Abstract
Endoscopic submucosal dissection (ESD) for early gastric cancer is a well-established procedure with the advantage of resection in an en bloc fashion, regardless of the size, shape, coexisting ulcer, and location of the lesion. However, gastric ESD is a more difficult and meticulous technique, and also requires a longer procedure time, than conventional endoscopic mucosal resection. These factors naturally increase the risk of various complications. The two most common complications accompanying gastric ESD are bleeding and perforation. These complications are known to occur both intraoperatively and postoperatively. However, there are other rare but serious complications related to gastric ESD, including aspiration pneumonia, stenosis, venous thromboembolism, and air embolism. Endoscopists should have sufficient knowledge about such complications and be prepared to deal with them appropriately, as successful management of complications is necessary for the successful completion of the entire ESD procedure.
Keywords: Endoscopic submucosal dissection, Complication, Hemorrhage, Perforation
INTRODUCTION
Endoscopic submucosal dissection (ESD) for early gastric cancer is a widely accepted and well-established procedure because of its curative potential and low invasiveness compared with surgical operative therapy.1 The major advantage of ESD over conventional endoscopic mucosal resection (EMR) lies in en bloc resection, regardless of the size, shape, coexisting ulcer, and location of the lesion. However, ESD is a more difficult and meticulous technique than EMR, and sometimes causes serious adverse events.2 Therefore endoscopists who perform ESD should have sufficient knowledge of the complications associated with the procedure. In this review article, we present an overview of these complications and the appropriate countermeasures.
MANAGEMENT OF THE MAJOR COMPLICATIONS
Bleeding
ESD operators often encounter bleeding from the site of the operation. This bleeding can be classified into two groups with respect to the time of onset. One is intraoperative bleeding, which is defined as any bleeding occurring during the ESD procedure. The other is postoperative bleeding, which occurs after the ESD procedure. Most cases of ESD-related bleeding can be controlled by means of endoscopic hemostasis through either the coagulation of blood vessels with an electrosurgical knife or hemostatic forceps, or suture with endoclips. However, massive bleeding may lead to serious life-threatening conditions, including hemorrhagic shock. If endoscopic hemostasis is not technically feasible, it is important not to hesitate to convert to emergency surgery or artery embolization with vascular interventional radiology.
Intraoperative bleeding
Although massive amounts of blood loss often result in critical conditions, it is difficult to accurately measure the total volume of bleeding during ESD. Therefore, the severity of bleeding can often only be determined postoperatively. Oda et al.3 defined "significant" intraoperative (immediate) bleeding as a dilution of >2 g/dL in hemoglobin (Hb) from the preprocedure level to the next-day level. On the basis of this definition, they reported that significant intraoperative (immediate) bleeding occurs at a rate of 7%,3 which may have been lower in recent years owing to the development of new devices.
However, intraoperative bleeding that does not meet these criteria occurs at a much higher rate. This does not mean that such "insignificant" bleeding can be ignored. The prevention and early control of any intraoperative bleeding is also important because bleeding can impair the endoscopic view, resulting in an increase in procedure time and other intraoperative complications.
To prevent intraoperative bleeding, it is necessary to perform ESD with a clear endoscopic view, which may be obtained by means of sufficient submucosal injection. Preventive hemostatic coagulation of visible blood vessels with the use of coagulation devices, dissection of the deep submucosal layer to an appropriate depth, and use of appropriate traction with an electrosurgical knife or other devices has also been reported to be effective.4
However, intraoperative bleeding during the ESD procedure cannot always be avoided. Therefore, rapid and accurate control of bleeding is important, with hemostasis through coagulation being the preferred strategy.4 During the hemostatic procedure, identification of the bleeding site or the responsible bleeding vessel is crucial. Use of a water jet is effective in detecting the bleeding site, by securing visibility through the irrigation of blood pooling, and helps operators find the bleeding site or responsible bleeding vessels faster, resulting in faster hemostasis. At our institute, we use endoscopes with water-jet systems for all ESD cases. If bleeding cannot be managed with coagulation, suture of the blood vessels by using endoclips is another option. However, the use of endoclips is technically difficult compared with coagulation; moreover, once an endoclip is deployed, the procedure is often irreversible. Operators should exercise care in deploying the endoclips at a location that will not interfere with the subsequent procedure, because this may increase the technical difficulty and procedure time of ESD.
