, and Dave Webb for logistic and technical assistance, Harvard Forest staff for facilitating the research, and Michael Burns, Herve Cochard, Andrea Nardini, Sebastiano Salleo, Matt Thompson, Mel Tyree, and two anonymous reviewers for helpful discussion or comments on the manuscript.
This work was supported by the Arnold Arboretum of Harvard University (Putnam Fellowship to L.S.), the Andrew W. Mellon Foundation, and the National Science Foundation (grant no. 0139495).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.031203.
References
- Aasamaa K, Sober A, Rahi M (2001) Leaf anatomical characteristics associated with shoot hydraulic conductance, stomatal conductance and stomatal sensitivity to changes of leaf water status in temperate deciduous trees. Aust J Plant Physiol 28: 765–774 [Google Scholar]
- Altus DP, Canny MJ (1985) Water pathways in wheat leaves. 1. The division of fluxes between different vein types. Aust J Plant Physiol 12: 173–181 [Google Scholar]
- Altus DP, Canny MJ, Blackman DR (1985) Water pathways in wheat leaves. 2. Water-conducting capacities and vessel diameters of different vein types, and the behavior of the integrated vein network. Aust J Plant Physiol 12: 183–199 [Google Scholar]
- Armacost RR (1944) The structure and function of the border parenchyma and vein-ribs of certain dicotyledon leaves. Proc Iowa Acad Sci 51: 157–169 [Google Scholar]
- Boyer JS (1974) Water transport in plants: mechanism of apparent changes in resistance during absorption. Planta 117: 187–207 [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM (2003) Changes in leaf hydraulic conductance during leaf shedding in seasonally dry tropical forest. New Phytol 158: 295–303 [Google Scholar]
- Canny MJ (1990) What becomes of the transpiration stream? New Phytol 114: 341–368 [DOI] [PubMed] [Google Scholar]
- Canny MJ (1993) The transpiration stream in the leaf apoplast: water and solutes. Philos Trans R Soc Lond B Biol Sci 341: 87–100 [Google Scholar]
- Cochard H, Coll L, Le Roux X, Ameglio T (2002) Unraveling the effects of plant hydraulics on stomatal closure during water stress in walnut. Plant Physiol 128: 282–290 [PMC free article] [PubMed] [Google Scholar]
- Cochard H, Martin R, Gross P, Bogeat-Triboulot MB (2000) Temperature effects on hydraulic conductance and water relations of Quercus robur L. J Exp Bot 51: 1255–1259 [PubMed] [Google Scholar]
- Colbert JT, Evert RF (1982) Leaf vasculature in sugarcane (Saccharum officinarum L.). Planta 156: 136–151 [DOI] [PubMed] [Google Scholar]
- Cuenca RH (1989) Irrigation System Design: An Engineering Approach. Prentice Hall, Englewood Cliffs, NJ
- Davies WJ (1986) Transpiration and the water balance of plants. In FC Steward, ed, Plant Physiology, Vol IX. Academic Press, Orlando, FL, pp 49–154
- Dengler N, Kang J (2001) Vascular patterning and leaf shape. Curr Opin Plant Biol 4: 50–56 [DOI] [PubMed] [Google Scholar]
- Dengler NG, Mackay LB (1975) Leaf anatomy of beech, Fagus grandifolia. Can J Bot 53: 2202–2211 [Google Scholar]
- Esau K (1965) Plant Anatomy, Ed 2. John Wiley, New York
- Fredeen AL, Sage RF (1999) Temperature and humidity effects on branchlet gas-exchange in white spruce: an explanation for the increase in transpiration with branchlet temperature. Trees 14: 161–168 [Google Scholar]
- Givnish TJ (1979) On the adaptive significance of leaf form. In OT Solbrig, S Jain, GB Johnson, PH Raven, eds, Topics in Plant Population Biology. Columbia University Press, New York, pp 375–407
- Gleason HA, Cronquist A (1991) Manual of Vascular Plants of Northeastern United States and Adjacent Canada, Ed 2. New York Botanical Garden, Bronx, NY
- Haines TH (1994) Water transport across biological membranes. FEBS Lett 346: 115–122 [DOI] [PubMed] [Google Scholar]
- Huve K, Remus R, Luttschwager D, Merbach W (2002) Water transport in impaired leaf vein systems. Plant Biol 4: 603–611 [Google Scholar]
- Jeje AA (1985) The flow and dispersion of water in the vascular network of dicotyledonous leaves. Biorheology 22: 285–302 [DOI] [PubMed] [Google Scholar]
- Judd WS, Campbell CS, Kellogg EA, Stevens PF, Donoghue MJ (2002) Plant Systematics: A Phylogenetic Approach. Sinauer, Sunderland, MA
