Abstract
Anemia is common in older persons and is associated with substantial morbidity and mortality. One third of anemic older adults have unexplained anemia of the elderly (UAE). We carried out a randomized, wait list control trial in outpatients with UAE and serum ferritin levels between 20 and 200 ng/mL. Intravenous iron sucrose was given as a 200-mg weekly dose for 5 weeks either immediately after enrollment (immediate intervention group) or following a 12-week wait list period (wait list control group). The primary outcome measure was change in 6-minute walk test (6MWT) distances from baseline to 12 weeks between the two groups. Hematologic, physical, cognitive, and quality of life parameters were also assessed. The study was terminated early after 19 subjects enrolled. The distance walked in the 6MWT increased a mean 8.05±55.48 m in the immediate intervention group and decreased a mean 11.45±49.46 m in the wait list control group (p=0.443). The hemoglobin increased a mean 0.39±0.46 g/dL in the immediate intervention group and declined a mean 0.39±0.85 g/dL in the wait list control group (p=0.026). Thus, a subgroup of adults with UAE may respond to intravenous iron. Enrollment of subjects into this type of study remains challenging.
Keywords: unexplained anemia, hemoglobin, intravenous iron, 6-minute walk test
Introduction
Anemia is common in older adults, with a prevalence of approximately 10% in community-dwelling men and women aged 65 and older, rising to 20–35% in those aged 85 and above.1, 2 Although on an individual basis anemia in older adults is frequently overlooked or ignored, studies from numerous older populations throughout the developed world have consistently demonstrated an association between anemia, which is typically mild, and poor clinical outcomes, including decreased physical performance and strength3, 4, decreased mobility function5, impairment in instrumental activities of daily living6, increased frailty7, impaired quality of life8, decreased cognitive function9, and increased mortality.10, 11
Anemia has many causes. Data from large population-based surveys have ascertained several broad etiologies of anemia in older adults: iron deficiency that is possibly nutritional but more often secondary to blood loss, anemia associated with inflammation, anemia due to renal insufficiency, anemia due to nutritional deficiencies, and unexplained anemia of the elderly (UAE). UAE, a relatively new diagnostic category, is consistently found in approximately 30–44% of older anemic subjects.1, 2, 12 Prospective studies incorporating a thorough clinical evaluation have demonstrated similar proportions of UAE.13, 14 Iron deficiency in older adults may be difficult to identify, with the diagnosis confirmed only by response to a trial of iron supplementation.13 In addition, patients who do not respond to oral iron may have a rise in hemoglobin following the administration of intravenous iron.15 The Partnership for Anemia: Clinical and Translational Trials in the Elderly (PACTTE) consortium was formed to investigate treatment strategies in subjects with UAE. This study was designed as the first PACTTE interventional study, utilizing intravenous iron sucrose (IVIS) in a subset of subjects with UAE.
Materials and methods
Study design
The study was designed as a randomized, wait list control trial. Subjects were randomized to receive IVIS either immediately after enrollment (immediate intervention group) or after an initial waiting period of 12 weeks (wait list control group). The protocol was approved by an independent data and safety monitoring board (DSMB) as well as the institutional review board at each participating institution, and the trial was conducted in accordance with the Declaration of Helsinki for biomedical research involving human subjects. Written informed consent was provided by all subjects. The trial was designed, implemented, and overseen by the PACTTE Steering Committee. An independent DSMB reviewed the safety data and study progress on an ongoing basis.
Participants
Outpatient men and women with the following criteria were eligible to enroll: age ≥65 years with a hemoglobin concentration of ≥9 g/dL and <11.5 g/dL for women or <12.7 g/dL for men with unexplained anemia; serum ferritin between 20 and 200 ng/mL (inclusive); ability to walk without the use of a walker or motorized device, or the assistance of another person; lack of significant cognitive impairment defined by a Montreal Cognitive Assessment score of 22 or higher; and ability to understand and speak English (Table 1). The protocol initially included subjects with a serum ferritin between 20 and 100 ng/mL (inclusive) but was modified on March 26, 2012, due to poor recruitment to allow serum ferritin levels between 20 and 200 ng/mL (inclusive). The protocol was additionally modified on August 20, 2012, at sites with Spanish-speaking study staff to include subjects who were able to speak and understand Spanish. Unexplained anemia was defined, similar to published criteria13, 14, as not meeting criteria for any known etiology of anemia, including vitamin B12, folate, or iron deficiency (defined as serum ferritin < 20 ng/mL); renal insufficiency (defined as glomerular filtration rate of less than 3016 using the four-variable Modification of Diet in Renal Disease equation17); thyroid dysfunction; myelodysplastic syndrome; anemia of inflammation; plasma cell dyscrasia; thalassemia trait; alcohol overuse; any prior history of hematologic malignancy; unexplained splenomegaly or lymphadenopathy; or the presence of any condition reasonably assumed to be causing anemia and not corrected for 3 months (Table 2). Subjects were excluded if they had received a red blood cell transfusion, intravenous iron, or an erythropoiesis stimulating agent within 3 months prior to enrollment; had had unstable angina, a myocardial infarction, a stroke, or a transient ischemic attack within 3 months prior to enrollment; had uncontrolled hypertension; had a positive fecal occult blood test during the screening period; had significant impairment in liver function; had a documented history of anaphylactic reaction to iron sucrose infusion; had recently initiated oral iron supplementation; or if the distance walked on the 6-minute walk test (6MWT) was above the median for age and sex, to avoid a ceiling effect (Table 1; Appendix A).
Table 1.
Study entry criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
Table 2.
