Abstract
In eukaryotes, the switch between alternative developmental pathways is mainly attributed to a switch in transcriptional programs. A major mode in this switch is the transition between histone deacetylation and acetylation. In budding yeast, early meiosis-specific genes (EMGs) are repressed in the mitotic cell cycle by active deacetylation of their histones. Transcriptional activation of these genes in response to the meiotic signals (i.e., glucose and nitrogen depletion) requires histone acetylation. Here we follow how this regulated switch is accomplished, demonstrating the existence of two parallel mechanisms. (i) We demonstrate that depletion of glucose and nitrogen leads to a transient replacement of the histone deacetylase (HDAC) complex on the promoters of EMG by the transcriptional activator Ime1. The occupancy by either component occurs independently of the presence or absence of the other. Removal of the HDAC complex depends on the protein kinase Rim15, whose activity in the presence of nutrients is inhibited by protein kinase A phosphorylation. (ii) In the absence of glucose, HDAC loses its ability to repress transcription, even if this repression complex is directly bound to a promoter. We show that this relief of repression depends on Ime1, as well as on the kinase activity of Rim11, a glycogen synthase kinase 3β homolog that phosphorylates Ime1. We further show that the glucose signal is transmitted through Rim11. In cells expressing the constitutive active rim11-3SA allele, HDAC repression in glucose medium is impaired.
Repression and regulated expression of genes by histone deacetylases (HDACs) and histone acetyltransferases (HATs) play pivotal roles in the well-being of all eukaryotic organisms. These processes are involved in the regulation of transcription, cell cycle progression, differentiation, and the development of specific tumors, as well as in controlling the life span of organisms (8, 10). Repression of specific genes is mediated by the recruitment of the HDAC complex to promoters following association with specific DNA-binding proteins (28). In addition, recruitment of HDACs to many promoters via a nontargeted, unknown mechanism leads to global deacetylation (44). A vast amount of information is emerging on repression and activation by the HDAC and HAT enzymes, but little is known about the mechanism(s) regulating the switch between deacetylation and acetylation.
In Saccharomyces cerevisiae, early meiosis-specific genes (EMGs) are repressed in a medium promoting vegetative growth with either glucose or acetate as the sole carbon source (16). Under meiotic conditions (i.e., upon nitrogen depletion and the presence of a nonfermentable carbon source such as acetate), these genes are transiently transcribed (16). Transcriptional repression, as well as activation, is mediated by a short element, URS1, present in the promoters of all EMGs (3, 42). In addition, these genes carry a UAS element that is required for high-level expression (3, 42). The URS1 element is present and active in additional genes, for instance INO1, whose functions are not restricted to meiosis. Ume6 is a Zn cluster DNA-binding protein that binds the URS1 element and is required for both transcriptional repression and activation of these genes (1, 40). In SD medium (synthetic glucose), these genes are not transcribed because Ume6 recruits the HDAC and the chromatin-remodeling Isw2 complexes (9, 14, 15). The HDAC function is present in a large protein complex that includes the HDAC Rpd3 and Sin3, which physically associates with both Ume6 and Rpd3 (14, 19). Repression is accomplished because histone deacetylation prevents the binding of TATA-binding protein to the TATA element (37).
Transcription of EMGs depends on the transcriptional activator Ime1 and the HAT Gcn5 (for review, see reference 16). Two-hybrid assays reveal an interaction between Ime1 and Ume6, suggesting that Ume6 recruits the repression as well as activation complexes to the promoters of EMGs (34). Multiple mechanisms ensure that transcriptional activation of EMGs will take place only under meiotic conditions. (i) In a medium promoting vegetative growth with glucose as the sole carbon source (SD), IME1 is not transcribed, while low levels of IME1 mRNA are observed in medium with acetate as the sole carbon source (SA medium). Upon nitrogen depletion in the presence of acetate as the sole carbon source (sporulation medium [SPM]), the transcription of IME1 is transiently induced, but only in MATa/MATα diploids (17). (ii) Efficient translation of IME1 mRNA requires nitrogen depletion (35). (iii) Under vegetative growth conditions, phosphorylation on Ime1 by the Cdc28/Cln cyclin-dependent kinase (CDK) sequesters it to the cytoplasm, whereas upon nitrogen depletion, when the G1 cyclins Cln1 to −3 are absent, Ime1 accumulates in the nuclei (5). (iv) In the absence of glucose, Ime1 phosphorylation on Tyr-359 by Rim11 promotes the two-hybrid interaction of Ime1 with Ume6 and initiation of meiosis (I. Rubin-Bejerano, S. Sagee, O. Friedman, L. Pnueli, and Y. Kassir, unpublished data). Rim11 is homologous to the glycogen synthase kinase 3β (GSK3-β) family, which is conserved in all eukaryotes (31). Currently, there are no reports on how and why, under meiotic conditions, the Sin3/Rpd3 complex does not interfere with transcriptional activation of EMGs. Lamb and Mitchell (23) reported that the Ume6/Sin3/Rpd3 complex is formed under meiotic conditions. However, this conclusion was based on a single time point, a time when cells had already initiated the transcription of EMGs. Thus, this complex may contribute to the repression rather than the activation of EMGs. Sin3 and Rpd3 are also required for the transcription of middle meiosis-specific genes (13), suggesting that these proteins must be present at middle meiotic times.
This report focuses on how transcriptional repression by histone deacetylation is relieved when cells are transferred to meiotic conditions. We show the existence of two distinct mechanisms that contribute to the relief of repression. (i) In SA medium, depending on Ime1, Rim11, and Ume6, repression by a DNA-bound Rpd3 is relieved. (ii) In SPM, a transient switch in the complexes formed on the URS1 elements takes place: Sin3 and Rpd3 are transiently removed from Ume6 and are replaced by Ime1. Removal of Sin3/Rpd3 depends on the activity of the protein kinase Rim15, whose activity in the presence of glucose and nitrogen is inhibited by protein kinase A (PKA) phosphorylation. We further show that phosphorylation on Tyr-359 in Ime1 by Rim11 is not required for tethering Ime1 by Ume6 to these promoters.
MATERIALS AND METHODS
Strains and plasmids.
The relevant genotypes of the strains used in this study are described in Table 1. A detailed description of how these strains were constructed is available upon request.
TABLE 1.
List of strains and relevant genotypes
| Strain | Relevant genotype | Description |
|---|---|---|
| Y422 | MATa/MATα ura3-52/ura3-52 trp1Δ/trp1Δ leu2-3,112/leu2-3,112 ade2-1/ade2-R8 his4-519/HIS4 his6-1/HIS6 can1-1/CAN1 | |
| Y449 | ime1Δ::hisG/ime1Δ::hisG | Isogenic to Y422 |
| Y1049 | gal80Δ::hisG/gal80Δ::hisG | Isogenic to Y422 |
| Y1064 | MATaura3-52 leu2-3,112 trp1Δ his3Δ::hisG ade2-1 gal80::hisG gal4::hisG | Isogenic to the MATa parent of Y422 |
| Y1065 | MATα ura3-52 trp1Δ leu2-3,112 his3::hisG ade2-R8 gal80::hisG gal4::hisG | Isogenic to the MATα parent of Y422 |
| Y1076 | ime1::hisG | Isogenic to Y1065 |
| Y1089 | rim11Δ::LEU2 | Isogenic to Y1065 |
| Y1256 | rim11Δ::LEU2 | Isogenic to Y1064 |
| Y1326 | ume6Δ::URA3 | Isogenic to Y1064 |
| Y1328 | ume6Δ::URA3 | Isogenic to Y1065 |
| Y1402 | leu2-3,112::LEU2-GAL1uas-HIS4uas-his4-lacZ ume6Δ::hisG ime1Δ::URA3 | Isogenic to Y1064 |
| Y1456 | rim::LEU2 ura3-52::URA3-rim113SA | Isogenic to Y1064 |
| Y1457 | rim15::loxP/rim15::loxP | Isogenic to Y422 |
| Y1458 | ura3-52::URA3- GAL1uas-HIS4uas-his4-lacZ rim11::LEU2 | Isogenic to Y1065 |
| Y23566 | MATa/MATα ume6::kanMX4/UME6 | EUROSCARFa |
| Y33566 | ume6::kanMX4/ume6::kanMX4 | EUROSCARF, isogenic to Y23566 |
European Saccharomyces cerevisiae Archive for Functional Analysis, University of Frankfurt, Frankfurt, Germany.