The most significant risk factor for intraoperative bleeding is reported to be the tumor location. ESD of the middle and upper thirds of the body, in which the submucosal layer is vascular rich, with thick vessels penetrating the muscle layer, is associated with a higher rate of intraoperative bleeding compared with the antrum. Therefore, operators must perform ESD with greater caution for lesions located in these regions.5,6,7
Postoperative bleeding
Postoperative bleeding is generally defined as one or more of the following signs of bleeding after the completion of the ESD procedure: hematemesis or melena, unstable vital signs or a dilution of >2 g/dL in Hb, and requirement for endoscopic hemostatic treatment.3,6,7,8
Postoperative bleeding is reported to occur in 5.3% to 15.6% of gastric ESD cases.3,6,7,8,9,10 At our institute, the rate of postoperative bleeding is 6.6% (36 of 546 cases between 2009 and 2013). Several risk factors such as resection size, tumor location (lower and middle thirds of the gastric body), insufficient operator experience (<50 cases of gastric ESD), and poor control of intraoperative bleeding during ESD for postoperative bleeding have been previously reported.6,7,9,10 Tsuji et al.7 reported that antiplatelet agents, anticoagulants, steroids, and nonsteroidal anti-inflammatory drugs were risk factors for postoperative bleeding. Koh et al.10 reported that oral antithrombotic drug therapy was an independent risk factor for delayed postoperative bleeding. On the other hand, Lim et al.11 reported that in ESD for antiplatelet users, continuous administration of the drugs did not have an independent significant association with bleeding. The possible influence of such drugs on postoperative bleeding is controversial, and further research is required.
Proton pump inhibitors (PPIs) are reported to be effective in the prevention of postoperative bleeding, and PPI administration may be discontinued after 2 weeks when the deteriorating factors for ESD ulcer are excluded.9 The application of second-look endoscopy may not necessarily be recommended in all cases because it does not seem to affect clinical outcomes, including bleeding and morbidity after ESD.12 The outcomes of postoperative bleeding after gastric ESD and peptic ulcer bleeding are similar, and most cases could be treated with endoscopic hemostasis mainly by using endoclips and/or coagulation (Fig. 1).13 However, as hematoma sometimes exists on the bleeding site, or ESD ulcer in case of postoperative bleeding, operators often need to perform water-jet irrigation, or sometimes use forceps, to eliminate the hematoma.
Fig. 1.
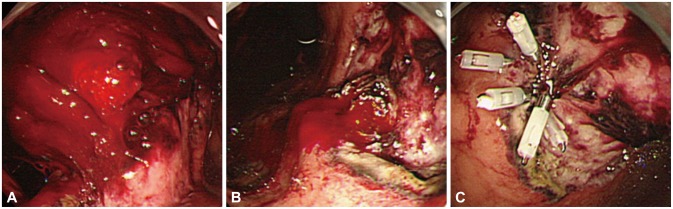
A case of postoperative bleeding. (A) An example of postoperative (day 1 after endoscopic submucosal dissection) bleeding with a large amount of hematoma. (B) Pulsating bleeding observed after the hematoma has been removed. (C) Successful hemostasis by using endoclips.
Perforation
Perforation associated with the ESD procedure is divided into two groups with respect to the time of onset. One is intraoperative perforation, which is mainly due to the penetration of an electrosurgical knife through the stomach wall during ESD. The other is postoperative perforation, which mainly occurs 1 to 2 days after the ESD procedure.
Intraoperative perforation
Intraoperative perforation occurs at a rate of 1.2% to 8.2% during gastric ESD.6,8,14,15 At our institute, the rate of intraoperative perforation is 0.5% (3 of the total of 546 cases between 2009 and 2013). The tumor location (middle and upper thirds of the gastric body), tumor diameter (larger size, e.g., >2 cm), ulcerative findings (presence), and longer operation time (e.g., >2 hours) are reported to be the independent risk factors for intraoperative perforation.6,14,15
Intraoperative perforation can be diagnosed through the endoscopic view as the fat or external organs observed through the muscle layer, and/or on the basis of the presence of free air on a plain radiograph or abdominal computed tomography (CT) just after the ESD procedure (Fig. 2).6 If the hole of the perforation is relatively large, it can be detected through the endoscopic view during the ESD procedure. Free air is also a significant sign of perforation; however, sometimes, free air close to the stomach is detected in abdominal CT on the day after ESD although no evidence of endoscopic perforation was seen during the ESD procedure, or of peritonitis. Watari et al.16 defined such free air without a visible endoscopic perforation as "silent" free air. According to their report, this silent free air was identified in 37.3% of patients. The tumor location (upper portion of the stomach), presence of a damaged muscular layer during ESD, and procedure time are reported to be significantly associated with silent free air, and the procedure time (≥105 minutes) is reported to be an independent predictor. There was no significant difference in inflammatory markers between silent free air-positive and -negative patients; therefore, the authors concluded that silent free air may not lead to clinically significant complications.16
Fig. 2.
