Abstract
2B4 is a SLAM-related receptor expressed on natural killer (NK) cells and cytotoxic T cells. It can regulate killing and gamma interferon secretion by NK cells, as well as T-cell-mediated cytotoxicity. There are conflicting data regarding the mechanism of action of 2B4. In these studies, we attempted to understand better the nature and basis of 2B4 signaling. Our studies showed that engagement of 2B4 on NK cells triggered a tyrosine phosphorylation signal implicating 2B4, Vav-1, and, to a lesser extent, SHIP-1 and c-Cbl. Structure-function analyses demonstrated that this response was defined by a series of tyrosine-based motifs in the cytoplasmic region of 2B4 and was not influenced by the extracellular or transmembrane segment of 2B4. In addition, the 2B4-induced signal was absolutely dependent on coexpression of SAP, a Src homology 2 (SH2) domain-containing adaptor associating with SLAM-related receptors and mutated in X-linked lymphoproliferative disease. It was also observed that 2B4 was detectably associated with the Src-related protein tyrosine kinase FynT in an immortalized NK cell line. Mutation of arginine 78 of SAP, a residue critical for binding of SAP to FynT, eliminated 2B4-mediated protein tyrosine phosphorylation, implying that SAP promotes 2B4 signaling most probably by recruiting FynT. Finally, despite the similarities in the signaling modalities of 2B4 and its relative SLAM, the natures of the tyrosine phosphorylation signals induced by these two receptors were found to be different. These differences were not caused by variations in the extent of binding to SAP but rather were dictated by the tyrosine-based sequences in the cytoplasmic domain of the receptors. Taken together, these data lead to a better understanding of 2B4 signaling. Furthermore, they provide firm evidence that the signals transduced by the various SLAM-related receptors are unique and that the specificity of these signals is defined by the distinctive arrays of intracytoplasmic tyrosines in the receptors.
2B4, also named CD244, is a member of the SLAM family of immune cell-specific receptors that also includes SLAM, CD84, Ly-9, NTB-A/Ly-108, and CRACC (12, 23, 32, 34). It is expressed in natural killer (NK) cells, cytotoxic CD8+ T cells, γδ T cells, and mast cells. Like its relatives, 2B4 possesses immunoglobulin (Ig)-like domains in its extracellular region, a single transmembrane domain, and an intracellular segment bearing several tyrosine-based motifs. The ligand for 2B4 is CD48, a glycosylphosphatidylinositol-linked receptor expressed on diverse types of immune cells (5, 16). While an analysis of mice lacking 2B4 has yet to be reported, engagement of 2B4 by anti-2B4 antibodies or CD48 was shown to promote cytotoxicity and gamma interferon (IFN-γ) secretion by NK cells (24). Furthermore, it augments antigen-induced IFN-γ secretion by activated CD8+ T cells (14, 21).
Like most other members of the SLAM family, 2B4 interacts through its cytoplasmic domain with SAP (or SH2D1A), a small intracellular Src homology 2 (SH2) domain-containing adaptor expressed in T cells, NK cells, and some B cells (8, 12, 19, 26, 28, 29). In the case of SLAM, a member of the family regulated by homotypic self-associations, the binding of SAP enables SLAM to mediate an intracellular tyrosine phosphorylation signal implicating SLAM, the 5′ inositol phosphatase SHIP-1, and the adaptor molecules Dok-1, Dok-2, and Shc (17). SAP expression also appears to be critical for the ability of SLAM-SLAM homotypic interactions to inhibit IFN-γ secretion during T-cell activation (17). Accumulating data show that SAP promotes SLAM signaling by recruiting the Src-related protein tyrosine kinase FynT. This reflects the capacity of SAP to bind directly to FynT by way of a second binding surface in the SAP SH2 domain and the FynT SH3 domain (7, 18). Whether a similar function is provided by SAP for the other SLAM family members is not known.
Confusing data exist regarding the mechanism by which 2B4 regulates immune cell functions. An early report indicated that 2B4 engagement triggered tyrosine phosphorylation of several polypeptides, including 2B4, the guanine nucleotide exchange factor Vav-1, and phospholipase C-γ1 (35). In subsequent studies, it was suggested that 2B4 signaling was dependent on the capacity of 2B4 to interact with the lipid raft-associated transmembrane adaptor molecule LAT by way of a dicysteine motif (CxC) located in the transmembrane portion of 2B4 (3, 15). It was also reported that the ability of 2B4 to undergo tyrosine phosphorylation in response to treatment with the protein tyrosine phosphatase inhibitor pervanadate was only minimally reduced in cells lacking SAP, suggesting that SAP may not be required for 2B4 signaling (27).
Nonetheless, an important role for SAP in 2B4 function was shown by analyses of NK cells from patients with X-linked lymphoproliferative (XLP) disease, a severe immunodeficiency characterized by an abnormal immune response to Epstein-Barr virus infection (2, 25, 27, 30). Patients with XLP disease carry inactivating mutations or deletions in the sap gene and consequently either lack SAP expression or express nonfunctional SAP proteins. Importantly, NK cells from these individuals were revealed to display a marked reduction in 2B4-mediated cytotoxicity. Thus, it seems likely that SAP is involved in critical aspects of 2B4 signaling.
In this report, we clarify the mechanism of 2B4-mediated signal transduction. The results of our studies show that ligation of 2B4 on NK cells evokes a specific protein tyrosine phosphorylation response involving 2B4, Vav-1, and, to a lesser extent, SHIP-1 and c-Cbl. This signal is absolutely dependent on coexpression of SAP and correlates with the capacity of SAP to associate with the Src-related protein tyrosine kinase FynT. Experiments with chimeric and mutated forms of 2B4 indicated that the 2B4-mediated signal is defined solely by the tyrosine-based motifs in the cytoplasmic domain of 2B4. It is not appreciably influenced by the transmembrane and extracellular segments of 2B4. Finally, a comparative analysis of 2B4 and SLAM signaling revealed that each receptor transduces a unique SAP-dependent signal, which is specified by the distinctive array of tyrosines located in the cytoplasmic domain of the receptor.
MATERIALS AND METHODS
Cells.
YT is a transformed human NK cell line. It expresses 2B4 and SAP but does not express the SAP-related protein EAT-2 (R. Roncagalli and A. Veillette, unpublished results). YT was propagated in RPMI 1640 medium supplemented with 15% fetal bovine serum (FBS), glutamine, antibiotics, and, if necessary, puromycin (2 μg/ml). BI-141 is an antigen-specific mouse T-cell line that does not normally express 2B4, SAP, or SLAM (data not shown). BI-141 derivatives expressing SAP alone or in combination with Tac-Tac-SLAM or SLAM were described previously (17). BI-141 cells were grown in RPMI 1640 medium containing 10% FBS, glutamine, antibiotics, and, if necessary, G418 (0.6 mg/ml) and/or puromycin (2 μg/ml). Normal mouse NK cells were isolated from the spleens of C57BL/6 mice (Harlan, Chicago, Ill.) by negative selection with an NK cell isolation cocktail (Stem Cell Technologies, Vancouver, British Columbia, Canada). About 90% of the purified cells were NK1.1+ and CD3− (data not shown). Cells were then propagated in RPMI 1640 medium supplemented with 10% FBS, antibiotics, β-mercaptoethanol, and interleukin-2 (IL-2; 1,000 U/ml) and used for experimentation after 8 to 10 days. At that time, greater than 95% of the cells were NK1.1+, 2B4+, DX-5+, and CD3− (data not shown). These cells expressed both SAP and EAT-2 (Roncagalli and Veillette, unpublished). Human NK cells were isolated from peripheral blood of a normal individual or from a previously described XLP patient, as detailed elsewhere (1, 2, 11). This XLP patient carries an arginine 55-to-leucine (R55L) mutation in the SAP protein. While he has not yet developed severe clinical manifestations of XLP, three male relatives carrying the same mutation died of the disease. After purification, cells were grown in RPMI 1640 medium containing 10% human serum, antibiotics, nonessential amino acids, β-mercaptoethanol, and human IL-2 (1,000 U/ml). Before experimentation, cells were incubated for 6 h in AIM-V serum-free medium (Invitrogen Canada, Burlington, Ontario). This human study was approved by the University of British Columbia Clinical Research Ethics Board, and informed consent was obtained from all subjects before blood collection.
Antibodies.
A rabbit antiserum directed against human 2B4 was kindly provided by Eric Long (National Institutes of Health, Rockville, Md.). An antiserum recognizing mouse 2B4 was generated by immunizing rabbits with a TrpE fusion protein encompassing the full cytoplasmic domain of mouse 2B4. This antiserum recognizes mouse 2B4 and does not react with other SLAM-related receptors such as SLAM, NTB-A/Ly-108, CD84, and CRACC (data not shown). C1.7 is a mouse monoclonal antibody (MAb) reacting with human 2B4 (32). The hybridoma producing this MAb was a generous gift from Giorgio Trinchieri (Schering-Plough Research Institute, Dardilly, France). Mouse MAb 2B4 is directed against mouse 2B4 and was purchased from BD Biosciences (Mississauga, Ontario, Canada). Anti-Tac mouse MAb 7G7 reacts against the human IL-2 receptor α chain and was purified from culture supernatant. In some experiments, biotinylated MAb 2B4 or 7G7 was used. Biotinylated MAb F23.1 is a mouse antibody directed against the T-cell receptor (TCR). Rabbit sera directed against SAP, FynT, Vav-1, SHIP-1, phospholipase C-γ1, c-Cbl, LAT, and phosphotyrosine were generated in our laboratory. Mouse MAb 4G10 is directed against phosphotyrosine and was purchased from Upstate Biotechnologies Inc. (Lake Placid, N.Y.). Rat MAb 12F12 is specific for mouse SLAM (6). It reacts against the extracellular domain of SLAM. A rabbit anti-Tac serum that works in immunoblots was purchased from Santa Cruz Biotechnology, Santa Cruz, Calif. Phosphospecific antibodies recognizing FynT molecules phosphorylated at tyrosine 417 (activated FynT) or Vav-1 phosphorylated at tyrosine 160 were purchased from Biosources (Camarillo, Calif.).
cDNAs, mutants, and constructs.