Criteria for common anemia etiologies
| Conditions | Criteria |
|---|---|
| Vitamin B12 deficiency | Vitamin B12 < 200 pg/mL Further diagnostic studies (e.g., methylmalonic acid) or a treatment trial may be considered for a B12 level of 200–300 pg/dL. Oral or parental B12 treatment recommended. Oral therapy should entail 1000 mcg or more daily. A 3-month trial of B12 therapy with normalization of B12 level and ongoing anemia will exclude B12 deficiency anemia and allow enrollment. |
| Folate deficiency | Folate level < lower limit of normal A 3-month trial of adequate folate correction and ongoing anemia will exclude folate deficiency and allow enrollment. |
| Renal insufficiency | Creatinine clearance by 4-variable MDRD of < 30 mL/min |
| Thyroid dysfunction | Thyrotropin < 0.1 mcU/mL or > 10 mcU/mL 3 months of thyroid therapy with a normal free T4 and ongoing anemia will exclude thyroid dysfunction and allow enrollment. |
| Myelodysplastic syndrome | Myelodysplastic syndrome by bone marrow evaluation using the WHO criteria A bone marrow examination should be performed if clinically indicated. Indications include an MCV ≥ 100 fL, a platelet count < 120 K/uL, or a neutrophil count < 1200 K/uL not attributable to another cause. A bone marrow evaluation should be considered if prior chemotherapy, radiation therapy, an abnormal peripheral blood smear, or if over time the MCV has been rising, platelets falling, or leukocyte count falling. |
| Anemia of chronic inflammation/disease | Anemia in association with any inflammatory condition, including:
|
| Plasma cell dyscrasia | Multiple myeloma or plasma cell dyscrasia that may be causing anemia Monoclonal gammopathy ≥ 1 g/dL. A monoclonal paraprotein of < 1 g/dL without bone lesions on skeletal survey, hypercalcemia, < 5% light chain restricted plasma cells, and no other evidence of progressive disease or multiple myeloma will exclude a plasma cell dyscrasia and be allowable. |
| Thalassemia trait | An MCV < 80 fL and red blood cell count within the normal reference range without iron deficiency in the appropriate ethnic group. An elevated hemoglobin A2 (> 3.5%) without iron deficiency on hemoglobin electrophoresis or demonstrated two deletions for alpha thalassemia confirms thalassemia trait. A single alpha thalassemia deletion will not be excluded on this protocol. A documented decline of hemoglobin of 1.5 g/dL over time without another cause except for baseline thalassemia will be acceptable to the protocol. |
| Alcohol | Evidence of alcohol overuse Overuse of alcohol will be determined by the clinician. Consider alcohol overuse if more than 3–4 drinks per occasion for woman and more than 4–5 drinks per occasion for men. A trial of 3 months of using < 3 drinks per week and ongoing anemia will exclude alcohol overuse. |
| Other | Physical exam showing lymphadenopathy ≥ 2 cm or splenomegaly without another cause. Any condition that can reasonably be assumed to be causing or a major contributor to the anemia and has not been corrected for 3 months. Any prior history of a hematologic malignancy. |
Abbreviations: MDRD, Modification of Diet in Renal Disease equation (186.3 × serum creatinine−1.154 × age−0.203 × [0.742 if female] × [1.212 if black]); WHO, World Health Organization; MCV, mean cell volume.
Randomization
Subjects were randomized to start IVIS either immediately (immediate intervention group) or after a 12-week wait list period (wait list control group) at a 1:1 ratio via an interactive voice and web response system. The randomization sequence was computer-generated with random block sizes. Neither subjects nor investigators were blinded.
Study therapy
The total administered dose of IVIS (Venofer®) supplied by Luitpold Pharmaceuticals was 1,000 mg given at a dose of 200 mg per week. The first dose was infused over at least 30 minutes; if there was no reaction encountered with administration of the first dose, each subsequent dose was administered over at least 15 minutes (or per local hospital pharmacy policy) in a maximum of 100 mL 0.9% sodium chloride. Monitoring included assessment of vital signs at baseline and every 15 minutes during the infusion and 15 minutes postinfusion.
Study outcomes
The primary outcome was change in 6MWT distances from baseline to 12 weeks. The primary outcome was chosen to assess whether iron repletion would improve functional impairment, which is of high importance in geriatric populations. Secondary outcomes included the change from baseline to 12 weeks for hemoglobin measurement, and quantification of the impact of anemia treatment on functional and self-report outcome measures as assessed by the Geriatric Evaluation Panel (GEP), consisting of the following:
Cognitive function based on the Trail Making Test and four CogState® cognitive subtests
Quality of life as measured by the 36-Item Short Form Health Survey (SF-36) physical component summary subscale and the Functional Assessment of Cancer Therapy–Anemia (FACT-An)
Four components of the frailty index: grip strength, 4-m walk speed, self-reported exhaustion, and self-reported activity level
For reporting purposes, the secondary outcomes based on the GEP are summarized as follows:
physical function: 4-m walk speed obtained as a component of the frailty index (see below);
cognitive function: primary cognitive outcome of Trail Making Test Part B in seconds per completed circle, with shorter time representing better executive function and cognitive flexibility, and secondary cognitive outcomes with three cognitive composite Z-scores, with higher score indicating better cognitive function (see Appendix B for definitions)—composite speed of processing Z-score, composite complex attention/executive processing Z-score, and composite learning and memory Z-score;
quality of life: SF-36 physical component summary score and FACT-An Total Score;
four binary components of frailty index as defined by the Women’s Health and Aging Studies criteria18 (see Appendix C): exhaustion (self-reported), low energy (based on self-reported activity level), weakness (based on grip strength), and slowness (based on 4-m walking speed). The overall frailty score based on the five components (including weight loss) of the frailty index was also used to describe the population at baseline.
The GEP was administered to each subject during the screening period and at weeks 12 and 24. The 6MWT was additionally measured at weeks 6 and 18. Safety outcomes included all clinical and reportable events.
Statistical considerations
We calculated that a sample size of 84 subjects, with 42 subjects per group, would provide 84% power to detect a clinical significant difference of 50 m in change of distances (the primary outcome) between the immediate intervention group and the wait list control group, with a type I error rate of 0.05. This calculation was based on a two-sample t-test by assuming a standard deviation of 115 m for the baseline 6MWT distance in both groups and correlations of 0.7 and 0.9 between distances at baseline and 12 weeks for the immediate intervention and wait list control groups, respectively. This sample size also took into account a 10% missing data rate.
Baseline characteristics were summarized using descriptive statistics, with categorical data presented as percentages and continuous data presented as the mean plus/minus standard deviation. Differences between treatment groups were assessed using a chi-square test or Fisher’s exact test (for small frequencies) for categorical data, and a t-test or Wilcoxon test (for non-normal data) for continuous data. The primary endpoint of change in 6MWT distances from baseline to 12 weeks between the two groups was tested using the two-sample t-test. All intent-to-treat patients were included in the primary analysis with an assumption that any missing data were missing completely at random. The impact of missing data in the primary analysis was examined by sensitivity analyses based on best and worst case scenarios for imputing the missing change. Per-protocol analysis was also performed on the primary outcome where the per-protocol population included only patients enrolled with eligible criteria who received the assigned treatment. All secondary outcomes were analyzed as exploratory analyses with a chi-square test or Fisher’s exact test for categorical data and a t-test or Wilcoxon test for continuous data. The level of statistical significance for all analyses was 0.05, and all analyses were two-sided. All analyses were performed with SAS software, version 9.2 (SAS, Cary, NC).
