Abstract
Limb synovial joints are intricate structures composed of articular cartilage, synovial membranes, ligaments and an articular capsule. Each joint has a unique shape, organization and biomechanical function, and articular cartilage itself is rather complex and organized in distinct zones, including the superficial zone that produces lubricans and contains stem/progenitor cells. There has been a great of interest for many years to decipher the mechanisms by which the joints form and come to acquire such unique structural features and diversity. Decades ago, classic embryologists discovered that the first overt sign of joint formation at each prescribed limb site is the appearance of a dense and compact population of mesenchymal cells collectively called the interzone. Work carried out since by several groups has provided evidence that the interzone cells do actively participate in joint tissue formation over developmental time. This minireview provides a succinct but comprehensive description of the many and important recent advances in this field of research. These includes: studies using various conditional reporter mice to genetically trace and track the origin, fate and possible function of joint progenitor cells; studies on the involvement and roles in signaling pathways and transcription factors in joint cell determination and functioning; and studies using advanced methods of gene expression analyses to uncover novel genetic determinants of joint formation and diversity. The overall advances are impressive, and the findings are not only of obvious interest and importance, but have major implications to conceive future translational medicine tools to repair and regenerate defective, overused or aging joints.
Keywords: Synovial joint formation, interzone, articular cartilage, extracellular matrix, limb development, skeletogenesis, progenitor cells, signaling pathways, transcription factors
Introduction
The synovial joints in the limbs – be it the elbow, the hip or interphalangeal joints- are intricate and diverse organs. They are composed of reciprocally-shaped surfaces covered by articular cartilage, are stabilized mechanically by intrajoint and peri-joint ligaments, and are insulated from the body environment by the synovial lining and a thick surrounding synovial capsule (Archer et al., 1999). Articular cartilage itself is rather complex and is organized in histologically and phenotypically distinct zones (Hunziker et al., 2007). The superficial zone contains elongated and flat-shaped cells oriented parallel to the articular surface, held together by a largely collagenous matrix, and producing lubricin, hyaluronate and other anti-adhesive macromolecules essential for frictionless joint movement (Jay et al., 2001). Articular chondrocytes in the middle zone are round in shape, are usually organized in small vertical rows, and produce and maintain important extracellular components – particularly collagen II and aggrecan that confer the tissue with its key biomechanical feature: resilience. The chondrocytes in the bottom zone tend to be larger in size, are also active in matrix production and maintenance, and face the critical tissue boundary – referred to as the tidemark- between articular cartilage and underlying subchondral bone (Broom and Poole, 1982). While the structure of the articular cartilage is similar throughout all synovial joints, the location and function of each joint defines its distinct architecture. For example, in the hip the nearly spherical and concave acetabulum articulates with the nearly spherical and convex femoral head. In the knee, the dual distal femoral condyles articulate with the relatively flat proximal tibial plateau, but make no direct contact with the fibula. The knee also contains unique fibrocartilaginous structures such as the meniscus and intrajoint ligaments, while the hip displays the centrally located teres and the interphalangeal joints have externally positioned collateral ligaments. This extraordinary variety of anatomical, histological and biomechanical features is all well documented and fairly well understood in terms of its multiple essential roles in joint function, maintenance, endurance, motion, and dissipation of mechanical loads (Li et al., 2013a). In comparison, what remains unclear is how the joints form and come to acquire such variety of structural, organizational and biomechanical features during embryogenesis and early postnatal life, each adept and adapted to specific anatomical locations and distinct function (Archer et al., 2003; Pacifici et al., 2005; Pitsillides and Ashhurst, 2008). For instance, what are the progenitor cells that give rise to the joints and their specialized tissues? How does articular cartilage acquire its stratified organization? How are the opposing sides of a joint molded in a reciprocal lock-and-key manner? Advances in these areas would be of obvious basic research value, but could also have critical biomedical implications, possibly leading one day to treatments for congenital disorders such as developmental dysplasia of the hip or acquired age-related disorders such as osteoarthritis. This mini-review then provides a concise description of important advances on joint development research obtained in the last few years.
Interzone origin and cell lineage tracing-tracking
Classic studies carried out decades ago in mammalian and avian embryos showed that the cartilaginous skeleton forming in the early limb is continuous and uninterrupted, as exemplified by the Y-shaped anlagen corresponding to the stylopod element (humerus or femur), the two zeugopod elements (radius-ulna and tibia-fibula), and the autopod rays corresponding to the phalangeal elements (Hamrick, 2001; Hinchliffe and Johnson, 1980). These and other studies led to the realization that the first overt histological sign of joint formation was the emergence of a compact avascular mesenchymal tissue layer at each prospective joint site, interrupting the adjacent cartilaginous elements and thus originally named the “interzone” (Holder, 1977; Mitrovic, 1978). The interzone is composed of flat-shaped cells oriented perpendicularly to the limb’s main axis, tightly bound via gap junctions and requiring the hypoxia regulator Hif-1α function (Archer et al., 2003; Provot et al., 2007). Because the interzone cells emerge at sites previously occupied by chondrocytes, this led to the suggestion that the interzone cells originate from, and are the direct descendants of, de-differentiated chondrocytes (Craig et al., 1987; Nalin et al., 1995). When Holder microsurgically removed the interzone at the prospective elbow site in early chick embryos in ovo, no joint formed at later stages (Holder, 1977). These data revealed for the first time that the interzone is needed for joint formation, but did not clarify whether the interzone represents a mere physical signpost demarcating the joint site or has additional and more direct roles.