A 2b4 cDNA (long form) from a C57BL/6 mouse was obtained from Marco Colonna (Washington University, St. Louis, Mo.). The wild-type and arginine 78-to-alanine (R78A) sap cDNAs were described elsewhere (18). cDNAs encoding chimeras possessing the extracellular region of Tac fused to the transmembrane and cytoplasmic domains of mouse 2B4 (Tac-2B4-2B4), or to the transmembrane region of Tac and the cytoplasmic domain of mouse 2B4 (Tac-Tac-2B4), were produced by PCR. A construct coding for Tac-Tac-SLAM-2B4, in which the first 27 amino acids of the cytoplasmic region of 2B4 were replaced with those of SLAM, was also generated by PCR. A variant of SLAM (SLAM-SLAM-2B4) in which the intracytoplasmic domain of Tac-Tac-SLAM-2B4 was fused to the extracellular and transmembrane segments of SLAM was further produced by PCR. Mutant forms of Tac-Tac-2B4 in which one or more cytoplasmic tyrosines were mutated to phenylalanines were created by using the Stratagene PCR mutagenesis kit (Stratagene, La Jolla, Calif.). All constructs were verified by sequencing to ensure that they contained no unwanted mutations (data not shown). For stable transfection into YT or BI-141 cells, the various 2B4-based constructs were inserted into the vector pSRα-puro, which contains the puromycin resistance gene. For retroviral infection in BI-141 cells, the sap cDNAs were inserted into the retroviral vector pMIG or pFB. Both vectors contain an internal ribosomal entry site and the gene for green fluorescent protein (GFP).
Transfections.
YT and BI-141 cells were transfected by electroporation in accordance with a previously described protocol (20). After selection in the presence of puromycin (2 μg/ml for YT and 1 μg/ml for BI-141), monoclonal cell lines were generated by limiting dilution. Clones expressing the various Tac chimeras were identified by flow cytometry with anti-Tac MAb 7G7. In some cases, polyclonal populations in which greater than 85% of the puromycin-resistant cells were positive for Tac were also used for experimentation. The YT-derived cell lines that were used in our experiments had unchanged amounts of human 2B4 at the surface, while the BI-141 derivatives expressed unaltered levels of TCR, CD3, CD45, and Thy-1 (data not shown).
Retroviral infections.
BI-141 derivatives were infected by “spinfection” with retroviruses encoding GFP alone or in combination with the indicated polypeptides. Following 4 to 5 days in culture, GFP+ cells were isolated by cell sorting and tested as described later in the text.
Cell stimulation.
For stimulation of human 2B4, YT cells (20 × 106/ml) or ex vivo human NK cells (20 × 106/ml) were incubated for the indicated periods of time at 37°C with mouse anti-2B4 MAb C1.7 and either rabbit anti-mouse (RAM) IgG or sheep anti-mouse (SAM) IgG. For ligation of Tac chimeras, YT cells (20 × 106/ml) or BI-141 derivatives (20 × 106/ml) were triggered for the indicated times at 37°C, either with biotinylated mouse anti-Tac MAb 7G7 and avidin or with MAb 7G7 and RAM IgG. Ex vivo normal mouse NK cells were ligated either with biotinylated mouse anti-2B4 MAb 2B4 and avidin or with MAb 2B4 and RAM IgG. After stimulation, cells were lysed in modified radioimmunoprecipitation assay buffer (1× modified radioimmunoprecipitation assay buffer is 50 mM HEPES [pH 7.4], 1% Triton X-100, 1% sodium deoxycholate, 0.2% n-dodecyl-β-d-maltoside, 2 mM EDTA, 150 mM NaCl, 10 mM sodium pyrophosphate, and 10% glycerol) supplemented with protease and phosphatase inhibitors, and postnuclear lysates were subjected to immunoprecipitation and/or immunoblot assays.
Immunoprecipitation and immunoblot assays.
Immunoprecipitation and immunoblot assays were performed as described in earlier reports (10, 33). Immunoreactive products were detected with either 125I-labeled protein A, horseradish peroxidase-coupled protein A, 125I-labeled RAM IgG, or horseradish peroxidase-coupled SAM IgG. All secondary reagents were purchased from Amersham Biosciences, Baie d'Urfé, Québec, Canada. Radioactive signals were quantitated with a Phosphorimager (BAS2000; Fuji).
NK cell-mediated cytotoxicity assays.
Human NK cell-mediated cytotoxicity was assayed as described elsewhere (1), by using the indicated cell lines as target cells. Briefly, 51Cr-labeled target cells were incubated for 4 h with NK cells at the indicated effector cell/target cell ratio, in the presence or absence of anti-2B4 MAb C1.7. The release of 51Cr was then measured in a γ counter and represented as outlined elsewhere (1).
IFN-γ production assays.
BI-141 cells were stimulated at 37°C with the indicated concentrations of anti-CD3 MAb 145-2C11, which were used to coat 96-well plastic plates. After 24 h, supernatants were harvested and assayed for IFN-γ production with an enzyme-linked immunosorbent assay as specified by the manufacturer (R&D Systems Inc., Minneapolis, Minn.). All assays were done in duplicate and repeated at least five times.
RESULTS
The cytoplasmic domain of 2B4 is sufficient to mediate 2B4-induced protein tyrosine phosphorylation.
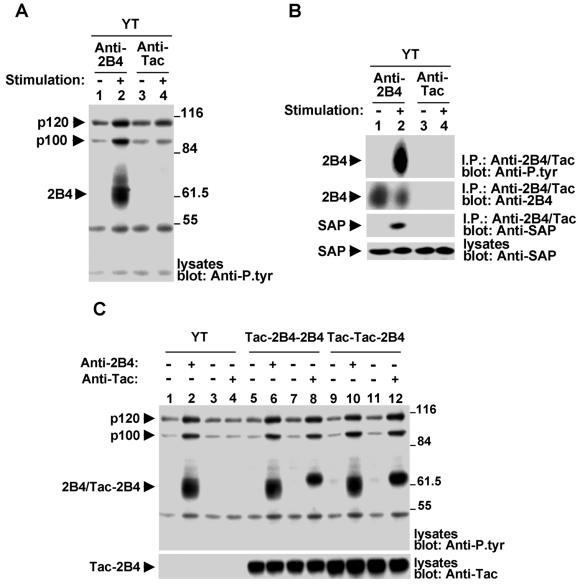
To understand the mechanism of 2B4 signal transduction, we began by examining the impact of 2B4 engagement on intracellular protein tyrosine phosphorylation (Fig. 1). The human NK cell line YT, which expresses both 2B4 and SAP, was stimulated for 10 min at 37°C with mouse anti-human 2B4 MAb C1.7 and RAM IgG. After lysis, changes in protein tyrosine phosphorylation were monitored by immunoblotting of total cell lysates with antiphosphotyrosine (anti-P.tyr) antibodies (Fig. 1A). This analysis revealed that 2B4 engagement (lane 2) induced prominent tyrosine phosphorylation of an ∼60- to 70-kDa substrate consistent with 2B4. An enhancement of the tyrosine phosphorylation of polypeptides of 120 (p120) and 100 (p100) kDa was also seen. In contrast, no response was noted in cells stimulated with an irrelevant (anti-Tac) antibody (lane 4).
FIG. 1.
Engagement of 2B4 on YT cells induces intracellular protein tyrosine phosphorylation. (A) Overall intracellular protein tyrosine phosphorylation. YT cells were left unstimulated or stimulated for 10 min at 37°C with mouse anti-human 2B4 MAb C1.7 or an irrelevant antibody (mouse anti-Tac MAb 7G7), followed by RAM IgG. Unstimulated controls were incubated with RAM IgG alone. The accumulation of phosphotyrosine-containing proteins was determined by immunoblotting of total cell lysates with anti-P.tyr antibody. (B) Tyrosine phosphorylation of 2B4 and its association with SAP. This experiment was performed in parallel with that shown in panel A. After lysis in detergent-containing buffer, lysates were immunoprecipitated (I.P.) with the indicated antibodies and probed by immunoblotting with anti-P.tyr antibody (first part). The abundance of 2B4 in the immunoprecipitates was verified by reprobing of the immunoblot membrane with rabbit anti-human 2B4 serum (second part). The association of 2B4 with SAP was ascertained by probing of parallel 2B4 immunoprecipitates with polyclonal rabbit anti-human SAP antibody (third part). Lastly, the abundance of SAP was monitored by immunoblotting of total cell lysates with anti-SAP antibody (fourth part). (C) The cytoplasmic domain of 2B4 is sufficient to mediate intracellular protein tyrosine phosphorylation. Parental YT cells and YT derivatives expressing the indicated Tac-2B4 chimeras were left unstimulated or stimulated with anti-2B4 MAb C1.7 or anti-Tac MAb 7G7, as described for panel A. Changes in protein tyrosine phosphorylation were monitored by anti-P.tyr antibody immunoblotting (top). The abundance of the Tac-2B4 chimeras was verified by reprobing of the membrane with a polyclonal rabbit serum recognizing the extracellular segment of Tac (bottom). All cells expressed equivalent amounts of SAP (data not shown). The values to the right of panels A and C are molecular sizes in kilodaltons.
