Abstract
Chlamydia trachomatis is a major bacterial pathogen throughout the world. Although antibiotic therapy can be implemented in the case of early detection, a majority of the infections are asymptomatic, requiring the development of preventive measures. Efforts have focused on the production of a vaccine using the C. trachomatis major outer membrane protein (MOMP). MOMP is purified in its native (n) trimeric form using the zwitterionic detergent Z3–14, but its stability in detergent solutions is limited. Amphipols (APols) are synthetic polymers that can stabilize membrane proteins (MPs) in detergent-free aqueous solutions. Preservation of protein structure and optimization of exposure of the most effective antigenic regions can avoid vaccination with misfolded, poorly protective protein. Previously, we showed that APols maintain nMOMP secondary structure and that nMOMP/APol vaccine formulations elicit better protection than formulations using either recombinant or nMOMP solubilized in Z3–14. To achieve a greater understanding of the structural behavior and stability of nMOMP in APols, we have used several spectroscopic techniques to characterize its secondary structure (circular dichroism), tertiary and quaternary structures (immunochemistry and gel electrophoresis) and aggregation state (light scattering) as a function of temperature and time. We have also recorded NMR spectra of 15N-labeled nMOMP and find that the exposed loops are detectable in APols but not in detergent. Our analyses show that APols protect nMOMP much better than Z3–14 against denaturation due to continuous heating, repeated freeze/thaw cycles, or extended storage at room temperature. These results indicate that APols can help improve MP-based vaccine formulations.
Keywords: Amphipols, Vaccine, Stability, MOMP, Chlamydia trachomatis, NMR
Introduction
Integral membrane proteins (MPs) are difficult to handle in vitro because they are not soluble in aqueous solutions unless they are complexed by surfactants, most often detergents. Detergents, however, destabilize most MPs (see e.g., refs. Bowie 2001; Garavito and Ferguson-Miller 2001; Gohon and Popot 2003; Rosenbusch 2001). In 1996, a new class of surfactants called “amphipols” (APols) was introduced as a less aggressive substitute to detergents (Tribet et al. 1996). APols are synthetic amphipathic polymers that adsorb onto the hydrophobic transmembrane surface of MPs and keep them both biochemically stable and water-soluble in the absence of detergent. Over 40 integral MPs have been demonstrated to be kept soluble in their native conformation using APols (Zoonens and Popot 2014). A8–35, a polyacrylate-based APol, has previously been shown to preserve the secondary structure of native MOMP (nMOMP) as assayed by circular dichroism (CD) (Tifrea et al. 2011). CD provides an easy measure of secondary structure content, allowing direct comparison of the conformational state of the protein in various environments. A8–35 was also observed to be advantageous as part of a vaccine formulation (Tifrea et al. 2011). Specifically, when groups of mice were immunized using nMOMP or misfolded, recombinant MOMP in complex with either detergent or A8–35, the mice vaccinated with nMOMP/A8–35 complexes were significantly better protected against an intranasal challenge with C. trachomatis than the other groups of animals. This higher protection most likely results from a better preservation of the native structure of nMOMP and/or from a more efficient presentation of the antigen to the immune system, rather than from any adjuvant effect of the APol (Tifrea et al. 2014, 2011). APols by themselves do not elicit antibodies [(Popot et al. 2003) and unpublished observations].
Many vaccines use denatured material, which is not optimal. Although heat killing renders microbes non-infective, it also damages components of the pathogen required to elicit the most robust immune response. In addition, most vaccines require refrigeration to prevent further degradation. The innately poor stability of many vaccines hampers their development and use (Patois et al. 2011; Webby and Sandbulte 2008). Perturbations, including temperature changes (e.g., exposure to heat and freeze/thaw cycles), as well as long storage time, may affect the stability and efficacy of a vaccine. Developing vaccines with high heat stability is essential to their implementation in the field (Kristensen et al. 2011). Freezing proteins is common in the development, manufacturing, and storage of protein-based therapeutics, in an attempt to slow down degradation (Kolhe et al. 2010). However, damage to the protein may occur at each freeze/thaw cycle, resulting in irreversible denaturation, and aggregation once the protein has been returned to the solution phase (Jiang and Nail 1998; Kueltzo et al. 2008; Strambini and Gabellieri 1996). Possible effects from freezing include: cold denaturation (Franks and Hatley 1985; Griko et al. 1988), generation of and exposure of the protein to ice-liquid interfaces (Chang et al. 1996; Kueltzo et al. 2008; Schwegman et al. 2009), and freezing-induced concentration of the protein and solutes (Kueltzo et al. 2008), which can potentially lead to crystallization and pH shifts due to crystalline water separating from the buffer (Akers et al. 1995; Kueltzo et al. 2008; Murase and Franks 1989). Freezing-induced increases in salt concentration can reduce intermolecular repulsion (i.e., colloidal stability) between protein molecules via charge shielding, resulting in more favorable intermolecular interactions that lead to aggregation (Kueltzo et al. 2008). In the particular case of MP/detergent solutions, freezing induces a concentration of the detergent in the liquid phase. This is deleterious, one of the most common causes of MP denaturation being an increase in the volume of the micellar phase, into which stabilizing factors like lipids become diluted (for an example, see ref. Breyton et al. 1997). In the subsequent thawing, additional damaging effects can be caused by ice re-crystallization, which introduces extra interfacial stresses (Cao et al. 2003; Zhang et al. 2011). The World Health Organization recommends that an ideal vaccine should maintain a shelf life of 2 to 3 years when stored between 2 and 8 °C (Kristensen et al. 2011). Storage time, however, increases the likelihood of protein denaturation and aggregation (Weiss et al. 2009), and very few MPs stand being kept in the presence of detergents for extended periods (see e.g., refs. Bowie 2001; Garavito and Ferguson-Miller 2001; Gohon and Popot 2003; Rosenbusch 2001). APols have shown a remarkable capacity at extending the lifetime of MPs in solution (see e.g., refs. Bazzacco et al. 2012; Champeil et al. 2000; Dahmane et al. 2009; Opacčić et al. 2014; Picard et al. 2006; Pocanschi et al. 2013). It is therefore of great interest to further characterize the structural features of APol-trapped nMOMP and to gather information about the preservation of the structure of the protein and the accessibility of its most effective antigenic regions.
In the present work, we have examined to which extent APol A8–35, the best characterized and most widely used APol (Gohon et al. 2006; Tribet et al. 1996; Zoonens and Popot 2014), can maintain nMOMP structure and solubility through exposure to heat, freeze/thaw cycles, and storage over extended time. Multiple analytical tools, such as CD, sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE), light scattering and UV spectroscopy, can contribute to a better understanding of vaccine stability by detecting and characterizing changes in the structure and aggregation state of antigenic proteins in complex formulations (Patois et al. 2011; Sun et al. 2007). The results presented here indicate that, as compared to the detergent-solubilized form, A8–35-trapped nMOMP is more efficiently protected against denaturation due to heating or repeated freeze/thaw cycles, and that its structure is preserved for much longer upon extended storage. The vaccine formulation containing nMOMP/A8–35 complexes is structurally and physically stable for at least 100 days at room temperature (RT). This represents a substantial improvement in shelf life, which, judging from the generality of the stabilizing effects of APols, is likely to hold for other MP-based vaccine formulations.
Materials and Methods
Chemicals
Amphipol A8–35 (batches FGH15, FGH20 and FGH29) was synthesized, purified, and characterized by F. Giusti (IBPC) as described in refs. (Gohon et al. 2006, 2004).
Preparation of Stocks of C. trachomatis MoPn
C. trachomatis biovar MoPn (strain Nigg II; ATCC VR 123 also called Chlamydia muridarum) was purchased from the American Type Culture Collection (Manassas, VA) (Nigg 1942). To prepare stocks, Chlamydia was grown in tissue culture flasks using McCoy cells. Eagle’s minimal essential medium was supplemented with 1 mg/ml of glucose, 50 μg/ml of gentamicin sulfate, and 1 μg/ml of cycloheximide. The stocks were stored at −70 °C in SPG (0.2 M sucrose, 0.02 M sodium phosphate, pH 7.2, and 0.005 M glutamic acid).
