Abstract
The Arp2/3 complex can be independently activated to initiate actin polymerization by the VCA domain of WASP family members and by the acidic N-terminal and F-actin-binding repeat region of cortactin, which possesses a C-terminal SH3 domain. Cortactin is a target for phosphorylation by Src tyrosine kinases and by serine/threonine kinases that include Erk. Here we demonstrate that cortactin binds N-WASP and WASP via its SH3 domain, induces in vitro N-WASP-mediated actin polymerization, and colocalizes with N-WASP and WASP at sites of active actin polymerization. Erk phosphorylation and a mimicking S405,418D double mutation enhanced cortactin binding and activation of N-WASP. In contrast, Src phosphorylation inhibited the ability of cortactin previously phosphorylated by Erk, and that of S405,418D double mutant cortactin, to bind and activate N-WASP. Furthermore, Y→D mutation of three tyrosine residues targeted by Src (Y421, Y466, and Y482) inhibited the ability of S405,418D cortactin to activate N-WASP. We propose that Erk phosphorylation liberates the SH3 domain of cortactin from intramolecular interactions with proline-rich regions, causing it to synergize with WASP and N-WASP in activating the Arp2/3 complex, and that Src phosphorylation terminates cortactin activation of N-WASP and WASP.
Actin assembly, the synthesis of filamentous actin (F-actin) from globular actin monomers (G-actin), underlies multiple cellular processes. Nucleation of actin polymerization is catalyzed by the Arp2/3 complex, which is composed of seven subunits, two of which, Arp2 and Arp3, show homology to actin (33). For actin nucleation to proceed, the Arp2/3 complex needs to be activated (30, 46). Members of the Wiskott-Aldrich syndrome family of proteins play an important role in activating the Arp2/3 complex in response to extracellular signals (19, 35). The founder member of this family, WASP, is encoded by the gene mutated in the Wiskott-Aldrich syndrome (9). WASP is expressed in hematopoietic cells, while its homologue N-WASP is ubiquitously expressed (37). Other WASP family members include the WAVE and Scar proteins (2, 38). All are modular proteins that possess a conserved C-terminal VCA (verprolin-cofilin homology-acidic) domain that recruits G-actin and interacts with the Arp2/3 complex (34). WASP and N-WASP have an N-terminal Ena/VASP homology domain 1 (EVH1) that binds WIP (36, 43), a Cdc42/Rac GTPase-binding domain (GBD), and a proline-rich domain that interacts with multiple SH3 domain-containing proteins, such as Nck (45) and Grb2 (52), followed by the VCA domain. WASP and N-WASP are thought to exist in cells in a closed inactive conformation mainly owing to intramolecular autoinhibitory interactions that involve the C-terminal acidic domain and the GBD and/or the basic region that precedes it (18, 24, 47). Disruption of these interactions by binding of the Cdc42, PIP2, or SH3 domain to N-WASP allows the VCA domain to interact with and activate the Arp2/3 complex (7, 49).
Cortactin is an actin-binding protein that was initially identified as a major Src substrate (66). Cortactin contains six and a half 37-amino-acid (aa) repeats, of which the fourth is required for F-actin-binding activity (64). These repeats are followed by a helical region and a proline-rich region that precede a C-terminal SH3 domain (65). Cortactin also harbors an N-terminal acidic (NTA) domain functionally equivalent to the VCA domain of WASP family proteins in that it binds, through a conserved DDW motif, the Arp2/3 complex and activates it in conjunction with the F-binding repeat region (57, 61, 63). A single cortactin gene has been identified, which is expressed in nearly all mammalian tissues (28, 66). A number of observations suggest that cortactin plays an important role in remodeling the actin cytoskeleton. Cortactin binds to the side of actin filaments and favors actin branching in vitro, possibly by recruiting the Arp2/3 complex. It has also been shown to synergize with the VCA domain of N-WASP in causing Arp2/3 complex-mediated actin polymerization, possibly by stabilizing the interaction of the Arp2/3 complex with the mother actin filament (57, 60, 61). Cortactin is translocated from the cytoplasm to the periphery following growth factor signaling, integrin activation, and bacterial entry (6, 55, 62). It is enriched in neuronal growth cones, podosomes, and lamellipodia induced by platelet-derived growth factor and epidermal growth factor (10, 27, 39, 65). Cortactin has been found to be overexpressed in carcinomas and is thought to play a role in the tissue invasiveness of these tumors (3, 31, 32).
Cortactin is a target for phosphorylation by tyrosine kinases, including Src, Fer, and Syk (14, 25, 65), and by serine/threonine kinases, including Erk and PAK (4, 58). The role of phosphorylation in cortactin function is not well understood (64). Here we show that cortactin binds to WASP and N-WASP and activates N-WASP-mediated Arp2/3 complex-dependent actin polymerization via its SH3 domain. The ability of cortactin to activate N-WASP via its SH3 domain is positively regulated by Erk phosphorylation and negatively regulated by Src phosphorylation.
MATERIALS AND METHODS
Cells, reagents, and antibodies.
Swiss 3T3 fibroblasts were a gift of Alan Hall (University College London, London, United Kingdom). Jurkat E6-1 T cells and NALM6 B cells were obtained from the American Type Culture Collection. Cells were cultured in Iscove's modified Dulbecco modified Eagle medium (fibroblasts) or RPMI 1640 medium (Jurkat, NALM6 B cells) with 10% fetal calf serum. The antibodies used were anti-cortactin monoclonal antibody (MAb) 4F11 (Upstate); rabbit polyclonal immunoglobulin G anti-WAVE (Upstate), which we find only recognizes WAVE-1; anti-WASP antibody H-250; MAb B9 (Santa Cruz); anti-WASP MAb 5A5 (23); anti-myc MAb 9E10 (Santa Cruz); affinity-purified anti-N-WASP rabbit antibody (47); anti-Src MAb GD11 (Upstate); anti-Erk MAb 7D8 (Zymed); and rabbit polyclonal phospho-cortactin-specific antibodies pY421 and pY466 (Biosource). Recombinant Src and Erk were purchased from Upstate and used in accordance with the manufacturer's instructions.
Protein preparation and pulldown assay.
Murine cortactin-glutathione (GSH) S-transferase (GST) fusion constructs (made with FL-cort [full-length cortactin], N-cort [N-terminal fragment of cortactin], C-cort [C-terminal fragment of cortactin], and SH3-cort [SH3 domain of cortactin]) were provided by Sheila M. Thomas (Cancer Biology Program, BIH/Harvard Medical School). After verification by sequencing, they were subcloned into the PGEX-6P2 vector (Amersham Pharmacia Biotech). The W22A, S405,418D, Y466,482D, S405,418D-Y466,482D, Y421D, S405,418D-Y421D, Y421,466,482D, S405,418D-Y421,466,482D, FLW525K, and SH3W525K mutant cortactin constructs were generated by PCR with a QuikChange site-directed mutagenesis kit (Stratagene) and the appropriate oligonucleotides. ΔSH3 truncated mutant forms of cortactin were generated by PCR with GST-FL-cort as the template. PCR amplicons were verified by DNA sequencing and cloned into pGEX-6P2. GST fusion proteins were induced, purified, and used in pulldown assays with GSH-Sepharose beads. After extensive washing, bound proteins were eluted by boiling in Laemmli buffer for 3 min, split into two aliquots, and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on duplicate 4 to 15% gradient gels (Bio-Rad). One gel was stained with Coomassie blue, and the other was analyzed by Western blotting. All pulldown assays were done at least three times. Affinity-purified, His-tagged, baculovirus-expressed N-WASP protein and the constructs used to generated N-WASP fragments were previously described (47, 48). Truncated N-WASP constructs were in vitro transcribed and translated with TNT-coupled reticulocyte lysate (Promega).
Immunoprecipitation and Western blotting.
Cells were lysed in ice-cold lysis buffer (10 mM Tris [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 50% glycerol, 1 mM sodium orthovanadate, 50 mM NaF, protease inhibitor cocktail [Sigma]). Immunoprecipitations (25 × 106 cells) and lysates (106 cells) were run on SDS-PAGE 4 to 15% gradient gels (Bio-Rad) and analyzed by Western blotting with the indicated antibodies, followed by secondary antibodies conjugated to horseradish peroxidase, and enhanced chemiluminescence detection (Perkin-Elmer).
Pyrene-labeled actin polymerization assay.