To verify the extent of tyrosine phosphorylation of 2B4, 2B4 was immunoprecipitated from these cell lysates and probed by immunoblotting with anti-P.tyr antibody (Fig. 1B, first part). While 2B4 lacked detectable phosphotyrosine content before stimulation (lane 1), it became tyrosine phosphorylated after engagement with anti-2B4 antibody (lane 2). No tyrosine-phosphorylated product was detected in immunoprecipitates obtained with the irrelevant (anti-Tac) antibody (lanes 3 and 4). The ability of 2B4 to associate with SAP was also tested in parallel by immunoblotting of 2B4 immunoprecipitates with anti-SAP antibody (third part). While little or no SAP was detected in 2B4 immunoprecipitates from unstimulated cells (lane 1), SAP was clearly associated with 2B4 in anti-2B4 antibody-stimulated cells (lane 2).
Next, we determined which domain(s) of 2B4 is needed for the ability to mediate a protein tyrosine phosphorylation signal (Fig. 1C). For this purpose, we created receptor chimeras consisting of the human IL-2 receptor α chain (Tac) and 2B4. A first chimera (Tac-2B4-2B4) contained the extracellular domain of Tac fused to the transmembrane and cytoplasmic segments of 2B4. A second one (Tac-Tac-2B4) encompassed the extracellular and transmembrane regions of Tac linked to the cytoplasmic domain of 2B4. Note that this last chimera lacked the two cysteines in the transmembrane portion of 2B4 that were proposed to mediate recruitment of 2B4 to lipid rafts (15). Constructs were stably transfected into YT cells, and cells expressing the chimeric proteins were selected by flow cytometry with anti-Tac MAb 7G7 (data not shown). When stimulated with anti-Tac antibody, either of the two 2B4 chimeras (lanes 8 and 12) induced a pattern of protein tyrosine phosphorylation similar to that observed after stimulation of endogenous 2B4 with anti-2B4 antibody (lanes 2, 6, and 10). Importantly, the presence of the 2B4 transmembrane segment had no appreciable influence on this signal (compare lanes 8 and 12). Likewise, it had no impact on the capacity of 2B4 to associate with SAP (data not shown). Hence, these observations implied that the ability of 2B4 to trigger protein tyrosine phosphorylation in YT cells was dependent exclusively on its cytoplasmic domain.
Identification of 2B4-regulated protein tyrosine phosphorylation substrates.
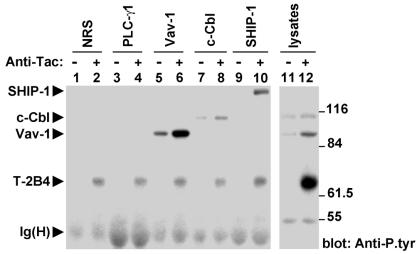
The substrates undergoing tyrosine phosphorylation in response to 2B4 stimulation were identified (Fig. 2). YT cells expressing Tac-Tac-2B4 were stimulated with biotinylated anti-Tac MAb 7G7 and avidin, and candidate substrates were immunoprecipitated and probed by anti-P.tyr antibody immunoblotting. This experiment showed that Vav-1 (lanes 5 and 6), which comigrates with p100 in cell lysates (lane 12), underwent an approximately fourfold increase in tyrosine phosphorylation upon 2B4 stimulation. This was in keeping with the earlier study of Watzl et al. (35). Ligation of 2B4 also evoked tyrosine phosphorylation of the 150-kDa 5′ inositol phosphatase SHIP-1 (lanes 9 and 10), although this substrate was not well seen in total cell lysates (lane 12). In addition, it triggered a lesser, albeit consistent, induction of the phosphotyrosine content of the E3 ubiquitin ligase c-Cbl (lanes 7 and 8). This protein comigrates with p120 (lane 12). Unlike Watzl et al. (35), we were unable to detect any tyrosine phosphorylation of PLC-γ1 (lanes 3 and 4). Likewise diverging from Bottino and colleagues (3), we saw no tyrosine phosphorylation of LAT (data not shown). A similar pattern of tyrosine phosphorylation was identified after engagement of Tac-2B4-2B4 or of endogenous 2B4 (data not shown).
FIG. 2.
Identification of 2B4-regulated protein tyrosine phosphorylation substrates. YT cells expressing Tac-Tac-2B4 were left unstimulated or stimulated with biotinylated anti-Tac MAb 7G7 and avidin. After preclearing of cell lysates, potential substrates were immunoprecipitated with specific antibodies and tyrosine phosphorylation was assessed by immunoblotting with anti-P.tyr antibody. Reprobing of the immunoblot membrane with antibodies directed against the various substrates confirmed that all of the polypeptides were adequately immunoprecipitated under all of the conditions used (data not shown). The 70-kDa tyrosine-phosphorylated product seen in all lanes from anti-Tac antibody-stimulated cells is Tac-Tac-2B4, which was nonspecifically immunoprecipitated via the stimulating antibody. NRS, normal rabbit serum. The values to the right are molecular sizes in kilodaltons.
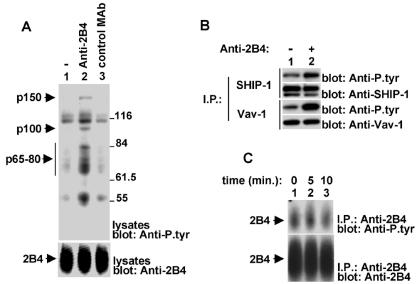
To ensure that this pathway is also relevant to 2B4 function in normal cells, 2B4-induced protein tyrosine phosphorylation was characterized in normal mouse NK cells (Fig. 3). These cells express 2B4 and SAP. Moreover, immunoblot analyses show that they contain the SAP-related protein EAT-2 (Roncagalli and Veillette, unpublished). IL-2-activated mouse NK cells were stimulated with biotinylated anti-2B4 MAb 2B4 and avidin, and changes in protein tyrosine phosphorylation were monitored by immunoblotting of cell lysates with anti-P.tyr antibody (Fig. 3A, top, lane 2). 2B4 engagement on mouse NK cells resulted in tyrosine phosphorylation of polypeptides of 150 (p150), 100 (p100), and 54 (p54) kDa. Tyrosine phosphorylation of polypeptides of 65 to 80 kDa (p65-80) was also seen. No tyrosine phosphorylation signal was evoked by an irrelevant control mouse MAb (F23.1; lane 3). Immunoprecipitation experiments (Fig. 3B) confirmed that SHIP-1 (first part) and Vav-1 (third part) underwent 2B4-elicited tyrosine phosphorylation in these cells. However, unlike in YT cells, 2B4 tyrosine phosphorylation was constitutive (Fig. 3C, top, lane 1) and was not appreciably altered by 2B4 ligation (lanes 2 and 3). This was possibly due to constitutive engagement of 2B4 by its ligand, CD48, which is present on mouse NK cells. Whether the 65- to 85-kDa substrates appearing in 2B4-stimulated cells (Fig. 3A, lane 2) represent modified forms of 2B4 that could not be immunoprecipitated or one or more unidentified substrates is not known. Similarly, the nature of the p110-130 and p54 substrates remains to be determined. Hence, these results support the notion that Vav-1 and SHIP-1 are physiological mediators of 2B4 signaling. Nevertheless, the detection of supplementary substrates in 2B4-triggered normal mouse NK cells also suggested that 2B4 signaling may be more complex in these cells than in YT cells. This is perhaps due to the additional presence of EAT-2.
FIG. 3.
Analysis of 2B4-mediated protein tyrosine phosphorylation in IL-2-activated ex vivo mouse NK cells. (A) Overall protein tyrosine phosphorylation. Normal NK cells were isolated from mouse spleen and propagated in the presence of high concentrations of IL-2. After 7 to 10 days, cells were stimulated for 3 min with biotinylated MAb 2B4 (lane 2) or anti-TCR MAb F23.1 (lane 3), followed by avidin. Changes in protein tyrosine phosphorylation were determined by anti-P.tyr antibody immunoblotting (top). The abundance of 2B4 was assessed by reprobing with RAM 2B4 serum (bottom). (B) Tyrosine phosphorylation of SHIP-1 and Vav-1. The experiment was done as outlined for that shown in panel A, except that cells were left unstimulated or stimulated for 10 min. SHIP-1 (first and second parts) and Vav-1 (third and fourth parts) were immunoprecipitated (I.P.) from cell lysates with specific rabbit antisera. Their phosphotyrosine content was assessed by immunoblotting with anti-P.tyr antibody (first and third parts), whereas their abundance was verified by reprobing with anti-SHIP-1 antibody (second part) and anti-Vav-1 antibody (fourth part), respectively. (C) Tyrosine phosphorylation of 2B4. Mouse NK cells were left untriggered or triggered for the indicated periods of time with anti-2B4 MAb 2B4 and RAM IgG. After lysis, 2B4 was recovered by immunoprecipitation and probed by immunoblotting with anti-P.tyr (top) or anti-2B4 (bottom) antibody. The values to the right of panel A are molecular sizes in kilodaltons.
The ability of 2B4 to regulate protein tyrosine phosphorylation is dependent on SAP.