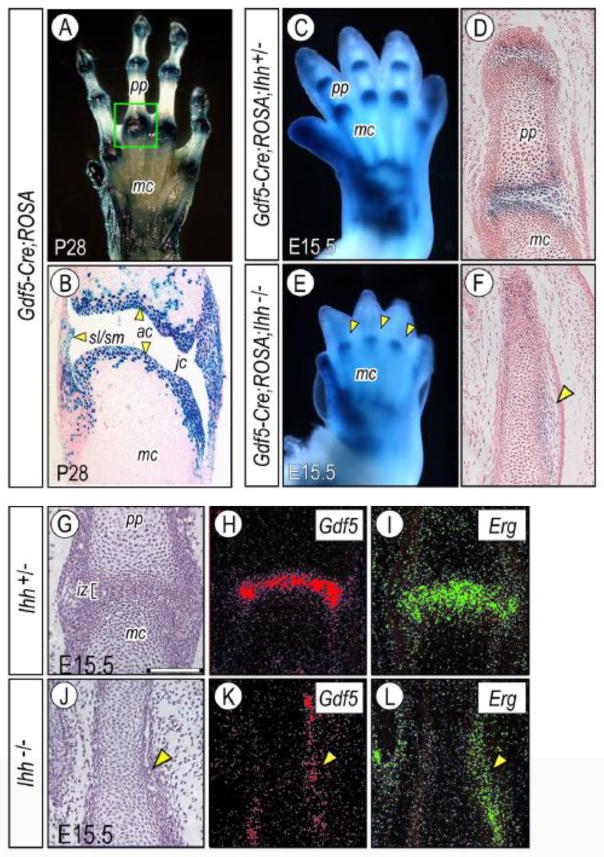
Exploiting the fact that incipient interzone cells express growth and differentiation factor 5 (Gdf5) (Storm and Kingsley, 1996), we and our collaborators carried out genetic cell lineage tracing-and-tracking experiments using compound Gdf5-Cre;ROSA R26R (LacZ) reporter mice (Koyama et al., 2008; Rountree et al., 2004). We found that LacZ-positive interzone cells and their progeny gave rise to many joint tissues over developmental time — including articular cartilage, synovial lining and intrajoint ligaments — that persisted into adulthood (Fig. 1A–1B). Thus the data showed that interzone cells are not transient, actively take part in joint tissue formation, and constitute a progenitor cell cohort endowed with joint-formation capacity. In concurrent studies, Hyde and collaborators carried out similar cell lineage tracing-and-tracking experiments in the developing knee using compound matrillin 1-Cre;R26R mice; matrillin 1 is normally expressed by all chondrocytes except articular chondrocytes (Hyde et al., 2007). They found that nascent articular chondrocytes emerging in the incipient knee joint at E13.5 were LacZ-negative, while the adjacent shaft chondrocytes were positive, and that this pattern persisted over time. In related experiments, the same group used Col2a1-Cre;R26R mice to track cells in developing knees and found that reporter-positive cells initially gave rise to articular cartilage, cruciate ligament and medial meniscus (Hyde et al., 2008). Reporter-negative cells appeared in the developing joint at E14.5 (termed intermediate zone cells) and were also present at later stages in the lateral portions of meniscus. The authors concluded that knee development involves cells present in the original anlagen with a Col2a1 history as well as invading Col2a1-negative cells recruited from the surroundings. Similarly, we had previously used in ovo fluorescent DiI cellular labeling-tracing in chick embryo limbs to provide evidence that surrounding cells migrate into developing joints (Pacifici et al., 2006). Likewise, we had studied Indian hedgehog-null (Ihh−/−) mouse embryos in which the limb skeletal elements remain wholly cartilaginous and lack joints (Fig. 1E–1F) that are well appreciable in age-matched control littermates (Fig. 1C–1D) (Koyama et al., 2007). Using compound Ihh−/−;Gdf5-Cre;R26R embryos, we found that LacZ-positive cells did form at prospective joint sites in the mutants (Fig. 1E, arrowheads), but flanked and surrounded the uninterrupted joint sites (Fig. 1F, arrowhead). These reporter positive cells expressed interzone marker genes including Gdf5 and Erg (Figs. 1J–1L, arrowheads), and phenotypically similar cells were present in prescribed patterns within the joints in control littermates as expected (Figs. 1C–1D and 1G–1I). We interpreted the data to indicate that prospective joint progenitor cells had emerged and were topographically specified in the Ihh-null mutants, but could not penetrate the fused joint sites. Recruitment and immigration of flanking cells into the interzone has also been suggested by Mundlos and coworkers in their study of joint development defects in the Short digits mouse mutant (Niedermaier et al., 2005).
Fig. 1.