Purification of C. trachomatis MoPn Native MOMP
The extraction and purification of the native C. trachomatis MoPn nMOMP have been described previously (Pal et al. 1997). Briefly, infected cell monolayers were centrifuged and washed with PBS (pH 7.4). The pellet was resuspended in a solution containing 0.02 M Tris (pH 7.4), 1.0 M NaCl, 0.012 M MgCl2, and 1 mM phenylmethylsulfonyl fluoride (PMSF; Calbiochem, La Jolla, CA); sonicated to resuspend the elementary bodies (EB); and incubated with 25 μg of DNase/ml for 2 h on ice with constant mixing. Following ultracentrifugation (100,000×g), the pellet was resuspended in 0.2 M sodium phosphate buffer (pH 5.5) containing 0.001 M each of EDTA and PMSF. The pellet was further extracted with 3-[(3-cholamidopropyl)-dimethyl-ammonio]-1-propanesulfonate (CHAPS; Anatrace, Maumee, OH) and dithiothreitol (DTT; Roche Applied Sciences, Indianapolis, IN) at final concentrations of 2 % and 0.1 M, respectively, for 2 h at 37 °C. Following centrifugation (100,000×g), the pellet was extracted a second time with CHAPS for 1 h at 37 °C. The pellet of the second CHAPS extraction was re-extracted with 2 % n-tetradecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate (Z3–14; Anatrace) (Blake and Gotschlich 1984; Jansen et al. 2000). After incubation at 37 °C for 2 h with constant mixing, the sample was centrifuged (100,000×g), and the nMOMP was recovered in the supernatant. The nMOMP was further purified using a 1 × 20 cm hydroxyapatite column, as described previously (Caldwell et al. 1981), with the following modifications. The column was equilibrated with 0.02 M phosphate buffer (pH 5.5) containing 0.1 % Z3–14 and 0.001 M each of EDTA, PMSF, and DTT. The column was eluted with a linear gradient of phosphate buffer from 0.02 M to 0.5 M containing 0.1 % Z3–14 and EDTA, PMSF, and DTT as indicated above. The purity of the nMOMP preparation was assessed by gel electrophoresis. After extraction and purification of the nMOMP with Z3–14, the detergent was exchanged for A8–35: APol A8–35 was added at a 2:1 mass ratio to protein, followed by the addition of Bio-Beads SM-2 (Bio-Rad) to remove the detergent.
Preparation and Purification of 15N-Labeled Native MOMP
To label the nMOMP with 15N, following infection, the McCoy monolayers were cultured with BioExpress® 2000 (U-15N) insect cell media (Cambridge Isotopes Laboratories, Inc) supplemented with 1 mg/ml of glucose, 50 μg/ml of gentamicin sulfate, and 1 μg/ml of cycloheximide. The nMOMP was extracted as described above and its trimeric state was confirmed by SDS-PAGE. The NMR samples were prepared in a volume of 300 μL with 10 % D2O, 100 mM sodium phosphate, pH 7.4; 300 μM nMOMP in 25 mM dodecylphosphocholine (DPC) and 300 μM nMOMP with 24 mg/ml APol A8–35.
Shelf Life
Samples were stored in inert, non-breathable scintillation vials. The scintillation vials were kept tightly sealed and in opaque boxes at RT or in a 4 °C refrigerator. Prior to opening, the vials were vortexed very briefly to homogenize the mixture. The sample was collected into the bottom of the vial by gentle tapping and carefully removed using a gel loading tip prior to being transferred quickly into the CD cuvette. After measurement, the sample was withdrawn from the cuvette again with a gel loading tip and quickly deposited back into the storage vial.
Freezing
Samples were stored in the same inert, non-breathable scintillation vials as in the shelf life study in an opaque box at −80 °C. The vials were allowed to come to RT by warming in hand. Prior to opening, the vials were vortexed very briefly to homogenize the mixture. The sample was collected into the bottom of the vial by gentle tapping and carefully removed using a gel loading tip prior to being transferred quickly into the CD cuvette. After measurement, the sample was withdrawn from the cuvette again with a gel loading tip and quickly deposited back into the storage vial.
Stability at 55 °C
The sample was stored as the shelf-life samples were (4 °C). For the experiment, the sample was placed in a heat block set to 55 °C. Prior to measurement, the vial was inverted and vortexed. An aliquot was removed and placed into the CD cuvette, with the CD temperature regulator set to RT. A CD spectrum was measured, and the sample was returned to vial in the heat block.
CD Spectroscopy
The secondary structure of the purified nMOMP trimer was analyzed by CD spectroscopy in the far-UV (185–270 nm) region (Minetti et al. 1997). The samples were analyzed at 1.0 nm wavelength intervals using a JASCO (Easton, MD) model 720 CD spectropolarimeter at a scan speed of 50 nm/min and an average response time of 2 s. A total of 10 consecutive scans were accumulated. The samples were analyzed at 22 °C using a 1-mm path-length cell (Hellma USA, Plainview, NY). Buffer alone (60 mM sodium phosphate, pH = 7.8) was used as a blank.
SDS-PAGE
The apparent Mr and purity of nMOMP were determined by 10 % tricine–SDS–PAGE, 25 mM DTT as previously described (Schägger and von Jagow 1987; Tifrea et al. 2011). Band intensities were quantified using the ImageJ software suite (Schneider et al. 2012).
Western Blots
Western blot analyses were performed using nitrocellulose membranes as previously described (Pal et al. 2002). nMOMP was loaded onto a 17.5-cm-wide minislab gel and resolved by 10 % SDS-PAGE. Ascite fluid containing monoclonal antibody (mAb) MoPn-18b was diluted with PBS containing 0.05 % Tween-20 and 10 % fetal bovine serum. mAb MoPn-18b, which binds to a conformational epitope, was used to probe the trimer of nMOMP (Sun et al. 2007).
UV/Visible Spectroscopy
The absorbance from 200–400 nm was measured at RT using a DU800 UV/Visible spectrophotometer (Beckman Coulter). The contribution at 320 nm (i.e., scattering) was subtracted from the absorbance at 280 nm, and the protein concentration was computed using the extinction coefficient 62380 M−1 cm−1.
NMR Spectroscopy
NMR 15N HSQC data were acquired on a Varian Inova 800 MHz spectrometer (160 scans, 1,024 points, 48 increments). Data processing was performed using NMR Pipe (Delaglio et al. 1995).
Results
nMOMP was prepared in Zwittergent 3–14 (Z3–14) and part of it transferred to APol A8–35. These two preparations were compared with regards to shelf-life at 4 °C and RT, resistance to freeze/thaw, and thermostability. In addition, NMR spectroscopy was used to characterize structural features of nMOMP in either A8–35 or the NMR-compatible zwitterionic detergent DPC.
Trapping with A8–35 Extends the Shelf Life of nMOMP
Duplicate samples of nMOMP/A8–35 and nMOMP/Z3–14 were kept at RT or at 4 °C for up to 100 days. Secondary structure was monitored by measuring CD spectra at regular intervals (twice daily for the first week, once weekly for a month, once monthly for the last 2 months). CD spectra for all samples were collected at 22 °C. Because the native trimer of nMOMP resists SDS at RT (Sun et al. 2007), preservation of the native tertiary and quaternary structures could be assayed, after 72 and 100 days, by SDS-PAGE followed by silver staining or labeling with a conformation-sensitive monoclonal antibody (mAb MoPn-18b) (Sun et al. 2007).