The following plasmids were used. YEp1791 carries IME2(−978 to +1586) on a LEU2 2μm vector. YEp1931 carries pIME1-gal4(dbd)-ime1(270-360) on a TRP1 2μm vector. YEp2050 carries pADH1-IME1 on a HIS3 2μm vector. YEp2149 carries pADH1-gal4(dbd) on a TRP1 2μm vector. YIp2218 carries GAL1uas-HIS4uas-his4-lacZ on a LEU2 vector. YIp2240 carries pSIN3-SIN3-3xHA on a LEU2 vector. YEp2543 carries pCDC28-gal4(dbd)-SIN3 on a TRP1 2μm vector. YEp2546 carries pRPD3-RPD3-13xmyc on a URA3 2μm vector. YEp2593 carries pADH1-gal4(dbd)-RPD3 on a TRP1 2μm vector. YCp2665 carries pGAL1-GST-rim15S709AS1094AS1416AS1463AS1621A on an ARS1 CEN4 LEU2 vector (30, 32). This allele was designated rim15-5SA. YEp2713 carries pIME1-gal4(dbd)-ime1(270-360)Y359FS306A on a TRP1 2μm vector. YEp2780 carries pADH1-gal4(dbd)-ime1(270-360) on a TRP1 2μm vector. YIp2801 carries GAL1uas-HIS4uas-his4-lacZ on a URA3 vector. A detailed description of how these plasmids were constructed is available upon request.
Growth conditions.
SD medium (synthetic dextrose), SA medium (previously designated PSP2, minimal acetate growth medium), and SPM have been described previously (18, 36). Meiosis was induced by shifting logarithmic-phase cells grown in SA medium to SPM as described previously (18).
Antibodies.
Mouse monoclonal antibodies directed against Gal4(dbd) (the DNA binding domain of Gal4, amino acids 1 to 147) (RK5C1), and rabbit polyclonal antibodies directed against the PSTAIRE epitope were purchased from Santa Cruz Biotechnology, Inc. Mouse monoclonal antibodies directed against c-myc (Ab-1, clone 9E11) were purchased from NeoMarkers. Mouse monoclonal antibodies directed against hemagglutinin (HA) (12CA5) were purchased from Boehringer Mannheim. Rabbit polyclonal antibodies against acetylated histone H3 (AHP412) were purchased from Serotec.
Primers for ChIP PCR.
The following primer pairs were used for chromatin immunoprecipitation (ChIP) PCR: GAL1F (GGAAATGTAAAGAGCCCC) and GAL1R (CGCATTATCATCCTATGGT), HOP1-R-106 (CATAGGAAACTGCAGTCA) and HOP1-260 (GCGCCAGGTTATCAAAAC), IME2-446R (CCAGCACTTGTCTGTGGCTT) and IME2-663 (CTGAGTGGCACAGCTTTTCC), INO1-254 (GGGGTTGGATGCGGAATC) and INO1-R-20 (GGAACCCGACAACAGAAC), and ARS305-S (TTTCAGAGCCTTCTTTGGAG) and ARS305-AS (CAAACTCCGTTTTTAGCCCC).
Oligonucleotides.
The oligonucleotides used in this study were HOP1F (ATGTCTAATAAACAACTAGT) and HOP1R (CTACCAGTTACTTTTCAAAG).
ChIP.
Fifty milliliters of cells at 107 cells/ml was washed twice with phosphate-buffered saline (PBS) and resuspended in 1 ml of 10 mM dimethyl adipimidate (DMA)-0.25% dimethyl sulfoxide (DMSO) in PBS for 45 min at room temperature. Cells were then washed twice with PBS and resuspended in 1 ml of PBS containing 1% formaldehyde. Cells were incubated for 15 min at room temperature. Cross-linking was stopped by the addition of glycine to a final concentration of 140 mM. We then carried out ChIP essentially as described previously (39). For histone-DNA cross-linking, formaldehyde was added directly to the 50 ml of cells to a final concentration of 1% for 15 min at room temperature. We then proceeded as described above.
In the experiments described, cells carried more than one tagged protein, allowing the concomitant ChIP analysis of the DNA/protein complexes. Cell lysates were split in two prior to the addition of antibodies. Each PCR was repeated several times. In cases in which the amount of PCR product was too high (nonlogarithmic reaction), all the samples in that experiment were diluted. This protocol promoted reliable comparisons between samples in a single experiment. In order to increase the efficiency in detecting the proteins bound to IME2 promoter, IME2 was placed on a 2μm vector. Similar results were obtained for the genomic IME2 gene (data not shown).
Northern analysis.
RNA was extracted from 3 ml of cells at a titer of 1 × 107 cells/ml, using an RNeasy Mini kit from QIAGEN. Northern analysis was performed according to standard protocols. DNA to make probes was isolated either from YEp1791 (IME2) or by PCR, using oligonucleotides HOP1F and HOP1R and yeast genomic DNA as a template.
Repression assay.
Proteins were extracted from at least three independent transformants and assayed for β-galactosidase activity as described previously (33). For reliable comparisons between strains, the reporter gene UASGAL1-UASHIS4-his4-lacZ was integrated into the LEU2 or URA3 of a parental haploid, which was then mated to various strains to create the isogenic heterozygote and homozygote diploid strains.
RESULTS
Under meiotic conditions, Sin3 and Rpd3 are transiently removed from the promoters of EMGs.
Three simple, nonexclusive mechanisms might be responsible for the relief of Rpd3 repression of EMGs in cells transferred to meiotic conditions: (i) lack of expression and/or induced degradation of Rpd3 and/or Sin3, (ii) sequestering of Rpd3 and/or Sin3 from the DNA and/or the nucleus, and (iii) masking or inhibiting of the HDAC activity of Rpd3. In this report, the validity of these three models was tested.
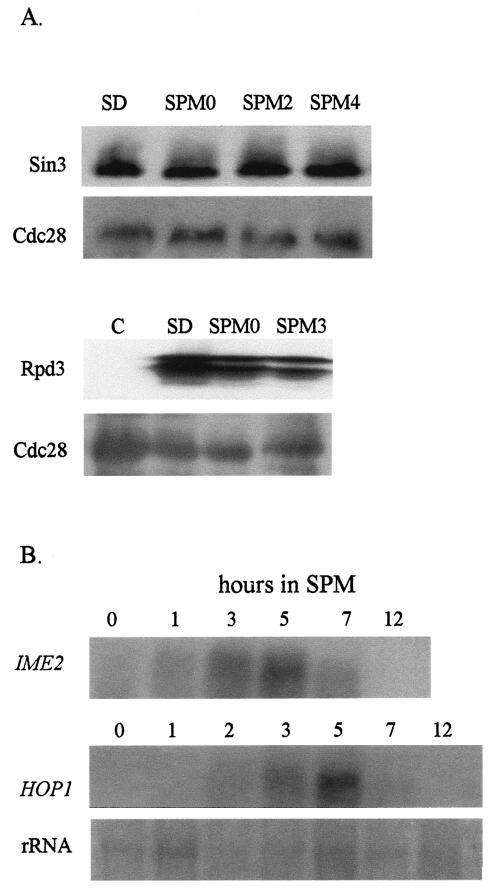
By Western analysis, we determined the availability of Sin3 and Rpd3 in cells incubated under meiotic conditions (SPM) in comparison to cells incubated in medium promoting vegetative growth with either glucose (SD medium) or acetate (SA medium) as the sole carbon source. In order to detect Rpd3 and Sin3, these proteins, expressed from their own promoters, were tagged with 13 copies of the myc epitope, and three copies of the HA epitope, respectively. In addition, for control, the level of the constitutively expressed Cdc28 protein (11) was determined by using antibodies directed against the PSTAIRE epitope. The level of Sin3 and Rpd3 was apparently not affected by glucose or nitrogen depletion, the two meiotic signals operating in MATa/MATα diploid cells (Fig. 1A). Figure 1B shows that the transcription of the early meiosis-specific genes IME2 and HOP1 is transiently induced following 1 and 2 h of incubation in SPM medium, respectively, as previously reported (for review, see reference 16 and the references therein). Premeiotic DNA replication took place between 5 and 12 h in SPM, and at 24 h, 81.6% asci were observed (data not shown). We conclude, therefore, that lack of Sin3 and/or Rpd3 proteins in meiotic cultures cannot explain the relief of Rpd3 repression and transcriptional induction.
FIG. 1.