Polymerization assays were performed essentially as previously described (47, 48). Briefly, the Arp 2/3 complex, N-WASP, and actin were purified as previously reported (48). G-actin was freshly thawed, kept at 4°C in G-actin buffer (48) overnight, and centrifuged at 400,000 × g for 1 h to remove residual filamentous actin. Actin (1 μM final concentration, actin/pyrene-actin ratio, 9:1), the Arp 2/3 complex (33 nM), N-WASP (90 nM), and VCA (100 nM) were used in a total volume of 60 μl of actin polymerization buffer (48). Proteins were mixed and preincubated for 5 min at room temperature, and the reaction was initiated by addition of actin; the increase in fluorescence was monitored in a spectrofluorimeter. The curves were shifted along the abscissa to account for the time delay between the actin addition and the first fluorescence reading. All experiments were repeated a minimum of three times with two different batches of expressed proteins.
Erk and Src in vitro phosphorylation assay.
The Erk and Src in vitro phosphorylation assay was done by following the manufacturer's recommendations and reference 21. Briefly, equal amounts of GST-cortactin and GST-cortactin mutants coupled to GSH-beads were incubated with constant tumbling at room temperature for 30 min with commercial Erk and/or Src in their respective buffers (Upstate). One small aliquot (approximately 1/10) of the reaction was carried out in parallel with [γ-32P]ATP. After the reaction was stopped by addition of Laemmli buffer, the samples were analyzed by SDS-PAGE, transferred to a nitrocellulose membrane, and developed by autoradiography to confirm the phosphorylation. Double Erk/Src phosphorylation was done sequentially. The reaction of the major aliquot containing the proteins to be used in the assays was terminated by extensive washing. GST-cortactin was eluted with GSH and further purified with a column with a cutoff of 50 ± 10 kDa.
T-cell-antigen-presenting cell conjugate formation and immunofluorescence microscopy.
Jurkat T cells and NALM6 B cells were conjugated as previously described (5). NALM6 B cells were labeled with 5 μM blue fluorescent cell tracker CMAC (Molecular Probes) for 20 min and then incubated with or without superantigen SEE (Toxin Technologies) at 5 μg/ml for 30 min at 37°C. They were washed twice and incubated with an equal number of T cells at 37°C for 20 min while they were allowed to settle on poly-l-lysine-coated coverslips (Sigma). Conjugates were scored visually. Immunofluorescence assay was performed with anti-cortactin MAb 4F11 and rabbit polyclonal anti-WASP antibody, followed by a tetramethyl rhodamine isothiocyanate (TRITC)- or Alexa green-labeled secondary antibody (Molecular Probes). F-actin staining was done with TRITC-phalloidin (Sigma) at 1 μg/ml. Fifty T-cell-B-cell conjugates were examined in each experiment.
RESULTS
Cortactin interacts with WASP and N-WASP.
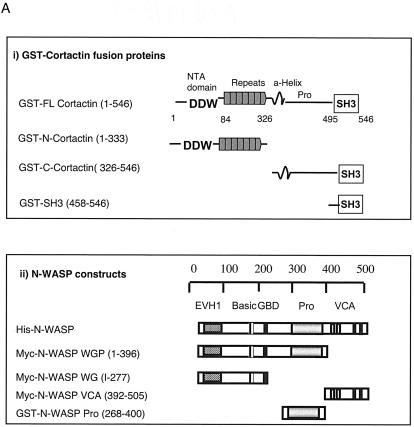
We performed a database search (67) for proteins that contain SH3 domains that may interact with the proline-rich regions in WASP. Of several potential candidates identified, we chose to examine cortactin because of its known role in the organization of the actin cytoskeleton. To assess the interaction of cortactin with WASP, we expressed FL-cort and three cortactin fragments as GST fusion proteins. These consisted of N-cort, which spans aa 1 to 333 and includes the NTA domain (aa 1 to 84) that binds the Arp2/3 complex and all six and a half repeats; C-cort, which spans aa 326 to 546 and contains the α-helical region, the proline-rich region, and the SH3 domain; and SH3-cort, which spans aa 458 to 546 (Fig. 1A). Pulldown assays with the GST fusion proteins and lysates of Jurkat T cells were performed. Both bound and unbound proteins were Western blotted with anti-WASP MAb 5A5 and with rabbit polyclonal anti-human WAVE-1 antibody as a control. Figure 1B shows that FL-cort, C-cort, and SH3-cort, but not N-cort or GST alone, bound WASP from Jurkat cell lysates. SH3-cort bound considerably more WASP than did FL-cort or C-cort and effectively depleted the cell lysates of WASP, suggesting that the SH3 domain may be partially masked in the native molecule. There was no detectable binding of any of the cortactin-GST fusion proteins to WAVE-1. These results suggest that cortactin binds WASP via its SH3 domain.
FIG. 1.
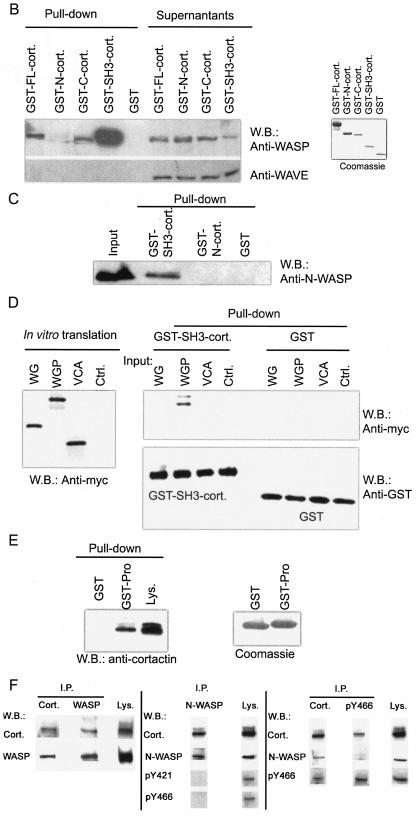
Cortactin interacts with WASP and N-WASP. (A, part i) Map of GST-cortactin constructs. (A, part ii) Map of N-WASP constructs. (B) Pulldown assay of cortactin binding to WASP from Jurkat T-cell lysates. (Left) Proteins that bound to GSH-beads (Pull-down) and unbound proteins (Supernatants) were probed with anti-WASP MAb 5A5 (23) and with anti-WAVE polyclonal antibody (Upstate) as a control. (Right) Coomassie blue staining of the GST fusion proteins used in the pulldown assay. W.B., Western blot. (C) Cortactin binds directly to N-WASP. Recombinant baculovirus-expressed N-WASP protein was pulled down by GST-cortactin fusion proteins coupled to GSH-beads. (D) Mapping of the N-WASP site for SH3-cort. Myc-tagged N-WASP fragments (N-WASP WG [EVH1 plus the GBD], WGP [EVH1 plus the GBD plus the proline-rich region], and VCA domains) were in vitro transcribed, translated, and used in a pulldown assay with GST-SH3-cort. Input (left) and bound (right top) proteins were Western blotted with an anti-Myc MAb (Santa Cruz). The membrane was reblotted with an anti-GST MAb (Santa Cruz) as a loading control (Ctrl.) (right bottom). (E) Pulldown of cortactin by the proline-rich region of N-WASP. A GST fusion protein containing the proline-rich region of N-WASP (right) was able to pull down cortactin, which was detected by MAb 4F11 (Upstate) (65), from 3T3 fibroblast lysates (Lys.) (left). (F) Coprecipitation of cortactin (Cort.) with WASP and N-WASP. (Left) Coprecipitation of cortactin and WASP from Jurkat cells. Cortactin immunoprecipitates (I.P.) obtained with MAb 4F11 (5 μg) were blotted with anti-WASP MAb B9 and reprobed with anti-cortactin MAb 4F11 (both MAbs were used at 1 μg/ml). WASP immunoprecipitates obtained with MAb B9 (5 μg; Santa Cruz) were Western blotted with anti-cortactin MAb 4F11 and reprobed with anti-WASP MAb B9. (Middle) Coprecipitation of N-WASP and cortactin in 3T3-Swiss fibroblasts. N-WASP immunoprecipitates (1;2,000 dilution of the antibody) were blotted for cortactin with MAb 4F11 and phosphospecific antibodies pY421 and pY466 (at a 1:800 dilution). (Right) Cortactin immunoprecipitates were blotted with N-WASP polyclonal antibody (1:10,000 dilution) (48) and reprobed with anti-cortactin MAb. Immunoprecipitates obtained with pY466 (5 μg) were Western blotted for cortactin, N-WASP, and pY466. All experiments were performed a minimum of three times with similar results.
WASP and N-WASP are highly homologous. To assess if cortactin also interacts with N-WASP, we used baculovirus-expressed, affinity-purified, recombinant, His-tagged N-WASP (48) and cortactin-GST fusion proteins in a pulldown assay. Figure 1C shows that SH3-cort, but not N-cort, bound N-WASP. Since the assay used recombinant proteins, we conclude that the SH3 domain of cortactin binds directly to N-WASP.