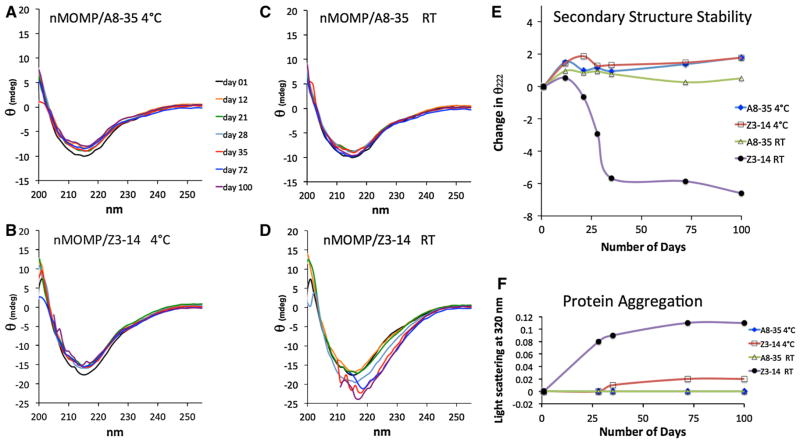
APols provide increased stabilization of nMOMP structure compared to detergent, especially at RT. The secondary structure of nMOMP stored at 4 °C is retained similarly in both APols and detergent. Specifically, at 4 °C the shape of the CD spectra of nMOMP in A8–35 does not change appreciably over a period of 100 days, suggesting that the β-sheet content does not vary (Fig. 1a). A similar effect is found in the CD spectra for nMOMP/Z3–14 stored at 4 °C (Fig. 1b). Upon storage at RT, however, CD spectra show a dramatic difference between APol-trapped and detergent-solubilized samples. Specifically, CD spectra of the nMOMP/Z3–14 sample stored at RT reveal the progressive formation of helical structure (Fig. 1d). It has been shown previously that nMOMP/Z3–14 acquires α-helicity upon denaturation by heating above 52 °C (Tifrea et al. 2011). Although aggregation causes light scattering that perturbs the CD spectra in Fig. 1d, data are reliable above 218 nm and denaturation can be monitored by measuring the extent of helix formation from the ellipticity at 222 nm. Figure 1e shows that substantial denaturation occurs within 1 month for the nMOMP/Z3–14 sample kept at RT. Whether kept at 4 °C or RT, the protein/detergent complexes show signs of aggregation, as reflected by their turbidity at 320 nm (Fig. 1f), this phenomenon being strongest for the sample stored at RT. In contrast, CD spectra of nMOMP in A8–35 at 4 °C or RT change only slightly, suggesting that the β-barrel structure of nMOMP is well retained over time (Fig. 1c). Absorbance at 320 nm does not reveal any aggregation in these samples. Together, these results indicate that the ability of APols to protect the secondary structure of nMOMP and prevent its aggregation clearly exceeds that of detergent when the sample is kept at RT for months.
Fig. 1.
Amphipols extend the shelf life of nMOMP at RT and 4 °C. CD spectra of nMOMP in A8–35 (a) and in Z3–14 (b); in both surfactants, nMOMP was kept at 4 °C except for approximately 10 min during each measurement at 22 °C. CD spectra of nMOMP in A8–35 (c) and in Z3–14 (d); in both surfactants, nMOMP was kept at RT. Protein aggregation resulted in light scattering in d; low-wavelength data were removed where light scattering was significant. Black 1 day, orange 12 days, green 21 days, blue-gray 28 days, red 35 days, dark blue 72 days, purple 100 days. e Denaturation of MOMP was previously shown to generate helical secondary structure with a decrease in ellipticity at 222 nm (Tifrea et al. 2011). Here, the ellipticity at 222 nm is plotted as a measure of the loss of β-barrel structure. f Protein aggregation of the shelf-life samples was assessed as light scattering at 320 nm. Blue diamonds A8–35, 4 °C; open squares Z3–14, 4 °C; open triangles A8–35, RT; black circles Z3–14, RT (Color figure online)
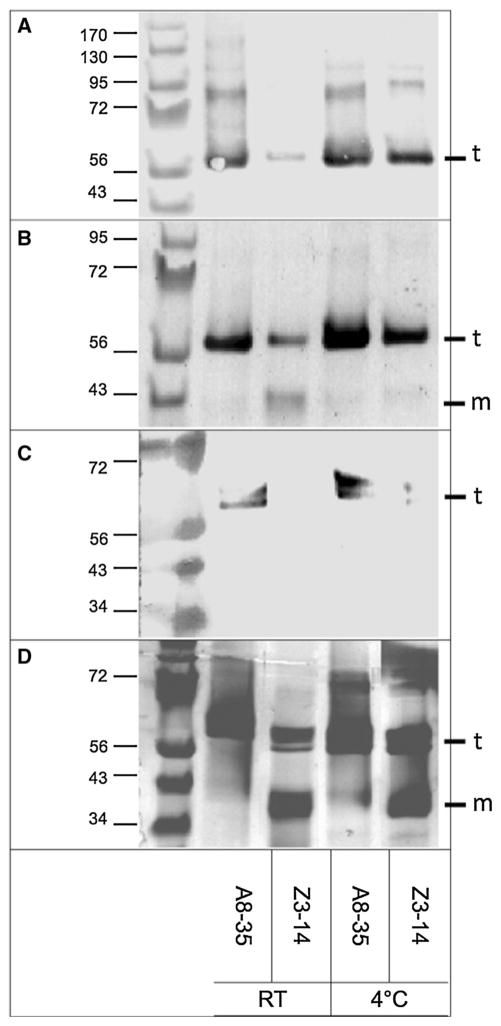
Electrophoresis and immunoblotting data indicate that nMOMP maintains its tertiary and quaternary structures better when in complex with A8–35 than in detergent solution. Over time, nMOMP/A8–35 stored at 4 °C consistently migrates as a trimer upon SDS-PAGE, forming a characteristic band at Mr ≈ 66 kDa (Fig. 2b, d). This band is detectable by a monoclonal antibody (mAb MoPn-18b) that recognizes specifically the native fold (Sun et al. 2007) (Fig. 2a, c) at 72 and 100 days. In contrast, for nMOMP/Z3–14 samples kept at 4 °C, SDS-PAGE performed at 72 and 100 days reveal the presence of monomer bands (Fig. 2b, d), which are not recognized by mAb MoPn-18b (Fig. 2a, c). Samples kept at RT show the largest differences between nMOMP/A8–35 and nMOMP/Z3–14: after 72 days, ~34 % of the latter sample has become monomer (Fig. 2b). This sample continues to degrade over time, with the predominant species being monomeric (~57 %) after 100 days. Remarkably, at the 100-day point, the trimer is no longer recognized by mAb MoPn-18b (Figs. 2c and S1). Thus, the native structure of the remaining trimer becomes altered when the protein is kept in detergent solution. In contrast, APol-trapped samples remain trimeric and are recognized by mAb MoPn-18b whether stored at 4 °C or RT throughout the 3.3-month period of study.
Fig. 2.
Integrity of the tertiary and quaternary structures of nMOMP samples in either A8–35 or Z3–14, stored either at room temperature or at 4 °C, as monitored by SDS-PAGE and Western blotting. Native trimers and denatured monomers are indicated by (t) and (m), respectively. Although the molecular mass of the MOMP monomer is ~40 kDa, it is well established that the trimer migrates as ~66 kDa in SDS (Sun et al. 2007). a Western blot probed with mAb MoPn-18b and b corresponding silver-stained gel of nMOMP at RT and at 4 °C after 72 days. c Western blot probed with mAb MoPn-18b and d corresponding silver-stained SDS-PAGE of nMOMP at RT and at 4 °C after 100 days. By 100 days, substantial monomer forms in both detergent samples but none is detected in the APol sample (d). c and d panels assembled from larger gel shown in supplemental Fig. S1. Samples were incubated with DTT prior to electrophoresis
APols Help nMOMP Retain β-Barrel and Trimeric Structure During Multiple −80 °C Freeze/Thaw Cycles
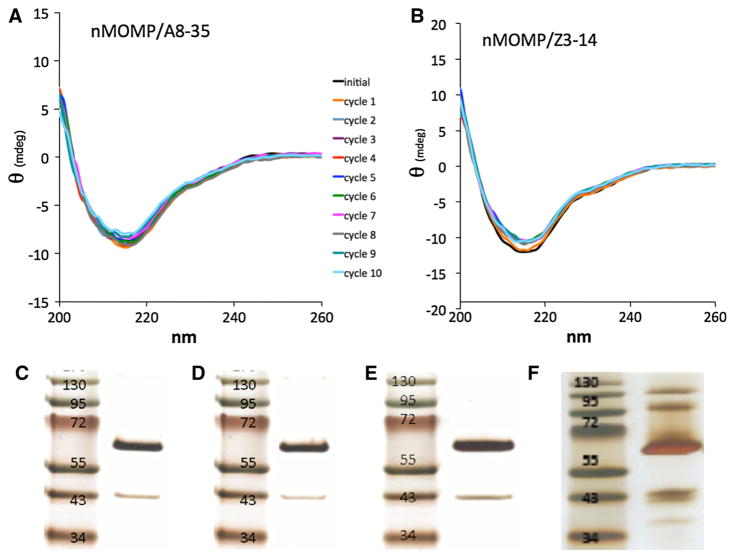
Freezing a protein sample can sometimes cause irreversible damage to the protein fold, as well as aggregation. This is particularly frequent for MPs kept in detergent solutions, presumably due to the increase of the detergent concentration in the fluid phase during freezing. A8–35 has been found to protect bacteriorhodopsin (BR) from denaturation during freezing and thawing (Gohon et al. 2008). To examine whether this would hold for nMOMP, samples of nMOMP/A8–35 and nMOMP/Z3–14 were frozen by placement in a −80 °C freezer (slow freeze, not flash frozen) and then thawed at RT. The secondary structure was monitored by CD (performed at 22 °C) after each cycle, at ≥4 h intervals, allowing the sample to completely refreeze (Fig. 3a, b). Tertiary and quaternary structures were assayed after ten cycles by silver-stained SDS-PAGE (Fig. 3c–f).