Sin3 and Rpd3 are available throughout the meiotic cycle. (A) Western analysis of Sin3-3xHA, Rpd3-13xmyc, and Cdc28. (B) Northern (RNA) analysis of HOP1 and IME2. RNA and proteins were extracted from cells grown in SD or SA medium and from cells transferred from SA medium to SPM for the indicated hours. The strain used is Y422 carrying pSIN3-SIN3-3xHA integrated at the LEU2 loci (plasmid YIp2240) and pRPD3-RPD3-13xmyc on a URA3 2μm vector (YEp2546). Immunoblot analysis was performed with antibodies directed against the HA, myc, and the PSTAIRE (for Cdc28) epitopes. For Northern analysis, we used a probe from either the HOP1 or IME2 gene. C, control (a strain without tagged proteins).
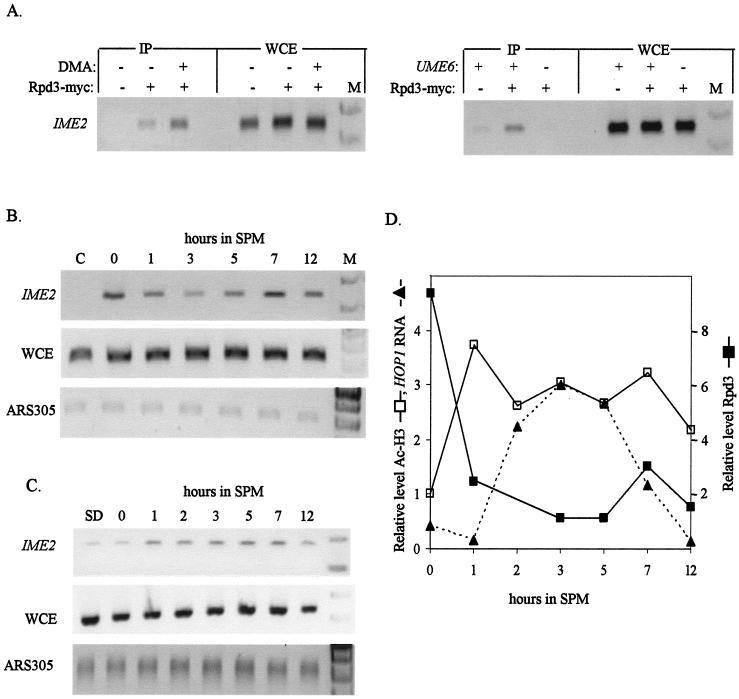
We used the ChIP assay to detect the presence of Rpd3 on the URS1 elements in diploid cells expressing Rpd3-13xmyc. In cells grown in SD medium, immunoprecipitation with antibodies directed against the myc epitope led to specific enrichment of the URS1 element carrying gene IME2 (Fig. 2A). A nonspecific DNA, that of ARS305, gave no PCR product (data not shown). However, low levels of PCR products were obtained when a 10-fold increase in the amount of DNA was used (Fig. 2B) (data not shown). Because Rpd3 is not directly bound to DNA but through Sin3 and Ume6, proteins and DNA were cross-linked with DMA in addition to formaldehyde. As reported (22), the use of DMA increased the efficiency of Rpd3-DNA cross-linking (Fig. 2A). Therefore, in all subsequent experiments, this protein-protein cross-linker was used. Figure 2A also shows that the binding of Rpd3 to the DNA depends on Ume6: in diploid cells with UME6 deleted, the PCR product was not obtained, confirming previous, indirect reports that Rpd3 is recruited by Ume6 to promoters (14).
FIG. 2.
Under meiotic conditions, Rpd3 is transiently removed from the promoters of EMGs. Samples for ChIP (Rpd3-13xmyc in panels A and B and acetylated histone H3 in panel C) were taken from cells incubated in SPM medium for the indicated number of hours or from SD-grown cells (panel A and as indicated). The PCRs amplified IME2 or the nonspecific ARS305 loci. Detection of ARS305 DNA required a 10-fold increase in the level of the input DNA. The strains used are wild type (Y422) or ume6(1-158) (Y1326 × Y1328), carrying on 2μm vectors IME2 (YEp1791) and RPD3-13xmyc (YEp2546). Antibodies directed against myc (A and B) or acetylated histone H3 (C) were used. The relative levels of Rpd3 occupying the promoter (solid squares), acetylated histone H3 (open squares), and HOP1 RNA (solid triangles and dashed line) calculated from the ChIP analysis in panels B and C and the Northern analysis in Fig. 1B are shown in panel D. C, control (a strain without tagged proteins); WCE, IME2 PCR product of whole-cell extract; M, marker.
ChIP analysis revealed that when diploid cells were transferred to meiotic conditions (SPM), Rpd3 was transiently removed from the promoter of IME2 (Fig. 2B). Figure 2D shows the relative level of PCR product in comparison to the nonspecific, ARS305 DNA, as calculated from Fig. 2B. The figure shows that following 1, 3, and 5 h of incubation in SPM, the level of the PCR product is reduced, and then at 7 and 12 h in SPM the level is increased. Similar results were observed for HOP1 and INO1, two additional genes carrying the URS1 element (data not shown) (see Fig. 4).
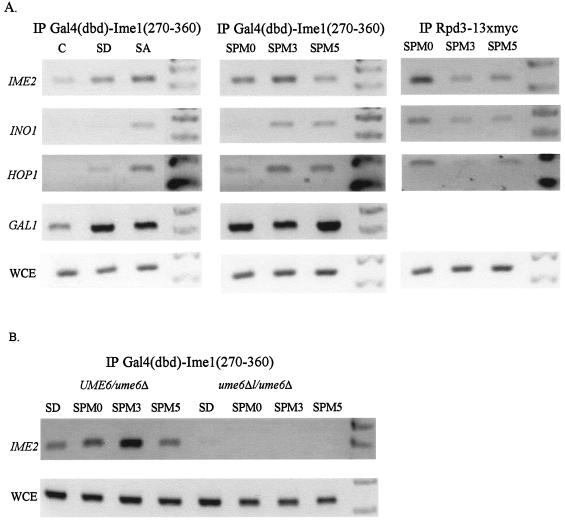
FIG. 4.
Ime1 is recruited by Ume6 to promoters of EMGs. Samples for ChIP analysis were taken from cells grown in SD (glucose) or SA (acetate) medium or incubated in sporulation medium for the indicated hours (SPM0 to SPM5). The following strains were used: a wild-type diploid, Y422 (A), and a ume6Δ/ume6Δ null allele diploid, Y33566, and its isogenic ume6Δ/UME6 strain, Y23566 (B). These strains carried on 2μm vectors the following chimeric genes: IME2 (YEp1791), RPD3-13xmyc (YEp2546), and pIME1-gal4(dbd)-ime1(270-360) (YEp1931). Antibodies directed against myc or Gal4(dbd) were used for IP, and the PCRs amplified the indicated genes. C, control (a strain without tagged proteins); WCE, whole-cell extract.
In order to determine whether the transient decrease in Rpd3 occupancy at the promoters of EMGs is correlated with increased acetylation of histone H3, samples were taken for immunoprecipitation with antibodies directed against acetylated histone H3. Figure 2C shows that under meiotic conditions there is a transient increase in histone acetylation. The relative level of PCR product in comparison to the nonspecific ARS305 DNA was calculated from Fig. 2C and is illustrated in Fig. 2D. Figure 2D shows that as expected (21), the transient removal of Rpd3 is correlated with a transient increase in histone acetylation. Furthermore, at later meiotic times, the reloading of Rpd3 to the DNA is correlated with a decline in histone acetylation (Fig. 2D). In this experiment, samples were also taken to measure the level of EMG RNA by Northern analysis (Fig. 1). The relative level of HOP1 mRNA was measured from Fig. 1B and is drawn in Fig. 2D. The transient removal of Rpd3 from the promoters of EMGs and the increase in histone acetylation are correlated with a transient induction in the transcription of these genes (Fig. 1B, 2, and 4).
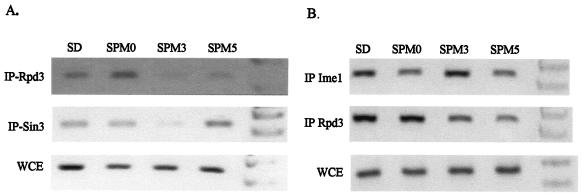
Sin3 mediates the recruitment of Rpd3 to Ume6. Therefore, the removal of Rpd3 from the URS1 elements can be attributed to the disruption in the physical association between either Sin3 and Rpd3 or Sin3 and Ume6. We determined, therefore, the binding of Sin3 to the URS1 elements throughout the meiotic cycle. Proteins for ChIP analysis were extracted from diploid cells expressing Sin3 tagged with the Gal4(dbd). In cells grown in SD medium, immunoprecipitation with antibodies directed against the Gal4(dbd) epitope led to specific enrichment of the URS1 carrying gene IME2 (Fig. 3). A nonspecific DNA, that of ARS305, gave no PCR product (data not shown). A shift of cells grown in SA medium to SPM led to a transient removal of Sin3 from the URS1 element of IME2 (Fig. 3 and see Fig. 5). At 3 h in SPM, the level of the PCR product was reduced, whereas at 5 h in SPM Sin3 was recruited again. These results demonstrate a similar behavior for Sin3 and Rpd3, suggesting that at early meiotic times the physical association between Sin3 and Ume6 is disrupted.
