Abstract
We present a programmable acoustofluidic pump that utilizes the acoustic streaming effects generated by the oscillation of tilted sharp-edge structures. This sharp-edge based acoustofluidic pump is capable of generating stable flow rates as high as 8 μL/min (~76 Pa of pumping pressure), and it can tune flow rates across a wide range (nL/min to μL/min). Along with its ability to reliably produce stable and tunable flow rates, the acoustofluidic pump is easy to operate and requires minimum hardware, showing great potential for a variety of applications.
In the past two decades, significant efforts have been made towards developing reliable and robust microfluidic pumps.1-3 These microfluidic pumps, based on their driving mechanisms, can be generally divided into two categories: passive pumps4-6 and active pumps.7-9 Passive pumps, particularly the surface-tension based microfluidic pumps,10,11 allow the manipulation of fluids without the need of peripheral equipment or moving parts, making them suitable to many portable analytical devices. Nevertheless, they are incapable of performing complex fluids manipulations as the flow direction and flow rate cannot be adjusted in real time and on demand, preventing their use in applications where multiple steps of fluid operations (e.g., immunoassay) are required. In contrast, active pumps, which use mechanical or electrical actuations, or other external forces to initiate fluid pumping, offer much more flexibility in terms of fluid manipulations, and can potentially provide solutions to the challenges faced by passive pumps.
Many active microfluidic pumps have been reported, including optically driven pumps,12,13 electroosmotic pumps,14,15 electrokinetic pumps,16,17 dielectric pumps,18,19 magnetic pumps,20,21 laser-driven pumps,22 pneumatic-membrane pumps,24,25 bio-hybrid pumps,26,27 and diffuser pumps.28,29 Despite these advances, the existing active micropumps suffer from drawbacks such as complicated device fabrication, involvement of moving structures, and/or unstable, unreliable performance. Recently, implementation of acoustic streaming effects29 in microfluidics have attracted great interest, and enabled numerous applications, including mixing,30-34 particle manipulation,35-39 and flow control.40,41 Microfluidic pumps powered by acoustically oscillating microbubbles has also been demonstrated.42,43 These bubble-based pumps are simple to fabricate and operate; however, the performance of these pumps suffer from bubble instability, temperature dependence, and inconvenience of the bubble-trapping process.
In this communication, we establish a simple, reliable microfluidic pumping mechanism using acoustically driven, solid, sidewall microstructures known as “sharp-edges”. This work is built upon our previous finding that acoustic streaming effects can be induced by acoustically oscillating sharp-edges.44,45 With the acoustically-induced acoustic streaming effects, we previously demonstrated rapid and homogeneous fluid mixing inside a microfluidic channel.44 In this work, we redesign the geometry and orientation of the sharp-edge structures and demonstrate that the sharp-edge-induced acoustic streaming can lead to applications beyond mixing. In particular, we demonstrate a highly effective, reliable, programmable microfluidic pump with minimum hardware. Our sharp-edge-based acoustofluidic micropump can generate a flow rate of approximately 8 μL/min, which corresponds to a pumping pressure of 76 Pa. Moreover, it is capable of generating flow rates ranging from nL/min to μL/min – a capability that most existing microfluidic pumps do not possess. Our acoustofluidic pump can be operated on-demand, and features advantages, such as simple fabrication and operation, high reliability, compactness, and tunable flow rates without complicated moving parts.