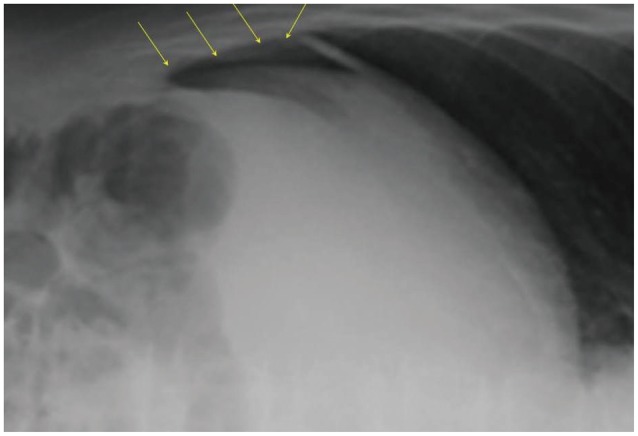
Intra-abdominal free air detected on plain radiograph after gastric endoscopic submucosal dissection (ESD). Free air (arrows) was observed on the surface of the liver after gastric ESD, on plain radiograph in the left lateral decubitus position.
To prevent intraoperative perforation, it is necessary to make a sufficient space in the submucosal layer by using hyaluronic acid solution for easier maneuverability.17 Appropriate sedation for the purpose of preventing body movement or gag reflex, and in some cases general anesthesia for longer procedures, may be effective for the prevention of intraoperative perforation. Recently, carbon dioxide insufflation has increasingly been used instead of air insufflation to minimize pneumoperitoneum caused by gastric perforation.18 At our institute, we perform ESD with carbon dioxide insufflation in all cases.
When perforation occurs or is suspected, the first priority is to close the hole by using endoclips. Subsequently, patients should be administered with antibiotics intravenously as soon as possible, ideally just after the perforation is confirmed, to reduce the risk of infection. Operators do not necessarily discontinue the ESD procedure because perforations during ESD are usually small and linear, allowing for simple closure by using several endoclips (Fig. 3).19 However, after the completion of ESD, the patient must be carefully observed to evaluate the severity of infection, pneumoperitoneum, and other adverse events. If severe pneumoperitoneum causes changes in vital signs, the gas in the abdominal cavity usually has to be released through peritoneocentesis after confirming that the intestine is not located at the puncture site, by using ultrasound as much as possible.15
Fig. 3.
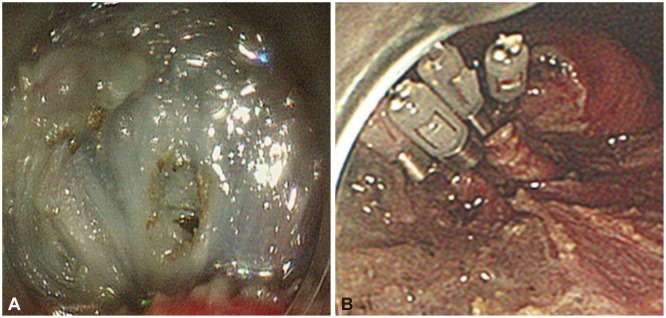
A case of intraoperative perforation. (A) A small perforation occurring during gastric endoscopic submucosal dissection. (B) The perforation site closed successfully by using endoclips.