FIG. 3.
Under meiotic conditions, Sin3 is transiently removed from the promoters of EMGs. Samples for ChIP analysis [Gal4(dbd)-Sin3] were taken from cells incubated in SPM for the indicated times or from SD-grown cells. The PCRs amplified the IME2 gene. The strain used is Y422, carrying on 2μm vectors the following chimeric genes: IME2 (YEp1791) and pCDC28-gal4(dbd)-SIN3 (YEp2543). Antibodies directed against Gal4(dbd) were used. C, control (a strain without tagged proteins); WCE, whole-cell extract; M, marker.
FIG. 5.
Binding of Ime1 and that of Rpd3 to the URS1 elements are independent. Samples for ChIP analysis were taken from cells grown in SD medium (glucose) or incubated in SPM for the indicated hours (SPM0 to SPM5). The following strains were used: for panel A, an ime1Δ/ime1Δ strain (Y449) carrying on 2μm vectors the chimeric genes IME2 (YEp1791), RPD3-13xmyc (YEp2546), and pCDC28-gal4(dbd)-SIN3 (YEp2543); and for panel B, a wild-type diploid, Y422, carrying on 2μm vectors the chimeric genes IME2 (YEp1791), RPD3-13xmyc (YEp2546), and pADH1-gal4(dbd)-ime1(id) (YEp2780). Antibodies directed against myc and Gal4(dbd) were used for IP, and the PCRs amplified IME2. WCE, whole-cell extract.
Under meiotic conditions, Ime1 is transiently recruited by Ume6 to EMGs.
IME1 encodes a transcriptional activator essential for the transcription of all EMGs (16). Two hybrid assays reveal that the C-terminal domain of Ime1, amino acids 270 to 360, interacts with Ume6 (16), suggesting that Ume6 recruits Ime1 to the promoters of EMGs. ChIP analysis was used to determine whether, and if so when, Ime1 is present on the promoters of EMGs. Figure 4 shows the specific binding of Gal4(dbd)-Ime1(270-360) to the URS1 carrying genes IME2, HOP1, and INO1, as well as, for a control, to the GAL1 promoter (left panel). Tagging of Ime1 with Gal4(dbd) provided an internal control for the efficiency of the immunoprecipitation, since the Gal4(dbd)-Ime1(270-360) protein is able to form a DNA/protein complex on both the EMG and GAL1 promoters. In addition, Gal4 expressed from the genomic GAL4 gene, binds the GAL1 promoter. Binding onto the EMG promoters was mainly observed in cells grown with acetate as the sole carbon source (SA medium) (Fig. 4), because in the presence of glucose (SD medium) the transcription of IME1 is repressed (17). The lower levels of binding observed in SD medium are due to low levels of transcription resulting from the increase in the copy number of the gene that is placed on a 2μm plasmid. Binding of Ime1 to the URS1 element was eliminated in cells carrying a complete deletion of the UME6 open reading frame (Fig. 4B), demonstrating that Ime1 is indeed tethered by Ume6 to the URS1 element. Transfer of cells to SPM led to a transient increase in the binding of Ime1 to these promoters (Fig. 4A, central panel). Following 3 h of incubation in SPM, there was an increased amount of Ime1/DNA complex, while at 5 h the amount declined. Ime1 is an unstable protein whose transient transcription results in transient availability (12), suggesting that the decline in Ime1 occupancy at promoters might be due to a decrease in its level. Nonetheless, it is also possible that at late meiotic times the efficiency of Ime1 binding is reduced. This hypothesis is supported by the observation that the binding of Gal4(dbd)-Ime1(270-360) to the GAL1 promoter was not decreased (Fig. 4A, middle panel). Thus, the transfer of cells to SPM leads, at early meiotic times, to a transient increase in the Ime1 occupancy at promoters. In this experiment samples were also taken to immunoprecipitate Rpd3-13xmyc. Figure 4A (right panel) shows that when Ime1 is present on the promoter, Rpd3 is absent, suggesting the possibility that the switch from repression to transcriptional activation is promoted by the elimination of the repression complex from the promoters and the formation of an activation complex. Moreover, the repression of EMGs at late meiotic times (16) (Fig. 1 and 2D) is correlated with the elimination of the activation complex and the formation of the repression complex. However, these results cannot exclude the possibility that Ime1 and Rpd3 can occupy, in the same cells, the URS1 element.
The binding of Ime1 and that of Sin3/Rpd3 to the URS1 elements are independent.
The above results raise the possibility that recruitment of Ime1 to the promoters might prevent the association of Sin3 with Ume6. We examined, therefore, the binding of Sin3 and Rpd3 to the URS1 elements in diploid cells with IME1 deleted. Figure 5A shows that in these diploid cells, similarly to the wild-type cells, both Sin3 and Rpd3 are transiently removed from the promoters. We conclude, therefore, that the binding of Ime1 to the URS1-bound Ume6 does not prevent the binding of Sin3/Rpd3 to this DNA/protein complex. In SD medium, the expression of Ime1 from a heterologous promoter fails to promote the expression of EMGs and meiosis (34), suggesting that glucose and/or the presence of Sin3/Rpd3 might preclude the physical association of Ime1 with Ume6. In order to test this hypothesis, ChIP analysis was performed on cells expressing the Gal4(dbd)-Ime1 fusion protein from the ADH1 promoter. Figure 5B shows that in either SD medium or SPM there is specific recruitment of Ime1 to the URS1 elements, suggesting that Ime1 can associate with Ume6 under all growth conditions.
Rim11 and Tyr-359 phosphorylation of Ime1 is not required for the recruitment of Ime1 to the promoters of EMGs.
The two-hybrid interaction between Ime1 and Ume6 depends on the GSK3-β homolog Rim11 (5, 25, 34). This result implies that Rim11 is required either for the physical association between Ime1 and Ume6 or for the ability of the formed complex to activate the transcription of the reporter gene. We used ChIP analysis to differentiate between these two possibilities. Figure 6A shows that Gal4(dbd)-Ime1(270-360) binds to the IME2 promoter in both wild-type and rim11Δ isogenic diploids. We have recently shown that Rim11 phosphorylates Ime1 on Tyr-359 and that this phosphorylation was required for the two-hybrid interaction of Ime1 with Ume6 as well as for entry into meiosis (Rubin-Bejerano et al., unpublished). Rim11 phosphorylated additional residues on Ime1, Ser-302, and/or Ser-306, but these phosphorylations had no apparent effect on Ime1 function (Rubin-Bejerano et al., unpublished). Figure 6B shows that the Gal4(dbd)-Ime1(270-360) protein carrying the S306A Y359F double mutations binds to the promoters of both HOP1 and IME2, with the same pattern of binding as the wild-type protein, namely a transient increase upon entry into sporulation conditions. We conclude, therefore, that phosphorylation on Tyr-359 in Ime1 by Rim11 is not required for the ability of Ime1 to physically associate with Ume6.
FIG. 6.
Binding of Ime1 to the URS1 elements is independent of Tyr-359 phosphorylation and Rim11. (A) ChIP of Gal4(dbd)-Ime1(270-360). (B) ChIP of Gal4(dbd)-Ime1(270-360)-S306AY359F. Samples for ChIP analysis were taken from cells incubated in SPM for the indicated hours. The wild-type strain used was Y422, and its isogenic rim11Δ strain was created by mating Y1089 to Y1256. These strains carried on 2μm vectors the following chimeric genes: IME2 (YEp1791) and either pIME1-gal4(dbd)-ime1(270-360) (YEp1931) (A) or pIME1-gal4(dbd)-ime1(270-360)S306AY359F (YEp2713) (B). Antibodies directed against Gal4(dbd) were used for IP, and the PCRs amplified IME2 (A) or the indicated genes (B). WCE, whole-cell extract.
Glucose depletion is required for the removal of the Sin3/Rpd3 repression complex from EMG promoters.