To map the binding site for cortactin on N-WASP, we used in vitro-translated constructs of Myc-tagged N-WASP (Fig. 1A) (47). Only the WGP fragment containing the proline-rich region bound GST-SH3-cort (Fig. 1D), suggesting that the binding site for cortactin maps to the proline-rich region of N-WASP. To confirm this, we examined the capacity of the proline-rich region of N-WASP fused to GST to pull cortactin down from 3T3 fibroblast lysates. Figure 1E shows that the proline-rich region of N-WASP fused to GST, but not GST alone, bound to cortactin. Thus, the cortactin binding site was mapped to the proline-rich region of N-WASP (aa 268 to 400).
To examine if cortactin interacts with WASP in cells, cortactin immunoprecipitates obtained from Jurkat T cells with MAb 4F11 were probed with the B9 anti-WASP MAb. Conversely, WASP immunoprecipitates from the same cells were Western blotted with cortactin-specific MAb 4F11. Figure 1F (left side) shows that cortactin and WASP were coprecipitated. To examine the association of cortactin with N-WASP, N-WASP immunoprecipitates from lysates of unstimulated 3T3 Swiss fibroblasts were probed for cortactin. Conversely, cortactin immunoprecipitates from the same cells were probed for N-WASP. Figure 1F (middle and right side) shows that cortactin and N-WASP were coprecipitated.
Cortactin colocalizes with N-WASP and WASP at areas of active actin polymerization.
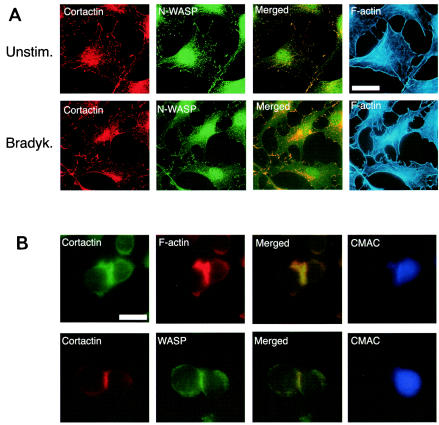
The fact that cortactin interacts with N-WASP and WASP prompted us to analyze whether it colocalizes with these proteins in cells. In subconfluent fibroblasts, bradykinin induces filopodia by activating Cdc42 (29) and lamellipodia as a result of the secondary activation of Rac by Cdc42 (42). To determine if cortactin and N-WASP are present within the same subcellular compartments, serum-starved, unstimulated 3T3 fibroblasts (Fig. 2A, top) and bradykinin-stimulated 3T3 fibroblasts (Fig. 2A, bottom) were fixed, permeabilized, and stained for cortactin (red), N-WASP (green), and F-actin (blue). The staining patterns of cortactin and N-WASP were similar. In unstimulated cells, both proteins were found in a punctate distribution and at the edges of the cell (Fig. 2A). In bradykinin-stimulated cells, there was accumulation of both proteins in areas of filopodium-lamellipodium formation, where F-actin accumulates. Merged images (yellow) demonstrated a large degree of overlap between cortactin staining and N-WASP staining, suggesting that the two proteins colocalize in cells. These results are consistent with previous observations that cortactin and N-WASP individually localize at areas of actin remodeling (41, 65).
FIG. 2.
Cortactin colocalizes with N-WASP and WASP at sites of active actin polymerization. (A) Cortactin colocalizes with N-WASP and F-actin at lamellipodia in bradykinin (Bradyk.)-stimulated 3T3 fibroblasts. Unstimulated (Unstim.) and bradykinin-stimulated cells were fixed, permeabilized, and then stained for F-actin with phalloidin-coumarin. Cortactin was detected with MAb 4F11, followed by goat anti-mouse-TRITC, and N-WASP was detected with rabbit anti-N-WASP antibody, followed by goat anti-rabbit antibody Alexa 488. Cortactin and N-WASP images were merged by using Adobe Photoshop. (B) Cortactin colocalizes with WASP and F-actin at the IS. Intracellular immunofluorescence staining for cortactin, F-actin, and WASP in Jurkat T cells stimulated by SEE (5 μg/ml) and NALM6 B cells preloaded with CMAC (blue). (Top) Cells were stained for cortactin with MAb 4F11 (green) and for F-actin with phalloidin-TRITC. (Bottom) Cells were stained for cortactin with MAb 4F11 (red) and for WASP with rabbit anti-WASP antibody H-250 (green). The images were merged (yellow) by using Adobe Photoshop. Scale bars, 10 μm. Control Jurkat T cells incubated with B cells in the absence of SEE showed very few conjugates.
Interaction between T lymphocytes and antigen-presenting cells induces the formation at the contact site of an immunological synapse (IS), which is enriched in newly formed F-actin (15) and WASP (5). We examined whether cortactin colocalizes with WASP at the IS in Jurkat T cells incubated with NALM6 B cells in the presence of the superantigen SEE. T-cell-B-cell conjugates were examined by immunofluorescence assay after 10 min. Cortactin colocalized with F-actin at the interface in 76% ± 9% of the conjugates. More importantly, cortactin colocalized with WASP at the interface in 71% ± 11% of these conjugates. In polarized cells, cortactin and WASP also accumulated at the pole opposite the IS. These results suggest that cortactin is recruited to the IS as well as to the uropod in polarized cells.
The SH3 domain of cortactin strongly activates N-WASP actin-nucleating activity.
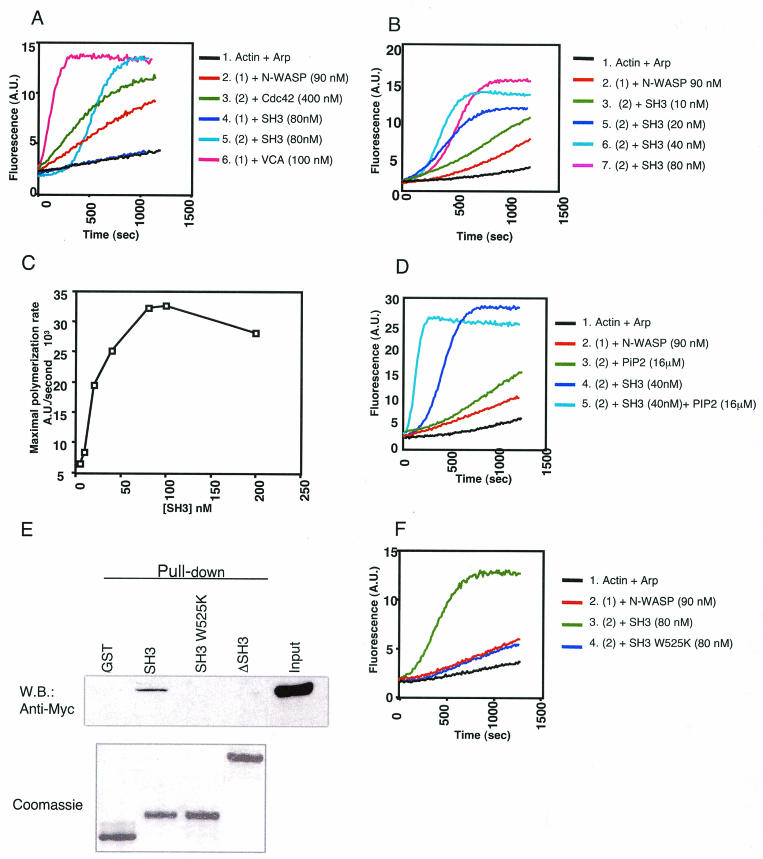
It was previously shown that the SH3 domains of Nck and Grb2 (7, 49) and the N-WASP-interacting protein WISH (13) activate N-WASP actin-nucleating activity. We used the in vitro pyrene-labeled actin polymerization assay (40) to investigate the effect of the SH3 domain of cortactin on the function of N-WASP. SH3-cort (80 nM) had no effect by itself on the Arp2/3 complex but caused vigorous activation of N-WASP (Fig. 3A). Figure 3B shows that the SH3 domain of cortactin activated N-WASP in a dose-dependent manner. The calculated half-maximal activation (Kact) of SH3-cort is ∼25 nM (Fig. 3C). PIP2 synergizes with the Nck SH3 domain to activate N-WASP (49). Figure 3D shows that there is synergy between PIP2 and SH3-cort. Introduction of a point mutation (W525K) into SH3-cort abolishes its ability to bind CortBP1 and dynamin (10, 50). Introduction of the same mutation into SH3-cort abolished its ability to bind N-WASP WGP (Fig. 3E). More importantly, it abolished its ability to activate N-WASP (Fig. 3F).
FIG. 3.
