Abstract
Background
Alopecia areata (AA) is a common form of localized, non-scarring hair loss. The cause of AA is unknown but reports suggest an autoimmune etiology, where oxygen free radicals play an important role.
Objective
The aim of this study was to investigate the role of a hydroxyl radicals (·OH)-modified antioxidant enzyme, superoxide dismutase (SOD), in AA autoimmunity.
Methods
SOD was modified by ·OH radicals. Binding characteristics of autoantibodies in AA patients (n=26) against ·OH-modified SOD (·OH-SOD) were evaluated by immunoassays and the results were compared with those of healthy, age-matched controls (n=30). The effects of ·OH radicals on immunoglobulin G (IgG) isolated from AA patients were studied.
Results
Highly specific binding to ·OH-SOD was observed in 32% of the samples of patient sera, whereas normal human sera showed negligible binding with either antigen. Competitive inhibition immunoassays reiterated the results from direct binding. Protein-A-purified IgG from AA patients (AA-IgG) also showed strong binding to ·OH-SOD as compared to IgG from normal human controls (p<0.001). In addition, AA-IgG from patients with alopecia universalis recognized ·OH-SOD to a greater extent than did AA-IgG from patients with the patchy, persistent type of alopecia. Furthermore, sera from AA patients had lower levels of SOD activity as compared to control sera.
Conclusion
This is the first report showing an association between ·OH-modified SOD and AA. These novel results demonstrate that ·OH radical-mediated changes in SOD present unique neo-epitopes that might contribute to antigen-driven antibody induction in AA.
Keywords: Alopecia areata, Autoimmunity, ·OH-SOD, Reactive oxygen species, Superoxide dismutase
INTRODUCTION
Alopecia areata (AA) is a non-scarring, autoimmune, inflammatory condition resulting in hair loss. It is relatively common, with an incidence of 0.15% worldwide1. Although the exact pathogenesis is unknown, autoimmune reactions have been implicated in AA2. Due to an aberrant T cells response observed against various self antigens, AA is now considered to be an autoimmune disease3. This etiology has been proposed on the basis of evidence such as its association with various autoimmune diseases4 and the presence of autoantibodies and various underlying immunologic abnormalities in the affected sites5. However, the precise mechanism of autoantibody generation in AA remains unclear5,6. It is well documented that oxidative stress plays a vital role in AA as well as other skin disorders7,8,9,10,11,12. Various studies have shown that AA is associated with increased formation of free radicals and a decrease in antioxidant potential7,8,9,10,13,14,15. This may result in oxidative damage to cell components, including proteins and nucleic acids, as seen in several other autoimmune diseases16,17,18.
Superoxide dismutase (SOD) is a prime antioxidant enzyme that eliminates the effects of superoxide, thus limiting the toxicity from reactive oxygen species (ROS)19. Hence, SOD is considered an important regulator of oxidative stress. SOD dysfunction has been reported in patients with AA7,8,9,15, while SOD antigenicity has been observed in various chronic conditions20. It is assumed that since SOD may be constantly exposed to oxidative stress, alterations may occur in the enzyme's conformation and function, resulting in modification of its biological properties. Therefore, this study was designed to test the hypothesis that oxidative by-products such as hydroxyl radicaldamaged SOD help to initiate autoimmunity in AA. To test this hypothesis, the presence of circulating autoantibodies in AA patients directed against ROS-modified SOD (ROS-SOD) was investigated. This novel study supports the association between ROS-SOD and AA pathogenesis and provides evidence that ROS-damaged SOD could be an important biomarker for the evaluation of oxidative stress in AA patients.
MATERIALS AND METHODS
Patient recruitment
The study was carried out in accordance with the Code of Ethics for Research Involving Humans by the World Medical Association (Declaration of Helsinki, as revised in Tokyo 2004). The study was approved by the local ethics committee of the College of Medicine, Qassim University, Kingdom of Saudi Arabia. Study subjects were recruited through the dermatology outpatient clinic of Qassim University, Buraidah, Kingdom of Saudi Arabia, and informed consent was obtained from each subject. The study group included 26 AA subjects (5 females and 21 males) and their age range was 19~45 years (mean±standard deviation [SD], 31.4±7.32 years). The duration of the disease ranged from 1 month to 18 years. Subjects were classified according to Alopecia Areata Foundation Clinical Assessment Guidelines21, where subjects with less than 100% scalp hair loss for ≥1 year were classified as AA patchy persistent (AAP; n=21), while those with 100% scalp and body hair loss were classified as alopecia universalis (AU; n=5). Except for one subject who was treated with systemic corticosteroids and 10 subjects who were treated with topical steroids, all other AA subjects were untreated at the time of sample collection. The control group comprised 30 healthy volunteers (7 female and 23 male; age range 20 to 47 years; mean age±SD, 33.3±10.8 years). The mean age was not significantly different between the groups. The racial/ethnic and gender compositions of the AA groups were comparable with those of the control group. Venous blood samples were collected from all subjects and stored in small aliquots at -80℃ until analysis.