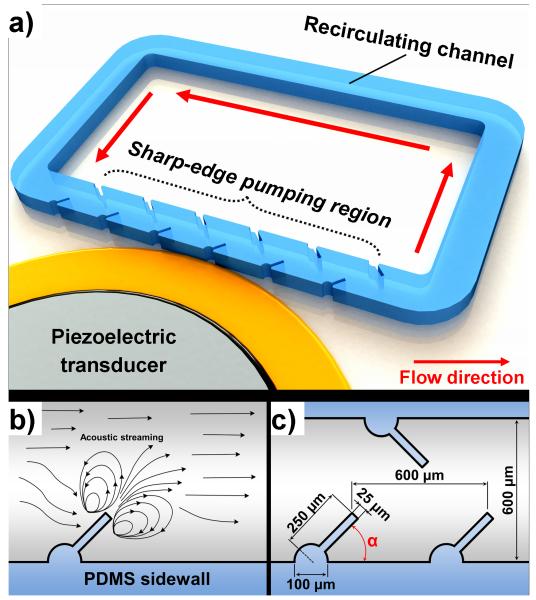
Figure 1 schematically shows the design and working mechanism of our acoustofluidic pump. The acoustofluidic pump, briefly, was made by bonding a single-layer polydimethylsiloxane (PDMS) channel onto a single glass slide and attaching a piezoelectric transducer (Part no. 81-7BB-27-4L0, Murata Electronics) adjacent to it using a thin layer of epoxy (PermaPoxy™ 5 Minute General Purpose, Permatex). To demonstrate pumping behavior, the PDMS channel was designed to be a rectangular recirculating (in a counter-clockwise direction) channel composed of four portions: left-channel, right-channel, upper-channel, and lower-channel. The lower channel, referred to as the pumping region, was designed with 20 tilted sharp-edge structures on its sidewall (10 on each side), while the other three channels were straight channels without any structures. The piezoelectric transducer, activated by amplified sine-wave signals from a function generator (AFG3011C, Tektronix) and an amplifier (25A250A, Amplifier Research), was used to acoustically oscillate the sharp-edge structures to generate acoustic streaming effects. Acoustically oscillated by the activation of the piezoelectric transducer, the tilted sharp-edge structure, as shown in Fig. 1b, generates an acoustic streaming pattern around its tip; since the sharp-edges are tilted to the right, the streaming results in a net force directed to the right. Fluid pumping occurs because the generated net forces push the bulk fluid. Figure 1c shows the design of our acoustofluidic device. The microchannel has a width of 600 μm and a depth of 100 μm, and each sharp-edge structure is identical. Different tilting angles (α) of the sharp-edge structures, including 30°, 45°, 60°, and 70°, were chosen to investigate the resulting pumping behavior, and determine the best angle for optimal pumping performance.
Fig. 1.
(a) Schematic of the sharp-edge-based acoustofluidic pumping device. This device includes a PDMS microfluidic channel and a piezoelectric transducer. (b) Schematic showing the acoustic streaming phenomenon around the tip of a tilted, oscillating sharp-edge structure. (c) Schematic showing the design of the channel and sharp-edge structure.
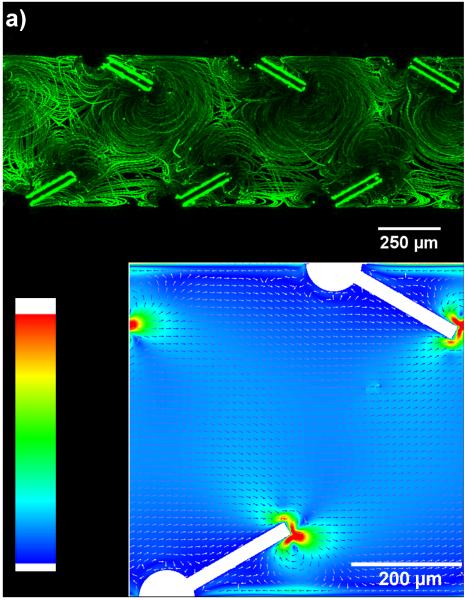
To prove our concept and determine the working frequency of the piezoelectric transducer, the pumping device with 30° tilted sharp-edge structures was first experimentally and numerically tested. A solution containing DI water and 1.9 μm diameter, dragon green fluorescent beads (Bangs Laboratory) was injected into the channel to characterize the acoustic streaming patterns induced by the oscillation of tilted sharp-edge structures. After injecting the solution, the inlet/outlet ports were sealed by two separate tubings connected to two separate syringes, enabling no pressure difference present between the inlet/outlet. By sweeping the frequency with a 50 Hz incremental from 1 kHz to 100 kHz, we observed that the acoustic streaming patterns, as shown in Fig. 2a, were developed around the tips of oscillating sharp-edge structures when the piezoelectric transducer was activated at 6.5 kHz. Based on these results, 6.5 kHz appeared to be the working frequency for the piezoelectric transducer to activate our pump. As a result, we used this frequency for all of the following experiments. Additionally, using the simulation approach we reported recently45 to model acoustically driven oscillating 30° tilted sharp-edge structures as shown in Fig. 2b, we found that the acoustic streaming effects produce a net flow of the fluid from the left to the right. These simulation results indicate pumping behavior and are also in good agreement with the experimental results we acquired. It should also be noted that in the simulations a flow singularity was observed at the sharp-edges, similar to that observed in our previous study,45 which is indicated by the maximum velocity at the sharp edge tips. This velocity increases with the mesh refinement and our solution is strictly valid only outside the boundary layer.