The SH3 domain of cortactin activates N-WASP-mediated Arp 2/3 complex-dependent actin polymerization. Actin (1 μM actin, actin/pyrene-actin ratio of 9:1) and the Arp 2/3 complex (33 nM) were kept constant. N-WASP at 90 nM and VCA at 100 nM were used throughout all of the experiments. All reactions were initiated by the addition of actin. (A) Plots of fluorescence intensity against time since initiation of the polymerization reaction. Consistent with previous observations (48), addition of Cdc42-GTPγS (400 nM) resulted in partial activation of N-WASP (trace 3). SH3-cort (80 nM) had no effect by itself on the Arp2/3 complex (trace 4) but caused vigorous activation of N-WASP (trace 5). A.U., arbitrary units. (B) Dose-response curve. (C) Maximal rate of actin polymerization calculated from the initial portion of the polymerization curve. Panels B and C represent different experiments. (D) PIP2 synergizes with SH3-cort in activating N-WASP. (E) Mutation (W525K) or deletion of SH3-cort abolished the binding of cortactin to N-WASP WGP. Pulldown assays with GST-cortactin and GST-cortactin mutants and reticulocyte lysates containing in vitro-translated, Myc-tagged N-WASP WGP (aa 1 to 396). W.B., Western blot. (F) The W525K mutation abolishes the ability of SH3-cort to activate N-WASP. The results shown are representative of three different experiments.
FL-cort weakly activates N-WASP.
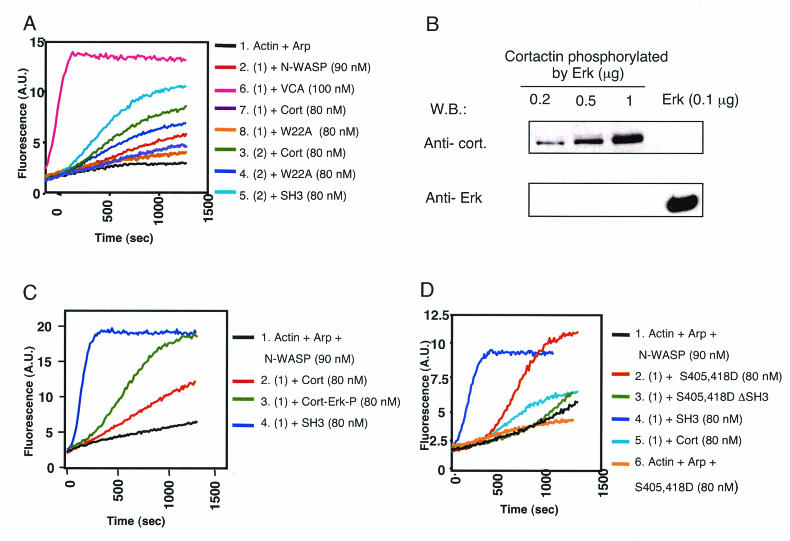
Cortactin is a relatively weak activator of the Arp2/3 complex (57, 61). This activation depends on a 20DDW22 motif located in the NTA domain of cortactin, which is critical for binding to Arp2/3 (63). Under the conditions we have used, we observed minimal activation of the Arp2/3 complex by FL-cort. This was diminished in the cortactin NTA domain mutant W22A (Fig. 4A), which fails to bind the Arp2/3 complex (57, 61). FL-cort (80 nM) caused modest N-WASP-dependent activation of the Arp2/3 complex, compared to the isolated SH3 domain (Fig. 4A). The ability of cortactin to activate N-WASP was dependent on its SH3 domain, because a cortactin SH3 deletion mutant (cortactin ΔSH3), which failed to bind N-WASP (Fig. 3E), caused no detectable activation of N-WASP (data not shown). Activation of N-WASP by FL-cort did not depend on cortactin binding to the Arp2/3 complex, because it was still observed with W22A mutant cortactin. However, W22A mutant cortactin was consistently less efficient than wild-type (WT) cortactin in activating N-WASP (Fig. 4A), although it bound N-WASP to an extent quite similar to that of the WT protein (Fig. 5A). These results suggest that the NTA and SH3 domains of cortactin may synergize in causing actin polymerization,
FIG. 4.
Effect of Erk phosphorylation of cortactin on N-WASP activation. (A) FL-cort weakly activates N-WASP. A.U., arbitrary units. (B) Erk enzyme was not present in the final cortactin preparations. Different amounts (0.2, 0.5, and 1 μg) of Erk-phosphorylated cortactin were run on a gel and Western blotted (W.B.) for cortactin (top) and for Erk (bottom). As a control, 0.1 μg of commercial Erk, which represents 10 times the amount used for phosphorylating 1 μg of cortactin, was loaded in a separated lane. (C) Erk-mediated phosphorylation of cortactin enhances its activation of N-WASP. (D) Introduction of an Erk phosphorylation-mimicking double mutation (S405,418D) into cortactin enhances its ability to activate N-WASP.
FIG. 5.
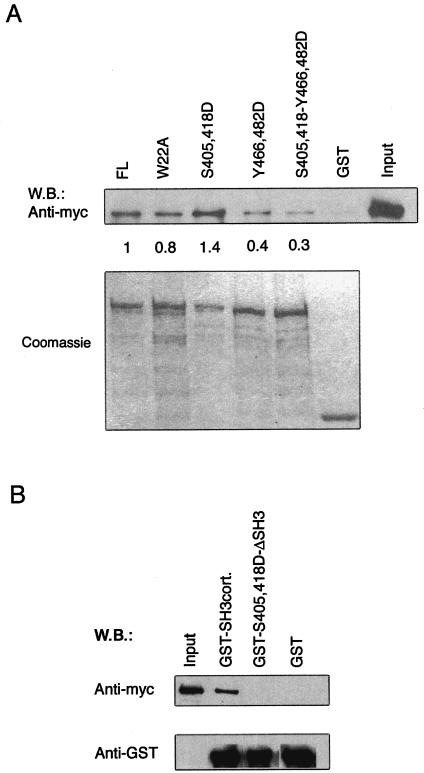
Effects of mutations in FL-cort on its ability to bind N-WASP. Pulldown assays with GST-cortactin and GST-cortactin mutants and reticulocyte lysates containing in vitro-translated, Myc-tagged N-WASP WGP (aa 1 to 396). Input: reticulocyte lysates from the in vitro translation reaction. (A, top) Western blot (W.B.) assay with anti-Myc MAb. (A, bottom) Coomassie blue staining of GST fusion proteins eluted from equivalent amounts of GSH beads used in the pulldown assay as loading controls. Values represent the N-WASP-binding capacity of the cortactin mutants relative to that of native cortactin. (B, top) Western blot assay with anti-Myc MAb. (B, bottom) Western blot assay with anti-GST MAb. All experiments were performed three times with similar results.
Erk phosphorylation of cortactin positively regulates its capacity to bind and activate N-WASP.
The SH3 domain of cortactin is thought to engage in intramolecular association with the proline-rich region of the molecule (4). The stronger binding and activation of N-WASP by SH3-cort compared to those by FL-cort prompted us to explore mechanisms by which the SH3 domain of cortactin becomes more accessible. Cortactin is a target for phosphorylation by the mitogen-activated protein kinase Erk at residues S405 and S418 (4). We first examined whether Erk phosphorylation of cortactin enhances its ability to activate N-WASP. GST-cortactin bound to GSH beads was phosphorylated with Erk, the beads were extensively washed, and cortactin was eluted and further purified with a column with a cutoff of 50 ± 10 kDa. There was no detectable contamination of the phosphorylated cortactin with Erk (Fig. 4B). Cortactin phosphorylated by Erk (Cort-Erk-P) activated N-WASP to a much greater extent than did unphosphorylated cortactin, and to an extent comparable to that observed with SH3-cort (Fig. 4C).
To confirm that the effect of Erk phosphorylation was due to the phosphorylation of S405 and S418, we mutated these two residues to aspartic acid to mimic the negative charge of phosphoserine. Figure 5A shows that the S405,418D double mutant form bound more WASP WGP than did the native protein. More importantly, this mutant protein was markedly more potent than native cortactin in activating N-WASP (Fig. 4D). Deletion of the SH3 domain from the S405,418D mutant protein abolished its abilities to bind Myc-tagged N-WASP WGP (Fig. 5B) and to activate N-WASP (Fig. 4D). These results suggest that Erk phosphorylation of cortactin at S405 and S418 liberates its SH3 domain, allowing it to bind and activate N-WASP.
Src phosphorylation of cortactin negatively regulates its capacity to bind and activate N-WASP.