Postoperative perforation
Postoperative perforation, which mainly occurs 1 to 2 days after the ESD procedure, is reported to be a rare complication; however, once it occurs, it can lead to serious conditions that often require emergency surgery.20,21 The frequency is reported about 0.45%.21 Ikezawa et al.20 reported that the shape of the postoperative perforation was round and the color of the surrounding muscle layer had become whitish, suggesting necrosis of the muscle layer, whereas intraoperative perforation was usually tear-like in shape. Because of its rare frequency, risk analyses have not been reported for postoperative perforation; however, theoretically, excessive thermal damage on the muscle layer might be one of the causes of postoperative perforation. The best precaution for thermal damage on the muscle layer is to avoid excessive coagulation of visible vessels.
Furthermore, although there have been a few reports about the conservative management of postoperative perforation with endoscopic closure,20 peritonitis caused by postoperative perforation can sometimes be managed only by surgery; thus, the timing for surgical treatment should not be missed.
Aspiration pneumonia
Aspiration pneumonia is reported to occur in 2.2% to 6.6% of patients who had undergone the ESD procedure.16,22 The risk factors are reported to be a longer procedure time (e.g., >2 hours), older age (e.g., >75 years), and male sex.22 Aspiration pneumonia is mainly diagnosed on the basis of physical findings such as fever, cough, and sputum. A plain radiograph or CT scan can also detect the signs of aspiration pneumonia. The body temperature, white blood cell count, and C-reactive protein level are reported to be significantly higher in patients with aspiration pneumonia than in those without aspiration pneumonia after ESD.16 Particularly, as most patients take the left lateral decubitus position during ESD, aspiration pneumonia often occurs in the left lung. To prevent aspiration pneumonia, adequate suction of the oral cavity to remove saliva during the ESD procedure may be effective.23 Avoidance of excessive air insufflation may also be effective to prevent vomiting, which can potentially cause the development of aspiration pneumonia. If the patient unfortunately develops aspiration pneumonia after ESD, a prompt CT scan is necessary and appropriate use of antibiotics is important, because Watari et al.16 reported that there is no significant difference in the duration of admission when appropriate antibiotics was administered.
Stenosis
Post-ESD stenosis is defined as a stricture that a standard endoscope could not pass through.24 Its incidence is reported to range from 0.9% to 1.9% in all gastric ESD cases.24,25 Most stenosis occurs a few weeks after the ESD procedure, during the healing process of the ESD ulcer. In particular, as a semicircumferential resection over 75% of the circumference by ESD in the prepylorus, antrum, and cardia is reported to be a risk factor for the occurrence of stricture,24 operators should pay attention to the possibility of stenosis after ESD for lesions located near the cardia and pylorus. For the treatment of stenosis after ESD, endoscopic balloon dilation (EBD) is an effective technique. Perforation has been reported as a complication related to EBD. Early intervention is recommended for patients with a high risk for stricture to avoid perforation during EBD, because the artificial ulcer made from ESD is reported to heal within 8 weeks with fibrosis in the stomach wall, and severe stricture and fibrosis in the stomach wall may be one of the reasons for the occurrence of a perforation.24 If the stenosis is not amenable to endoscopic intervention, surgical intervention is performed.24,25 Recently, steroid administration has also been reported to prevent stenosis after gastric ESD;26 however, further evaluation is needed.
Venous thromboembolism
Kusunoki et al.27 reported about venous thromboembolism (VTE) related to the ESD procedure. In their report, the overall frequency of asymptomatic VTE after ESD was 10.0%. Because staying in the same position for a prolonged period is often required during the ESD procedure, and sometimes patients need to keep lying on a bed for a few hours after ESD because of the intravenous sedation during the procedure, there is a risk of VTE in patients treated with ESD. VTE can potentially lead to pulmonary embolism; thus, preventing VTE is essential. The D-dimer level on the day after ESD, in particular, is reported to be potentially associated with the risk for VTE in ESD patients.27 To prevent VTE associated with ESD, a postural change after ESD or massage of the lower limbs might be effective. Elastic stockings may also be effective to prevent VTE, and at our institute, all patients are required to wear elastic stockings from the morning of the ESD procedure until at least the next morning.
Air embolism
Air embolism is a very rare complication; however, once it occurs, it has the potential to result in fatal conditions. There are no reports about air embolism related to ESD, but there are some reports about air embolism related to esophagogastroduodenoscopy.28 Systemic air embolism can cause cardiovascular symptoms, pulmonary symptoms, or neurological symptoms.29 However, as the initial neurological symptoms caused by air embolism are sometimes similar to sedation-related problems, endoscopists should pay sufficient attention to patients' signs such as the presence of arrhythmia, tachycardia, or ST-T change in the electrocardiogram, or symptoms such as the presence of dyspnea, tachypnea, breathlessness or prolonged altered mental status, dilated pupils, anisocoria, or coma.