Modification of SOD
SOD (catalog # S7571; Sigma-Aldrich, St. Louis, MO, USA) was modified in phosphate-buffered saline (PBS) (10 mM sodium phosphate buffer containing 150 mM NaCl, pH 7.4) as described previously20,22. Briefly, an aqueous solution of SOD (1 mg/ml) was modified by hydroxyl radicals generated by irradiating 30 µM hydrogen peroxide at 254 nm for 30 min. Excess hydrogen peroxide (Sigma, St. Louis, MO, USA) was removed from the samples by extensive dialysis (dialysis tubing was obtained from Sigma) with PBS, pH 7.4.
Purification of immunoglobulin G
Immunoglobulin G (IgG) was isolated from human serum by affinity chromatography using Protein A-Agarose affinity column (catalog # PA1-EA; Sigma-Aldrich) as described previously23. Serum (0.3 ml) diluted with an equal volume of PBS, pH 7.4, was applied to the column (12×45 mm) equilibrated with the same buffer. The flow-through was reloaded onto the column 2 to 3 times. Unbound proteins were removed by extensive washing with PBS, pH 7.4. The bound IgG was eluted with 0.58% acetic acid in 0.85% sodium chloride, neutralized with 1.0 ml Tris-HCl (1.0 M, pH 8.5), and 1-ml fractions were collected, which were read at 251 nm and 278 nm. The IgG concentration was determined considering that an optical density at 278 nm (OD278) of 1.38=1.0 mg IgG/ml24. The isolated IgG was purified by dialysis with PBS, pH 7.4, and stored at -20℃.
Enzyme-linked immunosorbent assay
Direct-binding enzyme-linked immunosorbent assay (ELISA) was performed using flat-bottomed, 96-well polystyrene maxiSorp immunoplates (catalog # P8616; Nunc-Immuno MicroWell; Sigma-Aldrich) as described previously25,26. Briefly, plates were coated with 100 µl of either native or modified SOD (10 µg/ml in 0.05 M carbonate buffer, pH 9.6) and incubated for 2 h at room temperature (RT) and then overnight at 4℃. Each SOD sample was coated in duplicate and half of the plates served as controls, devoid of antigen. Unbound antigen was washed with 10 mM PBS-T (150 mM NaCl, pH 7.4 containing 0.05% Tween-20; Sigma) and unoccupied sites were blocked with blocking buffer (PBS containing 1% bovine serum albumin) for 1~2 h at RT. After incubation, the plates were washed with PBS-T. The test serum (1 : 100) in PBS-T (100 µl/well) was applied and incubated for 2 h at RT and then overnight at 4℃. Bound antibodies were analyzed with anti-human horseradish peroxidase-linked conjugate (catalog # sc2769; Santa Cruz Biotechnology, Santa Cruz, CA, USA) using 3,3',5,5'-tetramethylbenzidine substrate (TMB, catalog # 206697A; Santa Cruz Biotechnology) and the reaction was stopped by the addition of stop solution (2 M H2SO4). The absorbance was recorded at 405 nm using an automatic microplate reader (Anthos Zenyth 3100 Multimode Detectors; Zenyth, Salzburg, Austria).
Competitive binding assays
Antibody specificity was determined by competitive inhibition ELISA as described previously27,28. Inhibitors (20 µg) were allowed to interact with a constant amount of serum sample for 2 h at 37℃ and then overnight at 4℃. The immune complexes formed were coated onto immunoplate wells in place of serum, while the rest of the steps were identical to those described for direct-binding ELISA. Percent inhibition was calculated using the formula: percent inhibition=1-[(Ainhibited/Auninhibited)]×100.
Determination of SOD activity
Activity of the antioxidant enzyme SOD in the serum samples from AA and control subjects was determined as described previously11. Enzyme activity was determined based on its ability to inhibit the autoxidation of epinephrine at pH 10.2. In a final volume of 3 ml, each cuvette contained 0.5 ml epinephrine (1.8 mM, freshly prepared), 0.5 ml ethylenediaminetetraacetic acid (0.6 mM), 0.5 ml sodium carbonate (0.3 M, pH 10.2), and serum samples. The reaction was initiated by the addition of epinephrine and the increase in absorbance was measured at 480 nm.
Statistical analysis
Results are expressed as mean±standard error of the mean (SEM) unless stated otherwise. One-way ANOVA followed by a Tukey-Kramer multiple comparisons test, and two-way ANOVA followed by a Bonferroni multiple comparisons test were performed; p<0.05 was considered significant. All statistical analyses were performed using GraphPad Prism version 5.0 (GraphPad Software Inc., San Diego, CA, USA) or Origin software package version 5.0 (Origin Lab Corporation, Northampton, MA, USA).