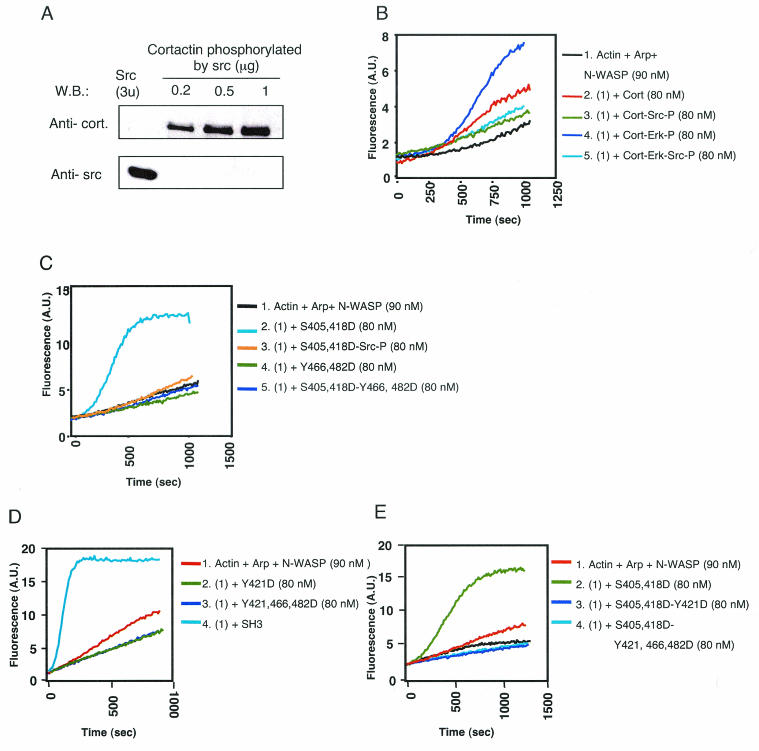
Cortactin was initially identified as a major Src substrate (66). We examined the effect of Src phosphorylation on the ability of cortactin to activate N-WASP. Src-phosphorylated cortactin was prepared and purified as described for Erk-phosphorylated cortactin. There was no detectable contamination of the phosphorylated cortactin with Src (Fig. 6A). Src-phosphorylated cortactin (Cort-Src-P) failed to activate N-WASP (Fig. 6B). In fact, the modest activation of N-WASP by unphosphorylated cortactin was lost upon Src phosphorylation. More importantly, Src phosphorylation inhibited the ability of Erk-phosphorylated cortactin and S405,418D mutant cortactin to activate N-WASP (Fig. 6B and C). These results suggest that Src phosphorylation inhibits cortactin activation of N-WASP.
FIG. 6.
Effect of Src phosphorylation of cortactin on N-WASP activation. (A) Src enzyme was not present in the final cortactin preparations. Different amounts (0.2, 0.5, and 1 μg) of Src-phosphorylated cortactin were run on a gel and Western blotted for cortactin (top) and Src (bottom). As a control, 3 U of commercial Src, which represents 20 times the amount used for phosphorylating 1 μg of cortactin, was loaded into a separate lane. W.B., Western blot. (B) Src-mediated phosphorylation of Erk-phosphorylated cortactin inhibits its ability to activate N-WASP. A.U., arbitrary units. (C) Src-mediated phosphorylation of S405,418D mutant cortactin inhibits its ability to activate N-WASP. Introduction of an Src-mimicking double mutation (Y466,482D) into Erk-mimicking S405,418D mutant cortactin inhibits its capacity to activate N-WASP. (D) Introduction of a single Src-mimicking mutation (Y421D) into Erk-mimicking S405,418D mutant cortactin inhibits its capacity to activate N-WASP. (E) Introduction of the Src-mimicking triple mutation Y421,Y466,Y482 into S405,418D mutant cortactin inhibits its activation of N-WASP. All experiments were performed three times with similar results.
Residues Y466 and Y482 of cortactin are known targets for Src phosphorylation (20). Because of their location immediately upstream of the SH3 domain, they are likely to be involved in Src-mediated inhibition of cortactin SH3 domain-mediated activation of N-WASP. We mutated Y466 and Y482 to aspartic acid to mimic their phosphorylation and examined the effect of the introduction of the double mutation on the binding of WT cortactin and S405,418D mutant cortactin to N-WASP. The Y466,482D double mutation reduced the capacity of WT cortactin and S405,418D mutant cortactin to bind N-WASP (Fig. 5A). More importantly, it inhibited the ability of S405,418D mutant cortactin to activate N-WASP (Fig. 6C). These results suggest that Src phosphorylation of cortactin at Y466 and Y482 inhibits its ability to bind N-WASP and activate it.
It is thought that Src phosphorylation of Y421, within the proline-rich region of cortactin, creates a docking site for the SH2 domain of Src that is essential for subsequent phosphorylation of Y466 and Y482 (17, 20). To investigate whether Y421 phosphorylation by itself affects the ability of cortactin to activate N-WASP, we mutated Y421 alone and in combination with Y466 and Y482 in cortactin and introduced the same mutations into S405,418D mutant cortactin. Both the single and triple Y→D mutants failed to activate N-WASP (Fig. 6D). More importantly, they inhibited the ability of S405,418D mutant cortactin to activate N-WASP (Fig. 6E).
DISCUSSION
We provide evidence for a novel mechanism of activation of N-WASP mediated by the SH3 domain of cortactin. Cortactin binding to and activation of N-WASP is promoted by Erk phosphorylation and inhibited by Src phosphorylation, suggesting that these kinases provide on and off signals that regulate cortactin activation of N-WASP following receptor signaling.
Cortactin was shown to bind WASP and N-WASP (Fig. 1). This was demonstrated in pulldown assays with GST-cortactin and by coimmunoprecipitation from cell lysates. This is consistent with the previously demonstrated association of cortactin with WASP in activated human platelets and of GST-SH3-cort binding to N-WASP from Src-transformed cells (16, 39). By using recombinant proteins, we have (i) established that N-WASP and cortactin directly interact and (ii) mapped the N-WASP-binding site to the SH3 domain of cortactin. SH3-cort bound more efficiently to WASP and N-WASP than did FL-cort, suggesting that the SH3 domain may not be fully accessible in the native molecule. The cortactin binding site was mapped to the proline-rich region of N-WASP (aa 268 to 400) that is distinct from the WIP binding site, which maps to the EVH1 domain spanning aa 26 to 147 (59). This suggests that WASP, cortactin, and WIP may form ternary complexes. Recently, cortactin was shown to bind via its SH3 domain to WIP, which, by virtue of its ability to bind G-actin, promotes actin polymerization by cortactin (26).
Cortactin colocalized with N-WASP in lamellipodia of 3T3 fibroblasts (Fig. 2A). It also colocalized with WASP at the IS between Jurkat T cells and superantigen-presenting B cells (Fig. 2B) and at the interface between Jurkat T cells and anti-CD3-coated beads (data not shown). These results suggest that these two proteins colocalize at areas of actin polymerization and that CD3 ligation is sufficient for the translocation of cortactin and its colocalization with WASP at the IS. WASP is critical for T-cell function (53), while N-WASP is essential for embryonic development (54). The role of cortactin in cellular development and function remains to be investigated.
The SH3 domain of cortactin potently activated N-WASP-mediated Arp2/3 complex-dependent actin (Fig. 3). The calculated Kact of SH3-cort was ∼25 nM, compared to ∼80 nM for Nck and 100 to 200 nM for Grb2 (7, 49). This activation required interaction of the SH3 domain with proline-rich sequences, because a point mutant (W525K) that disrupts SH3 domain binding to such sequences (10) abolished the ability of SH3-cort to bind and activate N-WASP. As has been reported for the SH3 domains of Nck and Grb2 (7, 49), PIP2 synergized with SH3-cort in activating N-WASP (Fig. 3D).
Consistent with its weaker binding to N-WASP, FL-cort activated N-WASP to a weaker degree than did SH3-cort (Fig. 4). Activation of N-WASP by FL-cort was dependent on its SH3 domain, because deleting this domain abolished the capacity of cortactin to activate N-WASP. Cortactin weakly activates the Arp/23 complex through an acidic motif in its N-terminal region in conjunction with the F-binding repeat region (57, 61). Under the conditions we have used in our assays, we did not detect activation of the Arp/23 complex by FL-cort. A cortactin W22A mutant that fails to bind and activate the Arp/23 complex activated N-WASP, albeit less potently than the WT molecule, although it bound normally to N-WASP. These results suggest that recruitment of the Arp2/3 complex by cortactin is not required for its activation of N-WASP. However, it may be required for optimal actin polymerization by the cortactin-N-WASP complex. Arp2/3 complex-mediated actin nucleation may proceed synergistically at two different sites in a cortactin-N-WASP complex, i.e., the NTA domain of cortactin and the VCA domain of WASP.