Fig. 2.
(a) Characterization of the acoustic streaming patterns developed around the tips of the 30° tilted sharp-edge structures. (b) Simulated results showing the streaming velocity in our pump in the presence of an acoustic field: a net flow of fluid from left to right is generated.
To further visualize and characterize the pumping behavior, DI water mixed with polystyrene beads of different diameters (10 μm and 0.9 μm) was injected into the channel. Figure 3 shows the movement of polystyrene beads over time in the upper channel when the piezoelectric transducer was ON (input voltage to piezoelectric transducer = 20 VPP), using the pumping device with 30° tilted sharp-edge structures. The results reveal that, with the piezoelectric transducer activated, the representative groups of beads (circled in red, yellow and blue) were moving from right to left, evidence that the fluid was being pumped and flowing along the recirculating channel.
Fig. 3.
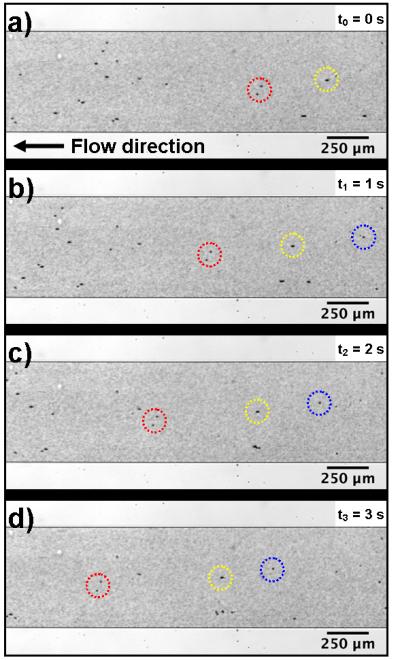
Experimental images showing the pumping behavior by indicating the movement of polystyrene beads at different time frames when (a) t0 = 0 s, (b) t1 = 1 s, (c) t2 = 2 s, and (d) t3 = 3 s. (Red, yellow, and blue circles indicate three representative groups of beads.)
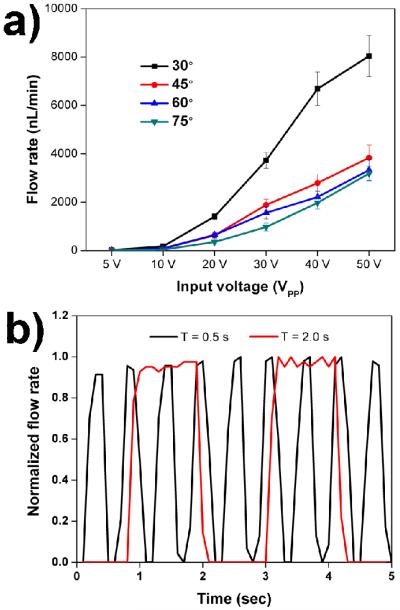
After proving the proposed pumping concept and determining the working frequency for the piezoelectric transducer, we further investigated the influence of the tilting angle of sharp-edge structures on the pumping performance. To quantitatively do so, we estimated the average flow rate inside the channel. The average flow rate was calculated by tracking average velocities of 10 μm beads in the upper channel, in which ~100 beads were randomly selected and tracked for each independent experiment. In addition to the effect of the tilting angle, pumping performance under different input voltages of the piezoelectric transducer was also characterized. Figure 4a shows the pumping performance for the four different tilting angles of sharp-edge structures under different input voltages. The results show that when the piezoelectric transducer was activated with voltages ranging from 5 VPP to 50 VPP, pumping took place in all of the devices, regardless of the tilting angle of the sharp-edge structures. As the tilting angle was decreased, the generated pumping flow rate increased. Of the four different tilting angles, the device with 30° tilted sharp-edge structures, as shown in Fig. 4a, generated a significantly larger pumping flow rate, and with a voltage of 50 VPP, it generated a flow rate as high as 8 μL/min. Using Poiseuille’s law43 and considering the channel length (25 mm), this flow rate corresponds to a calculated pumping pressure of 76 Pa. The lower pumping flow rates generated by the 45°, 60°, and 70° tilted sharp-edge structures can be attributed to the fact that, as the tilting angle is increased, the component of the generated net force that is perpendicular to flow direction also increases. As a result, the parallel component of the force that could push the bulk fluid to flow forward is decreased. Further work including different geometries of sharp-edge structure with smaller tilting angles is underway to further improve the efficiency of our acoustofluidic pumps. In addition, the results show a concurrent increase of flow rate with voltage, demonstrating that the pumping flow rate could be controlled by adjusting the input voltages.