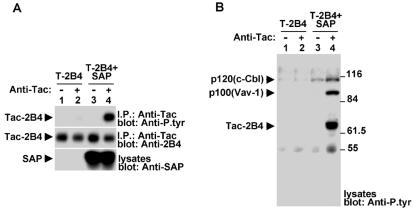
Previous studies indicated that the ability of 2B4 to undergo tyrosine phosphorylation in cells treated with pervanadate is not markedly influenced by SAP expression (27). Since pervanadate is a potent protein tyrosine phosphatase inhibitor that may bypass the need for SAP, we ascertained the impact of SAP under more physiological conditions. Two approaches were taken. First, Tac-Tac-2B4 was expressed alone or in combination with SAP in the mouse T-cell line BI-141, which lacks endogenous 2B4 and SAP (Fig. 4). Cells were stimulated with biotinylated anti-Tac MAb 7G7 and avidin, and the phosphotyrosine content of the chimera was examined by anti-P.tyr antibody immunoblotting of Tac immunoprecipitates (Fig. 4A, first part). In cells lacking SAP (lanes 1 and 2), Tac-Tac-2B4 exhibited no increase in tyrosine phosphorylation in response to anti-Tac antibody stimulation (lane 2). In contrast, it underwent robust tyrosine phosphorylation when SAP was expressed (lanes 3 and 4). The influence of SAP on tyrosine phosphorylation of downstream targets was also addressed (Fig. 4B). Once again, engagement of Tac-Tac-2B4 in the absence of SAP (lanes 1 and 2) had no impact on intracellular phosphotyrosine levels. However, in the presence of SAP (lanes 3 and 4), it provoked a striking increase in tyrosine phosphorylation of products of 100 and 70 kDa (lane 4), representing Vav-1 and Tac-Tac-2B4, respectively (data not shown). A moderate augmentation of the tyrosine phosphorylation of a 120-kDa substrate consistent with c-Cbl was also seen. The 55-kDa substrate observed in these experiments was a degraded form of Tac-Tac-2B4 (data not shown).
FIG. 4.
SAP expression is essential for 2B4-mediated protein tyrosine phosphorylation. Stable transfectants of BI-141 T cells expressing Tac-Tac-2B4, in the absence or presence of SAP, were stimulated for 10 min with anti-Tac MAb 7G7 and RAM IgG. (A) Tyrosine phosphorylation of Tac-Tac-2B4. The 2B4 chimera was immunoprecipitated (I.P.) from cell lysates and probed by immunoblotting with anti-P.tyr antibody (first part). The abundance of the chimera was verified by reprobing of the immunoblot membrane with rabbit anti-2B4 antibodies (second part), while the expression of SAP was confirmed by immunoblotting of total cell lysates with anti-SAP antibody (third part). (B) Overall protein tyrosine phosphorylation. Lysates from the experiment depicted in panel A were probed by anti-P.tyr antibody immunoblotting. The values to the right of panel B are molecular sizes in kilodaltons.
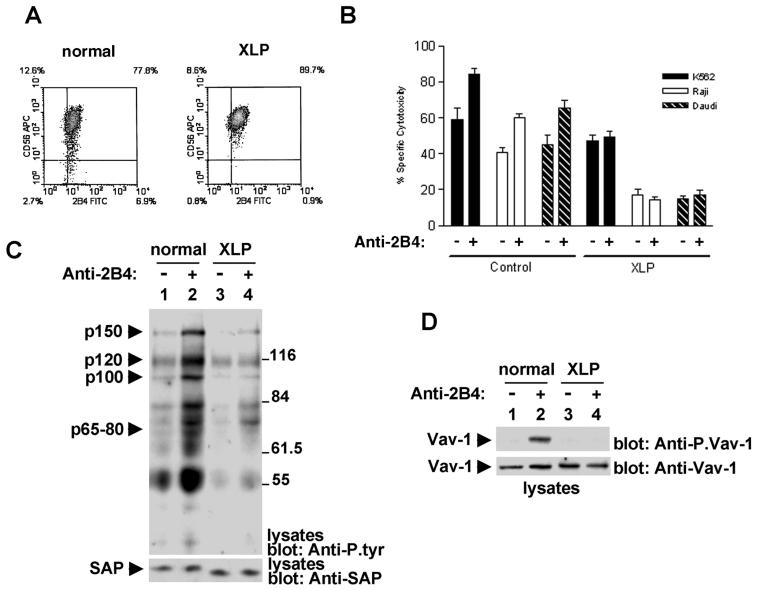
Second, the role of SAP in 2B4 signaling was assessed by examining 2B4-triggered protein tyrosine phosphorylation events in NK cells obtained from a patient with XLP disease (Fig. 5). This patient carries an arginine 55-to-leucine (R55L) mutation in the SAP SH2 domain (1, 2). Although this mutation does not affect the abundance of SAP, it precludes its binding to tyrosine-phosphorylated, SLAM-related receptors. As reported elsewhere (1), NK cells from this patient exhibited normal levels of 2B4 at the cell surface (Fig. 5A). Nevertheless, they demonstrated a defect in baseline killing of a variety of targets (K562, Raji, and Daudi cells) (Fig. 5B). This presumably reflected the involvement of SAP in cytotoxicity triggered by receptors such as 2B4, which has its ligand, CD48, expressed on target cells (2, 4). The ability of anti-2B4 antibodies to enhance NK cell-mediated cytotoxicity was also abolished.
FIG. 5.
Analysis of 2B4-mediated signaling and function in XLP-derived NK cells. IL-2-activated NK cells derived from a normal individual or an asymptomatic XLP patient with a SAP R55L mutation were analyzed. (A) Flow cytometry. Expression of 2B4 and CD56 was assessed with anti-2B4 MAb C1.7 and an anti-CD56 MAb. APC, allophycocyanin; FITC, fluorescein isothiocyanate. (B) Cytotoxicity assay. The ability of NK cells to kill various targets was examined with a standard 51Cr release assay in the absence (−) or presence (+) of anti-2B4 antibody. The effector cell/target cell ratio used was 10:1. The extent of 51Cr release is shown on the abscissa. Maximum release (defined by lysis of target cells in detergent-containing buffer) is 100%. (C) 2B4-mediated overall protein tyrosine phosphorylation. Cells were stimulated for 10 min with anti-2B4 MAb C1.7 and SAM IgG. Changes in intracellular protein tyrosine phosphorylation were ascertained by immunoblotting of total cell lysates with anti-P.tyr antibody (top). The abundance of SAP was confirmed by immunoblotting of lysates with anti-SAP antibody (bottom). (D) Vav-1 tyrosine phosphorylation. The experiment was done as described for panel C, except that tyrosine phosphorylation of Vav-1 was determined by immunoblotting of total cell lysates with a phosphospecific antibody recognizing Vav-1 molecules phosphorylated at Y160 (top). The abundance of Vav-1 was verified by reprobing of the immunoblot membrane with anti-Vav-1 antibody (bottom). The values to the right of panel C are molecular sizes in kilodaltons.
To ascertain 2B4-mediated signaling, cells were stimulated with anti-2B4 MAb C1.7 and overall tyrosine phosphorylation was measured by anti-P.tyr antibody immunoblotting of total cell lysates (Fig. 5C). In agreement with Fig. 1 to 4, 2B4 ligation evoked the tyrosine phosphorylation of polypeptides of 150, 120, 100, and 65 to 80 kDa in normal human NK cells (Fig. 5C, top, lanes 1 and 2). However, this signal was greatly compromised in NK cells from the XLP patient (lanes 3 and 4). The extent of tyrosine phosphorylation of Vav-1 was also specifically examined by immunoblotting of cell lysates with an antiserum recognizing tyrosine-phosphorylated Vav-1 (Fig. 5D, top). While Vav-1 became tyrosine phosphorylated in 2B4-stimulated normal human NK cells (lane 2), such an effect was not seen in XLP-derived NK cells (lane 4). This difference was not due to a discrepancy in the levels of Vav-1 (bottom). Although the results of Fig. 5C and D show that the ability of 2B4 to trigger intracellular protein tyrosine phosphorylation was markedly affected by the absence of a functional SAP protein, a weak tyrosine phosphorylation signal still existed in 2B4-stimulated XLP NK cells. While the basis for this finding is not established, it may reflect a residual activity of the mutated SAP protein or the presence of EAT-2.
2B4-mediated protein tyrosine phosphorylation requires multiple tyrosines in the 2B4 cytoplasmic domain.
To address the mechanism by which the cytoplasmic segment of 2B4 regulates protein tyrosine phosphorylation, the role of the tyrosines located in this domain was assessed (Fig. 6). The strategy used for these analyses was based on the results previously obtained with SLAM (17). It was reported that the first of three tyrosines in the cytoplasmic domain of SLAM with the consensus motif TxYxxV/I (where T is threonine, Y is tyrosine, V is valine, I is isoleucine, and x is any residue) was responsible for SAP binding, while the last two were involved in recruiting downstream substrates. Since mouse 2B4 bears four tyrosines having the same consensus sequence (Fig. 6A) (13), the following mutant constructs were engineered. The first one, termed Y1F, carried a tyrosine-to-phenylalanine (Y-to-F) mutation of the first tyrosine, tyrosine 267. The second one, named Y2,3,4F, was mutated at the tyrosines at positions 326, 345, and 370. Lastly, the third one, named Y1,2,3,4F, had mutations of all four tyrosines. The mutations were introduced into Tac-Tac-2B4, and the mutant constructs were stably expressed either in YT NK cells or in BI-141 T cells. While the results presented here pertain to YT cells, comparable findings were obtained with BI-141 cells (data not shown).
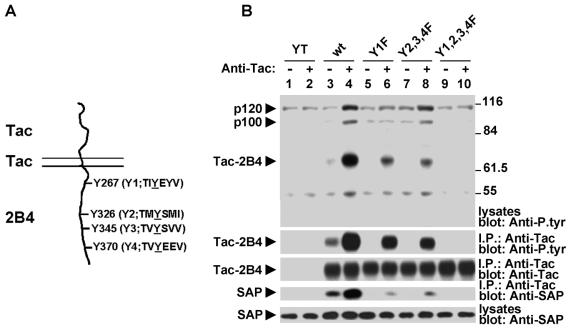
FIG. 6.