Our data show that Erk phosphorylation enhances the capacity of cortactin to activate N-WASP (Fig. 4C). Mutation to aspartic acid of two serine residues, S405 and S418, known to be phosphorylated by Erk, to mimic their phosphorylation, caused enhancement of cortactin binding and activation of N-WASP. This was dependent on the SH3 domain, because it was not observed when the SH3 domain was deleted from the S405,418D mutant protein. S405 and S418 are located in the middle of two adjacent minimal SH3 domain consensus motifs (PPxxP), 402PPASP406 and 415PPSSP419 (44). We propose that intramolecular interactions between the SH3 domain and these motifs in FL-cort limit its ability to interact with N-WASP. Phosphorylation of critical serines in these motifs by Erk may disrupt intramolecular SH3-proline-rich sequence interactions and render the SH3 domain fully accessible for binding N-WASP and WASP. A similar model of intramolecular interactions between SH3 domains and proline-rich sequences has been proposed for the Tec kinase ITK (1). We have failed to inhibit SH3-cort binding to and activation of N-WASP with peptides derived from the proline-rich region of cortactin (data not shown). This is possibly because the affinity of the SH3 domain for native N-WASP may be higher than its affinity for individual cortactin-derived peptides and/or because the region of cortactin that interacts with the SH3 domain of the molecule is not limited to the short peptides we used. In the absence of structural data, we cannot rule out the possibility that Erk phosphorylation induces a conformational change in the SH3 domain itself that enhances its ability to bind N-WASP and WASP.
In contrast to Erk phosphorylation, Src phosphorylation of cortactin did not enhance its ability to activate N-WASP. In fact, it inhibited the weak activation of N-WASP observed with FL-cort. More importantly, it inhibited the ability of Erk-phosphorylated cortactin and of S405,418D mutant cortactin to activate N-WASP (Fig. 6B and C). These results suggest that Src phosphorylation negatively regulates cortactin activation of N-WASP in cells. This is consistent with the observation that the Helicobacter pylori CagA protein causes Src inactivation, concomitant dephosphorylation of cortactin, and its redistribution to actin-rich cellular protrusions (51). It is also consistent with the observation that the fraction of cortactin that accumulates in the actin tail of pathogens is not tyrosine phosphorylated (12). Y466 and Y482, which are located immediately upstream of the SH3 domain, are phosphorylated by Src both in vitro and in vivo. Replacement of Y466 and Y482 with aspartic acid to mimic their phosphorylation inhibited the capacity of cortactin and S405,418D mutant cortactin to bind and activate N-WASP, suggesting that phosphorylation of these residues may cause a conformational change in the SH3 domain that inhibits its capacity to bind N-WASP and activate it. Phosphorylation of Y421 by Src is thought to be essential for the phosphorylation of Y466 and Y482 by Src (17, 20). Replacement of Y421 with aspartic acid inhibited the capacity of S405,418D mutant cortactin to activate N-WASP. This suggests an inhibitory effect of Src phosphorylation at Y421 independent of phosphorylation at Y466 and Y482. Tyrosine residues other than Y421, Y466, and Y482, including the tyrosine residues located in the SH3 domain of cortactin (20), may also be targets of Src phosphorylation and may contribute to the inhibition of N-WASP activation.
While this report was in preparation, antibodies that recognize cortactin phosphorylated at positions Y421 and Y466 became available. We used these antibodies to examine whether cortactin phosphorylated at these two positions is coprecipitated with N-WASP. We were unable to detect either phosphorylation of cortactin at position Y421 or Y466 in N-WASP immunoprecipitates (Fig. 1F, middle). Antibody to pY466 cortactin, but not pY421 cortactin, is an immunoprecipitating antibody, and therefore we were able to perform the converse immunoprecipitation with only the anti-pY466 antibody. We were unable to detect N-WASP in pY466 cortactin immunoprecipitates (Fig. 1F, right side). These results suggest that cortactin that associates with N-WASP is not phosphorylated at position Y421 or Y466. We were unable to examine the association of cortactin phosphorylated at residues that are targeted by Erk because antibodies specific to these phosphorylated residues are not available.
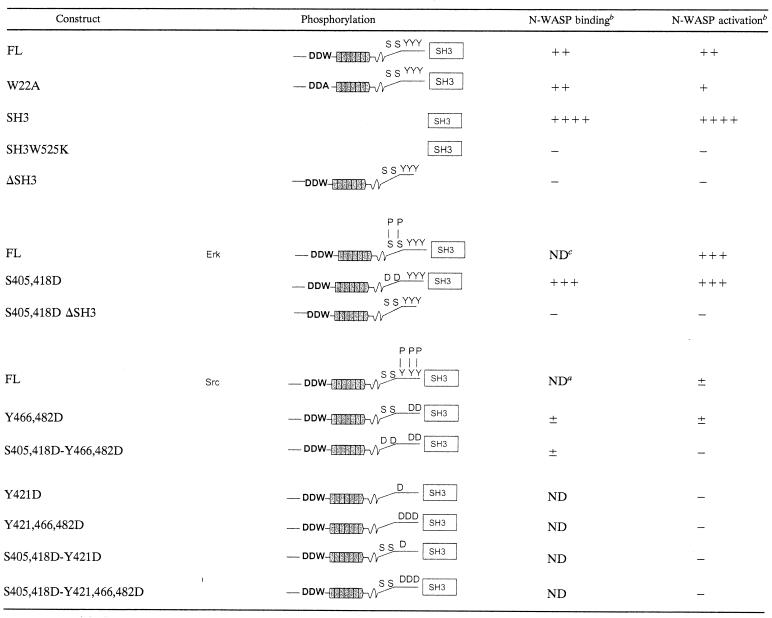
Our results, summarized in Table 1, suggest that the ability of SH3-cort to bind and activate WASP and N-WASP is subject to switch on-switch off regulation by Erk and Src kinases. We propose the following model for the role of the cortactin interaction with N-WASP and WASP in actin reorganization following receptor signaling. Engagement of surface receptors that activate Erk, e.g., G protein-coupled receptors, epidermal growth factor, T-cell receptor, and integrins, results in phosphorylation of cortactin and liberation of SH3-cort from intramolecular interaction with proline-rich regions. The liberated SH3-cort is then free to interact with N-WASP and WASP and could play a role in the recruitment of these proteins to sites of actin polymerization such as lamellipodia and the IS. More importantly, binding of cortactin activates N-WASP- and WASP-mediated actin nucleation. Cortactin, N-WASP, and WASP may also be bridged by the Arp2/3 complex and may synergize in causing actin polymerization. Engagement of the same surface receptors also activates Src kinases, which are anchored to the lipid layer of cell membranes and which accumulate at sites of actin polymerization, e.g., in lamellipodia and in the IS (8, 56). Src phosphorylation of cortactin at these sites inhibits its ability to bind and activate N-WASP and WASP. Furthermore, Src phosphorylation of cortactin decreases its F-actin-binding ability (21), which is important for its activation of the Arp2/3 complex (61). It also further decreases the ability of cortactin to activate the Arp2/3 complex by promoting its binding to myosin light-chain kinase, a key enzyme in the regulation of contractility (11). Finally, Src phosphorylation of cortactin promotes its degradation by calpain (22). This would effectively terminate both N-WASP-dependent and N-WASP-independent cortactin-mediated actin polymerization.
TABLE 1.
Summary of N-WASP binding and activation by cortactin constructs
Immunoprecipitation experiments (Fig. 1F) show no detectable binding of pY421 or pY466 cortactin to N-WASP.
−, none; +, weak; ++, strong; +++, very strong; ++++, robust; ±, very weak.
ND, not done.
Acknowledgments
We acknowledge the generosity of Lewis C. Cantley (Signal Transduction Laboratory, Beth Israel Deaconess Medical Center) for freely sharing his peptide library-based searching algorithm for protein domain interactions. We thank John Hartwig (Brigham and Woman's Hospital) for reagents.
This work was supported by NIH AI35714 and HL-59561 and March of Dimes grants.