Carbon dioxide insufflation instead of air during the procedure is reported to be expected to reduce the risk of air embolism because carbon dioxide can be easily absorbed.18
CONCLUSIONS
Although gastric ESD is a well-established procedure with the advantage of resection in an en bloc fashion, regardless of the size, shape, coexisting ulcer, and location of the lesion, it carries the risk of several complications. Although the occurrence rate of those complications is not very high, they sometimes result in critical conditions. Therefore, ESD operators should have sufficient knowledge and information of complications that could occur in association with the ESD procedure and should know how to manage them for the safe completion of ESD.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Fujishiro M. Endoscopic submucosal dissection for stomach neoplasms. World J Gastroenterol. 2006;12:5108–5112. doi: 10.3748/wjg.v12.i32.5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goda K, Fujishiro M, Hirasawa K, et al. How to teach and learn endoscopic submucosal dissection for upper gastrointestinal neoplasm in Japan. Dig Endosc. 2012;24(Suppl 1):136–142. doi: 10.1111/j.1443-1661.2012.01274.x. [DOI] [PubMed] [Google Scholar]
- 3.Oda I, Gotoda T, Hamanaka H, et al. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc. 2005;17:54–58. [Google Scholar]
- 4.Fujishiro M, Yahagi N, Kakushima N, et al. Management of bleeding concerning endoscopic submucosal dissection with the flex knife for stomach neoplasm. Dig Endosc. 2006;18(Suppl 1):S119–S122. [Google Scholar]
- 5.Toyonaga T, Nishino E, Hirooka T, Ueda C, Noda K. Intraoperative bleeding in endoscopic submucosal dissection in the stomach and strategy for prevention and treatment. Dig Endosc. 2006;18(Suppl 1):S123–S127. [Google Scholar]
- 6.Mannen K, Tsunada S, Hara M, et al. Risk factors for complications of endoscopic submucosal dissection in gastric tumors: analysis of 478 lesions. J Gastroenterol. 2010;45:30–36. doi: 10.1007/s00535-009-0137-4. [DOI] [PubMed] [Google Scholar]
- 7.Tsuji Y, Ohata K, Ito T, et al. Risk factors for bleeding after endoscopic submucosal dissection for gastric lesions. World J Gastroenterol. 2010;16:2913–2917. doi: 10.3748/wjg.v16.i23.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228–1235. doi: 10.1016/j.gie.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 9.Fujishiro M, Chiu PW, Wang HP. Role of antisecretory agents for gastric endoscopic submucosal dissection. Dig Endosc. 2013;25(Suppl 1):86–93. doi: 10.1111/j.1443-1661.2012.01370.x. [DOI] [PubMed] [Google Scholar]
- 10.Koh R, Hirasawa K, Yahara S, et al. Antithrombotic drugs are risk factors for delayed postoperative bleeding after endoscopic submucosal dissection for gastric neoplasms. Gastrointest Endosc. 2013;78:476–483. doi: 10.1016/j.gie.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Lim JH, Kim SG, Kim JW, et al. Do antiplatelets increase the risk of bleeding after endoscopic submucosal dissection of gastric neoplasms? Gastrointest Endosc. 2012;75:719–727. doi: 10.1016/j.gie.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 12.Ryu HY, Kim JW, Kim HS, et al. Second-look endoscopy is not associated with better clinical outcomes after gastric endoscopic submucosal dissection: a prospective, randomized, clinical trial analyzed on an as-treated basis. Gastrointest Endosc. 2013;78:285–294. doi: 10.1016/j.gie.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Fujishiro M, Abe N, Endo M, et al. Current managements and outcomes of peptic and artificial ulcer bleeding in Japan. Dig Endosc. 2010;22(Suppl 1):S9–S14. doi: 10.1111/j.1443-1661.2010.00961.x. [DOI] [PubMed] [Google Scholar]