Cell lineage tracing and joint development. (A–B) Whole mount and histochemical images showing that Gdf5-Cre;ROSA-R26R LacZ-positive cells were restricted to the limb joints in P28 mouse forelimbs (A) and constituted articular cartilage (ac) and synovial lining and associated layers (sl/sm) but were nearly absent in underling shaft (B). Boxed area in (A) corresponds to the joint sections shown in (B). (C–F) Whole mount and histochemical images from control heterozygous (Gdf5-Cre;ROSA;Ihh+/−) and mutant (Gdf5-Cre;ROSA;Ihh−/−) E15.5 mouse embryos showing that LacZ-positive cells were present at prescribed joint sites in both (C and E, arrowheads). In the mutants, however, the cells flanked and surrounded the fused joint site and were distributed longitudinally along it (F, arrowhead), whereas in controls they constituted the typical and transversely-oriented interzone (D). (G–L) Histological and in situ hybridization fluorescence images showing that the metacarpophalangeal joint in E15.5 control (Ihh+/−) embryos displayed a normal interzone (iz) (G) and characteristic Gdf5 and Erg expression patterns (H–I). In Ihh-null mutants, cells expressing those genes were present at prescribed joint sites, but longitudinally flanked the cartilaginous, still uninterrupted and fused site (J–L, arrowheads). In situ hybridization signal is presented in pseudo colors. ac, articular cartilage; jc, joint cavity; iz, interzone; mc, metacarpal element; pp, proximal phalangeal element; sl/sm, synovial lining and membrane.
Recent cell lineage-tracking experiments with Sox9CreERT2;R26R mouse embryos receiving tamoxifen at E11.5 and examined by E17.5 have indicated that Sox9-expressing cells are the precursors of both articular and growth plate chondrocytes, as well as ligaments and tendons (Soeda et al., 2010). In experiments using endogenous doublecortin (Dcx) to drive LacZ or eGFP expression in mouse embryos, Zhang et al. found that Dcx-expressing cells initially constitute much of limb mesenchyme, but later are restricted to the interzone and articular chondrocytes (Zhang et al., 2010). In line with the Sox9 study above, they concluded that articular and growth plate chondrocytes derive from common mesenchymal precursors originally expressing Dcx which then bifurcate into articular and growth plate chondrocytes. The TGFβ type II receptor (Tgfbr2) is essential for joint formation, particularly in the autopod (Spagnoli et al., 2007). Novel transgenic reporter mice bearing a Tgfbr2-βGal-GFP-BAC construct and expressing both β-galactosidase (βGal) and green fluorescence protein (GFP) as reporters were used to monitor the spatio-temporal distribution of Tgfbr2-expressing cells in developing digit joints over time (Li et al., 2013b). Tgfbr2-βGal-positive cells were first limited to dorsal and ventral regions of E13.5 joints and were undetectable in the central region of the interzone. By E16.5 and postnatally, positive cells were observed in synovial lining, meniscal surface, ligaments and groove of Ranvier, and BrdU-labeling showed that Tgfbr2-expressing cells also constitute slow cycling stem/progenitor cells. The authors reached the interesting conclusion that during interzone development, Tgfbr2-expressing cells would act as progenitors that orchestrate joint development within specified cell niches.
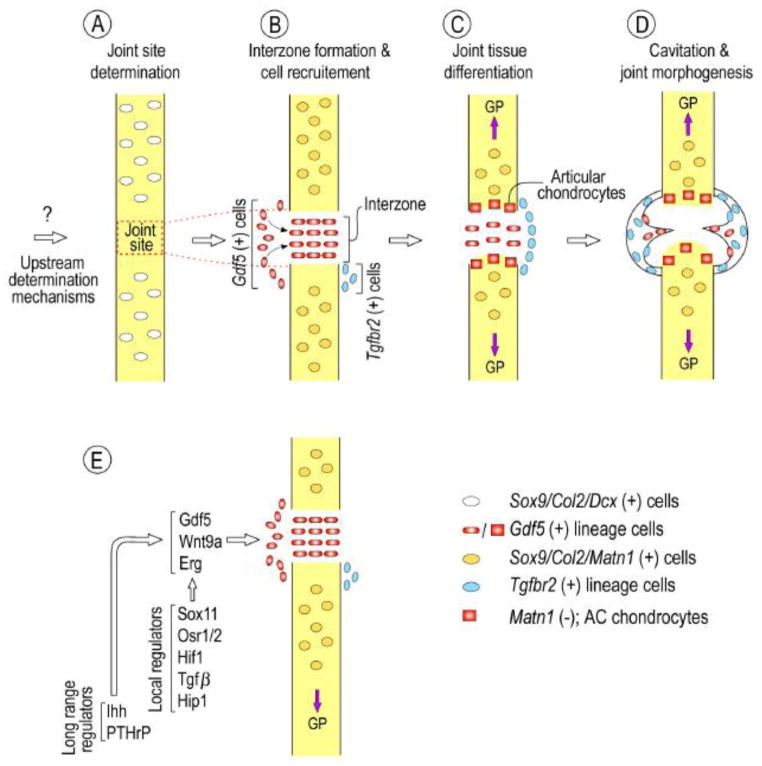
Taken together, the above studies provide a far better understanding of limb joint development as well as genesis, fate and roles of interzone cells. The data and insights can be summarized and synthesized into the following model (Fig. 2). Along the initial uninterrupted Sox9/Col2/Dcx-expressing cartilaginous anlagen, the location of prospective joint sites would be identified and determined by as yet unknown upstream morphogenetic and determination mechanisms. Soon after, expression of Gdf5 would be activated to define the initial interzone mesenchymal population with concurrent cell immigration and also maintenance of Dcx expression; cells flanking the interzone dorsally and ventrally would activate Tgfbr2 expression, while anlagen-bound chondrocytes would turn on matrillin-1 expression (Sox9/Col2/Matn1-positive cells) (Fig. 2B). Gdf5-positive cells adjacent their respective cartilaginous anlagen -with a Sox9/Col2 history but negative for matrillin-1 expression- would differentiate into articular chondrocytes that could involve the specific action of genes including Erg and PTHrP (Chen et al., 2008; Iwamoto et al., 2007) (Fig. 2C). Additional differentiation processes and mechanisms would bring about the genesis of ligaments and other joint-specific structures (i.e., meniscus in the knee) that would involve Gdf5- and Tgfbr2-positive and -negative cells (Fig. 2D). Tgfbr2-expressing cells would also emerge to constitute a slow-cycling, reserve progenitor cell population that could have roles in repair and regeneration.