Multiple tyrosines in the cytoplasmic domain of 2B4 are required for 2B4 signaling. (A) Schematic representation of Tac-Tac-2B4. The sequences of the four tyrosine-based motifs in mouse 2B4 with the consensus TxYxxV/I are indicated. (B) Impact of Y-to-F mutations on 2B4-mediated protein tyrosine phosphorylation. Parental YT cells or derivatives expressing the indicated Tac-Tac-2B4 variants were stimulated for 10 min with anti-Tac MAb 7G7 and RAM IgG. Changes in overall protein tyrosine phosphorylation were monitored by immunoblotting of total cell lysates with anti-P.tyr antibody (first part). The extent of tyrosine phosphorylation of the chimeras was also assessed by probing anti-Tac antibody immunoprecipitates (I.P.) with anti-P.tyr antibody (second part). The association of the chimeras with SAP was verified by immunoblotting of anti-Tac antibody immunoprecipitates with anti-SAP antibody (third part). The abundance of the Tac chimeras was confirmed by immunoblotting of anti-Tac antibody immunoprecipitates with anti-Tac antibody (fourth part), while the presence of SAP was assessed by immunoblotting of total cell lysates with anti-SAP antibody (fifth part). wt, wild type. The values to the right of panel B are molecular sizes in kilodaltons.
YT derivatives were stimulated with anti-Tac MAb 7G7, and changes in protein tyrosine phosphorylation were determined by anti-P.tyr antibody immunoblotting of total cell lysates (Fig. 6B). Compared to wild-type Tac-Tac-2B4 (lanes 3 and 4), the Tac-Tac-2B4 Y1F (lanes 5 and 6) and Y2,3,4F (lanes 7 and 8) mutant constructs mediated a weaker protein tyrosine phosphorylation signal. Tyrosine phosphorylation of p70, which corresponds to Tac-Tac-2B4 (second part), was partially reduced by either of these mutations, whereas phosphorylation of the downstream substrates p120 (c-Cbl) and p100 (Vav-1) was diminished mostly by the Y1F mutation (lane 6). Mutation of the last three tyrosines (lane 8) had a minimal effect on tyrosine phosphorylation of these substrates. Complete elimination of substrate tyrosine phosphorylation was achieved when all four tyrosines were mutated (lane 10).
The ability of the chimeras to associate with SAP was determined by immunoblotting of Tac immunoprecipitates with anti-SAP antibody (fourth part). In a manner analogous to SLAM (17), mutation of the first tyrosine (lane 5 and 6) severely reduced the ability of the 2B4 chimera to associate with SAP. While this effect was especially obvious after Tac stimulation (lane 6), it was also noted before receptor engagement (lane 5). Unlike SLAM, however, alteration of the three distal tyrosines of 2B4 (lanes 7 and 8) also reduced the ability to bind SAP. Moreover, replacement of all four tyrosines (lanes 9 and 10) was needed to eliminate the interaction completely. On the basis of these findings, we concluded that two or more tyrosines in the cytoplasmic domain of 2B4 were required for optimal association with SAP and for the induction of intracellular protein tyrosine phosphorylation. It is noteworthy, however, that while the Y1F and Y2,3,4F mutations severely compromised the ability of Tac-2B4 to associate with SAP, they still enabled the chimeras to mediate detectable protein tyrosine phosphorylation signals. This finding suggested that only a small amount of associated SAP may be needed to initiate 2B4 signaling.
The distinct signals mediated by 2B4 and SLAM are defined by their intracytoplasmic tyrosines and not by the extent of their association with SAP.
Although the functions of the various SLAM-related receptors are not well understood, it is presumed that they each mediate a unique biological effect. For instance, it was reported that, in the presence of SAP, 2B4 can augment target cell killing by NK cells (2, 25, 27, 30) while SLAM can inhibit IFN-γ production by activated T cells (17). To address the possible biochemical basis of these different effects, the protein tyrosine phosphorylation signals induced by 2B4 and SLAM were compared (Fig. 7). For these studies, we took advantage of the BI-141 system, since we had previously used this cell line to characterize SLAM-mediated signaling (17). The availability of BI-141 transfectants expressing Tac-Tac-SLAM, a chimera in which the extracellular and transmembrane segments of Tac are fused to the cytoplasmic domain of SLAM, also allowed a more direct comparison of 2B4- and SLAM-mediated signals.
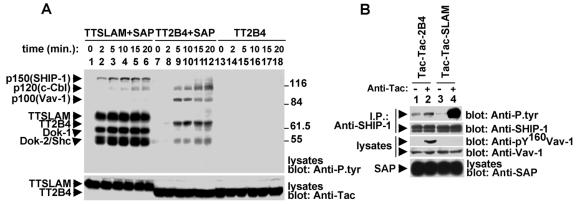
FIG. 7.
Different signals are transduced by the cytoplasmic domains of 2B4 and SLAM. (A) Overall protein tyrosine phosphorylation. BI-141 derivatives expressing the indicated Tac chimeras were stimulated for various times with biotinylated anti-Tac MAb 7G7 and avidin. Protein tyrosine phosphorylation was examined by immunoblotting of total cell lysates with anti-P.tyr antibody (top). The abundance of the chimeras was verified by reprobing of the immunoblot membrane with anti-Tac antibody (bottom). (B) Differential tyrosine phosphorylation of SHIP-1 and Vav-1. The experiment was as outlined for panel A, except that cells were stimulated for 5 min. Tyrosine phosphorylation of SHIP-1 was determined by probing of anti-SHIP-1 antibody immunoprecipitates (I.P.) with anti-P.tyr antibody (first part), whereas the abundance of SHIP-1 was verified by reprobing of the membrane with anti-SHIP-1 antibody (second part). The extent of tyrosine phosphorylation of Vav-1 was assessed by immunoblotting of total cell lysates with a phosphospecific antibody recognizing Vav-1 phosphorylated at Y160 (third part). The levels of Vav-1 were confirmed by reprobing of the membrane with anti-Vav-1 antibody (fourth part). Levels of SAP were compared by immunoblotting of total cell lysates with anti-SAP antibody (fifth part). The values to the right of panel A are molecular sizes in kilodaltons.
Cells were stimulated for various periods of time with anti-Tac MAb 7G7, and protein tyrosine phosphorylation was monitored by anti-P.tyr antibody immunoblotting of total cell lysates (Fig. 7A). This experiment revealed that the tyrosine phosphorylation signals generated by the two chimeras were qualitatively and quantitatively different. As reported earlier (17), ligation of Tac-Tac-SLAM (top, lanes 1 to 6) effected rapid and prominent tyrosine phosphorylation of polypeptides of 150, 75, 62, 56, and 52 kDa. These products correspond to SHIP-1, Tac-Tac-SLAM, Dok-1, Dok-2, and Shc, respectively. In contrast, engagement of Tac-Tac-2B4 (lanes 7 to 12) led to a slower and weaker phosphotyrosine signal, involving polypeptides of 120, 100, 70, and 55 kDa. These proteins are c-Cbl, Vav-1, and the 2B4 chimera (p70 and p55), respectively (see above). As expected, no protein tyrosine phosphorylation signal was generated by Tac-Tac-2B4 in cells lacking SAP (lanes 13 to 18).
The state of tyrosine phosphorylation of SHIP-1 and Vav-1 in these cells was also specifically examined (Fig. 7B). We found that engagement of Tac-Tac-2B4 (first part, lanes 1 and 2) resulted in much weaker tyrosine phosphorylation of SHIP-1 in comparison to ligation of Tac-Tac-SLAM (lanes 3 and 4). By opposition, triggering of the 2B4 chimera evoked much stronger tyrosine phosphorylation of Vav-1 (third part, lanes 1 and 2), in contrast to that of the SLAM chimera (lanes 3 and 4). Thus, the cytoplasmic domain of 2B4 triggered a signal that mostly involved Vav-1 and, to a lesser degree, SHIP-1 and c-Cbl, while the cytoplasmic region of SLAM was linked to a pathway principally implicating SHIP-1, Dok-related adaptors, and Shc.
There are two possible explanations for these differences. First, quantitative differences in the extent of association with SAP could somehow couple 2B4 and SLAM to qualitatively and quantitatively distinct signals. In support of this possibility, Tac-Tac-2B4 associated with much smaller amounts of SAP, compared to Tac-Tac-SLAM (Fig. 8B). As an alternative explanation, the cytoplasmic domains of 2B4 and SLAM could recruit, by way of their tyrosine-based motifs, distinct sets of intracellular effectors, yielding different signals. To investigate these scenarios, a modified Tac-Tac-2B4 chimera in which the first tyrosine-based motif of 2B4 was replaced with the equivalent motif from SLAM (Y288) (Fig. 8A) was created. Since Y288 mediates binding of SLAM to SAP (17), we reasoned that this receptor, named Tac-Tac-SLAM-2B4, would bind SAP as extensively as Tac-Tac-SLAM while preserving the last three tyrosines of 2B4. Tac-Tac-SLAM-2B4 was expressed with SAP in BI-141 cells, and its signaling properties were contrasted with those of Tac-Tac-2B4 and Tac-Tac-SLAM. Flow cytometric analyses confirmed that all three receptors were expressed in similar amounts at the cell surface (data not shown).
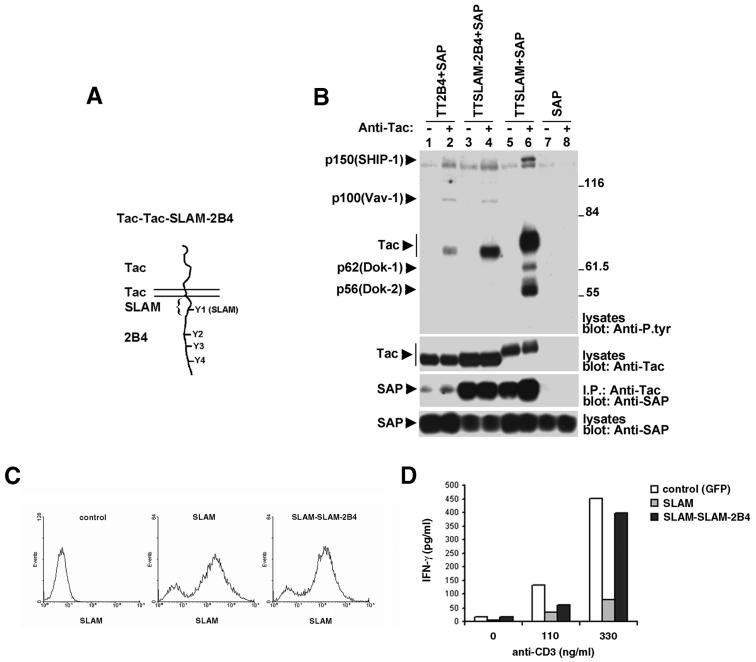
FIG. 8.