RESULTS
Characterization of ·OH-modified SOD
Native SOD (nSOD) was modified by hydroxyl radicals (·OH), generated by ultraviolet irradiation of hydrogen peroxide, and changes were induced in SOD as described previously20. The ·OH radical-modified SOD showed a marked hypochromicity at 280 nm, loss of tryptophan/tyrosine fluorescence, and increased protein carbonyl content as compared to its native analogue.
Detection of antibodies against ·OH-modified SOD in patients with AA
This is the first report regarding serum levels of ·OH-modified SOD (ROS-SOD)-specific antibodies in patients with AA, in an attempt to understand the role of oxidative damage of SOD in the pathogenesis of AA. Sera from 26 AA patients and 30 normal human (NH) subjects were tested for antibodies binding to SOD and ROS-SOD by specific ELISAs. The data showed that the AA sera showed a 32% stronger antibody binding to ROS-SOD as compared to NH sera, at 1 : 100 serum dilution (p<0.01) (Fig. 1A). The mean normalized absorbance at 405 nm (±SEM) of 26 AA serum samples and 30 NH serum samples binding to ROS-SOD was 0.38±0.11 and 0.28±0.09, respectively. On the other hand, AA and NH serum antibodies showed negligible binding to nSOD (Fig. 1B). The mean normalized absorbance at 405 nm (±SEM) of the 26 AA and 30 NH sera binding to nSOD was 0.21±0.07 and 0.19±0.09, respectively.
Fig. 1.
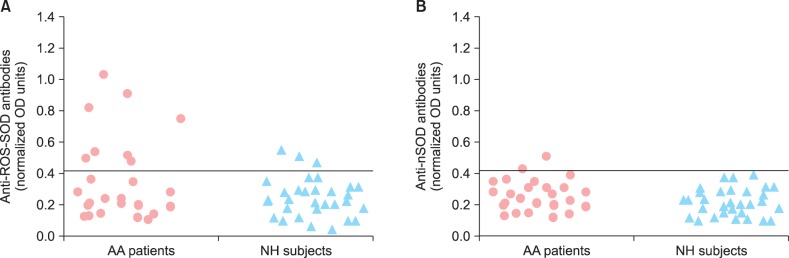
Direct binding of alopecia areata antibodies to reactive oxygen species-modified superoxide dismutase (ROS-SOD). (A) Levels of circulating antibodies in alopecia areata (AA) patients, binding to ROS-SOD and native SOD (nSOD). Anti-ROS-SOD antibodies versus anti-nSOD antibodies, p<0.01 in AA patients. (B) Levels of circulating antibodies in normal human (NH) subjects binding to ROS-SOD and nSOD. Anti-ROS-SOD antibodies versus anti-nSOD antibodies, p>0.05 in NH subjects. Microtiter plates were individually coated with ROS-SOD (10 µg/ml) and nSOD (10 µg/ml). OD: optical density or absorbance.
For further confirmation of the role of ROS-SOD and its associated autoimmunity in the pathogenesis of AA, 12 AA patients' sera and 12 NH sera were randomly selected and IgG was purified by affinity chromatography using a Protein-A agarose column. The purified IgG was eluted in a single symmetrical peak (Fig. 2). The interaction of ROS-SOD with affinity-purified AA-IgG and NH-IgG was ascertained by direct binding ELISA. Strong binding to ROS-SOD was observed in a majority of AA-IgG samples as compared to NH-IgG (Fig. 3A). The average normalized absorbance at 405 nm (±SEM) of AA-IgG and NH-IgG binding to ROS-SOD was 0.57±0.06 and 0.33±0.10, respectively. In the case of nSOD, both AA-IgG and NH-IgG showed negligible binding (Fig. 3B). The average normalized absorbance at 405 nm (±SEM) of AA-IgG and NH-IgG binding to nSOD was 0.23±0.04 and 0.19±0.05, respectively. The data reveal striking differences in the recognition of ROS-SOD and nSOD by AA autoantibodies (p<0.001).
Fig. 2.
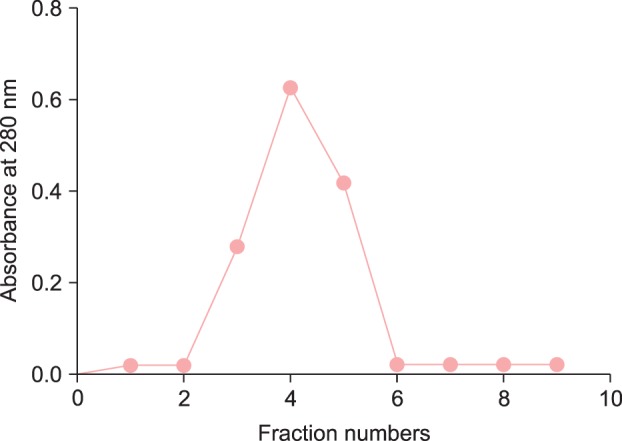
Affinity-purification of immunoglobulin G (IgG). Elution profile of IgG on a Protein A agarose affinity column.