Fig. 2.
Model of limb joint formation and morphogenesis. (A) At early developmental stages, as yet unknown upstream determination mechanisms would identify and prescribe the location of the joints along Sox9/Col2/Dcx-expressing anlagen. (B) Soon after, Gdf5 expression would be activated along with other interzone-specific genes (see E) that would define the initial interzone mesenchymal population within the (Sox9/Col2/Matn1-positive cartilaginous anlagen. This would be accompanied by cell immigration from the flank, and cells located dorsally and ventrally would activate Tgfbr2 expression. (C) Gdf5-positive cells adjacent their respective cartilaginous anlagen -with a Sox9/Col2 history but negative for matrillin-1 expression- would differentiate into articular chondrocytes. (D) Additional differentiation processes and mechanisms such as muscle movement would bring about cavitation and genesis of other joint tissues such as ligaments and other meniscus involving Gdf5- and Tgfbr2-positive and -negative cell progenies. Note that the above distinct spatio-temporal steps -presented here as distinct for illustration purposes- may actually occur more closely and involve overlapping events. Also, the model may not entirely apply to other joints -including intervertebral and temporomandibular joints- that involve additional and/or diverse mechanisms. (E) Schematic summarizing local and long-range regulators that converge to regulate interzone gene expression at early stages of joint formation. Note that this list is not exhaustive.
Regulators of interzone and joint formation
The Gdf5-positive interzone cells are initially mesenchymal in character and then contribute to the genesis of cartilaginous and fibrocartilaginous joint tissues. Thus, they must possess mechanisms that dictate their distinct phenotypic and differentiation capacity at specific times and sites within the developing joint over time (Pacifici et al., 2005). Indeed, joint ablation and cartilage fusion were originally observed in general or conditional mouse embryo mutants lacking Noggin, Wnt4/Wnt9a, Tgfbr2 or β-catenin, likely caused in each case by ectopic, excess and/or precocious chondrogenic cell differentiation at prospective joint sites (Brunet et al., 1998; Koyama et al., 2008; Spagnoli et al., 2007; Spater et al., 2006). This conclusion and explanation were also reached to account for joint defects and partial/complete fusion observed in mouse embryos deficient in TGFβ-activated kinase 1 (TAK1) (Gunnell et al., 2010) or heparan sulfate synthase Ext1 (Mundy et al., 2011). As pointed out above, joint fusion also occurs in mouse embryos lacking Ihh expression in pre-hypertrophic growth plate chondrocytes, a finding originally revealing that interzone formation and joint development are regulated in concert and close coordination with shaft and growth plate development (St-Jacques et al., 1999). This intriguing aspect of limb skeletogenesis was reiterated by the interesting observation that mouse embryo mutants lacking the master chondrogenic genes Sox5 and Sox6 display severe interzone, joint, meniscal as well as growth plate defects (Dy et al., 2010).