The specificity of SLAM-related receptor signaling is not defined by the extent of association with SAP. (A) Primary structure of Tac-Tac-SLAM-2B4. (B) Impact of the SAP-binding site from SLAM on 2B4 signaling. BI-141 cells expressing the indicated chimeras in the presence of SAP were stimulated for 10 min with anti-Tac MAb 7G7 as detailed in the legend to Fig. 7. The extent of intracellular protein tyrosine phosphorylation was measured by immunoblotting of total cell lysates with anti-P.tyr antibody (first part). The association of the chimeras with SAP was assessed by immunoblotting of anti-Tac antibody immunoprecipitates (I.P.) with anti-SAP antibodies (third part). The expression levels of the Tac chimeras and SAP were determined by immunoblotting of total cell lysates with anti-Tac (second part) and anti-SAP (fourth part) antibodies, respectively. (C) Expression of SLAM and SLAM-SLAM-2B4 on BI-141 cells. BI-141 cells expressing SAP were transduced with retroviruses encoding GFP alone (control) or in combination with full-length SLAM or a SLAM variant in which the cytoplasmic domain of SLAM was replaced with that of Tac-Tac-SLAM-2B4. GFP-positive cells were isolated by cell sorting and tested for cell surface expression of the two receptors with biotinylated anti-SLAM MAb 12F12 and streptavidin coupled to Quantum Red. Greater than 80% of the cells expressed SLAM or SLAM-SLAM-2B4. (D) Impact of SLAM and SLAM-SLAM-2B4 on TCR-induced IFN-γ production. Cells were stimulated overnight with the indicated concentrations of anti-CD3 MAb 145-2C11. The release of IFN-γ (in picograms per milliliter) in the supernatant was measured by enzyme-linked immunosorbent assay. Assays were done in duplicate, and the results shown are representative of at least five independent experiments. All cells expressed equivalent amounts of TCR complex at the cell surface (data not shown). The values to the right of panel B are molecular sizes in kilodaltons.
Cells were stimulated with or without anti-Tac antibody, and the extent of association with SAP was ascertained by probing anti-Tac antibody immunoprecipitates in an anti-SAP antibody immunoblot (Fig. 8B, third part). In agreement with our earlier report (17), we found that the association of Tac-Tac-SLAM with SAP was constitutive (lane 5). A small enhancement of this interaction was provoked by anti-Tac antibody stimulation (lane 6). In comparison, the binding of Tac-Tac-2B4 to SAP was much weaker (lanes 1 and 2). As expected, however, Tac-Tac-SLAM-2B4 (lanes 3 and 4) was associated with SAP as extensively as Tac-Tac-SLAM (lanes 5 and 6).
The nature of the signal triggered by Tac-Tac-SLAM-2B4 was assessed by anti-P.tyr antibody immunoblotting of total cell lysates (first part). In comparison to ligation of Tac-Tac-2B4 (lane 2), engagement of Tac-Tac-SLAM-2B4 (lane 4) led to stronger tyrosine phosphorylation of the chimera (Tac). Despite this, it evoked an overall protein tyrosine phosphorylation signal (lane 4) that was qualitatively and quantitatively identical to the one induced by Tac-Tac-2B4 (lane 2) and that remained distinct from the one triggered by Tac-Tac-SLAM (lane 6). In keeping with this, we also observed that, contrary to full-length SLAM, a variant of SLAM in which the cytoplasmic domain of SLAM was replaced with that of Tac-Tac-SLAM-2B4 was unable to inhibit IFN-γ production in antigen receptor-stimulated BI-141 cells (Fig. 8C and D) (17). Thus, although introduction of the SAP-binding site from SLAM allowed a more extensive association of 2B4 with SAP, it failed to modify the nature and intensity of the 2B4 signal. In the light of this, we concluded that the 2B4-induced protein tyrosine phosphorylation signal was specified by unique properties of the tyrosine-based motifs of 2B4 beyond the simple allowance of SAP binding.
Evidence supporting the involvement of Src-related protein tyrosine kinase FynT in 2B4 signaling.
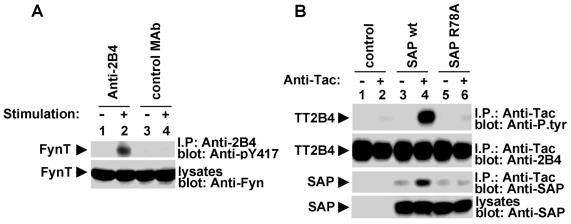
The capacity of SAP to allow SLAM-induced protein tyrosine phosphorylation appears to be due to its ability to associate with (and activate) FynT (7, 17, 18). This interaction involves a second binding surface in the SAP SH2 domain, centered around arginine 78 (R78), that directly contacts the FynT SH3 domain. To determine whether SAP promotes 2B4 signaling by a similar mechanism, we first examined whether 2B4 was associated with FynT (Fig. 9A). Parental YT cells were stimulated with anti-2B4 MAb C1.7 as outlined in Fig. 1, and the ability of 2B4 to interact with FynT was assessed by probing 2B4 immunoprecipitates with antibodies recognizing activated FynT (Fig. 9A, top, lanes 1 and 2). This experiment revealed that 2B4 engagement triggered the coimmunoprecipitation of 2B4 with activated FynT molecules (lane 2). No activated FynT was detected in 2B4 immunoprecipitates before 2B4 stimulation (lane 1) or in control immunoprecipitates obtained with an irrelevant antibody (lane 4).
FIG. 9.
Evidence implicating the Src-related protein tyrosine kinase FynT in 2B4 signaling. (A) Association of 2B4 with activated FynT in YT cells. The presence of activated FynT was detected by reprobing the immunoblot membrane from Fig. 1B (first part) with an antibody that recognizes FynT molecules phosphorylated at Y417, the positive regulatory site (top). The expression levels of FynT were ascertained by immunoblotting of total cell lysates with anti-FynT antibody (bottom). (B) Arginine 78 of SAP is required for 2B4 tyrosine phosphorylation. BI-141 cells expressing Tac-Tac-2B4 with GFP alone (control) or in combination with wild-type (wt) or SAP R78A were stimulated with anti-Tac antibody in accordance with the protocol used for Fig. 7. Tyrosine phosphorylation of the chimera was determined by immunoblotting of anti-Tac antibody immunoprecipitates (I.P.) with anti-P.tyr antibody (first part). The presence of Tac-Tac-2B4 was confirmed by reprobing of the membrane with anti-2B4 antibody (second part). The association of Tac-Tac-2B4 with SAP was examined by probing anti-Tac antibody immunoprecipitates with anti-SAP antibody (third part), whereas the abundance of SAP was monitored by immunoblotting of total cell lysates with anti-SAP antibody (fourth part). Greater than 95% of the cells used for experimentation were GFP+ (data not shown).
Whereas we could detect activated FynT in 2B4 immunoprecipitates from 2B4-triggered YT cells, it was difficult to show this association consistently in normal mouse NK cells or in 2B4-expressing BI-141 T cells (data not shown). One possible explanation for this result is that the stoichiometry of the 2B4-SAP-FynT association is much lower than that of the SLAM-SAP-FynT interaction, rendering the detection of 2B4-associated FynT more difficult. Alternatively, FynT may not play a central role in 2B4 signaling and thus may not be consistently recruited to 2B4.
To evaluate these issues, we assessed the ability of a mutant SAP protein that is unable to bind FynT (SAP with an arginine 78-to-alanine [R78A] mutation) (18) to promote 2B4 signaling (Fig. 9B). BI-141 cells expressing Tac-Tac-2B4 alone or in combination with wild-type or R78A mutant SAP were produced and tested for anti-Tac antibody-induced protein tyrosine phosphorylation (first part). This experiment showed that, unlike wild-type SAP (lanes 3 and 4), SAP R78A (lanes 5 and 6) was unable to promote tyrosine phosphorylation of the 2B4 chimera. Yet, both SAP polypeptides were able to associate with Tac-Tac-2B4 (lanes 3 to 6), in keeping with the finding that R78 is not required for binding of the SAP SH2 domain to the tyrosine-based sequence in SLAM (7, 18). Note, however, that no enhancement of this association in response to anti-Tac antibody stimulation was observed in cells expressing SAP R78A (lane 6). On the basis of these results, we concluded that the ability of SAP to support 2B4-mediated protein tyrosine phosphorylation correlated with its ability to interact with FynT and that, in all likelihood, FynT plays a critical role in SAP-dependent 2B4 signaling.
DISCUSSION
In this study, we attempted to understand the mechanism of 2B4 signaling. We found that ligation of full-length 2B4 by anti-2B4 MAb on NK cells resulted in a protein tyrosine phosphorylation response involving 2B4, Vav-1, and, to a lesser degree, c-Cbl and SHIP-1, as well as other, as yet unidentified, substrates. By creating chimeras between a heterologous receptor (Tac) and 2B4, it was revealed that this signal depended exclusively on the cytoplasmic domain of 2B4. No apparent contribution was made by the extracellular and transmembrane segments of 2B4. Analyses in transfected BI-141 T cells demonstrated that expression of SAP was absolutely required for 2B4-triggered protein tyrosine phosphorylation. Furthermore, studies of NK cells from an XLP patient carrying a SAP R55L mutation (which abolishes the ability of SAP to bind phosphotyrosine without affecting the abundance of SAP) indicated that the ability of SAP to interact with tyrosine-phosphorylated 2B4 is necessary for 2B4 signaling. Thus, put together, these data established that 2B4 signal transduction is a SAP-dependent process and that it is specified by the 2B4 cytoplasmic domain.