REFERENCES
- 1.Andreotti, A. H., S. C. Bunnell, S. Feng, L. J. Berg, and S. L. Schreiber. 1997. Regulatory intramolecular association in a tyrosine kinase of the Tec family. Nature 385:93-97. [DOI] [PubMed] [Google Scholar]
- 2.Bear, J. E., J. F. Rawls, and C. L. Saxe III. 1998. SCAR, a WASP-related protein, isolated as a suppressor of receptor defects in late Dictyostelium development. J. Cell Biol. 142:1325-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowden, E. T., M. Barth, D. Thomas, R. I. Glazer, and S. C. Mueller. 1999. An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene 18:4440-4449. [DOI] [PubMed] [Google Scholar]
- 4.Campbell, D. H., R. L. Sutherland, and R. J. Daly. 1999. Signaling pathways and structural domains required for phosphorylation of EMS1/cortactin. Cancer Res. 59:5376-5385. [PubMed] [Google Scholar]
- 5.Cannon, J. L., C. M. Labno, G. Bosco, A. Seth, M. H. McGavin, K. A. Siminovitch, M. K. Rosen, and J. K. Burkhardt. 2001. Wasp recruitment to the T cell: APC contact site occurs independently of Cdc42 activation. Immunity 15:249-259. [DOI] [PubMed] [Google Scholar]
- 6.Cantarelli, V. V., A. Takahashi, Y. Akeda, K. Nagayama, and T. Honda. 2000. Interaction of enteropathogenic or enterohemorrhagic Escherichia coli with HeLa cells results in translocation of cortactin to the bacterial adherence site. Infect. Immun. 68:382-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlier, M. F., P. Nioche, I. Broutin-L'Hermite, R. Boujemaa, C. Le Clainche, C. Egile, C. Garbay, A. Ducruix, P. Sansonetti, and D. Pantaloni. 2000. GRB2 links signaling to actin assembly by enhancing interaction of neural Wiskott-Aldrich syndrome protein (N-WASp) with actin-related protein (ARP2/3) complex. J. Biol. Chem. 275:21946-21952. [DOI] [PubMed] [Google Scholar]
- 8.Cinek, T., and V. Horejsi. 1992. The nature of large noncovalent complexes containing glycosyl-phosphatidylinositol-anchored membrane glycoproteins and protein tyrosine kinases. J. Immunol. 149:2262-2270. [PubMed] [Google Scholar]
- 9.Derry, J. M., H. D. Ochs, and U. Francke. 1994. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell 79:922-644. [PubMed] [Google Scholar]
- 10.Du, Y., S. A. Weed, W. C. Xiong, T. D. Marshall, and J. T. Parsons. 1998. Identification of a novel cortactin SH3 domain-binding protein and its localization to growth cones of cultured neurons. Mol. Cell. Biol. 18:5838-5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudek, S. M., K. G. Birukov, X. Zhan, and J. G. Garcia. 2002. Novel interaction of cortactin with endothelial cell myosin light chain kinase. Biochem. Biophys. Res. Commun. 298:511-519. [DOI] [PubMed] [Google Scholar]
- 12.Frischknecht, F., S. Cudmore, V. Moreau, I. Reckmann, S. Rottger, and M. Way. 1999. Tyrosine phosphorylation is required for actin-based motility of vaccinia but not Listeria or Shigella. Curr. Biol. 9:89-92. [DOI] [PubMed] [Google Scholar]
- 13.Fukuoka, M., S. Suetsugu, H. Miki, K. Fukami, T. Endo, and T. Takenawa. 2001. A novel neural Wiskott-Aldrich syndrome protein (N-WASP) binding protein, WISH, induces Arp2/3 complex activation independent of Cdc42. J. Cell Biol. 152:471-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallet, C., J. P. Rosa, A. Habib, M. Lebret, S. Levy-Toledano, and J. Maclouf. 1999. Tyrosine phosphorylation of cortactin associated with Syk accompanies thromboxane analogue-induced platelet shape change. J. Biol. Chem. 274:23610-23616. [DOI] [PubMed] [Google Scholar]
- 15.Grakoui, A., S. K. Bromley, C. Sumen, M. M. Davis, A. S. Shaw, P. M. Allen, and M. L. Dustin. 1999. The immunological synapse: a molecular machine controlling T cell activation. Science 285:221-227. [DOI] [PubMed] [Google Scholar]
- 16.Gross, B. S., J. I. Wilde, L. Quek, H. Chapel, D. L. Nelson, and S. P. Watson. 1999. Regulation and function of WASp in platelets by the collagen receptor, glycoprotein VI. Blood 94:4166-4176. [PubMed] [Google Scholar]
- 17.Head, J. A., D. Jiang, M. Li, L. J. Zorn, E. M. Schaefer, J. T. Parsons, and S. A. Weed. 2003. Cortactin tyrosine phosphorylation requires rac1 activity and association with the cortical actin cytoskeleton. Mol. Biol. Cell 14:3216-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgs, H. N., and T. D. Pollard. 2000. Activation by Cdc42 and PIP2 of Wiskott-Aldrich syndrome protein (WASp) stimulates actin nucleation by Arp2/3 complex. J. Cell Biol. 150:1311-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgs, H. N., and T. D. Pollard. 2001. Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu. Rev. Biochem. 70:649-676. [DOI] [PubMed] [Google Scholar]
- 20.Huang, C., J. Liu, C. C. Haudenschild, and X. Zhan. 1998. The role of tyrosine phosphorylation of cortactin in the locomotion of endothelial cells. J. Biol. Chem. 273:25770-25776. [DOI] [PubMed] [Google Scholar]
- 21.Huang, C., Y. Ni, T. Wang, Y. Gao, C. C. Haudenschild, and X. Zhan. 1997. Down-regulation of the filamentous actin cross-linking activity of cortactin by Src-mediated tyrosine phosphorylation. J. Biol. Chem. 272:13911-13915. [DOI] [PubMed] [Google Scholar]
- 22.Huang, C., N. N. Tandon, N. J. Greco, Y. Ni, T. Wang, and X. Zhan. 1997. Proteolysis of platelet cortactin by calpain. J. Biol. Chem. 272:19248-19252. [DOI] [PubMed] [Google Scholar]
- 23.Kawai, S., M. Minegishi, Y. Ohashi, Y. Sasahara, S. Kumaki, T. Konno, H. Miki, J. Derry, S. Nonoyama, T. Miyawaki, K. Horibe, N. Tachibana, E. Kudoh, Y. Yoshimura, Y. Izumikawa, M. Sako, and S. Tsuchiya. 2002. Flow cytometric determination of intracytoplasmic Wiskott-Aldrich syndrome protein in peripheral blood lymphocyte subpopulations. J. Immunol. Methods 260:195-205. [DOI] [PubMed] [Google Scholar]
- 24.Kim, A. S., L. T. Kakalis, N. Abdul-Manan, G. A. Liu, and M. K. Rosen. 2000. Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature 404:151-158. [DOI] [PubMed] [Google Scholar]
- 25.Kim, L., and T. W. Wong. 1998. Growth factor-dependent phosphorylation of the actin-binding protein cortactin is mediated by the cytoplasmic tyrosine kinase FER. J. Biol. Chem. 273:23542-23548. [DOI] [PubMed] [Google Scholar]
- 26.Kinley, A. W., S. A. Weed, A. M. Weaver, A. V. Karginov, E. Bissonette, J. A. Cooper, and J. T. Parsons. 2003. Cortactin interacts with WIP in regulating Arp2/3 activation and membrane protrusion. Curr. Biol. 13:384-393. [DOI] [PubMed] [Google Scholar]
- 27.Kinnunen, T., M. Kaksonen, J. Saarinen, N. Kalkkinen, H. B. Peng, and H. Rauvala. 1998. Cortactin-Src kinase signaling pathway is involved in N-syndecan-dependent neurite outgrowth. J. Biol. Chem. 273:10702-10708. [DOI] [PubMed] [Google Scholar]
- 28.Kitamura, D., H. Kaneko, Y. Miyagoe, T. Ariyasu, and T. Watanabe. 1989. Isolation and characterization of a novel human gene expressed specifically in the cells of hematopoietic lineage. Nucleic Acids Res. 17:9367-9379. [PMC free article] [PubMed] [Google Scholar]
- 29.Kozma, R., S. Ahmed, A. Best, and L. Lim. 1995. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol. Cell. Biol. 15:1942-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kreishman-Deltrick, M., and M. K. Rosen. 2002. Ignition of a cellular machine. Nat. Cell Biol. 4:E31-E33. [DOI] [PubMed] [Google Scholar]
- 31.Lagarkova, M. A., V. E. Boitchenko, A. A. Mescheryakov, U. A. Kashkarova, and S. A. Nedospasov. 2000. Human cortactin as putative cancer antigen. Oncogene 19:5204-5207. [DOI] [PubMed] [Google Scholar]
- 32.Li, Y., M. Tondravi, J. Liu, E. Smith, C. C. Haudenschild, M. Kaczmarek, and X. Zhan. 2001. Cortactin potentiates bone metastasis of breast cancer cells. Cancer Res. 61:6906-6911. [PubMed] [Google Scholar]