Additional insights into the regulation of interzone gene expression and phenotype come from very recent and important studies. Kan and collaborators found that Sox11 was initially expressed broadly in early limb prechondrogenic condensations, but became restricted to the interzone and when over-expressed, stimulated Gdf5 gene expression (Kan et al., 2013). Gao and coworkers showed that the zinc finger transcription factors Osr1 and Osr2 were not needed for onset of Gdf5, Wnt4 and Wnt9a expression in incipient limb interzones, but in their absence the expression of those interzone genes was not sustained and was followed by fusion of limb joints over embryonic time (Gao et al., 2011). Searching for upstream regulators of Wnt9a expression, Kan and Tabin identified c-Jun as a pivotal regulator acting at the enhancer level that when ablated, deranged both Wnt signaling and initiation and progression of joint formation (Kan and Tabin, 2013). Wnt signaling is also required to sustain the function and regulate the thickness of the joint’s superficial zone (Yasuhara et al., 2011; Yuasa et al., 2009). In a similar fashion, Longobardi and collaborators carried out laser capture-assisted gene arrays of tissue samples from E14.5 mouse embryo digits. They found that joint-forming interzone cells were characterized by low expression of chemokines, and in particular MCP-5, compared to adjacent growth plate chondrocytes, and that low MCP-5 expression was required for interzone and joint formation (Longobardi et al., 2012). Digit joint fusion occurring in Tgfbr2-deficient mouse embryos was rescued by concurrent blockade of the MCP-5 receptor CCR2. In line with studies cited above, the authors concluded that joint formation is closely linked to, and coordinately regulated with, shaft development, and that the TβRII/MCP-5 axis is an essential crossroad for this process. Jenner and colleagues recently completed transcriptional profiling of intermediate and flanking outer interzone layers of the mouse knee just prior to cavitation (Jenner et al., 2014). As in the study described above (Longobardi et al., 2012a), they found that genes related to inflammation and actin cytoskeletal organization were differentially regulated in the intermediate interzone. Interestingly, genes associated with cartilage hypertrophy and bone formation were highly upregulated in the flanking outer interzone layers, leading the authors to hypothesize that cells within these layers proceed to endochondral ossification while those within the intermediate interzone form articular cartilage. Clearly, many distinct effectors and molecular regulators acting at the local and long-range levels participate in the regulation of interzone gene expression patterns, fate and function (Fig. 2E).
During late embryogenesis and early postnatal life, the developing joints have to undergo cavitation to create a fluid and lubricant-filled synovial cavity that is needed for unhindered movement and body function (Fig. 2D). Given its fundamental nature, this process has attracted research interest for decades that continues unabated (Pitsillides and Ashhurst, 2008). Immobilization induced by experimental nerve damage has long been known to inhibit cavitation in developing limb joints (Fell and Canti, 1934; Osborne et al., 2002). This finding and implications were reinforced by studies indicating that movement stimulates the establishment of joint superficial cell phenotype, characterized by production of essential lubricants including hyaluronate and lubricin (Dowthwaite et al., 2003b). The importance of movement in joint formation and the specific pathways regulated by movement have been reassessed and illuminated by recent studies using muscleless mouse embryo mutants (Kahn et al., 2009). In the absence of muscles, the authors found that joint progenitor cell fate was not maintained, joint-associated Wnt/β-catenin signaling was not activated, and joints failed to form properly. These defects were observed in several limb joints including the elbow, but some joints appeared to be less or not at all affected, including the knee. These interesting findings raised the possibility that development of diverse limb joints may also involve differential responses to common cues such as muscle-driven movement. In a related and as interesting study, Pazin and collaborators carried out gene array-based molecular profiling to identify factors that direct the development of elbow and knee in mouse embryos (Pazin et al., 2012). They identified the period between E15 and E16 in which clear elbow- and knee-specific molecular identities could be uncovered. Intriguingly, they found that the elbow joints displayed enriched expression of muscle specification and development genes, in line with the derangement of elbow joint development in the muscleless mutants described above (Kahn et al., 2009). In comparison, the knee joints exhibited enrichment of genes belonging to the TGFβ signaling pathway consistent with knee defects seen in conditional limb Tgfbr2 mutants. The authors proposed that fundamentally different mechanisms contribute to the genesis of elbow and knee joints following interzone formation.
Prospectives
This brief synopsis does show that much progress has been made in the last few years toward a better understanding of limb joint formation. It is clear that joint formation and diversity appear to result from the combined interplays and complex orchestration amongst a multiplicity of players — including interzone and non-interzone cells, growth plate chondrocytes and skeletal muscles- and a multiplicity of growth factors, signaling proteins and transcription factors. Much, however, remains uncertain and challenging. Even the very definition of “interzone” is still vague and unclear, as there are no established criteria to define it and to identify its boundaries along the three major axes of development. The latter is not a trivial issue given studies such as the one by Li et al. (2013) identifying specific cell cohorts/niches within/near the interzone and our previous study showing an asymmetric distribution of Gdf5-positive interzone cells along the dorso-ventral axis in incipient digit joints (Koyama et al., 2007). A related and important lingering question is whether the “interzone” is made of similarly-endowed cells able to give rise to different joint tissues or whether the interzone is a mixture of predetermined cell subpopulations. Adult joints are found to contain stem/progenitor and slow-cycling cells (Candela et al., 2014; Dowthwaite et al., 2003a). Wu and colleagues recently used laser capture dissection and microarray analysis of human embryonic joint tissues to identify a surface phenotype (CD166low/negCD146low/negCD73+CD44lowBMPR1B+) of cells committed to a chondrocyte lineage (Wu et al., 2013). A subset of cells with this phenotype were maintained in the periarticular region of adult joints, and further analyses revealed the LIF, TGF-β, and BMP signaling pathways as potential regulators of their differentiation state. Furthermore, application of these findings allowed the authors to generate functional immature articular chondrocytes from pluripotent stem cell populations. These exciting findings will need to be followed by studies to clarify the nature of these stem/progenitor and slow-cycling cells in adult joints, whether they are related to — and descendant of — interzone cells, whether they constitute a single or multiple populations with similar/diverse capacities and roles in repair or joint inflammation, and how they could be mobilized to enhance the notoriously weak repair capacity of adult joints. Given that several transcription factors and signaling proteins converge to dictate the characteristic gene expression patterns and function of the interzone, what needs to be clarified and understood in the future is which specific developmental traits and processes each of those gene products controls, what hierarchies exist amongst them, how each gene is turn on and off, and whether the genes have developmental roles only or are reactivated and redeployed during repair or regeneration. By far, however, what remains both intriguing and obscure is how the three-dimensional architecture of different joints is brought about, how the two sides of each developing joint are able to form reciprocally-shaped surfaces, how each joint acquires distinct and unique components, and how articular cartilage remains permanent and long lasting and acquires its distinct zones. The road ahead is long, but the data and insights of the last few years are certainly paving the way toward effective progress in the near future.