These findings may seem to be at odds with those showing that the CxC motif in the 2B4 transmembrane domain is needed for localization of 2B4 to lipid rafts (15). While this possibility was not addressed in our studies, we established that the transmembrane segment of 2B4 is not specifically required for 2B4-triggered protein tyrosine phosphorylation. Of course, these data do not exclude the possibility that the 2B4 transmembrane region participates in some way in the function of 2B4. As previously suggested (3, 15), it may help target 2B4 to lipid rafts, thereby allowing 2B4 signaling to intersect more efficiently with activating signals initiated by immunoreceptors. However, it should be pointed out that we were unable to detect any evidence of a physical interaction between 2B4 and lipid raft-associated LAT or of induction of LAT tyrosine phosphorylation upon 2B4 stimulation (data not shown). Hence, the transmembrane domain of 2B4 may carry out its function through an as yet undetermined mechanism.
Although the function of 2B4 remains to be clarified by the creation of 2B4-deficient mice, stimulation experiments with anti-2B4 antibodies or CD48-expressing cells provided evidence that 2B4 is a costimulatory receptor involved in NK and T-cell activation (24). The possible involvement of the various 2B4-regulated substrates identified herein in these functions deserves consideration. Through its guanine nucleotide exchange activity, tyrosine-phosphorylated Vav-1 is known to activate Rac-1 and cdc42, thereby promoting cytoskeletal reorganization (31). Thus, it is attractive to speculate that Vav-1 may participate in 2B4-mediated target cell killing, a process known to require extensive cytoskeletal changes. In agreement with this, it was reported that anti-2B4 antibody-mediated cytotoxicity was compromised in NK cells derived from Vav-1−/− mice (9). Nonetheless, IFN-γ secretion was not affected, implying that other signaling effectors are responsible for this function. Whereas c-Cbl and/or SHIP-1 may be implicated in IFN-γ regulation, both polypeptides are usually negative regulators of cell signaling. Possibly, they are involved in turning off the function of Vav-1. Moreover, it is worth noting that anti-2B4 antibody-induced IFN-γ production was intact in NK cells from mice lacking either SHIP-1 or c-Cbl (Chen and Veillette, unpublished results). On these bases, we propose that other targets, perhaps not undergoing detectable tyrosine phosphorylation, may be responsible for the 2B4-mediated regulation of IFN-γ secretion.
Despite the similarities between 2B4 and SLAM, several differences were observed concerning their association with SAP. First, studies with Tac-based chimeras revealed that 2B4 interacted with SAP ∼10 times less efficiently than SLAM. This was presumably due to the fact that the first tyrosine of SLAM (Y288) has a much greater affinity for SAP than any of the tyrosines of 2B4. Second, whereas the interaction between SLAM and SAP was largely constitutive, the association between 2B4 and SAP was significantly inducible, at least in some systems. This likely reflects the previously described ability of SAP to bind tightly to the first tyrosine of SLAM even in the absence of tyrosine phosphorylation (22). By opposition, the interaction of SAP with the various tyrosine-based motifs of 2B4 was shown to be greatly dependent on tyrosine phosphorylation. Third, our site-directed mutagenesis experiments indicated that two or more tyrosines were cooperating to allow full association of 2B4 with SAP. In contrast, only one tyrosine is necessary for strong binding of SLAM to SAP (17).
What are the functional consequences of such differences? By studying Tac chimeras containing the cytoplasmic domain of 2B4 or SLAM, we observed that the substrates undergoing tyrosine phosphorylation in response to engagement of 2B4 or SLAM were different: 2B4 primarily targeted Vav-1 and, to a lesser degree, SHIP-1 and c-Cbl, while SLAM mostly recruited SHIP-1, Dok-related adaptors, and Shc. In addition, the signal triggered by 2B4 was weaker and more delayed than that evoked by SLAM. Obviously, these qualitative and quantitative differences may result from the disparity in the stoichiometry of association of the two receptors with SAP. To address this notion, we created a chimera in which the SAP-binding site from SLAM was inserted into 2B4. Although this receptor, Tac-Tac-SLAM-2B4, interacted as extensively with SAP as Tac-Tac-SLAM, the specificity and intensity of the signal transduced remained identical to that of Tac-Tac-2B4. Therefore, while the protein tyrosine phosphorylation signals induced by 2B4 and SLAM are both strictly dependent on SAP, their specific features are determined by unique sequences in their respective cytoplasmic domains (Fig. 10). Presumably, they are determined by the more membrane-distal tyrosine-based motifs in the receptors. In mouse SLAM, these motifs are TIY315VAA and TVY335ASV, while in mouse 2B4, they are TMY326SMI, TVY345SVV, and TVY370EEV.
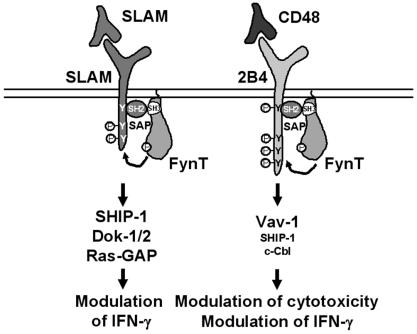
FIG. 10.
Model explaining the differential impact of 2B4 and SLAM on immune cell functions. A model for the different SAP-dependent impacts of two members of the SLAM family on immune cells is proposed. The ligand of SLAM is SLAM. In contrast, 2B4 interacts with CD48. See the text for further explanations.
Previously published data indicated that the ability of SAP to promote signaling by SLAM is mediated through recruitment of FynT, by way of a direct interaction between the SAP SH2 domain and the FynT SH3 domain (18). In agreement with this, we observed that engagement of 2B4 on YT NK cells triggered the association of 2B4 with activated FynT. Yet, it was difficult to detect this association in normal NK cells or BI-141 T cells (data not shown). Although the exact basis for this discrepancy is not known, this may indicate that 2B4 signaling does not involve FynT. Alternatively, it is possible that the stoichiometry of the 2B4-SAP-FynT interaction is low, thus rendering its detection difficult. In support of the latter possibility, we observed that SAP R78A, which is unable to bind FynT, was incapable of mediating Tac-Tac-2B4 signaling in BI-141 cells. While an analysis of 2B4 function in NK cells from FynT-deficient mice may help address the role of this kinase in 2B4 signaling, this experiment is hampered by the fact that NK cells express at least one other SAP-related adaptor, EAT-2, that may promote SLAM-related receptor signaling in a FynT-independent manner (19).
In summary, the data presented in this report show that 2B4 engagement results in a specific protein tyrosine phosphorylation signal involving Vav-1, c-Cbl, SHIP-1, and possibly others. Activation of this pathway is absolutely dependent on coexpression of SAP and correlates with the capacity of SAP to bind FynT. Our results also provide evidence that the nature of the tyrosine phosphorylation signals triggered by two different SLAM-related receptors, 2B4 and SLAM, is defined by the tyrosine-based motifs in their cytoplasmic domain rather than their simple association with SAP. SAP appears to act in these pathways as a “molecular switch” enabling the enlistment of FynT, which then permits tyrosine phosphorylation of the receptors and subsequent recruitment of the specific downstream effectors (Fig. 10). Since the other SLAM-related receptors (Ly-9, CD84, NTB-A/Ly-108, and possibly CRACC) also interact with SAP, it is likely that this signaling mechanism governs the function of the entire family.
Acknowledgments
We thank Eric Long and Marco Colonna for gifts of reagents.
This work was supported by grants from the Canadian Institutes of Health Research (to A.V. and R.T.), the National Cancer Institute of Canada (to A.V.), the CANVAC National Centre of Excellence (to A.V.), and the Institut National de la Santé et de la Recherche Médicale and the Association pour la Recherche sur le Cancer (France) (to S.L.). A.A. is supported by a Michael Smith Foundation for Health Research Scholarship, and R.T. is a Peter Wall Institute for Advanced Studies Scholar. R.R. holds a studentship from the National Cancer Institute of Canada. S.L. is a Scientist from the Centre National de la Recherche Scientifique (France). A.V. is a Senior Investigator of the Canadian Institutes of Health Research and holds the Canada Research Chair in Signaling in the Immune System.