- LaBarbera M (1990) Principles of design of fluid transport systems in zoology. Science 249: 992–1000 [DOI] [PubMed] [Google Scholar]
- Landsberg JJ, Fowkes ND (1978) Water movement through plant roots. Ann Bot (Lond) 42: 493–508 [Google Scholar]
- Lersten NR (1997) Occurrence of endodermis with a Casparian strip in stem and leaf. Bot Rev 63: 265–272 [Google Scholar]
- Martre P, Cochard H, Durand JL (2001) Hydraulic architecture and water flow in growing grass tillers (Festuca arundinacea Schreb.). Plant Cell Environ 24: 65–76 [Google Scholar]
- Martre P, Morillon R, Barrieu F, North GB, Nobel PS, Chrispeels MJ (2002) Plasma membrane aquaporins play a significant role during recovery from water deficit. Plant Physiol 130: 2101–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzner S, Comstock J (2001) The temperature dependence of shoot hydraulic resistance: implications for stomatal behaviour and hydraulic limitation. Plant Cell Environ 24: 1299–1307 [Google Scholar]
- McClendon JH (1992) Photographic survey of the occurrence of bundle sheath extensions in deciduous dicots. Plant Physiol 99: 1677–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloh KA, Sperry JS, Adler FR (2003) Water transport in plants obeys Murray's law. Nature 421: 939–942 [DOI] [PubMed] [Google Scholar]
- Meidner H, Mansfield TA (1968) Physiology of Stomata. McGraw-Hill, Maidenhead, UK
- Meinzer FC (2002) Co-ordination of vapour and liquid phase water transport properties in plants. Plant Cell Environ 25: 265–274 [DOI] [PubMed] [Google Scholar]
- Nardini A, Salleo S (2003) Effects of the experimental blockage of the major veins on hydraulics and gas exchange of Prunus laurocerasus L. leaves. J Exp Bot 54: 1213–1219 [DOI] [PubMed] [Google Scholar]
- Nardini A, Salleo S, Raimondo F (2003) Changes in leaf hydraulic conductance correlate with leaf vein embolism in Cercis siliquastrum L. Trees-Struct and Funct 17: 529–534 [Google Scholar]
- Nardini A, Tyree MT, Salleo S (2001) Xylem cavitation in the leaf of Prunus laurocerasus and its impact on leaf hydraulics. Plant Physiol 125: 1700–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklas KJ (1999) A mechanical perspective on foliage leaf form and function. New Phytol 143: 19–31 [Google Scholar]
- Nonami H, Schulze ED (1989) Cell water potential, osmotic potential, and turgor in the epidermis and mesophyll of transpiring leaves: combined measurements with the cell pressure probe and nanoliter osmometer. Planta 177: 35–46 [DOI] [PubMed] [Google Scholar]
- Nonami H, Schulze ED, Ziegler H (1991) Mechanisms of stomatal movement in response to air humidity, irradiance and xylem water potential. Planta 183: 57–64 [DOI] [PubMed] [Google Scholar]
- Plymale EL, Wylie RB (1944) The major veins of mesomorphic leaves. Am J Bot 31: 99–106 [Google Scholar]
- Pray TR (1954) Foliar venation of angiosperms. 1. Mature venation of Liriodendron. Am J Bot 41: 663–670 [Google Scholar]
- Rice WR (1989) Analyzing tables of statistical tests. Evolution 43: 223–225 [DOI] [PubMed] [Google Scholar]
- Roth-Nebelsick A, Uhl D, Mosbrugger V, Kerp H (2001) Evolution and function of leaf venation architecture: a review. Ann Bot (Lond) 87: 553–566 [Google Scholar]
- Russin WA, Evert RF (1984) Studies on the leaf of Populus deltoides (Salicaceae): morphology and anatomy. Am J Bot 71: 1398–1415 [Google Scholar]
- Sack L, Cowan PD, Holbrook NM (2003. a) The major veins of mesomorphic leaves revisited: tests for conductive overload in Acer saccharum (Aceraceae) and Quercus rubra (Fagaceae). Am J Bot 90: 32–39 [DOI] [PubMed] [Google Scholar]
- Sack L, Cowan PD, Jaikumar N, Holbrook NM (2003. b) The ‘hydrology’ of leaves: co-ordination of structure and function in temperate woody species. Plant Cell Environ 26: 1343–1356 [Google Scholar]
- Sack L, Melcher PJ, Zwieniecki MA, Holbrook NM (2002) The hydraulic conductance of the angiosperm leaf lamina: a comparison of three measurement methods. J Exp Bot 53: 2177–2184 [DOI] [PubMed] [Google Scholar]