Results
Disposition and baseline characteristics of the study subjects
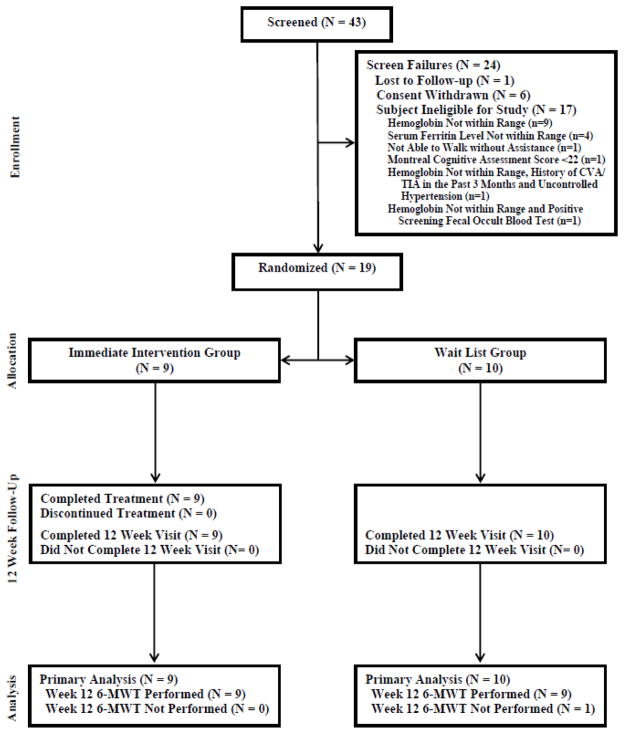
The study enrollment was from May 2011 to November 2012. The study was terminated early at DSMB recommendation after only 19 subjects were enrolled; poor enrollment persisted despite numerous recruitment initiatives. As shown in Figure 1, 43 subjects were identified as potentially eligible and underwent formal screening. Of these 43 subjects, 17 were ineligible, one was lost to follow up, and six withdrew consent. The remaining 19 subjects were randomized, nine to the immediate intervention group and 10 to the wait list control group. All subjects in the immediate intervention group completed the study. An additional patient in the wait list control group missed the 12-week 6MWT. The clinical characteristics of the 19 enrolled subjects are presented in Table 3. The mean age was 78.5 years, and 58% of enrolled subjects were female. The two randomized groups were similar with respect to key baseline clinical and laboratory characteristics, including serum ferritin, except for a few variables such as lower serum iron (55.3 vs. 84.5 mcg/dL, p=0.006), lower transferrin saturation (17.9% vs. 27.4%, p=0.015), and better composite learning and memory Z-score (0.69 vs. −0.48, p=0.012) in the immediate intervention group.
Fig. 1.
Flow of study subjects.
Table 3.
Characteristics of unexplained anemia subjects at baseline
| Demographic and clinical characteristics | Immediate intervention group (N=9) | Wait list control group (N=10) |
|---|---|---|
| Age at randomization (years) | 78.6±6.1 | 78.5±7.4 |
| Female sex, n (%) | 6 (66.7) | 5 (50.0) |
| Race, n (%) | ||
| White | 3 (33.3) | 6 (60.0) |
| Black or African American | 6 (66.7) | 3 (30.0) |
| Asian | 0 | 1 (10.0) |
| Medical history, n (%) | ||
| Hypertension | 7 (77.8) | 6 (60.0) |
| Diabetes | 2 (22.2) | 5 (50.0) |
| Medications, n (%) | ||
| Acetylsalicylic acid | 4 (44.4) | 8 (80.0) |
| Oral iron at screening | 1 (11.1) | 1 (10.0) |
| Vitamin B12 | 1 (11.1) | 1 (10.0) |
| MoCA score | 25.9 ±2.1 | 24.9 ±2.3 |
| Symptoms of anemia at enrollment, n (%) | ||
| Dizziness | 1 (11.1) | 0 |
| Pallor | 1 (11.1) | 3 (30) |
| Shortness of breath | 3 (33.3) | 1 (10) |
| Fatigue | 7 (77.8) | 5 (50) |
| No symptoms reported | 1 (11.1) | 0 |
| Baseline laboratory values | ||
| Hemoglobin (g/dL) | 11.19 ± 0.64 | 11.30 ±0.80 |
| Hematocrit (%) | 33.77 ± 1.86 | 35.62 ± 2.52 |
| Mean corpuscular volume (fL) | 89.94±6.84 | 92.48 ± 8.09 |
| Red cell distribution width (%) | N=9 14.76±1.31 |
N=9 14.78±0.99 |
| Reticulocyte count, absolute (109/L) | N=7 48.4±13.5 |
N=8 45.2±6.7 |
| Serum iron (mcg/dL) | 55.3±10.2 | 84.5±25.4 |
| Serum ferritin (ng/mL) | 79.1±54.1 | 53.0±26.1 |
| Total iron binding capacity (mcg/dL) | 313.3±49.1 | 321.3±71.7 |
| Transferrin saturation (%) | 17.9±4.0 | 27.4±9.7 |
| Serum erythropoietin (mIU/mL) | ||
| Creatinine (mg/dL) | 1.16±0.19 | 1.27±0.13 |
| eGFR (ml/min/1.73 m2)a | N=4 51.5±7.0 |
N=8 52.0±5.4 |
| eGFR category, n (%) | ||
| 30–59 | 4 (44.4) | 8 (80) |
| ≥ 60 | 5 (55.6) | 2 (20) |
| High-sensitivity C-reactive protein (mg/L) | N=9 4.712±5.258 |
N=8 1.961± 2.210 |
| Geriatric Evaluation Panel | ||
| Physical function | ||
| Baseline 6MWT results (m) | 351.40±67.01 | 344.80±90.30 |
| Baseline 4-m walk speed – usual walking speed (m/s) | N=6 0.736±0.0220 |
N=8 1.020±0.430 |
| Baseline 4-m walk speed – maximum walking speed (m/s) | N=7 1.054±0.224 |
N=8 1.325±0.393 |
| Cognitive function | ||
| Wechsler Test of Adult Reading standard score | N=7 107.6±21.4 |
N=10 100.0±23.6 |
| Trail Making Test Part B, seconds per completed circle | N = 9 6.37±3.18− |
N=6 14.43±14.82 |
| Composite speed of processing Z-score | N=8 0.16±0.97 |
N=10 −0.13±1.06 |
| Composite complex attention/executive processing Z-score | N=9 0.15±0.80 |
N=9 −0.15±1.20 |
| Composite learning and memory Z-score | N=7 0.69±0.67 |
N=10 −0.48±0.92 |
| Frailty, n/N (%) | ||
| Weight loss | 0/7 (0) | 2/9 (22.2) |
| Exhaustion | 1/9 (11.1) | 1/10 (10.0) |
| Low energy | 0/7 (0) | 0/8 (0) |
| Weakness based on grip strength | 1/6 (16.7) | 4/8 (50.0) |
| Slowness based on 4-m walk speed | 2/5 (40) | 1/8 (12.5) |
| Overall frailty score, n/N (%) | ||
| Robust | 1/4 (25) | 3/6 (50) |
| Pre-frail | 3/4 (75) | 2/6 (33.3) |
| Frail | 0/4 (0) | 1/6 (16.7) |
| Quality of life | ||
| Baseline SF-36 physical component summary score | 43.65±6.70 | 48.80±7.87 |
| Baseline FACT-An total score | N=9 147.9±28.7 |
N=8 156.4±23.9 |
There were no significant differences between the groups except serum iron (p=0.006), transferrin saturation (p=0.015), and the composite learning and memory Z-score (p=0.012).