Fig. 3.
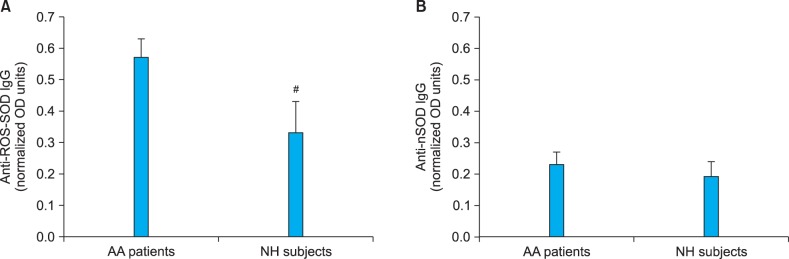
Binding of Protein A-purified alopecia areata (AA) immunoglobulin G (IgG) to reactive oxygen species-modified superoxide dismutase (ROS-SOD) and native superoxide dismutase. (A) Binding characteristic of AA-IgG and normal human (NH)-IgG to ROS-SOD. (B) Binding characteristics of AA-IgG and NH-IgG to native SOD (nSOD). IgG from 12 AA patients and 12 NH subjects were analyzed by direct-binding enzyme-linked immunosorbent assay. Microtiter plates were individually coated with ROS-SOD (10 µg/ml) and nSOD (10 µg/ml). For ROS-SOD: AA patients vs. #p<0.01; For nSOD: AA patients vs. NH subjects, p>0.05. OD: optical density or absorbance.
AA-IgGs directed to ROS-SOD were further evaluated by competitive binding assays. Table 1 shows the competitive inhibition of different AA-IgG samples by ROS-SOD and nSOD. All tested AA-IgGs showed strong recognition of ROS-SOD as compared to nSOD. NH-IgGs under identical experimental conditions showed negligible recognition with either of the inhibitors (Table 1). The data reveal marked differences in the recognition of native and ROS-modified SOD by AA IgG (p<0.001).
Table 1.
Competitive inhibition of AA-IgG and NH-IgG by ROS-SOD and nSOD
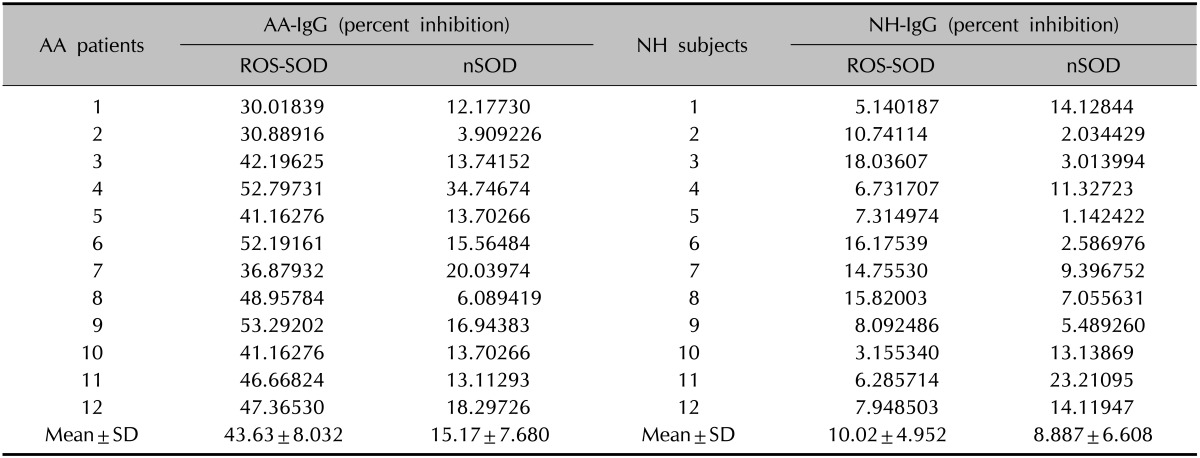
Microtitre plates were coated with ROS-SOD (10 µg/ml) and nSOD (10 µg/ml). Inhibitors concentration was 20 µg/ml. AA-IgG: ROS-SOD vs. nSOD, p<0.0001; NH-IgG: ROS-SOD vs. nSOD, p<>0.05. AA-IgG: alopecia areata-immunoglobin G, NH-IgG: normal humans-immunoglobin G, ROS-SOD: reactive oxygen species-modified superoxide dismutase, nSOD: native SOD, SD: standard deviation.