Acknowledgments
Work we originally carried out and summarized here was supported by NIH grants AR062908 and AR046000. R.S.D. is the recipient of a postdoctoral training grant (1F32AR064071) from the NIH. We express our gratitude to our several colleagues who contributed to the original studies described here, and apologize for not citing and describing the work of other relevant groups given the succinct format of this mini-review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Archer CW, Caterson B, Benjamin M, Ralphs JR. The Biology of the Synovial Joint. Harwood Academics; London: 1999. [Google Scholar]
- Archer CW, Dowthwaite GP, Francis-West P. Development of synovial joints. Birth Defects Res, Pt C. 2003;69:144–155. doi: 10.1002/bdrc.10015. [DOI] [PubMed] [Google Scholar]
- Broom ND, Poole CA. A functional-morphological study of the tidemark region of articular cartilage maintained in a non-viable physiological condition. J Anat. 1982;135:65–82. [PMC free article] [PubMed] [Google Scholar]
- Brunet LJ, McMahon JA, McMahon AP, Harland RM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280:1455–1457. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- Candela ME, Cantley L, Yasuhara R, Iwamoto M, Pacifici M, Enomoto-Iwamoto M. Distribution of slow-cycling cells in epiphyseal cartilage and requirement of β-catenin signaling for their maintenance in growth plate. J Ortho Res. 2014;32:661–668. doi: 10.1002/jor.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Macica CM, Nasiri A, Broadus AE. Regulation of articular chondrocyte proliferation and differentiation by indian hedgehog and parathyroid hormone-related proteins in mice. Arthr Rheum. 2008;58:3788–3797. doi: 10.1002/art.23985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig FM, Bentley G, Archer CW. The spatial and temporal pattern of collagens I and II and keratan sulphate in the developing chick metatarsophalangeal joint. Development. 1987;99:383–391. doi: 10.1242/dev.99.3.383. [DOI] [PubMed] [Google Scholar]
- Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJR, Haughton L, Bayram Z, Boyer S, Thomson B, Wolfe MS, Archer CW. The surface of articular cartilage contains a progeniotr cell population. J Cell Sci. 2003a;117:889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- Dowthwaite GP, Flannery CR, Flannelly J, Lewthwaite JC, Archer CW, Pitsillides AA. A mechanism underlying the movement requirement for synovial joint cavitation. Matrix Biol. 2003b;22:311–322. doi: 10.1016/s0945-053x(03)00037-4. [DOI] [PubMed] [Google Scholar]
- Dy P, Smits P, Silvester A, Penzo-Mendez A, Dumitriu B, Han Y, La Motte CD, Kingsley DM, Lefebvre V. Synovial joint morphogenesis requires the chondrogenic action of Sox5 and Sox6 in growth plate and articular cartilage. Dev Biol. 2010;341:346–359. doi: 10.1016/j.ydbio.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell HB, Canti RG. Experiments on the development in vitro of the avian knee joint. Proc R Soc London. 1934;116:316–351. [Google Scholar]
- Gao YH, Lan Y, Liu H, Jiang R. The zinc finger transcription factors Osr1 aand Osr2 control synovial joint formation. Dev Biol. 2011;352:83–91. doi: 10.1016/j.ydbio.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnell LM, Jonason JH, Loiselle AE, Kohn A, Schwarz EM, Hilton MJ, O’Keefe RJ. TAK1 regulates cartilage an djoint development via the MAPK and BMP signaling pathways. J Bone Min Res. 2010;25:1784–1797. doi: 10.1002/jbmr.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrick MW. Primate origins: evolutionary change in digital ray patterning and segmentation. J Hum Evol. 2001;40:339–351. doi: 10.1006/jhev.2001.0467. [DOI] [PubMed] [Google Scholar]
- Hinchliffe JR, Johnson DR. The Development of the Vertebrate Limb. Oxford University Press; New York: 1980. pp. 72–83. [Google Scholar]
- Holder N. An experimental investigation into the early development of the chick elbow joint. J Embryol Exp Morphol. 1977;39:115–127. [PubMed] [Google Scholar]