Plus-minus values are mean ± SD (N reported if at least one subject in either treatment group was missing a response for the baseline characteristic).
Abbreviations: MoCA, Montreal Cognitive Assessment; eGFR, estimated glomerular filtration rate; 6MWT, 6-minute walk test; SF-36, 36-Item Short Form Health Survey; FACT-AN, Functional Assessment of Cancer Therapy–Anemia.
Results < 60 only.
Safety
Overall, the study intervention was well tolerated. A total of seven subjects in the immediate intervention arm and four subjects in the wait list control group had at least one treatment emergent adverse event reported. Two subjects in the immediate intervention group experienced what were deemed possibly treatment-related events (one patient reported back pain, and one reported cough), while one patient in the wait list control group reported nausea and “feeling hot” as probably treatment-related events. All of the possibly or probably treatment-related events were reported as mild. Two subjects experienced serious adverse events. One 87-year-old subject had acute cholecystitis 95 days after the first dose of study drug, as well as a urinary tract infection 171 days after the first dose of study drug. Another 80-year-old subject suffered a pelvic fracture and syncope, both 62 days after the first dose of study drug. None of the serious adverse events was considered to be treatment-related, and all resolved.
Primary outcome
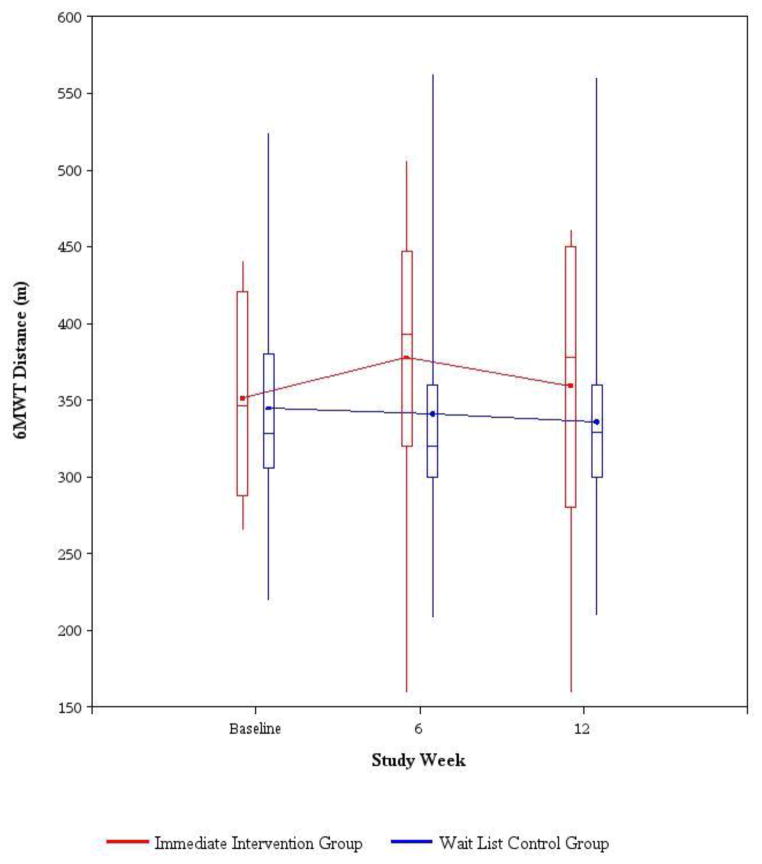
At baseline, the immediate intervention group walked a mean of 351.4±67.01 m and the wait list control group walked a mean of 344.80±90.30 m in 6 minutes (p=0.860), which were below the expected values for 75- to 79-year-old women and men (Appendix A). Primary analysis showed that the mean change in distance walked at 12 weeks was an increase of 8.05±55.48 m in the immediate intervention group and a decrease of 11.45±49.46 m in the wait list control group (p=0.443) (Table 4, Figure 2). Sensitivity analyses with imputation of data for the one subject in the wait list control group who had missing data on the 6MWT at 12 weeks were based on the best and worst scenarios, and yielded similar results (data not shown). The per-protocol analysis excluded one female subject who was incorrectly randomized but ineligible by hemoglobin criteria (with initial screening hemoglobin of 10.8 g/dL and a subsequent hemoglobin value drawn 12 days later of 11.5 g/dL, thus rendering her ineligible) and showed similar results (data not shown).
Table 4.