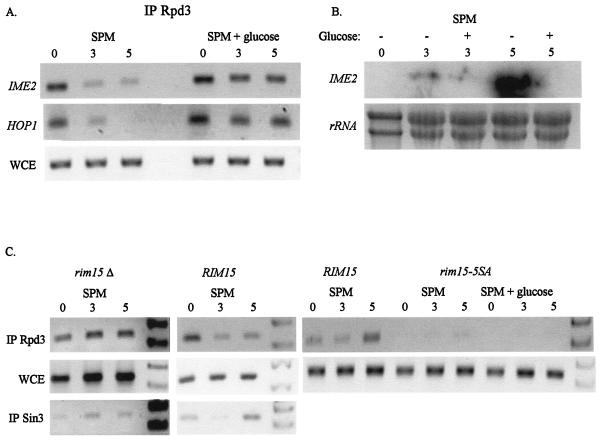
Under meiotic conditions, cells are depleted for both glucose and nitrogen. The results presented in Fig. 2 to 4 demonstrate that transfer of cells grown in the presence of acetate as the sole carbon source to nitrogen depletion leads to the transient removal of Sin3/Rpd3 from the promoters of EMGs. In order to examine whether exclusion of the Sin3/Rpd3 complex from the promoters also depends on glucose depletion, the binding of Rpd3 to the promoters was compared in cells transferred to either SPM or SPM supplemented with 2% glucose. Figure 7A demonstrates that nitrogen depletion in the presence of glucose leads to constitutive binding of Rpd3 to the IME2 and HOP1 promoters. Northern analysis shows that under these conditions (i.e., SPM supplemented with 2% glucose), the EMG IME2 is not transcribed (Fig. 7B). We conclude, therefore, that glucose is required to promote the physical association of the Sin3/Rpd3 repression complex with Ume6.
FIG. 7.
Role of glucose and Rim15 in the transient removal of Sin3/Rpd3 from the URS1 elements. Samples for ChIP (A and C) and Northern analysis (B) were taken from cells incubated in either SPM or SPM supplemented with 2% glucose for the indicated hours. The following strains were used: A wild-type RIM15 diploid (Y422), rim15Δ (Y1457), and rim15-5SA (Y157 carrying YCp2665). These strains carry on 2μm vectors the following chimeric genes: IME2 (YEp1791), RPD3-13xmyc (YEp2546), and pCDC28-gal4(dbd)-SIN3 (YEp2543). Antibodies directed against myc or Gal4(dbd) were used. The PCR products amplified the marked genes (A) or IME2 (C). WCE, whole-cell extract.
Rim15 regulates the dissociation of Ume6 from Sin3/Rpd3.
How do nutrients regulate the formation of the Ume6/Sin3/Rpd3 complex? The following considerations suggest that the protein kinase Rim15 might transmit this signal: (i) Rim15 stimulates the interaction of Ime1 with Ume6 (as revealed by a two-hybrid assay) (43), and (ii) Rim15 activity is inhibited by nutrients through the PKA signal transduction pathway (32). We determined whether Rim15 activity is required for removing Sin3/Rpd3 from the URS1-carrying promoters. ChIP analysis revealed that in diploid cells with RIM15 deleted the dissociation of Sin3 and Rpd3 from the promoters was prevented (Fig. 7C). Moreover, alanine substitution of the phosphate acceptors in Rim15 (as in the rim15-5SA allele) led to the construction of an in vitro constitutively active protein (32) and the elimination of Rpd3 from the URS1 element in both SPM and SPM supplemented with glucose (Fig. 7C, right panel). We conclude, therefore, that the glucose signal that inhibits dissociation of Ume6 from Sin3/Rpd3 is transmitted through Rim15.
In the absence of glucose, depending on Ume6, Ime1, and Rim11, Rpd3 repression is relieved.
The results presented in Fig. 5 and 6 demonstrate that in the presence of either glucose or acetate as the sole carbon source, Ime1 and Ume6 physically associate and that this association is independent on either Rim11 or Tyr-359 in Ime1. In contrast, the two-hybrid interaction between Ime1 and Ume6 required the absence of glucose and phosphorylation on Y359 in Ime1, as well as Rim11, the kinase phosphorylating this residue on Ime1 (34; Rubin-Bejerano et al., unpublished). These results imply that the Ume6/Ime1 complex that is formed in the presence of glucose functions as a repression complex or that it is defective in transcriptional activation. It is possible that in the presence of glucose, the Sin3/Rpd3 repression complex may be part of the Ume6/Ime1 complex, thus contributing to transcriptional repression. This hypothesis is supported by the observation that the repression activity of Sin3 did not promote transcriptional activation when a heterologous transcriptional activation domain was fused to Sin3 (20). We assume that in the absence of glucose, phosphorylation on Y359 in Ime1 may be required to override the repression activity of the Sin3/Rpd3 complex and thus to promote transcriptional activation by the activation domain of either Gal4 or Ime1.
The above hypothesis yields the prediction that direct recruitment of Rpd3 to promoters will lead to repression in the presence of glucose and that, in its absence, depending on both Ime1 and Rim11, repression will be relieved. We examined, therefore, the ability of a Gal4(dbd)-Rpd3 fusion protein to repress the transcription of a UASGAL1-UASHIS4-his4-lacZ reporter in gal4Δ gal80Δ diploid cells grown in either SD or SA medium. The level of expression was compared to that obtained in cells expressing only the Gal4(dbd) domain. Both proteins were expressed from the ADH1 promoter. Expression of Gal4(dbd)-Rpd3 led to a reduction in the transcription of the reporter gene in SD medium (Fig. 8), confirming previous results assayed in haploid cells (14). However, in SA medium, recruitment of Rpd3 did not lead to transcriptional repression (Fig. 8). In diploid cells with IME1, RIM11, or UME6 deleted, expression of Gal4(dbd)-Rpd3 led to complete repression in SD medium and only partial relief in SA medium (Fig. 8), indicating that complete relief of repression in the absence of glucose depends on these proteins. In accord, the constitutively active rim11-3SA allele resulted in a substantial reduction in the repression activity of Gal4(dbd)-Rpd3 in cells grown in SD medium (Fig. 8). On the other hand, expression of Ime1 from a heterologous promoter in SD medium did not relieve repression in wild-type cells (Fig. 8). These results suggest that in SA medium relief of repression by Ime1 may depend on posttranslational modifications (phosphorylation), events that normally take place only in the absence of glucose (Rubin-Bejerano et al., unpublished).
FIG. 8.
Repression activity of Rpd3 is relieved in the absence of glucose, depending on Ime1, Rim11, and Ume6. Proteins were extracted from at least three independent transformants. Cells were grown in either SD or SA medium to 107 cells/ml. The level of β-galactosidase was measured. The percentage of expression in cells expressing Gal4(dbd)-Rpd3 in comparison to that of the controlled cells expressing only Gal4(dbd) is given. The following diploid strains were used: wild type (the results are the average of the following diploid strains constructed by mating Y1402 to Y1065 and Y1458 to Y1064) (wt), ume6Δ (Y1402 × Y1328) (ume6), ime1Δ (Y1402 × Y1076) (ime1), rim11Δ (Y1458 × Y1256) (rim11), rim11-3SA (Y1458 × Y1456) (rim113SA), and a wild-type strain (Y1402 × Y1065) carrying pADH1-IME1 on a 2μm vector (YEp2050) ([IME1]). These strains carried the UASGAL1-UASHIS4-his4-lacZ reporter gene and on a 2μm vector either pADH1-gal4(dbd)-RPD3 (YEp2593) or pADH1-gal4(dbd) (control) (YEp2149).
DISCUSSION
In eukaryotes transcriptional activation of repressed genes is mediated by the recruitment of specific multiprotein complexes, including the Swi/Snf chromatin-remodeling complex and the SAGA complex with HAT activity. The resulting changes in nucleosome conformation promote the binding and/or activity of specific transcriptional activators (7, 29). Transcriptional repression is also mediated by the recruitment of multiprotein complexes with chromatin remodeling and HDAC activities. This report focuses on the mechanisms by which cells switch from transcriptional repression to activation. Using EMGs in budding yeast, which manifest a regulated switch from repression to transcriptional activation, we demonstrate the existence of two mechanisms that operate to relieve repression. (i) When cells are transferred to meiotic conditions, the Sin3/Rpd3 HDAC complex is transiently removed from the promoters of EMGs (Fig. 2 to 7), and the transcriptional activator, Ime1, is transiently tethered to these promoters (Fig. 4 to 6). This transient, regulated switch between repression and activation complexes formed on the promoters is correlated with a transient increase in histone acetylation, and the transcription of EMGs (Fig. 1 and 2). (ii) Using a repression assay, we demonstrate that the repression activity of the HDAC Rpd3 is regulated by glucose and that in the absence of glucose, depending on Ime1 and Rim11, Rpd3 repression is relieved (Fig. 8).