Fig. 3.
Amphipols help nMOMP retain β-barrel and trimeric structure during multiple freeze (−80 °C)/thaw cycles. The CD spectra taken at regular intervals for a, nMOMP/A8–35 and b, nMOMP/Z3–14 are shown. The sample was kept at 22 °C for approximately 10 min. during each measurement. Black = no freezing; orange = 1 freeze/thaw cycle, blue-gray = 2 cycles, purple = 3 cycles, red = 4 cycles, dark blue = 5 cycles, green = 6 cycles, magenta = 7 cycles, gray = 8 cycles, turquoise = 9 cycles, light blue = 10 cycles, silver-stained SDS-PAGE of nMOMP/A8–35 before (c) and after 10 freeze/thaw cycles (d); the trimer migrates at ~66 kDa; a small amount of monomer ~40 kDa is present at the beginning of the experiment; silver-stained gel of nMOMP/Z3–14 before (e) and after 10 freeze/thaw cycles (f); monomer band at 40 kDa and higher-order aggregates become prominent for the detergent sample after repeated freeze/thaw (f) but are not apparent in the APol-trapped sample (d) (Color figure online)
CD spectra for the freezing/thawing of nMOMP in either APol or detergent (Fig. 3a, b) overlap fairly well, indicating that repeated freeze/thaw cycles have very little effect on the total secondary structure. In addition, we saw no evidence for aggregation (absorbance at 320 nm) for either sample. However, SDS-PAGE reveals an increase in the amount of monomeric species and higher-order aggregates in the nMOMP/Z3–14 sample, but not in the nMOMP/A8–35 one, after 10 freeze/thaw cycles (Fig. 3d, f). In fact, we have noticed that more concentrated nMOMP/Z3–14 solutions stored frozen for vaccine preparation lose the ability to bind mAb MoPn-18b antibodies upon repeated freeze/thaw cycles. Thus, the damaging effects of freezing become more pronounced for solutions with high protein concentrations. In comparison to detergent solutions of the same protein concentration, APol-trapped nMOMP retains its secondary, tertiary, and quaternary structures despite multiple freeze/thaw cycles.
APols Increase the Resistance of nMOMP to Heating
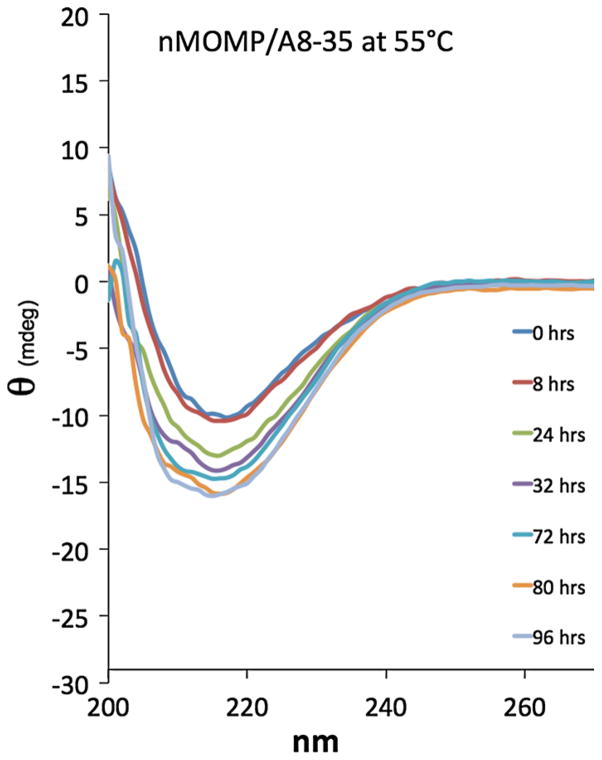
Some climates incur very hot temperatures and samples inadvertently left in vehicles can also be warmed significantly above RT. Previously, we measured the denaturation temperature of nMOMP solubilized in Z3–14 and found that denaturation becomes detectable around 44 °C, whereas A8–35-trapped nMOMP is stable up to at least 78 °C (Tifrea et al. 2011). To examine whether APols could help maintain nMOMP structure over extended periods of exposure to moderate heat, a sample of nMOMP/A8–35 was kept at 55 °C (131 °F) for 4 days. CD spectra were collected at regular intervals at 22 °C (once in the morning and once in the afternoon, resulting in intervals spaced at alternating periods of 8 and 16 h). Figure 4 shows that heating over time causes a shift of the spectra to more negative ellipticity and wider troughs consistent with the formation of α-helical conformation. An increase of helical content has been similarly observed during thermal denaturation of nMOMP/Z3–14 (Tifrea et al. 2011). Notably, there is little loss of structure at the first time point; thus APols are able to maintain the nMOMP fold for at least 8 h at 55 °C.
Fig. 4.
Amphipols help nMOMP retain β-structure during prolonged heating. CD spectra of nMOMP/A8–35 kept at 55 °C over time. For approximately 10 min during each measurement, the sample temperature was 22 °C, then returned promptly to 55 °C. Blue = t0, red = 8 h, green = 24 h, purple = 32 h, cyan = 72 h, orange = 80 h, blue-gray = 96 h. If a MOMP vaccine formulated with APols is inadvertently heated, these data show that the protein structure will be maintained for at least 8 h (Color figure online)
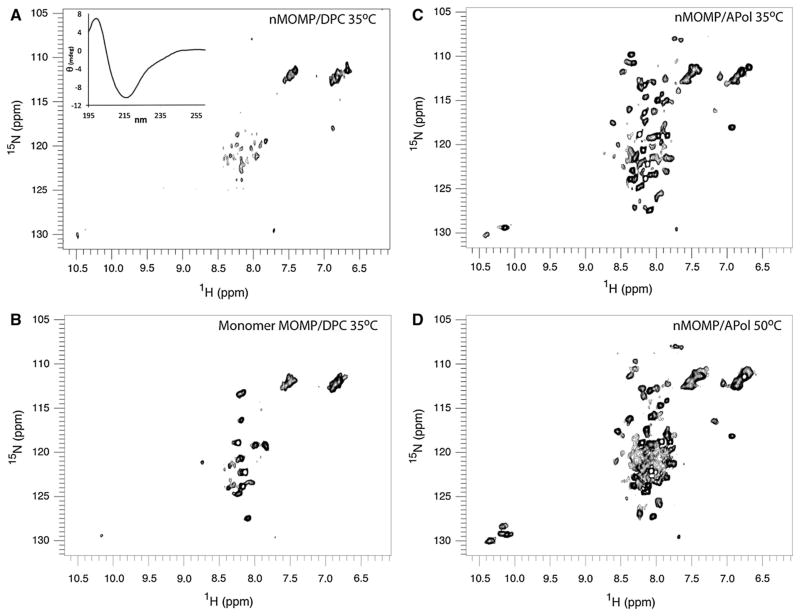
APols Provide Better Solution NMR Spectra of nMOMP than Detergents
NMR can be used to assess structural features of proteins in solution. For large proteins, this requires that the sample be labeled with an appropriate stable isotope. To incorporate 15N labels into C. trachomatis proteins requires growing mammalian cells in media containing suitably labeled amino acids. Chlamydiae are obligate intracellular parasites; they invade and reproduce within a host cell. In contrast to other bacteria, Chlamydiae require metabolites and amino acids to be provided by their host (Allan and Pearce 1983; Moulder 1974). In fact, various Chlamydiae species modulate host cell membranes so that the host will absorb amino acids more readily from the surrounding media (Harper et al. 2000). Consequently, we have found it possible to produce C. trachomatis in mammalian cell culture using commercially available insect cell media containing labeled amino acids and obtain very good ([95 %) incorporation of label into nMOMP. Using this strategy, we prepared fully 15N-labeled nMOMP. The protein was purified as described for unlabeled nMOMP, after which Z3–14 was exchanged either for DPC, a detergent classically used for solution NMR studies of MPs, or for A8–35.