- Hunziker EB, Kapfinger E, Geiss MD. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthr Cart. 2007;15:403–413. doi: 10.1016/j.joca.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Hyde G, Boot-Handford RP, Wallis GA. Col2a1 lineage tracing reveals that the meniscus of the knee joint has a complex cellular origin. J Anat. 2008;213:531–538. doi: 10.1111/j.1469-7580.2008.00966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde G, Dover S, Aszodi A, Wallis GA, Boot-Handford RP. Lineage tracing using matrilin-1 gene expression reveals that articular chondrocytes exist as the joint interzone forms. Dev Biol. 2007;304:825–833. doi: 10.1016/j.ydbio.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto M, Tamamura Y, Koyama E, Komori T, Takeshita N, Williams JA, Nakamura T, Enomoto-Iwamoto M, Pacifici M. Transcription factor ERG and joint and articular cartilage formation during mouse limb and spine skeletogenesis. Dev Biol. 2007;305:40–51. doi: 10.1016/j.ydbio.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay GD, Tantravahi U, Britt DE, Barrach HJ, Cha CJ. Homology of lubricin and superficial zone protein (SZP): products of megakaryocyte stimulating factor (MSF) gene expression by human synovial fibroblasts and articular chondrocytes localized to chromosome 1q25. J Orthop Res. 2001;19:677–687. doi: 10.1016/S0736-0266(00)00040-1. [DOI] [PubMed] [Google Scholar]
- Jenner F, IJpma A, Cleary M, Heijsman D, Narcisi R, van der Spek PJ, Kremer A, van Weeren R, Brama P, van Osch GJ. Differential Gene Expression of the Intermediate and Outer Interzone layers of developing articular cartilage in murine embryos. Stem Cells and Dev 2014 doi: 10.1089/scd.2013.0235. -Not available-, ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn J, Shwartz Y, Blitz E, Krief S, Sharir A, Breitel DA, Rattenbach R, Relaix F, Maire P, Roundtree RB, Kingsley DM, Zelzer E. Muscle contraction is necessary to maintain joint progenitor cell fate. Dev Cell. 2009;16:734–743. doi: 10.1016/j.devcel.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Kan A, Ikeda T, Fukai A, Nakagawa T, Nakamura K, Chung UI, Kawaguchi H, Tabin CJ. SOX11 contributes to the regulation of GDF5 in joint maintenance. BMC Dev Biol. 2013;13:4. doi: 10.1186/1471-213X-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan A, Tabin CJ. c-Jun is required for specification of joint cell fates. Genes Dev. 2013;27:514–524. doi: 10.1101/gad.209239.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama E, Ochiai T, Rountree RB, Kingsley DM, ENOMOTO-IWAMOTO M, Iwamoto M, Pacifici M. Synovial Joint Formation during Mouse Limb Skeletogenesis. Ann NY Acad Sci. 2007;1116:100–112. doi: 10.1196/annals.1402.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama E, Shibukawa Y, Nagayama M, Sugito H, Young B, Yuasa T, Okabe T, Ochiai T, Kamiya N, Rountree RB, Kingsley DM, Iwamoto M, Enomoto-Iwamoto M, Pacifici M. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev Biol. 2008;316:62–73. doi: 10.1016/j.ydbio.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J-S, Hosseini A, Gadikoda HR, Li G. Kinesiology of the knee joint. In: O’Keefe RJ, Jacobs JJ, Chu CR, Einhorn TA, editors. Orthopaedic Basic Science: Foundations of Clinical Practice. Amer. Acad. Orthop. Surgeons; Rosemont, IL: 2013a. pp. 261–278. [Google Scholar]
- Li TF, Longobardi L, Myers TJ, Temple JD, Chandler RL, Ozkan H, Contaldo C, Spagnoli A. Joint TGF-β type II receptor-expressing cells: ontogeny and characterization as joint progenitors. Stem Cells Dev. 2013b;22:1342–1359. doi: 10.1089/scd.2012.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longobardi L, Li T, Myers TJ, O’Rear L, Ozkan H, Li Y, Contaldo C, Spagnoli A. TGF-beta type II receptor/MCP-5 axis: at the crossroad between joint and growth plate development. Dev Cell. 2012a;23:71–81. doi: 10.1016/j.devcel.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrovic D. Development of the diathrodial joints in the rat embryo. Am J Anat. 1978;151:475–485. doi: 10.1002/aja.1001510403. [DOI] [PubMed] [Google Scholar]
- Mundy C, Yasuda T, Kinumatsu T, Yamaguchi Y, Iwamoto M, Enomoto-Iwamoto M, Koyama E, Pacifici M. Synovial jint formation requires local Ext1 expression and heparan sulftae production in developing mous eembryo limbs and spine. Dev Biol. 2011;351:70–81. doi: 10.1016/j.ydbio.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalin AM, Greenlee TK, Sandell LJ. Collagen gene expression during development of avian synovial joints: transient expression of type II and XI collagen genes in the joint capsule. Dev Dyn. 1995;203:352–362. doi: 10.1002/aja.1002030307. [DOI] [PubMed] [Google Scholar]