Rim15 is the kinase regulating the dissociation of Sin3/Rpd3 from Ume6.
Depletion of both glucose and nitrogen is required for the transient removal of Sin3 and Rpd3 from the URS1 element present in the promoters of all EMGs. This is evident from the following results (i) In the absence of glucose, depletion of nitrogen (shift from SA medium to SPM) leads to a substantial decline in the level of the URS1-DNA/Rpd3/Sin3 complex (Fig. 2 to 5 and 7). (ii) In the presence of glucose, nitrogen depletion (SA medium to SPM medium plus glucose) does not result in the removal of Rpd3 from the promoters (Fig. 7). The use of two opposing alleles of RIM15—a null allele, rim15Δ, and a constitutively active allele, rim15-5SA—reveals that Rim15 transmits both the glucose and nitrogen signals. The nitrogen effect on Rim15 is evident from the observation that in rim15Δ cells Rpd3 occupies the promoters of EMGs in cells transferred from SA medium to SPM (Fig. 7C). The glucose effect is evident from the observation that in cells carrying the rim15-5SA allele, Rpd3 does not occupy the URS1 elements, even in the presence of glucose (Fig. 7C).
What is the target of Rim15 whose phosphorylation leads to the disruption of the Sin3/Ume6 complex formation? Currently, three putative proteins, Sin3, Ume6, and Ime1, are considered. A two-hybrid assay revealed that Rim15 is required for the efficient interaction of Ime1 with Ume6 (43) and that Rim15 is required for phosphorylation on Ume6(1-232) (46), the domain in Ume6 that suffices for interaction with Ime1 (34, 43). These results suggest that phosphorylation on Ume6 by Rim15 might be required for its physical association with Ime1 and for the dissociation from Sin3. However, this suggestion is rejected by the observations that removal of Sin3/Rpd3 from the URS1 elements is independent of Ime1 (Fig. 5) and that Sin3 and Ime1 associate with different and distinct regions of Ume6, amino acids 515 to 530 and 1 to 232, respectively (34, 45). We suggest, therefore, that the exclusion of Sin3/Rpd3 from the promoters at early meiotic times is not due to competition between Ime1 and Sin3 for binding with Ume6. Further analysis is required to determine whether the effect of Rim15 on Ume6/Sin3 dissociation is mediated through phosphorylation on an additional domain in Ume6 or through a different target, for instance, Sin3.
The following reported results demonstrate that the cyclic AMP (cAMP)-dependent PKA signal transduction pathway transmits the glucose and nitrogen signals to Rim15: (i) PKA activity transmits both glucose and nitrogen signals (16, 24, 41; Rubin-Bejerano et al., unpublished). (ii) Deletion of RIM15 suppresses the lethality resulting from a lack of the three catalytic subunits of PKA, TPK1 to −3, as well as the temperature sensitivity of a mutation in the adenylate cyclase gene (32). (iii) The in vitro kinase activity of Rim15 is inhibited by the addition of PKA (32). (iv) The predicted amino acid sequence of Rim15 contains five Arg-Arg-X-Ser consensus sites for PKA phosphorylation. Serine-to-alanine mutations of these sites (rim15-5SA) results in a constitutively active Rim15, whose in vitro kinase activity is not inhibited by PKA (32). (v) In cells expressing the rim15-5SA allele Rpd3 is not bound to the EMG promoters in either SPM or SPM supplemented with 2% glucose (Fig. 7).
Ume6 recruits Ime1(270-360) to the promoters of EMGs under all growth conditions.
Prior studies, using a two-hybrid assay, showed that Ime1 interacts with Ume6 (34). The observation that IME1 encodes a transcriptional activator (27, 38) has led to the suggestion that Ume6 tethers Ime1 to the promoters of EMGs. This hypothesis is confirmed in this report, Ime1 is found bound to the URS1 elements and this binding depends on Ume6 (Fig. 4). Interestingly, in cells expressing only Ume6(1-158), Ime1 is still tethered to the EMG promoters (data not shown), suggesting that amino acids 1 to 158, rather than 1 to 232, suffice for this association.
The two-hybrid interaction between Ime1 and Ume6 is regulated by glucose and nitrogen depletion (34). In this report, we show that nitrogen depletion does indeed increase the physical association between Ime1 and Ume6 (Fig. 5). However, glucose, which has a major effect on the two-hybrid interaction between these two proteins (34), does not prevent the physical association of Ime1 with Ume6. Ime1 is bound to the URS1 element under all growth conditions, i.e., SD and SA media and SPM (Fig. 5). Moreover, Ime1(270-360) occupies the EMG promoters even in cells with RIM11 deleted or when it carries the S306AY359F mutations (Fig. 6). Recently, we have shown that these residues are phosphorylated by Rim11 and that in the presence of these mutations the two-hybrid interaction of Ime1 with Ume6 is abolished (Rubin-Bejerano et al., unpublished). We conclude, therefore, that the physical association of Ime1 with Ume6 is constitutive; it is not regulated by nutrients and Rim11. We also suggest that Rim15 is not required for Ime1/Ume6 association, because Ime1 occupies the promoters under conditions in which Rim15 is not active (i.e., SD medium). The role of these proteins in the transcription of EMGs is discussed below.
Ime1 is required to relieve Rpd3 repression.
The transcription of EMGs requires the relief of Rpd3 repression activity, as well as transcriptional activation. We suggest that these are two distinct functions and that the relief of Rpd3 repression depends on Ime1(270-360) as well as glucose depletion. This hypothesis is based on the following observations. (i) Fusion of a hetrologous transcriptional activation domain to Ime1(270-360) suppressed ime1Δ, promoting the transcription of EMGs and sporulation in SPM (27). (ii) Fusion of a transcriptional activation domain to Sin3 did not convert it into a transcriptional activator (20). Because Sin3 repression depends on Rpd3 (19), these results suggest that a transcriptional activation domain cannot relieve Rpd3 repression. (iii) Fusion of a transcriptional activation domain to Ume6 does not promote the transcription of EMGs in cells with IME1 deleted (45). It was suggested that the Gal4(ad)-Ume6 chimeric protein could not relieve Rpd3 repression, because Ume6 repression activity was dependent on Sin3 and Rpd3 (14) and because a gal4(ad)-ume6-6 allele that carried a mutation preventing association with Sin3 partially suppressed ime1Δ (45). (iv) Ectopic expression of Ime1 in SD medium does not promote the transcription of EMGs (35; Rubin-Bejerano et al., unpublished), even though Ime1 functions as a potent transcriptional activator under all growth conditions (27). (v) In this report, using ChIP analysis we demonstrate that Ime1 and Ume6 associate under all growth conditions. Nevertheless, a two-hybrid analysis reveals that the interaction between these two proteins is regulated by nutrients (34), Rim11 (34; Rubin-Bejerano et al., unpublished), Rim15 (43), and phosphorylation on Tyr-359 in Ime1 (Rubin-Bejerano et al., unpublished). This “discrepancy” suggests that in the presence of glucose the Gal4(dbd)-Ime1/Gal4(ad)-Ume6 complex is impaired in transcriptional activation. We suggest that in the two-hybrid assay, a large complex that includes Ime1, Ume6, Sin3, and Rpd3 is formed on the promoters and that the presence of Rpd3 leads to transcriptional repression, but only in SD medium. In the absence of glucose, when Rim11 is active, phosphorylation on Tyr-359 in Ime1 by Rim11 activates Ime1. The activated Ime1 relieves Rpd3 repression, even when the protein occupies the DNA. We further suggest that in cells with RIM15 deleted, the continuous, elevated binding of Sin3/Rpd3 to Ume6, leads to a decline in the two-hybrid interaction between Ime1 and Ume6 (43).