Levels of anti-ROS-SOD antibodies in patients with AAP and alopecia universalis
To further evaluate the significance of anti-ROS-SOD antibodies in AA patients, the relationship between the increase in the serum levels of anti-ROS-SOD antibodies in patients with AAP and AU was analyzed. Table 2 summarizes the direct binding and competitive binding ELISA results of nSOD and ROS-SOD with affinity-purified IgGs from AAP and AU patients. Direct binding ELISA results show that anti-ROS-SOD IgG was significantly higher in AU patients in comparison to AAP (p<0.05), whereas nSOD showed negligible binding with either of the IgG samples. Results from competitive ELISA reiterated results from direct binding ELISA. NH-IgG was used as negative control, which showed negligible binding to either of the antigens.
Table 2.
Immunological studies of affinity purified IgG from alopecia areata patients with alopecia universalis and alopecia areata patchy persistent binding to native and ROS-SOD
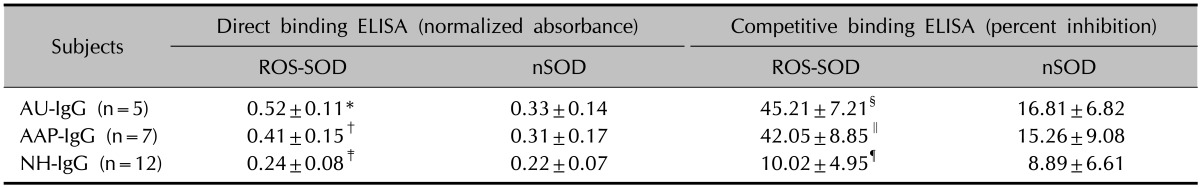
ELISA plates were coated with ROS-SOD (10 µg/ml) and nSOD (10 µg/ml). Inhibitors concentration was 20 µg/ml. *p<0.001 versus direct binding of AU-IgG with nSOD; †p<0.05 versus direct binding of AU-IgG with ROS-SOD; ‡p<0.0001 versus direct binding of AU-IgG with ROS-SOD; §p<0.0001 versus competitive inhibition of AU-IgG with nSOD; ∥ p<0.05 versus competitive inhibition of AU-IgG with ROS-SOD; ¶p<0.0001 versus competitive inhibition of AU-IgG with ROS-SOD. IgG: immunoglobulin G, ROS-SOD: reactive oxygen species-modified superoxide dismutase, ELISA: enzyme-linked immunosorbent assay, nSOD: native SOD dismutase, AU: alopecia universalis, AAP: alopecia areata patchy persistent, NH: normal human, n: number of samples tested.
SOD activity in patients with AA
To provide further support to the proposed hypothesis and assess the contribution of oxidative-damaged SOD protein in AA, the anti-oxidant function of SOD was analyzed in the serum. As shown in Fig. 4, the SOD activity in AA patient sera was significantly decreased as compared to that in sera from healthy controls (p<0.05).
Fig. 4.
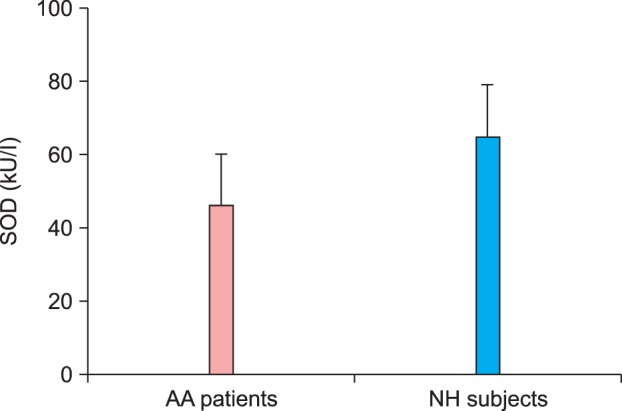
Disease-related decrease in superoxide dismutase (SOD) activity in patients with alopecia areata (AA). Serum levels of SOD activity in AA patients (n=25) compared with normal human (NH) subjects (n=26). Each histogram represents the mean±standard error of the mean. SOD activity in AA patients vs. SOD activity in NH subjects, p<0.05.