Fig. 4.
Experimental results illustrating the controllability and tunability of our acoustofluidic pump. (a) Comparison of generated pumping flow rates with various tilting angles of sharp-edge structures as a function of the voltages applied to the piezoelectric transducer. The 30° tilted sharp-edge structures could generate a flow rate as high as 8 μl/min under 50 VPP. (b) Characterization of flow rate profiles when alternately switching the piezoelectric transducer ON and OFF with different burst frequencies: (Black) burst frequency of 2 Hz, and (Red) burst frequency of 0.5 Hz.
We discovered that our acoustofluidic pumps can conveniently achieve a wide-range of pumping flow rates, from nL/min to μL/min, by adjusting the input voltage to the piezoelectric transducer. Video 1 in the ESI shows the real-time pumping behavior using the pumping device with 30° tilted sharp-edge structures under different voltages. Aside from the function of continuous fluid pumping, we also demonstrated (Fig. 4b) that by alternately switching the piezoelectric transducer ON and OFF at various burst frequencies (e.g., 0.5 and 2 Hz), our acoustofluidic pump can achieve pulsatile fluid pumping. These results imply that the profile of pumping flow rate can possibly be modulated by programming the input signal to the piezoelectric transducer. A video showing the pulsed pumping behavior can be found in the ESI (Video 2). Future work should include evaluation and optimization of pumping performance within different microchannel designs (e.g., a straight, non-circulating microchannel).
In summary, we demonstrate a new class of acoustically driven, reliable, programmable microfluidic pumps based on the oscillating tilted sharp-edge structures. Our acoustofluidic pump could generate a pumping flow rate as high as 8 μL/min, and with more sharp-edge structures, the generation of higher flow rates can be expected. By tuning the input voltage, a wide-range of pumping flow rates, from nL/min to μL/min, could also be generated by a single pump. Moreover, by programming the input signal to the piezoelectric transducer, we could modulate the profiles of pumping flow rates. In fact, it is possible to achieve various kinds of flow operations by programming the input signals to the piezoelectric transducer. With such features, our acoustofluidic pump offers advantages over other microfluidic pumps in terms of not only simplicity, stability, reliability, and cost-effectiveness, but also controllability and flexibility, which, when combined, make it valuable in many lab-on-a-chip applications.
Supplementary Material
Acknowledgement
The research was supported by National Institutes of Health (Director’s New Innovator Award, 1DP2OD007209-01), National Science Foundation (CBET-1438126 and IIP-1346440), and the Penn State Center for Nanoscale Science (MRSEC) under grant DMR-0820404. Components of this work were conducted at the Penn State node of the NSF-funded National Nanotechnology Infrastructure Network.