Change from baseline for outcome measures at week 12
| Outcome measures | Immediate intervention group | Wait list control group | Mean difference between groups (95% confidence interval) | p valuea |
|---|---|---|---|---|
| Primary outcome | ||||
| 6MWT (m) | N = 9 8.05±55.48 |
N= 9 −11.45±49.46 |
19.50 (−33.02–72.02) | 0.443 |
| Secondary outcomes | ||||
| Hemoglobin (g/dL) | N = 9 0.39±0.46 |
N = 10 −0.39±0.85 |
0.78 (0.11–1.45) | 0.026 |
| Geriatric Evaluation Panel | ||||
| Physical function: | ||||
| 4-m walk speed – usual walking speed (m/s) | N = 5 0.420±0.590 |
N = 6 −0.026±0.081 |
0.45 (−0.28–1.17) | 0.167 |
| 4-m walk speed maximum walking speed (m/s) | N = 6 0.413±1.107 |
N = 6 0.093±0.303 |
0.32 (−0.84–1.48) | 0.744 |
| Cognitive function: | ||||
| Trail Making Test Part B seconds per completed circle | N = 9 −0.77±3.54 |
N = 5 −4.96±8.14 |
4.18 (−5.74–14.11) | 0.325 |
| Composite speed of processing Z-score | N = 8 0.62±1.00 |
N = 9 1.08±0.88 |
−0.46 (−1.43–0.51) | 0.330 |
| Composite complex attention/executive processing Z-score | N = 9 0.36±1.28 |
N = 8 0.69±1.19 |
−0.33 (−1.61–0.96) | 0.595 |
| Composite learning and memory Z-score | N = 7 0.41±1.50 |
N = 7 1.39±2.36 |
−0.98 (−3.28–1.33) | 0.374 |
| Quality of life | ||||
| SF-36 physical component summary score | N = 8 −1.89±2.44 |
N = 9 −3.17±10.95 |
1.28 (−7.22–9.78) | 0.741 |
| FACT-An total score | N = 8 10.6±10.0 |
N = 6 0.0±34.7 |
10.6 (−25.7–47.0) | 0.495 |
| Frailty change from frail at baseline to not frail at week 12 | ||||
| Exhaustion | 1/9 (11.1) | 1/8 (12.5) | NA | >0.999 |
| Low energy | 0/7 (0) | 0/5 (0) | NA | NA |
| Weakness | 0/6 (0) | 0/7 (0) | NA | NA |
| Slowness | 0/4 (0) | 0/6 (0) | NA | NA |
Plus-minus values are mean ± SD.
Abbreviations: 6MWT, 6-minute walk test; SF-36, 36-Item Short Form Health Survey; FACT-An, Functional Assessment of Cancer Therapy – Anemia.
For the continuous variables, p-values are based on t-tests except for data that were not normally distributed, where Wilcoxon test p-values are reported; for categorical variables, p-values are based on Fisher’s exact tests.
Fig. 2.
6-minute walk test (6MWT) distance by study week. Solid line joins the average 6MWT distance (meters) for each treatment group. The bottom and top of the boxes are the 25th and 75th percentiles, the line in the box is the median, and the ends of the whiskers are the 5th and 95th percentiles.
Secondary outcomes
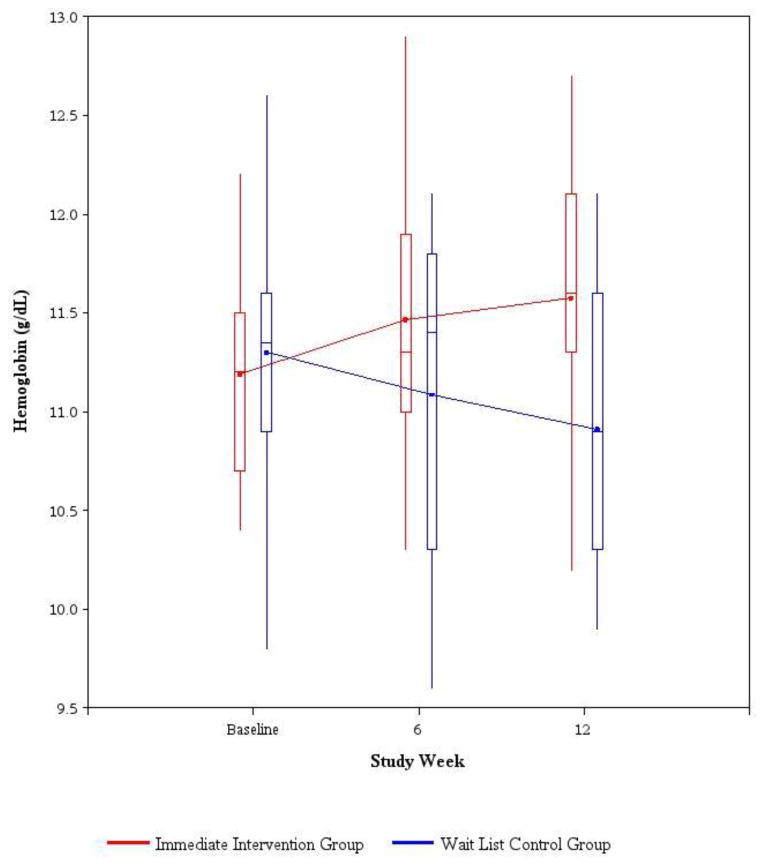
There was a small but statistically significant increase in hemoglobin of 0.39±0.46 g/dL at 12 weeks in the immediate intervention group compared to a decline in hemoglobin of 0.39±0.85 g/dL in the wait list control group (p=0.026, Table 4). One patient in each group had an increase in hemoglobin of at least 1 g/dL 12 weeks after receiving the first dose of IVIS (received immediately after screening in the immediate intervention group and after 12 weeks in the wait list control group). Over time, mean hemoglobin levels rose in the immediate intervention group but decreased in the wait list control group (Figure 3). Mean hemoglobin levels rose slightly in each study group 12 weeks after initiation of treatment with IVIS (from 11.19 g/dL to 11.58 g/dL in the immediate intervention group, and from 10.91 g/dL to 12.01 g/dL in the wait list control group). There were no significant differences in the two groups in change at 12 weeks for other secondary outcomes of physical, cognitive function, quality of life, and frailty (Table 4). There were no statistically significant correlations between the week 12 change in hemoglobin from baseline and any of the iron indices at baseline in either the immediate intervention group or the wait list control group (data not shown).
Fig. 3.