DISCUSSION
AA is a disease resulting in non-scarring hair loss and ranges in severity from patchy loss of scalp hair (AAP) to loss of all scalp and body hair (AU)29. The exact etiopathogenesis of AA is not fully understood, although several factors, including autoimmune reactions and environmental conditions, have been implicated2,3,4,5,6,30. Achieving a better understanding of the etiopathogenesis may help in preventing treatment failure and determining new treatment strategies for AA. Many studies have recently proposed the role of oxidative stress in AA7,8,9,10,13,14,15,31. A study by Abdel Fattah et al.8 demonstrated lower enzymatic antioxidant activity and higher levels of free radicals in patients with AA, which clearly supports evidence for an association between AA and oxidative stress. In AA patients, several inconsistencies have also been reported with respect to disease duration and the pattern and extent of hair loss. With regards to disease duration, significantly higher antioxidant activity and lower oxidant levels were reported in patients with shorter disease duration, irrespective of age8. This may be attributed to the ability of the antioxidant system to counteract harmful effects of ROS in the case of short disease duration, thus maintaining low oxidant levels. With regards to the pattern of hair loss, the lowest anti-oxidant enzymatic activity and the highest oxidant levels were noted in patients with AAP, followed by patients with poly-AA (patients with three or more lesions of AA) and then patients with monoAA (patients with one or two lesions of AA), with statistically insignificant differences between groups in terms of pattern of hair loss and age of patients8. Therefore, changes in oxidative stress parameters cannot be simply attributed to age and seem to be instead associated with disease severity. Regarding the extent of AA, a significant negative correlation was reported previously between anti-oxidant enzymatic activity and severity of AA, in addition to a positive correlation between oxidant levels and disease activity8.
In comparison to other reports9,16,31, these findings correlate with those of Naziroglu and Kokcam31 and Koca et al.15, who demonstrated lower enzymatic antioxidant activity in the blood of patients with AA as compared to healthy controls. However, these findings disagreed with those of Akar et al.9 who demonstrated higher antioxidant enzymatic activity in the scalp of patients with AA as compared to controls, suggesting that the antioxidant defense system is not impaired in AA. These discrepancies may be attributed to inconsistencies among patients studied, such as disease duration and the pattern or severity of hair loss. Higher enzymatic antioxidant activity in patients with AA of shorter duration or lesser severity may be sufficient to act as a defense against excess production of ROS. With prolonged duration, disease progression, or with increased severity of the disease, the protective mechanism of enzymatic antioxidation may become inadequate, with lower activity resulting in increased production of oxidants and oxidative by-products overwhelming the enzyme.
SOD is a major antioxidant enzyme and is considered to be a first line of defense against ROS in various pathological conditions32,33. It is also well documented that SOD is vulnerable to ROS, and ROS-modified SOD was found to be an antigenic stimulus, inducing antibodies in various autoimmune diseases20. SOD dysfunction has also been reported in patients with AA7,8,9,15,31. In this context, it is expected that the characterization of oxidative status of SOD would provide useful information regarding the redox state of the human body and also regarding alterations in the conformation and function of SOD, which may result in modification of its biological properties. Therefore, it is thought that SOD is continuously exposed to oxidative stress, causing alterations to its conformation and function, which may result in generation of autoimmunity against ROS-SOD in AA.
In the present study, the binding characteristics of naturally occurring AA antibodies to ROS-damaged SOD were investigated. Sera from 26 AA patients and 30 NH subjects were collected. Of these, 32% AA sera showed preferentially high binding to ROS-SOD as compared to its native analogue. No appreciable binding was observed in samples from the normal subjects. For better insight into the interaction of AA antibodies with native and ROS-modified SOD, IgGs from AA patients were purified by a Protein A affinity column. Direct-binding ELISA showed that IgG from AA patients recognized ROS-SOD to a greater extent as compared to its native form, nSOD. Results from competitive ELISA with AA-IgG reiterated the results from direct-binding ELISA, where the ROS-modified SOD was found to be an effective inhibitor, showing substantial difference in the recognition of modified SOD over nSOD. Thus, by employing various immunological techniques, the data clearly demonstrated a substantial increase in the recognition of ROS-modified SOD over nSOD by circulating AA autoantibodies. Furthermore, the level of anti-ROS-SOD antibodies was found to be higher in AU patients as compared to AAP patients. This clearly suggests that anti-ROS-SOD antibodies have a positive association with disease activity. The enhanced anti-ROS-SOD antibodies observed in AA patients in this study motivated further evaluation of the anti-oxidant activity of SOD in these subjects. The data showed that SOD activity was significantly lower in the AA patients as compared with the healthy controls. This enzymatic dysfunction of SOD in AA patients strongly supports the hypothesis proposed for this work.
These findings suggest that in AA patients with increased oxidative stress, the oxidative modification of blood proteins is greatly enhanced. As SOD is the most potent antioxidant blood enzyme, it is a likely candidate for extensive damage and this may be responsible for pathological conditions associated with AA. Results from this study suggest that SOD is continuously exposed to oxidative stress, so much so that alterations in its biological properties could result from conformational changes. This study demonstrates that ROS-induced SOD damage occurs in AA patients, which might play an active role in disease progression. We propose that, in addition to serum concentration of SOD, structural changes to SOD molecules may not only affect its protective function but also convert it to a pro-oxidant in AA patients. This novel study concludes that after modification with ROS, SOD presents unique epitopes for the production of AA antibodies. The present study also provides evidence to suggest that in AA patients, the oxidation of SOD leads to alterations in its conformation as well as its biological properties.