REFERENCES
- 1.Aoukaty, A., and R. Tan. 2002. Association of the X-linked lymphoproliferative disease gene product SAP/SH2D1A with 2B4, a natural killer cell-activating molecule, is dependent on phosphoinositide 3-kinase. J. Biol. Chem. 277:13331-13337. [DOI] [PubMed] [Google Scholar]
- 2.Benoit, L., X. Wang, H. F. Pabst, J. Dutz, and R. Tan. 2000. Defective NK cell activation in X-linked lymphoproliferative disease. J. Immunol. 165:3549-3553. [DOI] [PubMed] [Google Scholar]
- 3.Bottino, C., R. Augugliaro, R. Castriconi, M. Nanni, R. Biassoni, L. Moretta, and A. Moretta. 2000. Analysis of the molecular mechanism involved in 2B4-mediated NK cell activation: evidence that human 2B4 is physically and functionally associated with the linker for activation of T cells. Eur. J. Immunol. 30:3718-3722. [DOI] [PubMed] [Google Scholar]
- 4.Bottino, C., M. Falco, S. Parolini, E. Marcenaro, R. Augugliaro, S. Sivori, E. Landi, R. Biassoni, L. D. Notarangelo, L. Moretta, and A. Moretta. 2001. NTB-A, a novel SH2D1A-associated surface molecule contributing to the inability of natural killer cells to kill Epstein-Barr virus-infected B cells in X-linked lymphoproliferative disease. J. Exp. Med. 194:235-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, M. H., K. Boles, P. A. van der Merwe, V. Kumar, P. A. Mathew, and A. N. Barclay. 1998. 2B4, the natural killer and T cell immunoglobulin superfamily surface protein, is a ligand for CD48. J. Exp. Med. 188:2083-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro, A. G., T. M. Hauser, B. G. Cocks, J. Abrams, S. Zurawski, T. Churakova, F. Zonin, D. Robinson, S. G. Tangye, G. Aversa, K. E. Nichols, J. E. de Vries, L. L. Lanier, and A. O'Garra. 1999. Molecular and functional characterization of mouse signaling lymphocytic activation molecule (SLAM): differential expression and responsiveness in Th1 and Th2 cells. J. Immunol. 163:5860-5870. [PubMed] [Google Scholar]
- 7.Chan, B., A. Lanyi, H. K. Song, J. Griesbach, M. Simarro-Grande, F. Poy, D. Howie, J. Sumegi, C. Terhorst, and M. J. Eck. 2003. SAP couples Fyn to SLAM immune receptors. Nat. Cell Biol. 5:155-160. [DOI] [PubMed] [Google Scholar]
- 8.Coffey, A. J., R. A. Brooksbank, O. Brandau, T. Oohashi, G. R. Howell, J. M. Bye, A. P. Cahn, J. Durham, P. Heath, P. Wray, R. Pavitt, J. Wilkinson, M. Leversha, E. Huckle, C. J. Shaw-Smith, A. Dunham, S. Rhodes, V. Schuster, G. Porta, L. Yin, P. Serafini, B. Sylla, M. Zollo, B. Franco, D. R. Bentley, et al. 1998. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat. Genet. 20:129-135. [DOI] [PubMed] [Google Scholar]
- 9.Colucci, F., E. Rosmaraki, S. Bregenholt, S. I. Samson, B. Di, V., M. Turner, L. Vanes, V. Tybulewicz, and J. P. Di Santo. 2001. Functional dichotomy in natural killer cell signaling: Vav1-dependent and -independent mechanisms. J. Exp. Med. 193:1413-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson, D., L. M. Chow, M. Fournel, and A. Veillette. 1992. Differential regulation of T cell antigen responsiveness by isoforms of the src-related tyrosine protein kinase p59fyn. J. Exp Med. 175:1483-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutz, J. P., L. Benoit, X. Wang, D. J. Demetrick, A. Junker, D. de Sa, and R. Tan. 2001. Lymphocytic vasculitis in X-linked lymphoproliferative disease. Blood 97:95-100. [DOI] [PubMed] [Google Scholar]
- 12.Engel, P., M. J. Eck, and C. Terhorst. 2003. The SAP and SLAM families in immune responses and X-linked lymphoproliferative disease. Nat. Rev. Immunol. 3:813-821. [DOI] [PubMed] [Google Scholar]
- 13.Garni-Wagner, B. A., A. Purohit, P. A. Mathew, M. Bennett, and V. Kumar. 1993. A novel function-associated molecule related to non-MHC-restricted cytotoxicity mediated by activated natural killer cells and T cells. J. Immunol. 151:60-70. [PubMed] [Google Scholar]
- 14.Kambayashi, T., E. Assarsson, B. J. Chambers, and H. G. Ljunggren. 2001. Cutting edge: regulation of CD8+ T cell proliferation by 2B4/CD48 interactions. J. Immunol. 167:6706-6710. [DOI] [PubMed] [Google Scholar]
- 15.Klem, J., P. C. Verrett, V. Kumar, and J. D. Schatzle. 2002. 2B4 is constitutively associated with linker for the activation of T cells in glycolipid-enriched microdomains: properties required for 2B4 lytic function. J. Immunol. 169:55-62. [DOI] [PubMed] [Google Scholar]
- 16.Latchman, Y., P. F. McKay, and H. Reiser. 1998. Identification of the 2B4 molecule as a counter-receptor for CD48. J. Immunol. 161:5809-5812. [PubMed] [Google Scholar]
- 17.Latour, S., G. Gish, C. D. Helgason, R. K. Humphries, T. Pawson, and A. Veillette. 2001. Regulation of SLAM-mediated signal transduction by SAP, the X-linked lymphoproliferative gene product. Nat. Immunol. 2:681-690. [DOI] [PubMed] [Google Scholar]
- 18.Latour, S., R. Roncagalli, R. Chen, M. Bakinowski, X. Shi, P. L. Schwartzberg, D. Davidson, and A. Veillette. 2003. Binding of SAP SH2 domain to FynT SH3 domain reveals a novel mechanism of receptor signalling in immune regulation. Nat. Cell Biol. 5:149-154. [DOI] [PubMed] [Google Scholar]
- 19.Latour, S., and A. Veillette. 2003. Molecular and immunological basis of X-linked lymphoproliferative disease. Immunol. Rev. 192:212-224. [DOI] [PubMed] [Google Scholar]
- 20.Latour, S., J. Zhang, R. P. Siraganian, and A. Veillette. 1998. A unique insert in the linker domain of Syk is necessary for its function in immunoreceptor signalling. EMBO J. 17:2584-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, K. M., S. Bhawan, T. Majima, H. Wei, M. I. Nishimura, H. Yagita, and V. Kumar. 2003. Cutting edge: the NK cell receptor 2B4 augments antigen-specific T cell cytotoxicity through CD48 ligation on neighboring T cells. J. Immunol. 170:4881-4885. [DOI] [PubMed] [Google Scholar]
- 22.Li, S. C., G. Gish, D. Yang, A. J. Coffey, J. D. Forman-Kay, I. Ernberg, L. E. Kay, and T. Pawson. 1999. Novel mode of ligand binding by the SH2 domain of the human XLP disease gene product SAP/SH2D1A. Curr. Biol. 9:1355-1362. [DOI] [PubMed] [Google Scholar]
- 23.Mathew, P. A., B. A. Garni-Wagner, K. Land, A. Takashima, E. Stoneman, M. Bennett, and V. Kumar. 1993. Cloning and characterization of the 2B4 gene encoding a molecule associated with non-MHC-restricted killing mediated by activated natural killer cells and T cells. J. Immunol. 151:5328-5337. [PubMed] [Google Scholar]
- 24.Moretta, A., C. Bottino, M. Vitale, D. Pende, C. Cantoni, M. C. Mingari, R. Biassoni, and L. Moretta. 2001. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 19:197-223. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima, H., M. Cella, A. Bouchon, H. L. Grierson, J. Lewis, C. S. Duckett, J. I. Cohen, and M. Colonna. 2000. Patients with X-linked lymphoproliferative disease have a defect in 2B4 receptor-mediated NK cell cytotoxicity. Eur. J. Immunol. 30:3309-3318. [DOI] [PubMed] [Google Scholar]
- 26.Nichols, K. E., D. P. Harkin, S. Levitz, M. Krainer, K. A. Kolquist, C. Genovese, A. Bernard, M. Ferguson, L. Zuo, E. Snyder, A. J. Buckler, C. Wise, J. Ashley, M. Lovett, M. B. Valentine, A. T. Look, W. Gerald, D. E. Housman, and D. A. Haber. 1998. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc. Natl. Acad. Sci. USA 95:13765-13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parolini, S., C. Bottino, M. Falco, R. Augugliaro, S. Giliani, R. Franceschini, H. D. Ochs, H. Wolf, J. Y. Bonnefoy, R. Biassoni, L. Moretta, L. D. Notarangelo, and A. Moretta. 2000. X-linked lymphoproliferative disease. 2B4 molecules displaying inhibitory rather than activating function are responsible for the inability of natural killer cells to kill Epstein-Barr virus-infected cells. J. Exp. Med. 192:337-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sayos, J., C. Wu, M. Morra, N. Wang, X. Zhang, D. Allen, S. van Schaik, L. Notarangelo, R. Geha, M. G. Roncarolo, H. Oettgen, J. E. de Vries, G. Aversa, and C. Terhorst. 1998. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature 395:462-469. [DOI] [PubMed] [Google Scholar]
- 29.Tangye, S. G., S. Lazetic, E. Woollatt, G. R. Sutherland, L. L. Lanier, and J. H. Phillips. 1999. Cutting edge: human 2B4, an activating NK cell receptor, recruits the protein tyrosine phosphatase SHP-2 and the adaptor signaling protein SAP. J. Immunol. 162:6981-6985. [PubMed] [Google Scholar]
- 30.Tangye, S. G., J. H. Phillips, L. L. Lanier, and K. E. Nichols. 2000. Functional requirement for SAP in 2B4-mediated activation of human natural killer cells as revealed by the X-linked lymphoproliferative syndrome. J. Immunol. 165:2932-2936. [DOI] [PubMed] [Google Scholar]
- 31.Turner, M., and D. D. Billadeau. 2002. VAV proteins as signal integrators for multi-subunit immune-recognition receptors. Nat. Rev. Immunol. 2:476-486. [DOI] [PubMed] [Google Scholar]
- 32.Valiante, N. M., and G. Trinchieri. 1993. Identification of a novel signal transduction surface molecule on human cytotoxic lymphocytes. J. Exp. Med. 178:1397-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veillette, A., M. A. Bookman, E. M. Horak, and J. B. Bolen. 1988. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell 55:301-308. [DOI] [PubMed] [Google Scholar]
- 34.Veillette, A., and S. Latour. 2003. The SLAM family of immune-cell receptors. Curr. Opin. Immunol. 15:277-285. [DOI] [PubMed] [Google Scholar]
- 35.Watzl, C., C. C. Stebbins, and E. O. Long. 2000. NK cell inhibitory receptors prevent tyrosine phosphorylation of the activation receptor 2B4 (CD244). J. Immunol. 165:3545-3548. [DOI] [PubMed] [Google Scholar]