Hemoglobin (g/dL) by study week. Solid line joins the average hemoglobin for each treatment group. The bottom and top of the boxes are the 25th and 75th percentiles, the line in the box is the median, and the ends of the whiskers are the 5th and 95th percentiles
Discussion
This is the first exploratory intervention study aimed solely at treating older adults with UAE utilizing intravenous iron. We treated subjects with UAE and serum ferritin levels between 20 and 200 ng/mL (inclusive) with five weekly 200-mg doses of IVIS. Unfortunately, because of early termination of the study due to poor recruitment, the study is substantially underpowered to detect differences in the primary outcome. Thus, although the direction of changes in 6MWT results were as hypothesized—that is, the 6MWT improved in the immediate intervention group compared to the wait list control group the differences between the groups—were not significant. These results are compatible with a trial in older adults with heart failure and similar ferritin levels treated with intravenous ferric carboxymaltose.19 In that randomized, placebo-controlled study, the 304 subjects assigned to the ferric carboxymaltose arm had a mean increase in 6MWT results from 274 m to 312 m at 12-week follow-up, significantly higher than seen in the placebo group. The differing statistical significance of the results between the two studies may be explained by the very low numbers in our study hampering our ability to detect a significant difference in 6MWT results. Alternative possibilities include the differing study populations (unexplained anemia vs. congestive heart failure), dose and formulation of intravenous iron given, and baseline 6MWT results, which were higher in our study.
The study intervention was well tolerated, and there were no serious adverse events considered to be related to the study drug. With regard to secondary outcomes, a modest increase in hemoglobin was seen in the immediate intervention group compared to the wait list control group at 12 weeks. In addition, one patient in each group had an increase of at least 1 g/dL in hemoglobin at 12 weeks following initiation of IVIS. This suggests the possibility that a subgroup of subjects with UAE may respond to parenteral iron therapy. Interestingly, the increase in hemoglobin was not correlated with iron indices, although again, small numbers preclude making more definitive observations about these findings.
One of the lessons learned was the great difficulty in recruiting subjects to this type of study. All of the participating institutions were well-established clinical trial sites with histories of robust accrual to clinical trials. Subjects were vigorously recruited through multiple mechanisms, including specialty clinic and primary care referrals, the placement of study flyers at hospital and clinic sites, newspaper advertisements, the mailing of thousands of flyers to targeted population areas, electronic medical record searching, chart reviews, and investigator-led anemia lectures at local community and senior centers. Approximately 1,000 subjects were voluntarily reported by the sites to have been prescreened for the study. Nonetheless, despite intense recruitment efforts, including targeted mailing, which in some studies of the elderly has been shown to be the most effective recruitment maneuver20, 21, enrollment remained poor and the study was terminated early. Poor recruitment was likely driven by multiple factors, including the general clinician tendency to ignore typically mild anemia in older adults in the face of more prominent medical issues, the complex requirements for this study, including extensive functional testing, and the logistical difficulties for older adults in participating in interventional studies with involved follow-up. One of the most important barriers to recruitment was the overly restrictive eligibility criteria, which led to the exclusion of many subjects. In addition, the negative results from studies using erythropoietic agents may have blunted enthusiasm for anemia trials in general.22–24
Nonetheless, the issues remain of clinical importance. Although it is unknown whether anemia itself causes the extensive morbidity or mortality seen in older anemic adults, it is plausible to suggest that anemia, potentially leading to local tissue hypoxia, could aggravate functional decline and, furthermore, that treatment of anemia has the potential to ameliorate some, if not all, of these significant negative effects.
This trial involved performing a comprehensive battery of physical, cognitive, quality of life, and frailty tests. Although the findings were modest, this study shows that it is feasible to perform comprehensive evaluations in this population. Such investigation could form the basis for future studies in older anemic adults to better ascertain the exact benefits across relevant domains of physical function, cognition, quality of life, and frailty. Future trials focusing on treatment of UAE should utilize streamlined, patient-friendly design, minimal entry criteria, and intensive recruitment efforts tailored toward older adults. It will also be critical for future studies of UAE to significantly expand the patient recruitment base by increasing the numbers of participating institutions.
Acknowledgments
The authors thank Kerstin McHutchinson, Carrie Elliott, Kimberly Hickman, Joselene Sipin-Sayno, Ora White, Karina Ramirez, Mat Nelson, Nyesha Smith, Lisa Pape, Irene Flores, and Lani Krauz.
This work was funded by National Institutes of Health (NIH) Grant 5U01AG034661. IVIS was provided by Luitpold Pharmaceuticals. Neither the NIH nor Luitpold was involved in the study design; collection, analysis and interpretation of data; writing of the report; or the decision to submit the article for publication.
Appendix A: Upper 6MWT distances for exclusion as specified by age and sex
| Age (years) | Women (meters) | Men (meters) |
|---|---|---|
| 65–69 | 521 | 576 |
| 70–74 | 503 | 558 |
| 75–79 | 466 | 512 |
| 80–84 | 421 | 485 |
| 85–89 | 375 | 439 |
| >90 | 311 | 366 |
Appendix B: Cognitive function outcomes
Eight cognitive measures based on the Trail Making Test (TMT) Parts A and B and the CogState assessments are defined as follows:
-
TMT
Seconds per completed circle in TMT Part A (seconds per completed circle calculated from the time to complete the task [25 circles] or the number of circles completed in 180 seconds)
Seconds per completed circle in TMT Part B (seconds per completed circle calculated from the time to complete the task [25 circles] or the number of circles completed in 300 seconds)
-
CogState assessments (unit of measurement)
Simple reaction time from the CogState Detection task (log10 ms)
Choice reaction time from the CogState Identification task (log10 ms)
Time score from the CogState One-Back task (log10 ms)
Accuracy score from the CogState One-Back task (arcsine transformation of the square root of the proportion of correct responses)
Immediate recall score from the CogState International Shopping List (ISL) (number of correct responses)
Delayed recall score from the CogState ISL (number of correct responses)
In this paper, seconds per completed circle in TMT Part B is the primary cognitive outcome, with shorter time indicating better cognitive function.
In addition, three composite scores will be derived using the Z-scores specified below for each composite:
Speed of processing: (1) TMT Part A seconds per completed circle, (2) simple reaction time from the CogState Detection Task, and (3) choice reaction time from the CogState Identification Task
Complex attention/executive processing: (1) TMT Part B seconds per completed circle, (2) time score from the CogState One Back Task, and (3) accuracy score from the CogState One Back Task
Learning and memory: (1) CogState ISL immediate recall score (total score from three learning trials), (2) CogState ISL immediate recall score from the first learning trial, and (3) CogState ISL delayed recall scores.