- 14.Watari J, Tomita T, Toyoshima F, et al. Clinical outcomes and risk factors for perforation in gastric endoscopic submucosal dissection: a prospective pilot study. World J Gastrointest Endosc. 2013;5:281–287. doi: 10.4253/wjge.v5.i6.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nonaka K, Kita H. Endoscopic submucosal dissection for early gastric cancer. J Cancer Ther. 2013;4:26–32. [Google Scholar]
- 16.Watari J, Tomita T, Toyoshima F, et al. The incidence of "silent" free air and aspiration pneumonia detected by CT after gastric endoscopic submucosal dissection. Gastrointest Endosc. 2012;76:1116–1123. doi: 10.1016/j.gie.2012.07.043. [DOI] [PubMed] [Google Scholar]
- 17.Fujishiro M, Yahagi N, Nakamura M, et al. Successful outcomes of a novel endoscopic treatment for GI tumors: endoscopic submucosal dissection with a mixture of high-molecular-weight hyaluronic acid, glycerin, and sugar. Gastrointest Endosc. 2006;63:243–249. doi: 10.1016/j.gie.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Nonaka S, Saito Y, Takisawa H, Kim Y, Kikuchi T, Oda I. Safety of carbon dioxide insufflation for upper gastrointestinal tract endoscopic treatment of patients under deep sedation. Surg Endosc. 2010;24:1638–1645. doi: 10.1007/s00464-009-0824-5. [DOI] [PubMed] [Google Scholar]
- 19.Fujishiro M, Yahagi N, Kakushima N, et al. Successful nonsurgical management of perforation complicating endoscopic submucosal dissection of gastrointestinal epithelial neoplasms. Endoscopy. 2006;38:1001–1006. doi: 10.1055/s-2006-944775. [DOI] [PubMed] [Google Scholar]
- 20.Ikezawa K, Michida T, Iwahashi K, et al. Delayed perforation occurring after endoscopic submucosal dissection for early gastric cancer. Gastric Cancer. 2012;15:111–114. doi: 10.1007/s10120-011-0089-2. [DOI] [PubMed] [Google Scholar]
- 21.Hanaoka N, Uedo N, Ishihara R, et al. Clinical features and outcomes of delayed perforation after endoscopic submucosal dissection for early gastric cancer. Endoscopy. 2010;42:1112–1115. doi: 10.1055/s-0030-1255932. [DOI] [PubMed] [Google Scholar]
- 22.Park CH, Kim H, Kang YA, et al. Risk factors and prognosis of pulmonary complications after endoscopic submucosal dissection for gastric neoplasia. Dig Dis Sci. 2013;58:540–546. doi: 10.1007/s10620-012-2376-0. [DOI] [PubMed] [Google Scholar]
- 23.Onozato Y, Kakizaki S, Ishihara H, et al. Feasibility of endoscopic submucosal dissection for elderly patients with early gastric cancer and adenomas. Dig Endosc. 2008;20:12–16. [Google Scholar]
- 24.Iizuka H, Kakizaki S, Sohara N, et al. Stricture after endoscopic submucosal dissection for early gastric cancers and adenomas. Dig Endosc. 2010;22:282–288. doi: 10.1111/j.1443-1661.2010.01008.x. [DOI] [PubMed] [Google Scholar]
- 25.Tsunada S, Ogata S, Mannen K, et al. Case series of endoscopic balloon dilation to treat a stricture caused by circumferential resection of the gastric antrum by endoscopic submucosal dissection. Gastrointest Endosc. 2008;67:979–983. doi: 10.1016/j.gie.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 26.Shoji H, Yamaguchi N, Isomoto H, et al. Oral prednisolone and triamcinolone injection for gastric stricture after endoscopic submucosal dissection. Ann Transl Med. 2014;2:22. doi: 10.3978/j.issn.2305-5839.2014.02.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kusunoki M, Miyake K, Shindo T, et al. The incidence of deep vein thrombosis in Japanese patients undergoing endoscopic submucosal dissection. Gastrointest Endosc. 2011;74:798–804. doi: 10.1016/j.gie.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 28.Donepudi S, Chavalitdhamrong D, Pu L, Draganov PV. Air embolism complicating gastrointestinal endoscopy: a systematic review. World J Gastrointest Endosc. 2013;5:359–365. doi: 10.4253/wjge.v5.i8.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mirski MA, Lele AV, Fitzsimmons L, Toung TJ. Diagnosis and treatment of vascular air embolism. Anesthesiology. 2007;106:164–177. doi: 10.1097/00000542-200701000-00026. [DOI] [PubMed] [Google Scholar]