- Sack L, Tyree MT (2004) Leaf hydraulics and its implications in plant structure and function. In NM Holbrook, MA Zwieniecki, eds, Movement of Water and Carbohydrates in Plants: Integration of Long Distance Transport Processes. Academic Press, San Diego, in press
- Salleo S, Nardini A, Pitt F, Lo Gullo MA (2000) Xylem cavitation and hydraulic control of stomatal conductance in laurel (Laurus nobilis L.). Plant Cell Environ 23: 71–79 [Google Scholar]
- Salleo S, Raimondo F, Trifilo P, Nardini A (2003) Axial-to-radial water permeability of leaf major veins: a possible determinant of the impact of vein embolism on leaf hydraulics? Plant Cell Environ 26: 1749–1758 [Google Scholar]
- Shackel KA, Brinckmann E (1985) In situ measurement of epidermal cell turgor, leaf water potential, and gas exchange in Tradescantia virginiana L. Plant Physiol 78: 66–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman TF (1981) On connecting large vessels to small: the meaning of Murray's Law. J Gen Physiol 78: 431–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Tyree MT (2000) Plant hydraulic conductance measured by the high pressure flow meter in crop plants. J Exp Bot 51: 823–828 [PubMed] [Google Scholar]
- Tyree MT (2003) Hydraulic properties of roots. In EJW Visser, H de Kroon, eds, Root Ecology. Springer, Berlin, 125–150
- Tyree MT, Benis M, Dainty J (1973) Water relations of hemlock (Tsuga canadensis). 3. Temperature dependence of water exchange in a pressure bomb. Can J Bot 51: 1537–1543 [Google Scholar]
- Tyree MT, Caldwell C, Dainty J (1975) Water relations of hemlock (Tsuga canadensis). 5. Localization of resistances to bulk water flow. Can J Bot 53: 1078–1084 [Google Scholar]
- Tyree MT, Cheung YNS (1977) Resistance to water flow in Fagus grandifolia leaves. Can J Bot 55: 2591–2599 [Google Scholar]
- Tyree MT, Nardini A, Salleo S (2001) Hydraulic architecture of whole plants and single leaves. In M Labrecque, ed, L'Arbre 2000 The Tree. Isabelle Quentin Publisher, Montreal, pp 215–221
- Tyree MT, Sinclair B, Lu P, Granier A (1993) Whole shoot hydraulic resistance in Quercus species measured with a new high-pressure flowmeter. Ann Sci Forest 50: 417–423 [Google Scholar]
- Tyree MT, Sobrado MA, Stratton LJ, Becker P (1999) Diversity of hydraulic conductance in leaves of temperate and tropical species: possible causes and consequences. J Trop For Sci 11: 47–60 [Google Scholar]
- Tyree MT, Yianoulis P (1980) The site of water evaporation from sub-stomatal cavities, liquid path resistances and hydroactive stomatal closure. Ann Bot (Lond) 46: 175–193 [Google Scholar]
- Tyree MT, Zimmermann MH (2002) Xylem Structure and the Ascent of Sap. Springer, Berlin
- Van Fleet DS (1950) The cell forms, and their common substance reactions, in the parenchyma-vascular boundary. Bull Torrey Bot Club 77: 340–353 [Google Scholar]
- Wei CF, Tyree MT, Steudle E (1999) Direct measurement of xylem pressure in leaves of intact maize plants. A test of the cohesion-tension theory taking hydraulic architecture into consideration. Plant Physiol 121: 1191–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S, Clephan AL, Davies WJ (2001) Rapid low temperature-induced stomatal closure occurs in cold-tolerant Commelina communis leaves but not in cold-sensitive tobacco leaves, via a mechanism that involves apoplastic calcium but not abscisic acid. Plant Physiol 126: 1566–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmer C, Fricker M (1996) Stomata, Ed 2. Chapman & Hall, London
- Wylie RB (1943) The role of the epidermis in foliar organization and its relations to the minor venation. Am J Bot 30: 273–280 [Google Scholar]
- Wylie RB (1946) Conduction in dicotyledon leaves. Proc Iowa Acad Sci 53: 195–202 [Google Scholar]
- Yang S, Liu X, Tyree MT (1998) A model of stomatal conductance in sugar maple (Acer saccharum Marsh). J Theor Biol 191: 197–211 [Google Scholar]
- Yang SD, Tyree MT (1994) Hydraulic architecture of Acer saccharum and A. rubrum: comparison of branches to whole trees and the contribution of leaves to hydraulic resistance. J Exp Bot 45: 179–186 [Google Scholar]
- Zwieniecki MA, Melcher PJ, Boyce CK, Sack L, Holbrook NM (2002) Hydraulic architecture of leaf venation in Laurus nobilis L. Plant Cell Environ 25: 1445–1450 [Google Scholar]