We previously assessed the quality of nMOMP NMR spectra in other detergents; side chain tryptophan signals were not observable in either Z3–14 (Tifrea et al. 2014) or in octyl-β-glucoside (OG) at 35 °C. Although DPC is often a convenient surfactant for MP NMR studies, spectra of 15N-labeled nMOMP in DPC contained only a few peaks corresponding to side-chain resonances in the HSQC spectrum and a few weak backbone amide peaks (Fig. 5a). A CD spectrum of this sample confirms that the protein β-structure is maintained in DPC (Fig 5a inset). The NMR data shown in Fig. 5a were measured at 35 °C to optimize tumbling time but maintain a temperature below the melting temperature of the protein in a similar detergent (Z3–14). Heating this nMOMP/DPC sample to 70 °C for 10 min, then cooling back to 35 °C, converted the trimer to monomer. About 19 backbone signals can be recognized in the HSQC of heated monomeric nMOMP in DPC (Fig. 5b). In contrast, 15N-labeled nMOMP in A8–35 gave much better spectra at the same temperature where nMOMP/DPC spectra were collected, 35 °C (Fig. 5c). These signals fall within the random coil region and are likely to arise from accessible loops on the exposed surface of nMOMP. Since APols confer substantial thermal stabilization to this protein, we were able to heat the nMOMP/A8–35 sample and perform NMR measurements at 50 °C (Fig. 5d). We found improved spectra under these conditions where ~90 signals are apparent, reporting on ~25 % of the protein. Although increasing temperature causes some peaks to disappear because of increased exchange with the solvent, new, broader peaks appear, most likely providing a glimpse of the structured region of the protein in proximity to the loops. Signals apparent at 50 °C, therefore, probably report on regions most likely to be recognized by the immune system.
Fig. 5.
800-MHz NMR 15N HSQC spectra of 0.3 mM nMOMP solubilized in DPC or trapped with A8–35, 90 % H2O/10 % D2O, 100 mM sodium phosphate, pH 7.4. A single 15N-labeled nMOMP preparation was split and transferred into either DPC or A8–35. a Trimeric nMOMP/DPC, 35 °C. Inset: CD spectrum of this sample. b nMOMP/DPC, 35 °C after heating to 70 °C for 10 min to generate the monomer; NMR HSQC collected at 35 °C. c HSQC of nMOMP/A8–35, 35 °C. d HSQC of nMOMP/A8–35, 50 °C. Trp indole NH signals resonate between 10.0–10.5 ppm in the 1H dimension. Complex with APols results in better nMOMP spectra. Since APols stabilize the protein, NMR experiments can be performed at higher temperatures resulting in further improvement
Discussion
Identifying conditions that improve the solubility and stability of vaccine components can be time-consuming, but making this investment early in the process of vaccine development can limit the risk of reformulation, extra clinical trials, and additional regulatory approvals. Furthermore, once deployed in the field, structurally stabilized vaccines promote expanded immunization coverage, reliable protection, reduced waste, and lowered inventory turnover (Kristensen et al. 2011). As mentioned above, there are several conditions that may affect the stability and solubility of a vaccine. Specifically, both freezing and heat are detrimental to the stability of most vaccines (Kristensen et al. 2011; Patois et al. 2011), and, over long time periods, aggregation is a factor that may affect the solubility of any vaccine (Cai et al. 2009). To better understand and assess the structural properties and stability of proteins as pharmaceuticals, biophysical methods have been widely employed (Fan et al. 2007; Salnikova et al. 2008). In the present study, a combination of methods including CD, electrophoresis, Western blotting, and light scattering measurements was used to gain critical information concerning the stability of Chlamydia nMOMP, an important vaccine candidate. In addition, conditions were explored for studying the structure of nMOMP by solution NMR spectroscopy.
By all metrics assessed here, APol A8–35 confers superior stability to nMOMP as compared to the detergent Zwittergent 3–14. Most significantly, nMOMP/A8–35 samples can be kept at RT for at least 100 days, whereas nMOMP/Z3–14 samples denature and aggregate within 1 month. CD spectra reveal that APol-trapped nMOMP stored at RT retains native-like secondary structure content, and Western blots using the conformation-specific antibody mAb MoPn-18b confirm that the protein retains its native fold. The two kinds of measurements are highly complementary, because CD is likely to report mainly on the stability of the transmembrane β-barrel, whereas recognition by mAb MoPn-18b is more likely to depend on the conformation of extramembrane loops. Trapping with APols also allows for sample freezing with improved stability compared to detergents, a feature that is particularly useful for long-term storage. In addition, nMOMP/A8–35 samples can be heated to 55 °C for at least 8 h without change in secondary structure. In contrast, nMOMP/Z3–14 samples start denaturing at ~44 °C. The data presented here support and expand previously published data, both for nMOMP and APols. For instance, earlier studies have shown that the conformation of nMOMP solubilized in detergent contains predominantly (~40 %) β-sheet secondary structure (Sun et al. 2007), and that, when transferred to APols, the protein retains the same secondary, tertiary and quaternary structures, with improved thermal stability (Tifrea et al. 2011).
MP stabilization by APols is a rather general phenomenon, which has been observed, for instance, for the β-barrel outer membrane protein OmpA from Escherichia coli (Pocanschi et al. 2013), as well as for α-helical MPs such as the sarcoplasmic calcium pump (Champeil et al. 2000; Picard et al. 2006), BR (Dahmane et al. 2013; Gohon et al. 2008; Tribet et al. 1996), and several G protein-coupled receptors (Dahmane et al. 2009). In brief, three factors are thought to contribute to the mildness of APols toward MPs (for a more extended discussion, see refs. Popot 2010; Popot et al. 2011): (i) because the critical association concentration of APols is very low (Giusti et al. 2012), APols can keep MPs soluble at very low concentrations of free APol, which, as compared to detergents, lowers the volume of surfactant particles into which stabilizing cofactors such as lipids can become diluted (Zoonens et al. 2007); (ii) at a given concentration, APols are less inactivating than detergents, probably in part because they interfere less with stabilizing protein/protein and protein/lipid interactions (Popot et al. 2011); and (iii) APols appear to damp conformational excursions of MP transmembrane domains that can initiate denaturation (Picard et al. 2006; Popot et al. 2003, 2011). In keeping with this view, it has been shown that the stabilization of OmpA by A8–35 against denaturation by urea is a kinetic, not a thermodynamic phenomenon: the native state is not intrinsically more stable, but the kinetics of denaturation is severely slowed down after trapping by the APol, due to a higher free-energy activation barrier (Pocanschi et al. 2013). Also consistent with this scenario is the fact that, in molecular dynamics simulation of OmpX complexed either by A8–35 or by the detergent dihexanoylphosphatidylcholine, conformational excursions of the β-barrel and loops are restricted in the presence of APol as compared to the detergent (Perlmutter et al. 2014).
As a result of these various effects, transferring a MP from a detergent solution to APols generally improves its stability, often in a dramatic fashion. For instance, the lifetime of the calcium ATPase in the absence of calcium ions (whose removal destabilizes the transmembrane helix bundle) increases by ~60 × upon transfer from the detergent C8E12 to APol A8–35 (Champeil et al. 2000). The thermostability of the leukotriene B4 LTB1 receptor is increased by ~11 °C upon transfer from a detergent/lipid to an A8–35/lipid environment, with the result that it retains full activity over a period of 3 weeks at 4 °C, instead of losing half of it (Dahmane et al. 2009). At 40 °C, BR is highly stabilized by A8–35, denaturing by <10 % over a week, while under the same conditions the protein in octylthioglucoside is completely inactivated in a few hours (Popot 2010; Popot et al. 2003, 2011). The extent of thermostabilization of BR by A8–35 has not been precisely measured, but it is probably ~10–15 °C (Dahmane et al. 2013). That of nMOMP being at least 30 °C (Tifrea et al. 2011)—the highest observed to-date for any MP—, one can expect a dramatic improvement of its shelf-life, as indeed observed in the present work. BR/A8–35 complexes have also been subjected to repeated freeze/thaw cycles, without denaturing the protein [ref. (Gohon et al. 2008), and unpublished data], which also tallies with the present observations on nMOMP.