Footnotes
Electronic Supplementary Information (ESI) available: Video1-pumping behavior under different voltages. Video2: pulsed pumping behavior. See DOI: 10.1039/c000000x/
References
- 1.Iverson BD, Garimella SV. Microfluid. Nanofluidics. 2008;5:145–74. [Google Scholar]
- 2.Au AK, Lai H, Utela BR, Folch A. Micromachines. 2011;2:179–220. [Google Scholar]
- 3.Lai H, Folch A. Lab Chip. 2011;11:336–42. doi: 10.1039/c0lc00023j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmermann M, Schmid H, Hunziker P, Delamarche E. Lab Chip. 2007;7:119–25. doi: 10.1039/b609813d. [DOI] [PubMed] [Google Scholar]
- 5.Chao S-H, Meldrum DR. Lab Chip. 2009;9:867–9. doi: 10.1039/b819887j. [DOI] [PubMed] [Google Scholar]
- 6.Walker GM, Beebe DJ. Lab Chip. 2002;2:131–4. doi: 10.1039/b204381e. [DOI] [PubMed] [Google Scholar]
- 7.Unger MA, Chou HP, Thorsen T, Scherer a, Quake SR. Science. 2000;288:113–6. doi: 10.1126/science.288.5463.113. [DOI] [PubMed] [Google Scholar]
- 8.Brask A, Snakenborg D, Kutter JP, Bruus H. Lab Chip. 2006;6:280–8. doi: 10.1039/b509997h. [DOI] [PubMed] [Google Scholar]
- 9.Huang S, Li C, Qin J. Lab Chip. 2010;10:2925–31. doi: 10.1039/c005227b. [DOI] [PubMed] [Google Scholar]
- 10.Resto PJ, Berthier E, Beebe DJ, Williams JC. Lab Chip. 2012;12:2221–8. doi: 10.1039/c2lc20858j. [DOI] [PubMed] [Google Scholar]
- 11.Berthier E, Beebe DJ. Lab Chip. 2007;7:1475–8. doi: 10.1039/b707637a. [DOI] [PubMed] [Google Scholar]
- 12.Leach J, Mushfique H, di Leonardo R, Padgett M, Cooper J. Lab Chip. 2006;6:735–9. doi: 10.1039/b601886f. [DOI] [PubMed] [Google Scholar]
- 13.Maruo S, Inoue H. Appl. Phys. Lett. 2007;91:084101. [Google Scholar]
- 14.Wang C, Wang L, Zhu X, Wang Y, Xue J. Lab Chip. 2012;12:1710–6. doi: 10.1039/c2lc40059f. [DOI] [PubMed] [Google Scholar]
- 15.Gao M, Gui L. Lab Chip. 2014;14:1866–72. doi: 10.1039/c4lc00111g. [DOI] [PubMed] [Google Scholar]
- 16.Bazant M, Squires T. Phys. Rev. Lett. 2004;92:066101. doi: 10.1103/PhysRevLett.92.066101. [DOI] [PubMed] [Google Scholar]
- 17.Gregersen MM, Brask A, Hansen MF, Bruus H. Phys. Rev. E. 2007;76:056305. doi: 10.1103/PhysRevE.76.056305. [DOI] [PubMed] [Google Scholar]
- 18.Ren H, Xu S, Wu S-T. Lab Chip. 2013;13:100–5. doi: 10.1039/c2lc40953d. [DOI] [PubMed] [Google Scholar]
- 19.Fan S-K, Chen W-J, Lin T-H, Wang T-T, Lin Y-C. Lab Chip. 2009;9:1590–5. doi: 10.1039/b900790c. [DOI] [PubMed] [Google Scholar]
- 20.Atencia J, Beebe DJ. Lab Chip. 2004;4:598–602. doi: 10.1039/b407710e. [DOI] [PubMed] [Google Scholar]
- 21.Malouin BA, Vogel MJ, Olles JD, Cheng L, Hirsa AH. Lab Chip. 2011;11:393–7. doi: 10.1039/c0lc00397b. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Wu T-H, Chiou P-Y. Lab Chip. 2012;12:2–5. doi: 10.1039/c2lc40079k. [DOI] [PubMed] [Google Scholar]
- 23.Tseng H-Y, Wang C-H, Lin W-Y, Lee G-B. Biomed. Microdevices. 2007;9:545–54. doi: 10.1007/s10544-007-9062-6. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y-N, Hsiung S-K, Lee G-B. Microfluid. Nanofluidics. 2008;6:823–33. [Google Scholar]