The composite score for a subject at each time point will be defined as the mean of the Z-scores specified above for each composite at the time point. If one of the scores specified is missing, the mean was based on the available scores. The Z-score for each item at each time was derived by subtracting the overall baseline mean and then divided by the overall baseline standard deviation of this item. In order for all positive Z-scores to indicate a better cognitive function compared to the baseline average, a negative sign was added to the Z-scores for the TMT seconds per completed circle and the CogState time score from the One-Back Task, simple reaction time from the Detection Task, and choice reaction time from the Identification Task.
Appendix C: Definition of frailty (yes/no) based on the five components of frailty index
Based on Women’s Health and Aging Studies (WHAS) criteria*18:
-
Weight loss: If any of the following three criteria are met, the subject will be classified as frail for weight loss:
Body mass index at baseline is < 18.5 kg/m2.
“In the last year, have you lost more than 10 pounds unintentionally (i.e., not due to dieting or exercise)?” is answered “yes.”
Weight loss defined as (Weight in previous year baseline measured weight)/(weight in previous year) ≥ 0.05 and the loss was unintentional.
-
Subjective fatigue/exhaustion: If any of the following three criteria are met, the patient will be classified as frail for fatigue/exhaustion:
“In the past month, on average, have you been feeling unusually tired during the day?” is answered “yes” and indicated as “all of the time” or “most of the time.”
“In the past month, on average, have you felt unusually week?” is answered “yes” and indicated as “all of the time” or “most of the time.”
Energy level on a scale of 0 (no energy) to 10 (most energy) reported as ≤ 3.
-
Physical activity: Using six physical activities (walking for exercise, moderately strenuous household chores, moderately strenuous outdoor chores, dancing, bowling, regular exercise) condensed from the original 18 activities in the short version of the Minnesota Leisure Time Activity questionnaire, kilocalories (Kcals) per week expended are calculated utilizing a standardized algorithm. This variable is stratified by gender:
Men with Kcals of physical activity per week < 128 are frail.
Women with Kcals per week < 90 are frail.
-
4-meter walking speed (converted from cutpoints based on 15-foot walking time), stratified by gender and height (gender-specific cutoff = a medium height):
Cutoff for 4-meter usual walking speed criterion for frailty Men Height ≤ 173 cm ≤0.65 m/s Height > 173 cm ≤0.76 m/s Women Height ≤ 159 cm ≤0.65 m/s Height > 159 cm ≤0.76 m/s
If no walking speed is reported, subjects will be classified as frail for walking speed if they tried but were unable to walk, even with support, or if the examiner/subject felt it was unsafe to walk.
-
Grip strength, stratified by gender and body mass index (BMI) quartiles:
Cutoff for grip strength criterion for frailty25 Men BMI ≤ 24 ≤29 BMI 24.1–26 ≤30 BMI 26.1–28 ≤30 BMI > 28 ≤32 Women BMI ≤ 23 ≤17 BMI 23.1–26 ≤17.3 BMI 26.1–29 ≤18 BMI > 29 ≤21 The frailty criteria above will be used to define frailty for each of the five components (weight loss at baseline only). This will result in five binary outcomes for the five components of frailty. An overall frailty score (range from 0 to 5) will be calculated at baseline by summing the five binary outcomes. The combined binary frailty outcome (Yes/No) will be defined with cutpoint of 3 where subjects with an overall score of 3 or higher are considered as frail. Score of 1–2 is considered as pre-frail, and 0 is considered as robust. Only four components (grip strength, walking speed, fatigue/exhaustion, and physical activity) will be measured postbaseline as outcomes.
Footnotes
Frailty index was derived using the WHAS criteria except for weight loss. In this study, weight is only collected at baseline. Therefore, frailty for weight loss is based on BMI< 18.5 kg/m2 at baseline from WHAS and from the Cardiovascular Health Study (CHS), unintentional weight loss of > 10 pounds in the last year assessed at baseline as well as ≥ 5% unintentional weight loss at baseline compared to the previous year (instead of at follow-up compared to the previous year as specified by the WHAS and CHS criteria).
Conflict of interest disclosure
None of the authors had any financial, personal, or other interest in this work or perceived conflict of interest.
Author contributions
The PACTTE Steering Committee designed the study. HB and SS analyzed the data. EP, ASA, HB, SS, GC, SLS, and HJC prepared the manuscript. All authors critically revised the manuscript and approved the final version. The principal investigators have full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elizabeth Price, Email: eaprice@stanford.edu.
Andrew S. Artz, Email: aartz@medicine.bsd.uchicago.edu.
Huiman Barnhart, Email: huiman.barnhart@duke.edu.
Shelly Sapp, Email: shelly.sapp@duke.edu.
Gordon Chelune, Email: gordon.chelune@hsc.utah.edu.
William B. Ershler, Email: wershler@iasia.org.
Jeremy D. Walston, Email: jwalston@jhmi.edu.
Victor R. Gordeuk, Email: vgordeuk@uic.edu.
Nathan A. Berger, Email: nab@case.edu.
David Reuben, Email: dreuben@mednet.ucla.edu.
Josef Prchal, Email: josef.prchal@hsc.utah.edu.
Sunil V. Rao, Email: sunil.rao@dm.duke.edu.
Cindy N. Roy, Email: croy6@jhmi.edu.
Mark A. Supiano, Email: mark.supiano@utah.edu.
Stanley L. Schrier, Email: sschrier@stanford.edu.
Harvey Jay Cohen, Email: harvey.cohen@dm.duke.edu.