APols have proven remarkable tools for studying MPs by solution NMR (reviewed in refs. Elter et al. 2014; Planchard et al. 2014). As of today, the approach has been applied to five MPs, three β-barrel ones, OmpX (Bazzacco et al. 2012; Catoire et al. 2010b, 2009; Etzkorn et al. 2014) and the transmembrane domains of OmpA from E. coli (Dahmane et al. 2011; Zoonens et al. 2005) and from Klebsiella pneumoniae (Planchard et al. 2014; Renault 2008), and two α-helical ones, BR (Etzkorn et al. 2013, 2014; Raschle et al. 2010) and the LTB2 leukotriene receptor (Catoire et al. 2011, 2010a).Whereas MP/APol complexes are slightly bigger objects than the smallest MP/detergent ones, and therefore solution NMR spectra at a given temperature can be slightly less well resolved (Planchard et al. 2014; Zoonens et al. 2005), this is more than compensated for by the higher stability of APol-trapped MPs, as well as by the relative ease with which A8–35 can be partially (Gohon et al. 2004) or totally (Giusti et al. 2014) deuterated, giving access, for instance, to the determination of 1H–1H distances by nuclear Overhauser effect measurements (Catoire et al. 2010b; Planchard et al. 2014).
The lack of signals in the NMR spectrum of nMOMP/DPC complexes most likely results from a combination of slow trimer tumbling and unfortunate protein dynamics; detergent micelles are very dynamic environments that can allow for conformational heterogeneity. Membrane protein NMR spectra can vary significantly depending on the detergent used. Among the three detergents we have tested so far, spectra of nMOMP in DPC showed improvement compared to Z3–14 or OG, but spectra of nMOMP/A8–35 are far superior to these three detergents. Similarly, BR/A8–35 complexes give slightly better spectra than BR/DDM ones (Etzkorn et al. 2013). Furthermore, MD simulations of OmpX/A8–35 vs. OmpX/DHPC complexes show the effect of the APol on protein dynamics as a decrease in range of motion of the β-barrel and loops (Perlmutter et al. 2014). The adsorption of APols onto the hydrophobic protein surface normally imbedded in the lipid-bilayer could similarly restrict nMOMP protein dynamics; a decrease in conformational exchange could account for the improved NMR spectra compared to detergent. Moreover, with APols it is possible to collect NMR data on nMOMP at a higher temperature, which shortens the relatively long motional correlation times for the trimer in solution. Marked improvements of nMOMP/A8–35 NMR spectra are indeed seen at higher temperatures not attainable with the detergent sample.
Regions of the protein that are most accessible to solvent are most likely to have increased flexibility and give observable signals in solution NMR spectra; it is expected that the sharp signals we observe arise from exposed loops. The NMR spectra of nMOMP solubilized in APols are encouraging in defining protein loops that serve as the epitopes in developing immunity. Wagner and colleagues, who have compared the signals from BR in nanodisks, A8–35 and DDM, conclude that the transmembrane region is essentially the same, but the loops are slightly different (Etzkorn et al. 2013). In comparing spectra of nMOMP in detergent and APols, it appears that more protein is solvent-accessible in APol preparations. Five of the eight Trp indole NH signals are visible in APols, but no strong Trp signals appear in the spectra of the detergent-solubilized preparations we have tested. The increased exposure we see for Trp rings is most interesting, since these aromatic groups are known to partition within the headgroup region of phospholipids. The APols used here do not contain phosphocholine and would not be expected to interact with aromatic groups as detergents or lipids that contain phosphocholine can. This is reminiscent of the observation that a polyhistidine tag fused to the N-terminus of BR partitions into the polar head region of DDM micelles or DMPC-based nanodisks, but not in that of A8–35 (Etzkorn et al. 2014). This is a fortunate situation, as increased exposure of membrane protein surface could prove highly advantageous in vaccine development.
Summary
In conclusion, APol A8–35 has been demonstrated to be effective both at keeping Chlamydia nMOMP soluble and at stabilizing its secondary, tertiary and quaternary structures through extreme changes in temperature, as well as being superior to the zwitterionic detergent Z3–14 for maintaining structural integrity and solubility over extended storage time. Therefore, the use of APols should be considered for the formulation of membrane protein vaccines and for other applications that require stabilizing the structure of MPs. In addition, APols seem to offer interesting perspectives for the structural study of nMOMP by solution NMR.
Supplementary Material
Acknowledgments
We thank Stephen White and Wytze van der Veer (UC Irvine) for the use of the circular dichroism spectrophotometers and Fabrice Giusti (IBPC) for the synthesis of amphipol A8–35. We also thank Cambridge Isotopes for the gift of insect cell media for pilot expression studies. This work was supported by grant R01AI092129 from the National Institute of Allergy and Infectious Diseases, by the Centre National de la Recherche Scientifique, by University Paris-7, and by the “Initiative d’Excellence” program from the French State (Grant “DYNAMO”, ANR-11-LABX-0011-01).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00232-014-9693-5) contains supplementary material, which is available to authorized users.
Contributor Information
H. Eric Feinstein, Department of Pathology and Laboratory Medicine, University of California, Irvine, CA, USA.
Delia Tifrea, Department of Pathology and Laboratory Medicine, University of California, Irvine, CA, USA.
Guifeng Sun, Department of Pathology and Laboratory Medicine, University of California, Irvine, CA, USA.
Jean-Luc Popot, UMR 7099, Centre National de la Recherche Scientifique and Paris–7 University, Institut de Biologie Physico-Chimique (CNRS FRC 550), 13, rue Pierre-et-Marie-Curie, 75005 Paris, France.
Luis M. de la Maza, Department of Pathology and Laboratory Medicine, University of California, Irvine, CA, USA
Melanie J. Cocco, Email: mcocco@uci.edu, Department of Molecular Biology and Biochemistry, University of California, Irvine, CA, USA
References
- Akers MJ, Milton N, Byrn SR, Nail SL. Glycine crystallization during freezing: the effects of salt form, pH, and ionic strength. Pharm Res. 1995;12:1457–1461. doi: 10.1023/a:1016223101872. [DOI] [PubMed] [Google Scholar]
- Allan I, Pearce JH. Amino acid requirements of strains of Chlamydia trachomatis and C. psittaci growing in McCoy cells: relationship with clinical syndrome and host origin. J Gen Microbiol. 1983;129:2001–2007. doi: 10.1099/00221287-129-7-2001. [DOI] [PubMed] [Google Scholar]
- Bazzacco P, Billon-Denis E, Sharma KS, Catoire LJ, Mary S, Le Bon C, Point E, Banères JL, Durand G, Zito F, Pucci B, Popot J-L. Nonionic homopolymeric amphipols: application to membrane protein folding, cell-free synthesis, and solution nuclear magnetic resonance. Biochemistry. 2012;51:1416–1430. doi: 10.1021/bi201862v. [DOI] [PubMed] [Google Scholar]
- Blake MS, Gotschlich EC. Purification and partial characterization of the opacity-associated proteins of Neisseria gonorrhoeae. J Exp Med. 1984;159:452–462. doi: 10.1084/jem.159.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie JU. Stabilizing membrane proteins. Curr Opin Struct Biol. 2001;11:397–402. doi: 10.1016/s0959-440x(00)00223-2. [DOI] [PubMed] [Google Scholar]
- Breyton C, Tribet C, Olive J, Dubacq JP, Popot J-L. Dimer to monomer conversion of the cytochrome b6 f complex. Causes and consequences. J Biol Chem. 1997;272:21892–21900. doi: 10.1074/jbc.272.35.21892. [DOI] [PubMed] [Google Scholar]