- Niedermaier M, Schwabe GC, Fees S, Helmrich A, Brieske N, Seeman P, Hecht JT, Seitz V, Stricker S, Leschik G, Schrock E, Selby B, Mundlos S. An inversion involving the mouse Shh locus results in brachydactyly through dysregulation of Shh expression. J Clin Invest. 2005;115:900–909. doi: 10.1172/JCI200523675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne AC, Lamb KJ, Lewthwaite JC, Dowthwaite GP, Wheeler-Jones CPD, Pitsillides AA. Short-term rigid and flaccid paralysis diminish growth of embryonic chick limbs and abrogate joint cavity formation but differentially preserve pre-cavitated joints. J Musculosk Neuron Interactions. 2002;2:448–456. [PubMed] [Google Scholar]
- Pacifici M, Koyama E, Iwamoto M. Mechanisms of synovial joint and articular cartilage formation: recent advances, but many lingering mysteries. Birth Def Res Pt C. 2005;75:237–248. doi: 10.1002/bdrc.20050. [DOI] [PubMed] [Google Scholar]
- Pacifici M, Koyama E, Shibukawa Y, Wu C, Tamamura Y, Enomoto-Iwamoto M, Iwamoto M. Cellular and molecular mechanisms of synovial joint and articular cartilage formation. Ann NY Acad Sci. 2006;1068:74–86. doi: 10.1196/annals.1346.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazin DE, Gamer LW, Cox KA, Rosen V. Molecular profiling of synovial joints: use of microarray analysis to identify factors that direct the development of the knee and elbow. Dev Dyn. 2012;241:1816–1826. doi: 10.1002/dvdy.23861. [DOI] [PubMed] [Google Scholar]
- Pitsillides AA, Ashhurst DE. A critical evaluation of specific aspects of joint development. Dev Dyn. 2008;237:2284–2294. doi: 10.1002/dvdy.21654. [DOI] [PubMed] [Google Scholar]
- Provot S, Zinyk DL, Gunes Y, Kathri R, Le Q, Kronenberg HM, Johnson RS, Longaker MT, Giaccia AJ, Schipani E. Hif–1alpha regulates differentiation of limb bud mesenchyme and joint development. J Cell Biol. 2007;177:451–464. doi: 10.1083/jcb.200612023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rountree RB, Schoor M, Chen H, Marks ME, Harley V, Mishina Y, Kingsley DM. BMP receptor signaling is required for postnatal maintenance of articular cartilage. PLoS Biology. 2004;2:1815–1827. doi: 10.1371/journal.pbio.0020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeda T, Deng JM, de Crombrugghe B, Behringer RR, Nakamura T, Akiyama H. Sox9-expressing precursors are the cellular origin of the cruciate liament of the knee joint and the limb tendons. Genesis. 2010;48:635–644. doi: 10.1002/dvg.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnoli A, O’Rear L, Chandler RL, Granero-Molto F, Mortlock DP, Gorska AE, Weis JA, Longobardi L, Chytil A, Shimer K, Moses HL. TGF-β signaling is essential for joint morphogenesis. J Cell Biol. 2007;177:1105–1117. doi: 10.1083/jcb.200611031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spater D, Hill TP, Gruber M, Hartmann C. Role of canonical Wnt-signaling in joint formation. Eur Cells Materials. 2006;12:71–80. doi: 10.22203/ecm.v012a09. [DOI] [PubMed] [Google Scholar]
- St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2076–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm EE, Kingsley DM. Joint patterning defects caused by single and double mutations in members of the bone morphogenetic protein (BMP) family. Development. 1996;122:3969–3979. doi: 10.1242/dev.122.12.3969. [DOI] [PubMed] [Google Scholar]
- Wu L, Bluguermann C, Kyupelyan L, Latour B, Gonzalez S, Shah S, Galic Z, Ge S, Zhu Y, Petrigliano Frank A, Nsair A, Miriuka Santiago G, Li X, Lyons Karen M, Crooks Gay M, McAllister David R, Van Handel B, Adams John S, Evseenko D. Human Developmental Chondrogenesis as a Basis for Engineering Chondrocytes from Pluripotent Stem Cells. Stem Cell Rep. 2013;1:575–589. doi: 10.1016/j.stemcr.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara R, Ohta Y, Yuasa T, Kondo N, Hoang T, Addya S, Fortina P, Pacifici M, Iwamoto M, Enomoto-Iwamoto M. Roles of β-catenin signaling in phenotypic expression and proliferation of articular cartilage superficial zone cells. Lab Invest. 2011;91:1739–1752. doi: 10.1038/labinvest.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa T, Kondo N, Yasuhara R, Shimono K, Mackem S, Pacifici M, Iwamoto M, Enomoto-Iwamoto M. Transient Activation of Wnt/β-Catenin Signaling Induces Abnormal Growth Plate Closure and Articular Cartilage Thickening in Postnatal Mice. Am J Pathol. 2009;175:1993–2003. doi: 10.2353/ajpath.2009.081173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Cigan AD, Marrero L, Lopreore C, Liu S, Ge D, Savoie FH, You Z. Expression of doublecortin reveals articular chondrocyte lineage in mouse embryonic limbs. Genesis. 2010;49:75–82. doi: 10.1002/dvg.20702. [DOI] [PubMed] [Google Scholar]