- 33.Machesky, L. M., S. J. Atkinson, C. Ampe, J. Vandekerckhove, and T. D. Pollard. 1994. Purification of a cortical complex containing two unconventional actins from Acanthamoeba by affinity chromatography on profilin-agarose. J. Cell Biol. 127:107-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Machesky, L. M., and R. H. Insall. 1998. Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr. Biol. 8:1347-1356. [DOI] [PubMed] [Google Scholar]
- 35.Marchand, J. B., D. A. Kaiser, T. D. Pollard, and H. N. Higgs. 2001. Interaction of WASP/Scar proteins with actin and vertebrate Arp2/3 complex. Nat. Cell Biol. 3:76-82. [DOI] [PubMed] [Google Scholar]
- 36.Martinez-Quiles, N., R. Rohatgi, I. M. Anton, M. Medina, S. P. Saville, H. Miki, H. Yamaguchi, T. Takenawa, J. H. Hartwig, R. S. Geha, and N. Ramesh. 2001. WIP regulates N-WASP-mediated actin polymerization and filopodium formation. Nat. Cell Biol. 3:484-491. [DOI] [PubMed] [Google Scholar]
- 37.Miki, H., K. Miura, and T. Takenawa. 1996. N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J. 15:5326-5335. [PMC free article] [PubMed] [Google Scholar]
- 38.Miki, H., S. Suetsugu, and T. Takenawa. 1998. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 17:6932-6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mizutani, K., H. Miki, H. He, H. Maruta, and T. Takenawa. 2002. Essential role of neural Wiskott-Aldrich syndrome protein in podosome formation and degradation of extracellular matrix in src-transformed fibroblasts. Cancer Res. 62:669-674. [PubMed] [Google Scholar]
- 40.Mullins, R. D., and L. M. Machesky. 2000. Actin assembly mediated by Arp2/3 complex and WASP family proteins. Methods Enzymol. 325:214-237. [DOI] [PubMed] [Google Scholar]
- 41.Nakagawa, H., H. Miki, M. Ito, K. Ohashi, T. Takenawa, and S. Miyamoto. 2001. N-WASP, WAVE and Mena play different roles in the organization of actin cytoskeleton in lamellipodia. J. Cell Sci. 114:1555-1565. [DOI] [PubMed] [Google Scholar]
- 42.Nobes, C. D., and A. Hall. 1995. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81:53-62. [DOI] [PubMed] [Google Scholar]
- 43.Ramesh, N., I. M. Anton, J. H. Hartwig, and R. S. Geha. 1997. WIP, a protein associated with Wiskott-Aldrich syndrome protein, induces actin polymerization and redistribution in lymphoid cells. Proc. Natl. Acad. Sci. USA 94:14671-14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ren, R., B. J. Mayer, P. Cicchetti, and D. Baltimore. 1993. Identification of a ten-amino acid proline-rich SH3 binding site. Science 259:1157-1161. [DOI] [PubMed] [Google Scholar]
- 45.Rivero-Lezcano, O. M., A. Marcilla, J. H. Sameshima, and K. C. Robbins. 1995. Wiskott-Aldrich syndrome protein physically associates with Nck through Src homology 3 domains. Mol. Cell. Biol. 15:5725-5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson, R. C., K. Turbedsky, D. A. Kaiser, J. B. Marchand, H. N. Higgs, S. Choe, and T. D. Pollard. 2001. Crystal structure of Arp2/3 complex. Science 294:1679-1684. [DOI] [PubMed] [Google Scholar]
- 47.Rohatgi, R., H. Y. Ho, and M. W. Kirschner. 2000. Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4,5-bisphosphate. J. Cell Biol. 150:1299-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rohatgi, R., L. Ma, H. Miki, M. Lopez, T. Kirchhausen, T. Takenawa, and M. W. Kirschner. 1999. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97:221-231. [DOI] [PubMed] [Google Scholar]
- 49.Rohatgi, R., P. Nollau, H. Y. Ho, M. W. Kirschner, and B. J. Mayer. 2001. Nck and phosphatidylinositol 4,5-bisphosphate synergistically activate actin polymerization through the N-WASP-Arp2/3 pathway. J. Biol. Chem. 276:26448-26452. [DOI] [PubMed] [Google Scholar]
- 50.Schafer, D. A., S. A. Weed, D. Binns, A. V. Karginov, J. T. Parsons, and J. A. Cooper. 2002. Dynamin2 and cortactin regulate actin assembly and filament organization. Curr. Biol. 12:1852-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Selbach, M., S. Moese, R. Hurwitz, C. R. Hauck, T. F. Meyer, and S. Backert. 2003. The Helicobacter pylori CagA protein induces cortactin dephosphorylation and actin rearrangement by c-Src inactivation. EMBO J. 22:515-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.She, H. Y., S. Rockow, J. Tang, R. Nishimura, E. Y. Skolnik, M. Chen, B. Margolis, and W. Li. 1997. Wiskott-Aldrich syndrome protein is associated with the adapter protein Grb2 and the epidermal growth factor receptor in living cells. Mol. Biol. Cell 8:1709-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Snapper, S. B., F. S. Rosen, E. Mizoguchi, P. Cohen, W. Khan, C. H. Liu, T. L. Hagemann, S. P. Kwan, R. Ferrini, L. Davidson, A. K. Bhan, and F. W. Alt. 1998. Wiskott-Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity 9:81-91. [DOI] [PubMed] [Google Scholar]
- 54.Snapper, S. B., F. Takeshima, I. Anton, C. H. Liu, S. M. Thomas, D. Nguyen, D. Dudley, H. Fraser, D. Purich, M. Lopez-Ilasaca, C. Klein, L. Davidson, R. Bronson, R. C. Mulligan, F. Southwick, R. Geha, M. B. Goldberg, F. S. Rosen, J. H. Hartwig, and F. W. Alt. 2001. N-WASP deficiency reveals distinct pathways for cell surface projections and microbial actin-based motility. Nat. Cell Biol. 3:897-904. [DOI] [PubMed] [Google Scholar]
- 55.Tilghman, R. W., and R. L. Hoover. 2002. The Src-cortactin pathway is required for clustering of E-selectin and ICAM-1 in endothelial cells. FASEB J. 16:1257-1259. [DOI] [PubMed] [Google Scholar]
- 56.Timpson, P., G. E. Jones, M. C. Frame, and V. G. Brunton. 2001. Coordination of cell polarization and migration by the Rho family GTPases requires Src tyrosine kinase activity. Curr. Biol. 11:1836-1846. [DOI] [PubMed] [Google Scholar]
- 57.Uruno, T., J. Liu, P. Zhang, Y. Fan, C. Egile, R. Li, S. C. Mueller, and X. Zhan. 2001. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat. Cell Biol. 3:259-266. [DOI] [PubMed] [Google Scholar]
- 58.Vidal, C., B. Geny, J. Melle, M. Jandrot-Perrus, and M. Fontenay-Roupie. 2002. Cdc42/Rac1-dependent activation of the p21-activated kinase (PAK) regulates human platelet lamellipodia spreading: implication of the cortical-actin binding protein cortactin. Blood 100:4462-4469. [DOI] [PubMed] [Google Scholar]
- 59.Volkman, B. F., K. E. Prehoda, J. A. Scott, F. C. Peterson, and W. A. Lim. 2002. Structure of the N-WASP EVH1 domain-WIP complex: insight into the molecular basis of Wiskott-Aldrich syndrome. Cell 111:565-576. [DOI] [PubMed] [Google Scholar]
- 60.Weaver, A., J. Heuser, A. Karginov, W. Lee, J. Parsons, and J. Cooper. 2002. Interaction of cortactin and N-wasp with arp2/3 complex. Curr. Biol. 12:1270. [DOI] [PubMed] [Google Scholar]
- 61.Weaver, A. M., A. V. Karginov, A. W. Kinley, S. A. Weed, Y. Li, J. T. Parsons, and J. A. Cooper. 2001. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr. Biol. 11:370-374. [DOI] [PubMed] [Google Scholar]
- 62.Weed, S. A., Y. Du, and J. T. Parsons. 1998. Translocation of cortactin to the cell periphery is mediated by the small GTPase Rac1. J. Cell Sci. 111:2433-2443. [DOI] [PubMed] [Google Scholar]
- 63.Weed, S. A., A. V. Karginov, D. A. Schafer, A. M. Weaver, A. W. Kinley, J. A. Cooper, and J. T. Parsons. 2000. Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. J. Cell Biol. 151:29-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weed, S. A., and J. T. Parsons. 2001. Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene 20:6418-6434. [DOI] [PubMed] [Google Scholar]
- 65.Wu, H., and J. T. Parsons. 1993. Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J. Cell Biol. 120:1417-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu, H., A. B. Reynolds, S. B. Kanner, R. R. Vines, and J. T. Parsons. 1991. Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol. Cell. Biol. 11:5113-5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yaffe, M. B., G. G. Leparc, J. Lai, T. Obata, S. Volinia, and L. C. Cantley. 2001. A motif-based profile scanning approach for genome-wide prediction of signaling pathways. Nat. Biotechnol. 19:348-353. [DOI] [PubMed] [Google Scholar]