References
- 1.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the united states: Evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 2.Tettamanti M, Lucca U, Gandini F, Recchia A, Mosconi P, Apolone G, Nobili A, Tallone MV, Detoma P, Giacomin A, Clerico M, Tempia P, Savoia L, Fasolo G, Ponchio L, Della Porta MG, Riva E. Prevalence, incidence and types of mild anemia in the elderly: The “health and anemia” population-based study. Haematologica. 2010;95:1849–1856. doi: 10.3324/haematol.2010.023101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Penninx BW, Guralnik JM, Onder G, Ferrucci L, Wallace RB, Pahor M. Anemia and decline in physical performance among older persons. Am J Med. 2003;115:104–110. doi: 10.1016/s0002-9343(03)00263-8. [DOI] [PubMed] [Google Scholar]
- 4.Penninx BW, Pahor M, Cesari M, Corsi AM, Woodman RC, Bandinelli S, Guralnik JM, Ferrucci L. Anemia is associated with disability and decreased physical performance and muscle strength in the elderly. J Am Geriatr Soc. 2004;52:719–724. doi: 10.1111/j.1532-5415.2004.52208.x. [DOI] [PubMed] [Google Scholar]
- 5.Chaves PH, Ashar B, Guralnik JM, Fried LP. Looking at the relationship between hemoglobin concentration and prevalent mobility difficulty in older women. Should the criteria currently used to define anemia in older people be reevaluated? J Am Geriatr Soc. 2002;50:1257–1264. doi: 10.1046/j.1532-5415.2002.50313.x. [DOI] [PubMed] [Google Scholar]
- 6.Bang SM, Lee JO, Kim YJ, Lee KW, Lim S, Kim JH, Park YJ, Chin HJ, Kim KW, Jang HC, Lee JS. Anemia and activities of daily living in the korean urban elderly population: Results from the korean longitudinal study on health and aging (klosha) Ann Hematol. 2013;92:59–65. doi: 10.1007/s00277-012-1563-6. [DOI] [PubMed] [Google Scholar]
- 7.Chaves PH, Semba RD, Leng SX, Woodman RC, Ferrucci L, Guralnik JM, Fried LP. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: The women’s health and aging studies i and ii. J Gerontol A Biol Sci Med Sci. 2005;60:729–735. doi: 10.1093/gerona/60.6.729. [DOI] [PubMed] [Google Scholar]
- 8.Thein M, Ershler WB, Artz AS, Tecson J, Robinson BE, Rothstein G, Liede A, Gylys-Colwell I, Lu ZJ, Robbins S. Diminished quality of life and physical function in community-dwelling elderly with anemia. Medicine (Baltimore) 2009;88:107–114. doi: 10.1097/MD.0b013e31819d89d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andro M, Le Squere P, Estivin S, Gentric A. Anaemia and cognitive performances in the elderly: A systematic review. Eur J Neurol. 2013;20:1234–1240. doi: 10.1111/ene.12175. [DOI] [PubMed] [Google Scholar]
- 10.Riva E, Tettamanti M, Mosconi P, Apolone G, Gandini F, Nobili A, Tallone MV, Detoma P, Giacomin A, Clerico M, Tempia P, Guala A, Fasolo G, Lucca U. Association of mild anemia with hospitalization and mortality in the elderly: The health and anemia population-based study. Haematologica. 2009;94:22–28. doi: 10.3324/haematol.13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zakai NA, Katz R, Hirsch C, Shlipak MG, Chaves PH, Newman AB, Cushman M. A prospective study of anemia status, hemoglobin concentration, and mortality in an elderly cohort: The cardiovascular health study. Arch Intern Med. 2005;165:2214–2220. doi: 10.1001/archinte.165.19.2214. [DOI] [PubMed] [Google Scholar]
- 12.Ferrucci L, Guralnik JM, Bandinelli S, Semba RD, Lauretani F, Corsi A, Ruggiero C, Ershler WB, Longo DL. Unexplained anaemia in older persons is characterised by low erythropoietin and low levels of pro-inflammatory markers. Br J Haematol. 2007;136:849–855. doi: 10.1111/j.1365-2141.2007.06502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price EA, Mehra R, Holmes TH, Schrier SL. Anemia in older persons: Etiology and evaluation. Blood Cells Mol Dis. 2011;46:159–165. doi: 10.1016/j.bcmd.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Artz AS, Thirman MJ. Unexplained anemia predominates despite an intensive evaluation in a racially diverse cohort of older adults from a referral anemia clinic. J Gerontol A Biol Sci Med Sci. 2011;66:925–932. doi: 10.1093/gerona/glr090. [DOI] [PubMed] [Google Scholar]
- 15.Goodnough LT, Morris D, Koch T, He A, Bregman D. Hepcidin levels predict non-responsiveness to oral iron therapy in patients with iron deficiency anemia. American Society of Hematology Annual Conference. 2012;120:484. doi: 10.1002/ajh.23354. [DOI] [PubMed] [Google Scholar]
- 16.Ble A, Fink JC, Woodman RC, Klausner MA, Windham BG, Guralnik JM, Ferrucci L. Renal function, erythropoietin, and anemia of older persons: The inchianti study. Arch Intern Med. 2005;165:2222–2227. doi: 10.1001/archinte.165.19.2222. [DOI] [PubMed] [Google Scholar]
- 17.National Kidney Foundation. Calculators for health care professionals. www.Kidney.Org/professionals/kdoqi/gfr_calculator.Cfm.
- 18.Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, Zeger SL, Fried LP. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 19.Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Luscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart Rothe B, Pocock SJ, Poole-Wilson PA, Ponikowski P. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 20.Forster SE, Jones L, Saxton JM, Flower DJ, Foulds G, Powers HJ, Parker SG, Pockley AG, Williams EA. Recruiting older people to a randomised controlled dietary intervention trial--how hard can it be? BMC Med Res Methodol. 2010;10:17. doi: 10.1186/1471-2288-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanders KM, Stuart AL, Merriman EN, Read ML, Kotowicz MA, Young D, Taylor R, Blair-Holt I, Mander AG, Nicholson GC. Trials and tribulations of recruiting 2,000 older women onto a clinical trial investigating falls and fractures: Vital d study. BMC Med Res Methodol. 2009;9:78. doi: 10.1186/1471-2288-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizzo JD, Brouwers M, Hurley P, Seidenfeld J, Arcasoy MO, Spivak JL, Bennett CL, Bohlius J, Evanchuk D, Goode MJ, Jakubowski AA, Regan DH, Somerfield MR. American society of clinical oncology/american society of hematology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. J Clin Oncol. 2010;28:4996–5010. doi: 10.1200/JCO.2010.29.2201. [DOI] [PubMed] [Google Scholar]
- 23.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 24.Swedberg K, Young JB, Anand IS, Cheng S, Desai AS, Diaz R, Maggioni AP, McMurray JJ, O’Connor C, Pfeffer MA, Solomon SD, Sun Y, Tendera M, van Veldhuisen DJ. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med. 2013;368:1210–1219. doi: 10.1056/NEJMoa1214865. [DOI] [PubMed] [Google Scholar]
- 25.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol. 2001;56A:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]