ACKNOWLEDGMENT
This work was supported by funds from College of Medicine, Qassim University.
References
- 1.Delamere FM, Sladden MM, Dobbins HM, Leonardi-Bee J. Interventions for alopecia areata. Cochrane Database Syst Rev. 2008;(2):CD004413. doi: 10.1002/14651858.CD004413.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Alkhalifah A. Alopecia areata update. Dermatol Clin. 2013;31:93–108. doi: 10.1016/j.det.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Bertolini M, Gilhar A, Paus R. Alopecia areata as a model for T cell-dependent autoimmune diseases. Exp Dermatol. 2012;21:477–479. doi: 10.1111/j.1600-0625.2011.01427.x. [DOI] [PubMed] [Google Scholar]
- 4.Seetharam KA. Alopecia areata: an update. Indian J Dermatol Venereol Leprol. 2013;79:563–575. doi: 10.4103/0378-6323.116725. [DOI] [PubMed] [Google Scholar]
- 5.Thomas EA, Kadyan RS. Alopecia areata and autoimmunity: a clinical study. Indian J Dermatol. 2008;53:70–74. doi: 10.4103/0019-5154.41650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hordinsky M, Ericson M. Autoimmunity: alopecia areata. J Investig Dermatol Symp Proc. 2004;9:73–78. doi: 10.1111/j.1087-0024.2004.00835.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim SW, Kim BJ, Youn SW, Park KC, Huh CH. Evaluation of free oxygen radical and antioxidant capacity in alopecia areata. J Dermatol. 2010;37:762–764. doi: 10.1111/j.1346-8138.2010.00868.x. [DOI] [PubMed] [Google Scholar]
- 8.Abdel Fattah NS, Ebrahim AA, El Okda ES. Lipid peroxidation/antioxidant activity in patients with alopecia areata. J Eur Acad Dermatol Venereol. 2011;25:403–408. doi: 10.1111/j.1468-3083.2010.03799.x. [DOI] [PubMed] [Google Scholar]
- 9.Akar A, Arca E, Erbil H, Akay C, Sayal A, Gür AR. Antioxidant enzymes and lipid peroxidation in the scalp of patients with alopecia areata. J Dermatol Sci. 2002;29:85–90. doi: 10.1016/s0923-1811(02)00015-4. [DOI] [PubMed] [Google Scholar]
- 10.Bilgili SG, Ozkol H, Karadag AS, Ozkol HU, Seker A, Calka O, et al. Serum paraoxonase activity and oxidative status in subjects with alopecia areata. Cutan Ocul Toxicol. 2013;32:290–293. doi: 10.3109/15569527.2013.781616. [DOI] [PubMed] [Google Scholar]
- 11.Al-Shobaili HA, Alzolibani AA, Al Robaee AA, Meki AR, Rasheed Z. Biochemical markers of oxidative and nitrosative stress in acne vulgaris: correlation with disease activity. J Clin Lab Anal. 2013;27:45–52. doi: 10.1002/jcla.21560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasheed Z, Al-Shobaili HA, Al Robaee AA, Alzolibani AA, Wadi WI, Khan MI, et al. Preferential recognition of peroxynitrite damaged thymidine-monophosphate by anti-DNA autoantibodies in systemic lupus erythematosus. Nucleosides Nucleotides Nucleic Acids. 2012;31:736–751. doi: 10.1080/15257770.2012.724135. [DOI] [PubMed] [Google Scholar]
- 13.Bakry OA, Elshazly RM, Shoeib MA, Gooda A. Oxidative stress in alopecia areata: a case-control study. Am J Clin Dermatol. 2014;15:57–64. doi: 10.1007/s40257-013-0036-6. [DOI] [PubMed] [Google Scholar]
- 14.Namazi MR. Nitric oxide donors as potential additions to anti-alopecia areata armamentarium. Inflamm Res. 2003;52:227–229. doi: 10.1007/s00011-003-1175-7. [DOI] [PubMed] [Google Scholar]
- 15.Koca R, Armutcu F, Altinyazar C, Gürel A. Evaluation of lipid peroxidation, oxidant/antioxidant status, and serum nitric oxide levels in alopecia areata. Med Sci Monit. 2005;11:CR296–CR299. [PubMed] [Google Scholar]
- 16.Al-Shobaili HA, Al Robaee AA, Alzolibani AA, Rasheed Z. Antibodies against 4-hydroxy-2-nonenal modified epitopes recognized chromatin and its oxidized forms: role of chromatin, oxidized forms of chromatin and 4-hydroxy-2-nonenal modified epitopes in the etiopathogenesis of SLE. Dis Markers. 2012;33:19–34. doi: 10.3233/DMA-2012-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasheed Z, Ahmad R, Rasheed N, Ali R. Reactive oxygen species damaged human serum albumin in patients with hepatocellular carcinoma. J Exp Clin Cancer Res. 2007;26:395–404. [PubMed] [Google Scholar]