In this report, we used a repression assay to test this hypothesis. We show that Rpd3 functions as a carbon source-regulated repressor. As reported previously (14), in glucose-grown cells direct recruitment of Rpd3 to promoters leads to transcriptional repression (Fig. 8). However, in the absence of glucose and the presence of acetate as the sole carbon source, Rpd3 repression is relieved (Fig. 8). We further show that relief of Rpd3 repression depends on Ume6, Ime1, and Rim11 (Fig. 8). In a medium promoting vegetative growth with glucose as the sole carbon source (SD), IME1 is not transcribed (17), explaining why only in the absence of glucose (SA medium) is Rpd3 repression relieved. We suggest that glucose also regulate the activity of Ime1, because ectopic expression of Ime1 in SD medium does not impair Rpd3 function (Fig. 8). We further suggest that activation of Ime1 depends on phosphorylation by Rim11. This hypothesis is based on the following observations. (i) In vivo, Rim11 phosphorylates Ime1 only in the absence of glucose (Rubin-Bejerano et al., unpublished). (ii) In SD medium-grown cells that carry the constitutive active rim11-3SA allele, Ime1 is phosphorylated (Rubin-Bejerano et al., unpublished) and Rpd3 repression is impaired (Fig. 8). (iii) In cells expressing the ime1L321F allele EMGs are not transcribed and cells are sporulation deficient (4). This defect is attributed to the lack in Ime1 phosphorylation, because this mutant protein is impaired in association with Rim11 and in vitro it is not phosphorylated by Rim11 (26). It was suggested, therefore, that the interaction between Rim11 and Ime1 might be required for the activity of the Ime1/Ume6 complex rather than its formation (26). The above considerations suggest that the effect of Rim11 on the relief of Rpd3 repression is mediated through Ime1. Nevertheless, our results do not exclude the possibility that an additional target of Rim11, for instance Ume6, might contribute to the relief of Rpd3 function.
What might be the function of Ume6? We assume that Ume6 is required to recruit Ime1 and Rim11 to Rpd3, because it associates with these proteins as well as with Sin3, which associates with Rpd3. Deletion of UME6 leads, in SA medium, to only a partial relief of repression (Fig. 8), probably reflecting, on one hand the requirement for Ume6 to tether Ime1 to Rpd3 and on the other hand the exclusion of the Isw2 repression complex from the DNA. This is based on the observation that Ume6 recruits both the HDAC complex and the Isw2 chromatin remodeling complex to EMGs (9) and that in cells with both SIN3 and ISW2 deleted the expression of EMGs is highly induced in comparison to the single mutants (9).
A model for how repression by histone deacetylation is relieved upon transfer to meiotic conditions.
The schematic illustration in Fig. 9 summarizes our results and suggests a model for the switch in transcription of EMGs. In the presence of glucose and nitrogen, PKA inactivates two kinases, Rim11 (Rubin-Bejerano et al., unpublished) and Rim15 (32). The kinase activity of both Rim11 and Rim15 is inhibited by phosphorylation. Rim15 is a direct substrate of PKA, and the effect of PKA on Rim11 is most probably indirect (32; Rubin-Bejerano et al., unpublished). Under these conditions, the association of Ume6 with Sin3 leads to the recruitment of the HDAC complex to the DNA, histone deacetylation, and transcriptional repression (Fig. 9A). In the presence of glucose IME1 is not transcribed (17). However, ectopic expression of Ime1 does not relieve Rpd3 repression (Fig. 8) and promote the transcription of EMGs and sporulation (6, 34, 35; Rubin-Bejerano et al., unpublished), even though Ime1 is recruited by Ume6 to the EMG promoters (Fig. 5). The absence of glucose and nitrogen, through the PKA signal transduction pathway, leads to the following events (Fig. 9B). (i) The kinase-active, nonphosphorylated Rim15, phosphorylates Ume6, Sin3, or an unknown protein. This event is required for the dissociation of Sin3 from Ume6. As a result, the HDAC complex is removed from the DNA and a concomitant increase in histone acetylation is observed (Fig. 2). (ii) The transcription of IME1 is induced (17). (iii) Ime1 is localized in the nucleus (5) and is transiently recruited to the URS1 elements (Fig. 4 and 5). (iv) The kinase-active, nonphosphorylated Rim11 phosphorylates Ime1 on Tyr-359 and Ser-302 and/or Ser-306 (Rubin-Bejerano et al., unpublished). We assume that phosphorylation on Ime1 on Tyr-359 is required to relieve Rpd3 repression and by this to activate the transcription of EMGs and to promote meiosis and sporulation (Rubin-Bejerano et al., unpublished). The role of Rim11 in transmitting the glucose and nitrogen signals was revealed by the use of two opposing alleles of RIM11. In cells with RIM11 deleted, phosphorylation on Tyr-359 and Ser-302 or Ser-306 in Ime1 was impaired, there was no detectable two-hybrid interaction between Ime1 and Ume6, EMGs were not transcribed, and cells were sporulation deficient (5, 34, 38; Rubin-Bejerano et al., unpublished). On the other hand, cells carrying the constitutively active rim11-3SA allele promoted almost complete phosphorylation on Ime1 in SD medium, Rpd3 repression was impaired, and cells were sporulation proficient when incubated in SPM medium supplemented with 2% glucose (Rubin-Bejerano et al., unpublished). (v) Transcription of EMGs also depends on a specific transcriptional activation domain supplied by Ime1 (27, 38). The transcription of EMGs is transient, and prior to its decline, the activation complex present on the DNA is replaced by the repression complex. We suggest that this replacement is accomplished by regulating both the rerecruitment of Sin3/Rpd3 to the promoters and the concomitant removal of Ime1. When cells are transferred to SPM, RIM15 is not transcribed and the Rim15 protein gradually disappears (43), a condition promoting the association of Sin3 with Ume6. Ime1 is absent at late meiotic times, because the meiosis-specific kinase, Ime2 (an EMG), phosphorylates Ime1, sending it to degradation by the proteosome (12).
FIG. 9.
Transcriptional repression of EMGs (A [with glucose]) and its relief in the absence of glucose and nitrogen (B [acetate without nitrogen]) depend on the cAMP/PKA signal transduction pathway through two protein kinases, Rim15 and Rim11, a GSK3-β homolog. The results are represented schematically. See the text for details.
It is likely that the use of multiple regulated mechanisms to relieve repression and promote transcriptional activation is required to ensure that entry into a developmental pathway takes place only under the correct conditions. Higher eukaryotic cells also use repression by HDACs and its regulated relief as a way of controlling differentiation. In humans, the development of specific tumors is correlated with the failure of repression due to unscheduled expression of HDAC (2, 8). Future analysis will reveal whether higher eukaryotes use similar mechanisms to regulate the recruitment, as well as function, of the HDAC complexes.
Acknowledgments
We thank E. Lifschitz for helpful discussions. We thank K. Struhl and M. Foiani for technical assistance. We thank G. Fink, B. Horowitz, D. Rave, and G. Simchen for critical reading of the manuscript. We thank C. De Virgilio for kindly providing plasmids.
This work was supported by a grant from the Israel Science Foundation.