- 25.Chen H, Cornwell J, Zhang H, Lim T, Resurreccion R, Port T, Rosengarten G, Nordon RE. Lab Chip. 2013;13:2999–3007. doi: 10.1039/c3lc50123j. [DOI] [PubMed] [Google Scholar]
- 26.Park J, Kim IC, Baek J, Cha M, Kim J, Park S, Lee J, Kim B. Lab Chip. 2007;7:1367–70. doi: 10.1039/b703900j. [DOI] [PubMed] [Google Scholar]
- 27.Andersson H, Van Der Wijngaart W, Nilsson P, Enoksson P, Stemme G. Sens. Actuators, B. 2001;72:259–65. [Google Scholar]
- 28.Nguyen N, Huang X. Sensors Actuators A Phys. 2001;88:104–11. [Google Scholar]
- 29.Bruus H, Dual J, Hawkes J, Hill M, Laurell T, Nilsson J, Radel S, Sadhal S, Wiklund M. Lab Chip. 2011;11:3579–80. doi: 10.1039/c1lc90058g. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed D, Mao X, Juluri BK, Huang TJ. Microfluid. Nanofluidics. 2009;7:727–31. [Google Scholar]
- 31.Ahmed D, Mao X, Shi J, Juluri BK, Huang TJ. Lab Chip. 2009;9:2738–41. doi: 10.1039/b903687c. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed D, Chan CY, Lin S-CS, Muddana HS, Nama N, Benkovic SJ, Huang TJ. Lab Chip. 2013;13:328–31. doi: 10.1039/c2lc40923b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang P-H, Lapsley MI, Ahmed D, Chen Y, Wang L, Huang TJ. Appl. Phys. Lett. 2012;101:141101. doi: 10.1063/1.4742864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Destgeer G, Im S, Ha BH, Jung JH, Ansari MA, Sung HJ. Appl. Phys. Lett. 2014;104:023506. [Google Scholar]
- 35.Rogers P, Neild A. Lab Chip. 2011;11:3710–5. doi: 10.1039/c1lc20459a. [DOI] [PubMed] [Google Scholar]
- 36.Schmid L, Weitz DA, Franke T. Lab Chip. 2014 doi: 10.1039/c4lc00588k. DOI: 10.1039/C4LC00588K. [DOI] [PubMed] [Google Scholar]
- 37.Destgeer G, Lee KH, Jung JH, Alazzam A, Sung HJ. Lab Chip. 2013;13:4210–6. doi: 10.1039/c3lc50451d. [DOI] [PubMed] [Google Scholar]
- 38.Ding X, Li P, Lin S-CS, Stratton ZS, Nama N, Guo F, Slotcavage D, Mao X, Shi J, Costanzo F, Huang TJ. Lab Chip. 2013;13:3626–49. doi: 10.1039/c3lc50361e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin S-CS, Mao X, Huang TJ. Lab Chip. 2012;12:2766–70. doi: 10.1039/c2lc90076a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmid L, Wixforth A, Weitz DA, Franke T. Microfluid. Nanofluidics. 2011;12:229–35. [Google Scholar]
- 41.Hashmi A, Heiman G, Yu G, Lewis M, Kwon H-J, Xu J. Microfluid. Nanofluidics. 2012;14:591–6. [Google Scholar]
- 42.Tovar AR, Lee AP. Lab Chip. 2009;9:41–3. doi: 10.1039/b812435c. [DOI] [PubMed] [Google Scholar]
- 43.Tovar AR, Patel MV, Lee AP. Microfluid. Nanofluidics. 2011;10:1269–1278. [Google Scholar]
- 44.Huang P-H, Xie Y, Ahmed D, Rufo J, Nama N, Chen Y, Chan CY, Huang TJ. Lab Chip. 2013;13:3847–52. doi: 10.1039/c3lc50568e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nama N, Huang P-H, Huang TJ, Costanzo F. Lab Chip. 2014;43:2824–36. doi: 10.1039/c4lc00191e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.