- Cai S, He F, Samra HS, de la Maza LM, Bottazzi ME, Joshi SB, Middaugh CR. Biophysical and stabilization studies of the Chlamydia trachomatis mouse pneumonitis major outer membrane protein. Mol Pharm. 2009;6:1553–1561. doi: 10.1021/mp900110q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao E, Chen Y, Cui Z, Foster PR. Effect of freezing and thawing rates on denaturation of proteins in aqueous solutions. Biotechnol Bioeng. 2003;82:684–690. doi: 10.1002/bit.10612. [DOI] [PubMed] [Google Scholar]
- Catoire LJ, Zoonens M, van Heijenoort C, Giusti F, Popot J-L, Guittet E. Inter-and intramolecular contacts in a membrane protein/surfactant complex observed by heteronuclear dipole-to-dipole cross-relaxation. J Magn Reson. 2009;197:91–95. doi: 10.1016/j.jmr.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Catoire LJ, Damian M, Giusti F, Martin A, van Heijenoort C, Popot J-L, Guittet E, Banères JL. Structure of a GPCR ligand in its receptor-bound state: leukotriene B4 adopts a highly constrained conformation when associated to human BLT2. J Am Chem Soc. 2010a;132:9049–9057. doi: 10.1021/ja101868c. [DOI] [PubMed] [Google Scholar]
- Catoire LJ, Zoonens M, van Heijenoort C, Giusti F, Guittet E, Popot J-L. Solution NMR mapping of water-accessible residues in the transmembrane β-barrel of OmpX. Eur Biophys J. 2010b;39:623–630. doi: 10.1007/s00249-009-0513-2. [DOI] [PubMed] [Google Scholar]
- Catoire LJ, Damian M, Baaden M, Guittet E, Banères JL. Electrostatically-driven fast association and perdeuteration allow detection of transferred cross-relaxation for G protein-coupled receptor ligands with equilibrium dissociation constants in the high-to-low nanomolar range. J Biomol NMR. 2011;50:191–195. doi: 10.1007/s10858-011-9523-3. [DOI] [PubMed] [Google Scholar]
- Champeil P, Menguy T, Tribet C, Popot J-L, le Maire M. Interaction of amphipols with sarcoplasmic reticulum Ca2+-ATPase. J Biol Chem. 2000;275:18623–18637. doi: 10.1074/jbc.M000470200. [DOI] [PubMed] [Google Scholar]
- Chang BS, Kendrick BS, Carpenter JF. Surface-induced denaturation of proteins during freezing and its inhibition by surfactants. J Pharm Sci. 1996;85:1325–1330. doi: 10.1021/js960080y. [DOI] [PubMed] [Google Scholar]
- Dahmane T, Damian M, Mary S, Popot J-L, Banères JL. Amphipol-assisted in vitro folding of G protein-coupled receptors. Biochemistry. 2009;48:6516–6521. doi: 10.1021/bi801729z. [DOI] [PubMed] [Google Scholar]
- Dahmane T, Giusti F, Catoire LJ, Popot J-L. Sulfonated amphipols: synthesis, properties, and applications. Biopolymers. 2011;95:811–823. doi: 10.1002/bip.21683. [DOI] [PubMed] [Google Scholar]
- Dahmane T, Rappaport F, Popot J-L. Amphipol-assisted folding of bacteriorhodopsin in the presence or absence of lipids: functional consequences. Eur Biophys J. 2013;42:85–101. doi: 10.1007/s00249-012-0839-z. [DOI] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- Elter S, Raschle T, Arens S, Viegas A, Gelev V, Etzkorn M, Wagner G. Use of amphipols for the NMR structural characterization of 7-TM receptors. J Membr Biol. 2014 doi: 10.1007/s00232-014-9669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzkorn M, Raschle T, Hagn F, Gelev V, Rice AJ, Walz T, Wagner G. Cell-free expressed bacteriorhodopsin in different soluble membrane mimetics: biophysical properties and NMR accessibility. Structure. 2013;21:394–401. doi: 10.1016/j.str.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzkorn M, Zoonens M, Catoire LJ, Popot J-L, Hiller S. How amphipols embed membrane proteins: global solvent accessibility and interaction with a flexible protein terminus. J Membr Biol. 2014 doi: 10.1007/s00232-014-9657-9. [DOI] [PubMed] [Google Scholar]
- Fan H, Li H, Zhang M, Middaugh CR. Effects of solutes on empirical phase diagrams of human fibroblast growth factor 1. J Pharm Sci. 2007;96:1490–1503. doi: 10.1002/jps.20796. [DOI] [PubMed] [Google Scholar]
- Franks F, Hatley RH. Low-temperature unfolding of chymotrypsinogen. Cryobiology. 1985;22:608. [Google Scholar]
- Garavito RM, Ferguson-Miller S. Detergents as tools in membrane biochemistry. J Biol Chem. 2001;276:32403–32406. doi: 10.1074/jbc.R100031200. [DOI] [PubMed] [Google Scholar]
- Giusti F, Popot J-L, Tribet C. Well-defined critical association concentration and rapid adsorption at the air/water interface of a short amphiphilic polymer, amphipol A8-35: a study by Förster resonance energy transfer and dynamic surface tension measurements. Langmuir. 2012;28:10372–10380. doi: 10.1021/la300774d. [DOI] [PubMed] [Google Scholar]
- Giusti F, Rieger J, Catoire LJ, Qian S, Calabrese AN, Watkinson TG, Casiraghi M, Radford SE, Ashcroft AE, Popot J-L. Synthesis, characterization and applications of a perdeuterated amphipol. J Membr Biol. 2014 doi: 10.1007/s00232-014-9656-x. [DOI] [PubMed] [Google Scholar]
- Gohon Y, Popot J-L. Membrane protein–surfactant complexes. Curr Opin Colloid Interface Sci. 2003;8:15–22. [Google Scholar]
- Gohon Y, Pavlov G, Timmins P, Tribet C, Popot J-L, Ebel C. Partial specific volume and solvent interactions of amphipol A8-35. Anal Biochem. 2004;334:318–334. doi: 10.1016/j.ab.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Gohon Y, Giusti F, Prata C, Charvolin D, Timmins P, Ebel C, Tribet C, Popot J-L. Well-defined nanoparticles formed by hydrophobic assembly of a short and polydisperse random terpolymer, amphipol A8-35. Langmuir. 2006;22:1281–1290. doi: 10.1021/la052243g. [DOI] [PubMed] [Google Scholar]
- Gohon Y, Dahmane T, Ruigrok RW, Schuck P, Charvolin D, Rappaport F, Timmins P, Engelman DM, Tribet C, Popot J-L, Ebel C. Bacteriorhodopsin/amphipol complexes: structural and functional properties. Biophys J. 2008;94:3523–3537. doi: 10.1529/biophysj.107.121848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griko YV, Privalov PL, Sturtevant JM, Venyaminov S. Cold denaturation of staphylococcal nuclease. Proc Natl Acad Sci USA. 1988;85:3343–3347. doi: 10.1073/pnas.85.10.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper A, Pogson CI, Pearce JH. Amino acid transport into cultured McCoy cells infected with Chlamydia trachomatis. Infect Immun. 2000;68:5439–5442. doi: 10.1128/iai.68.9.5439-5442.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen C, Wiese A, Reubsaet L, Dekker N, de Cock H, Seydel U, Tommassen J. Biochemical and biophysical characterization of in vitro folded outer membrane porin PorA of Neisseria meningitidis. Biochim Biophys Acta. 2000;1464:284–298. doi: 10.1016/s0005-2736(00)00155-3. [DOI] [PubMed] [Google Scholar]
- Jiang S, Nail SL. Effect of process conditions on recovery of protein activity after freezing and freeze-drying. Eur J Pharm Biopharm. 1998;45:249–257. doi: 10.1016/s0939-6411(98)00007-1. [DOI] [PubMed] [Google Scholar]
- Kolhe P, Amend E, Singh SK. Impact of freezing on pH of buffered solutions and consequences for monoclonal antibody aggregation. Biotechnol Prog. 2010;26:727–733. doi: 10.1002/btpr.377. [DOI] [PubMed] [Google Scholar]
- Kristensen D, Chen D, Cummings R. Vaccine stabilization: research, commercialization, and potential impact. Vaccine. 2011;29:7122–7124. doi: 10.1016/j.vaccine.2011.05.070. [DOI] [PubMed] [Google Scholar]
- Kueltzo LA, Wang W, Randolph TW, Carpenter JF. Effects of solution conditions, processing parameters, and container materials on aggregation of a monoclonal antibody during freeze-thawing. J Pharm Sci. 2008;97:1801–1812. doi: 10.1002/jps.21110. [DOI] [PubMed] [Google Scholar]
- Minetti CA, Tai JY, Blake MS, Pullen JK, Liang SM, Remeta DP. Structural and functional characterization of a recombinant PorB class 2 protein from Neisseria meningitidis. Conformational stability and porin activity. J Biol Chem. 1997;272:10710–10720. doi: 10.1074/jbc.272.16.10710. [DOI] [PubMed] [Google Scholar]