REFERENCES
- 1.Anderson, S. F., C. M. Steber, R. E. Esposito, and J. E. Coleman. 1995. UME6, a negative regulator of meiosis in Saccharomyces cerevisiae, contains a C-terminal Zn2Cys6 binuclear cluster that binds the URS1 DNA sequence in a zinc-dependent manner. Protein Sci. 4:1832-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archer, S. Y., and R. A. Hodin. 1999. Histone acetylation and cancer. Curr. Opin. Genet. Dev. 9:171-174. [DOI] [PubMed] [Google Scholar]
- 3.Bowdish, K. S., and A. P. Mitchell. 1993. Bipartite structure of an early meiotic upstream activation sequence from Saccharomyces cerevisiae. Mol. Cell. Biol. 13:2172-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowdish, K. S., H. E. Yuan, and A. P. Mitchell. 1994. Analysis of RIM11, a yeast protein kinase that phosphorylates the meiotic activator IME1. Mol. Cell. Biol. 14:7909-7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colomina, N., E. Gari, C. Gallego, E. Herrero, and M. Aldea. 1999. G1 cyclins block the Ime1 pathway to make mitosis and meiosis incompatible in budding yeast. EMBO J. 18:320-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colomina, N., Y. Liu, M. Aldea, and E. Gari. 2003. TOR regulates the subcellular localization of Ime1, a transcriptional activator of meiotic development in budding yeast. Mol. Cell. Biol. 23:7415-7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosma, M. P., T. Tanaka, and K. Nasmyth. 1999. Ordered recruitment of transcription and chromatin remodeling factors to a cell cycle- and developmentally regulated promoter. Cell 97:299-311. [DOI] [PubMed] [Google Scholar]
- 8.Cress, W. D., and E. Seto. 2000. Histone deacetylases, transcriptional control, and cancer. J. Cell Physiol. 184:1-16. [DOI] [PubMed] [Google Scholar]
- 9.Goldmark, J. P., T. G. Fazzio, P. W. Estep, G. M. Church, and T. Tsukiyama. 2000. The Isw2 chromatin remodeling complex represses early meiotic genes upon recruitment by Ume6p. Cell 103:423-433. [DOI] [PubMed] [Google Scholar]
- 10.Guarente, L. 2001. SIR2 and aging—the exception that proves the rule. Trends Genet. 17:391-392. [DOI] [PubMed] [Google Scholar]
- 11.Guttmann-Raviv, N., E. Boger-Nadjar, I. Edri, and Y. Kassir. 2001. Cdc28 and Ime2 possess redundant functions in promoting entry into premeiotic DNA replication in Saccharomyces cerevisiae. Genetics 159:1547-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guttmann-Raviv, N., S. Martin, and Y. Kassir. 2002. Ime2, a meiosis-specific kinase in yeast, is required for destabilization of its transcriptional activator, Ime1. Mol. Cell. Biol. 22:2047-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hepworth, S. R., H. Friesen, and J. Segall. 1998. NDT80 and the meiotic recombination checkpoint regulate expression of middle sporulation-specific genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 18:5750-5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadosh, D., and K. Struhl. 1997. Repression by Ume6 involves recruitment of a complex containing Sin3 corepressor and Rpd3 histone deacetylase to target promoters. Cell 89:365-371. [DOI] [PubMed] [Google Scholar]
- 15.Kadosh, D., and K. Struhl. 1998. Targeted recruitment of the Sin3-Rpd3 histone deacetylase complex generates a highly localized domain of repressed chromatin in vivo. Mol. Cell. Biol. 18:5121-5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kassir, Y., N. Adir, E. Boger-Nadja, N. Guttmann-Raviv, I. Rubin-Bejerano, S. Sagee, and G. Shenhar. 2003. Transcriptional regulation of meiosis in budding yeast. Int. J. Cytol. Surv. Cell Biol. 224:111-171. [DOI] [PubMed] [Google Scholar]
- 17.Kassir, Y., D. Granot, and G. Simchen. 1988. IME1, a positive regulator gene of meiosis in S. cerevisiae. Cell 52:853-862. [DOI] [PubMed] [Google Scholar]
- 18.Kassir, Y., and G. Simchen. 1991. Monitoring meiosis and sporulation in Saccharomyces cerevisiae. Methods Enzymol. 194:94-110. [DOI] [PubMed] [Google Scholar]
- 19.Kasten, M. M., S. Dorland, and D. J. Stillman. 1997. A large protein complex containing the yeast Sin3p and Rpd3p transcriptional regulators. Mol. Cell. Biol. 17:4852-4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasten, M. M., and D. J. Stillman. 1997. Identification of the Saccharomyces cerevisiae genes STB1-STB5 encoding Sin3p binding proteins. Mol. Gen. Genet. 256:376-386. [DOI] [PubMed] [Google Scholar]
- 21.Katan-Khaykovich, Y., and K. Struhl. 2002. Dynamics of global histone acetylation and deacetylation in vivo: rapid restoration of normal histone acetylation status upon removal of activators and repressors. Genes Dev. 16:743-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurdistani, S. K., D. Robyr, S. Tavazoie, and M. Grunstein. 2002. Genome-wide binding map of the histone deacetylase Rpd3 in yeast. Nat. Genet. 31:248-254. [DOI] [PubMed] [Google Scholar]
- 23.Lamb, T. M., and A. P. Mitchell. 2001. Coupling of Saccharomyces cerevisiae early meiotic gene expression to DNA replication depends upon RPD3 and SIN3. Genetics 157:545-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lengeler, K. B., R. C. Davidson, C. D'Souza, T. Harashima, W.-C. Shen, P. Wang, X. Pan, M. Waugh, and J. Heitman. 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64:746-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malathi, K., Y. Xiao, and A. P. Mitchell. 1999. Catalytic roles of yeast GSK3beta/Shaggy homolog Rim11p in meiotic activation. Genetics 153:1145-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malathi, K., Y. Xiao, and A. P. Mitchell. 1997. Interaction of yeast repressor-activator protein Ume6p with glycogen synthase kinase 3 homolog Rim11p. Mol. Cell. Biol. 17:7230-7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandel, S., K. Robzyk, and Y. Kassir. 1994. IME1 gene encodes a transcription factor which is required to induce meiosis in Saccharomyces cerevisiae. Dev. Genet. 15:139-147. [DOI] [PubMed] [Google Scholar]
- 28.Ng, H. H., and A. Bird. 2000. Histone deacetylases: silencers for hire. Trends Biochem. Sci. 25:121-126. [DOI] [PubMed] [Google Scholar]
- 29.Norton, V. G., K. W. Marvin, P. Yau, and E. M. Bradbury. 1990. Nucleosome linking number change controlled by acetylation of histones H3 and H4. J. Biol. Chem. 265:19848-19852. [PubMed] [Google Scholar]
- 30.Pedruzzi, I., N. Burckert, P. Egger, and C. De Virgilio. 2000. Saccharomyces cerevisiae Ras/cAMP pathway controls post-diauxic shift element-dependent transcription through the zinc finger protein Gis1. EMBO J. 19:2569-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puziss, J. W., T. A. Hardy, R. B. Johnson, P. J. Roach, and P. Hieter. 1994. MDS1, a dosage suppressor of an mck1 mutant, encodes a putative yeast homolog of glycogen synthase kinase 3. Mol. Cell. Biol. 14:831-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reinders, A., N. Burckert, T. Boller, A. Wiemken, and C. De Virgilio. 1998. Saccharomyces cerevisiae cAMP-dependent protein kinase controls entry into stationary phase through the Rim15p protein kinase. Genes Dev. 12:2943-2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rose, M., and D. Botstein. 1983. Construction and use of gene fusions to lacZ (beta-galactosidase) that are expressed in yeast. Methods Enzymol. 101:167-180. [DOI] [PubMed] [Google Scholar]
- 34.Rubin-Bejerano, I., S. Mandel, K. Robzyk, and Y. Kassir. 1996. Induction of meiosis in Saccharomyces cerevisiae depends on conversion of the transcriptional repressor Ume6 to a positive regulator by its regulated association with the transcriptional activator Ime1. Mol. Cell. Biol. 16:2518-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherman, A., M. Shefer, S. Sagee, and Y. Kassir. 1993. Post-transcriptional regulation of IME1 determines initiation of meiosis in Saccharomyces cerevisiae. Mol. Gen. Genet. 237:375-384. [DOI] [PubMed] [Google Scholar]
- 36.Sherman, F. 1991. Getting started with yeast. Methods Enzymol. 194:3-21. [DOI] [PubMed] [Google Scholar]
- 37.Shimizu, M., K. Takahashi, T. M. Lamb, H. Shindo, and A. P. Mitchell. 2003. Yeast Ume6p repressor permits activator binding but restricts TBP binding at the HOP1 promoter. Nucleic Acids Res. 31:3033-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith, H. E., S. E. Driscoll, R. A. Sia, H. E. Yuan, and A. P. Mitchell. 1993. Genetic evidence for transcriptional activation by the yeast IME1 gene product. Genetics 133:775-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strahl-Bolsinger, S., A. Hecht, K. Luo, and M. Grunstein. 1997. SIR2 and SIR4 interactions differ in core and extended telomeric heterochromatin in yeast. Genes Dev. 11:83-93. [DOI] [PubMed] [Google Scholar]
- 40.Strich, R., R. T. Surosky, C. Steber, E. Dubois, F. Messenguy, and R. E. Esposito. 1994. UME6 is a key regulator of nitrogen repression and meiotic development. Genes Dev. 8:796-810. [DOI] [PubMed] [Google Scholar]
- 41.Thevelein, J. M., and J. H. de Winde. 1999. Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 33:904-918. [DOI] [PubMed] [Google Scholar]
- 42.Vershon, A. K., N. M. Hollingsworth, and A. D. Johnson. 1992. Meiotic induction of the yeast HOP1 gene is controlled by positive and negative regulatory sites. Mol. Cell. Biol. 12:3706-3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vidan, S., and A. P. Mitchell. 1997. Stimulation of yeast meiotic gene expression by the glucose-repressible protein kinase Rim15p. Mol. Cell. Biol. 17:2688-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vogelauer, M., J. Wu, N. Suka, and M. Grunstein. 2000. Global histone acetylation and deacetylation in yeast. Nature 408:495-498. [DOI] [PubMed] [Google Scholar]
- 45.Washburn, B. K., and R. E. Esposito. 2001. Identification of the Sin3-binding site in Ume6 defines a two-step process for conversion of Ume6 from a transcriptional repressor to an activator in yeast. Mol. Cell. Biol. 21:2057-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao, Y., and A. P. Mitchell. 2000. Shared roles of yeast glycogen synthase kinase 3 family members in nitrogen-responsive phosphorylation of meiotic regulator Ume6p. Mol. Cell. Biol. 20:5447-5453. [DOI] [PMC free article] [PubMed] [Google Scholar]