- 18.Rasheed Z, Ahmad R, Rasheed N, Ali R. Enhanced recognition of reactive oxygen species damaged human serum albumin by circulating systemic lupus erythematosus autoantibodies. Autoimmunity. 2007;40:512–520. doi: 10.1080/08916930701574331. [DOI] [PubMed] [Google Scholar]
- 19.Peixoto EB, Pessoa BS, Biswas SK, Lopes de Faria JB. Antioxidant SOD mimetic prevents NADPH oxidase-induced oxidative stress and renal damage in the early stage of experimental diabetes and hypertension. Am J Nephrol. 2009;29:309–318. doi: 10.1159/000163767. [DOI] [PubMed] [Google Scholar]
- 20.Al-Shobaili HA, Rasheed Z. Immunological studies of oxidized superoxide dismutase in patients with systemic lupus erythematosus. Correlation with disease induction and progression. Saudi Med J. 2012;33:1177–1184. [PubMed] [Google Scholar]
- 21.Olsen E, Hordinsky M, McDonald-Hull S, Price V, Roberts J, Shapiro J, et al. Alopecia areata investigational assessment guidelines. National Alopecia Areata Foundation. J Am Acad Dermatol. 1999;40:242–246. doi: 10.1016/s0190-9622(99)70195-7. [DOI] [PubMed] [Google Scholar]
- 22.Rasheed Z. Hydroxyl radical damaged immunoglobulin G in patients with rheumatoid arthritis: biochemical and immunological studies. Clin Biochem. 2008;41:663–669. doi: 10.1016/j.clinbiochem.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Goding JW. Use of staphylococcal protein A as an immunological reagent. J Immunol Methods. 1978;20:241–253. doi: 10.1016/0022-1759(78)90259-4. [DOI] [PubMed] [Google Scholar]
- 24.Alzolibani AA, Al Robaee AA, Al-Shobaili HA, Rasheed Z. 4-Hydroxy-2-nonenal modified histone-H2A: a possible antigenic stimulus for systemic lupus erythematosus autoantibodies. Cell Immunol. 2013;284:154–162. doi: 10.1016/j.cellimm.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Rasheed Z, Al-Shobaili HA, Alzolibani AA, Ismail Khan M, Tariq Ayub M, Khan MI, et al. Immunological functions of oxidized human immunoglobulin G in type 1diabetes mellitus: its potential role in diabetic smokers as a biomarker of elevated oxidative stress. Dis Markers. 2011;31:47–54. doi: 10.3233/DMA-2011-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Shobaili HA, Al Robaee AA, Alzolibani AA, Rasheed Z. Immunological studies of reactive oxygen species damaged catalase in patients with systemic lupus erythematosus: correlation with disease activity index. Immunol Invest. 2013;42:191–203. doi: 10.3109/08820139.2012.751396. [DOI] [PubMed] [Google Scholar]
- 27.Rasheed Z, Ali R. Reactive oxygen species damaged human serum albumin in patients with type 1 diabetes mellitus: biochemical and immunological studies. Life Sci. 2006;79:2320–2328. doi: 10.1016/j.lfs.2006.07.041. [DOI] [PubMed] [Google Scholar]
- 28.Rasheed Z, Ahmad R, Ali R. Structure and immunological function of oxidised albumin in lung cancer: its potential role as a biomarker of elevated oxidative stress. Br J Biomed Sci. 2009;66:67–73. doi: 10.1080/09674845.2009.11730247. [DOI] [PubMed] [Google Scholar]
- 29.Kutner A, Friedman A. Hair loss in the dermatology office: an update on alopecia areata. J Drugs Dermatol. 2013;12:588–593. [PubMed] [Google Scholar]
- 30.Tosti A, Duque-Estrada B. Treatment strategies for alopecia. Expert Opin Pharmacother. 2009;10:1017–1026. doi: 10.1517/14656560902876368. [DOI] [PubMed] [Google Scholar]
- 31.Naziroglu M, Kokcam I. Antioxidants and lipid peroxidation status in the blood of patients with alopecia. Cell Biochem Funct. 2000;18:169–173. doi: 10.1002/1099-0844(200009)18:3<169::AID-CBF870>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 32.Batinić-Haberle I, Rebouças JS, Spasojević I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid Redox Signal. 2010;13:877–918. doi: 10.1089/ars.2009.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson F, Giulivi C. Superoxide dismutases and their impact upon human health. Mol Aspects Med. 2005;26:340–352. doi: 10.1016/j.mam.2005.07.006. [DOI] [PubMed] [Google Scholar]