- Moulder JW. Intracellular parasitism: life in an extreme environment. J Infect Dis. 1974;130:300–306. doi: 10.1093/infdis/130.3.300. [DOI] [PubMed] [Google Scholar]
- Murase N, Franks F. Salt precipitation during the freeze-concentration of phosphate buffer solutions. Biophys Chem. 1989;34:293–300. doi: 10.1016/0301-4622(89)80066-3. [DOI] [PubMed] [Google Scholar]
- Nigg C. An unidentified virus which produces pneumonia and systemic infection in mice. Science. 1942;95:49–50. doi: 10.1126/science.95.2454.49-a. [DOI] [PubMed] [Google Scholar]
- Opačić M, Durand G, Bosco M, Polidori A, Popot J-L. Amphipols and photosynthetic pigment-protein complexes. J Membr Biol. 2014 doi: 10.1007/s00232-014-9712-6. submitted. [DOI] [PubMed] [Google Scholar]
- Pal S, Theodor I, Peterson EM, de la Maza LM. Immunization with an acellular vaccine consisting of the outer membrane complex of Chlamydia trachomatis induces protection against a genital challenge. Infect Immun. 1997;65:3361–3369. doi: 10.1128/iai.65.8.3361-3369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Davis HL, Peterson EM, de la Maza LM. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein by use of CpG oligodeoxynucleotides as an adjuvant induces a protective immune response against an intranasal chlamydial challenge. Infect Immun. 2002;70:4812–4817. doi: 10.1128/IAI.70.9.4812-4817.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patois E, Capelle MA, Gurny R, Arvinte T. Stability of seasonal influenza vaccines investigated by spectroscopy and microscopy methods. Vaccine. 2011;29:7404–7413. doi: 10.1016/j.vaccine.2011.07.067. [DOI] [PubMed] [Google Scholar]
- Perlmutter J, Popot J-L, Sachs J. Molecular dynamics simulations of a membrane protein/amphipol complex. J Membr Biol. 2014 doi: 10.1007/s00232-014-9690-8. [DOI] [PubMed] [Google Scholar]
- Picard M, Dahmane T, Garrigos M, Gauron C, Giusti F, le Maire M, Popot J-L, Champeil P. Protective and inhibitory effects of various types of amphipols on the Ca2+-ATPase from sarcoplasmic reticulum: a comparative study. Biochemistry. 2006;45:1861–1869. doi: 10.1021/bi051954a. [DOI] [PubMed] [Google Scholar]
- Planchard N, Point E, Dahmane T, Giusti F, Renault M, Le Bon C, Durand G, Milon A, Guittet E, Zoonens M, Popot J-L, Catoire LJ. The use of amphipols for solution NMR studies of membrane proteins: advantages and constraints as compared to other solubilizing media. J Membr Biol. 2014 doi: 10.1007/s00232-014-9654-z. [DOI] [PubMed] [Google Scholar]
- Pocanschi CL, Popot J-L, Kleinschmidt JH. Folding and stability of outer membrane protein A (OmpA) from Escherichia coli in an amphipathic polymer, amphipol A8-35. Eur Biophys J. 2013;42:103–118. doi: 10.1007/s00249-013-0887-z. [DOI] [PubMed] [Google Scholar]
- Popot J-L. Amphipols, nanodiscs, and fluorinated surfactants: three nonconventional approaches to studying membrane proteins in aqueous solutions. Annu Rev Biochem. 2010;79:737–775. doi: 10.1146/annurev.biochem.052208.114057. [DOI] [PubMed] [Google Scholar]
- Popot J-L, Berry EA, Charvolin D, Creuzenet C, Ebel C, Engelman DM, Flötenmeyer M, Giusti F, Gohon Y, Hervé P, Hong Q, Lakey JH, Leonard K, Shuman HA, Timmins P, Warschawski DE, Zito F, Zoonens M, Pucci B, Tribet C. Amphipols: polymeric surfactants for membrane biology research. Cell Mol Life Sci. 2003;60:1559–1574. doi: 10.1007/s00018-003-3169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popot J-L, Althoff T, Bagnard D, Baneres JL, Bazzacco P, Billon-Denis E, Catoire LJ, Champeil P, Charvolin D, Cocco MJ, Cremel G, Dahmane T, de la Maza LM, Ebel C, Gabel F, Giusti F, Gohon Y, Goormaghtigh E, Guittet E, Kleinschmidt JH, Kuhlbrandt W, Le Bon C, Martinez KL, Picard M, Pucci B, Sachs JN, Tribet C, van Heijenoort C, Wien F, Zito F, Zoonens M. Amphipols from A to Z. Annu Rev Biophys. 2011;40(40):379–408. doi: 10.1146/annurev-biophys-042910-155219. [DOI] [PubMed] [Google Scholar]
- Raschle T, Hiller S, Etzkorn M, Wagner G. Nonmicellar systems for solution NMR spectroscopy of membrane proteins. Curr Opin Struct Biol. 2010;20:471–479. doi: 10.1016/j.sbi.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault M. PhD Thesis. Université Paul Sabatier; Toulouse: 2008. Etudes structurales et dynamiques de la protéine membranaire KpOmpA par RMN en phase liquide et solide. [Google Scholar]
- Rosenbusch JP. Stability of membrane proteins: relevance for the selection of appropriate methods for high-resolution structure determinations. J Struct Biol. 2001;136:144–157. doi: 10.1006/jsbi.2001.4431. [DOI] [PubMed] [Google Scholar]
- Salnikova MS, Joshi SB, Rytting JH, Warny M, Middaugh CR. Physical characterization of Clostridium difficile toxins and toxoids: effect of the formaldehyde crosslinking on thermal stability. J Pharm Sci. 2008;97:3735–3752. doi: 10.1002/jps.21261. [DOI] [PubMed] [Google Scholar]
- Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwegman JJ, Carpenter JF, Nail SL. Evidence of partial unfolding of proteins at the ice/freeze-concentrate interface by infrared microscopy. J Pharm Sci. 2009;98:3239–3246. doi: 10.1002/jps.21843. [DOI] [PubMed] [Google Scholar]
- Strambini GB, Gabellieri E. Proteins in frozen solutions: evidence of ice-induced partial unfolding. Biophys J. 1996;70:971–976. doi: 10.1016/S0006-3495(96)79640-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Pal S, Sarcon AK, Kim S, Sugawara E, Nikaido H, Cocco MJ, Peterson EM, de la Maza LM. Structural and functional analyses of the major outer membrane protein of Chlamydia trachomatis. J Bacteriol. 2007;189:6222–6235. doi: 10.1128/JB.00552-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tifrea DF, Sun G, Pal S, Zardeneta G, Cocco MJ, Popot J-L, de la Maza LM. Amphipols stabilize the Chlamydia major outer membrane protein and enhance its protective ability as a vaccine. Vaccine. 2011;29:4623–4631. doi: 10.1016/j.vaccine.2011.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tifrea DF, Pal S, Popot J-L, Cocco MJ, de la Maza LM. Increased immunoaccessibility of MOMP epitopes in a vaccine formulated with amphipols may account for the very robust protection elicited against a vaginal challenge with Chlamydia muridarum. J Immunol. 2014;192:5201–5213. doi: 10.4049/jimmunol.1303392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribet C, Audebert R, Popot J-L. Amphipols: polymers that keep membrane proteins soluble in aqueous solutions. Proc Natl Acad Sci USA. 1996;93:15047–15050. doi: 10.1073/pnas.93.26.15047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webby RJ, Sandbulte MR. Influenza vaccines. Front Biosci. 2008;13:4912–4924. doi: 10.2741/3050. [DOI] [PubMed] [Google Scholar]
- Weiss WFt, Young TM, Roberts CJ. Principles, approaches, and challenges for predicting protein aggregation rates and shelf life. J Pharm Sci. 2009;98:1246–1277. doi: 10.1002/jps.21521. [DOI] [PubMed] [Google Scholar]
- Zhang A, Qi W, Singh SK, Fernandez EJ. A new approach to explore the impact of freeze-thaw cycling on protein structure: hydrogen/deuterium exchange mass spectrometry (HX-MS) Pharm Res. 2011;28:1179–1193. doi: 10.1007/s11095-011-0383-z. [DOI] [PubMed] [Google Scholar]
- Zoonens M, Popot J-L. Amphipols for each season. J Membr Biol. 2014 doi: 10.1007/s00232-014-9666-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoonens M, Catoire LJ, Giusti F, Popot J-L. NMR study of a membrane protein in detergent-free aqueous solution. Proc Natl Acad Sci USA. 2005;102:8893–8898. doi: 10.1073/pnas.0503750102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoonens M, Giusti F, Zito F, Popot J-L. Dynamics of membrane protein/amphipol association studied by Förster resonance energy transfer: implications for in vitro studies of amphipol-stabilized membrane proteins. Biochemistry. 2007;46:10392–10404. doi: 10.1021/bi7007596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.